Abstract
A two-dimensional (2D) gas chromatography/electron impact-mass spectrometry (GC/EI-MS) method for simultaneous quantification of Δ9-tetrahydrocannabinol (THC), 11-hydroxy-Δ9-tetrahydrocannabinol (11-OH-THC), and 11-nor-Δ9-tetrahydrocannabinol-9-carboxylic acid (THCCOOH) in human plasma was developed and validated. The method employs 2D capillary GC and cryofocusing for enhanced resolution and sensitivity. THC, 11-OH-THC, and THCCOOH were extracted by precipitation with acetonitrile followed by solid-phase extraction. GC separation of trimethylsilyl derivatives of analytes was accomplished with two capillary columns in series coupled via a pneumatic Deans switch system. Detection and quantification were accomplished with a bench-top single quadrupole mass spectrometer operated in electron impact-selected ion monitoring mode. Limits of quantification (LOQ) were 0.125, 0.25 and 0.125 ng/mL for THC, 11-OH-THC, and THCCOOH, respectively. Accuracy ranged from 86.0 to 113.0% for all analytes. Intra- and inter-assay precision, as percent relative standard deviation, was less than 14.1% for THC, 11-OH-THC, and THCCOOH. The method was successfully applied to quantification of THC and its 11-OH-THC and THCCOOH metabolites in plasma specimens following controlled administration of THC.
Keywords: Tetrahydrocannabinol, Plasma, Gas chromatography–mass spectrometry, Two-dimensional chromatography, Cryofocusing
1. Introduction
Currently, there is increasing interest in the chemistry, pharmacology, and toxicology of cannabinoids [1–3] and in the development of potential cannabinoid medications [4,5]. Therapeutic benefit of oral and oromucosal delivery of cannabinoids is being investigated for analgesia [6], treatment of muscle spasticity in motor disease [7–9], nausea and vomiting [10], incontinence [11], and many other conditions [12–14]. Cannabis also is one of the most commonly used illicit drugs with subsequent impairment of cognitive function [15–21]. Identification of potential impairment is highly relevant in forensic applications, law enforcement, and driving under the influence cases. Cannabinoid pharmacokinetics influence the onset, magnitude, and duration of pharmacodynamic effects and are essential to interpreting cannabinoid test results. Therefore, highly accurate and sensitive cannabinoid assays are needed for pharmacological research, development of new pharmacotherapies, and for determining the contribution of cannabis to accident causation. Analysis of cannabinoids in plasma is challenging due to the different physiochemical nature of cannabinoid analytes, low concentrations, and difficulty in separating target analytes from complex biological matrices [22].
Δ9-Tetrahydrocannabinol (THC) is the major psychoactive constituent in cannabis. THC is metabolized to 11-hydroxy-Δ9-tetrahydrocannabinol (11-OH-THC), also pharmacologically active, and to the inactive 11-nor-Δ9-tetrahydrocannabinol-9-carboxylic acid (THCCOOH). Accurate measurement of THC and THC metabolites is important in pharmacokinetic studies, determining dose/effect relationships for clinical applications, and also in evaluation of time of last use [23–25]. A variety of gas chromatography/mass spectrometry (GC/MS) methods for quantification of cannabinoid analytes in various matrices are available [26–32]. The goal in most recent methods has been improved limits of detection (LOD) and quantification (LOQ), particularly for THC and THCCOOH. Specialized positive and negative chemical ionization (CI) detection is often applied to achieve lower detection limits; however, CI techniques may not provide sufficient qualifier ion fragmentation for most forensic applications. Newer chromatographic techniques have included the use of two-dimensional gas chromatography (2D-GC) to resolve matrix interference and improve detection limits in hair and oral fluid specimens [33,34]. One recent manuscript utilized 2D-GC for quantification of THC and THCCOOH in whole blood; however, the method does not include detection and quantification of 11-OH-THC [35]. Another recent published method [28] includes THC, 11-OH-THC, and THCCOOH and reports similar LOD and LOQ data. The method reported here provides advanced opportunity in resolving interferences and should be applicable to other difficult matrices, including meconium, whole blood, and oral fluid.
Our approach was to develop an efficient and reliable extraction and derivatization of the principal cannabinoid analytes (THC, 11-OH-THC, and THCCOOH) and combine 2D-GC with cryogenic focusing to improve both resolution and sensitivity. The goal was a rugged, flexible method with enhanced resolution power and lower detection and quantification limits. The method utilizes relatively inexpensive upgrades to readily available GC/MS hardware and can potentially be applied to various complex matrices such as blood, urine, meconium, among others. This manuscript presents the development and validation of an improved method for the simultaneous extraction and quantification of low concentrations of THC, 11-OH-THC, and THCCOOH from human plasma. 2D-GC and cryogenic trapping achieved adequate resolution of analyte from complex matrix components and electron impact-mass spectrometry (EI-MS) was used to quantify analytes in the picogram/mL concentration range.
2. Experimental
2.1. Instrumentation
An Agilent 6890 gas chromatograph configured with Agilent 7683 automated liquid sampler, microfluidic Deans switch, flame ionization detector (FID), and interfaced to an Agilent 5973 mass selective detector (Agilent Technologies, Wilmington, DE) was used for specimen analysis. The GC was also equipped with a cryogenic focusing trap (Joint Analytical Systems, Marlton, NJ). The Deans switch enables 2D-GC with two capillary chromatographic columns in series. The pneumatic switch system directs the output of the primary column to either the FID or the inlet of the secondary column. The inlet end of the secondary column was inserted through the cryogenic trap and the outlet directed to the MSD. The air-cooled cryogenic trap focuses time-programmed “cuts” from the primary capillary column to the head of the secondary capillary column, and then re-vaporizes the trapped eluent with rapid heating of the trapping zone. The cryogenic trap was mounted inside the GC oven and controlled with Agilent ChemStation software. GC front (injection) inlet, back (cryotrap) inlet, oven, Deans switch, post-run, and FID operating parameters are listed in Table 1.
Table 1.
