Abstract
BACKGROUND
Understanding methamphetamine (MAMP) and amphetamine (AMP) excretion in sweat is important for interpreting sweat and hair testing results in judicial, workplace, and drug treatment settings.
METHODS
Participants (n = 8) received 4 10-mg (low) oral doses of sustained-release S-(+)-MAMP HCl (d-MAMP HCl) within 1 week in a double-blind, institutional review board–approved study. Five participants also received 4 20-mg (high) doses 3 weeks later. PharmChek sweat patches (n = 682) were worn for periods of 2 h to 1 week during and up to 3 weeks after dosing. The mass of MAMP and AMP in each patch was measured by GC-MS, with a limit of quantification of 2.5 ng/patch.
RESULTS
MAMP was measurable in sweat within 2 h of dosing. After low and high doses, 92.9% and 62.5% of weekly sweat patches were positive, with a median (range) MAMP of 63.0 (16.8 – 175) and 307 (199 – 607) ng MAMP/patch, respectively; AMP values were 15.5 (6.5 – 40.5) and 53.8 (34.0 – 83.4) ng AMP/patch. Patches applied 2 weeks after the drug administration week had no measurable MAMP following the low doses, and only 1 positive result following the high doses. Using criteria proposed by the Substance Abuse Mental Health Services Administration, 85.7% (low) and 62.5% (high) weekly sweat patches from the dosing week were positive for MAMP, and all patches applied after the dosing week were negative.
CONCLUSIONS
These data characterize the excretion of MAMP and AMP after controlled MAMP administration and provide a framework for interpretation of MAMP sweat test results in clinical and forensic settings.
Methamphetamine (MAMP)4 abuse in the US continues to increase (1), with more MAMP-related arrests and a higher number of treatment admissions. Routes of administration include intravenous, insufflation, smoking, and oral. Recent reports have described an oral ingestion technique called “parachuting,” in which a swallowed tissue containing MAMP unfolds for time-released doses (2). Criminal justice and treatment programs detect prohibited drug use with sweat, hair, oral fluid, and/or urine testing (3–6). Understanding the excretion of MAMP and its metabolite amphetamine (AMP) in sweat is important for interpreting results of sweat and hair monitoring methods. The importance of hair testing derives from transfer of MAMP in sweat to hair (7).
Sweat testing is conducted using patches cleared for use by the Food and Drug Administration, which are usually worn for 1 week (8, 9). Patches provide a qualitative record of an individual’s drug use over the period of observation. Sweat patches are a less invasive means of specimen collection than blood testing and circumvent the privacy issues of urine collection. Disadvantages are the possibility of time-dependent drug loss from the patch by drug degradation on the patch or skin, reabsorption into the skin, and volatile losses through the covering membrane of the patch (10). There also are reports of patch contamination by cocaine, heroin, or MAMP remaining on the skin before patch application (11). Despite these limitations, sweat testing can be useful if appropriate wash procedures are used before application, and patch removal is properly timed. A number of investigators have reported clinical study results for opiates (4, 12, 13), cocaine (8, 9, 14–17), MAMP (18), and 3,4-methylenedioxymethamphetamine (MDMA) (19).
There have been no well-controlled studies of the excretion of MAMP or AMP into sweat. In an early report, investigators administered S-(+)-dimethylamphetamine to 2 individuals and collected sweat after physical exertion (20). They reported that the parent drug and its metabolite MAMP appeared in sweat as early as 1.5 h. Using immunoassay and GC-MS, Fay et al. (18) analyzed weekly sweat patches from known MAMP users and individuals given MAMP. The study was designed to validate the analytical method and did not fully describe the individuals or their drug use history. Pichini et al. (19) administered a single oral dose of 100 mg MDMA, a congener of MAMP, to 9 individuals and found that the parent drug appeared in sweat after 1.5 h and peaked at 24 h. The between-individual mass of MDMA varied from 3.2 to 1326 ng/patch. The metabolite 3,4-methylenedioxyamphetamine (MDA) was present in trace amounts in the sweat of 7 of 9 individuals.
In this comprehensive double-blind, placebo-controlled, multiple-dose study, we examined sweat collected from 8 individuals before, during, and after daily oral administration of 4 low (10 mg) and high (20 mg) doses of sustained-release MAMP administered within 7 days. Times of 1st detection, peak and duration of excretion, and expected mass/patch of MAMP and AMP in sweat were measured by GC-MS. Data were analyzed using the limit of quantification (LOQ) of 2.5 ng/patch and the Substance Abuse and Mental Health Services Administration (SAMHSA) proposed guidelines for MAMP and AMP sweat testing (3). These proposed guidelines establish a confirmation cutoff of 25 ng/patch for each compound, with an additional requirement forAMPto be present at or above the method’s limit of detection to report a positive MAMP result.
Materials and Methods
PARTICIPANTS AND DRUG ADMINISTRATION
Four male (2 Hispanic, 2 non-Hispanic white; ages 26 – 39 years; weight 61.5–106.5 kg) and 4 female (4 African Americans, ages 34 – 43 years; weight 56.6 – 75 kg) volunteers provided informed consent and were financially compensated for their time and effort during participation in this investigation, which was approved by the National Institute on Drug Abuse Institutional Review Board. Before admission, individuals with a history of stimulant and opioid use underwent thorough medical (physical exam, electrocardiography, and blood and urine chemistries) and psychological evaluations. For the duration of the study (10 weeks), participants resided on the secure clinical research unit, under 24-h medical surveillance, to ensure safety and to prevent additional drug use. The participants were free to conduct normal activities, including exercise in the air-conditioned facility and basketball and volleyball outside in a walled courtyard. The study was conducted over multiple years at all seasons of the year.
