Summary
Alcoholism is a progressive disorder that involves the amygdala. Mice lacking protein kinase C epsilon (PKCε) show reduced ethanol consumption, sensitivity and reward. We therefore investigated whether PKCε signaling in the amygdala is involved in ethanol consumption. Local knockdown of PKCε in the amygdala reduced ethanol consumption and preference in a limited access paradigm. Further, mice which are heterozygous for the PKCε allele consume less ethanol compared to wild type mice in this paradigm. These mice have a >50% reduction in the abundance of PKCε in the amygdala compared with wild-type mice. We conclude that amygdala PKCε is important for ethanol consumption in mice.
Introduction
Alcoholism is a common form of substance abuse with an estimated annual economic burden in the U.S. exceeding $170 billion dollars (Rice, 1999). The anatomical and molecular basis for alcoholism remains unresolved. The amygdala, located in the medial temporal lobe, is a collection of nuclei that are involved in emotional processing, reward and learning (LeDoux, 2007; Murray, 2007). Current evidence suggests that the amygdala plays a role in the development and maintenance of alcoholism. Exposure of alcoholics to ethanol odor induces a craving that is associated with amygdala activation (Schneider et al., 2001) and alcoholics exhibit a small reduction in amygdala volume (Makris et al., 2008). These clinical findings are supported by studies in the amygdala of rodents, where acute ethanol exposure inhibits spontaneous neuronal activity (Perra et al., 2008), induces changes in gene expression (Pandey et al., 2006; Pandey et al., 2008) and enhances GABAergic neurotransmission in the amygdala (Roberto et al., 2003). Furthermore, the amygdala has been implicated in enhanced ethanol self-administration in dependent rats (Funk et al., 2006a).
Mice lacking protein kinase C epsilon (PKCε) show reduced ethanol self-administration and reduced signs of alcohol withdrawal (Hodge et al., 1999; Olive et al., 2000). PKCε null mice also show reduced anxiety-like behavior (Hodge et al., 2002) and we recently determined that this phenotype can be replicated in wild-type mice by lentiviral-mediated knockdown of PKCε in the amygdala (Lesscher et al., 2008). Because ethanol-induced GABA release in the amygdala requires PKCε (Bajo et al., 2008) and GABA antagonists infused into the amygdala reduce the reinforcing effects of ethanol (Hyytia & Koob, 1995), amygdala PKCε may be important for ethanol intake. Here we determined whether local PKCε knockdown in the amygdala could reduce ethanol intake in a mouse limited-access paradigm.
Materials and Methods
Animals
All experiments used 8 to 10 week old F2 generation male C57BL/6J ×129S4/SvJae wild type and PKCε (+/−) mice. These mice were derived from an F1 generation PKCε (+/−) C57BL/6J × 129S4/SvJae intercross, as described previously (Khasar et al., 1999). The mice treated with PKCε or control shRNA expressing lentivirus had been used in a previous study examining the role of amygdala PKCε in anxiety-like behavior (Lesscher et al 2008). The mice were maintained on a 12-h light/dark cycle (lights off at 12:00 PM for limited access ethanol consumption). Food and water were available ad libitum. The Institutional Animal Care and Use Committee of the Ernest Gallo Clinic and Research Center approved all experimental procedures. Separate groups of naïve animals were used for all experiments.
Limited access ethanol consumption
To determine the role of PKCε in voluntary ethanol consumption, we first sought to develop a paradigm that evokes high levels of ethanol preference drinking in mice. We adapted a dark-phase, limited access paradigm that has previously been validated in C57BL/6 mice (Lopez & Becker, 2005; Rhodes et al., 2005), to a limited free-choice preference paradigm. Each day, 3h into the dark cycle at 3:00 PM, mice were placed into a separate cage with access to two drinking tubes, i.e. 10 ml polysterene pipettes fitted with a stainless steel ball-bearing sipper tube. One tube delivered tap water and the other 15% ethanol (v/v in tap water). During the initial 7 days of training, the water and ethanol bottles were on fixed locations. Thereafter, the bottle positions were switched daily to avoid side-preference. The mice were never food- or water-deprived. Fluid volumes were measured to the nearest 0.05 ml prior to and after each drinking session by reading the scale of the 10 ml pipette. During the sessions, the drinking tubes were fixed to the cages using black clips to prevent spillage. Daily limited access drinking sessions were repeated, using separate groups of animals, for a total period of 10 days (naltrexone validation experiment) or 4 weeks (local PKCε knockdown and heterozygotes). Ethanol intake (g/kg/2h) and ethanol preference were calculated for individual data points. For local PKCε knockdown and heterozygotes experiments, intake, preference and total fluid data were averaged per animal for each week.
