Abstract
Rationale: A well-known clinical paradox is that severe bacterial infections persist in the lungs of patients with cystic fibrosis (CF) despite the abundance of polymorphonuclear neutrophils (PMN) and the presence of a high concentration of human neutrophil peptides (HNP), both of which are expected to kill the bacteria but fail to do so. The mechanisms remain unknown.
Objectives: This study examined several possible mechanisms to understand this paradox.
Methods: PMN were isolated from sputum and blood of subjects with and without CF or non-CF bronchiectasis for phagocytic assays. HNP isolated from patients with CF were used to stimulate healthy PMN followed by phagocytic tests.
Measurements and Main Results: PMN isolated from the sputum of the bronchiectatic patients display defective phagocytosis that correlated with high concentrations of HNP in the lung. When healthy PMN were incubated with HNP, decreased phagocytic capacity was observed in association with depressed surface Fcγ RIII, actin-filament remodeling, enhanced intracellular Ca2+, and degranulation. Treatment of PMN with an intracellular Ca2+ blocker or α1-proteinase inhibitor to attenuate the activity of HNP largely prevented the HNP-induced phagocytic deficiency. Intratracheal instillation of HNP in Pallid mice (genetically deficient in α1-proteinase inhibitor) resulted in a greater PMN lung infiltration and phagocytic deficiency compared with wild-type mice.
Conclusions: HNP or PMN alone exert antimicrobial ability, which was lost as a result of their interaction. These effects of HNP may help explain the clinical paradox seen in patients with inflammatory lung diseases, suggesting HNP as a novel target for clinical therapy.
Keywords: inflammation, innate immunity, lung injury
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Severe bacterial infections persist in bronchiectatic lungs despite the abundance of neutrophils and the presence of human neutrophil peptides (HNP), both of which are expected to kill the bacteria but fail to do so. The mechanisms remain unknown.
What This Study Adds to the Field
HNP or neutrophils alone exert antimicrobial ability, which was lost as a result of their interaction. This finding may explain the clinical paradox and suggests HNP as a novel target for clinical therapy in inflammatory diseases.
A well-known clinical paradox in the lungs of patients with cystic fibrosis (CF) is that severe bacterial infections may persist despite the abundance of polymorphonuclear neutrophils (PMN), which are expected to kill the bacteria but fail to do so (1–4). Pulmonary PMN normally exhibit an avid capacity for phagocytosis of microorganisms. During phagocytosis, or in response to a variety of stimuli, PMN release large amounts of proteins into the extracellular milieu as a consequence of exocytosis of the contents of intracellular granules (degranulation) and leakage during phagosome formation. Human neutrophil peptides (HNP)−1,-2 and -3, or α-defensins, are the most abundant proteins stored in the PMN azurophilic granules (5) and they are highly homologous, exerting broad antimicrobial activities (6). HNP-1 and HNP-2 constitute approximately 90% of the total HNP content within human neutrophils, and HNP-1 and HNP-3 are 30 residues in length and identical in sequence in all but their amino terminal residues (6). The levels of HNP in the plasma of healthy volunteers range from undetectable to 0.2 μg/ml, but in in patients with bacterial infections they increase to concentrations as high as 170 μg/ml (7, 8). Extremely high levels of HNP in sputum (>1,600 μg/ml) have been reported in patients with CF (9).
Because CF transmembrane conductance regulator (CFTR) expression is very low or undetectable in PMN isolated from the lung and blood, the reduced phagocytic activity may not be the direct result of a CFTR mutation in phagocytes but rather a consequence of the milieu of the CF lung (10, 11). This concept is further supported by the fact that circulating PMNs from patients with CF have similar phagocytotic properties as those isolated from normal control subjects (11). Furthermore, recent evidence suggests that the abnormality of antimicrobial activity in the CF lung is salt-independent (12, 13). However, a detailed understanding of the mechanisms underlying the deficiency of bacterial killing by PMN in the CF lung is currently lacking.
To investigate the mechanisms of the clinical paradox described above, we speculated that the HNP released from activated PMN are not bystanders and they exert important immunological properties in the CF lung. We tested the hypothesis that HNP interact with their activated host PMN through a negative feedback mechanism resulting in impairment of their antimicrobial function in inflammatory conditions. Our study demonstrates that the PMN isolated from the sputum of patients with CF and non-CF bronchiectasis display defective phagocytosis that correlated with high concentrations of HNP in the lung. HNP or PMN alone exert antimicrobial ability, which was lost as a result of their interaction, suggesting that HNP could be a useful inflammatory marker for bronchiectatic lungs and a novel target for clinical therapy.
METHODS
All studies reported here were approved by the institutional human Research Ethics Board and Animal Care Committee. Informed consent was obtained from all healthy subjects and patients with CF or non-CF bronchiectasis involved in the study.
Patients
Forty-five patients with CF, 8 patients with non-CF bronchiectasis, and 15 healthy control subjects were included in the study. The patients with CF included 26 male (58%) and 19 female (42%) patients between 18 and 46 years of age. Inclusion criteria were the diagnosis of CF by clinical symptoms and positive sweat test or two disease-associated mutations in the Cftr gene, FEV1 greater than 25% of predicted value, and being on concomitant therapy for at least 2 weeks before the study. Exclusion criteria were a history of lung transplantation or awaiting lung transplantation, any lung surgery within the previous 2 years, severe biliary cirrhosis with ascites, current cigarette use, and current pregnancy. Fifty percent of the patients with CF were delta F508 homozygotes. Therapy in the patients with CF consisted of inhaled corticosteroids in 69%, oral corticosteroids in 6%, and inhaled mucolytics in 18%. Although 96% of patients had positive sputum cultures, almost half of the patients had two or more organisms identified: 65% grew Pseudomonas aeruginosa, 24% each grew Burkholderia cepacia and Aspergillus spp., and 22% grew Staphylococcus aureus. Stenotrophomonas, Alcaligenes, and Burkholderia gladioli were each present in less than 10% of the patients.
