Abstract
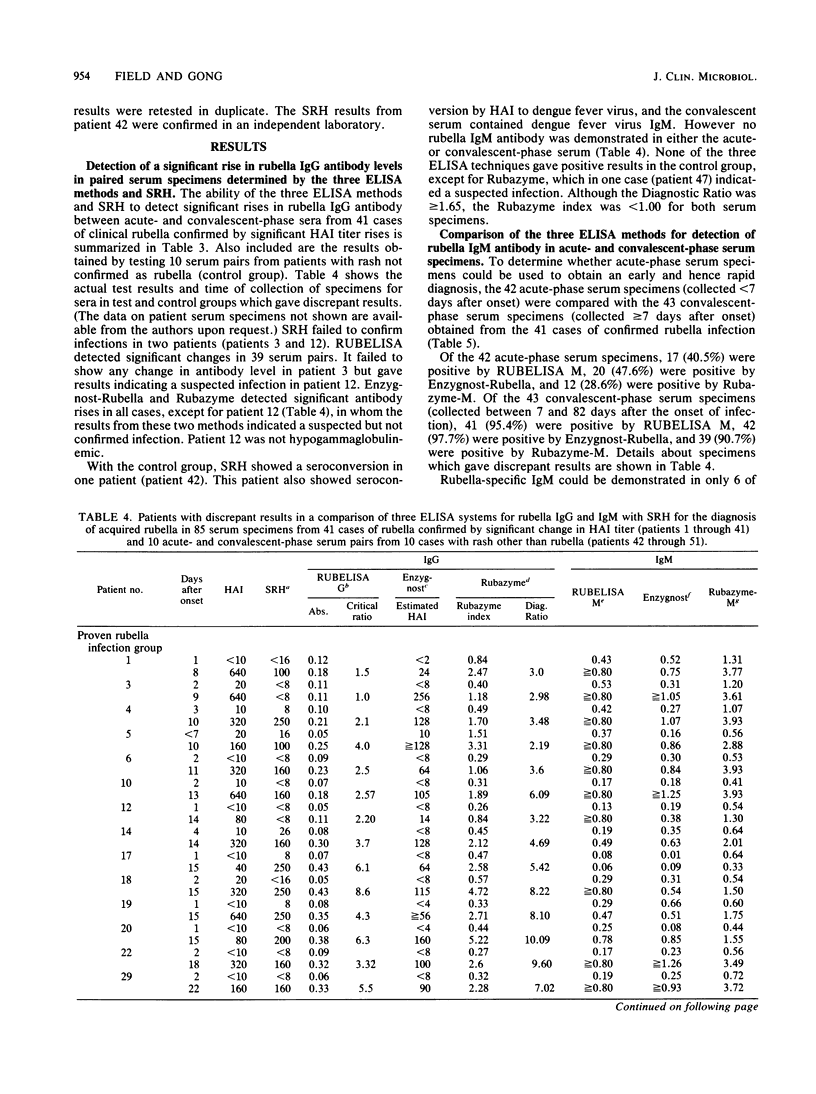
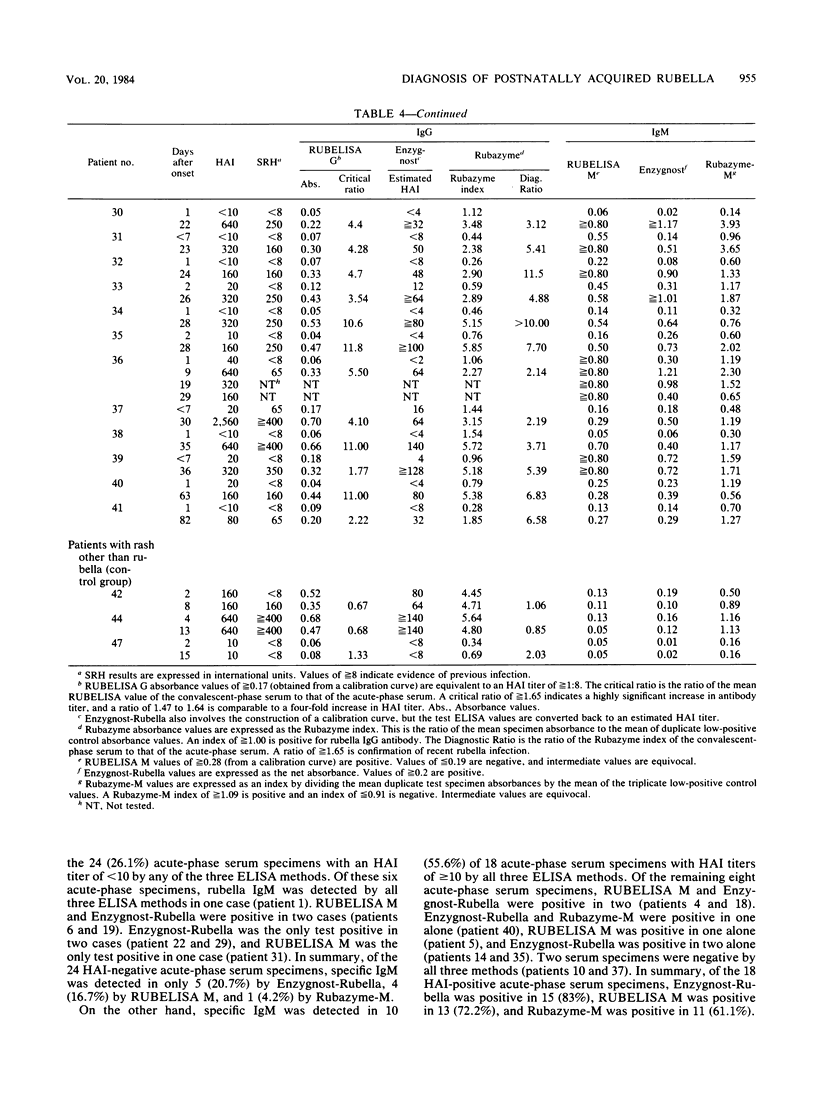
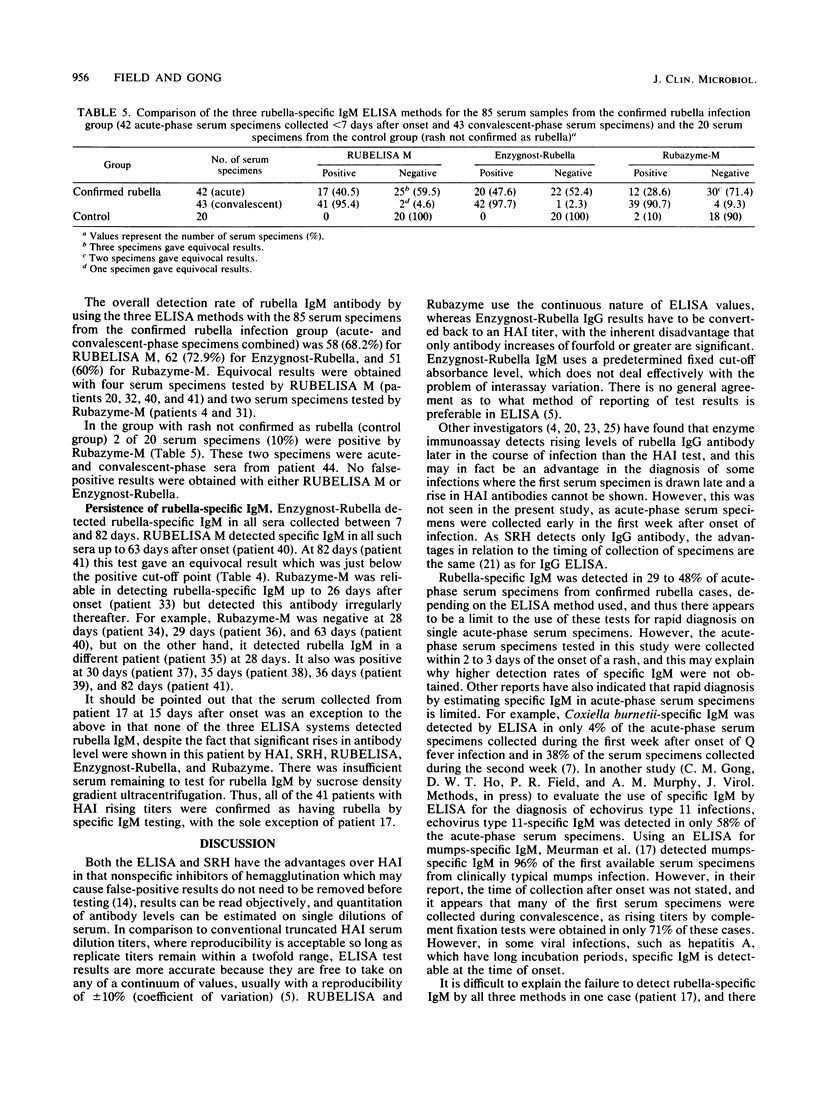
Three commercial indirect enzyme-linked immunosorbent assays (ELISAs) (RUBELISA, Enzygnost-Rubella, and Rubazyme) and a commercial single radial hemolysis (SRH) test (Rubazone) were evaluated for the diagnosis of acute rubella by testing 41 acute- (less than 7 days postonset) and convalescent-phase (8 to 82 days postonset) serum pairs from cases of rubella previously confirmed by significant change in the hemagglutination inhibition test titer. Specificity was tested by using 10 acute- and convalescent-phase serum samples from patients with rash not confirmed as rubella (control group). In testing for rubella-specific immunoglobulin G (IgG) antibody, Enzygnost-Rubella and Rubazyme confirmed infection in 40 and RUBELISA in 39 of the 41 proven rubella patients. For one patient all three ELISAs failed to show a significant titer rise. No false-positive diagnoses occurred in the control group, although a suspected infection was shown by Rubazyme in one patient. No specific IgM could be detected in this case. Single radial hemolysis confirmed infection in 39 of the 41 proven rubella patients, and one false-positive diagnosis occurred in the control group patients. Of the 43 convalescent-phase serum samples, rubella-specific IgM was detected in 42 by Enzygnost-Rubella, in 41 by RUBELISA M, and in 39 by Rubazyme-M. For a rapid diagnosis with acute-phase sera, specific IgM detection by ELISA was most reliable in hemagglutination inhibition test-positive sera; of 18 such serum samples IgM was shown in 15 by Enzygnost-Rubella, in 13 by RUBELISA M, and in 11 by Rubazyme-M. False-positive specific IgM results were shown by Rubazyme-M in two serum samples from one patient in the control group. These serum samples were negative with the other two ELISA methods.