Abstract
Before implantation, the porcine endometrium and trophoblast synthesize elevated amounts of luteoprotective prostaglandin E2 (PGE2). We hypothesized that embryo signal, estradiol-17β (E2) and PGE2 modulate expression of key enzymes in PG synthesis: prostaglandin-endoperoxide synthase-2 (PTGS2), PGE synthase (mPGES-1), PGF synthase (PGFS), and prostaglandin 9-ketoreductase (CBR1); as well as PGE2 receptor (PTGER2 and 4) expression and signaling within the endometrium. We determinated the site of action of PGE2 in endometrium during the estrous cycle and pregnancy. Endometrial tissue explants obtained from gilts (n=6) on days 11-12 of the estrous cycle were treated with vehicle (control), PGE2 (100 nM), E2 (1-100 nM) or phorbol 12-myristate 13-acetate (100 nM, positive control). E2 increased PGE2 secretion through elevating expression of mPGES-1 mRNA and PTGS2 and mPGES-1 protein in endometrial explants. By contrast, E2 decreased PGFS and CBR1 protein expression. E2 also stimulated PTGER2 but not PTGER4 protein content. PGE2 enhanced mPGES-1 and PTGER2 mRNA as well as PTGS2, mPGES-1 and PTGER2 protein expression. PGE2 had no effect on PGFS, CBR1 and PTGER4 expression and PGF2α release. Treatment of endometrial tissue with PGE2 increased cAMP production. Co-treatment with PTGER2 antagonist (AH6809) but not PTGER4 antagonist (GW 627368X) inhibited significantly PGE2-mediated cAMP production. PTGER2 protein was localized in luminal and glandular epithelium and blood vessels of endometrium, and was significantly up-regulated on days 11-12 of pregnancy. Our results suggest that E2, prevents luteolysis through enzymatic modification of PG synthesis and that E2, PGE2 and endometrial PTGER2 are involved in PGE2 positive feedback loop in porcine endometrium.
Keywords: endometrium, pig, prostaglandin synthesis, PGE2 receptors, estradiol-17β
INTRODUCTION
Porcine embryos secrete enhanced levels of estrogens, mainly estradiol-17β (E2) between days 10 and 13 after fertilization, just before implantation in the process of the maternal recognition of pregnancy (1-4). It has been demonstrated that systemic estrogen administration between days 11 and 13 of the estrous cycle prevents corpus luteum (CL) regression and extends this gland life span in the pig (5). The action of estrogens may be luteoprotective and antiluteolytic (3, 4, 6).
However, estrogen alone, does not fully exert the effect of the embryo on growth and development of CL (7). It has been suggested that besides estrogens, prostaglandin E2 (PGE2) could be involved in the maternal recognition of pregnancy as a luteoprotective/antiluteolytic factor (8, 9). Endometrial PGE2, and prostaglandin F2α (PGF2α) which has an opposite luteolytic effect, are involved in reproduction processes in many species (10, 11). It was demonstrated that inhibition of prostaglandin (PG) synthesis before implantation causes pregnancy failure in different species, including the pig (11, 12).
PGs are synthesized by prostaglandin-endoperoxide synthase (PTGS) and specific terminal prostaglandin synthases: PGE synthase and PGF synthase (PGFS) (13, 14). Moreover, PGE2 can be converted into PGF2α by prostaglandin 9-ketoreductase/carbonyl reductase (CBR1) (15-17). It was found that highly inducible forms of prostaglandin-endoperoxide synthase and PGE synthase in the porcine endometrium are PTGS2, (known also as PGHS-2 or COX-2) (18, 19) and microsomal PGE synthase-1 (mPGES-1), respectively (14).
Before implantation, the endometrium and trophoblast synthesize elevated amounts of PGE2 which results in high content of this prostanoid in the uterine lumen and/or utero-ovarian circulation in the pig (9, 14, 17, 20). Our recent study has demonstrated that expression of prostaglandin synthesis pathway enzymes is altered in the porcine conceptus and endometrium to favor luteoprotective PGE2 synthesis between days 10 and 13 of pregnancy (14, 17).
PGE2 mediates its effect via binding to either of four subtypes of G-protein-coupled receptors (PTGER1-4 also known as EP1-4), which are encoded by four separate genes (21). The effect of PGE2 in endometrial angiogenesis, uterine receptivity, and decidualization is mediated by cAMP-dependent mechanisms in rats (22), rabbits (23) and human (24). Only two subtypes of PGE2 receptors - PTGER2 and PTGER4 are coupled to adenylate cyclase and generate cAMP that activates the protein kinase A signaling pathway (21). Knockout studies in mice indicated that PTGER2, but not PTGER1, PTGER3, or PTGER4 is associated with reproductive dysfunction (25). PTGER2-deficient mice demonstrate impaired ovulation and dramatic reduction in litter size (25). In pigs, the PGE2-binding sites were detected in both cycling and pregnant endometrium but their location within endometrium was not determined (26). Moreover, no mRNA or protein expression of any PGE2 receptor subtype has been identified in the porcine endometrium yet. To-date, regulation of expression of PGE2 receptors in porcine endometrium remains unknown. Therefore, the objective of the present study was to elucidate whether the well established porcine embryo signal, E2 and the putative embryo signal, PGE2 regulate expression of prostaglandin synthesis pathway enzymes: PTGS2, mPGES-1, PGFS and CBR1, as well as PGE2 receptors (PTGER2 and PTGER4) in the porcine endometrium. Moreover, the aim of study was to determine the site of action of PGE2 in the porcine endometrium during the estrous cycle and early pregnancy.
MATERIALS AND METHODS
Tissue Collection
Peripubertal crossbred gilts of similar age (approximately 5-5.5 months) were observed daily for onset of estrus. After exhibiting two natural estrous cycle gilts were assigned into two groups: pregnant and cycling. Gilts assigned to the pregnant groups were artificially inseminated at 12 h after onset of estrus (day 0) and 24 h later. Gilts were slaughtered at a local abattoir on either day 9, 11, 12 or 15 of pregnancy or the estrous cycle (n = 4-6 per every group). The stage of the estrous cycle was verified by utero-ovarian morphology (14) and pregnancy was confirmed by the presence of morphologically normal conceptuses. Uterine tissues from all groups were fixed in 4% neutral buffered formaldehyde overnight at 4 C, stored in 70% ethanol, and wax embedded (for immunohistochemical analyses). The endometrium was snap-frozen and stored until further use (for RNA and protein extraction). Additionally, uteri collected from gilts on days 11-12 of the estrous cycle were immediately placed in ice-cold phosphate-buffered saline (PBS) and transported to the laboratory within 1 to 1.5 h for in vitro explant tissue and cell culture.
To determine if there are differences in expression of mPGES-1 protein between regions near the conceptus and without proximity to the conceptus, endometrial samples were collected from the implantation sites (n=4) and interimplantation sites (n=4) in day 25 pregnant gilts (n=4).
All procedures involving animals were approved by the Local Research Ethics Committee and were conducted in accordance with the national guidelines for agricultural animal care.
Endometrial Explant Culture
Uteri collected from gilts on days 11-12 of the estrous cycle (n=6) were washed three times in sterile PBS. The endometrial tissue from middle of uterine horn was dissected and cut into small pieces (2 × 3 mm) and then washed three times in medium M199 (Sigma-Aldrich Co., St Louis, MO, USA). A total of 100 mg of tissue was placed into each glass vial with 2 ml of M199 medium containing 0.1 % bovine serum albumin (ICN Biomedicals, Inc., Costa Mesa, CA, USA), 5% dextran/charcoal-stripped newborn calf serum (Sigma-Aldrich), penicillin (100 IU/ml) and streptomycin (100 μg/ml). The endometrial tissue explants were preincubated in a shaking water bath at 37 C in a humified atmosphere of 5% CO2 in air for 2 h.