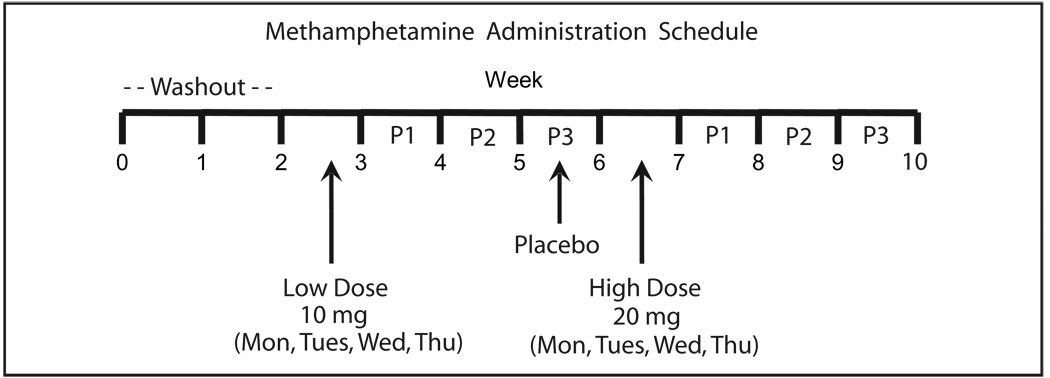
The 1st 2 weeks of the study served as a washout period to permit elimination of previously self-administered drugs. In the 3rd week, participants (n = 8) received 4 daily sustained-release doses of 10 mg (low) oral S-(+)-methamphetamine HCl (d-methamphetamine HCl) within a 7-day period (see Fig. 1). The study design included 4 consecutive daily doses; however, some administrations were not on consecutive days because of either increased baseline heart rate or research unit schedules, but all were within 7 days. After at least a 3-week interval that included administration of placebo, 5 of 8 participants also received 4 daily 20 mg (high) oral doses. Two participants were disqualified for medical reasons and a 3rd for personal issues. Participants were administered a single capsule containing 1 or 2 Desoxyn Gradumet 10-mg sustained-release tablets (Abbott Laboratories) with lactose (Amend Drug & Chemical Co., Inc.) as the filler. This formulation was developed to sustain slow release of drug after oral administration. For placebo treatments, the capsule contained only lactose filler. Additional information about the participants and administered drug and simultaneously obtained plasma, oral fluid, and urine data may be found in previous publications (21–23).
Fig. 1. Methamphetamine administration schedule for 4 10-mg (low) and 4 20-mg (high) oral MAMP doses.
Washout refers to the 2 weeks before drug administration that permitted excretion of previously self-administered illicit drugs. P1, P2, and P3 refer to the 1st, 2nd, and 3rd weeks after the low and high doses to monitor residual drug excretion after the dosing week.
SWEAT COLLECTION
Weekly sweat patches
PharmChek® (PharmChem Inc.) sweat patches were applied to participants upon admission, 1 on the back and 1 on the abdomen, and removed at the end of 1 week. Duplicate patches also were applied in the same manner every week for the duration of the protocol (10 weeks). The sweat patch device consists of an adhesive layer on a thin transparent film of surgical dressing and a rectangular, absorbent, cellulose pad (14 cm2). The surgical dressing film allows oxygen, carbon dioxide, and water vapor to escape while the nonvolatile constituents in sweat are retained in the absorbent pad. Patches applied and removed in the 1st 2 weeks before dosing were termed washout patches. Dosing patches were applied before the 1st dose and removed up to 7 days later. Weekly patches applied after the end of drug administration were defined as postdose patches. Data for duplicate patches were reported individually, and median values were determined using all data, not using mean data for the replicates. Five (0.6%) of the 786 sweat patches did not adhere throughout the wear period. Unacceptable data were obtained for 3 patches (0.4%) owing to the lack of addition of internal standard. Nineteen patches (2.4%) were not applied owing to clinical or administration issues, and 28 (3.6%) were removed because of skin irritation. The most regrettable was the loss of 48 short-term and 20 weekly patches between collection and analysis. Further detail (whether the loss occurred during short- or long-term storage on the research unit) is unavailable.
SHORT-TERM SWEAT PATCHES
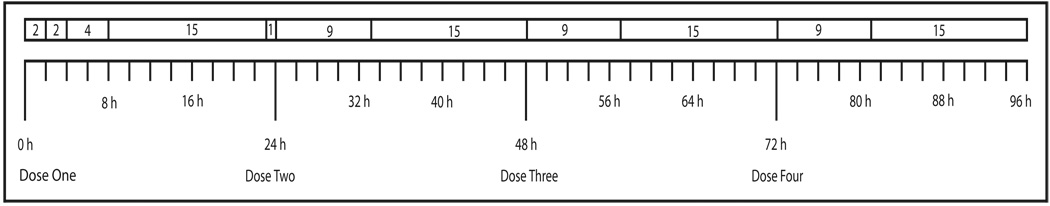
Single patches were applied to monitor excretion throughout each day that drug was administered (Fig. 2). The patches after the 1st dose covered the time periods 0–2 h, 2–4 h, 4–8 h, 8–23 h (15-h patch), and 23–24 h (baseline patch for the next day’s dosing). On subsequent dosing days, short-term sweat patches covered the periods of 0–9 h and 9–24 h (15-h patch).
Fig. 2. Short-term sweat patch application and removal schedule.
Short-term sweat patches were applied for 0–2, 2–4, 4–8, 8–23, and 23–24 h after the first low- and high-dose methamphetamine administration, and 0–9 and 9–24 h for the other 3 dosing days.
