Abstract
In this study, we tested the hypothesis that, in the male Fischer 344 × Brown Norway (F344xBN) rat, aging would be associated with an increase in sympathetic nervous system activity and a decrease in skeletal muscle β-adrenergic-receptor (β-AR) density and function. Radioligand-binding studies using [125I]iodocyanopindolol were done to evaluate β-AR density (Bmax) and antagonist-binding affinity in gastrocnemius and cardiac muscle from 6-, 18-, and 28-mo-old male F344xBN rats. β-AR function was measured as adenylyl cyclase (AC) activity stimulated by the β-AR agonist isoproterenol (Iso, 10−4 M). Basal arterial plasma norepinephrine (pNE) concentrations were higher in the 28-than in the 6- and 18-mo-old rats. Bmax was greatest and Iso-stimulated AC activity was unchanged in gastrocnemius muscle of the 28-mo-old age group. In contrast, there was an age-associated decrease in Bmax and Iso-stimulated AC activity in cardiac muscle. In conclusion, there was an age-associated increase in pNE concentrations in male F344xBN rats, suggesting an increase in sympathetic nervous system activity. In addition, there was an age-associated increase in skeletal muscle β-AR density, whereas in skeletal muscle β-AR-stimulated AC activity remained unchanged with age.
Keywords: adenylyl cyclase, catecholamines, sympathetic nervous system, isoproterenol, forskolin
Studies in humans and rodents indicate that aging is associated with increased sympathetic nervous system activity, as assessed by plasma norepinephrine concentrations (11, 16, 18), increased release of norepinephrine in radioisotope kinetic studies (8, 28, 29), and direct recording of muscle sympathetic nervous system activity (10). In addition, an age-associated decrease in β-adrenergic-receptor (β-AR) density and/or response in several tissues (including cardiac muscle, brown adipose tissue, and lung) has been observed in previous studies (23, 25, 26). In cardiac muscle, a decline in β-AR-mediated stimulation of adenylyl cyclase (AC) activity with age has been demonstrated without a decrease in β-AR density, suggesting an age-associated uncoupling of the β-AR-AC complex (1). To date, β-AR density and function in skeletal muscle of aging rodents have not been characterized. However, data from Fischer 344 (F344) rats suggest that skeletal muscle adrenergic responsiveness is not diminished with age in response to exogenous epinephrine infusion (13). Maintenance of β-AR responsiveness in skeletal muscle would be important for the aging organism during metabolically stressful situations such as shivering thermogenesis and fighting or fleeing dangerous situations, because adrenergic stimulation helps to mobilize fuel by stimulating glycogenolysis in skeletal muscle. A previous investigation indicated an age-associated decrease in glucose metabolism in skeletal muscle during metabolically stressful situations in male F344 rats (14). A decline in β-AR density and/or function in skeletal muscle with age could account for the age-associated decline in stress-induced glucose metabolism. Therefore, we tested the hypothesis that, in the male F344 × Brown Norway (F344xBN) rat, aging would be associated with an increase in sympathetic nervous system activity and a decrease in skeletal muscle β-AR density and/or function.
The F344xBN rat has been proposed as a new model for the study of aging rodents by the National Institute on Aging because the F344xBN rat is devoid of many of the age-associated complications observed in the highly inbred F344 rat (3). Little is known about age-associated changes in sympathetic nervous system activity or cardiac or skeletal muscle β-AR density and function in this rat strain. Because an age-related decrease in cardiac β-AR density and/or function is well characterized in other rat strains, we also measured cardiac β-AR density and function in the uncharacterized F344xBN rat to compare with skeletal muscle β-AR function.