After this period, explants were treated with vehicle only (control), or PGE2 (100 nM, ICN), or E2 (1, 10, 100 nM; Sigma-Aldrich Co.), or phorbol 12-myristate 13-acetate (PMA; 100 nM; Sigma-Aldrich Co.) and incubated for an additional 24 h period. The structure of PMA is analogous to diacylglycerol and serves as an agonist for activation of protein kinase C. Since it is known that PMA increases PG secretion, it was used as a positive control (27, 28).
All treatments were performed in duplicate in six independent experiments. After culture the medium were collected and stored at −20 C until PG concentrations were measured by EIA. Furthermore, endometrial tissue explants were snap-frozen in liquid nitrogen (for RNA and protein extraction) and stored at -80 C until further use.
Real-time PCR Quantitation
Total RNA was extracted from endometrial samples and tissue explants collected after in vitro experiment using the Total RNA Prep Plus kit (A&A Biotechnology, Gdansk, Poland) and treated with DNase I (Invitrogen Life Technology Inc., Carlsbad, CA, USA) according to the manufacturer’s protocol. Real time PCR was performed with the Applied Biosystems 7300 Real-Time PCR System (Applied Biosystems, CA, USA) using QuantiTect SYBR Green PCR master mix (Qiagen GmbH, Hilden, Germany), as described previously (14, 17). Briefly, total RNA was reverse transcribed using oligo(dT) primer and avian myeloblastosis virus reverse transcriptase (Promega, Madison, WI, USA). Real-time PCR reaction (25 μl) included 12.5 μl QuantiTect SYBR Green PCR master mix, 0.5 μM sense and antisense primers each and reverse transcribed cDNA (3.5 μl of diluted RT product). The specific primers were used to evaluate the mRNA levels (Fig 1G). For quantification, standard curves consisting of serial dilutions of the appropriate purified cDNA were included. Before amplification, an initial denaturation (15 min at 95 C) step was used. The PCR programs for each gene were performed as follows: 36 cycles of denaturation (15 s at 95 C), annealing (30 s at 52.5 C for PGFS; or at 55 C for PTGS2, mPGES-1, CBR1 and β-actin) and elongation (60 s at 72 C). The PCR programs for PTGER2 and PTGER4 genes were performed as follows: 36 cycles of denaturation (15 s at 95 C), annealing (60 s at 60 C). After PCR, melting curves were acquired by stepwise increases in the temperature from 60-95 C to ensure that a single product was amplified in the reaction. Data obtained from the real-time PCR for PTGS2, mPGES-1, PGFS, CBR1, PTGER2 and PTGER4 were normalized against β-actin. Control reactions in absence of reverse transcriptase were performed to test for genomic DNA contamination.
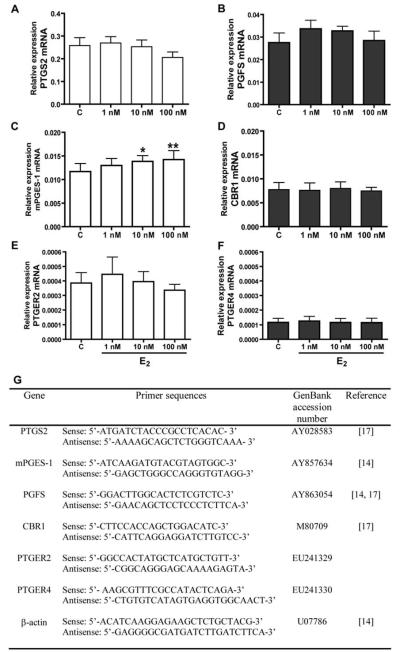
Figure 1.
Effect of E2 (1, 10 or 100 nM) on mRNA expression of prostaglandin-endoperoxide 2 (A), PGF synthase (B), PGE synthase (C), prostaglandin 9-ketoreductase (D) and PGE2 receptors: PTGER2 (E) and PTGER4 (F) in endometrial tissue explants in vitro. Asterisks indicate significant differences between control (C) and experimental groups (* p<0.05; ** p<0.01). (G) Primers used for Real-time PCR.
Protein extraction
Endometrial samples and tissue explants were homogenized on ice in buffer containing 50 mM Tris-HCl, pH 8.0; 150 mM NaCl, 1mM EDTA and supplemented with protease inhibitor cocktail (Sigma-Aldrich Co.). Homogenates were then centrifuged for 15 min at 800 x g at 4 C and stored at −70 C for further analysis. The protein concentration was determined by the Bradford (29) method.
Western blot analysis
Western blot analysis was performed as we previously described (14, 17). Protein extracts (30 μg) were dissolved in SDS gel-loading buffer (50 mM Tris-HCl, pH 6.8; 4% SDS, 20% glycerol and 2% β-mercaptoethanol), heated to 95 C for 4 min and separated on 15% (for mPGES-1), 12% (for PGFS and CBR1) and 10% (for PTGS2, PTGER2 and PTGER4) SDS-PAGE. Separated proteins were electroblotted onto 0.2 μm nitrocellulose membrane in transfer buffer (20 mM Tris-HCl buffer, pH 8.2; 150 mM glycine, 20% methanol). After blocking in 5% non-fat dry milk in TBS-T buffer (Tris-buffered saline, containing 0.1% Tween-20) for 1.5 h at 25.6 C, the membranes were incubated overnight with 1:750 anti-COX-2 antibody (anti-PTGS2 antibody; Cayman Chemical, Ann Arbor, MI, USA), or 1:1000 polyclonal anti-mPGES-1 antibody (Cayman Chemical), or 1:2000 anti-lung-type PGFS antiserum (kindly donated from Prof. Kikuko Watanabe, University of East Asia, Yamaguchi, Japan), or 1:2000 polyclonal anti-human carbonyl reductase 1 antibody (Abcam, Cambridge, UK), or 1:200 rabbit polyclonal antibodies against human EP2 (anti-PTGER2 antibody; Cayman Chemical), or 1:50 rabbit polyclonal antibodies against human EP4 (anti-PTGER4 antibody; Cayman Chemical) at 4 C. Subsequently, the studied proteins were detected by incubating the membrane with 1:20000 dilution of secondary polyclonal anti-rabbit alkaline phosphatase-conjugated antibodies (for PTGS2, mPGES-1, PGFS, PTGER2 and PTGER4; Sigma-Aldrich Co.) and 1:2000 dilution of anti-goat alkaline phosphatase-conjugated antibodies (for CBR1, Abcam) for 1.5 h at 25.6 C. Immune complexes were visualized using alkaline phosphatase visualization procedure. Western blots were quantitated using Kodak 1D program (Eastman Kodak, Rochester, NY, USA). Sample loading was standardized to expression of β-actin using specific antibodies (1:3000; Abcam). Control experiments were performed for PTGER receptors by incubating the membranes with anti-PTGER2 or anti-PTGER4 antibodies preabsorbed with the corresponding immunogenic blocking peptide (Cayman Chemical). Western blot analyses for PTGER receptors by incubating the membranes with the primary antibodies preabsorbed with the related blocking peptides did not give any signal. Specificity of other antibodies used was confirmed previously (14, 17, 30).