Local PKCε knockdown by RNA interference
To examine the role of PKCε in alcohol consumption, we used RNA interference to selectively reduce levels of PKCε in the amygdala. The design and validation of the lentiviruses used here has been described previously (Lesscher et al., 2008). Briefly, short hairpin RNA constructs were designed and incorporated into a pLentiLox 3.7 vector (kindly provided by L. van Parijs, MIT, Cambridge, MA) (Rubinson et al., 2003). Lentivirus was produced using Virapower packaging vectors (Invitrogen Carlsbad, CA, USA) for long-lasting PKCε knockdown in vivo. The shRNA construct used for this study was selected out of three constructs based on a high PKCε knockdown efficiency (>60%) in Neuro2A cells and in vivo in the amygdala (Lesscher et al., 2008). A sequence that did not recognize any known mammalian gene in a BLAST search was used as a control.
For microinjection of lentivirus, male F2 C57BL/6J ×129S4/SvJae wild type mice were anaesthetized with xylazine (7 mg/kg i.p.) and ketamine (100 mg/kg i.p.) and placed in a digital stereotaxic alignment system (Model 1900, David Kopf Instruments, Tujunga, CA, USA). The injectors (33 gauge, 0.2 mm outside diameter) were targeted to the central nucleus of the amygdala using the coordinates: −0.85 mm posterior to bregma, +/− 3.1 mm lateral to midline and −4.9 mm ventral from bregma. Lentivirus (2 μl, 80 × 106 pg p24 antigen/ml) was infused at a rate of 0.2 μl/min. The mice were allowed to recover for two weeks post-surgery. The animals used in this study had been used in a previous study (Lesscher et al 2008) examining the role of amygdala PKCε in anxiety-like behavior. No pharmacological treatments were given in the previous study and the animals were ethanol-naïve at the beginning of the current study. At the completion of the anxiety tests in the previous study, mice were adapted to the altered light-dark cycle (12:00 PM lights off) for two weeks.
Quantitative Western Blotting
Mice were anesthetized with CO2 and rapidly decapitated. The cortex and amygdala were isolated and homogenized separately in ice-cold lysis buffer containing 25 mM Tris (pH 7.6), 150 mM NaCl, 1% NP-40, 1% Sodium deoxycholate, 0.1% SDS (pH 7.6) (G-Biosciences, St. Louis, MO) and 1x ProteCease-50 plus EDTA protease inhibitor cocktail (G-Biosciences, St. Louis, MO). Proteins were separated in 10% tris-glycine, polyacrylamide gels (Invitrogen, Carlsbad, CA), transferred to nitrocellulose membranes, and analyzed using antibodies against PKCε (Choi et al., 2002). Following incubation with primary antibody, blots were incubated with goat anti-rabbit-peroxidase (1:1000; Chemicon) and protein bands were visualized by enhanced chemiluminescence (Pierce Biotechnology, Rockford, IL, USA) according to the manufacturer’s instructions. Autoradiograms were scanned using a flatbed scanner and the optical density of each immunoreactive band was calculated using the program ImageJ (http://rsb.info.nih.gov/ij). The amount of immunoreactivity in each sample was measured as the slope of the line determined by optical density values measured at four different protein amounts (5, 10, 15 and 20 μgs). Slopes from wild type animals were average to calculate a mean. Slopes from all animals (wild type and PKCε (+/−)) were then expressed as a percentage of this mean.
Statistical Analysis
All results are shown as mean ± SEM values. Pairs of means for limited access drinking data were compared by ANOVA with repeated measurements (time) and genotype or treatment as the between-subjects factor. Post hoc Bonferroni tests were applied where appropriate. The effects of naltrexone on ethanol intake and preference were analyzed by one-tailed paired t-tests. Two-tailed independent t-tests were applied to analyze the data for quinine and sucrose taste preference tests and for PKCε expression. All statistical analyses were conducted using Prism 4.0 (GraphPad Software, San Diego, CA) or Sigmastat v3.0 (Systat, San Jose, CA). Differences between pairs of means were considered significant with α= 0.05.
