Abstract
Background: Several strategies to reduce construct stiffness have been proposed to promote secondary bone healing following fracture fixation with locked bridge plating constructs. However, stiffness reduction is typically gained at the cost of construct strength. In the present study, we tested whether a novel strategy for stiffness reduction, termed far cortical locking, can significantly reduce the stiffness of a locked plating construct while retaining its strength.
Methods: Locked plating constructs and far cortical locking constructs were tested in a diaphyseal bridge plating model of the non-osteoporotic femoral diaphysis to determine construct stiffness in axial compression, torsion, and bending. Subsequently, constructs were dynamically loaded until failure in each loading mode to determine construct strength and failure modes. Finally, failure tests were repeated in a validated model of the osteoporotic femoral diaphysis to determine construct strength and failure modes in a worst-case scenario of bridge plating in osteoporotic bone.
Results: Compared with the locked plating constructs, the initial stiffness of far cortical locking constructs was 88% lower in axial compression (p < 0.001), 58% lower in torsion (p < 0.001), and 29% lower in bending (p < 0.001). Compared with the locked plating constructs, the strength of far cortical locking constructs was 7% lower (p = 0.005) and 16% lower (p < 0.001) under axial compression in the non-osteoporotic and osteoporotic diaphysis, respectively. However, far cortical locking constructs were 54% stronger (p < 0.001) and 9% stronger (p = 0.04) under torsion and 21% stronger (p < 0.001) and 20% stronger (p = 0.02) under bending than locked plating constructs in the non-osteoporotic and osteoporotic diaphysis, respectively. Within the initial stiffness range, far cortical locking constructs generated nearly parallel interfragmentary motion. Locked plating constructs generated significantly less motion at the near cortex adjacent to the plate than at the far cortex (p < 0.01).
Conclusions: Far cortical locking significantly reduces the axial stiffness of a locked plating construct. This gain in flexibility causes only a modest reduction in axial strength and increased torsional and bending strength.
Clinical Relevance: Far cortical locking may provide a novel bridge plating strategy to enhance interfragmentary motion for the promotion of secondary bone healing while retaining sufficient construct strength.
The stiffness of a fixation construct is a principal determinant of fracture-site motion and thereby affects the mechanism and progression by which a fracture heals1. Traditionally, conventional compression plates have been used to promote primary bone healing by delivering absolute stability at the fracture site2. The introduction of locking plates has improved the fixation strength of plate constructs, expanding their indications to bridge plating of comminuted fractures3-5. Furthermore, locking plates allow for the use of biological fixation techniques that emphasize preservation of blood supply and functional reduction over anatomic reduction and interfragmentary compression. However, in the absence of anatomic reduction and interfragmentary compression, locked plating constructs rely on secondary bone healing6,7. Secondary bone healing is induced by interfragmentary motion in the millimeter range1,8,9 and can be enhanced by passive or active dynamization10-12. Clinically, secondary bone healing is expected to occur in association with the use of external fixators and intramedullary nails. While locked plating constructs have been termed internal fixators13, they can be severalfold stiffer than external fixators14. Furthermore, they can be as stiff as conventional plating constructs15 designed to induce primary bone healing, which requires interfragmentary motion to remain <0.15 mm16. The relatively high stiffness of locked constructs may therefore suppress interfragmentary motion to a level insufficient for optimal promotion of secondary bone healing6,17,18. This theoretical concern is supported by early case studies on locked plating that have described deficient callus formation, delayed union, and nonunion with late hardware failure5,19,20.
On the basis of these theoretical and clinically emerging concerns, several strategies to decrease the stiffness of locked plating constructs have been investigated21-23. These strategies include decreasing the plate thickness, increasing the plate elevation, and increasing the plate span. While these strategies are effective for reducing the stiffness of locked plating constructs to varying degrees, they also reduce their strength.
The present study investigated a novel strategy, termed far cortical locking, designed to reduce the stiffness of locked plating constructs while retaining construct strength. In far cortical locking, locking screws with a reduced midshaft diameter provide unicortical fixation in the far cortex of the diaphysis without being rigidly fixed in the near cortex underlying the plate. The middle part of the screw shaft decreases the stiffness of the plating construct by acting as an elastic cantilever beam, similar to a half-pin of an external fixator.
The present study tested the hypothesis that far cortical locking can significantly reduce the stiffness of a locked plating construct while retaining its strength. Such a less-stiff yet strong far cortical locking construct potentially could enhance secondary bone healing by promoting early interfragmentary motion.
Materials and Methods
Locked plating constructs and far cortical locking constructs were tested in a diaphyseal bridge plating configuration under axial compression, torsion, and bending. First, the stiffness of locked plating and far cortical locking constructs was determined for each principal loading mode in surrogates of the non-osteoporotic femoral diaphysis. Subsequently, constructs were tested to failure in each loading mode to determine their strength and failure modes. Finally, failure tests were repeated in a validated model of the osteoporotic femoral diaphysis to determine construct strength and failure modes in a worst-case scenario of bridge plating in osteoporotic bone.