METHODS
Animals and animal care
Male F344xBN Fl hybrid rats, ages 6, 18, and 28 mo, were obtained from the National Institute on Aging's animal colony maintained by Harlan Sprague Dawley Laboratory (Indianapolis, IN). According to life-span data for the F344xBN rodent strain (personal communication, National Institute on Aging), the age groups chosen represent recently mature (6 mo), middle-aged (18 mo), and senescent (28 mo) rats. Rats were acclimated to the colony conditions of the University of Michigan's Core Facility for Aged Rodents, i.e., light cycle, temperature, etc., for 2 wk prior to experimentation. Rats were housed individually in hanging plastic cages (28 × 56 cm) and kept on a 12:12-h light-dark cycle at a temperature of 20–22°C. The rats were fed Purina Rodent 5001 Laboratory Chow and water ad libitum. Although necropsy and microbiological examinations were not performed routinely, animals were inspected daily for external signs of disease. Only rats with no external signs of disease were used in this study. No rats were excluded from this study because of disease.
Experimental procedures
Animals were briefly anesthetized with halothane, and a cannula was implanted in the left carotid artery. Survivors of the carotid artery cannulation (6 mo, n = 12; 18 mo, n = 16; and 28 mo, n = 14) were awake and active within 20 min of initiation of halothane anesthetic. The animals were allowed to recover from surgery for 24 h without food (to obtain fasting blood values). Beginning at 0700, intra-arterial blood pressure and heart rate were continuously monitored for 30 min with a Statham P23 pressure transducer and a Gould pressure recording device and expressed as the average for the 30-min period. A 2-ml arterial blood sample was obtained via the carotid cannula and placed into prechilled tubes that contained ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid and glutathione. The sample was immediately centrifuged at 4°C, and plasma was stored at −70°C for subsequent analysis of epinephrine and norepinephrine. The number of animals with nonpatent cannulas is reflected in the decreased data collected for both heart rate and blood pressure (6 mo, n = 10; 18 mo, n = 15; and 28 mo, n = 12). The animals were then killed with pentobarbital sodium, delivered through the carotid cannula. The ventricles of the heart and the left gastrocnemius muscle were quickly dissected, freeze clamped, weighed, and stored at −70°C for subsequent analysis of β-AR density, binding affinity, AC activity, and myosin content. Because of equipment failure during the isolation of β-AR from a small number of cardiac muscle samples, not all assays could be performed on all tissues.
Preparation of muscle membranes
Frozen tissues (whole left gastrocnemius muscle or right and left ventricles) were powdered in a mortar and pestle cooled in liquid N2 and analyzed for β-AR density and function, with the use of a modification of methods described by Liggett et al. (15). Briefly, powdered tissues were suspended in 20 vol (wt/vol) of ice-cold buffer [10 mM tris(hydroxymethyl)aminomethane (Tris), 5 mM EDTA, pH 7.4] and homogenized twice for 10 s with a Brinkman Polytron (setting 7). Homogenates were centrifuged at 500 g for 15 min at 4°C, supernatants were saved, and pellets were resuspended in 10 ml of ice-cold buffer (10 mM Tris, 5 mM EDTA, pH 7.4) and centrifuged at 500 g for 15 min at 4°C. Supernatants from both 500-g spins were combined and centrifuged at 40,000 g for 33 min at 4°C. Pellets were then resuspended in ice-cold buffer (75 mM Tris·HCl, 25 mM MgCl2, and 5 mM EDTA, pH 7.4) and recentrifuged at 40,000 g for 33 min at 4°C. Final pellets were resuspended in 2 ml (cardiac) or 1 ml (gastrocnemius) of ice-cold buffer (75 mM Tris·HCl, 25 mM MgCl2, and 5 mM EDTA, pH 7.4) and stored at −70°C.
For analysis of myosin content, frozen tissues (200 mg) were powdered in a mortar and pestle cooled in liquid N2. Powdered tissues were suspended in 5.0 ml of ice-cold SSS buffer (10 mM NaHC03, 0.25 µ sucrose, and 5.0 mM NaN3) and homogenized twice for 10 s with a Brinkman Polytron (setting 7). Homogenates were centrifuged at 1,200 g for 10 min at 4°C, and pellets were resuspended in 5 ml of ice-cold SSS buffer and centrifuged at 1,200 g for 10 min at 4°C. Supernatants were combined and centrifuged at 190,000 g for 66 min at 4°C. Pellets were then resuspended in 500 µl of ice-cold SSS buffer and stored at −70°C.