Results
Limited Access Ethanol Intake
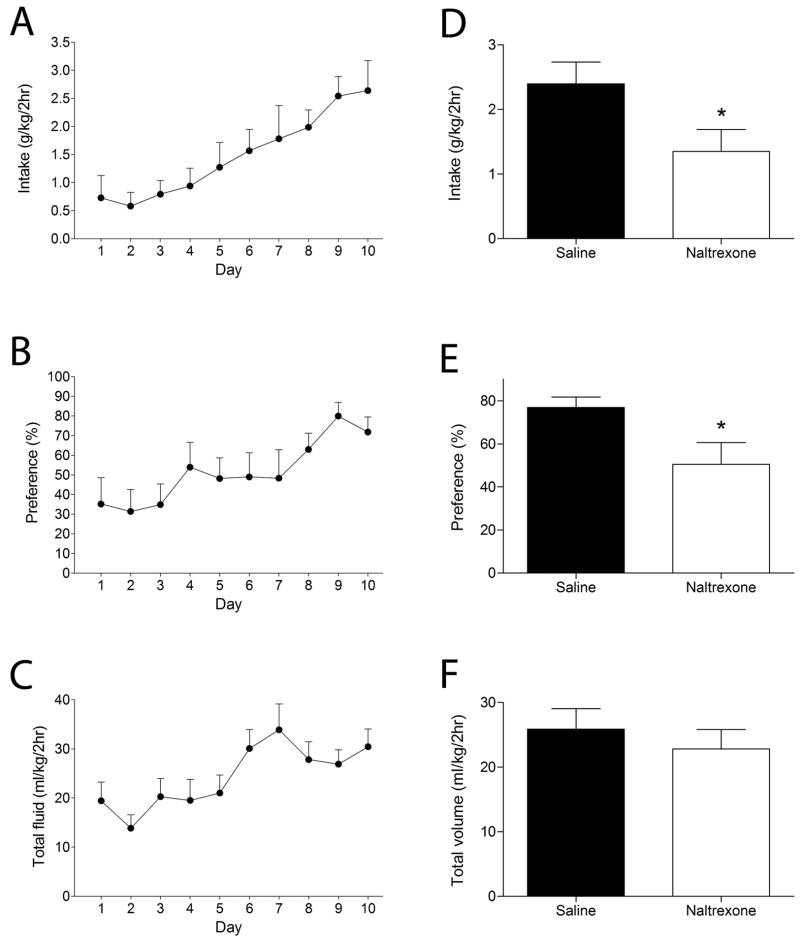
With repeated sessions, wild-type C57BL/6J × 129S4/SVJae mice increased their ethanol consumption and preference such that after 10 sessions mice consumed 2.64 ± 0.53 g/kg ethanol during the 2-h period (Fig. 1A). One-way, repeated measures ANOVA showed an increase in ethanol intake (F12,96 = 6.0, P < 0.001), ethanol preference (F12,94 = 4.1, P < 0.001), and total fluid intake (F12,96 =4.2, P < 0.001) over time. Water consumption in this paradigm was minimal. By day 10, mice showed a high preference for ethanol, exceeding 75% (Fig. 1B) and total volume consumed averaged 30ml/kg/2h (Fig. 1C). Blood ethanol concentrations, measured by tail vein bleed after the tenth session, were 51.2 ± 12.3 mg/dl (AM-1 alcohol analyzer, Analox Instruments, Luneburg, MA, USA).
Figure 1. High levels of ethanol consumption under conditions of limited access are naltrexone-sensitive.
Shown are mean ± S.E.M. values from 9 male, wild type mice. Ethanol intake (A), ethanol preference (B) and total fluid intake (C) increased over the 10-day period. Mice were allowed to drink for an additional 4 days. Injection of naltrexone (NTX, 1 mg/kg) i.p. immediately prior to the 2-h session on day 14 reduced ethanol intake (D) and preference (E) without affecting total fluid intake (F). * P < 0.05 compared with vehicle treatment on day 13, by one-tailed, paired t-test.
Limited Access Ethanol Intake is Reduced by Naltrexone
Naltrexone reduces the reported desire of humans to drink alcohol and is an FDA approved drug for the treatment of alcoholism (Anton, 2008). Therefore, to validate our results we determined the effects of naltrexone on ethanol intake in the adjusted limited access paradigm. When administered naltrexone (1 mg/kg i.p) mice showed a 40% reduction in ethanol intake (t8 = 2.5, P =0.017, Fig 1D) and preference (t8 = 2.3, P = 0.023, Fig 1E) with no change in total fluid intake (Fig. 1F).
Amygdala PKCε and ethanol consumption
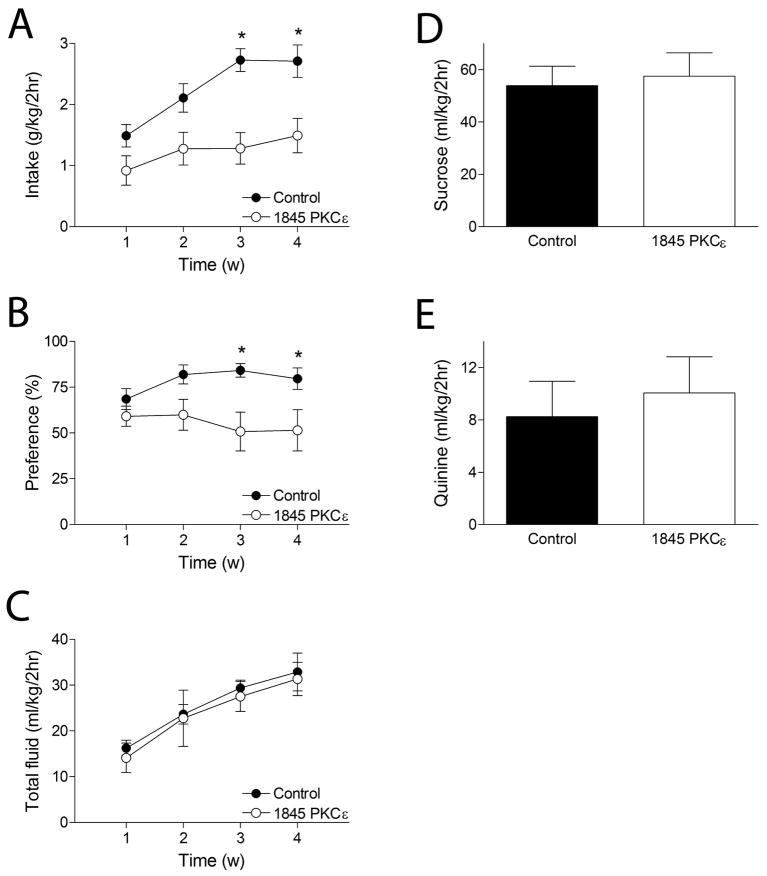
Local knockdown of PKCε in the amygdala was achieved by RNA interference as described previously (Lesscher et al., 2008). Control animals were injected with a virus that expresses a control shRNA. Ethanol consumption was assessed using the limited access paradigm described above. Data from each animal were averaged for each week. Mice with amygdala PKCε knockdown showed significantly reduced ethanol consumption and preference compared with controls (Fig. 2A–B). Two-way repeated measures ANOVA across four consecutive weeks of daily ethanol drinking showed main effects of treatment (F1,48 = 18.5, P < 0.001) and time (F3,48 = 8.0, P < 0.001) for ethanol consumption and a main effect of treatment (F1,48 = 6.9, P < 0.02) for ethanol preference. There was no significant