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Best J. M., Palmer S. J., Morgan-Capner P., Hodgson J. A comparison of Rubazyme-M and MACRIA for the detection of rubella-specific IgM. J Virol Methods. 1984 Feb;8(1-2):99–109. doi: 10.1016/0166-0934(84)90044-2. [DOI] [PubMed] [Google Scholar]
- Birch C. J., Glaun B. P., Hunt V., Irving L. G., Gust I. D. Comparison of passive haemagglutination and haemagglutination-inhibition techniques for detection of antibodies to rubella virus. J Clin Pathol. 1979 Feb;32(2):128–131. doi: 10.1136/jcp.32.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champsaur H., Dussaix E., Tournier P. Hemagglutination inhibition, single radial hemolysis, and ELISA tests for the detection of IgG and IgM to rubella virus. J Med Virol. 1980;5(4):273–286. doi: 10.1002/1096-9071(1980)5:4<273::aid-jmv1890050403>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Field P. R., Hunt J. G., Murphy A. M. Detection and persistence of specific IgM antibody to Coxiella burnetii by enzyme-linked immunosorbent assay: a comparison with immunofluorescence and complement fixation tests. J Infect Dis. 1983 Sep;148(3):477–487. doi: 10.1093/infdis/148.3.477. [DOI] [PubMed] [Google Scholar]
- Field P. R., Murphy A. M. The role of specific IgM globulin estimations in the diagnosis of acquired rubella. Med J Aust. 1972 Nov 25;2(22):1244–1248. doi: 10.5694/j.1326-5377.1972.tb47544.x. [DOI] [PubMed] [Google Scholar]