Gas chromatography/Deans switch/cryotrap method parameters for the analysis of Δ9-tetrahydrocannabinol (THC), 11-hydroxy-Δ9-tetrahydrocannabinol (11-OH-THC), and 11-nor-Δ9-tetrahydrocannabinol-9-carboxylic acid (THCCOOH) in human plasma
| Front inlet | Back inlet (cryofocusing trap) | ||
|---|---|---|---|
| Mode | Constant pressure | Initial temp | 100 °C |
| Inlet temp | 275 °C | Initial time | 7.00 min |
| Injection mode | Splitless | Ramp #1: 800 °C/min | Final temp: 275 °C |
| Purge flow | 50.0 mL/min | Final time: 0.20 min | |
| Purge time | 0.80 min | Ramp #2: 800 °C/min | Final temp: 100 °C |
| Pressure | 21.6 psi | Final time: 2.10 min | |
| Total flow | 55.7 mL/min | Ramp #3: 800 °C/min | Final temp: 275 °C |
| Final time: 0.00 min | |||
| Oven | Deans switch | ||
| Initial oven temp | 150 °C | FID restrictor length | 20 cm |
| Initial oven hold | 0.0 min | FID restrictor i.d. | 0.100 |
| Ramp #1: 25 °C/min | Final temp: 200 °C | Aux 3 pressure | 12.9 psi |
| Final time: 0.50 min | THC cut time start | 6.65 min | |
| Ramp #2: 15 °C/min | Final temp: 275 °C | THC cut time end | 6.90 min |
| Final time: 1.00 min | OH-THC cut time start | 8.15 min | |
| Ramp #3: 75 °C/min | Final temp: 225 °C | OH-THC cut time end | 8.40 min |
| Final time: 0.00 min | THCCOOH cut time start | 9.05 min | |
| Ramp #4: 15 °C/min | Final temp: 275 °C | THCCOOH cut time end | 9.45 min |
| Final time: 2.00 min | |||
| Post-run | Flame ionization detector | ||
| Post temp | 300 °C | FID temp | 275 °C |
| Post time | 0.50 min | Hydrogen flow | 40 mL/min |
| Column 1 pressure | 1.0 psi | Air flow | 400 mL/min |
| Column 2 pressure | 65.0 psi | Nitrogen makeup flow | 20 mL/min |
2.2. Reagents
THC, 11-OH-THC, and THCCOOH and deuterated internal standards (Table 2) were purchased from Cerilliant (Austin, TX). Anhydrous sodium acetate, dibasic and monobasic potassium phosphate, hydrochloric acid, glacial acetic acid, sodium hydroxide, methanol, hexane, and acetonitrile were obtained from Mallinckrodt Baker (Phillipsburg, NJ). All chemicals were of ACS reagent grade and solvents were of HPLC grade. β-Glucuronidase (type IX-A from E. coli) from Sigma–Aldrich (St. Louis, MO), N,O-bis(trimethylsilyl) trifluoroacetamide (BSTFA) containing 1% trimethylchlorosilane (TMCS) (Pierce Biotechnology, Rockford, IL) and Clean Screen solid-phase extraction columns (part no. ZSTHC020, 200 mg of sorbent, 10 mL tube volume), United Chemical Technologies (Bristol, PA) were utilized in specimen preparation.
Table 2.
Mass selective detector parameters for Δ9-tetrahydrocannabinol, 11-hydroxy-Δ9-tetrahydrocannabinol, and 11-nor-Δ9-tetrahydrocannabinol-9-carboxylic acid
| Compound | Quantification ion (m/z) | Qualifier ion (m/z) | Qualifier ion (m/z) | RT (min) |
|---|---|---|---|---|
| Δ9-Tetrahydrocannabinol-d3 | 389.2 | 374.2 | 8.54 | |
| Δ9-Tetrahydrocannabinol | 386.2 | 371.2 | 303.2 | 8.55 |
| 11-Hydroxy-Δ9-tetrahydrocannabinol-d3 | 374.2 | 477.3 | 12.99 | |
| 11-Hydroxy-Δ9-tetrahydrocannabinol | 371.2 | 474.3 | 459.3 | 13.01 |
| 11-nor-Δ9-Tetrahydrocannabinol-9-carboxylic acid-d3 | 374.2 | 491.3 | 14.05 | |
| 11-nor-Δ9-Tetrahydrocannabinol-9-carboxylic acid | 371.2 | 488.3 | 473.3 | 14.07 |
Sodium acetate buffer (pH 4.0 ± 0.1) was prepared with 2.0 M sodium acetate and 2.0 M acetic acid. 0.1 M potassium phosphate buffer was prepared by adjustment of 0.1 M monobasic potassium phosphate to pH 6.8 with 0.1 M dibasic potassium phosphate. The elution solvent was hexane:ethyl acetate, 80:20 by volume. Working β-glucuronidase (20,000 units/mL) was prepared by dilution of stock enzyme with 0.1 M pH 6.8 phosphate buffer. Blank human plasma for development and validation of the method was obtained from the National Institutes of Health Clinical Center. Blank plasma matrices were verified to be drug free prior to preparation of calibrators and controls.
2.3. Preparation of standard solutions
Intermediate standard containing 10 mcg/mL THC, 11-OH-THC, and THCCOOH was made by diluting 1.0 mg/mL stock solutions with methanol and 10, 100, and 1000 ng/mL calibrators were prepared by diluting 10 mcg/mL intermediate standards with methanol (stored at −20 °C). Working plasma calibrators (0.125, 0.25, 0.5, 1.0, 2.5, 5.0, 10, 25, 50, 75, and 100 ng/mL) were prepared daily by addition of appropriate amounts of 10, 100, or 1000 ng/mL calibration standard to 1.0 mL blank plasma.
Methanolic quality control solutions were prepared with different lot numbers of stock standards than for preparation of calibrators. Intermediate standard containing 1000 ng/mL THC, 11-OH-THC, and THCCOOH in methanol was prepared from 1.0 mg/mL stock solutions. Methanolic quality control solutions contained 10, 100 and 500 ng/mL of each analyte (stored at −20 °C). Plasma quality control samples (0.35, 0.75, 2.0, 20, 30, 60, and 90 ng/mL) were prepared by addition of appropriate amounts of methanolic quality control solutions to 1.0 mL blank plasma.
Stock 10 mcg/mL internal standard solution containing three deuterated analogs (THC-d3, 11-OH-THC-d3, and THCCOOH-d3) was made by diluting 100 mcg/mL stock solutions with methanol. Working methanolic internal standards containing 200 ng/mL of each deuterated analyte were prepared by diluting 10 mcg/mL stock (stored at −20 °C). Twenty-five microliters working (200 ng/mL) internal standard was added to 1.0 mL plasma, yielding a final deuterated internal standard concentration of 5 ng/mL.
2.4. Clinical specimens
Clinical specimens were collected from volunteer participants enrolled in a study of THC and metabolite concentrations in chronic cannabis users. Participants provided written informed consent for this NIDA Institutional Review Board approved study. Plasma specimens were stored at −20 °C until analysis.
2.5. Specimen hydrolysis
One milliliter of each plasma specimen, quality control sample, or calibrator was combined with 25 µL working internal standard, 1 mL 0.1 M pH 6.8 phosphate buffer, and 0.25 mL working β-glucuronidase. Tubes were capped, vortexed gently, and incubated at 37 °C for 16 h in a shaking water bath. Following hydrolysis, proteins were precipitated with 2.0 mL cold acetonitrile, added in 0.5 mL increments, with vortexing. Tubes were centrifuged at 1800 × g for 10 min to pellet protein. Supernatants were decanted into tubes containing 3.0 mL 2N pH 4.0 sodium acetate buffer and vortex mixed.