Implants
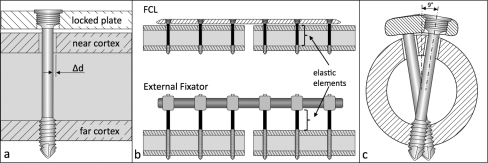
Generic locked plating and far cortical locking implants were designed to resemble standard broad 4.5-mm locking plates and screws. Plates were 17.5 mm wide and 200 mm long and had eleven holes with a space of 18 mm between holes. Locking screws had a 4.5-mm-diameter bone thread with 1-mm pitch and a four-fluted self-tapping feature. Far cortical locking screws for unicortical fixation in the far cortex had a smooth screw shaft with a 3.2-mm diameter to bypass the near cortex, allowing for elastic cantilever bending of the screw shaft within a controlled motion envelope in the near cortex (Fig. 1, a). Analogous to external fixator pins, this feature enabled far cortical locking constructs to derive a low stiffness by elastic bending of screw shafts (Fig. 1, b). Under elevated axial loading of the far cortical locking construct, contact between the screw shaft and the near cortex provided additional support and prevented far cortical locking screw shaft bending beyond the elastic range. To compensate for the lower bending strength of far cortical locking screws caused by the shaft diameter reduction, far cortical locking screws were arranged in a staggered 9° converging pattern (Fig. 1, c). All other dimensions of the far cortical locking implants were identical to those of the locked plating implants. Implants were custom manufactured from surgical grade titanium alloy (Ti-6Al-4V) by a company specializing in the production of orthopaedic implants (Thortex, Portland, Oregon). Locked plating and far cortical locking constructs were evaluated in a standard bridge plating configuration in femoral diaphysis surrogates with a 10-mm fracture gap. Plates were applied with three screws, which were placed in the first, third, and fifth holes from the fracture site. All screws were tightened to 4 Nm with the plate at 1 mm of elevation from the surrogate surface with use of temporary spacers to simulate biological fixation with preservation of periosteal perfusion15. One hole was left empty over the fracture gap, yielding a plate span of 36 mm that was bridging the gap.
Fig. 1.
a: Illustration depicting the far cortical locking screw for unicortical fixation in the far cortex, enabling elastic flexion of the screw shaft within the motion envelope (Δd) in the near cortex. b: Mechanically, the far cortical locking (FCL) construct functions as an internal fixator that derives axial flexibility by cantilever bending of the far cortical locking screw shafts similar to an external fixator that derives elasticity from fixation pin flexion. c: A staggered and converging far cortical locking screw arrangement was implemented to improve construct strength in torsion.
Specimens
Implants were evaluated in surrogate specimens of the femoral diaphysis to minimize interspecimen variability. For implant evaluation in non-osteoporotic bone, cylindrical bone surrogates with a diameter of 27 mm and a wall thickness of 7 mm were manufactured with the same material and dimensions as the diaphysis of the validated medium-size fourth-generation composite Sawbones femur (#3403; Pacific Research Laboratories, Vashon, Washington)24. For the evaluation of implants in weak bone, a validated model of the osteoporotic femoral diaphysis was used25. This model consisted of a 27-mm-diameter and 2-mm-thick cortex made of reinforced epoxy and a trabecular core machined from 10 pcf (0.16 g/cm3) solid rigid polyurethane foam (Pacific Research Laboratories). Previous research demonstrated that five structural properties of this bone surrogate (torsional rigidity and strength, bending rigidity and strength, and screw pull-out strength) matched the lower 16% of the cumulative range reported for cadaver femora25. Therefore, this osteoporotic bone surrogate reflected the diminished structural properties seen in osteoporotic femora.
Loading
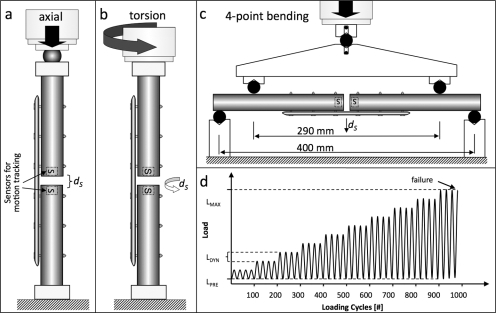
Locked plating and far cortical locking constructs were tested in axial compression, torsion, and bending with a biaxial materials testing system (Instron 8874; Instron, Canton, Massachusetts) (Fig. 2). Both constructs were tested to failure under each loading mode in five non-osteoporotic and five osteoporotic bone specimens, requiring a total of sixty test specimens. Axial compression was applied through a spherical bearing proximally while the distal end of the specimen was rigidly mounted to the load cell to replicate the axial loading configurations in previous studies (Fig. 2, a)15,26. Torsion was applied around the diaphyseal shaft axis (Fig. 2, b). Bending was applied under four-point bending to generate a constant bending moment over the entire plate length (Fig. 2, c). The upper and lower cylindrical supports were separated by 290 and 400 mm, respectively. The plate was located on the tension side to induce bending in a gap-closing mode.
Fig. 2.
a, b, and c: Construct stiffness and strength were evaluated under three loading conditions: axial compression (a), torsion (b), and four-point bending in a gap-closing direction (c). Motion-tracking sensors (S) captured subsidence (ds). d: A progressive dynamic loading protocol was used to ensure that construct failure was attained for each construct and loading mode within a reasonable number of load cycles (<10,000 cycles). After application of a static pre-load (LPRE), dynamic loading (LDYN) was applied and was increased until constructs failed at peak load LMAX.
First, construct stiffness in non-osteoporotic bone surrogates was assessed under axial compression, torsion, and bending by loading to 1 kN, 10 Nm, and 10 Nm, respectively. In addition to actuator displacement, interfragmentary motion under axial compression was recorded at the near and far cortices with use of two digital calipers with 0.01-mm resolution. Subsequently, construct strength was determined by progressive dynamic loading to failure (Fig. 2, d)26,27. After the application of a static preload (LPRE), sinusoidal loading with a load amplitude of LDYN was applied at 2 Hz. Every 100 loading cycles, this load amplitude was increased stepwise by LDYN until construct failure occurred. For axial compression, torsion, and bending, preloads (LPRE) of 50 N, 1 Nm, and 1 Nm and stepwise load amplitudes (LDYN) of 100 N, 1 Nm, and 1 Nm were selected, respectively. This stepwise load increase enabled dynamic loading to failure while ensuring that failure was attained for each construct within a reasonable number of load cycles (<10,000)27.