β-AR assay
Membrane preparations were analyzed for β-AR density and antagonist-binding affinity with the use of the Scatchard transformation of [125I]iodocyanopindolol (ICYP; specific activity 2,200 Ci/mmol; New England Nuclear, Boston, MA) specific binding data (15). Briefly, 100-µg aliquots of membrane homogenates and seven concentrations of [125I]ICYP spanning a range of 5–150 pM were incubated with and without 1 µM propranolol (to define nonspecific binding) for 90 min at 25°C in a total volume of 200 µl. The binding reaction was stopped by addition of ice-cold buffer and rapid filtration under vacuum onto Whatman GF/C filters with the use of a Brandel Cell Harvester (Brandel Laboratories, Gaithersburg, MD). Filters were washed twice with ice-cold buffer, allowed to air dry, and counted in a gamma counter (Tm Analytic, Elk Village, IL). Nonspecific binding was consistently <30% of total binding, and linear Scatchard curves with r values >0.76 were obtained for each preparation.
AC assay
A 20-µg aliquot of each membrane preparation was analyzed for AC activity by measuring the conversion of ATP to adenosine 3′,5′-cyclic monophosphate (cAMP) during a 15-min incubation with 10 8–10−4 M isoproterenol (Iso), 25 mM NaF, or 40 µM forskolin (Fsk) at 30°C. AC activity was stopped by placing reaction tubes in a boiling water bath for 3 min and then quickly submerging the tubes into liquid N2. Frozen tubes were then stored at −20°C until they were assayed for cAMP concentration with the use of a radioimmunoassay, as described previously (28). The results are expressed both as the percent and absolute increase above basal AC activity. Basal AC activity was determined by incubation of membranes for 15 min without stimulation and expressed as picomoles of cAMP produced per milligram protein during the 15-min incubation period.
Myosin Western blotting
Vertical slab Polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate was performed on molecular weight markers (Amersham, Arlington Heights, IL) and aliquots of skeletal muscle homogenates, each containing 30 µg protein (21). Because of the limited amount of cardiac tissue available, we were unable to determine cardiac myosin content. An aliquot of control protein (30 µg protein) obtained from combined muscle homogenates from 6-mo-old animals was run on each gel. Samples were separated on a 10% polyacrylamide-resolving gel and then electrophoretically transferred onto Immobilon polyvinylidene fluoride (PVDF) membrane (Millipore, Milford, MA). Immobilon PVDF membranes were then blocked for 60 min with Tris-buffered saline with Tween 20 (100 mM Tris, 0.9% NaCl, 2.0 ml Tween 20, pH 7.5) and 5% Carnation nonfat dry milk. Immunoblotting was performed using a mouse monoclonal antibody against both slow and fast myosin protein (monoclonal anti-pan myosin; Amersham). Myosin-antibody binding to the transfer membrane was visualized by incubating with species-specific, 125I-labeled anti-mouse immunoglobulin (Amersham) and exposing the transfer membrane to Kodak X-OMAT film at −80°C for 24 h. Bands of the appropriate molecular weight were then cut from the 125I-labeled transfer membranes and counted in a gamma counter (Tm Analytic). Total counts per minute were corrected for background and then specific counts were normalized to that blot's control sample.
Analytic procedures
Plasma catecholamines were determined by radioenzymatic assay (4). Muscle protein content was determined with the use of a Bradford protein assay kit from Bio-Rad (Richmond, CA).