interaction between the factors for ethanol consumption (F3,48 = 1.9, N.S.) or ethanol preference (F3,48 = 2.4, N.S.) Separate analyses of the data for each treatment group by one way repeated measures ANOVA showed that mice treated with the control shRNA increased their intake over 4 weeks (F3,24 = 9.5, P < 0.001) whereas mice treated with the 1845 shRNA against PKCε did not (F3,24 = 1.3, N.S.). Thus, knockdown of PKCε signaling in the amygdala reduces ethanol intake and preference. Total fluid intake was not different between the two treatment groups (Fig. 2C); a two-way repeated measures ANOVA with between-subjects factor for treatment and a repeated measure for time showed a significant effect of time (F3,48 = 11.0, P < 0.001) but not treatment (F1,48 = 0.2508, P > 0.05) with no significant interaction between the two (F3,48 = 0.015, P > 0.05).
Figure 2. Local knockdown of PKCε in the amygdala reduces ethanol intake under limited gaccess conditions.
Over 4 consecutive weeks of daily ethanol consumption under conditions of daily limited access, mice treated with control shRNA (n = 9) increased their ethanol intake (A) and ethanol preference (B), whereas mice treated with the 1845 PKCε shRNA (n = 9) did not. (C) Total fluid intake increased over 4 weeks similarly in both treatment groups. Control and PKCε shRNA treated mice consumed equal amounts of 20% sucrose (D) and 0.03 mM quinine (E) in 2-h sessions. * P < 0.05 compared with 1845 PKCε shRNA-treated mice by post-hoc Bonferroni test for intake in week 3 (t16 = 4.2, P < 0.001) and week 4 (t16 = 3.5, P < 0.01) and for preference in week 3 (t16 = 3.1, P < 0.01) and week 4 (t16 = 2.6, P < 0.05).
Because differential taste reactivity or caloric intake may influence ethanol intake, we also measured the intake of both sweet (20% sucrose, isocaloric to 15% ethanol) and bitter (0.03 mM quinine) solutions in 2-h sessions. Mice treated with the 1845 PKCε shRNA consumed similar amounts of the 20% sucrose and 0.03 mM quinine solutions when compared with mice treated with the control shRNA (Fig. 2D–E), which is in agreement with previous findings for PKCε −/− mice (Hodge et al., 1999). The observed reduction in ethanol intake in 1845 PKCε shRNA-treated mice is therefore unlikely due to alterations in taste reactivity.
Post-mortem histological analysis confirmed infection of the central amygdala with the main infection site located between −1.2 and −1.8 mm from bregma (Paxinos & Franklin, 2001) and an average spread of 0.25 mm along the sagittal plane. There was also occasional infection of the basolateral nucleus of the amygdala in 30% of animals and less extensive infection in the caudate putamen along the injection tract in 60% of the mice. Histological analysis of these animals has been published previously (Lesscher et al., 2008).