- Forghani B., Schmidt N. J. Antigen requirements, sensitivity, and specificity of enzyme immunoassays for measles and rubella viral antibodies. J Clin Microbiol. 1979 Jun;9(6):657–664. doi: 10.1128/jcm.9.6.657-664.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner L., Strannegård O. Evaluation of the hemolysis-in-gel test for the screening of rubella immunity and the demonstration of recent infection. J Clin Microbiol. 1976 Feb;3(2):86–90. doi: 10.1128/jcm.3.2.86-90.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnett G. B., Palmer C. A., Mackay-Scollay E. M. Single-radial-hemolysis test for the assay of rubella antibody in antenatal, vaccinated, and rubella virus-infected patients. J Infect Dis. 1979 Dec;140(6):937–944. doi: 10.1093/infdis/140.6.937. [DOI] [PubMed] [Google Scholar]
- Haukenes G., Blom H. False positive rubella virus haemagglutination inhibition reactions: occurrence and disclosure. Med Microbiol Immunol. 1975;161(2):99–106. doi: 10.1007/BF02121750. [DOI] [PubMed] [Google Scholar]
- Henle G., Lennette E. T., Alspaugh M. A., Henle W. Rheumatoid factor as a cause of positive reactions in tests for Epstein-Barr virus-specific IgM antibodies. Clin Exp Immunol. 1979 Jun;36(3):415–422. [PMC free article] [PubMed] [Google Scholar]
- Meurman O. H. Antibody responses in patients with rubella infection determined by passive hemagglutination, hemagglutination inhibition, complement fixation, and solid-phase radioimmunoassay tests. Infect Immun. 1978 Feb;19(2):369–372. doi: 10.1128/iai.19.2.369-372.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meurman O., Hänninen P., Krishna R. V., Ziegler T. Determination of IgG- and IgM-class antibodies to mumps virus by solid-phase enzyme immunoassay. J Virol Methods. 1982 May;4(4-5):249–256. doi: 10.1016/0166-0934(82)90071-4. [DOI] [PubMed] [Google Scholar]