Eight patients with non-CF bronchiectasis (six females and two males aged between 21 and 81 yr) were included with CT scan confirmation. The inclusion criteria were persistent colonization with P. aeruginosa and the ability to spontaneously expectorate sputum with no underlying immune deficiency and negative results on CF testing. One patient had persistent growth also of Aspergillus. The volumes of FEV1 were greater than 25% of predicted value. All patients except one received inhaled corticosteroids and regular inhaled antibiotics.
HNP
Purified HNP from the sputum of patients with CF were a mixture of HNP-1, -2, and -3 and were confirmed by acid-urea polyacrylamide gel electrophoresis and mass spectroscopy (Mass Spectrometry Laboratory, Molecular Medicine Research Center, University of Toronto, Toronto, ON) as previously described (14–19). (See online supplement for details.) The purified HNP was used for all in vitro and in vivo experiments. Synthetic HNP-1 and HNP-2 serving as controls in some experiments were purchased from Sigma (St. Louis, MO).
PMN Isolation
Mouse PMN were isolated from bone marrow and lung lavage fluids. Human PMN were isolated from peripheral blood of nonsmoking healthy donors and patients with CF or non-CF bronchiectasis at clinic visit, and from induced sputum of healthy donors or spontaneously expectorated sputum from the same patients with CF or non-CF bronchiectasis who donated blood. (See online supplement for details.)
Flow Cytometry
Flow cytometry was performed on a FACSCanto controlled by FACSdiva software (BD Biosciences, Mississauga, ON) to detect surface markers using mouse anti-human monoclonal anti-CR1, anti-CR3, anti-Fcγ RIII, anti-CD63, and anti-66b antibodies (Serotec Inc., Raleigh, NC), and 10,000 events were analyzed.
Phagocytosis Assays
The phagocytic assays included flow cytometry assays and slide assays using latex beads and labeled Escherichia coli and live P. aeruginosa. (See online supplement for details.)
HNP ELISA and Human Neutrophil Elastase Assay
HNP concentration was measured by ELISA. (See online supplement for details.) The concentrations of human neutrophil elastase in PMN cell culture medium were measured in the presence and absence of HNP stimulation by using a Human Elastase ELISA Kit (Hbt, HyCult Biotechnology, Uden, The Netherlands).
Lung Permeability Assessment
Pallid mice (deficient in α1-PI) and background strain C57BL/6J of wild-type mice weighing 25 to 28 g were purchased from Jackson Laboratories (Bar Harbor, ME). Electron microscopy estimates of the thickness of the alveolar surface liquid give an average value of about 0.4 μm in mice (20, 21). According to the morphometric data analysis (20), the mean alveolar surface area is approximately 0.1 m2 in a 25-g mouse. Based on these values, the total alveolar surface liquid is about 40 μl in a 25-g mouse. To estimate changes of lung permeability, we administered HNP intratracheally at a dose of 200 pg in mice. The dose of HNP used would thus roughly correspond to an initial concentration of 5 μg/ml in the lungs of the mice. Evans blue dye technique was used to evaluate lung injury. (See online supplement for details.)
Statistical Analysis
Data are reported as mean ± SE. Statistical significance was determined using the Scheffe method for complex contrasts or the Bonferroni/Dunn multiple comparisons approach (StatView 5.0.1; SAS Institute Inc., Cary, NC). Correlation between phagocytosis and HNP concentration and associated two-tailed P values were determined using the Spearman correlation test (GraphPad Prism 4.0; GraphPad Software Inc., San Diego, CA). A P value less than 0.05 was considered as statistical significance.
RESULTS
We isolated PMN from the sputum of patients with CF and non-CF bronchiectasis. The number of PMN in the induced sputum from patients with CF was greater than from patients with non-CF bronchiectasis and both were greater than PMN from healthy individuals (see Figure E1a in the online supplement). Peripheral blood PMN isolated from patients with either CF or non-CF bronchiectasis and from healthy volunteers demonstrated an identical phagocytic capacity for E. coli. However, PMN isolated from the sputum of patients with CF and non-CF bronchiectasis exhibited decreased phagocytic capacity compared with PMN isolated from the induced sputum of healthy control subjects (Figures E1B and E1C).
To examine the mechanisms underlying this apparent phagocytic deficiency, we focused on patients with CF and assessed expression of three surface receptors known to be pivotal in phagocytosis, including CR1 (the complement receptor type 1 or CD35), CR3 (the phagocyte integrin CD11b/CD18), and IgG Fc receptor Fcγ RIII (CD16). The expression of CR1, CR3, and Fcγ RIII was normal in the PMN isolated from blood, but Fcγ RIII was lower in the PMN isolated from CF sputum than from the healthy donors (Figure E2).
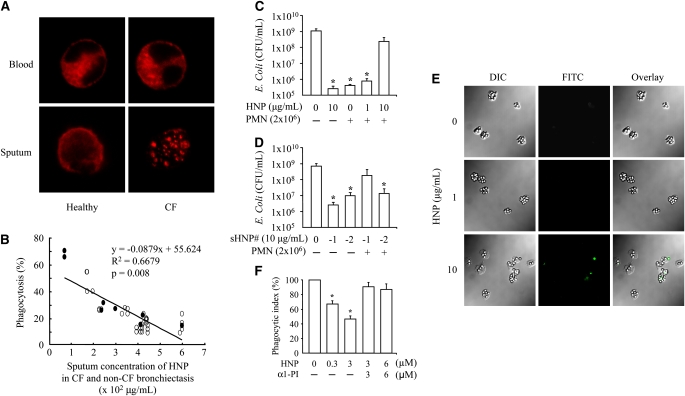
Because the actin cytoskeleton is known to be critical in the phagocytic process and because Fcγ RIII can modulate actin-filament (F-actin) redistribution (22, 23), we stained and observed an altered F-actin distribution in the PMN isolated from sputum of the patients with CF compared with the diffuse distribution of F-actin in PMN isolated from blood or from induced sputum of healthy control subjects (Figure 1A).
Figure 1.