The skin was thoroughly cleaned with an isopropyl alcohol prep wipe (70% vol/vol) before patch application. After removal, patches were placed into plastic specimen bags along with a clean index card with identification information, sealed, and stored at −20 °C until analysis.
REAGENTS AND MATERIALS
We purchased MAMP, AMP,MAMP-d11, and AMP-d10 from Cerilliant; N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) with 1% trimethylchlorosilane (TMCS) and N-methyl-N-(tert-butyldimethylsilyl)trifluoroacetamide (MTBSTFA) with 1% tert-butyldimethylchlorosilane (TBDMCS) from Pierce Chemical; and filtration columns (RFV02F4P) and solid-phase extraction (SPE) columns (Clean Screen CSDAU020) from United Chemical Technologies.
GC/MS ANALYSIS
We analyzed sweat patches for MAMP and AMP by modification of a published SPE GC/MS procedure (14, 24). These modifications permitted simultaneous quantification of MAMP, AMP, opiates, cocaine, and metabolites in a single analysis. Briefly, we added calibrators and control solutions to drug-free patches and added deuterated internal standards directly to calibrator, control, and participants’ sweat patches. Patches were folded and placed into 12-mL filtration columns fitted with stopcocks. A 4-mL aliquot of 0.5 mol/L sodium acetate buffer (pH 4.0) was added, and the sweat patch remained immersed in this solution for 30 min at room temperature. We collected buffered eluates in disposable 16 by 100mm glass test tubes. This step was repeated twice using 2 mL buffer and 30-min immersion intervals. We applied the combined buffered extracts (8 mL) to SPE columns preconditioned with 1 mL freshly prepared elution solvent; methylene chloride: 2-propanol:ammonium hydroxide (80:20:2, vol/vol/vol), methanol (1 mL), distilled water (3 mL), and 1.5 mL of 2.0 mol/L sodium acetate buffer (pH 4.0). The columns were washed with distilled water (3 mL), 0.2 mol/L hydrochloric acid (1.5 mL), and methanol (3 mL) and dried for 5min under full vacuum. Analytes of interest were eluted using 5 aliquots (1 mL) of elution solvent. We added 20 µL MTBSTFA with 1% TBD MCS to each tube to reduce MAMP and AMP volatility (24) and evaporated the eluates to dryness under nitrogen. Extracts were reconstituted in acetonitrile and subjected to dual derivatization with MTBSTFA with 1% TBDMCS and BSTFA with 1% TMCS. Derivatives were analyzed in splitless mode on an Agilent 6890 gas chromatograph/5973 quadrupole mass selective detector operated in electron ionization mode. The temperatures of the quadrupole, ion source, and mass selective detector interface were 150, 250, and 295 °C, respectively. The injection port temperature was maintained at 250 °C. The initial oven temperature was maintained at 70 °C for 1 min, followed by ramps of 30 °C/min to 175 °C, 23 °C/min to 250 °C, and 18 °C/min to a final temperature of 310 °C, which was held for 3 min.
In each analytical run, we constructed 2 calibration curves for each analyte to establish extended calibration ranges. Low (2.5–50 ng/patch) and high (50–500 ng/patch) calibration curves (n = 10) were constructed for MAMP and AMP, with coefficients of determination (R2) ≥0.989. LOQs for MAMP and AMP were 2.5 ng/patch. We calculated estimates of imprecision using duplicate controls from 10 analytical runs (n = 20) according to Krouwer and Rabinowitz (25). For all runs, the pooled within-run component of imprecision, expressed as %CV, was < 12.4% for all control concentrations (3.75, 12.5, 125, and 375 ng/patch). Between-run imprecision (%CV) for all analytes, at all concentrations, was <12.1% for methamphetamine and <10.6% for amphetamine. Total imprecision of the method (%CV) was reported as <17.8% and <20.8% for MAMP and AMP, respectively. Recoveries were within 10.2% of target concentrations.
Results
We collected 682 sweat patches from 8 participants throughout the 10-week study. No weekly patch (n = 38) collected during the washout period had detectable MAMP or AMP. After controlled administration of 40 and 80 mg sustained-release MAMP (4 10- or 20-mg doses within 1 week), parent drug was the primary analyte detected in sweat. With the exception of 6 short-term patches, AMP was also detected in all patches with MAMP values >25 ng/patch. In addition, 10 patches (1.5%) contained AMP above the method LOQ without concurrent MAMP; 5 of these were weekly patches.
MAMP was the only analyte detected in any short-term patch (n = 32) worn at intervals (0 –2, 2–4, 4–8, 8–23 h) after the first low-dose administration (Table 1). Twelve of these short-term patches (37.5%) had MAMP above the LOQ. Because of the absence of AMP, no patch met the SAMHSA criteria for a positive result. After the first 20-mg MAMP administration, 75% of short-term sweat patches (n = 16) were positive for MAMP above the assay LOQ and 25% satisfied SAMHSA requirements for a positive specimen. MAMP was 1st detected in short-term sweat patches applied just before dosing and removed 2 h later (0–2 h). AMP was detected in short-term patches only after the high dose (Table 1).
Table 1.