2.6. Solid-phase extraction and derivatization
Clean Screen extraction columns were conditioned with 1 mL elution solvent (hexane:ethyl acetate, 80:20), 3 mL methanol, 3 mL deionized water, and 2 mL 0.1 N HCl. Buffered supernatants were added to conditioned columns. Columns were washed with 3 mL distilled water and 2 mL 0.1 N HCl/acetonitrile (70:30) and dried by vacuum for 10 min. After priming the sorbent bed with 0.2 mL hexane, analytes were eluted with 5 mL elution solvent into 10 mL centrifuge tubes containing 0.5 mL absolute ethanol. Eluates were dried under nitrogen at 40 °C.
Dried extracts were reconstituted in 25 µL BSTFA, capped, derivatized at 70 °C for 30 min and transferred to autosampler vials.
2.7. Two-dimensional gas chromatography
2D chromatographic separation was achieved with a primary DB-1MS capillary column (15 m × 0.25 mm i.d., 0.25 µm film thickness; Agilent Technologies, Wilmington, DE) and a secondary ZB-50 capillary column (30 m × 0.32 mm i.d., 0.25 µm film thickness; Phenomenex, Torrance, CA). Three microliters derivatized extract was introduced in splitless injection mode. Analyte elution times from the primary column were determined by injection of high concentration standards with the Deans switch control directing effluent via the restrictor to the FID. Subsequently, the Deans switch valve was programmed to actuate 0.125 min prior to and 0.125 min after each analyte retention time to divert a “cut” of the analyte elution band to the secondary GC column for further chromatographic resolution. The secondary column was inserted through the cryogenic trap and the effluent end interfaced to the MSD for detection and quantification.
2.8. Cryogenic focusing
The air-cooled cryogenic trap permits cold-trapping of analyte bands entering the head of the secondary capillary column. The cryogenic trap was programmed (via the GC back inlet electronics) to maintain 100 °C from the beginning of the chromatographic run through the completion of the “cut” window for the first analyte (THC). Immediately after the cut window for THC, the 100 °C cryogenic trap was ramped at maximum rate (800 °C/min) to 275 °C and the 0.25 min primary column effluent “cut” was re-vaporized for migration through the secondary column. After maintaining re-vaporization temperature (275 °C) for 0.2 min, the cryogenic trap was returned to 100 °C for a second trapping sequence. In the second trapping sequence, the elution “cut” windows of 11-OH-THC and THCCOOH were combined by maintaining the trap at 100 °C until both analyte “cuts” were condensed. A third GC oven ramp was employed to optimize 11-OH-THC and THCCOOH resolution within the secondary column. Following trapping of effluents for 11-OH-THC and THCCOOH, the GC oven temperature was dropped to 225 °C and analytes released by ramping to 275 °C. Focusing of the analyte band at the head of the secondary column enhances the chromatographic signal-to-noise (S/N), improving sensitivity.
2.9. Mass spectrometry
The mass selective detector was operated in electron impact-selected ion monitoring (SIM) mode with a dwell time of 10 ms. Three ions for each analyte and two for each internal standard were acquired. Quantitative and qualifier ions, and representative retention times are listed in Table 2. MS interface, source and quadrupole temperatures were 280, 230, and 150 °C, respectively.
2.10. Data analysis
Data were acquired and analyzed using Agilent Enhanced ChemStation G1701DA software version D. Analytes were identified by comparing retention time (±2%) and relative abundance of qualifier ions. Qualifier ions exhibiting relative intensity greater than 10% of target ion were required to be ±20% (relative) of the corresponding average values of calibrators assayed in the same run. Qualifier ions with relative intensity less than 10% of the abundance of the target ion were required to be ±5% (absolute) of average calibrator values. Quantification was based upon ratios of target ion peak areas of native analyte to the corresponding deuterated internal standard. Calibration with internal standardization was performed with linear regression curve fits with 1/X weighting.
In each analytical run, two calibration curves were constructed for each analyte in order to extend the dynamic range of the assay. Multianalyte working calibration standards at 0.125, 0.25, 0.50, 1.0, 2.5, 5.0, 10, 25, 50, 75, and 100 ng/mL were assayed with each batch. Calibrator concentrations were required to be ±20% of target when calculated against the full calibration curve. Low calibration curves were constructed from 0.125 to 25 ng/mL for THC and THCCOOH and 0.125–10 ng/mL for 11-OH-THC; high calibration curves were from 25 to 100 ng/mL for THC and THCCOOH and 10–75 ng/mL for 11-OH-THC.
2.11. Validation
Linearity, limits of detection and quantification, intra- and inter-batch precision, accuracy, extraction efficiency, and stability were investigated to evaluate method integrity. Matrix effects and method specificity were evaluated by assaying 6 different human plasma sources and 23 potential interfering compounds. To assess potential interferences, low quality control samples were spiked to contain 10,000 ng/mL pseudoephedrine, methamphetamine, amphetamine, methylenedioxymethamphetamine, phentermine, phenylpropanolamine, fenfluramine, ketamine, acetaminophen, ibuprofen, acetylsalicylic acid, dextromethorphan, chlorpheniramine, pentazocine, methadone, nicotine, caffeine, cocaine, morphine, codeine, oxycodone, oxymorphone, or hydrocodone. Interference from cannabinol and cannabidiol at 100 ng/mL also was evaluated.
LOD and LOQ were determined by assaying a series of decreasing concentrations of drug-fortified human plasma. LOD was the lowest analyte concentration with S/N ratio of at least 3 for all ions, acceptable chromatographic peak shape, retention time, and qualifier ion ratios. LOQ was the lowest concentration with target ion S/N ratio of ≥ 10, acceptable chromatographic peak shape, retention time, and qualifier ion ratios, and concentration within ±20% of target.
Linearity of the method was investigated by calculation of the regression line by the method of least squares and expressed as the coefficient of determination (r2). Linearity of each analyte was determined with at least seven concentrations for the low curve (0.125–25 ng/mL) and at least four concentrations for the high curve (10–100 ng/mL).
Precision and accuracy were evaluated over the linear range of each curve with appropriate quality control samples at target concentrations of 0.35, 0.75, 2.0, 20, 30, 60, and 90 ng/mL. Inter-batch precision was evaluated for five replicates at each concentration on 4 days (ntotal = 20). Inter-batch precision was expressed as percent relative standard deviation of 20 individual values, equally weighted over four batches. Intra-batch precision was evaluated from five determinations per concentration over four batches. Data were evaluated for normal distribution by a one-sample Kolmogorov–Smirnov test for normality and a two-way analysis of variance (ANOVA) was used to evaluate inter-assay variability. Accuracy was determined by comparison of mean measured concentrations to target values over four assay runs (n = 20) and expressed as percent of target concentration.