Human neutrophil peptides (HNP) inhibited phagocytosis of polymorphonuclear neutrophils (PMN). (A) Altered distribution of F-actin in PMN isolated from cystic fibrosis (CF) sputum. The PMN from blood and sputum of healthy donors and patients with CF were seeded on fibronectin-coated coverslips, fixed and permeabilized before staining with rhodamine-phalloidin (red). n = 9; a representative image is shown in each group. (B) Inverse correlation between PMN phagocytosis and HNP concentration in sputum of CF (open circle) and non-CF bronchiectasis (filled circle), where the x represents HNP concentration, and y the percentage of phagocytosis in the regression equation. (C) PMN isolated from blood of healthy donors were incubated with HNP for 30 minutes before Escherichia coli exposure for 2 hours. The supernatants were plated for E. coli colony counting. *P < 0.05 versus the far left and the right bar, respectively. (D) PMN isolated from blood of healthy donors were incubated with synthetic HNP-1 or HNP-2 for 30 minutes before E. coli exposure for 2 hours. The supernatants were plated for E. coli colony counting. sHNP = synthetic HNP. *P < 0.05 versus the far left bar, respectively. (E) Healthy human PMN were incubated with HNP for 30 minutes, followed by exposure to IgG-opsonized latex beads for 60 minutes. The extracellular beads were stained with fluorescein isothiocyanate anti-IgG antibody (green). DIC = differential interference contrast. (F) Phagocytic index defined as the number of intracellular beads per cell. A complex of HNP (10 μg/ml HNP is equal to 2.8 μM) and an equimolar Prolastin was used to treat PMN 30 minutes before exposure to the beads. n = 10 per group, *P < 0.05 versus 0 μM HNP and the two groups at the far right, respectively.
To investigate the relationship between HNP and phagocytic function of PMN, we measured the sputum concentration of HNP, which was greater in the patients with CF (413 ± 22 μg/ml) and non-CF bronchiectasis (297 ± 71 μg/ml) than in the healthy control subjects (0.14 ± 0.01 μg/ml, both P < 0.05). We observed an inverse correlation between the phagocytic capacity of PMN and the concentration of HNP in the sputum of CF and non-CF bronchiectasis patients (Figure 1B).
To test the specific effect of HNP on PMN phagocytic dysfunction seen in patients with CF and non-CF bronchiectasis, we next isolated PMN from healthy volunteers and exposed them to HNP purified from CF sputum and then added E. coli as phagocytic prey. We chose to use E. coli but not P. aeruginosa in this assay because CR1 and the complement protein fragment C3bi on PMN can be cleaved by opsonized P. aerugonisa, creating an opsonin-receptor mismatch that impairs the complement-mediated phagocytosis against the bacteria (24, 25). We observed that the purified HNP and the isolated healthy PMN alone each exhibited E. coli killing activity; however, the bacterial killing capacity decreased when PMN were treated with HNP for 30 minutes before exposure to E. coli (Figure 1C). To further examine the individual effect of HNP on PMN phagocytic function, synthetic HNP-1 and HNP-2, the two major HNP that are commercially available, were used to repeat the E. coli killing assay. Both synthetic HNP-1 and HNP-2 showed ability to kill bacteria (Figure 1D). Furthermore, the synthetic HNP-1–treated but not HNP-2–treated PMN from healthy control subjects showed a decreased killing activity against E. coli (Figure 1D). We repeated the assays using IgG-opsonized latex beads and observed a dose-dependent decrease in phagocytic index in the PMN treated with HNP (Figure 1E).
Through protein–protein interactions, HNP form complexes with serine proteinase inhibitors (serpins), including α1-PI, α1-antichymotrypsin, α2-antiplasmin, and antithrombin III, leading to mutual inactivation (26). α1-PI has been shown to inhibit HNP activity in human lung cell line A549. We observed that the treatment of PMN with equimolar amounts of purified human α1-PI (Prolastin) largely reversed the HNP-induced phagocytic defect (Figure 1F).
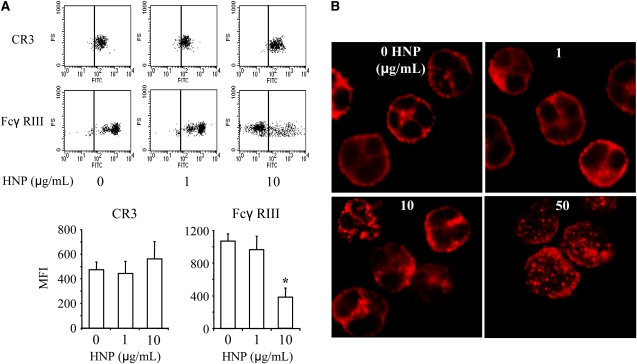
In PMN incubated with HNP, the surface expression of CR3 did not change significantly but Fcγ RIII expression dramatically decreased (Figure 2A). An F-actin network was disrupted into punctate or globular structures in the cortical region and in the cytoplasm after stimulation with HNP (Figure 2B), which was similar to the phenotypic alteration observed in the PMN isolated from sputum of the patients with CF (Figure 1A). These effects were dose-dependent.
Figure 2.
Effects of human neutrophil peptides (HNP) on surface expression of CD markers and F-actin distribution of polymorphonuclear neutrophils (PMN). (A) HNP decreased surface Fcγ RIII expression. Healthy PMN were treated with the indicated concentrations of HNP for 1 hour and stained with anti-human CR3–fluorescein isothiocyanate or Fcγ RIII–fluorescein isothiocyanate antibodies for 30 minutes, followed by analysis with flow cytometry. Analysis markers in lines indicate gating for unstained cells. MFI = mean fluorescence intensity. n = 10, *P < 0.05 versus other groups, respectively, at identical conditions. (B) HNP altered F-actin distribution. PMN from healthy donors were incubated with indicated concentrations of HNP for 1 hour. Cells were washed, fixed, and permeabilized with 0.2% Triton-X for 15 minutes before staining of F-actin with rhodamine-phalloidin (red). A representative image of F-actin is illustrated from seven experiments per group.