Methamphetamine and amphetamine results for hourly (2, 4, and 15 h) patches worn during or after a single low-dose (10 mg) or high-dose (20 mg) oral MAMP administration.
| Time after dose, h |
Time worn, h |
MAMP ≥LOQ, na |
Median MAMP, ng/patch (range) |
AMP ≥LOQ, na |
Median AMP, ng/patch (range) |
≥SAMHSA, nb |
|---|---|---|---|---|---|---|
|
Low dose (n =8) |
||||||
| 0–2 | 2 | 1 | 4.8 | 0 | Not detectedc | 0 |
| 2–4 | 2 | 1 | 3.1 | 0 | Not detectedc | 0 |
| 4–8 | 4 | 5 | 5.8 (3.0–23.3) | 0 | Not detectedc | 0 |
| 8–23 | 15 | 5 | 11.2 (5.3–42.3) | 0 | Not detectedc | 0 |
|
High dose (n = 5) |
||||||
| 0–2 | 2 | 2 | 46.5 (6.9–86.0) | 1 | 18.6 | 1 |
| 2–4 | 2 | 3 | 9.8 (6.1–21.4) | 0 | Not detectedc | 0 |
| 4–8 | 4 | 3 | 34.2 (33.1–41.4) | 1 | 4.4 | 1 |
| 8–23 | 15 | 4 | 28.3 (18.9–103.4) | 3 | 5.0 (3.3–8.4) | 2 |
LOQ 2.5 ng/patch.
Criteria for MAMP positive result: MAMP ≥25 ng/patch, AMP ≥ limit of detection.
≥2.5 ng/patch.
Results for 15-h patches worn at the end of each day of MAMP administration are presented in Table 2. After 4 low and high doses, respectively, 67.7% of patches (n = 31) and 92.9% of patches (n = 14) were above the LOQ for MAMP, vs 25.8% and 78.6% for AMP. Five of 31 15-h patches after low MAMP doses were positive by SAMHSA guidelines, vs 7 of 14 after high doses. AMP was only present without MAMP in 2 of 49 patches, and never at ≥25 ng/patch to fulfill SAMHSA requirements for a positive AMP sweat test.
Table 2.
Methamphetamine and amphetamine mass per sweat patch worn for 15 h at the end of each day after administration of 4 daily low (10 mg) or high (20 mg) MAMP doses.
| MAMP | AMP | MAMP | AMP | MAMP | AMP | MAMP | AMP | |
|---|---|---|---|---|---|---|---|---|
| Low dose | ||||||||
| n | 8 | 8 | 7 | 7 | 8 | 8 | 8 | 8 |
| ≥LOQ, na | 5 | 0 | 5 | 3 | 5 | 2 | 6 | 3 |
| Median ng/patch (range) |
11.2 (5.3–42.3) |
ND | 32.5 (5.0–82.1) |
9.5 (2.9–9.8) |
5.9 (2.8–72.4) |
10.2 (8.0–12.4) |
23.7 (16.8–104) |
4.8 (2.8–20.1) |
| ≥25 ng/patch, n | 1 | 0 | 3 | 0 | 1 | 0 | 3 | 0 |
| ≥SAMHSA, nb | 0 | 0 | 2 | 0 | 1 | 0 | 2 | 0 |
| High dose | ||||||||
| n | 4 | 4 | 4 | 4 | 3 | 3 | 3 | 3 |
| ≥LOQ, na | 4 | 3 | 3 | 3 | 3 | 3 | 3 | 2 |
| Median ng/patch (range) |
28.3 (18.9–103) |
5 (3.3–8.4) |
70.6 (22.7–215) |
15.8 (5.1–21.5) |
68.5 (15.8–148) |
8.5 (3.9–19.4) |
17.1 (11.5–74.8) |
8.1 (5.2–11.0) |
| ≥25 ng/patch, n | 2 | 0 | 2 | 0 | 2 | 0 | 1 | 0 |
| ≥SAMHSA, nb | 2 | 0 | 2 | 0 | 2 | 0 | 1 | 0 |
LOQ 2.5 ng/patch.
Criteria for MAMP positive result: MAMP ≥25 ng/patch, AMP ≥ limit of detection.
Weekly patches were worn during the 40- and 80-mg cumulative MAMP dosing week. During the low dose, 13 of 14 weekly patches had MAMP above the LOQ, with a median (range) for positive patches of 63.0 (16.8 – 175) ng/patch. All of these patches were positive for AMP, with a median mass/patch of 15.5 (6.5–40.5) ng/patch (Table 3). Twelve of 14 were positive for MAMP by SAMHSA criteria. During the cumulative 80-mg administration period, 5 of 8 weekly patches were above the assay’s LOQ for MAMP, with 6 of 8 positive for AMP. For positive weekly sweat patches, the median (range) mass/patch values were 307 (199–607) ng/patch and 53.8 (34.0–83.4) ng/patch for MAMP and AMP, respectively. All MAMP-positive weekly sweat patches worn during the high-dose condition also contained AMP above the SAMHSA cutoff of 25 ng/patch, and 1 of 8 weekly patches contained AMP without concurrent MAMP. A larger percentage of weekly patches (85.7%) were positive by SAMHSA criteria during the low compared to the high doses (62.5%). However, this was not statistically significant using χ2 analysis (P > 0.05), potentially owing to the small number of patches or to the differences in MAMP sweat excretion in the individuals receiving the low and high doses. For the weeks after either the low or high dosing week, there were no positive sweat patches when applying SAMHSA criteria (Table 3). The week after the low-dose week (n = 13), 61.5% and 30.8% of patches were above the LOQ for MAMP and AMP, respectively. Both analytes were below the LOQ for all weekly patches collected 2 and 3 weeks after low dose. Note that many of the missing data points were for weeks that followed a week when patches were negative at the LOQ. One would expect these patches to also be negative. Detection rates increased after the high doses. In the week after the high MAMP doses (n = 7), 100.0% (MAMP) and 71.4% (AMP) of patches exceeded the LOQ. In the same individuals, when using a 2.5µg/L urinary cutoff (LOQ), Oyler et al. (23) reported last detection times up to 169 h from the last MAMP dose. Our results show positive sweat patches during this time frame (after dose week 1). In addition, 1 weekly patch collected in week 2 after high dose contained MAMP(15.2 ng). There were no positive urine tests at this time. All other week 2 and week 3 postdose patches had no detectable drug. There were no positive sweat patches with the proposed federally mandated cutoffs. Of course it is not expected that drug test results will be identical between matrices due to different analytes and analytical cutoffs, different periods of time for collection—i.e., sweat patches accumulate drug over 1 week vs a single urine sample representing only a few hours of excretion (26).