Extraction efficiency for each analyte was assessed by adding analyte control solution to blank matrix at low and high control concentrations (2.0 and 60 ng/mL) before solid phase extraction and to a second set after extraction, but prior to the evaporation step. Samples were derivatized and analyzed. The relative extraction efficiency was calculated by comparing the mean analyte peak area (n = 6) of each compound in the first set with the appropriate mean analyte peak area in the second.
Dilution integrity was investigated by diluting quality control samples with 0.1 M pH 6.8 phosphate buffer. Ninety and 50% dilutions (v/v) were prepared in quadruplicate. Assayed concentrations of diluted samples were corrected by dilution factor (× 2 or × 10) and compared to mean undiluted quality control concentrations (n = 4).
Analyte stability was evaluated using human plasma fortified with THC, 11-OH-THC, and THCCOOH at 2.0, 20, and 60 ng/mL (n = 3). Short-term temperature stability was tested for plasma stored for 16 h at room temperature and at 4 °C. Freeze–thaw stability also was determined after three freeze–thaw cycles at 24 h intervals. THC, 11-OH-THC, and THCCOOH concentrations in the samples stored under all three storage conditions were compared to freshly fortified samples.
Stability of derivatized extracts maintained at ambient temperature was evaluated over 48 h. Extracted low and high quality control samples (2.0 and 60 ng/mL) were analyzed immediately after extraction along with calibration standards and then reinjected and analyzed at 24 and 48 h intervals. All samples were quantified using the initial calibration curve.
3. Results
3.1. Two-dimensional chromatographic optimization
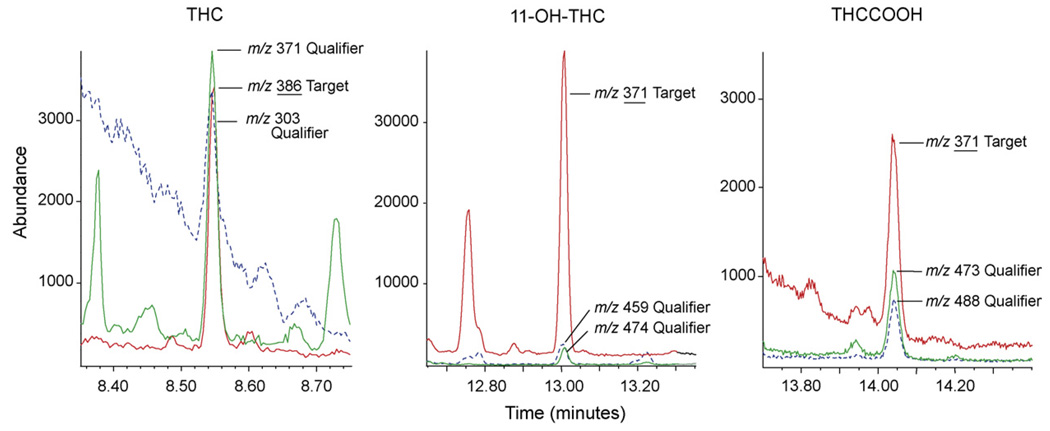
The most reliable resolution of plasma matrix interference was achieved with a 15m DB-1 (100% dimethylpolysiloxane) primary and 30m ZB-50 [(50% phenyl)-methylpolysiloxane] secondary column combination. This column pairing effectively separated each analyte from matrix and also improved consistency of low concentration qualifier ion ratios. As shown in Fig. 1, THC, 11-OH-THC, and THCCOOH were effectively separated from matrix interferences at LOQ concentrations. This was particularly true for THC and THCCOOH, where evaluation of blank plasma pools and clinical specimens did not demonstrate interferences in ion chromatograms and ion ratios were consistent to as low as 0.125 ng/mL.
Fig. 1.
Extracted ion chromatograms for Δ9-tetrahydrocannabinol (THC) [m/z 386, 371, 303], 11-hydroxy-Δ9-tetrahydrocannabinol (11-OH-THC) [m/z 371, 459, 474], and 11-nor-Δ9-tetrahydrocannabinol-9-carboxylic acid (THCCOOH) [m/z 371, 473, 488] in human plasma fortified at the limit of quantification (LOQ) for each analyte. The LOQ concentration is 0.125 ng/mL for THC and THCCOOH and 0.25 ng/mL for 11-OH-THC. Quantification ions are underlined.
3.2. Calibration and validation
The method was validated according to criteria presented in Section 2. Multianalyte working calibrators at 0.125, 0.25, 0.50, 1.0, 2.5, 5.0, 10, 25, 50, 75, and 100 ng/mL were assayed with each batch. A total of six calibration curves were prepared for method validation. Low concentration calibration curves were constructed with eight standards ranging from 0.125 to 25 ng/mL for THC and THCCOOH. 11-OH-THC low calibration curves were constructed using seven concentrations, 0.125–10 ng/mL. Improved curve fits and r2 values were obtained by limiting 11-OH-THC low and high curve upper limits of linearity to 10 and 75 ng/mL, respectively. Four standards were utilized for high calibration curves for THC and THCCOOH (25–100 ng/mL) and 11-OH-THC (10–75 ng/mL). Low and high calibration curves coefficients of determination always exceeded 0.990. Calculated concentration of calibrators was assayed against the full calibration curve and was required to be ±20% of target. LOD and LOQ for both THC and THCCOOH were 0.125 ng/mL, and LOD and LOQ for 11-OH-THC were 0.25 ng/mL. Failure of signal to noise and qualifier ion ratio criteria and quantification failure (>20% of target) occurred at the same concentration for all three analytes; therefore LOD and LOQ concentrations are identical. A summary of calibration data over the dynamic range of the assay is presented in Table 3.
Table 3.