Reduced alcohol consumption by PKCε+/− mice
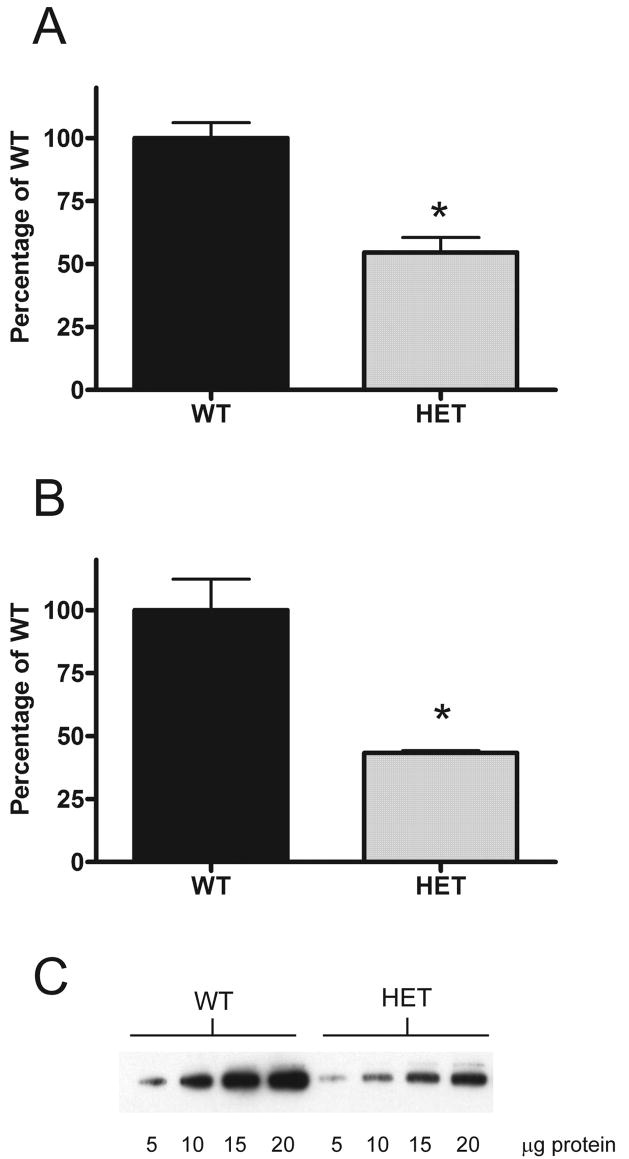
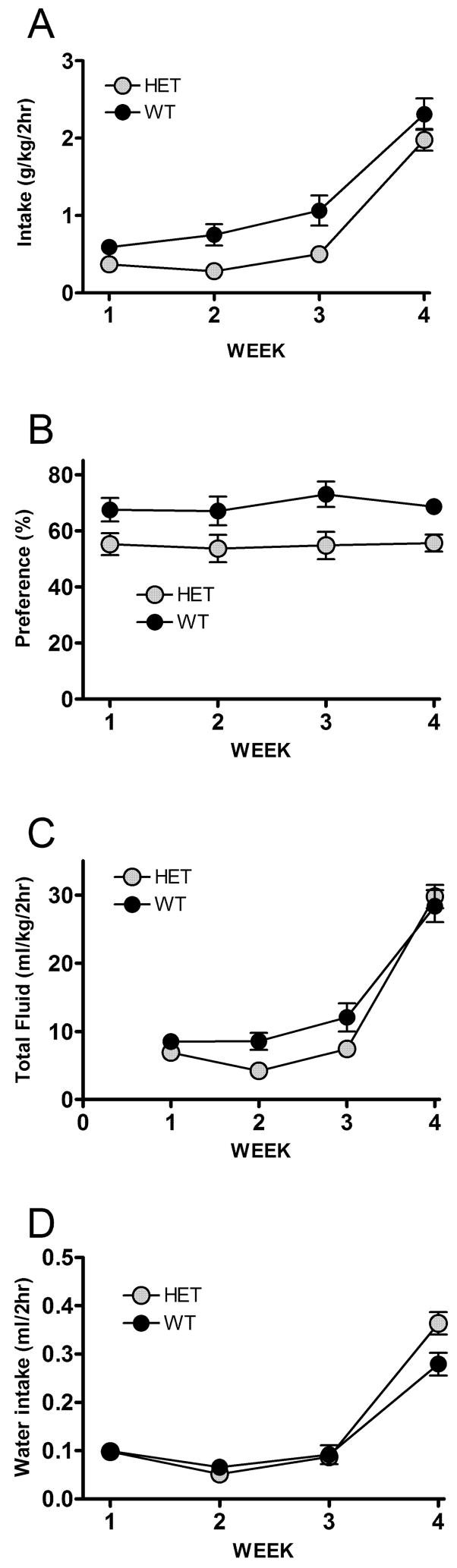
We have previously shown reduced ethanol consumption by PKCε-null mice in an unlimited access two-bottle choice (Hodge et al., 1999) and operant (Olive et al., 2000) paradigms. PKCε levels in the amygdala in mice treated with the 1845 PKCε shRNA are reduced by 51–62% (Lesscher et al., 2008). We hypothesized that mice heterozygous for the PKCε allele would show a similar reduction in PKCε protein abundance. To confirm this, we first measured the abundance of PKCε in the amygdala of PKCε+/− mice by western blotting. We observed that the abundance of PKCε in heterozygous mice was 43.3 +/− 1% of that in wild-type mice (Fig 3), equivalent to the abundance in mice treated with the 1845 PKCε shRNA (Lesscher et al., 2008). As an important control for non-specific effects of the RNAi construct, we therefore also determined ethanol intake in the limited access paradigm for mice heterozygous for the PKCε allele. Compared to wild type mice, heterozygotes showed reduced ethanol intake and preference over a four week period (Fig 4). Two-way repeated measures ANOVA across four consecutive weeks of daily ethanol drinking showed main effects of genotype (F1,63 = 14.51, P = 0.01) and time (F3,63 = 70.29, P < 0.0001) for ethanol consumption (Fig 4A) and a main effect of genotype (F1,63 = 15.53, P < 0.001) for ethanol preference (Fig 4B). No interactions were found in either analysis. There was no difference in total volume consumed by the PKCε +/− mice (Fig 4C), a two-way repeated measures ANOVA showed no main effect of genotype (F1,63 = 4.237, P >0.05) but there was a significant effect of time (F3,63 = 105.8, P < 0.001). The “P” value for genotype in the analysis was close to significance (0.0522). This might be expected if the animals show a reduction in ethanol intake. To confirm that the PKCε heterozygous animals exhibit a selective reduction in ethanol consumption, we analyzed water intake. A two-way repeated measures ANOVA revealed a significant effect of week (F3,63 = 111.8, P < 0.001) but not genotype (F1,63 = 2.125, P < 0.05). There was a significant interaction between week and genotype (F3,63 = 4.038, P < 0.05). Post hoc Bonferroni tests revealed that the difference between genotypes was significant in week 4, where the heterozygous animals drank significantly more water (t21 = 3.7, P < 0.01, Fig 4D).