Table 3.
Mass of MAMP and AMP in weekly sweat patches during (week 1) and after administration of 4 10-mg (low) or 4 20-mg (high) doses of MAMP within 1 week.
| Subject | During Week 1 MAMP |
AMP | After Week 1 MAMP |
AMP | Week 2 MAMP |
AMP | Week 3 MAMP |
AMP |
|---|---|---|---|---|---|---|---|---|
| Low dose | ||||||||
| 1 | 56.9 | 21.0 | 22.9 | 13.7 | <2.5 | <2.5 | <2.5 | <2.5 |
| 1 | a | a | <2.5 | <2.5 | a | a | a | a |
| 2 | 175 | 40.5 | a | a | a | a | a | a |
| 2 | 121 | 21.7 | a | a | a | a | a | a |
| 3 | 73.2 | 32.5 | <2.5 | <2.5 | <2.5 | <2.5 | a | a |
| 3 | 58.3 | 27.3 | <2.5 | <2.5 | <2.5 | <2.5 | a | a |
| 4 | 16.8 | 6.5 | <2.5 | <2.5 | a | a | a | a |
| 5 | 38.2 | 7.1 | 4.2 | <2.5 | <2.5 | <2.5 | a | a |
| 5 | 28.3 | 8.6 | 4.2 | <2.5 | <2.5 | <2.5 | a | a |
| 6 | 44.9 | 7.6 | <2.5 | <2.5 | <2.5 | <2.5 | <2.5 | <2.5 |
| 6 | 63.0 | 10.9 | 8.2 | 2.6 | <2.5 | <2.5 | <2.5 | <2.5 |
| 7 | 130 | 15.9 | 9.9 | 2.6 | <2.5 | <2.5 | a | a |
| 7 | 115 | 15.0 | 8.7 | 2.5 | <2.5 | <2.5 | a | a |
| 8 | <2.5 | 16.1 | 4.3 | <2.5 | <2.5 | <2.5 | a | a |
| 8 | 72.9 | 13.3 | 5.0 | <2.5 | <2.5 | <2.5 | a | a |
| ≥LOQb | 13 | 14 | 8 | 4 | 0 | 0 | 0 | 0 |
| ≥SAMHSAc | 12 | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 14 | 14 | 13 | 13 | 11 | 11 | 3 | 3 |
| Median | 63.0 | 15.5 | 5.0 | 2.6 | ||||
| Minimum | 16.8 | 6.5 | 4.2 | 2.5 | ||||
| Maximum | 175 | 40.5 | 22.9 | 13.7 | ||||
| High dose | ||||||||
| 1 | 199 | 34.0 | 24.9 | 14.2 | 15.2 | <2.5 | <2.5 | <2.5 |
| 3 | 306 | 83.4 | 7.8 | 3.7 | <2.5 | <2.5 | a | a |
| 3 | 303 | 82.3 | 4.4 | 2.9 | <2.5 | <2.5 | a | a |
| 5 | <2.5 | <2.5 | 3.8 | <2.5 | a | a | a | a |
| 5 | <2.5 | <2.5 | 2.8 | <2.5 | a | a | a | a |
| 7 | 607 | 66.6 | 12.4 | 4.0 | <2.5 | <2.5 | <2.5 | <2.5 |
| 7 | 322 | 40.9 | 11.5 | 3.8 | <2.5 | <2.5 | <2.5 | <2.5 |
| 8 | <2.5 | 40.9 | a | a | <2.5 | <2.5 | a | a |
| 8 | a | a | a | a | <2.5 | <2.5 | a | a |
| ≥LOQb | 5 | 6 | 7 | 5 | 1 | 0 | 0 | 0 |
| ≥SAMHSAc | 5 | 6 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 8 | 8 | 7 | 7 | 7 | 7 | 3 | 3 |
| Median | 307 | 53.8 | 7.8 | 3.8 | 0.0 | |||
| Minimum | 199 | 34.0 | 2.8 | 2.9 | 15.2 | |||
| Maximum | 607 | 83.4 | 24.9 | 14.2 | 15.2 | |||
Missing data.
LOQ 2.5 ng/patch.
Criteria for MAMP positive result: MAMP ≥25 ng/patch, AMP ≥ limit of detection.
Table 4 compares the cumulative mass/patch of MAMP and AMP in short-term patches to comparable weekly patches. For 9 of 12 dosing occasions, the cumulative mass/patch was greater than the average weekly mass/patch of MAMP. Some differences were large, with cumulative mass/patch more than twice that of the average weekly patches. Differences for AMP between cumulative short-term and weekly patches were less frequent and of smaller magnitude.
Table 4.