Construct failure was defined either by catastrophic fracture or by a subsidence threshold at the fracture site, whichever occurred first28,29. Subsidence (dS) represents the nonrecoverable collapse at the fracture site after load removal and is caused by implant bending or implant loosening. A dS threshold of 1 mm in compression, 5° in torsion29, and 1 mm in bending was deemed indicative of the onset of construct failure in the absence of a catastrophic fracture. Subsidence by 5° nominally correlated with a 1-mm shear displacement between cortices at the fracture site. Subsidence was assessed with two miniature electromagnetic motion sensors (pcBIRD; Ascension Technology, Burlington, Vermont). The sensors were centered in the medullary canal at each side of the fracture gap and recorded the motion of the bone ends at the fracture site in six degrees of freedom with a resolution of 0.1 mm and 0.1° after filtering of raw data acquired at a 100-Hz sampling rate. To eliminate errors in electromagnetic motion sensing due to interference from ferromagnetic objects, all testing components in the vicinity of the test specimen were machined from nonmagnetic materials.
Outcome Evaluation
The performance of the locked plating and far cortical locking constructs was described by their axial, torsional, and bending stiffnesses, failure strengths, and failure mechanisms. Construct stiffness was calculated from load-displacement data. Axial stiffness was calculated by dividing the axial load amplitude by the actuator displacement amplitude. Torsional stiffness was calculated by dividing the torsion amplitude by the amplitude of actuator rotation (α) around the diaphyseal axis. Torsional stiffness was multiplied by the unsupported specimen length to derive torsional rigidity. Bending stiffness was expressed in terms of flexural rigidity as EI = Fa2 (3l -4a)/12y, where F is the total applied force, l is the distance between the lower supports (400 mm), a is the distance between the lower and upper supports (55 mm), and y is the displacement of the upper supports. Failure strength was defined as the peak load (LMAX) during progressive dynamic loading to failure under each loading mode. Failure modes were visually analyzed for the presence of hardware failure, fixation failure, and bone fracture.
For statistical analysis, the stiffness and strength results were compared between the far cortical locking and locked plating groups individually for each loading mode. For axial compression, interfragmentary motion results at the near and far cortices were also compared. Two-tailed, unpaired Student t tests at a level of significance of α = 0.05 were used to detect significant differences.
Source of Funding
Financial support for salaries and supplies for the present study was provided by the National Institutes of Health (NIH/NIAMS grant R21 AR053611).
Results
Construct Stiffness (Table I)
TABLE I.
Stiffness and Strength of Locked Plating and Far Cortical Locking Constructs
| Locked Plating* | Far Cortical Locking*† | P Value‡ | |
|---|---|---|---|
| Stiffness (strong bone) | |||
| Axial stiffness (kN/mm) | 2.9 ± 0.13 | 0.36 ± 0.05/2.26 ± 0.08 | <0.001/<0.001 |
| Torsional rigidity (Nm2/deg) | 0.40 ± 0.03 | 0.17 ± 0.04/0.32 ± 0.01 | <0.001/<0.001 |
| Bending rigidity (Nm2) | 82.9 ± 1.96 | 59.0 ± 1.3/68.1 ± 3.3 | <0.001/<0.001 |
| Strength (strong bone) | |||
| Axial (kN) | 5.9 ± 0.2 | 5.5 ± 0.2 | 0.005 |
| Torsion (Nm) | 19.8 ± 1.1 | 30.4 ± 1.5 | <0.001 |
| Bending (Nm) | 75.0 ± 3.1 | 90.8 ± 5.0 | <0.001 |
| Strength (osteoporotic bone) | |||
| Axial (kN) | 4.4 ± 0.1 | 3.7 ± 0.2 | <0.001 |
| Torsion (Nm) | 19.6 ± 1.0 | 21.3 ± 1.1 | 0.036 |
| Bending (Nm) | 30.4 ± 3.4 | 36.5 ± 3.2 | 0.02 |
N = 5 for each testing group. The values are given as the mean and the standard deviation.
The stiffness data are given as the initial value followed by the secondary value.
The first p value pertains to the comparison between the initial far cortical locking value and the locked plating value, and the second p value pertains to the comparison between the secondary far cortical locking value and the locked plating value.
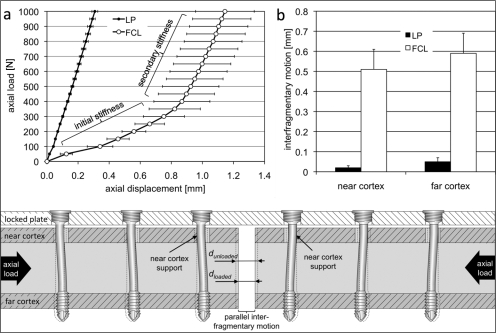
The locked plating construct had a nominally constant stiffness over the elastic loading range, whereas the far cortical locking construct exhibited a biphasic stiffness profile with an initial stiffness and a secondary stiffness (Fig. 3, a). The initial stiffness of the far cortical locking construct increased to a higher secondary stiffness for axial loading above 400 N. Axial loading above 400 N induced near-cortex contact of the far cortical locking screw shaft and provided the additional structural support responsible for the increased secondary stiffness. Within the initial stiffness range of the far cortical locking constructs, 200 N of axial loading induced nearly parallel motion at the fracture site, with similar displacement magnitudes at the near cortex (0.51 ± 0.08 mm) and the far cortex (0.59 ± 0.10 mm) (p = 0.24) (Fig. 3, b). In the locked plating constructs, the corresponding motion was significantly smaller at the near cortex (0.02 ± 0.01 mm) than at the far cortex (0.05 ± 0.02 mm) (p < 0.01).
Fig. 3.
Stiffness comparison between locked plating (LP) and far cortical locking (FCL) constructs. a: Far cortical locking constructs exhibited a biphasic stiffness profile. In axial loading, far cortical locking constructs had a low initial stiffness within the near cortex motion envelope that allowed for approximately 0.8 mm of axial motion before reaching the secondary stiffness due to near-cortex support. b: At 200 N of loading, the initial stiffness of far cortical locking constructs induced comparable amounts of interfragmentary motion at the near and the far cortex. This fracture-site motion was one order of magnitude greater than that in locked plating constructs. The cross-sectional view of a far cortical locking construct at the bottom of the figure illustrates elastic deformation of far cortical locking screws and the resulting parallel interfragmentary motion.