Δ9-Tetrahydrocannabinol (THC), 11-hydroxy-Δ9-tetrahydrocannabinol (11-OH-THC), and 11-nor-Δ9-tetrahydrocannabinol-9-carboxylic acid (THCCOOH) in human plasma by two-dimensional gas chromatography/electron impact-mass spectrometry: limits of detection (LOD), limits of quantification (LOQ), and mean (±SD) slope, intercept, and linear range (n = 6) for low and high curve calibration results
| Compound | Internal standard | LOD (ng/mL) | LOQ (ng/mL) | Slope (mean ± SD) | Intercept (mean ± SD) | Linear range (ng/mL) |
|---|---|---|---|---|---|---|
| THC | THC-d3 | 0.125 | 0.125 | Low: 0.184 (±0.007) | 0.010 (±0.005) | 0.125–25 |
| High: 0.140 (±0.003) | 0.897 (±0.221) | 25–100 | ||||
| 11-OH-THC | 11-OH-THC-d3 | 0.25 | 0.25 | Low: 0.221 (±0.003) | 0.004 (±0.001) | 0.25–10 |
| High: 0.143 (±0.009) | 0.884 (±0.096) | 10–75 | ||||
| THCCOOH | THCCOOH-d3 | 0.125 | 0.125 | Low: 0.208 (±0.021) | 0.007 (±0.002) | 0.125–25 |
| High: 0.126 (±0.005) | 1.425 (±0.635) | 25–100 |
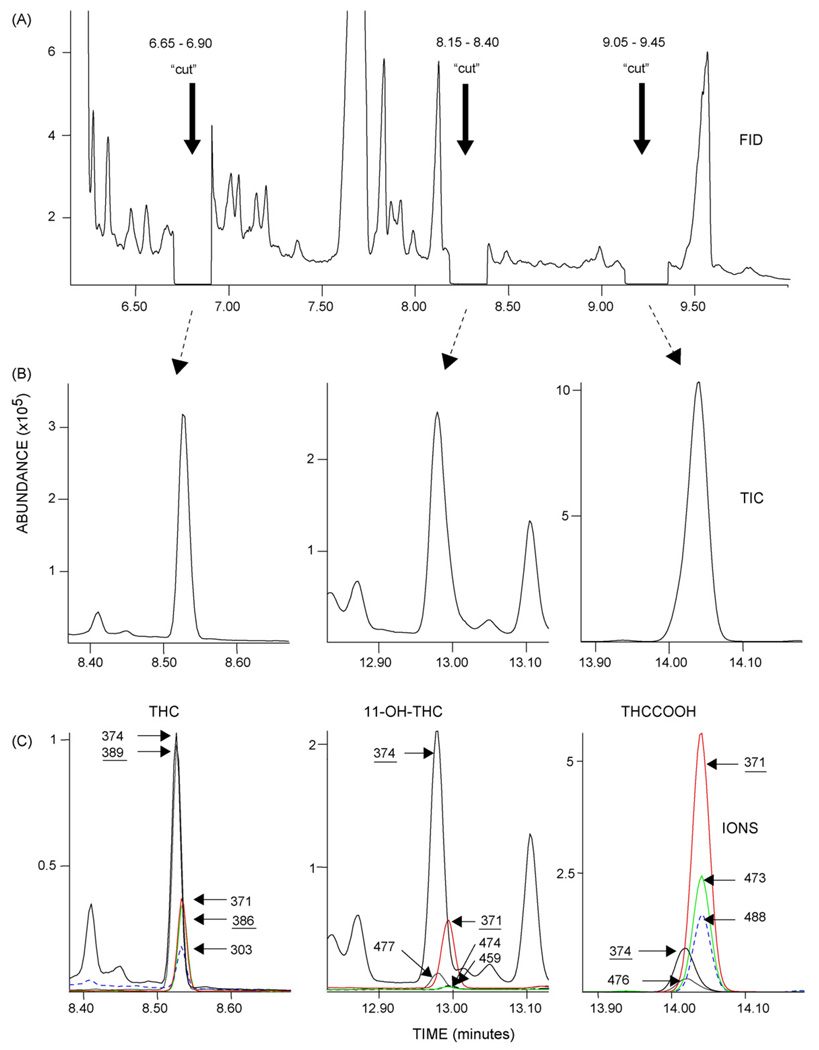
Matrix effects and method specificity were evaluated by assay of 6 different human plasma pools, 16 clinical specimens and 23 potential interfering drugs. No endogenous signal was observed in human plasma pools, and clinical specimens did not exhibit chromatographic interference (Fig. 2). To assess potential exogenous interference, quality control samples were spiked to contain 10,000 ng/mL of potential interfering drug. Interference from 100 ng/mL concentrations of cannabinol (CBN) and cannabidiol (CBD) also was evaluated. Quality control concentrations were within 20% of target and met ion ratio criteria for all analytes. Interconversion of analytes was assessed by spiking an elevated concentration of single analyte into blank plasma. A 500 ng/mL concentration of THC, 11-OH-THC, or THCCOOH did not show quantifiable amounts of the other two analytes.
Fig. 2.
Chromatograms demonstrating two-dimensional separation of Δ9-tetrahydrocannabinol (THC), 11-hydroxy-Δ9-tetrahydrocannabinol (11-OH-THC), and 11-nor-Δ9-tetrahydrocannabinol-9-carboxylic acid (THCCOOH) from a participant plasma specimen containing 1.9 ng/mL THC, 1.2 ng/mL 11-OH-THC, and 25.4 ng/mL THCCOOH. (A) The flame ionization detector signal of eluent from the primary DB-1 column showing time-cut diversion to secondary capillary column. (B) The MSD total ion chromatograms (TIC) of THC, 11-OH-THC, and THCCOOH following resolution on ZB-50 secondary capillary column. (C) The merged ion chromatograms for d0 and d3 THC (m/z 386, 371, 303; 389, 374), 11-OH-THC (m/z 371, 474, 459; 374, 477), and THCCOOH (m/z 371, 473, 488; 374, 491). Quantification ions are underlined.
Precision and accuracy were evaluated at seven concentrations (0.35, 0.75, 2.0, 20, 30, 60, 90 ng/mL; (n = 20)) across the linear dynamic ranges of the appropriate calibration curve for THC and THCCOOH. Six concentrations (0.35, 0.75, 2.0, 20, 30, 60 ng/mL) were evaluated for 11-OH-THC. Table 4 details concentrations tested and accuracy and precision data. Inter-assay precision ranged from 1.5 to 13.6% (% relative standard deviation) for all analytes (n = 20). Intra-assay precision (n = 5; assays = 4) was less than 10.7%. Evaluation of inter-assay variability using two-way analysis of variance (two-way ANOVA) demonstrated significant differences between days (p = 0.05); however, differences in daily mean analyte concentrations for all controls (except THC at 0.35 ng/mL) did not exceed 13.4% and were clinically insignificant. Intra-assay maximum mean difference for THC at 0.35 ng/mL was 26.1%. Accuracy calculated as the percent of target concentration of each analyte ranged from 86.0 to 113.0%.
Table 4.
Δ9-Tetrahydrocannabinol (THC), 11-hydroxy-Δ9-tetrahydrocannabinol (11-OH-THC), and 11-nor-Δ9-tetrahydrocannabinol-9-carboxylic acid (THCCOOH) in human plasma by two-dimensional gas chromatography/electron impact-mass spectrometry: precision and accuracy dataa
| Target (ng/mL) | Precision | Accuracy (% target) (n = 20) | ||
|---|---|---|---|---|
| Inter-assay (% RSD, n = 20) | Intra-assay (average % RSD, n = 5, assays = 4) | |||
| THC | 0.35 | 13.6 | 10.6 | 91.9 |
| 0.75 | 3.4 | 2.6 | 96.9 | |
| 2.0 | 4.6 | 4.2 | 93.7 | |
| 20 | 1.9 | 1.8 | 91.6 | |
| 30 | 3.6 | 2.0 | 99.9 | |
| 60 | 2.8 | 1.7 | 101.3 | |
| 90 | 4.9 | 0.9 | 93.9 | |
| 11-OH-THC | 0.35 | 3.6 | 3.0 | 97.7 |
| 0.75 | 1.5 | 1.3 | 99.8 | |
| 2.0 | 3.9 | 3.8 | 94.5 | |
| 20 | 2.3 | 1.7 | 105.9 | |
| 30 | 3.4 | 1.9 | 113.0 | |
| 60 | 3.4 | 3.2 | 96.6 | |
| THCCOOH | 0.35 | 4.3 | 3.0 | 86.0 |
| 0.75 | 1.8 | 1.8 | 90.8 | |
| 2.0 | 4.2 | 4.0 | 88.2 | |
| 20 | 1.8 | 1.6 | 87.6 | |
| 30 | 4.1 | 1.9 | 96.5 | |
| 60 | 2.9 | 1.7 | 98.9 | |
| 90 | 4.9 | 0.9 | 91.1 | |
Precision is expressed as percent relative standard deviation (% RSD) and accuracy as percent of target concentration. Inter- and intra-assay precision and accuracy were evaluated across four runs with each run containing five replicates of each quality control.