Comparison of MAMP and AMP mass per patch in duplicate weekly sweat patches with corresponding cumulative mass per patch in hourly patches covering the same MAMP administration period.
| Methamphetamine, ng/patch | Amphetamine, ng/patch | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subject | Weekly patch 1 |
Weekly patch 2 |
Mean | Hourly sum |
Difference | Weekly patch 1 |
Weekly patch 2 |
Mean | Hourly sum |
Difference |
| Low dose | ||||||||||
| 1 | 56.9 | — | — | 69.6 | 12.7 | 21.0 | — | — | 6.5 | −14.5 |
| 2 | 175 | 121 | 148 | 431 | 283 | 40.5 | 21.7 | 31.1 | 64.9 | 24.4 |
| 3 | 73.2 | 58.3 | 65.8 | 103 | 36.8 | 32.5 | 27.3 | 29.9 | 38.8 | 6.3 |
| 4 | 16.8 | — | — | 11.2 | −5.6 | 6.5 | — | — | 0.0 | −6.5 |
| 5 | 38.2 | 28.3 | 33.3 | 27.1 | −6.2 | 7.1 | 8.6 | 7.9 | 0.0 | −7.1 |
| 6 | 44.9 | 63.0 | 54.0 | 73.2 | 19.3 | 7.6 | 10.9 | 9.3 | 0.0 | −7.6 |
| 7 | 131 | 115 | 123 | 207 | 83.8 | 15.9 | 15.0 | 15.5 | 16.0 | 0.1 |
| 8 | 0.0 | 72.9 | 36.5 | 102 | 66.0 | 16.1 | 13.3 | 14.7 | 10.4 | −5.7 |
| High dose | ||||||||||
| 1 | 199 | — | — | 250 | 50.8 | 34.0 | — | — | 19.8 | −14.2 |
| 3 | 307 | 303 | 305 | 149 | −156 | 83.4 | 82.3 | 82.9 | 189 | 106 |
| 5 | 0.0 | 0.0 | 0.0 | 229 | 229 | 0.0 | 0.0 | 0.0 | 42.4 | 42.4 |
| 7 | 607 | 322 | 465 | 1106 | 641 | 66.6 | 40.9 | 53.8 | 133 | 66.1 |
Discussion
This comprehensive, controlled, multiple oral dose MAMP administration study addressed the disposition of MAMP and its metabolite AMP in human sweat. The study was conducted on a closed research unit, and included a 2-week washout period to ensure that drug detected was not from prior self-administration. Sweat patches (n = 682) were applied and removed at various times to monitor excretion of drug into sweat during hourly and weekly timeframes. MAMP was the principal analyte identified after MAMP administration. This also was reported by Fay et al. (18), as well as a review of the clinical pharmacokinetics of amphetamines (27). MAMP in our study appeared as early as 2 h after oral dosing, consistent with the findings of Vree et al. (20), who reported the appearance of parent drug in sweat in 2 participants 1.5 h after oral administration of 20 mg S-(+)-dimethylamphetamine. Pichini et al. (19) observed the appearance of MDMA in sweat 1.5 h after administration and demonstrated high interindividual variability in mass MDMA/patch values in weekly patches, consistent with our findings of weekly MAMP mass/patch from <2.5 to 175 ng/patch during administration of 40 mg MAMP, and <2.5 to 607 ng/patch for 80 mg MAMP. Some variability is explained by different sweat excretion rates (10) and differences in bioavailability and metabolism between individuals. Other possibilities are loss of drug through degradation on the patch or skin, reabsorption through the skin, and potential loss through the outer sweat patch covering to the environment. We found that for most drug administration occasions, cumulative short-term mass MAMP/patch values were greater than the weekly mass MAMP/patch values covering the same periods (Table 4). This could indicate that there was some loss of MAMP from the patches worn for 1 week through the mechanisms discussed by Uemura et al. (10). This group found that deuterated cocaine placed on sweat patches could be absorbed through skin over a period of time. They also observed up to 8-fold differences in mass cocaine/patch with patch location. We did not observe these differences for MAMP on sweat patches placed on the back and abdomen. Loss from patches did not appear to be significant for AMP.
Mass MAMP/sweat patch values were higher on the 2nd day of administration, but median mass/patch did not continue to increase on subsequent dosing days. This also was true for mass MAMP/sweat patch in the subset of patches worn the last 15 h of each dosing day. These sweat patches showed that the median mass MAMP/patch was greater for the higher doses, but the number of patches positive using an LOQ cutoff or SAMHSA criteria were not significantly different between doses.
AMP did not appear in measurable amounts in sweat the 1st day after a 10-mg dose of MAMP, but was excreted within the first 2 h for 1 individual after the 20-mg dose. Vree et al. (20) found that the metabolite S-(+)-methylamphetamine, after a 20-mg oral dose of S(+)-dimethylamphetamine, peaked in 5 and 7 h for 2 participants. One might expect in our study to see the highest mass/patch of the metabolite AMP in this time-frame. AMP also was present in 3 of 4 patches collected the last 15 h of the 1st high-dose day and in amounts that were about 10% of those for MAMP. On days 2 through 4 of the high-dose administration, AMP was detectable in most short-term patches, but was <25 ng/patch.
It is interesting that a larger percentage of weekly sweat patches worn during low drug administration had MAMP above the LOQ for the low (92.9%) vs the high (62.5%) dose, despite the median mass MAMP/patch being >4 times higher. Using SAMHSA criteria yielded similar positive rates, 85.7% vs 62.5%. The differences are large but not statistically significant given the small sample size (χ2, P > 0.05). These results indicate that, with the recommended cutoff mass/patch, detection rates may vary independent of administered dose.
Our findings have implications for interpreting hair test results. Contamination of hair by drugs in sweat is well documented (7, 28–33), and hair-testing laboratories have different methods for removing external contamination (29, 34, 35). Our results demonstrate that MAMP may be present in sweat within 2 h of oral ingestion and may be excreted for >1 week after cessation of multiple uses. It is possible for MAMP in sweat to become incorporated into hair during this period of time.
Based on our results, clinicians who wish to monitor individuals in drug treatment programs can expect to detect a cumulative dose of MAMP as small as 40 mg with a weekly sweat patch. For individuals taking a total dose of 80 mg MAMP, one would expect weekly patches worn during administration to be positive but patches applied in the weeks after drug cessation to be negative using SAMHSA criteria. These data provide a scientific database for interpreting MAMP and AMP sweat test results and contribute to improved clinical monitoring of MAMP use.