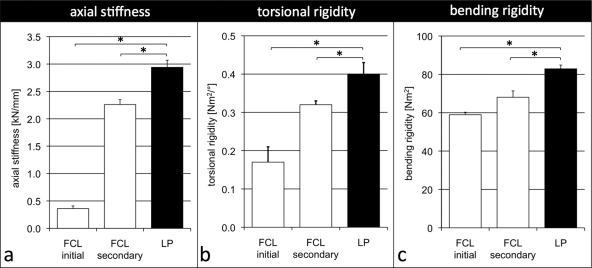
In axial compression, the initial stiffness of the far cortical locking construct was 88% lower than that of the locked plating construct (0.36 ± 0.05 compared with 2.94 ± 0.13 kN/mm; p < 0.001) (Fig. 4, a). For axial loads of >400 N, the secondary stiffness of the far cortical locking construct was 2.26 ± 0.08 kN/mm and remained 22% below that of the locked plating construct (p < 0.001). In torsion, the initial torsional rigidity of the far cortical locking construct was 58% lower than the torsional rigidity of the locked plating construct (0.17 ± 0.04 compared with 0.40 ± 0.03 Nm2/deg; p < 0.001) (Fig. 4, b). For torsion of >1 Nm, the secondary rigidity of the far cortical locking construct increased to 0.32 ± 0.01 Nm2/deg and remained 20% below that of the locked plating construct (p < 0.001). In bending, the initial bending rigidity of the far cortical locking construct was 29% lower than the bending rigidity of the locked plating construct (59.0 ± 1.3 compared with 82.9 ± 2.0 Nm2; p < 0.001) (Fig. 4, c). For bending moments of >1 Nm, the secondary rigidity of far cortical locking constructs increased to 68.1 ± 3.3 Nm2 and remained 18% below that of the locked plating construct (p < 0.001).
Fig. 4.
The initial stiffness of far cortical locking (FCL) constructs was 88% lower in axial compression (a), 58% lower in torsion (b), and 29% lower in bending (c) compared with locked plating (LP) constructs. At elevated loading, the far cortical locking construct stiffness increased to within 22%, 20%, and 18% of the locked plating construct stiffness in compression, torsion, and bending, respectively. *Significant (p < 0.001).
Construct Strength in the Non-Osteoporotic Diaphysis (Table I)
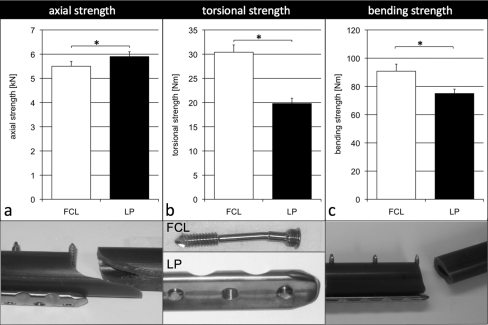
In axial compression, the far cortical locking construct was 6.8% weaker than the locked plating construct (p = 0.005) (Fig. 5, a). Both constructs failed as a result of fracture of the diaphysis through the screw hole at the plate end. After fracture, far cortical locking constructs showed screw bending in three specimens and screw breakage and plate bending in two specimens. Locked plating constructs showed no hardware failure in two specimens and screw bending and plate bending in three specimens. In torsion, the far cortical locking construct was 54% stronger than the locked plating construct (p < 0.001) (Fig. 5, b). Far cortical locking constructs failed as a result of subsidence due to screw shaft bending. Locked plating constructs failed as a result of screw breakage between the elevated plate and the bone due to repetitive screw bending during cyclic torsion. In bending, the far cortical locking construct was 21% stronger than the locked plating construct (p < 0.001) (Fig. 5, c). Both constructs failed as a result of fracture through the screw hole at the plate end. After fracture, all locked plating specimens and four of five far cortical locking specimens showed plate bending.
Fig. 5.
In the non-osteoporotic diaphysis, the strength of the far cortical locking (FCL) constructs in axial compression (a) was 7% less than that of locked plating (LP) constructs. In torsion (b) and bending (c), far cortical locking constructs were 54% and 21% stronger than locked plating constructs, respectively. In axial compression, both constructs failed as a result of fracture of the diaphysis through the screw hole at the plate end. In torsion, far cortical locking constructs failed as a result of subsidence due to screw shaft bending whereas locked plating constructs failed as a result of screw breakage between the plate and the bone. In bending, both constructs failed as a result of fracture through the screw hole at the plate end. *Significant (p ≤ 0.005).
Construct Strength in the Osteoporotic Diaphysis (Table I)
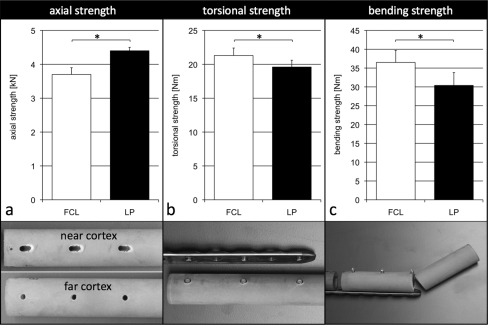
In axial compression, the far cortical locking construct was 16% weaker than the locked plating construct (p < 0.001) (Fig. 6, a). Both constructs failed as a result of subsidence due to screw bending and migration in the near cortex. In torsion, the far cortical locking construct was 9% stronger than the locked plating construct (p = 0.036) (Fig. 6, b). Far cortical locking constructs failed as a result of subsidence due to screw shaft bending. Locked plating constructs failed as a result of screw breakage between the elevated plate and the bone. In bending, the far cortical locking construct was 20% stronger than the locked plating construct (p = 0.02) (Fig. 6, c). Both constructs failed as a result of fracture through the screw hole at the plate end in the absence of hardware bending or breakage.