Dilution accuracy was studied by assay of diluted plasma quality control samples. Results of high concentration quality control plasma samples diluted 50 and 90% (v/v) in 0.1 M pH 6.8 phosphate buffer (replicates = 4) yielded mean measured concentrations 94.7–104.5% of target concentrations with all observations falling within 5.3% of the mean undiluted concentration. These dilution studies indicate that accurate measurement of THC, 11-OH-THC, and THCCOOH can be made by dilution of concentrated specimens with 0.1 M pH 6.8 phosphate buffer.
Extraction efficiency, evaluated at 2.0 and 60 ng/mL, was variable for analytes. THC had extraction efficiencies of 50.6 and 49.7% at 2.0 and 60 ng/mL, respectively. 11-OH-THC and THCCOOH extraction efficiencies ranged from 72.8 to 82.5%.
Analyte stability evaluations are presented in Table 5. THC, 11-OH-THC, and THCCOOH concentrations in low, medium, and high quality control samples after 16 h at room temperature, 16 h at 4 °C, and after three freeze–thaw cycles over 72 h were compared to freshly fortified samples. THC and 11-OH-THC mean (n = 3) concentrations of stability samples were within 5.3% of freshly prepared samples. Low, medium, and high THCCOOH concentrations, however, were 7.2, 26.3, and 28.2% lower than freshly prepared samples after 16 h storage at room temperature (Table 5), and within 15.9% with storage at 4 °C and after three freeze–thaw cycles.
Table 5.
Δ9-tetrahydrocannabinol (THC), 11-hydroxy-Δ9-tetrahydrocannabinol (11-OH-THC), and 11-nor-Δ9-tetrahydrocannabinol-9-carboxylic acid (THCCOOH) in human plasma by two-dimensional gas chromatography/electron impact-mass spectrometry: stability data
| Analyte | Target (ng/mL) |
Stabilitya | ||
|---|---|---|---|---|
| R.T. 16 h |
4 °C 16 h |
−20 °C/72 h Three freeze/thaw cycles |
||
| THC | 2.0 | +4.9% | +4.4% | 0.0% |
| 20 | +3.3% | +5.3% | −3.2% | |
| 60 | +2.0% | +0.1% | −0.7% | |
| 11-OH-THC | 2.0 | 0.0% | +4.6% | −2.5% |
| 20 | +2.0% | +5.1% | −2.2% | |
| 60 | +0.9% | −0.7% | −0.9% | |
| THCCOOH | 2.0 | −7.2% | +15.9% | +7.0% |
| 20 | −26.3% | +1.3% | +12.0% | |
| 60 | −28.2% | −14.8% | +3.2% | |
Stability is mean percent difference (n = 3) from freshly prepared samples after 16 h at room temperature (R.T.), 16 h at 4 °C, and 72 h at−20 °C with three freeze/thaw cycles.
Stability of derivatized extracts at ambient temperature was evaluated by reinjection after 24 and 48 h. All analytes were stable, differing from samples injected immediately by less than 6.8%.
3.3. Clinical specimens
The method was employed to quantify THC, 11-OH-THC, and THCCOOH in plasma specimens collected from different volunteers enrolled in a study of THC and metabolite concentrations in chronic cannabis users during monitored abstinence. Merged ion chromatograms demonstrating two-dimensional separation of analytes from a participant’s specimen containing 1.9 ng/mL THC, 1.2 ng/mL 11-OH-THC, and 25.4 ng/mL THCCOOH are shown in Fig. 2.
4. Discussion
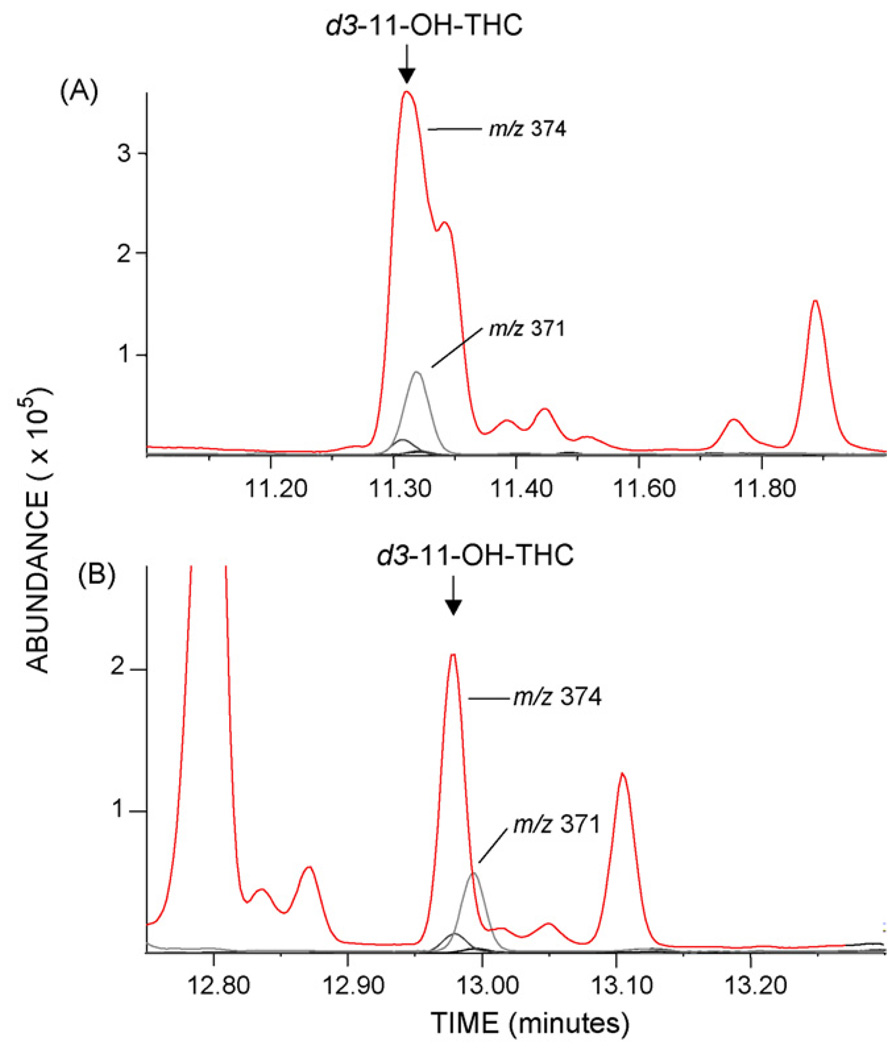
Capillary GC columns of various polarities and lengths were evaluated to optimize resolution of analytes from matrix interferences. Column combinations included DB-1 (100% dimethylpolysiloxane), HP-5 [(5% phenyl)-methylpolysiloxane], DB-35 [(35% phenyl)-methylpolysiloxane], DB-17 (Agilent Technologies), ZB-50 (Phenomenex) [(50% phenyl)-methylpolysiloxane], and RTX-200 (Restek) [(trifluoropropylmethyl-polysiloxane). Two-dimensional resolution was expected to be optimized by maximum stationary phase polarity difference [33]. In most combinations, the non-polar DB-1 was the first column providing satisfactory initial resolution of derivatized THC, 11-OH-THC, and THCCOOH from most matrix interferences. A 1.5 min separation of THC/11-OH-THC and a 1.0 min separation of 11-OH-THC/THCCOOH were achieved with the 15 m DB-1 primary column. The DB-1/DB-35 stationary phase combination provided excellent resolution of THC and THCCOOH ions; however, there were interferences with the 11-OH-THC m/z 374 (d3 target ion) and m/z 459 qualifier ion in some plasma specimens. This interference was resolved by substitution of a 30m 50% phenylmethylpolysiloxane phase (ZB-50) secondary column (Fig. 3).