Acknowledgments
Grant/funding Support: The research was supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health, and the American Registry of Pathology. We would also like to thank PharmChem for generously providing sweat patches for this study.
Footnotes
Nonstandard abbreviations: MAMP, methamphetamine; AMP, amphetamine; MDMA, 3,4-methylenedioxymethamphetamine; MDA, 3,4-methylenedioxyamphetamine; LOQ, limit of quantification; SAMHSA, Substance Abuse and Mental Health Services Administration; BSTFA, N,O-bis(trimethylsilyl)trifluoroacetamide; MTBSTFA, N-methyl-N-(tert-butyldimethylsilyl)trifluoroacetamide; TBDMCS, tert-butyldimethylchlorosilane; SPE, solid-phase extraction.
Financial Disclosures: None declared.
The opinions in this article are those of the authors and do not necessarily reflect the views of the National Institute on Drug Abuse or the Department of Defense.
References
- 1.Office of Applied Statistics, Substance Abuse and Mental Health Services Administration, US Department of Health and Human Services. State estimates of past year methamphetamine use. National Survey on Drug Use and Health, The NSDUH Report: Issue 37. 2006
- 2.Hendrickson RG, Horowitz BZ, Norton RL, Noten-boom H. “Parachuting” meth: a novel delivery method for methamphetamine and delayed-onset toxicity from “body stuffing. ”. Clin Toxicol (Phila) 2006;44:379–382. doi: 10.1080/15563650600671746. [DOI] [PubMed] [Google Scholar]
- 3.US Department of Health and Human Services. Mandatory guidelines and proposed revisions to mandatory guidelines for federal workplace drug testing programs. Federal Register. 2004;69:19644–19673.
- 4.Huestis MA, Cone EJ, Wong CJ, Umbricht A, Preston KL. Monitoring opiate use in substance abuse treatment patients with sweat and urine drug testing. J Anal Toxicol. 2000;24:509–521. doi: 10.1093/jat/24.7.509. [DOI] [PubMed] [Google Scholar]
- 5.Huestis MA, Choo RE. Drug abuse’s smallest victims: in utero drug exposure. Forensic Sci Int. 2002;128:20–30. doi: 10.1016/s0379-0738(02)00160-3. [DOI] [PubMed] [Google Scholar]
- 6.Dolan K, Rouen D, Kimber J. An overview of the use of urine, hair, sweat and saliva to detect drug use. Drug Alcohol Rev. 2004;23:213–217. doi: 10.1080/09595230410001704208. [DOI] [PubMed] [Google Scholar]
- 7.Cone EJ. Mechanisms of drug incorporation into hair. Ther Drug Monit. 1996;18:438–443. doi: 10.1097/00007691-199608000-00022. [DOI] [PubMed] [Google Scholar]
- 8.Liberty HJ, Johnson BD, Fortner N, Randolph D. Detecting crack and other cocaine use with fast-patches. Addict Biol. 2003;8:191–200. doi: 10.1080/1355621031000117428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liberty HJ, Johnson BD, Fortner N. Detecting cocaine use through sweat testing: multilevel modeling of sweat patch length-of-wear data. J Anal Toxicol. 2004;28:667–673. doi: 10.1093/jat/28.8.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uemura N, Nath RP, Harkey MR, Henderson GL, Mendelson J, Jones RT. Cocaine levels in sweat collection patches vary by location of patch placement and decline over time. J Anal Toxicol. 2004;28:253–259. doi: 10.1093/jat/28.4.253. [DOI] [PubMed] [Google Scholar]
- 11.Kidwell DA, Smith FP. Susceptibility of Pharm-Chek drugs of abuse patch to environmental contamination. Forensic Sci Int. 2001;116:89–106. doi: 10.1016/s0379-0738(00)00353-4. [DOI] [PubMed] [Google Scholar]
- 12.Fogerson R, Schoendorfer D, Fay J, Spiehler V. Qualitative detection of opiates in sweat by EIA and GC-MS. J Anal Toxicol. 1997;21:451–458. doi: 10.1093/jat/21.6.451. [DOI] [PubMed] [Google Scholar]
- 13.Schwilke EW, Barnes AJ, Kacinko SL, Cone EJ, Moolchan ET, Huestis MA. Opioid disposition in human sweat after controlled oral codeine administration. Clin Chem. 2006;52:1539–1545. doi: 10.1373/clinchem.2006.067983. [DOI] [PubMed] [Google Scholar]
- 14.Huestis MA, Oyler JM, Cone EJ, Wstadik AT, Schoendorfer D, Joseph RE., Jr Sweat testing for cocaine, codeine and metabolites by gas chromatography-mass spectrometry. J Chromatogr B Biomed Sci Appl. 1999;733:247–264. doi: 10.1016/s0378-4347(99)00246-7. [DOI] [PubMed] [Google Scholar]
- 15.Winhusen TM, Somoza EC, Singal B, Kim S, Horn PS, Rotrosen J. Measuring outcome in cocaine clinical trials: a comparison of sweat patches with urine toxicology and participant self-report. Addiction. 2003;98:317–324. doi: 10.1046/j.1360-0443.2003.00311.x. [DOI] [PubMed] [Google Scholar]
- 16.Kidwell DA, Kidwell JD, Shinohara F, Harper C, Roarty K, Bernadt K, et al. Comparison of daily urine, sweat, and skin swabs among cocaine users. Forensic Sci Int. 2003;133:63–78. doi: 10.1016/s0379-0738(03)00051-3. [DOI] [PubMed] [Google Scholar]