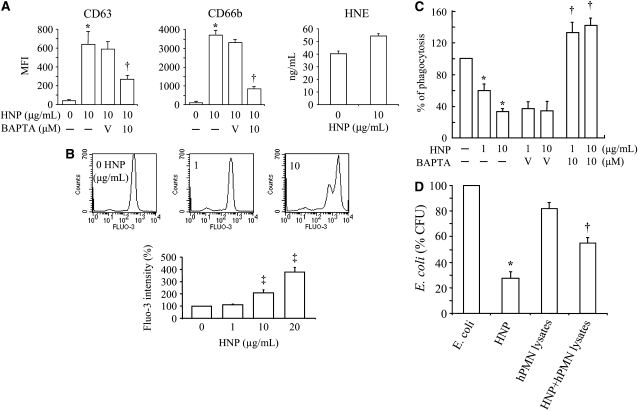
Because the disassembly of F-actin can facilitate degranulation in PMN (27, 28), the expression of the azurophilic-granule membrane marker CD63 (29) and the specific granule marker CD66b (28) was thus examined. Degranulation of both granule types was observed 30 minutes after stimulation with HNP (Figure 3A). Ca2+ has been shown to play many roles in cytoskeletal changes as well as being a cofactor for cell degranulation (27, 30, 31); thus, we loaded healthy PMN with an intracellular indicator Fluo-3 AM and observed a rapid, dose-dependent increase in intracellular Ca2+ concentration on exposure to HNP (Figure 3B). When the PMN were treated with the intracellular Ca2+ chelator BAPTA-AM, the surface expression of CD63 and CD66b was significantly attenuated in response to HNP (Figure 3A). Treatment of PMN with BAPTA-AM also largely restored their phagocytic capacity after incubation with HNP (Figure 3C).
Figure 3.
Mechanisms of human neutrophil peptides (HNP)–induced deficiency of polymorphonuclear neutrophils (PMN) phagocytosis. (A) HNP increased PMN degranulation. PMN from healthy donors were incubated with HNP for 30 minutes and stained with anti-human antibody against CD63 or CD66b, in the presence and absence of an intracellular Ca2+ chelator BAPTA. MFI = mean fluorescence intensity. n = 10. Healthy PMN (1 × 106/ml) in 24-well plates were treated with HNP at 10 μg/ml or vehicle control solution for 1 hour. The concentrations of human neutrophil elastase (HNE) were measured in cell culture medium. (B) HNP increased intracellular Ca2+. Healthy PMN were loaded with fluo-3 AM for 1 hour in phosphate-buffered saline free of Ca2+ and Mg2+, exposed to HNP followed by analysis over 60 seconds (n = 5). (C) Effects of BAPTA on the phagocytic index. Healthy PMN were incubated with BAPTA and HNP for 30 minutes, followed by exposure to IgG opsonized latex beads for 1 hour. *P < 0.05 versus 0 HNP, †P < 0.05 versus vehicle (V, 0.01% dimethyl sulfoxide) in the presence of HNP at identical conditions, respectively. ‡P < 0.05 versus 0 and 1 μg/ml of HNP at identical conditions, respectively. (D) PMN disables the antibacterial activity of HNP. Escherichia coli was incubated for 3 hours with and without HNP (10 μg/ml), with and without PMN lysates (1 × 106 cells isolated from blood of healthy volunteers [hPMN lysates] or bone marrow of C57BL/6J mice [mPMN lysates]), or with and without the combination of HNP and the human or murine PMN lysates, respectively. n = 3 experiments. *P < 0.05 versus E. coli, †P < 0.05 versus E. coli and HNP, respectively.
To elucidate whether exposure to HNP triggers the release of proteases that may in turn impair PMN function, we observed a small increase in the release of human PMN elastase after HNP stimulation for 1 hour. However, this increase did not reach statistical significance (Figure 3A, panel 3). To examine whether the concentration of HNP used in our phagocytic assays altered cell viability, PMN apoptosis was assessed using Annexin V staining after stimulation with HNP (0–20 μg/ml) for 30 minutes. No significant apoptosis was observed under these conditions (Figure E3). However, high concentrations of HNP (50 μg/ml) increased apoptosis by 30% (Figure E3).
An important question was whether PMN could disable the microbicidal activity of HNP. We demonstrated that HNP at 10 μg/ml was able to kill E. coli efficiently (75% killing at 60 minutes). The PMN lysates obtained from healthy human PMN showed bacterial killing activity (20% killing at 2 hours). However, when human PMN lysates were mixed with HNP, the bacterial killing activity of HNP was diminished by approximately 30% compared with HNP alone (P < 0.05, Figure 3D). These results suggest that soluble mediators in the human PMN lysates can alter the antimicrobial properties of HNP.
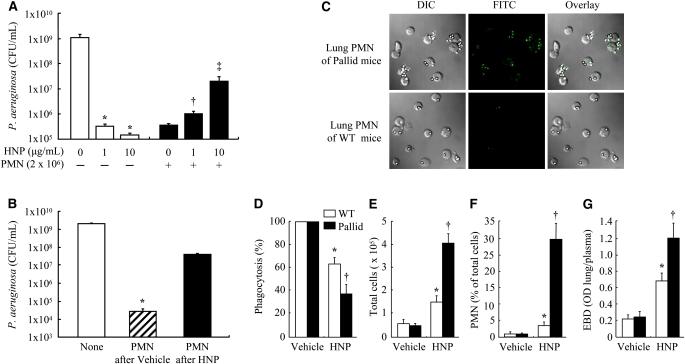
Similar to the results obtained from human cell studies, we observed that the PMN isolated from bone marrow of C57/BL6 mice exhibited P. aeruginosa killing activity that was impaired in the presence of both HNP and PMN (Figure 4A). Furthermore, the PMN recovered from lung lavage fluid had a decreased bacterial killing capacity in the mice who received intratracheal HNP compared with the mice receiving vehicle control solution (Figure 4B). To test the hypothesis that PMN from Pallid mice (deficient in α1-antitrypsin) would show impaired phagocytic function in response to HNP challenge, we studied the phagocytic function of PMN obtained from lung lavage from these mice and compared this to PMN from wild-type control mice. We observed that lung PMN from Pallid mice after HNP instillation had decreased capacity to internalize IgG opsonized latex beads compared with the PMN obtained from wild-type mice (Figures 4C and 4D). The model of HNP-induced PMN phagocytic deficiency in the lung was characterized by greater lung PMN sequestration and pulmonary permeability in the Pallid mice than in the wild-type control subjects (Figures 4E–4G).