Fig. 6.
In the osteoporotic diaphysis, the strength of far cortical locking (FCL) constructs in axial compression (a) was 16% less than that of locked plating (LP) constructs. In torsion (b) and bending (c), far cortical locking constructs were 9% and 20% stronger than locked plating constructs, respectively. In axial compression, both constructs failed as a result of subsidence due to screw bending and migration in the near cortex. In torsion, far cortical locking constructs failed as a result of subsidence due to screw shaft bending and locked plating constructs failed as a result of screw breakage between the plate and the bone. In bending, both constructs failed as a result of fracture through the screw hole at the plate end. *Significant (p ≤ 0.05).
Discussion
The results of the present study support the hypothesis that far cortical locking can significantly reduce the stiffness of a locked plating construct while retaining its strength. Stiffness reduction was most pronounced under axial loading. The axial stiffness of the locked plating construct (2.9 kN/mm) was comparable with that reported for broad 4.5-mm conventional plating constructs (2.6 to 3.2 kN/mm) and locked plating constructs (2.1 to 2.7 kN/mm) tested under similar loading conditions and bridge plating configurations15. In contrast, the initial axial stiffness of the far cortical locking construct was 0.36 kN/mm, 88% lower than that of the locked plating construct and comparable with that of an external monolateral fixator, reported to be in the range of 0.05 to 0.4 kN/mm30-32.
The stiffness reduction provided by far cortical locking may be desirable for bridge plating osteosynthesis, which relies on secondary, not primary, bone healing6,33. Secondary bone healing requires flexible fixation7 and relative stability34 to enable interfragmentary motion to stimulate callus formation. While locked plating constructs have been referred to as “internal fixators,” the screw lengths for locked plates are ten to fifteen times shorter than external fixator pins, greatly increasing construct rigidity6. Therefore, locking plates are believed to act as extremely rigid internal fixators that could cause nonunions because of their high stiffness and close proximity to the bone6,17,18. This theoretical concern has been supported by in vivo studies documenting improved fracture-healing with less rigid fixators35 and plates36,37 and by a recent systematic review of twenty-nine case series of supracondylar femoral fractures that demonstrated a 3.5-fold increase in the rate of nonunions associated with locking plates (5.3%) as compared with intramedullary nailing (1.5%)38.
The stiffness of locked plating constructs may be reduced by increasing the plate span or by plate elevation21-23. However, the reported efficacy in terms of stiffness reduction is inconsistent and is gained at the cost of construct strength. Stoffel et al. reported that increasing the plate span by omitting one screw hole on either side of the fracture made a locked plating construct (4.5 mm titanium LCP; Synthes, Paoli, Pennsylvania) almost twice as flexible in both compression and torsion but also led to a 33% reduction in strength under axial compression23. In contrast, Field et al. reported that omitting two screws proximal and distal to the fracture had no significant effect on either bending or torsional stiffness of a conventional plate construct (4.5 mm DCP; Synthes) in a comparable bridge plating configuration39. Alternatively, increasing the plate elevation from 2 to 6 mm has been reported to yield a 10% to 15% decrease in both axial and torsional rigidity23. However, 5 mm of plate elevation decreased construct strength in axial compression by 63%21.
The far cortical locking construct achieved flexible fixation through elastic cantilever bending of the far cortical locking screw shaft, similar to external fixators that derive flexibility from elastic deformation of fixation pins. With elevated loading, the far cortical locking shaft contacted the near cortex, providing a sixfold increase in construct stiffness for progressive stabilization of the fracture site. This biphasic stiffness profile resembles the nonlinear behavior of Ilizarov fixators that become progressively stiffer for increasing loads. Caja et al. reported an axial stiffness of approximately 0.05 kN/mm for Ilizarov fixators at loads of <200 N that increased to >0.14 kN/mm for loads of >800 N30. The benefit of a low initial stiffness has been supported by the clinical success of the Ilizarov method40 and by the original work by Goodship and Kenwright9. Those investigators found that a decrease in fixation stiffness from 0.7 to 0.5 kN/mm caused a significant increase in the rate of fracture-healing in a sheep model.
Low initial stiffness allows fracture-site motion in the early postoperative phase under reduced weight-bearing conditions41. In this low-stiffness range, the far cortical locking construct delivered similar axial motion at the near and far cortices of the fracture gap. Assuming that 200-N loading is representative of toe-touch weight-bearing recommended for the immediate postoperative period42, the far cortical locking construct delivered interfragmentary motion of between 0.51 mm (near cortex) and 0.59 mm (far cortex). The amount of interfragmentary motion attainable under the initial far cortical locking stiffness was limited to approximately 0.8 mm by the near-cortex motion envelope and was within the 0.2 to 1-mm stimulus range of axial interfragmentary motion established for the promotion of secondary bone healing1,9,12,43.
In torsion and bending, the stiffness reduction of the far cortical locking construct relative to the locked plating construct was less pronounced than in axial compression. Nevertheless, the 20% lower torsional rigidity of the far cortical locking construct will increase shear displacement at the fracture site. The effect of interfragmentary shear on fracture-healing remains controversial. Augat et al. found that large shear movements of 1.5 mm delayed healing relative to axial movement of the same magnitude in a 3-mm osteotomy gap44. Others found that torsion-induced shear movement stimulated callus formation and improved strength as compared with rigid fixation45,46.
The present study also investigated the strength of far cortical locking constructs relative to locked plating constructs in both non-osteoporotic and osteoporotic bone as fixation strength and failure modes are highly affected by bone quality47. Under axial compression, the far cortical locking construct was weaker than the locked plating construct in both the non-osteoporotic and the osteoporotic diaphysis. However, the axial strength of the far cortical locking construct in osteoporotic bone (3.7 kN) remained above the strength reported for broad 4.5-mm periarticular nonlocked plates (1.9 kN) and locked plates (2.6 kN) tested in human cadaver femora under comparable loading conditions15.