EIA of PGE2 and PGF2α
Concentrations of PGE2 in medium were determined by an enzyme immunoassay (EIA) as described previously (31). Cross-reactivities of the anti-PGE2 antiserum (donated by Dr. Seiji Ito, Kansai Medical University, Osaka, Japan) were as follows: PGE1 18%, PGA1 10%, PGA2 4.6%, PGB2 6.7%, PGD2 0.13%, PGF2α 2.8%, PGJ2 14% and 15-keto-PGE2 0.05%. Assay sensitivity was 0.19 ng/ml and the intra- and interassay coefficients of variation were 4.9% and 8.5%, respectively. Concentrations of PGF2α were determined by EIA test as described previously (31). Cross-reactivities of the anti-PGF2α antiserum (Sigma-Aldrich Co.) were as follows: PGF1α 60%, PGE1 and PGE2 <0.1%, and PGA1, PGA2, PGB1 and PGB2 <0.01%. Assay sensitivity was 0.23 ng/ml and the intra- and interassay coefficients of variation were 8.9% and 11.9%, respectively.
cAMP assay
Endometrial tissue (100 mg) collected from gilts on days 11-12 of the estrous cycle (n = 6) were minced finely into small pieces (2 × 3 mm). The tissue explants were incubated overnight at 37 C in a humidified 5% CO2 incubator in 2 ml M199 medium (Sigma-Aldrich Co.) containing 100 IU penicillin and 100 μg streptomycin and 3 μg/ml indomethacin (Sigma-Aldrich Co.). Following overnight treatment, the tissue was incubated in M199 medium, containing 100 IU/ml penicillin and 100 μg/ml streptomycin, 3 μg/ml indomethacin and 1 mM 1-methyl-3-iso-butylxanthine (Sigma-Aldrich Co.) in the absence or presence of the appropriate receptor antagonist as shown in Figure 5C legend at 37 C for 30 min. It was then treated with vehicle, or 100 nM PGE2 in presence/or absence of 10 μM AH6809 (PTGER2 antagonist; Sigma-Aldrich Co.) or 5 μM GW 627368X (PTGER4 antagonist, Cayman Chemical) (32) for 10 min at 37 C and washed in ice-cold PBS and snap-frozen. Tissue explants were homogenized in Lysis Buffer (100 mg of tissue per 1 ml of the buffer; R&D Systems, Abingdon, UK) and cAMP concentration was quantified in the homogenates by ELISA using a cAMP kit (R&D Systems) according to the manufacturer’s protocol. The results were normalized to the protein concentration which was determined using protein assay kit (Bio-Rad, Hemel Hempstead, Herts, UK).
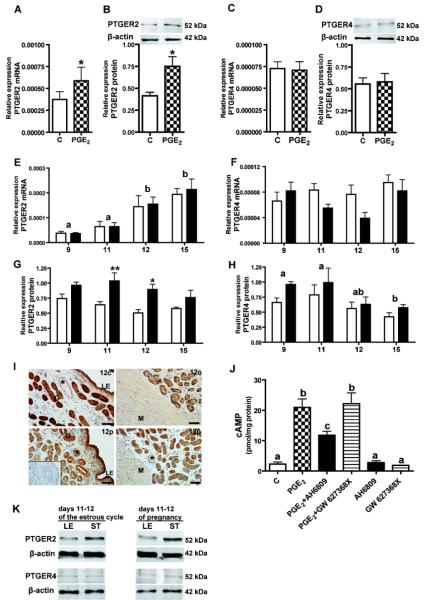
Figure 5.
Regulation of PTGER2 and PTGER4 expression, cAMP signaling and localization of PTGER2 expression in the porcine endometrium.
Effect of PGE2 (100 nM) on mRNA and protein expression of PGE2 receptors: PTGER2 (A, B) and PTGER4 (C, D) in endometrial tissue explants in vitro. Asterisks indicate significant differences between control (C) and experimental group (* p<0.05). Expression of PTGER2 (E) and PTGER4 (F) mRNA during the estrous cycle (white bar) and early pregnancy (black bar). Expression of PTGER2 (G) and PTGER4 (H) protein during days 9-15 of the estrous cycle (white bar) and early pregnancy (black bar). Days with different letters for expression of PTGER2 mRNA and PTGER4 protein are statistically different. PTGER4 mRNA is not affected by day or reproductive status (p>0.05). The asterisk represents differences (* p<0.05; ** p<0.01) for PTGER2 protein abundance between cyclic and pregnant gilts on days 11 and 12 of the estrous cycle and pregnancy.
I, Immunolocalization of PTGER2 protein in luminal (LE) and glandular epithelium (G) endometrium, blood vessels (*) and myometrium (M) in the uterus of pregnant and cycling gilts. Representative immunohistochemical analyses are presented: 12c – day 12 of the estrous cycle; 12p – day 12 of pregnancy. Insets: negative control with preabsorbed antibodies. Scale bar, 50 μm. J, cAMP accumulation in endometrial tissue explants in response to treatment with 100 nM PGE2 in the presence/absence of PTGER2 antagonist (AH6809), or PTGER4 antagonist (GW 627368X). Additionally, endometrial explants were treated with PTGER2 or PTGER4 antagonists alone. Means with different letters indicate significant differences (p<0.05). K, Protein expression of PTGER2 and PTGER4 in luminal epithelial (LE) and stromal cells (ST) isolated from uterus on days 11-12 of the estrous cycle and pregnancy.
Immunohistochemistry
Immunohistochemical localization of PTGER2 in the endometrium on days 9, 11, 12, 15 of the estrous cycle and pregnancy (n=3-4 in every group) was performed using Bond-X automated immunostaining machine (Vision Biosystems, Newcastle, UK). The method on the Bond-X automated machine utilizes a specific polymer high contrast program. Briefly, cross-sections were made in the middle portion of the uterine horn. Five micron paraffin sections of uterine tissue were cut, dewaxed in xylene and dehydrated using decreasing grades of ethanol. Antigen retrieval was performed by pressure-cooking in 0.01 M sodium citrate (pH 6) before being place on the Bond-X machine. Then, endogenous peroxidase activity was removed by fixing sections in 3% hydrogen peroxide for 5 min in methanol. Afterwards, slides were incubated with rabbit polyclonal anti-PTGER2 (1:1000; Cayman Chemical) antibody for 2 h, and treated with the polymer reagent (Vision Biosystems) for 15 min. Detection step was performed using 3,3’-diaminobenzidine tetrahydrochloride for 10 min and sections were counterstained in haematoxylin for 5 min. Slides were then removed from the machine and dehydrated and mounted using Pertex. To confirm antibody specificity, negative controls were performed by incubating sections with a control rabbit IgG at the matched concentrations to the primary antibodies and with the primary antibody preabsorbed with the peptide it was raised (Cayman Chemical). Immunoreactivity was negligible with both preabsorbed antibody and with a control rabbit IgG.
Endometrial cell culture in vitro
Endometrial epithelial and stromal cells were isolated from uteri on days 11-12 of the estrous cycle (n=5) and pregnancy (n=3), as described previously (31), and seeded in 6-well plates. Cells cultures were incubated in M199 medium containing 2% bovine serum albumin (ICN Biomedicals), 10% newborn calf serum (Sigma-Aldrich Co.), penicillin (100 IU/ml) and streptomycin (100 μg/ml) at 37 C in atmosphere of 95% air and 5% CO2. At 80-90% cell confluence medium was replaced with serum-free M199. After 24 h cells were harvested and lysed in RIPA buffer (50 mM Tris; pH 7.4; 150 mM NaCl; 1% Triton X-100; 0.5% sodium deoxycholate; 0.1% SDS; 1 mM EDTA; protease inhibitor cocktail) and the obtained protein extracts were used to study the expression of PTGER2 and PTGER4 by Western blot analyses.