- 17.Kacinko SL, Barnes AJ, Schwilke EW, Cone EJ, Moolchan ET, Huestis MA. Disposition of cocaine and its metabolites in human sweat after controlled cocaine administration. Clin Chem. 2005;51:2085–2094. doi: 10.1373/clinchem.2005.054338. [DOI] [PubMed] [Google Scholar]
- 18.Fay J, Fogerson R, Schoendorfer D, Niedbala RS, Spiehler V. Detection of methamphetamine in sweat by EIA and GC-MS. J Anal Toxicol. 1996;20:398–403. doi: 10.1093/jat/20.6.398. [DOI] [PubMed] [Google Scholar]
- 19.Pichini S, Navarro M, Pacifici R, Zuccaro P, Ortuno J, Farre M, et al. Usefulness of sweat testing for the detection of MDMA after a single-dose administration. J Anal Toxicol. 2003;27:294–303. doi: 10.1093/jat/27.5.294. [DOI] [PubMed] [Google Scholar]
- 20.Vree TB, Muskens AT, van Rossum JM. Excretion of amphetamines in human sweat. Arch Int Pharmacodyn Ther. 1972;199:311–317. [PubMed] [Google Scholar]
- 21.Schepers RJ, Oyler JM, Joseph RE, Jr, Cone EJ, Moolchan ET, Huestis MA. Methamphetamine and amphetamine pharmacokinetics in oral fluid and plasma after controlled oral methamphetamine administration to human volunteers. Clin Chem. 2003;49:121–132. doi: 10.1373/49.1.121. [DOI] [PubMed] [Google Scholar]
- 22.Kim I, Oyler JM, Moolchan ET, Cone EJ, Huestis MA. Urinary pharmacokinetics of methamphetamine and its metabolite, amphetamine following controlled oral administration to humans. Ther Drug Monit. 2004;26:664–672. doi: 10.1097/00007691-200412000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Oyler JM, Cone EJ, Joseph RE, Jr, Moolchan ET, Huestis MA. Duration of detectable methamphetamine and amphetamine excretion in urine after controlled oral administration of methamphetamine to humans. Clin Chem. 2002;48:1703–1714. [PubMed] [Google Scholar]
- 24.Yang W, Barnes AJ, Ripple MG, Fowler DR, Cone EJ, Moolchan ET, et al. Simultaneous quantification of methamphetamine, cocaine, codeine, and metabolites in skin by positive chemical ionization gas chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;833:210–218. doi: 10.1016/j.jchromb.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Krouwer JS, Rabinowitz R. How to improve estimates of imprecision. Clin Chem. 1984;30:290–292. [PubMed] [Google Scholar]
- 26.Cone EJ, Sampson-Cone A, Huestis MA. Interpreting alternative matrix test results. In: Karch SB, editor. Drug Abuse Handbook, Vol. Boca Raton: CRC Press; 2006. pp. 814–828. [Google Scholar]
- 27.de la Torre R, Farre M, Navarro M, Pacifici R, Zuccaro P, Pichini S. Clinical pharmacokinetics of amfetamine and related substances: monitoring in conventional and non-conventional matrices. Clin Pharmacokinet. 2004;43:157–185. doi: 10.2165/00003088-200443030-00002. [DOI] [PubMed] [Google Scholar]
- 28.Blank DL, Kidwell DA. Decontamination procedures for drugs of abuse in hair: are they sufficient? Forensic Sci Int. 1995;70:13–38. doi: 10.1016/0379-0738(94)01617-e. [DOI] [PubMed] [Google Scholar]
- 29.Cairns T, Hill V, Schaffer M, Thistle W. Removing and identifying drug contamination in the analysis of human hair. Forensic Sci Int. 2004;145:97–108. doi: 10.1016/j.forsciint.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 30.Stout PR, Ropero-Miller JD, Baylor MR, Mitchell JM. External contamination of hair with cocaine: evaluation of external cocaine contamination and development of performance-testing materials. J Anal Toxicol. 2006;30:490–500. doi: 10.1093/jat/30.8.490. [DOI] [PubMed] [Google Scholar]
- 31.Joseph RE, Jr, Hold KM, Wilkins DG, Rollins DE, Cone EJ. Drug testing with alternative matrices II Mechanisms of cocaine and codeine deposition in hair. J Anal Toxicol. 1999;23:396–408. doi: 10.1093/jat/23.6.396. [DOI] [PubMed] [Google Scholar]
- 32.Henderson GL, Harkey MR, Zhou C, Jones RT, Jacob P., 3rd Incorporation of isotopically labeled cocaine and metabolites into human hair: 1. Dose-response relationships. J Anal Toxicol. 1996;20:1–12. doi: 10.1093/jat/20.1.1. [DOI] [PubMed] [Google Scholar]
- 33.Rothe M, Pragst F, Spiegel K, Harrach T, Fischer K, Kunkel J. Hair concentrations and self-reported abuse history of 20 amphetamine and ecstasy users. Forensic Sci Int. 1997;89:111–128. doi: 10.1016/s0379-0738(97)00123-0. [DOI] [PubMed] [Google Scholar]
- 34.Jurado C, Sachs H. Proficiency test for the analysis of hair for drugs of abuse, organized by the Society of Hair Testing. Forensic Sci Int. 2003;133:175–178. doi: 10.1016/s0379-0738(03)00065-3. [DOI] [PubMed] [Google Scholar]
- 35.Schaffer M, Hill V, Cairns T. Hair analysis for cocaine: the requirement for effective wash procedures and effects of drug concentration and hair porosity in contamination and decontamination. J Anal Toxicol. 2005;29:319–326. doi: 10.1093/jat/29.5.319. [DOI] [PubMed] [Google Scholar]