In torsion, the far cortical locking construct was stronger than the locked plating construct in both the non-osteoporotic and the osteoporotic diaphysis. In the locked plating construct, torsion-induced toggle of the elevated plate around its plane of fixation resulted in fatigue fracture of the screw shaft between the plate and bone at 19.8 ± 1.1 Nm. This failure mode correlated with the findings of a previous study in which locking plates applied to synthetic femora at 1 mm of elevation failed in torsion as a result of screw breakage at approximately 20 Nm15. This failure mode was prevented in far cortical locking constructs by multiplanar fixation with a staggered far cortical locking screw arrangement. However, the present findings are limited to locked plating with plate elevation. Locked plating without plate elevation can improve torsional construct strength48 but may also adversely affect periosteal perfusion and biological fixation targeted with locked plating.
In bending, far cortical locking and locked plating constructs failed by fracture at the plate end, whereby far cortical locking constructs tolerated a higher load to failure in both the non-osteoporotic and the osteoporotic diaphysis. The superior bending strength of far cortical locking constructs relative to locked plating constructs is likely due to improved load distribution by elastic far cortical locking screw fixation that can reduce stress concentrations and subsequent fracture at the plate end. Fracture at the plate end is a well-recognized complication associated with conventional plate fixation in osteoporotic bone with an incidence rate of 1% to 3%49,50. In bending tests of plate constructs applied to the cadaveric tibial diaphysis, all constructs failed as a result of a transverse fracture at the end screw51. A recent case series on locked plating demonstrated a 2.6% incidence rate of fractures at the plate end20. The results of the present study suggest that far cortical locking could theoretically reduce this fracture risk in addition to providing less-rigid internal fixation to promote secondary bone healing.
The results of the present study are limited to the use of surrogate specimens. Validated synthetic bone models were employed to extract relative differences between constructs under highly reproducible test conditions24,25. Given the large deviation in structural properties of cadaver specimens and the considerable number of experimental variables under investigation, this comprehensive evaluation benefited from the use of reproducible surrogates.
Far cortical locking performance was evaluated in the femoral diaphysis, which accommodated far cortical locking screws of sufficient length to achieve the desired stiffness reduction while retaining sufficient strength. Scaling the far cortical locking concept to smaller-diameter bones may require a reduction of the far cortical locking screw diameter that could severely compromise screw strength.
The results represent the performance of implants made of titanium alloy. The use of stainless steel implants would likely increase the stiffness of locked plating and far cortical locking constructs because of the higher elastic modulus of stainless steel as compared with titanium.
Stiffness and strength results were investigated individually for the principal forces that a fracture construct might experience, namely, axial loading, torsion, and bending. This approach was vital to develop a comprehensive understanding of the relative benefits and weaknesses of far cortical locking constructs under specific loading modes. In clinical applications, fracture constructs are loaded with some combination of these forces, making the true failure mechanism more complex than described in the present study.
While insufficient interfragmentary motion can suppress callus formation, excessive interfragmentary motion can lead to hypertrophic callus formation and nonunion52. To be clinically effective, far cortical locking has to be dimensioned to target the appropriate stiffness range for secondary fracture-healing. Therefore, despite the theoretical benefits of the biphasic far cortical locking stiffness profile, future in vivo studies will be required to evaluate whether appropriately configured far cortical locking constructs can better promote formation and maturation of a fracture callus than contemporary locking plates.
In conclusion, the far cortical locking construct was significantly less stiff than the locked plating construct. Its axial stiffness was comparable with that of an external fixator, and its biphasic stiffness resembled the progressive stiffening behavior characteristic of Ilizarov fixators. It delivered nearly parallel fracture site motion under initial axial compression. Furthermore, the far cortical locking construct retained at least 80% of the strength of the locked plating construct in axial loading and was stronger than the locked plating construct in bending and torsion. Therefore, far cortical locking may provide an attractive alternative to reduce the stiffness while retaining the strength of bridge plating constructs when interfragmentary motion is desired to promote secondary bone healing. Additional studies are required to assess far cortical locking performance in combined loading modes and to determine if far cortical locking constructs effectively promote secondary bone healing in vivo. 
Acknowledgments
Note: The authors thank Sebastian Boldhaus for his technical support.
Disclosure: In support of their research for or preparation of this work, one or more of the authors received, in any one year, outside funding or grants in excess of $10,000 from the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (R21 AR053611). In addition, one or more of the authors or a member of his or her immediate family received, in any one year, payments or other benefits in excess of $10,000 or a commitment or agreement to provide such benefits from commercial entities (Synthes CMF and Zimmer).