Statistics
Values are presented as means ± SE. Statistical analysis was performed with Statview 4.01 (Abacus Concepts, Berkeley, CA). The nonparametric Mann-Whitney test and analysis of variance (ANOVA) were used to compare differences between the rats at the various age groups. When a significant main effect was found, the Fishers least-significant difference post hoc test was used to determine differences between the age groups. Differences were considered significant at P < 0.05.
RESULTS
Physiological characteristics
Body, heart, and skeletal muscle weight for each age group are summarized in Table 1. With increasing age, body weight and cardiac weight significantly increased. Gastrocnemius muscle weight was increased in the 18- compared with the 6-mo-old animals, and the 28-mo-old rats had gastrocnemius muscle weights lower than either the 6-or 18-mo-old animals. In the gastrocnemius muscle, neither total protein (mg/g tissue) nor myosin content (expressed as a percentage of a 6-mo-old control sample) differed significantly between the age groups. Therefore, the increase in muscle weight in the 18-mo-old animals includes a proportionate increase in contractile tissue.
Table 1.
Physiological characteristics of male F344xBN rats ages 6, 18, and 28 months
| Age, mo | ||||
|---|---|---|---|---|
| 6 | 18 | 28 | Effect of Age | |
| Body wt, g | 451.9 ±10.4 | 556.7 ±9.1* | 595.3 ± 12.5*, † | P< 0.0001 |
| (n = 12) | (n = 16) | (n = 14) | ||
| Cardiac wt, g | 0.97 ±0.02 | 1.20 ±0.02* | 1.44 ±0.04*, † | P< 0.0001 |
| (n = 12) | (n = 16) | (n = 14) | ||
| Gastrocnemius wt, g | 2.64 ±0.04 | 2.80 ± 0.04* | 2.38±0.04*, † | P< 0.0001 |
| (n = 12) | (n = 16) | (n = 14) | ||
| Gastrocnemius | ||||
| Protein, mg/g tissue | 86.4 ±2.3 | 85.4 ±2.6 | 84.0 ±1.8 | NSD |
| (n = 11) | (n = 9) | (n = 13) | ||
| Myosin, % 6 mo | 100.0 ±8.6 | 102.1 ±5.0 | 109.3 ±5.6 | NSD |
| (n = 11) | (n = 7) | (n = 13) | ||
Values are means ± SE; n no. of rats in each group. Gastrocnemius myosin data are presented as a percentage of 6-mo-old control animal results. F344xBN, Fischer 344 × Brown Norway; NSD, no significant difference.
Significant difference (P<0.05) vs. 6-mo-old rats.
Significant difference (P<0.05) 18- vs. 28-mo-old rats.
Plasma catecholamine concentrations
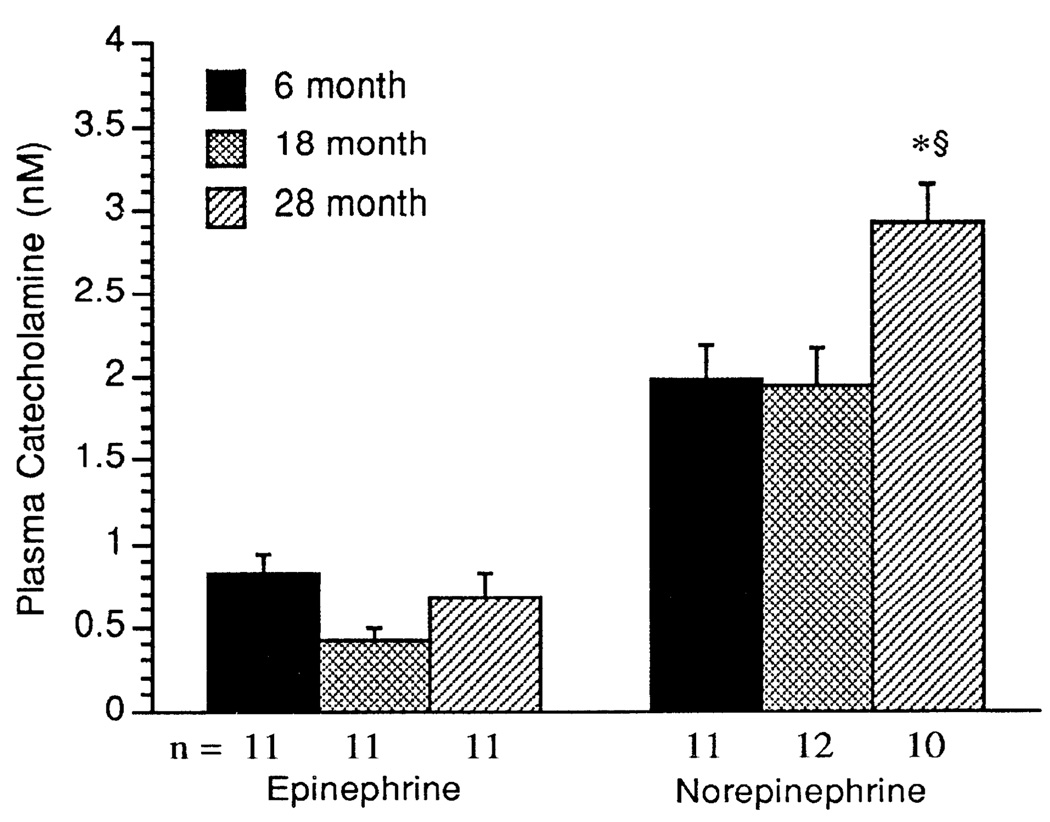
As shown in Fig. 1, plasma epinephrine concentrations showed no age-related differences. In contrast, plasma norepinephrine concentrations were significantly greater in the 28- than in the 6- and 18-mo-old age groups (P < 0.0001).
Fig. 1.
Arterial plasma concentrations of epinephrine (nmol/1) and norepinephrine (nmol/1). Values are means + SE; n = no. of rats in each group. * Significant difference (P < 0.05) vs. 6-mo-old rats. § Significant difference (P < 0.05) 18- vs. 28-mo-old rats.