Statistical analysis
Statistical analyses were performed using Repeated measures one-way ANOVA or Paired t-Test (GraphPad Prism 4.0, Graphpad Software, San Diego, CA, USA). Two-way ANOVA was used to evaluate changes in abundance of PTGER2 and PTGER4 mRNA and protein due to day of the estrous cycle and pregnancy as well as an effect of reproductive status. All numerical data are presented as the mean ± SEM and differences were considered as statistically significant when p<0.05.
RESULTS
Effect of E2 on PTGS2, mPGES-1, PGFS, CBR1, PTGER2 and PTGER4 expression and PGE2 and PGF2α secretion in endometrial tissue explants
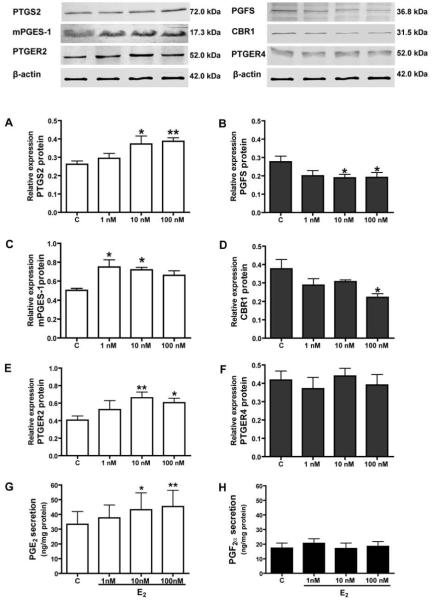
E2 elevated expression of mPGES-1 mRNA (10 nM and 100 nM, p<0.05 and p<0.01, respectively) but had no effect on PTGS2, PGFS, CBR1, PTGER2 and PTGER4 mRNA content in endometrial tissue explants (Fig. 1). E2 stimulated expression of PTGS2 (10 and 100 nM; p<0.05 and p<0.01, respectively), mPGES-1 (1 nM and 10 nM, p<0.05) and PTGER2 protein (10 nM and 100 nM) but decreased (p<0.05) expression of PGFS (10 nM and 100 nM) and CBR1 protein (100 nM) and had no effect on PTGER4 protein content (Fig. 2, A-F). E2 treatment increased PGE2 secretion in a dose dependent manner (Fig. 2G). However, there was no effect of E2 treatment on PGF2α secretion by endometrial tissue explants (Fig. 2H).
Figure 2.
Effect of E2 (1, 10 or 100 nM) on protein expression of prostaglandin-endoperoxide 2 (A), PGF synthase (B), PGE synthase (C), prostaglandin 9-ketoreductase (D) and PGE2 receptors: PTGER2 (E) and PTGER4 (F) in endometrial tissue explants in vitro. The representative samples of Western blots are shown in upper panels. Effect of E2 (1, 10 or 100 nM) on PGE2 (G) and PGF2α secretion (H) in endometrial tissue explants in vitro. Asterisks indicate significant differences between control (C) and experimental groups (* p<0.05; ** p<0.01).
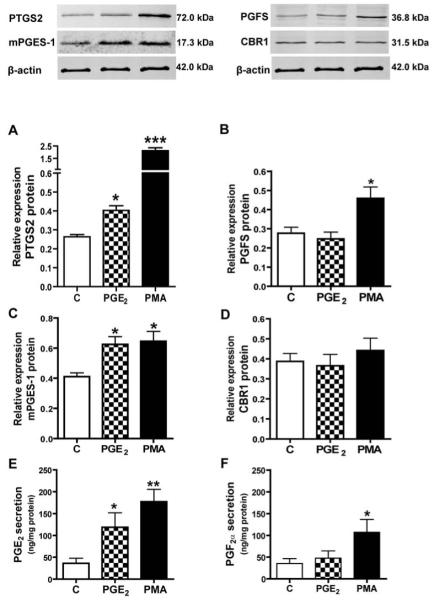
Effect of PGE2 on PTGS2, mPGES-1, PGFS, CBR1, PTGER2 and PTGER4 expression and PGE2 and PGF2α secretion in endometrial tissue explants
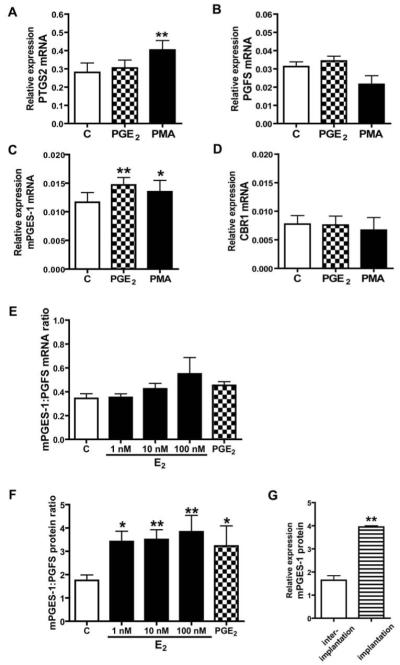
PGE2 stimulated mPGES-1 (p<0.01; Fig. 3C) and PTGER2 mRNA expression (p<0.05; Fig. 5A) and had no effect on PTGS2, PGFS and CBR1 mRNA content in endometrial explants (Fig. 3, A, B and D). PGE2 enhanced PTGS2 (Fig. 4A), mPGES-1 (p<0.05, Fig. 4C) and PTGER2 protein expression (p<0.5, Fig. 5B) as well as PGE2 secretion by endometrial tissue explants (p<0.05, Fig. 4E). By contrast, PGE2 had no effect on PGFS and CBR1 expression and PGF2α release (Fig 4, B, D and F) and PTGER4 mRNA and protein expression (Fig. 5, C and D). PMA used as a positive control stimulated both PTGS2 and mPGES-1 mRNA and protein expression (p<0.05, Fig. 3A, C and Fig. 4, A and C) and PGFS protein levels (p<0.05, Fig. 4B) but had no effect on CBR1 expression (Fig. 3D and Fig. 4D). Moreover, PMA increased secretion of PGE2 (p<0.01, Fig. 4E) and PGF2α (p<0.05, Fig. 4F).
Figure 3.
Effect of PGE2 (100 nM) and phorbol-12-myristate-13-acetate (100 nM) on mRNA expression of prostaglandin-endoperoxide 2 (A), PGF synthase (B), PGE synthase (C) and prostaglandin 9-ketoreductase (D) in endometrial tissue explants in vitro. Effect of PGE2 and E2 on the ratios of mPGES-1:PGFS mRNA (E) and protein (F). Expression of mPGES-1 protein in implantation and interimplantation sites. Asterisks indicate significant differences between control (C) and experimental groups (* p<0.05; ** p<0.01).
Figure 4.
Effect of PGE2 (100 nM) and phorbol-12-myristate-13-acetate (100 nM) on protein expression of prostaglandin-endoperoxide 2 (A), PGF synthase (B), PGE synthase (C) and prostaglandin 9-ketoreductase (D) in endometrial tissue explants in vitro. The representative samples of Western blots are shown in upper panels. Effect of PGE2 (100 nM) and phorbol-12-myristate-13-acetate (100 nM) on PGE2 (E) and PGF2α secretion (F) in endometrial tissue explants in vitro. Asterisks indicate significant differences between control (C) and experimental groups (* p<0.05; ** p<0.01; *** p<0.001).