Figure 3. Reduced abundance of PKC ε in the amygdala of PKCε+/−mice.
Abundance of PKC ε was determined by quantitative western blot analysis. Values are expressed as a percentage of the mean abundance in wild type whole brain (A) (n=3 for both genotypes) and amygdala (B) (n=4 for both genotypes). A representative blot for amygdala samples is also shown (C). * P < 0.05 compared to wild type mice for whole brain (t4 = 5.3, P < 0.01) and amygdala (t6 = 4.5, P < 0.01).
Figure 4.
PKCε+/−mice show reduced ethanol intake under limited access conditions.
Over 4 consecutive weeks of daily ethanol consumption under conditions of limited access, PKCε+/− mice (n = 11) showed reduced ethanol intake (A) and preference (B), compared with wild-type mice (n=12), with no difference in total volume consumed (C) or water consumed (D).
Discussion
Our findings provide evidence for a PKCε-dependent signaling pathway within the amygdala that is important for ethanol consumption in mice. These results provide a rationale for the development of PKCε inhibitors to treat alcohol use disorders.
For this study, we adapted a dark-phase, limited access single bottle paradigm (Lopez & Becker, 2005; Rhodes et al., 2005) to include a free-choice between water and ethanol using two bottles. With the conditions used here, hybrid mice consumed amounts of ethanol associated with mild intoxication in humans. Moreover, the mice showed a high preference (> 75%) for the ethanol solution over water. By comparison, ethanol preference is approximately 40% in C57BL/6J × 129S4/SvJae mice when given 24-h access to water and ethanol (Hodge et al., 1999). This striking increase in preference for ethanol was not due to a preference for one side of the cage since the bottle position was changed every day. Therefore, restricting access to ethanol and providing ethanol during the early dark phase of the light-dark cycle increases the motivation of mice to consume ethanol.
PKCε null mice show reduced ethanol consumption and reward in unlimited access (Besheer et al., 2006; Hodge et al., 1999; Wallace et al., 2007), operant self-administration (Olive et al., 2000) and place preference (Newton & Messing, 2007) paradigms. Here, using RNA interference, we identify the amygdala as an anatomical site of action for PKCε that regulates ethanol intake and preference. Heterozygous mice with a ~55% reduction in the abundance of PKCε in the amygdala, equivalent to that obtained using the lentiviral vector (Lesscher et al., 2008), also showed a reduction in ethanol intake thus supporting our findings with the lentiviral vector. The size and pattern of effect is however different from the behavioral changes observed for PKCε knockdown mice. While local knockdown of PKCε in the amygdala reduces ethanol intake and preference and prevented the development of high ethanol intake and ethanol preference, heterozygous mice show an overall reduction in ethanol intake and preference but increase their drinking behavior over time. This apparent discrepancy can likely be explained by the fact that the reduction in PKCε abundance in PKCε+/− mice is not confined to the amygdala. Thus far we have observed similar reductions in prefrontal cortex and whole brain lysates (P.M.N and R.O.M., unpublished observations), suggesting that PKCε+/− mice have a reduced abundance of PKCε throughout the central nervous system. Our findings suggest that PKCε signaling, particularly in the amygdala, is important for ethanol intake and ethanol preference in mice.
Ethanol stimulates GABA release in the amygdala and we have recently shown that this requires PKCε (Bajo et al., 2008). GABA antagonists infused in the amygdala reduce operant responding for ethanol (Hyytia & Koob, 1995) suggesting that ethanol stimulation of GABA release in the amygdala acts as a feed-forward signal to promote ethanol self-administration. PKCε signaling in the amygdala may therefore facilitate ethanol intake through regulation of GABA release in the amygdala.