Fig. 3.
Merged ion chromatograms of plasma extract illustrating resolution of deuterated 11-hydroxy-Δ9-tetrahydrocannabinol (d3-11-OH-THC) target ion (m/z 374) from matrix interference. Ion chromatogram A is chromatography of d3-11-OH-THC ion on a DB-1/DB-35 2D column configuration. Chromatogram B demonstrates resolution of the same plasma extract on DB-1/ZB-50.
The pneumatic Deans switch configuration also permits reversal of carrier gas flow within post-run parameters. Reversing the carrier flow to back flush the capillary column markedly reduced the frequency of column, inlet, and source maintenance.
The combination of 2D-GC and cryogenic focusing was first applied in the separation of petroleum products and in the fragrance industry. The ability to selectively cold-trap analytes and release to the secondary column condenses elution bands and yields sharper chromatographic peaks and improved signal-to-noise ratio. Recent application of cryogenic focusing with 2D-GC to drug quantification demonstrated enhanced resolution and detection of a single analyte (THCCOOH) in hair [33] and THC in oral fluid [34]. Another published method includes both THC and THCCOOH, but does not use cryogenic focusing [35]. Our method incorporates 2D-GC with cryogenic focusing and quantifies THC and principal metabolites 11-OHTHC and THCCOOH at lower concentrations in plasma than previously reported methods.
Separate low and high calibration curves significantly extended the dynamic calibration range of the assay. Effective calibration from 0.125 to 100 ng/mL enables quantification of both low concentration and peak concentration plasma specimens frequently encountered in our controlled administration studies and with unknown forensic specimens. Merged ion chromatograms for each analyte at the LOQ are presented in Fig. 1. Target ion retention times and S/N ratios for THC and THCCOOH at 0.062 ng/mL concentrations were acceptable; but quantification criteria, peak shape and qualifier ion ratios were not met. Therefore, LOD and LOQ for THC and THCCOOH were 0.125 ng/mL. 11-OH-THC target ion quantification was acceptable at concentrations less than 0.25 ng/mL; however, ion ratios failed acceptance criteria at concentrations below 0.25 ng/mL. The m/z 474 and 459 qualifier ions for 11-OH-THC traditionally exhibit low abundance ratios (4–6%) relative to m/z 371 target ion. The m/z 474 (M+) ion maintained consistent ion ratios throughout the linear range; however, m/z 459 (qualifier #2) did not provide consistent ion ratios at concentrations less that 1.0 ng/mL due to occasional matrix interference. World Anti-Doping Agency (WADA) EI-GC/MS criteria for maximum tolerance windows for relative ion intensities are ±5% (absolute) for ions with relative abundance less than 25%. WADA criteria (WADA Technical Document TD2003IDCR) were applied to m/z 474 and 459 qualifier ions. LOD and LOQ for 11-OH-THC were determined to be 0.25 ng/mL, a concentration at which these criteria were consistently fulfilled. Other ion ratio criteria for GC/MS may require a ±20% of the mean ion ratio for all calibrators or of a single calibrator. Validation data for 11-OH-THC demonstrate a 1.0 ng/mL LOQ when ±20% (relative) forensic criteria are used for both m/z 474 and 459 qualifier ions.
This validated method enables concurrent quantification of THC and both principle metabolites (11-OH-THC and THCCOOH) in human plasma at lower concentrations than previously reported.
5. Conclusion
A validated method for the simultaneous detection and quantification of THC, 11-OH-THC, and THCCOOH in human plasma is presented. The method employs a rapid and economical SPE and utilizes readily available single quadrupole GC/MS instrumentation. The 2D capillary chromatography system, in combination with cryofocusing, proved to be a versatile and powerful analyte resolution tool. Required hardware upgrades are relatively inexpensive and cost-effective compared to tandem MS instrumentation. Acceptable assay characteristics and enhanced analytical sensitivity with improved S/N and detection limits extending to picogram concentrations were achieved. This assay was developed to simultaneously extract, identify, and quantify THC, 11-OH-THC, and THCCOOH in human plasma. This 2D capillary chromatography/mass spectrometry assay could also be applied to the analysis of cannabinoids in other complex matrices.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health. The authors would like to acknowledge Bruce D. Quimby and Fred Feyerherm of Agilent Technologies and Karl Scheidweiler and Dave Darwin of NIDA for their technical assistance.