PGE2 and E2 elevated about 2-fold the mPGES-1/PGFS protein ratios in endometrial tissue explants (Fig. 3F).
Endometrial expression of mPGES-1 protein in implantation and interimplantation sites
Expression of mPGES-1 protein was up-regulated in endometrium collected from the implantation sites when compared to interimplantaion sites (p<0.01; Fig 3G).
Effect of PGE2 on cAMP accumulation in endometrial explants
Treatment with PGE2 increased cAMP production in endometrial tissue explants in absence of any PTGER antagonists (p<0.001; Fig. 5J). Co-treatment with PTGER2 antagonist (AH6809) inhibited significantly the PGE2-mediated cAMP production. However, the response to PGE2 was not suppressed by co-treatment with the PTGER4 antagonist (GW 627368X). No significant alteration in basal levels of cAMP was observed in endometrial tissue treated with the antagonists alone.
Expression of PTGER2 and PTGER4 in the endometrium during the estrous cycle and early pregnancy
No effect of day or reproductive status was detected for PTGER4 mRNA expression (Fig. 5F). PTGER4 protein and PTGER2 mRNA expression was affected by day but not reproductive status (Fig. 5H and E). PTGER2 mRNA content was significantly higher on day 12 and 15 in cyclic and pregnant gilts when compared to days of 9 and 11 the estrous cycle and pregnancy (Fig 5E). Expression of PTGER2 protein was affected by reproductive status but not day. PTGER2 protein expression was up-regulated on day 11and 12 of pregnancy when compared to corresponding days of the estrous cycle (p<0.05; Fig 5G).
PTGER2 protein was highly expressed in luminal and glandular epithelium of endometrium and in blood vessels, and was present in myometrium (Fig. 5I). Protein expression of PTGER2 was detected on days 9 to 15 of the estrous cycle and pregnancy. Moreover, protein expression of PTGER2 and PTGER4 was confirmed in both luminal epithelial and stromal cells isolated from the uterus on days 11-12 of the estrous cycle and days 11-12 of pregnancy (Fig. 5K).
DISCUSSION
Present hypotheses about the antilutolytic mechanism and recognition of pregnancy in the pig are mainly focused on way of the PGF2α secretion (2, 4, 33). Although PGE2 has been reported to have luteoprotective effects (8, 9), currently there are no studies describing the molecular mechanisms regulating PGE2 synthesis in the endometrium during early pregnancy. To our knowledge, this is the first study to address the effect of PGE2 and embryo signal, E2 on the prostaglandin biosynthesis pathway and PGE2 signaling in the porcine endometrium. PTGS2 is an enzyme involved in nonselective production of PGs, whereas PG synthases (mPGES-1 and PGFS) and CBR1 control the selective production and associated specific functions of PGE2 and PGF2α. We have found that PGE2 biosynthetic pathways are selectively activated in the porcine endometrium (14). Our hypothesis was that selective changes in expression of enzymes involved in PG synthesis and in the porcine endometrium are a result of embryo signals.
Even spherical day 10-11 blastocycts sized 5-7 mm in diameter are capable for enhanced estrogen synthesis (34, 35). Synthesis and secretion of estrogens by conceptuses dramatically increase during trophoblast elongation on days 11-12 of pregnancy, decline rapidly on day 13, and then initiate a second more sustained increase on days 16-25 of pregnancy (4, 36). In the present studies, we demonstrated that E2 stimulated PGE2 synthesis through increase of PTGS2 protein and mPGES-1 mRNA and protein expression and decreased the protein content of enzymes involved in PGF2α production – PGFS and CBR1 in the porcine endometrium on days 11-12 after estrus. Stimulation of PTGS2 protein expression in endometrial explants by estradiol-17β in vitro observed by us is in agreement with the observation that administration of estrogen in vivo affects expression pattern of PTGS2 (18). The data presented herein are also consistent with our previous findings that mPGES-1 expression is higher and PGFS and CBR1 - lower in the porcine endometrium between days 10 and 13 of pregnancy when compared to following days of gestation (14, 17). The observations in the explant cultures after treatment with conceptus factors corresponds also with comparison of CBR1 and PGFS protein expression in pregnant versus cyclic animals on days 10-11 and 10-13, respectively (14, 17). In contrast, our previous data showed no differences in mRNA and protein expression of mPGES-1 in the endometrium on days 11-12 of pregnancy and days 11-12 of the estrous cycle (14). This discrepancy between stimulation of mPGES-1 expression in explants by conceptus factors (E2 and PGE2) and lack of effect of pregnancy on mPGES-1 mRNA and protein can be a result of local changes induced in the endometrium by the conceptus. It is not possible to distinguish which endometrial samples collected on day 11-12 of pregnancy had direct contact with the embryo since implantation begins on day 14. It is tempting to speculate that effect of conceptus factors on endometrial explants can mimic local changes in the endometrium which is near the conceptus. This hypothesis, although needs further study, is supported by results concerning later stage of pregnancy and indicating two-fold up-regulation of mPGES-1 protein expression in the endometrium in the implantation sites when compared with interimplantation sites (Fig. 3G). It is likely that effects of estrogen on the endometrium are restricted to regions in close proximity to the conceptus due to metabolic activity of trophectoderm. During pregnancy, pig endometrium rapidly converts estradiol-17β to the biologically inactive estrone sulfate which is present in high concentrations within the uterine lumen of pregnant pigs (37). The trophectoderm has sulfatase enzyme activity that restores the biological activity of estrogen, allowing for a localized effect of estrogen to up-regulate genes such as SPP1 in luminal epithelium (38).
Profile of estrogen secretion by conceptus (36) highly corresponds with selective and dramatic changes in PG synthesis enzymes during early pregnancy, reported previously by us (14, 17). Endometrial mPGES-1 expression exhibits a biphasic profile, similar to estrogen synthesized and secreted by conceptuses. By contrast, rapid increase of PGFS and CBR1 after initiation of implantation corresponds with the decline in conceptus estrogen synthesis. Our findings are in agreement with in vivo observations which demonstrate that estrogens increase PGE2 content in the porcine uterus (5) and therefore may be an important factor in increase of the PGE2:PGF2α ratio during early pregnancy.
CBR1 is the enzyme which can modulate both PG concentrations since it converts PGE2 into PGF2α (15, 16). In the present study, E2 inhibited CBR1 protein expression. This is consistent with reports demonstrating inhibition of prostaglandin 9-ketoreductase activity in the sheep endometrium during the maternal recognition of pregnancy (39). Similarly, interferon-τ, the conceptus signal in ruminants, down-regulates expression of prostaglandin 9-ketoreductase gene and increases PGE2 secretion in epithelial cells of bovine endometrium (40). Moreover, E2 inhibits prostaglandin 9-ketoreductase activity in human placenta (41).
E2 had a significant effect mainly on protein levels but did not influence the mRNA content of most factors investigated (except of mPGES-1). This can be explained by the fact that steroids affect stability of many mRNAs that are important in reproductive processes and in this way may influence gene expression levels and function (42).
In vivo studies demonstrated that timing of endometrial exposure to estrogen is critical to establishment of pregnancy (18). Early administration of estrogen on days 9 and 10 of pregnancy probably desynchronizes the uterine environment for conceptus implantation resulting in later embryonic loss as well as alters the pattern of many gene expression, including endometrial PTGS2 mRNA and protein expression (18, 43). Therefore, in our experiment we used gilts on days 11-12 of the estrous cycle that corresponds to the normal period of conceptus E2 secretion. We did not choose the 11-12 day pregnant gilts because their endometrium would have been exposed already to embryo signal and hence resulted in altered expression of target genes of interest.