Figure 4.
Effects of human neutrophil peptides (HNP) on polymorphonuclear neutrophils (PMN) phagocytosis in mice. (A) PMN isolated from bone marrow of C57BL/6J mice (n = 5) were incubated with the indicated concentrations of HNP for 30 minutes before Pseudomonas aeruginosa exposure for 3 hours for bacterial killing assay. *P < 0.05 versus 0 HNP in the absence of PMN; †P < 0.05 versus 0 HNP in the presence of PMN; ‡P < 0.05 versus other groups in the presence of PMN, respectively. (B) Mice received intratracheal instillation of HNP (200 pg) or vehicle control in 30 μl phosphate-buffered saline and observed for 5 hours (n = 7 each). PMN were then isolated from lung lavage fluids and exposed to P. aeruginosa for 3 hours for bacterial killing assay. *P < 0.05 versus other groups. (C and D) In a separate assay to evaluate phagocytic function, PMN recovered from lavage fluids of the wild-type (WT) and the Pallid mice (n = 7 each group) 5 hours after HNP instillation were exposed to IgG opsonized beads for 30 minutes. After fixation, the extracellular beads were labeled with fluorescein isothiocyanate (green). Thus for a given number of beads and PMN in the assays, the greater the number of extracellular green beads, the less efficient was phagocytosis (as quantified by the phagocytic index) as seen in the Pallid mice. Phagocytosis was defined by the number of beads internalized per cell was calculated as a percentage of controls. (E and F) Total cell count and differentiation in lung lavage fluids was performed in the wild-type (WT) and Pallid mice. (G) In separate experiments, mice (n = 5 each) received Evans blue dye (EBD) via tail vein 30 minutes before the end of a 5-hour experiment after HNP instillation. EBD concentration in lung homogenate supernatant was quantified by a dual wavelength spectrophotometer for correction of contaminating heme pigments. *P < 0.05 versus WT in Vehicle control; †P < 0.05 versus Pallid in Vehicle and WT in HNP group, respectively.
DISCUSSION
In the present study, we observed that the phagocytic ability of PMN isolated from the sputum of patients with CF and non-CF bronchiectasis was impaired in part due to the presence of high concentrations of HNP. Our results are in agreement with more recent studies demonstrating that PMN recovered from CF lung had blunted phagocytic capacity (11, 12, 32). We provide strong evidence that part of the defect in the antimicrobial function of PMN is related to the interactive inhibitory effects of the high concentrations of HNP and their host PMN in bronchiectatic lungs.
Bronchiectatic lung is characterized by frequent bacterial infection and the presence of large numbers of PMN (1–4). The PMN that have transmigrated into the lung are believed to kill bacteria by means of a complex series of events that include ingestion, fusion of phagosomes with lysosomes, and finally killing and digestion of the ingested microorganisms. During these processes, there is sometimes release of the contents of the phagosome, such as HNP, outside the confines of the cell into the extracellular space. We report here that PMN isolated from sputum of patients with CF and non-CF bronchiectasis display defective phagocytosis that correlated with high concentrations of HNP in the lung.
We demonstrated that there is ongoing active inflammation in conjunction with PMN degranulation in the CF lung as reflected by increased numbers of PMN and a higher concentration of HNP in the sputum of the patients with CF. A previous study has reported concentrations of HNP up to greater than 1,600 μg/ml (9), whereas we detected a mean value of 400 μg/ml in sputum of patients with CF. This difference may be because hospitalized patients with unstable severe CF were included in the former study (9), whereas relatively stable patients with CF were included at clinic visits in our study.
Because the phagocytic activity of PMN in blood from patients with CF was normal and there was an inverse correlation between the phagocytic capacity and the concentration of HNP in the CF sputum, we considered the possibility that the high concentration of HNP in the sputum of patients with CF may exert inhibitory effects on PMN phagocytosis. Our studies show that PMN alone and HNP alone in vitro are able to kill bacteria, but fail to do so when mixed together. We further demonstrated that incubation of PMN isolated from healthy control subjects with HNP, especially HNP-1, can induce phagocytic deficiency, although the specific domain (i.e., C- or N-terminal) of HNP responsible for the deficiency is yet to be identified.
The Fcγ RIII represents the largest proportion of FcR that participate in bacterial phagocytosis by recognizing and binding opsonized particles and modulating F-actin redistribution (11, 22, 23). We showed that the expression of Fcγ RIII in otherwise healthy PMN was significantly reduced in response to HNP exposure, which reproduced the features seen in the PMN obtained from CF sputum. Our results are consistent with a previous study demonstrating a lower PMN surface expression of Fcγ RIII and no significant differences in CR1, CR3, Fcγ RII (CD32), and Fcγ RI (CD64) in patients with CF compared with non-CF control subjects (4, 33, 34). The downregulation of Fcγ RIII may be involved in several mechanisms, including shedding of FcRIII from the cell surface on PMN activation (34, 35), cellular apoptosis (36), cleavage of the receptor by proteinases such as elastase (37), and high levels of IL-8 (38). The shedding of Fcγ RIII can be stopped in the presence of inhibitors of metalloproteinases but not by inhibition of serine proteinases (37).
A decreased expression of Fcγ RIII can affect F-actin structure and polymerization (39). We observed that a normal cortical F-actin structure was disrupted in PMN from healthy donors after exposure to purified HNP, consistent with the observation that an altered F-actin organization was present in the PMN isolated from CF sputum. Degranulation of PMN induced by a rapid intracellular Ca2+ burst may induce additional alterations in the actin cytoskeleton (27, 30, 31, 40). Indeed, when PMN were treated with the intracellular Ca2+ chelator BAPTA-AM, the HNP-induced degranulation was attenuated and the PMN phagocytic activity was largely restored.