Investigation performed at Legacy Biomechanics Laboratory, Portland, Oregon
References
- 1.Claes LE, Heigele CA, Neidlinger-Wilke C, Kaspar D, Seidl W, Margevicius KJ, Augat P. Effects of mechanical factors on the fracture healing process. Clin Orthop Relat Res. 1998;355 Suppl:S132-47. [DOI] [PubMed]
- 2.Perren SM, Allgöwer M, Cordey J, Russenberger M. Developments of compression plate techniques for internal fixation of fractures. Prog Surg. 1973;12:152-79. [DOI] [PubMed] [Google Scholar]
- 3.Kolodziej P, Lee FS, Patel A, Kassab SS, Shen KL, Yang KH, Mast JW. Biomechanical evaluation of the schuhli nut. Clin Orthop Relat Res. 1998;347:79-85. [PubMed] [Google Scholar]
- 4.Ramotowski W, Granowski R. Zespol. An original method of stable osteosynthesis. Clin Orthop Relat Res. 1991;272:67-75. [PubMed] [Google Scholar]
- 5.Ring D, Kloen P, Kadzielski J, Helfet D, Jupiter JB. Locking compression plates for osteoporotic nonunions of the diaphyseal humerus. Clin Orthop Relat Res. 2004;425:50-4. [DOI] [PubMed] [Google Scholar]
- 6.Egol KA, Kubiak EN, Fulkerson E, Kummer FJ, Koval KJ. Biomechanics of locked plates and screws. J Orthop Trauma. 2004;18:488-93. [DOI] [PubMed] [Google Scholar]
- 7.Perren SM. Backgrounds of the technology of internal fixators. Injury. 2003;34 Suppl 2:B1-3. [DOI] [PubMed]
- 8.Duda GN, Sollmann M, Sporrer S, Hoffmann JE, Kassi JP, Khodadadyan C, Raschke M. Interfragmentary motion in tibial osteotomies stabilized with ring fixators. Clin Orthop Relat Res. 2002;396:163-72. [DOI] [PubMed] [Google Scholar]
- 9.Goodship AE, Kenwright J. The influence of induced micromovement upon the healing of experimental tibial fractures. J Bone Joint Surg Br. 1985;67:650-5. [DOI] [PubMed] [Google Scholar]
- 10.Claes LE, Wilke HJ, Augat P, Rubenacker S, Margevicius KJ. Effect of dynamization on gap healing of diaphyseal fractures under external fixation. Clin Biomech (Bristol, Avon). 1995;10:227-34. [DOI] [PubMed] [Google Scholar]
- 11.Hente R, Fuchtmeier B, Schlegel U, Ernstberger A, Perren SM. The influence of cyclic compression and distraction on the healing of experimental tibial fractures. J Orthop Res. 2004;22:709-15. [DOI] [PubMed] [Google Scholar]
- 12.Kershaw CJ, Cunningham JL, Kenwright J. Tibial external fixation, weight bearing, and fracture movement. Clin Orthop Relat Res. 1993;293:28-36. [PubMed] [Google Scholar]
- 13.Wagner M. General principles for the clinical use of the LCP. Injury. 2003;34 Suppl 2:B31-42. [DOI] [PubMed] [Google Scholar]
- 14.Peindl RD, Zura RD, Vincent A, Coley ER, Bosse MJ, Sims SH. Unstable proximal extraarticular tibia fractures: a biomechanical evaluation of four methods of fixation. J Orthop Trauma. 2004;18:540-5. [DOI] [PubMed] [Google Scholar]
- 15.Stoffel K, Booth G, Rohrl SM, Kuster M. A comparison of conventional versus locking plates in intraarticular calcaneus fractures: a biomechanical study in human cadavers. Clin Biomech (Bristol, Avon). 2007;22:100-5. [DOI] [PubMed] [Google Scholar]
- 16.Perren SM. Physical and biological aspects of fracture healing with special reference to internal fixation. Clin Orthop Relat Res. 1979;138:175-96. [PubMed] [Google Scholar]
- 17.Kubiak EN, Fulkerson E, Strauss E, Egol KA. The evolution of locked plates. J Bone Joint Surg Am. 2006;88 Suppl 4:189-200. [DOI] [PubMed] [Google Scholar]
- 18.Uhthoff HK, Poitras P, Backman DS. Internal plate fixation of fractures: short history and recent developments. J Orthop Sci. 2006;11:118-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Button G, Wolinsky P, Hak D. Failure of less invasive stabilization system plates in the distal femur: a report of four cases. J Orthop Trauma. 2004;18:565-70. [DOI] [PubMed] [Google Scholar]
- 20.Sommer C, Gautier E, Müller M, Helfet DL, Wagner M. First clinical results of the Locking Compression Plate (LCP). Injury. 2003;34 Suppl 2:B43-54. [DOI] [PubMed] [Google Scholar]
- 21.Ahmad M, Nanda R, Bajwa AS, Candal-Couto J, Green S, Hui AC. Biomechanical testing of the locking compression plate: when does the distance between bone and implant significantly reduce construct stability? Injury. 2007;38:358-64. [DOI] [PubMed] [Google Scholar]
- 22.Kowalski MJ, Schemitsch EH, Harrington RM, Chapman JR, Swiontkowski MF. A comparative biomechanical evaluation of a noncontacting plate and currently used devices for tibial fixation. J Trauma. 1996;40:5-9. [DOI] [PubMed] [Google Scholar]
- 23.Stoffel K, Dieter U, Stachowiak G, Gächter A, Kuster MS. Biomechanical testing of the LCP—how can stability in locked internal fixators be controlled? Injury. 2003;34 Suppl 2:B11-9. [DOI] [PubMed] [Google Scholar]
- 24.Chong AC, Miller F, Buxton M, Friis EA. Fracture toughness and fatigue crack propagation rate of short fiber reinforced epoxy composites for analogue cortical bone. J Biomech Eng. 2007;129:487-93. [DOI] [PubMed] [Google Scholar]
- 25.Sommers MB, Fitzpatrick DC, Madey SM, Vande Zanderschulp C, Bottlang M. A surrogate long-bone model with osteoporotic material properties for biomechanical testing of fracture implants. J Biomech. 2007;40:3297-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marti A, Fankhauser C, Frenk A, Cordey J, Gasser B. Biomechanical evaluation of the less invasive stabilization system for the internal fixation of distal femur fractures. J Orthop Trauma. 2001;15:482-7. [DOI] [PubMed] [Google Scholar]