β-AR density and binding affinity
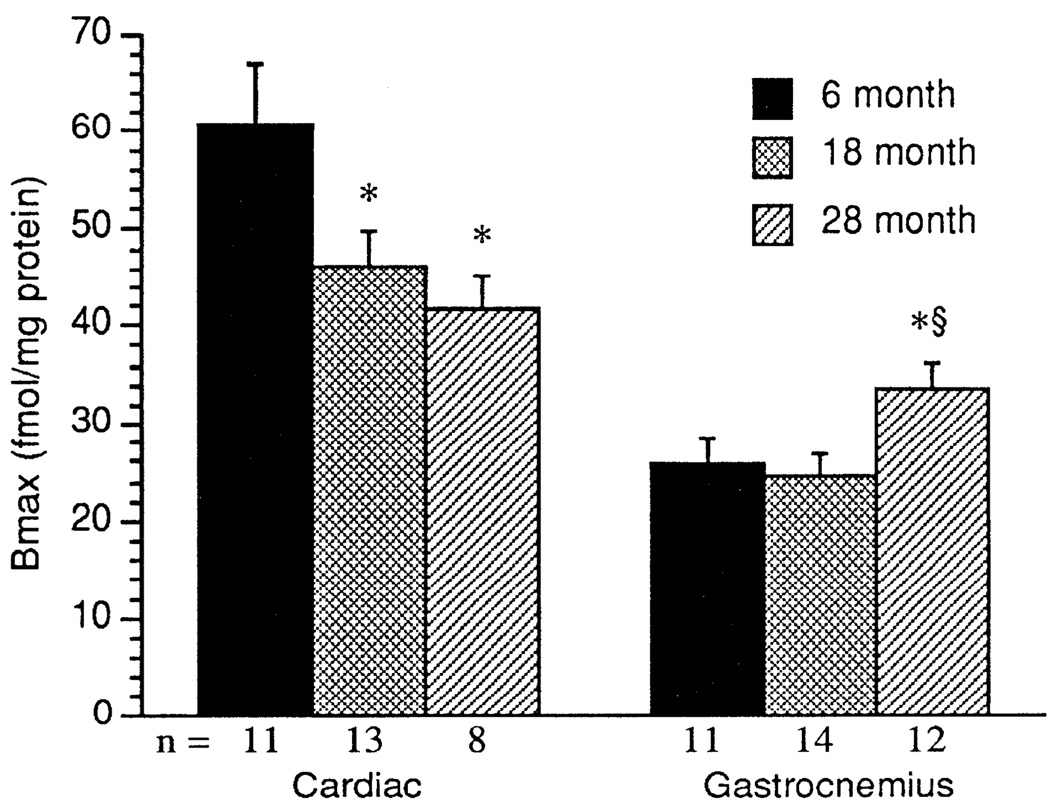
Equilibrium binding studies using [125I]ICYP in the presence and absence of 1 µM propranolol yielded saturable binding. Scatchard transformation of the specific-binding data yielded linear plots from which receptor density (Bmax) and antagonist-binding affinity (Kd) were determined. As shown in Fig. 2, in cardiac tissue, β-AR density significantly decreased with increasing age. The β-AR density was significantly lower in the cardiac tissue from the 18- and 28- than from the 6-mo-old rats (P < 0.025). Cardiac muscle had significantly greater β-AR density than the gastrocnemius muscle in all age groups. In contrast to the age-associated decrease in cardiac β-adrenergic density β-AR density in the gastrocnemius muscle was significantly higher in the 28-than in the 6- and 18-mo-old age groups (P < 0.032; Fig. 2). There was no effect of age on the antagonist-binding affinity for [125I]ICYP in any of the tissues studied, although the Kd in gastrocnemius was lower than in cardiac muscle (cardiac muscle: 6 mo, 42.5 ± 3.6; 18 mo, 38.9 ± 2.7; and 28 mo, 35.2 ± 3.6 pM; gastrocnemius muscle: 6 mo, 22.3 ± 1.7; 18 mo, 21.7 ± 1.7; and 28 mo, 25.0 ± 1.3 pM).