There are additional possible mechanisms by which the antimicrobial activity of HNP can be altered in CF conditions. For example, antimicrobial compounds such as HNP in the CF sputum may become glycosylated and rendered inactive thus contributing to the sustained inflammatory response in the CF airways (40, 41). However, we consider this mechanism to be unlikely because HNP lack consensus sequences for N-linked glycosylation (42). Interestingly, we found that the contents of human PMN can dramatically affect the antibacterial activity of HNP. These data suggest that degranulation of human PMN, by releasing intracellular contents, can disable HNP and compromise microbial killing during inflammation. This notion merits further investigation.
It is noteworthy that we chose to use low concentrations (1–10 μg/ml) of HNP for short periods of time of stimulation. Although higher concentrations of HNP are reported in blood and lung of patients with inflammation (7, 8, 43–45) they may include both free HNP and HNP that may be functionally inactive because they are either complexed with other proteins, such as α1-PI and albumin, or bound to cells (9, 26, 46). Also, the milieu of the bronchiectatic lungs in which HNP interact with PMN is likely considerably more complex than in our experimental model systems. Finally, we cannot exclude the possibility that the impaired PMN function in sputum of the patients with CF was a result of the combined effects of CF disease and medication. However, because the PMN from sputum of patients with non-CF bronchiectasis also showed a similar defect in bacterial killing, it suggests that the defective PMN phagocytosis is unlikely due to medication in the CF lung.
To reproduce the results obtained from human cell studies, the murine model was chosen because murine PMN do not produce endogenous HNP (47), although they do respond to HNP stimulation (19, 48) and thus provide a clean background to study HNP. The intratracheal instillation of HNP resulted in a significant infiltration of PMN into the lung, which can be explained by the observation that HNP stimulate lung epithelial cells to produce IL-8 (or KC/MIP-2 in murine systems), a potent chemoattractant for PMN (17, 19, 49). We found that the PMN isolated from healthy mice after HNP priming exhibited an impaired P. aeruginosa killing activity. Also, the transmigrated PMN recovered from lung lavage fluid demonstrated impaired ability to kill P. aeruginosa or to internalize opsonized latex beads after HNP instillation into the lung (14, 17, 18, 49). When the in vivo experiments were repeated in Pallid mice that have genetic α1-antitrypsin deficiency, the phagocytic function of the PMN recovered from the lung decreased further in the Pallid mice in response to HNP instillation. Our results suggest that PMN are more susceptible to develop phagocytic dysfunction in conditions of serpin deficiency. Indeed, although concentrations of HNP in plasma are undetectable in volunteers, they are markedly increased to around 50 μg/ml levels in patients with α1-PI deficiency and moderate to severe lung disease (50, 51).
The present study examines the interaction between PMN and HNP that might have been previously overlooked in the context of defective phagocytosis in bronchiectatic lungs. Another known problem in the CF lung is the physical barriers to phagocytosis resulting from bacterial glycoconjugates contributing to biofilm formation. An inhibition of bacterial neuraminidases could reduce the biofilm formation and thus bacterial infection (52).
In conclusion, we demonstrate that several interrelated intracellular signals are involved in the HNP-induced phagocytic defect; thus HNP may play a crucial role in the clinical paradox seen in bronchiectatic lungs. HNP could also be a useful inflammatory marker and a novel target for clinical therapy in other inflammatory lung diseases where PMN sequestration and high concentrations of HNP are found (7, 8, 43–46).
Supplementary Material
Acknowledgments
The authors thank Dr. Tom Ganz, Department of Cellular and Molecular Pathology, University of California Los Angeles, for providing protocol and supervision of HNP purification. They thank Dr. Sergio Grinstein, Cell Biology, the Hospital for Sick Children, University of Toronto, for critical discussions and comments on the manuscript.
Supported by operating grants from the Canadian Institutes of Health Research to A.S., G.D., and H.Z. (MOP-8588, MT-10994, and MOP-69042), NIH operating grant to G.D. (R01 HL090669-01), the Ontario Thoracic Society to H.Z., and in part by the Garfield Weston Foundation to A.S. S.V. is a recipient of the Canadian Cystic Fibrosis Foundation Graduate Studentship. H.Z. is a recipient of the Ontario Premier Research Excellence Award.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200808-1250OC on April 30, 2009
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Elston C, Geddes D. Inflammation in cystic fibrosis–when and why? Friend or foe? Semin Respir Crit Care Med 2007;28:286–294. [DOI] [PubMed] [Google Scholar]
- 2.Sagel SD, Chmiel JF, Konstan MW. Sputum biomarkers of inflammation in cystic fibrosis lung disease. Proc Am Thorac Soc 2007;4:406–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Terheggen-Lagro SW, Rijkers GT, van der Ent CK. The role of airway epithelium and blood neutrophils in the inflammatory response in cystic fibrosis. J Cyst Fibros 2005;4:15–23. [DOI] [PubMed] [Google Scholar]