- 27.Zlowodzki M, Williamson S, Cole PA, Zardiackas LD, Kregor PJ. Biomechanical evaluation of the less invasive stabilization system, angled blade plate, and retrograde intramedullary nail for the internal fixation of distal femur fractures. J Orthop Trauma. 2004;18:494-502. [DOI] [PubMed] [Google Scholar]
- 28.Gösling T, Schandelmaier P, Marti A, Hufner T, Partenheimer A, Krettek C. Less invasive stabilization of complex tibial plateau fractures: a biomechanical evaluation of a unilateral locked screw plate and double plating. J Orthop Trauma. 2004;18:546-51. [DOI] [PubMed] [Google Scholar]
- 29.Hasenboehler E, Smith WR, Laudicina L, Philips GC, Stahel PF, Morgan SJ. Fatigue behavior of Ilizarov frame versus tibial interlocking nail in a comminuted tibial fracture model: a biomechanical study. J Orthop Surg Res. 2006;1:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caja V, Kim W, Larsson S, Chao EY. Comparison of the mechanical performance of three types of external fixators: linear, circular and hybrid. Clin Biomech (Bristol, Avon). 1995;10:401-6. [DOI] [PubMed] [Google Scholar]
- 31.Finlay JB, Moroz TK, Rorabeck CH, Davey JR, Bourne RB. Stability of ten configurations of the Hoffmann external-fixation frame. J Bone Joint Surg Am. 1987;69:734-44. [PubMed] [Google Scholar]
- 32.Moroz TK, Finlay JB, Rorabeck CH, Bourne RB. External skeletal fixation: choosing a system based on biomechanical stability. J Orthop Trauma. 1988;2:284-96. [PubMed] [Google Scholar]
- 33.Perren SM. Evolution of the internal fixation of long bone fractures. The scientific basis of biological internal fixation: choosing a new balance between stability and biology. J Bone Joint Surg Br. 2002;84:1093-110. [DOI] [PubMed] [Google Scholar]
- 34.Giannoudis PV, Einhorn TA, Marsh D. Fracture healing: the diamond concept. Injury. 2007;38 Suppl 4:S3-6. [DOI] [PubMed] [Google Scholar]
- 35.Goodship AE, Watkins PE, Rigby HS, Kenwright J. The role of fixator frame stiffness in the control of fracture healing. An experimental study. J Biomech. 1993;26:1027-35. [DOI] [PubMed] [Google Scholar]
- 36.Foux A, Yeadon AJ, Uhthoff HK. Improved fracture healing with less rigid plates. A biomechanical study in dogs. Clin Orthop Relat Res. 1997;339:232-45. [DOI] [PubMed] [Google Scholar]
- 37.Panagiotopoulos E, Fortis AP, Lambiris E, Kostopoulos V. Rigid or sliding plate. A mechanical evaluation of osteotomy fixation in sheep. Clin Orthop Relat Res. 1999;358:244-9. [PubMed] [Google Scholar]
- 38.Herrera DA, Kregor PJ, Cole PA, Levy BA, Jönsson A, Zlowodzki M. Treatment of acute distal femur fractures above a total knee arthroplasty: systematic review of 415 cases (1981-2006). Acta Orthop. 2008;79:22-7. [DOI] [PubMed] [Google Scholar]
- 39.Field JR, Törnkvist H, Hearn TC, Sumner-Smith G, Woodside TD. The influence of screw omission on construction stiffness and bone surface strain in the application of bone plates to cadaveric bone. Injury. 1999;30:591-8. [DOI] [PubMed] [Google Scholar]
- 40.Ilizarov GA. Clinical application of the tension-stress effect for limb lengthening. Clin Orthop Relat Res. 1990;250:8-26. [PubMed] [Google Scholar]
- 41.Jagodzinski M, Krettek C. Effect of mechanical stability on fracture healing—an update. Injury. 2007;38 Suppl 1:S3-10. [DOI] [PubMed] [Google Scholar]
- 42.Rüedi T, Leutenegger A. [After-care of fractures, especially following osteosynthesis]. Ther Umsch. 1989;46:435-40. German. [PubMed] [Google Scholar]
- 43.Kenwright J, Richardson JB, Cunningham JL, White SH, Goodship AE, Adams MA, Magnussen PA, Newman JH. Axial movement and tibial fractures. A controlled randomised trial of treatment. J Bone Joint Surg Br. 1991;73:654-9. [DOI] [PubMed] [Google Scholar]
- 44.Augat P, Burger J, Schorlemmer S, Henke T, Peraus M, Claes L. Shear movement at the fracture site delays healing in a diaphyseal fracture model. J Orthop Res. 2003;21:1011-7. [DOI] [PubMed] [Google Scholar]
- 45.Bishop NE, van Rhijn M, Tami I, Corveleijn R, Schneider E, Ito K. Shear does not necessarily inhibit bone healing. Clin Orthop Relat Res. 2006;443:307-14. [DOI] [PubMed] [Google Scholar]
- 46.Park SH, O'Connor K, McKellop H, Sarmiento A. The influence of active shear or compressive motion on fracture-healing. J Bone Joint Surg Am. 1998;80:868-78. [DOI] [PubMed] [Google Scholar]
- 47.Schneider E, Goldhahn J, Burckhardt P. The challenge: fracture treatment in osteoporotic bone. Osteoporos Int. 2005;16 Suppl 2:S1-2. [DOI] [PubMed] [Google Scholar]
- 48.Gardner MJ, Griffith MH, Demetrakopoulos D, Brophy RH, Grose A, Helfet DL, Lorich DG. Hybrid locked plating of osteoporotic fractures of the humerus. J Bone Joint Surg Am. 2006;88:1962-7. [DOI] [PubMed] [Google Scholar]
- 49.Beaupré GS, Giori NJ, Caler WE, Csongradi J. A comparison of unicortical and bicortical end screw attachment of fracture fixation plates. J Orthop Trauma. 1992;6:294-300. [DOI] [PubMed] [Google Scholar]
- 50.Stern PJ, Drury WJ. Complications of plate fixation of forearm fractures. Clin Orthop Relat Res. 1983;175:25-9. [PubMed] [Google Scholar]
- 51.Miclau T, Remiger A, Tepic S, Lindsey R, McIff T. A mechanical comparison of the dynamic compression plate, limited contact-dynamic compression plate, and point contact fixator. J Orthop Trauma. 1995;9:17-22. [DOI] [PubMed] [Google Scholar]
- 52.Epari DR, Kassi JP, Schell H, Duda GN. Timely fracture-healing requires optimization of axial fixation stability. J Bone Joint Surg Am. 2007;89:1575-85. [DOI] [PubMed] [Google Scholar]