- Morgan-Capner P., Tedder R. S., Mace J. E. Rubella-specific IgM reactivity in sera from cases of infectious mononucleosis. J Hyg (Lond) 1983 Jun;90(3):407–413. doi: 10.1017/s0022172400029041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann P. W., Weber J. M. Single radial hemolysis test for rubella immunity and recent infection. J Clin Microbiol. 1983 Jan;17(1):28–34. doi: 10.1128/jcm.17.1.28-34.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekarchi I. C., Sever J. L., Tzan N., Ley A., Ward L. C., Madden D. Comparison of hemagglutination inhibition test and enzyme-linked immunosorbent assay for determining antibody to rubella virus. J Clin Microbiol. 1981 May;13(5):850–854. doi: 10.1128/jcm.13.5.850-854.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaheri A., Saksela O., Vänänen P. Rubella screening by hemolysis-in-gel test. Lancet. 1979 Sep 22;2(8143):644–644. [PubMed] [Google Scholar]
- Vejtorp M., Fanøe E., Leerhoy J. Diagnosis of postnatal rubella by the enzyme-linked immunosorbent assay for rubella IgM and IgG antibodies. Acta Pathol Microbiol Scand B. 1979 Jun;87B(3):155–160. doi: 10.1111/j.1699-0463.1979.tb02419.x. [DOI] [PubMed] [Google Scholar]
- Vejtorp M., Leerhoy J. Comparison of the sensitivity of ELISA and the haemagglutination-inhibition test for routine diagnosis of rubella. Acta Pathol Microbiol Scand B. 1980 Dec;88(6):349–350. doi: 10.1111/j.1699-0463.1980.tb02655.x. [DOI] [PubMed] [Google Scholar]
- Vejtorp M. Serodiagnosis of postnatal rubella. A survey of methods with special reference to the enzyme-linked immunosorbent assay. Dan Med Bull. 1983 Mar;30(2):53–66. [PubMed] [Google Scholar]
- Vänänen P., Vaheri A. Hemolysis-in-gel test in immunity surveys and diagnosis of rubella. J Med Virol. 1979;3(4):245–252. doi: 10.1002/jmv.1890030402. [DOI] [PubMed] [Google Scholar]
- de Savigny D., Voller A. The communication of ELISA data from laboratory to clinician. J Immunoassay. 1980;1(1):105–128. doi: 10.1080/01971528008055779. [DOI] [PubMed] [Google Scholar]