- 4.Tirouvanziam R, Gernez Y, Conrad CK, Moss RB, Schrijver I, Dunn CE, Davies ZA, Herzenberg LA. Profound functional and signaling changes in viable inflammatory neutrophils homing to cystic fibrosis airways. Proc Natl Acad Sci USA 2008;105:4335–4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol 2003;3:710–720. [DOI] [PubMed] [Google Scholar]
- 6.Selsted ME, Harwig SS, Ganz T, Schilling JW, Lehrer RI. Primary structures of three human neutrophil defensins. J Clin Invest 1985;76:1436–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ihi T, Nakazato M, Mukae H, Matsukura S. Elevated concentrations of human neutrophil peptides in plasma, blood, and body fluids from patients with infections. Clin Infect Dis 1997;25:1134–1140. [DOI] [PubMed] [Google Scholar]
- 8.Panyutich AV, Panyutich EA, Krapivin VA, Baturevich EA, Ganz T. Plasma defensin concentrations are elevated in patients with septicemia or bacterial meningitis. J Lab Clin Med 1993;122:202–207. [PubMed] [Google Scholar]
- 9.Soong LB, Ganz T, Ellison A, Caughey GH. Purification and characterization of defensins from cystic fibrosis sputum. Inflamm Res 1997;46:98–102. [DOI] [PubMed] [Google Scholar]
- 10.Crawford I, Maloney PC, Zeitlin PL, Guggino WB, Hyde SC, Turley H, Gatter KC, Harris A, Higgins CF. Immunocytochemical localization of the cystic fibrosis gene product cftr. Proc Natl Acad Sci USA 1991;88:9262–9266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris MR, Doull IJ, Dewitt S, Hallett MB. Reduced ic3b-mediated phagocytotic capacity of pulmonary neutrophils in cystic fibrosis. Clin Exp Immunol 2005;142:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bals R, Weiner DJ, Meegalla RL, Accurso F, Wilson JM. Salt-independent abnormality of antimicrobial activity in cystic fibrosis airway surface fluid. Am J Respir Cell Mol Biol 2001;25:21–25. [DOI] [PubMed] [Google Scholar]
- 13.Clunes MT, Boucher RC. Front-runners for pharmacotherapeutic correction of the airway ion transport defect in cystic fibrosis. Curr Opin Pharmacol 2008;8:292–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khine AA, Del Sorbo L, Vaschetto R, Voglis S, Tullis E, Slutsky AS, Downey GP, Zhang H. Human neutrophil peptides induce interleukin-8 production through the p2y6 signaling pathway. Blood 2006;107:2936–2942. [DOI] [PubMed] [Google Scholar]
- 15.Porro GA, Lee JH, de Azavedo J, Crandall I, Whitehead T, Tullis E, Ganz T, Liu M, Slutsky AS, Zhang H. Direct and indirect bacterial killing functions of neutrophil defensins in lung explants. Am J Physiol Lung Cell Mol Physiol 2001;281:L1240–L1247. [DOI] [PubMed] [Google Scholar]
- 16.Syeda F, Liu HY, Tullis E, Liu M, Slutsky AS, Zhang H. Differential signaling mechanisms of hnp-induced IL-8 production in human lung epithelial cells and monocytes. J Cell Physiol 2008;214:820–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Syeda F, Tullis E, Slutsky AS, Zhang H. Human neutrophil peptides upregulate expression of cox-2 and endothelin-1 by inducing oxidative stress. Am J Physiol Heart Circ Physiol 2008;294:H2769– H2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaschetto R, Grinstein J, Del Sorbo L, Khine AA, Voglis S, Tullis E, Slutsky AS, Zhang H. Role of human neutrophil peptides in the initial interaction between lung epithelial cells and CD4+ lymphocytes. J Leukoc Biol 2007;81:1022–1031. [DOI] [PubMed] [Google Scholar]
- 19.Zhang H, Porro G, Orzech N, Mullen B, Liu M, Slutsky AS. Neutrophil defensins mediate acute inflammatory response and lung dysfunction in dose-related fashion. Am J Physiol Lung Cell Mol Physiol 2001;280:L947–L954. [DOI] [PubMed] [Google Scholar]
- 20.Gehr P, Mwangi DK, Ammann A, Maloiy GM, Taylor CR, Weibel ER. Design of the mammalian respiratory system. V. Scaling morphometric pulmonary diffusing capacity to body mass: wild and domestic mammals. Respir Physiol 1981;44:61–86. [DOI] [PubMed] [Google Scholar]
- 21.Weibel ER, Gil J. Electron microscopic demonstration of an extracellular duplex lining layer of alveoli. Respir Physiol 1968;4:42–57. [DOI] [PubMed] [Google Scholar]
- 22.May RC, Machesky LM. Phagocytosis and the actin cytoskeleton. J Cell Sci 2001;114:1061–1077. [DOI] [PubMed] [Google Scholar]
- 23.Selvaraj P, Rosse WF, Silber R, Springer TA. The major Fc receptor in blood has a phosphatidylinositol anchor and is deficient in paroxysmal nocturnal haemoglobinuria. Nature 1988;333:565–567. [DOI] [PubMed] [Google Scholar]
- 24.Bainbridge T, Fick RB Jr. Functional importance of cystic fibrosis immunoglobulin g fragments generated by pseudomonas aeruginosa elastase. J Lab Clin Med 1989;114:728–733. [PubMed] [Google Scholar]
- 25.Tosi MF, Zakem H, Berger M. Neutrophil elastase cleaves c3bi on opsonized pseudomonas as well as cr1 on neutrophils to create a functionally important opsonin receptor mismatch. J Clin Invest 1990;86:300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Panyutich AV, Hiemstra PS, van Wetering S, Ganz T. Human neutrophil defensin and serpins form complexes and inactivate each other. Am J Respir Cell Mol Biol 1995;12:351–357. [DOI] [PubMed] [Google Scholar]
- 27.Muallem S, Kwiatkowska K, Xu X, Yin HL. Actin filament disassembly is a sufficient final trigger for exocytosis in nonexcitable cells. J Cell Biol 1995;128:589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naucler C, Grinstein S, Sundler R, Tapper H. Signaling to localized degranulation in neutrophils adherent to immune complexes. J Leukoc Biol 2002;71:701–710. [PubMed] [Google Scholar]