PKCε could also regulate alcohol intake through its actions at GABAA receptors containing γ2 subunits. Chronic ethanol intake by nonhuman primates is associated with a decrease in the abundance of mRNA for γ2, and a decrease in sensitivity of amygdala GABAA receptors to benzodiazepines (Anderson et al., 2007). We have recently shown that the γ2 subunit of GABAA receptors is a substrate for PKCε, and that phosphorylation by PKCε decreases the sensitivity of GABAA receptors to benzodiazepines and ethanol (Qi et al., 2007). Further studies will be necessary to determine if a PKCε-mediated alteration in the sensitivity of amygdala GABAA receptors to ethanol regulates voluntary ethanol consumption.
Another possible mechanism by which PKCε could regulate alcohol intake may involve amygdala corticotropin-releasing factor (CRF). Amygdala CRF has been implicated in ethanol consumption, particularly in alcohol-dependent animals. Upon withdrawal from ethanol, CRF levels in the amygdala are elevated (Merlo Pich et al., 1995). These changes are thought to drive ethanol consumption because CRF antagonists can reverse the increase in ethanol intake observed in ethanol-withdrawn rats (Valdez et al., 2002). Recently, we have shown that PKCε regulates levels of amygdala CRF (Lesscher et al., 2008). PKCε−/− mice show a 50% reduction in CRF peptide and mRNA in the amygdala. Furthermore, activation of PKCε increases CRF levels in primary amygdala neurons. Therefore it is possible that PKCε signaling in the amygdala controls voluntary ethanol consumption through regulation of amygdala CRF. Ongoing experiments with CRF1 receptor antagonists, which reduce ethanol consumption in rats (Funk et al., 2006b; Marinelli et al., 2007), should address this hypothesis. However, in rats, CRF antagonists, given systemically or infused into the amygdala, reduce ethanol consumption only in alcohol-dependent animals withdrawn from chronic intermittent ethanol exposure. These antagonists are without effect on voluntary ethanol consumption in non-dependent rats. Under the conditions of our limited access paradigm, mice drank moderate levels of alcohol and achieved blood alcohol levels (~50 mg/dl) that are not expected to result in dependence. Therefore it is likely that there are other PKCε-dependent mechanisms at work in this model that are CRF-independent and are yet to be elucidated.
Identification of downstream targets of PKCε in the amygdala that control voluntary ethanol consumption is an important challenge for future studies and will provide further insight in the neurobiological processes that increase ethanol consumption after repeated exposure.
Acknowledgments
The authors thank A. Lasek for technical advice. This work was supported by Public Health Services grant AA013588 and U.S. Army contract DAMD17-03-1-0058 to R.O.M. and by E.U. Marie Curie Fellowship MOIF-CT-2004-002812 to H.M.B.L.
References
- Anderson NJ, Daunais JB, Friedman DP, Grant KA, McCool BA. Long-term ethanol self-administration by the nonhuman primate, Macaca fascicularis, decreases the benzodiazepine sensitivity of amygdala GABA(A) receptors. Alcohol Clin Exp Res. 2007;31:1061–1070. doi: 10.1111/j.1530-0277.2007.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton RF. Naltrexone for the management of alcohol dependence. N Engl J Med. 2008;359:715–721. doi: 10.1056/NEJMct0801733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo M, Cruz MT, Siggins GR, Messing R, Roberto M. Protein kinase C epsilon mediation of CRF- and ethanol-induced GABA release in central amygdala. Proc Natl Acad Sci U S A. 2008;105:8410–8415. doi: 10.1073/pnas.0802302105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Lepoutre V, Mole B, Hodge CW. GABA(A) receptor regulation of voluntary ethanol drinking requires PKCepsilon. Synapse. 2006;60:411–419. doi: 10.1002/syn.20314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DS, Wang D, Dadgar J, Chang WS, Messing RO. Conditional rescue of protein kinase C epsilon regulates ethanol preference and hypnotic sensitivity in adult mice. J Neurosci. 2002;22:9905–9911. doi: 10.1523/JNEUROSCI.22-22-09905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, O’Dell LE, Crawford EF, Koob GF. Corticotropin-Releasing Factor within the Central Nucleus of the Amygdala Mediates Enhanced Ethanol Self-Administration in Withdrawn, Ethanol-Dependent Rats. J Neurosci. 2006a;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, Zorrilla EP, Lee MJ, Rice KC, Koob GF. Corticotropin-Releasing Factor 1 Antagonists Selectively Reduce Ethanol Self-Administration in Ethanol-Dependent Rats. Biol Psychiatry. 2006b doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge CW, Mehmert KK, Kelley SP, McMahon T, Haywood A, Olive MF, Wang D, Sanchez-Perez AM, Messing RO. Supersensitivity to allosteric GABA(A) receptor modulators and alcohol in mice lacking PKCepsilon. Nat Neurosci. 1999;2:997–1002. doi: 10.1038/14795. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Raber J, McMahon T, Walter H, Sanchez-Perez AM, Olive MF, Mehmert K, Morrow AL, Messing RO. Decreased anxiety-like behavior, reduced stress hormones, and neurosteroid supersensitivity in mice lacking protein kinase Cepsilon. J Clin Invest. 2002;110:1003–1010. doi: 10.1172/JCI15903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyytia P, Koob GF. GABAA receptor antagonism in the extended amygdala decreases ethanol self-administration in rats. Eur J Pharmacol. 1995;283:151–159. doi: 10.1016/0014-2999(95)00314-b. [DOI] [PubMed] [Google Scholar]