These studies indicate for the first time, the existence of PGE2 autoamplification loop in the porcine endometrium that can be involved in the increase of PGE2:PGF2α ratio during the maternal recognition of pregnancy. Porcine endometrial tissue explants produced cAMP in response to PGE2 treatment. Increase of PGE2 mediated-cAMP production occurred via PTGER2 since cAMP production in response to PGE2 was inhibited following co-treatment with the PTGER2 specific antagonist. Our study suggests that PGE2 acts through endometrial PTGER2 receptor, activates cAMP signaling pathway and elevates the mRNA and protein expression of enzymes involved in PGE2 synthesis (namely PTGS2 and mPGES-1) and secretion of this prostanoid by the endometrium (Fig. 6). It is consistent with the reports that PGE2 itself may induce its own secretion in other cell types (44-46). Both PGE2 and estradiol-17β increased the mPGES-1:PGFS protein ratios which are indicative of the relative PGE2 to PGF2α production in the endometrium. Moreover, E2 and PGE2 stimulated endometrial PTGER2 expression that corresponds to up-regulation of PTGER2 protein content in the endometrium on days 11-12 of pregnancy when compared to corresponding days of the estrous cycle. These data provide a novel mechanism of positive feedback loop that contributes to increased PGE2 synthesis in the porcine uterus during the peri-implantation window (Fig. 6). Similar observations have been reported for the bovine embryo recognition signal, interferon-τ (47).
Figure 6.
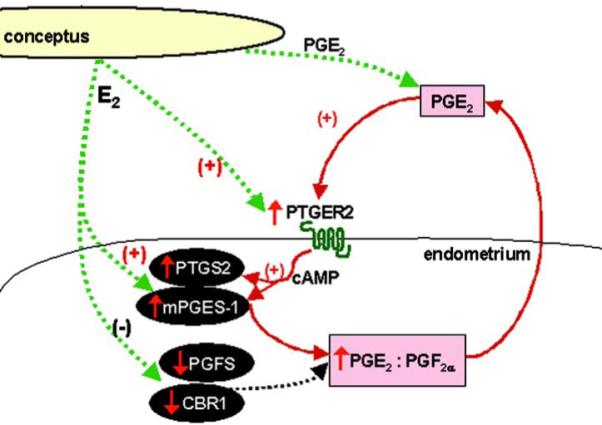
Potential mechanism involved in increasing luteoprotective PGE2 level and the PGE2:PGF2α ratio during the maternal recognition of pregnancy in the pig - involvement of estradiol-17β, PGE2 and endometrial PGE2 receptors (PTGER2) in PGE2 positive feedback loop in the porcine endometrium.
The primary conceptus signal, E2 and another conceptus factor - PGE2 regulate the protein expression of endometrial PG synthesis pathway enzymes to favor production of luteoprotective PGE2. E2 up-regulates PTGS2 and PGE synthase protein expression and down-regulates endometrial PGFS and CBR1 protein concentrations increasing PGE2 secretion and the PGE2:PGF2α ratio. PGE2 acts through endometrial PTGER2 receptor, activates cAMP signaling pathway and elevates the mRNA and protein expression of enzymes involved in PGE2 synthesis (PTGS2 and mPGES-1) and its own secretion in the endometrium.
It is likely that PGE2 produced in the uterus acts in autocrine/paracrine manner via PGE2 receptors, resulting in the local increase of endometrial vascular permeability and preparation for angiogenesis and implantation (11, 48). We have identified the mRNA and protein expression of two subtypes of PGE2 receptors coupled with cAMP signaling pathway, in the porcine endometrium, for the first time. PTGER2 protein was highly expressed in the luminal and glandular epithelium of endometrium and in uterine blood vessels, and moderately in myometrium in both pregnant and cycling gilts. Both epithelial and stromal cells isolated from the uterus of cycling and pregnant gilts expressed PTGER2 and PTGER4 protein. On basis of our present and previous results (14), it may be concluded that the site of PGE2 action in the porcine endometrium corresponds highly to localization of PGE2 synthesis. The present results are also consistent with localization of PTGER2 in bovine and human endometrium (49, 50). It is suggested that in the rat and mouse PTGER2 is involved in implantation, whereas PTGER4 is important in the process of decidualization (11). Role of PTGER4 could vary in different species. This hypothesis is supported by fact that in contrast to rodents and human, in the bovine endometrium PTGER4 expression is very low (50). The specific roles of these receptors in the porcine endometrium remains to be elucidated. However, the present results indicate that expression of PTGER4 is not affected by reproductive status or by factors secreted by conceptus during the maternal recognition of pregnancy. On the other hand, our studies suggest an important role for PTGER2 in the porcine maternal-embryo communication.
In contrast to PTGER2 and PGE synthase, expression of the enzymes involved in PGF2α production (PGFS and CBR1) was not affected by PGE2 treatment. As predicted, expression of PTGS2, mPGES-1 and PGFS as well as PGE2 and PGF2α secretion from endometrial explants was stimulated by PMA, used in the present study as a positive control. PMA mimics diacylglycerol activation of protein kinase C and it was demonstrated that this phorbol ester stimulates both PG secretion and expression of PG synthesis enzymes (27, 28).
In summary, the present study provides the first direct evidence that primary conceptus signal, E2 and another conceptus factor - PGE2 regulate the protein expression of endometrial PG synthesis pathway enzymes to favor production of luteoprotective PGE2 in pigs. Our novel findings indicate that one potential mechanism of inhibition of luteolysis in the pig is the up-regulation of PTGS2 and PGE2 synthase protein expression and the down-regulation of endometrial PGFS and CBR1 protein concentrations by E2. Our results suggest that E2 produced by the porcine trophoblast prevents luteolysis through enzymatic modification of PG synthesis in endometrial cells.
PGE2 could exert a luteoprotective effect as well as it may act locally through endometrial PGE2 receptors, especially PTGER2. Moreover, endometrial PTGER2 may be involved in a positive feedback loop during increased PGE2 synthesis in the porcine uterus in peri-implantation window.
ACKNOWLEDGEMENTS
We are very grateful to Jan Klos, Katarzyna Gromadzka-Hliwa, Sheila Wright and Nancy Evans for technical assistance. We thank Michal Blitek for help in care and handling of animals. Moreover, the authors would like to acknowledge Prof. Kikuko Watanabe (University of East Asia, Yamaguchi, Japan) for providing anti-PGFS antibody. This research was supported by grant N N311 319135 from the State Committee for Scientific Research in Poland and by COST (GEMINI)-STSM-FA0702-03685 grant (AW). Agnieszka Waclawik was awarded the Domestic Grant for Young Scientists from the Foundation for Polish Sciences.
Footnotes
Disclosure statement: The authors of this manuscript have nothing to disclose.
Summary Sentence: Estradiol-17β, prostaglandin E2 (PGE2) and the PTGER2 are involved in PGE2 positive feedback loop in the porcine endometrium.