- 29.Metzelaar MJ, Wijngaard PL, Peters PJ, Sixma JJ, Nieuwenhuis HK, Clevers HC. CD63 antigen. A novel lysosomal membrane glycoprotein, cloned by a screening procedure for intracellular antigens in eukaryotic cells. J Biol Chem 1991;266:3239–3245. [PubMed] [Google Scholar]
- 30.Downey GP, Chan CK, Trudel S, Grinstein S. Actin assembly in electropermeabilized neutrophils: role of intracellular calcium. J Cell Biol 1990;110:1975–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nanamori M, Chen J, Du X, Ye RD. Regulation of leukocyte degranulation by CGMP-dependent protein kinase and phosphoinositide 3-kinase: potential roles in phosphorylation of target membrane snare complex proteins in rat mast cells. J Immunol 2007;178:416–427. [DOI] [PubMed] [Google Scholar]
- 32.Alexis NE, Muhlebach MS, Peden DB, Noah TL. Attenuation of host defense function of lung phagocytes in young cystic fibrosis patients. J Cyst Fibros 2006;5:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petit-Bertron AF, Tabary O, Corvol H, Jacquot J, Clement A, Cavaillon JM, Adib-Conquy M. Circulating and airway neutrophils in cystic fibrosis display different tlr expression and responsiveness to interleukin-10. Cytokine 2008;41:54–60. [DOI] [PubMed] [Google Scholar]
- 34.Witko-Sarsat V, Halbwachs-Mecarelli L, Sermet-Gaudelus I, Bessou G, Lenoir G, Allen RC, Descamps-Latscha B. Priming of blood neutrophils in children with cystic fibrosis: correlation between functional and phenotypic expression of opsonin receptors before and after platelet-activating factor priming. J Infect Dis 1999;179:151–162. [DOI] [PubMed] [Google Scholar]
- 35.Huizinga TW, van der Schoot CE, Jost C, Klaassen R, Kleijer M, von dem Borne AE, Roos D, Tetteroo PA. The pi-linked receptor FcRIII is released on stimulation of neutrophils. Nature 1988;333:667–669. [DOI] [PubMed] [Google Scholar]
- 36.Nusbaum P, Laine C, Seveau S, Lesavre P, Halbwachs-Mecarelli L. Early membrane events in polymorphonuclear cell (PMN) apoptosis: membrane blebbing and vesicle release, cd43 and cd16 down-regulation and phosphatidylserine externalization. Biochem Soc Trans 2004;32:477–479. [DOI] [PubMed] [Google Scholar]
- 37.Middelhoven PJ, Van Buul JD, Hordijk PL, Roos D. Different proteolytic mechanisms involved in Fcγ RIIIb shedding from human neutrophils. Clin Exp Immunol 2001;125:169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ralston DR, Marsh CB, Lowe MP, Wewers MD. Antineutrophil cytoplasmic antibodies induce monocyte IL-8 release. Role of surface proteinase-3, alpha1-antitrypsin, and Fcγ receptors. J Clin Invest 1997;100:1416–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aderem A. How to eat something bigger than your head. Cell 2002;110:5–8. [DOI] [PubMed] [Google Scholar]
- 40.Marshall LJ, Perks B, Bodey K, Suri R, Bush A, Shute JK. Free secretory component from cystic fibrosis sputa displays the cystic fibrosis glycosylation phenotype. Am J Respir Crit Care Med 2004;169:399–406. [DOI] [PubMed] [Google Scholar]
- 41.Mehta A, Bush A. Beyond chloride transport: CFTR in the 21st century—introductory remarks to a new state of the art series. Pediatr Pulmonol 2005;39:289–291. [DOI] [PubMed] [Google Scholar]
- 42.Michaelson D, Rayner J, Couto M, Ganz T. Cationic defensins arise from charge-neutralized propeptides: a mechanism for avoiding leukocyte autocytotoxicity? J Leukoc Biol 1992;51:634–639. [DOI] [PubMed] [Google Scholar]
- 43.Ashitani J, Mukae H, Arimura Y, Sano A, Tokojima M, Nakazato M. High concentrations of alpha-defensins in plasma and bronchoalveolar lavage fluid of patients with acute respiratory distress syndrome. Life Sci 2004;75:1123–1134. [DOI] [PubMed] [Google Scholar]
- 44.Ashitani J, Mukae H, Hiratsuka T, Nakazato M, Kumamoto K, Matsukura S. Elevated levels of alpha-defensins in plasma and BAL fluid of patients with active pulmonary tuberculosis. Chest 2002;121:519–526. [DOI] [PubMed] [Google Scholar]
- 45.Ashitani J, Mukae H, Nakazato M, Ihi T, Mashimoto H, Kadota J, Kohno S, Matsukura S. Elevated concentrations of defensins in bronchoalveolar lavage fluid in diffuse panbronchiolitis. Eur Respir J 1998;11:104–111. [DOI] [PubMed] [Google Scholar]
- 46.Panyutich A, Ganz T. Activated alpha 2-macroglobulin is a principal defensin-binding protein. Am J Respir Cell Mol Biol 1991;5:101–106. [DOI] [PubMed] [Google Scholar]
- 47.Eisenhauer PB, Lehrer RI. Mouse neutrophils lack defensins. Infect Immun 1992;60:3446–3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ayabe T, Satchell DP, Pesendorfer P, Tanabe H, Wilson CL, Hagen SJ, Ouellette AJ. Activation of paneth cell alpha-defensins in mouse small intestine. J Biol Chem 2002;277:5219–5228. [DOI] [PubMed] [Google Scholar]
- 49.Van Wetering S, Mannesse-Lazeroms SP, Van Sterkenburg MA, Daha MR, Dijkman JH, Hiemstra PS. Effect of defensins on interleukin-8 synthesis in airway epithelial cells. Am J Physiol 1997;272:L888–L896. [DOI] [PubMed] [Google Scholar]
- 50.Spencer LT, Paone G, Krein PM, Rouhani FN, Rivera-Nieves J, Brantly ML. Role of human neutrophil peptides in lung inflammation associated with alpha1-antitrypsin deficiency. Am J Physiol Lung Cell Mol Physiol 2004;286:L514–L520. [DOI] [PubMed] [Google Scholar]
- 51.Wencker M, Brantly ML. Cytotoxic concentrations of alpha-defensins in the lungs of individuals with alpha 1-antitrypsin deficiency and moderate to severe lung disease. Cytokine 2005;32:1–6. [DOI] [PubMed] [Google Scholar]
- 52.Wagner T, Soong G, Sokol S, Saiman L, Prince A. Effects of azithromycin on clinical isolates of pseudomonas aeruginosa from cystic fibrosis patients. Chest 2005;128:912–919. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.