Fig. 2.
β-Adrenergic receptor (β-AR) density (Bmax; fmol/mg protein) in cardiac and gastrocnemius muscle. Values are means + SE. * Significant difference (P < 0.05) vs. 6-mo-old rats. § Significant difference (P < 0.05) 18- vs. 28-mo-old rats.
AC activity
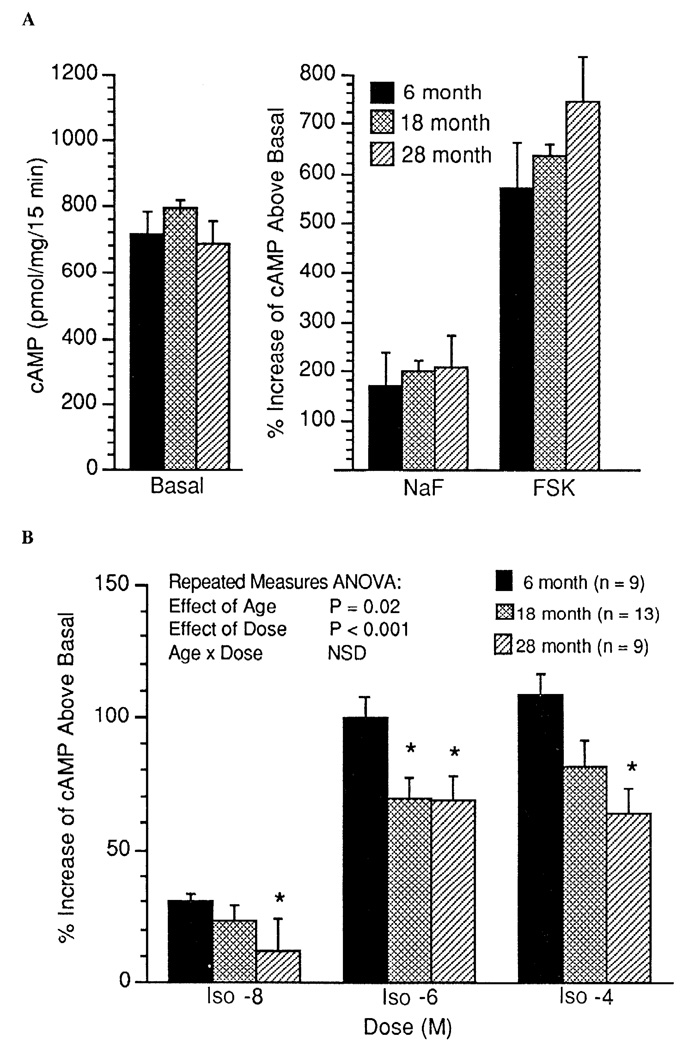
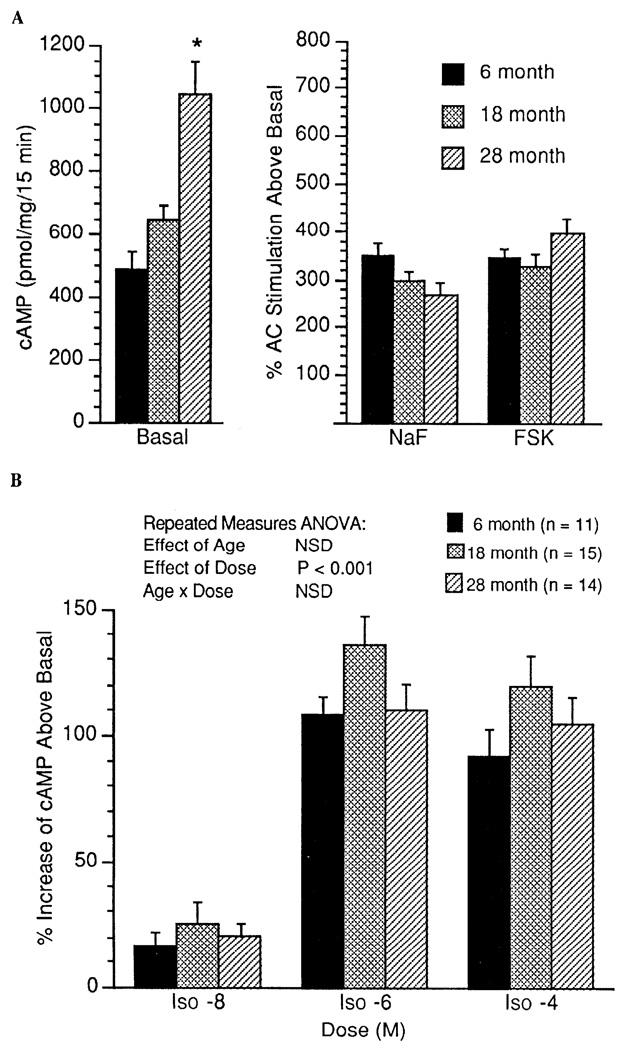
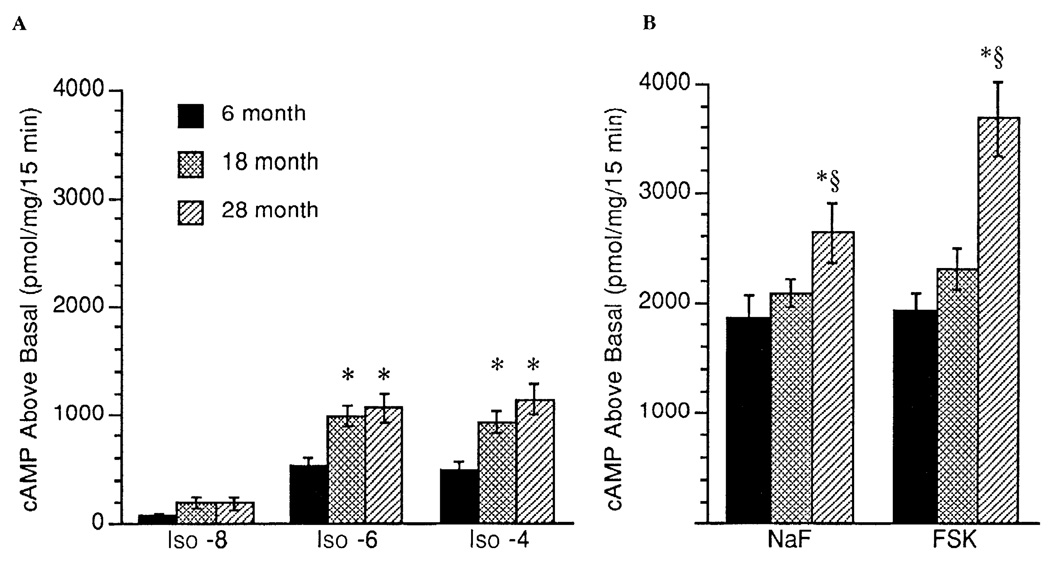
In cardiac tissue, no age-associated differences were observed for basal (unstimulated), NaF-stimulated, or Fsk-stimulated AC activity (expressed as percent stimulation above basal; Fig. 3A). By repeated-measures ANOVA, there was a significant age-associated decrease in the percentage of Iso (10−8–10−4 M)-stimulated AC activity in cardiac tissue (P = 0.02, Fig. 3B). In addition, in cardiac tissue, when data are expressed as absolute cAMP produced above basal (pmol·mg−1·15 min−1), a similar age-associated decrease in Iso-stimulated AC activity was observed (P = 0.10). There was a marginal positive correlation between Bmax and basal (unstimulated) AC activity in the cardiac muscle (r = 0.36, P = 0.06). Basal (unstimulated) AC activity in the gastrocnemius muscle was higher in the 28-mo-old animals than in the two younger groups (Fig. 4A). There was a significant positive correlation between Bmax and basal (unstimulated) AC activity in the gastrocnemius