REFERENCES
- 1.Perry JS, Heap RB, Amoroso EC. Steroid hormone production by pig blastocysts. Nature. 1973;245:45–47. doi: 10.1038/245045a0. [DOI] [PubMed] [Google Scholar]
- 2.Bazer FW, Thatcher WW. Theory of maternal recognition of pregnancy in swine based on estrogen controlled endocrine versus exocrine secretion of prostaglandin F2alpha by the uterine endometrium. Prostaglandins. 1977;14:397–400. doi: 10.1016/0090-6980(77)90185-x. [DOI] [PubMed] [Google Scholar]
- 3.Ford SP, Magness RR, Farley DB, Van Orden DE. Local and systemic effects of intrauterine estradiol-17 beta on luteal function of nonpregnant sows. J Anim Sci. 1982;55:657–664. doi: 10.2527/jas1982.553657x. [DOI] [PubMed] [Google Scholar]
- 4.Spencer TE, Bazer FW. Conceptus signals for establishment and maintenance of pregnancy. Reprod Biol Endocrinol. 2004;2:49. doi: 10.1186/1477-7827-2-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geisert RD, Thatcher WW, Roberts RM, Bazer FW. Establishment of pregnancy in the pig III. Endometrial secretory response to estradiol valerate administered on day 11 of the estrous cycle. Biol Reprod. 1982;27:957–965. doi: 10.1095/biolreprod27.4.957. [DOI] [PubMed] [Google Scholar]
- 6.Conley AJ, Ford SP. Direct luteotrophic effect of oestradiol-17 beta on pig corpora lutea. J Reprod Fertil. 1989;87:125–131. doi: 10.1530/jrf.0.0870125. [DOI] [PubMed] [Google Scholar]
- 7.Christenson LK, Ford SP. Comparison of prostaglandin F2 alpha-induced luteolysis in early pregnant and estrogen-treated ‘pseudopregnant’ gilts. Anim Reprod Sci. 1995;38:239–249. [Google Scholar]
- 8.Akinlosotu BA, Diehl JR, Gimenez T. Sparing effects of intrauterine treatment with prostaglandin E2 on luteal function in cycling gilts. Prostaglandins. 1986;32:291–299. doi: 10.1016/0090-6980(86)90132-2. [DOI] [PubMed] [Google Scholar]
- 9.Christenson LK, Farley DB, Anderson LH, Ford SP. Luteal maintenance during early pregnancy in the pig role for prostaglandin E2. Prostaglandins. 1994;47:61–75. doi: 10.1016/0090-6980(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 10.Weems CW, Weems YS, Randel RD. Prostaglandins and reproduction in female farm animals. Vet J. 2006;171:206–228. doi: 10.1016/j.tvjl.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy TG, Gillio-Meina C, Phang SH. Prostaglandins and the initiation of blastocyst implantation and decidualization. Reproduction. 2007;134:635–643. doi: 10.1530/REP-07-0328. [DOI] [PubMed] [Google Scholar]
- 12.Kraeling RR, Rampacek GB, Fiorello NA. Inhibition of pregnancy with indomethacin in mature gilts and prepuberal gilts induced to ovulate. Biol Reprod. 1985;32:105–110. doi: 10.1095/biolreprod32.1.105. [DOI] [PubMed] [Google Scholar]
- 13.Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J Biol Chem. 1996;271:33157–33160. doi: 10.1074/jbc.271.52.33157. [DOI] [PubMed] [Google Scholar]
- 14.Waclawik A, Rivero-Muller A, Blitek A, Kaczmarek MM, Brokken LJ, Watanabe K, Rahman NA, Ziecik AJ. Molecular cloning and spatiotemporal expression of prostaglandin F synthase and microsomal prostaglandin E synthase-1 in porcine endometrium. Endocrinology. 2006;147:210–221. doi: 10.1210/en.2005-0880. [DOI] [PubMed] [Google Scholar]
- 15.Schieber A, Frank RW, Ghisla S. Purification and properties of prostaglandin 9-ketoreductase from pig and human kidney. Identity with human carbonyl reductase. Eur J Biochem. 1992;206:491–502. doi: 10.1111/j.1432-1033.1992.tb16952.x. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh D, Sawicki M, Pletnev V, Erman M, Ohno S, Nakajin S, Duax WL. Porcine carbonyl reductase. structural basis for a functional monomer in short chain dehydrogenases/reductases. J Biol Chem. 2001;276:18457–18463. doi: 10.1074/jbc.M100538200. [DOI] [PubMed] [Google Scholar]
- 17.Waclawik A, Ziecik AJ. Differentional expression of prostaglandin synthesis enzymes in conceptus during periimplantation period and endometrial expression of carbonyl reductase/prostaglandin 9-ketoreductase in the pig. J Endocrinol. 2007;194:1–13. doi: 10.1677/JOE-07-0155. [DOI] [PubMed] [Google Scholar]
- 18.Ashworth MD, Ross JW, Hu J, White FJ, Stein DR, Desilva U, Johnson GA, Spencer TE, Geisert RD. Expression of porcine endometrial prostaglandin synthase during the estrous cycle and early pregnancy, and following endocrine disruption of pregnancy. Biol Reprod. 2006;74:1007–1015. doi: 10.1095/biolreprod.105.046557. [DOI] [PubMed] [Google Scholar]
- 19.Blitek A, Waclawik A, Kaczmarek MM, Stadejek T, Pejsak Z, Ziecik AJ. Expression of cyclooxygenase-1 and -2 in the porcine endometrium during the oestrous cycle and early pregnancy. Reprod Domest Anim. 2006;41:251–257. doi: 10.1111/j.1439-0531.2006.00646.x. [DOI] [PubMed] [Google Scholar]
- 20.Geisert RD, Renegar RH, Thatcher WW, Roberts RM, Bazer FW. Establishment of pregnancy in the pig: I. Interrelationships between preimplantation development of the pig blastocyst and uterine endometrial secretions. Biol Reprod. 1982;27:925–939. doi: 10.1095/biolreprod27.4.925. [DOI] [PubMed] [Google Scholar]
- 21.Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- 22.Papay KD, Kennedy TG. Characterization of temporal and cell-specific changes in transcripts for prostaglandin E(2) receptors in pseudopregnant rat endometrium. Biol Reprod. 2000;62:1515–1525. doi: 10.1095/biolreprod62.6.1515. [DOI] [PubMed] [Google Scholar]
- 23.Fortier MA, Boulet AP, Dugré FJ, Lambert RD. Local alteration in adenylate cyclase activity and stimulation response at implantation site in rabbit endometrium during early pregnancy. Biol Reprod. 1990;42:106–113. doi: 10.1095/biolreprod42.1.106. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka N, Miyazaki K, Tashiro H, Mizutani H, Okamura H. Changes in adenylyl cyclase activity in human endometrium during the menstrual cycle and in human decidua during pregnancy. J Reprod Fertil. 1993;98:33–39. doi: 10.1530/jrf.0.0980033. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi T, Narumiya S. Function of prostanoid receptors: studies on knockout mice. Prostaglandins Other Lipid Mediat. 2002;68-69:557–573. doi: 10.1016/s0090-6980(02)00055-2. [DOI] [PubMed] [Google Scholar]
- 26.Kennedy TG, Keys JL, King GJ. Endometrial prostaglandin E2-binding sites in the pig characterization and changes during the estrous cycle and early pregnancy. Biol Reprod. 1986;35:624–632. doi: 10.1095/biolreprod35.3.624. [DOI] [PubMed] [Google Scholar]