References
- 1.Nadulski T, Pragst F, Weinberg G, Roser P, Schnelle M, Fronk EM, Stadelmann AM. Ther. Drug Monit. 2005;27:799. doi: 10.1097/01.ftd.0000177223.19294.5c. [DOI] [PubMed] [Google Scholar]
- 2.Pertwee RG. Int. J. Obes. 2006;30 Suppl. 1:S13. doi: 10.1038/sj.ijo.0803272. [DOI] [PubMed] [Google Scholar]
- 3.Maurer HH, Sauer C, Theobald DS. Ther. Drug Monit. 2006;28:447. doi: 10.1097/01.ftd.0000211812.27558.6e. [DOI] [PubMed] [Google Scholar]
- 4.Guy GW, Whittle BA, Robson PJ. The Medicinal Uses of Cannabis and Cannabinoids. London: Pharmaceutical Press; 2004. [Google Scholar]
- 5.Blake DR, Robson P, Ho M, Jubb RW, McCabe CS. Rheumatology (Oxford) 2005 doi: 10.1093/rheumatology/kei183. [DOI] [PubMed] [Google Scholar]
- 6.Notcutt W, Price M, Miller R, Newport S, Phillips C, Simmons S, Sansom C. Anaesthesia. 2004;59:440. doi: 10.1111/j.1365-2044.2004.03674.x. [DOI] [PubMed] [Google Scholar]
- 7.Rog DJ, Nurmikko TJ, Friede T, Young CA. Neurology. 2005;65:812. doi: 10.1212/01.wnl.0000176753.45410.8b. [DOI] [PubMed] [Google Scholar]
- 8.Wade DT, Makela PM, House H, Bateman C, Robson P. Mult. Scler. 2006;12:639. doi: 10.1177/1352458505070618. [DOI] [PubMed] [Google Scholar]
- 9.Wade DT, Makela P, Robson P, House H, Bateman C. Mult. Scler. 2004;10:434. doi: 10.1191/1352458504ms1082oa. [DOI] [PubMed] [Google Scholar]
- 10.Mechoulam R, Hanu L. Pain Res. Manage. 2001;6:67. doi: 10.1155/2001/183057. [DOI] [PubMed] [Google Scholar]
- 11.Brady CM, DasGupta R, Dalton C, Wiseman OJ, Berkley KJ, Fowler CJ. Mult. Scler. 2004;10:425. doi: 10.1191/1352458504ms1063oa. [DOI] [PubMed] [Google Scholar]
- 12.Russo E, Guy GW. Med. Hypotheses. 2006;66:234. doi: 10.1016/j.mehy.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 13.Sidney S. J. Clin. Pharmacol. 2002;42:64S. doi: 10.1002/j.1552-4604.2002.tb06005.x. [DOI] [PubMed] [Google Scholar]
- 14.Abrams DI, Hilton JF, Leiser RJ, Shade SB, Elbeik TA, Aweeka FT, Benowitz NL, Bredt BM, Kosel B, Abert JA, Deeks SG, Mitchell TF, Mulligan K, Bacchetti P, McCune JM, Schambelan M. Ann. Intern. Med. 2003;139:258. doi: 10.7326/0003-4819-139-4-200308190-00008. [DOI] [PubMed] [Google Scholar]
- 15.Curran HV, Brignell C, Fletcher S, Middleton P, Henry J. Psychopharmacology (Berl) 2002;164:61. doi: 10.1007/s00213-002-1169-0. [DOI] [PubMed] [Google Scholar]
- 16.Lundqvist T. Pharmacol. Biochem. Behav. 2005;81:319. doi: 10.1016/j.pbb.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 17.Chang L, Yakupov R, Cloak C, Ernst T. Brain. 2006;129:1096. doi: 10.1093/brain/awl064. [DOI] [PubMed] [Google Scholar]
- 18.Rogers RD, Robbins TW. Curr. Opin. Neurobiol. 2001;11:250. doi: 10.1016/s0959-4388(00)00204-x. [DOI] [PubMed] [Google Scholar]
- 19.Ashton CH. Br. J. Anaesth. 1999;83(4):637. doi: 10.1093/bja/83.4.637. [DOI] [PubMed] [Google Scholar]
- 20.Wachtel SR, ElSohly MA, Ross SA, Ambre J, deWit H. Psychopharmacology (Berl) 2002;161:331. doi: 10.1007/s00213-002-1033-2. [DOI] [PubMed] [Google Scholar]
- 21.Nicholson AN, Turner C, Stone BM, Robson PJ. J. Clin. Psychopharmacol. 2004;24:305. doi: 10.1097/01.jcp.0000125688.05091.8f. [DOI] [PubMed] [Google Scholar]
- 22.Huestis MA. In: Handbook of Experimental Pharmacology. Pertwee RG, editor. vol. 168. New York: Springer; 2005. p. 657. [Google Scholar]
- 23.Huestis MA, Elsohly M, Nebro W, Barnes A, Gustafson RA, Smith ML. Ther. Drug Monit. 2006;28:540. doi: 10.1097/00007691-200608000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Musshoff F, Madea B. Ther. Drug Monit. 2006;28:155. doi: 10.1097/01.ftd.0000197091.07807.22. [DOI] [PubMed] [Google Scholar]
- 25.Huestis MA, Cone EJ. Int. Assoc. Forensic Toxicologists. 1992:334. [Google Scholar]
- 26.Huang W, Moody DE, Andrenyak DM, Smith EK, Foltz RL, Huestis MA, Newton JF. J. Anal. Toxicol. 2001;25:531. doi: 10.1093/jat/25.7.531. [DOI] [PubMed] [Google Scholar]
- 27.Moore C, Guzaldo F, Donahue T. J. Anal. Toxicol. 2001;25:555. doi: 10.1093/jat/25.7.555. [DOI] [PubMed] [Google Scholar]
- 28.Nadulski T, Sporkert F, Schnelle M, Stadelmann AM, Roser P, Schefter T, Pragst F. J. Anal. Toxicol. 2005;29:782. doi: 10.1093/jat/29.8.782. [DOI] [PubMed] [Google Scholar]
- 29.Gustafson RA, Moolchan ET, Barnes A, Levine B, Huestis MA. J. Chromatogr. B. 2003;798:145. doi: 10.1016/j.jchromb.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 30.Steinmeyer S, Bregel D, Warth S, Kraemer T, Moeller MR. J. Chromatogr. B. 2002;772:239. doi: 10.1016/s1570-0232(02)00102-2. [DOI] [PubMed] [Google Scholar]
- 31.Saito T, Wtsadik A, Scheidweiler KB, Fortner N, Takeichi S, Huestis MA. Clin. Chem. 2004;50:2083. doi: 10.1373/clinchem.2004.034868. [DOI] [PubMed] [Google Scholar]
- 32.ElSohly MA, deWit H, Wachtel SR, Feng S, Murphy TP. J. Anal. Toxicol. 2001;25:565. doi: 10.1093/jat/25.7.565. [DOI] [PubMed] [Google Scholar]
- 33.Moore C, Rana S, Coulter C, Feyerherm F, Prest H. J. Anal. Toxicol. 2006;30:171. doi: 10.1093/jat/30.3.171. [DOI] [PubMed] [Google Scholar]
- 34.Fritch DF, Quimby BD. Application Note, Agilent Technologies. 2006:1. [Google Scholar]
- 35.Scurlock RD, Ohlson GB, Worthen DK. J. Anal. Toxicol. 2006;30:262. doi: 10.1093/jat/30.4.262. [DOI] [PubMed] [Google Scholar]