- Khasar SG, Lin YH, Martin A, Dadgar J, McMahon T, Wang D, Hundle B, Aley KO, Isenberg W, McCarter G, Green PG, Hodge CW, Levine JD, Messing RO. A novel nociceptor signaling pathway revealed in protein kinase C epsilon mutant mice. Neuron. 1999;24:253–260. doi: 10.1016/s0896-6273(00)80837-5. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The amygdala. Curr Biol. 2007;17:R868–R874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Lesscher HM, McMahon T, Lasek AW, Chou WH, Connolly J, Kharazia V, Messing RO. Amygdala protein kinase C epsilon regulates corticotropin-releasing factor and anxiety-like behavior. Genes Brain Behav. 2008;7:323–333. doi: 10.1111/j.1601-183X.2007.00356.x. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Becker HC. Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology (Berl) 2005;181:688–696. doi: 10.1007/s00213-005-0026-3. [DOI] [PubMed] [Google Scholar]
- Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, Marinkovic K, Breiter HC, Gasic GP, Harris GJ. Decreased volume of the brain reward system in alcoholism. Biol Psychiatry. 2008;64:192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Juzytsch W, Harding S, Rice KC, Shaham Y, Le AD. The CRF(1) receptor antagonist antalarmin attenuates yohimbine-induced increases in operant alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 2007 doi: 10.1007/s00213-007-0905-x. [DOI] [PubMed] [Google Scholar]
- Merlo Pich E, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF, Weiss F. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA. The amygdala, reward and emotion. Trends Cogn Sci. 2007;11:489–497. doi: 10.1016/j.tics.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Newton PM, Messing RO. Increased sensitivity to the aversive effects of ethanol in PKCepsilon null mice revealed by place conditioning. Behav Neurosci. 2007;121:439–442. doi: 10.1037/0735-7044.121.2.439. [DOI] [PubMed] [Google Scholar]
- Olive MF, Mehmert KK, Messing RO, Hodge CW. Reduced operant ethanol self-administration and in vivo mesolimbic dopamine responses to ethanol in PKCepsilon-deficient mice. Eur J Neurosci. 2000;12:4131–4140. doi: 10.1046/j.1460-9568.2000.00297.x. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Roy A, Misra K. Central and medial amygdaloid brain-derived neurotrophic factor signaling plays a critical role in alcohol-drinking and anxiety-like behaviors. J Neurosci. 2006;26:8320–8331. doi: 10.1523/JNEUROSCI.4988-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Ugale R, Prakash A, Xu T, Misra K. Effector immediate-early gene arc in the amygdala plays a critical role in alcoholism. J Neurosci. 2008;28:2589–2600. doi: 10.1523/JNEUROSCI.4752-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KB. The Mouse Brain in Stereotaxic Coordinates. Academic Press; San Diego: 2001. [Google Scholar]
- Perra S, Pillolla G, Luchicchi A, Pistis M. Alcohol inhibits spontaneous activity of basolateral amygdala projection neurons in the rat: involvement of the endocannabinoid system. Alcohol Clin Exp Res. 2008;32:443–449. doi: 10.1111/j.1530-0277.2007.00588.x. [DOI] [PubMed] [Google Scholar]
- Qi ZH, Song M, Wallace MJ, Wang D, Newton PM, McMahon T, Chou WH, Zhang C, Shokat KM, Messing RO. Protein kinase C epsilon regulates gamma-aminobutyrate type A receptor sensitivity to ethanol and benzodiazepines through phosphorylation of gamma2 subunits. J Biol Chem. 2007;282:33052–33063. doi: 10.1074/jbc.M707233200. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rice DP. Economic costs of substance abuse, 1995. Proc Assoc Am Physicians. 1999;111:119–125. doi: 10.1046/j.1525-1381.1999.09254.x. [DOI] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR. Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:2053–2058. doi: 10.1073/pnas.0437926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinson DA, Dillon CP, Kwiatkowski AV, Sievers C, Yang L, Kopinja J, Rooney DL, Ihrig MM, McManus MT, Gertler FB, Scott ML, Van Parijs L. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat Genet. 2003;33:401–406. doi: 10.1038/ng1117. [DOI] [PubMed] [Google Scholar]
- Schneider F, Habel U, Wagner M, Franke P, Salloum JB, Shah NJ, Toni I, Sulzbach C, Honig K, Maier W, Gaebel W, Zilles K. Subcortical correlates of craving in recently abstinent alcoholic patients. Am J Psychiatry. 2001;158:1075–1083. doi: 10.1176/appi.ajp.158.7.1075. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Wallace MJ, Newton PM, Oyasu M, McMahon T, Chou WH, Connolly J, Messing RO. Acute functional tolerance to ethanol mediated by protein kinase Cepsilon. Neuropsychopharmacology. 2007;32:127–136. doi: 10.1038/sj.npp.1301059. [DOI] [PubMed] [Google Scholar]