- 27.Tysseling KA, Uzumcu M, Hoagland TA, Crain RC, Mirando MA. The role of phosphoinositide-derived second messengers in oxytocin-stimulated prostaglandin F2 alpha release from endometrium of pigs. Domest Anim Endocrinol. 1996;13:411–420. doi: 10.1016/0739-7240(96)00071-9. [DOI] [PubMed] [Google Scholar]
- 28.Parent J, Fortier MA. Expression and contribution of three different isoforms of prostaglandin E synthase in the bovine endometrium. Biol Reprod. 2005;73:36–44. doi: 10.1095/biolreprod.104.037036. [DOI] [PubMed] [Google Scholar]
- 29.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 30.Waclawik A, Kaczmarek MM, Kowalczyk AE, Bogacki M, Ziecik AJ. Expression of prostaglandin synthesis pathway enzymes in the porcine corpus luteum during the oestrous cycle and early pregnancy. Theriogenology. 2008;70:145–152. doi: 10.1016/j.theriogenology.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 31.Blitek A, Ziecik AJ. Prostaglandins F and E secretion by porcine epithelial and stromal endometrial cells on different days of the oestrous cycle. Reprod Domest Anim. 2004;39:340–346. doi: 10.1111/j.1439-0531.2004.00523.x. [DOI] [PubMed] [Google Scholar]
- 32.Wilson RJ, Giblin GM, Roomans S, Rhodes SA, Cartwright KA, Shield VJ, Brown J, Wise A, Chowdhury J, Pritchard S, Coote J, Noel LS, Kenakin T, Burns-Kurtis CL, Morrison V, Gray DW, Giles H. GW627368X ((N-{2-[4-(4,9-diethoxy-1-oxo-1,3-dihydro-2H-benzo[f]isoindol-2-yl)phenyl]acetyl} benzene sulphonamide): a novel, potent and selective prostanoid EP4 receptor antagonist. Br J Pharmacol. 2006;148:326–339. doi: 10.1038/sj.bjp.0706726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krzymowski T, Kotwica J, Stefanczyk-Krzymowska S. Uterine and ovarian countercurrent pathways in the control of ovarian function in the pig. J Reprod Fertil Suppl. 1990;40:179–191. [PubMed] [Google Scholar]
- 34.Fischer HE, Bazer FW, Fields MJ. Steroid metabolism by endometrial and conceptus tissues during early pregnancy and pseudopregnancy in gilts. J Reprod Fertll. 1985;75:69–78. doi: 10.1530/jrf.0.0750069. [DOI] [PubMed] [Google Scholar]
- 35.Pusateri AE, Rothschild MF, Warner CM, Ford SP. Changes in morphology, cell number, cell size and cellular estrogen content of individual littermate pig conceptuses on days 9 to 13 of gestation. J Anim Sci. 1990;68:3727–3735. doi: 10.2527/1990.68113727x. [DOI] [PubMed] [Google Scholar]
- 36.Geisert RD, Yelich JV. Regulation of conceptus development and attachment in pigs. J Reprod Fertil Suppl. 1997;52:133–149. [PubMed] [Google Scholar]
- 37.Flood PF. Steroid-metabolizing enzymes in the early pig conceptus and in the related endometrium. J Endocrinol. 1974;63:413–414. doi: 10.1677/joe.0.0630413. [DOI] [PubMed] [Google Scholar]
- 38.White FJ, Ross JW, Joyce MM, Geisert RD, Burghardt RC, Johnson GA. Steroid regulation of cell specific secreted phosphoprotein 1 (osteopontin) expression in the pregnant porcine uterus. Biol Reprod. 2005;73:1294–1301. doi: 10.1095/biolreprod.105.045153. [DOI] [PubMed] [Google Scholar]
- 39.Beaver CJ, Murdoch WJ. Ovarian and uterine prostaglandin E2-9-ketoreductase activity in cyclic and pregnant ewes. Prostaglandins. 1992;44:37–42. doi: 10.1016/0090-6980(92)90105-3. [DOI] [PubMed] [Google Scholar]
- 40.Asselin E, Fortier MA. Detection and regulation of the messenger for a putative bovine endometrial 9-keto-prostaglandin E(2) reductase effect of oxytocin and interferon-tau. Biol Reprod. 2000;62:125–131. doi: 10.1095/biolreprod62.1.125. [DOI] [PubMed] [Google Scholar]
- 41.Schlegel W, Krüger S, Korte K. Purification of prostaglandin E2-9-oxoreductase from human decidua vera. FEBS Lett. 1984;171:141–144. doi: 10.1016/0014-5793(84)80475-5. [DOI] [PubMed] [Google Scholar]
- 42.Ing NH. Steroid hormones regulate gene expression posttranscriptionally by altering the stabilities of messenger RNAs. Biol Reprod. 2005;72:1290–1296. doi: 10.1095/biolreprod.105.040014. [DOI] [PubMed] [Google Scholar]
- 43.Geisert RD, Ross JW, Ashworth MD, White FJ, Johnson GA, DeSilva U. Maternal recognition of pregnancy signal or endocrine disruptor: the two faces of oestrogen during establishment of pregnancy in the pig. Soc Reprod Fertil Suppl. 2006;62:131–145. [PubMed] [Google Scholar]
- 44.Weems YS, Lammoglia MA, Lewis AW, Randel RD, Sasser RG, Morita I, Weems CW. PGE2 induces its own secretion in vitro by bovine 270-day placenta but not by 200-day placenta. Prostaglandins Other Lipid Mediat. 1999;57:189–205. doi: 10.1016/s0090-6980(99)00003-9. [DOI] [PubMed] [Google Scholar]
- 45.Jabbour HN, Milne SA, Williams AR, Anderson RA, Boddy SC. Expression of COX-2 and PGE synthase and synthesis of PGE(2)in endometrial adenocarcinoma: a possible autocrine/paracrine regulation of neoplastic cell function via EP2/EP4 receptors. Br J Cancer. 2001;85:1023–1031. doi: 10.1054/bjoc.2001.2033. [DOI] [PubMed] [Google Scholar]
- 46.Sales KJ, Katz AA, Davis M, Hinz S, Soeters RP, Hofmeyr MD, Millar RP, Jabbour HN. Cyclooxygenase-2 expression and prostaglandin E(2) synthesis are up-regulated in carcinomas of the cervix: a possible autocrine/paracrine regulation of neoplastic cell function via EP2/EP4 receptors. J Clin Endocrinol Metab. 2001;86:2243–2249. doi: 10.1210/jcem.86.5.7442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arosh JA, Banu SK, Kimmins S, Chapdelaine P, Maclaren LA, Fortier MA. Effect of interferon-tau on prostaglandin biosynthesis, transport, and signaling at the time of maternal recognition of pregnancy in cattle: evidence of polycrine actions of prostaglandin E2. Endocrinology. 2004;145:5280–5293. doi: 10.1210/en.2004-0587. [DOI] [PubMed] [Google Scholar]
- 48.Hamilton GS, Kennedy TG. Uterine vascular changes after unilateral intrauterine infusion of indomethacin and prostaglandin E2 to rats sensitized for the decidual cell reaction. Biol Reprod. 1994;50:757–764. doi: 10.1095/biolreprod50.4.757. [DOI] [PubMed] [Google Scholar]
- 49.Milne SA, Perchick GB, Boddy SC, Jabbour HN. Expression, localization, and signaling of PGE(2) and EP2/EP4 receptors in human nonpregnant endometrium across the menstrual cycle. J Clin Endocrinol Metab. 2001;86:4453–4459. doi: 10.1210/jcem.86.9.7856. [DOI] [PubMed] [Google Scholar]
- 50.Arosh JA, Banu SK, Chapdelaine P, Emond V, Kim JJ, MacLaren LA, Fortier MA. Molecular cloning and characterization of bovine prostaglandin E2 receptors EP2 and EP4: expression and regulation in endometrium and myometrium during the estrous cycle and early pregnancy. Endocrinology. 2003;144:3076–3091. doi: 10.1210/en.2002-0088. [DOI] [PubMed] [Google Scholar]