muscle (r = 0.41, P = 0.013). However, there was no age-associated increase in the percent AC stimulation above basal by NaF and Fsk in gastrocnemius muscle (Fig. 4A). Similarly, as illustrated in Fig. 4B, there was no age-associated alteration in Iso-stimulated AC activity (expressed as percent stimulation above basal) in the gastrocnemius muscle. In contrast, reflecting the increase in basal activity, absolute cAMP produced above basal was greater in the gastrocnemius muscle of the 18- and 28-mo-old groups after Iso (10 −6 and 10 4 M) and greater in the 28-mo-old group after NaF and Fsk stimulation (Fig. 5).
Fig. 3.
Adenylyl cyclase (AC) activity measured as percent increase above basal in cAMP produced in cardiac muscle during stimulation with NaF and forskolin (Fsk) (A) and stimulation with the β-AR agonist isoproterenol (Iso; 10−8, 10−6, and 10−4 M) (B). Values are means + SE. ANOVA, analysis of variance; NSD, no significant difference. * Significant difference (P < 0.05) vs. 6-mo-old rats.
Fig. 4.
AC activity measured as percent increase above basal in cAMP produced in gastrocnemius muscle during stimulation with NaF and Fsk (A) and stimulation with the β-AR agonist Iso (10−8, 10−6, and 10−4 M) (B). Values are means + SE. * Significant difference (P < 0.05) vs. 6-mo-old rats.
Fig. 5.
AC activity measured as cAMP produced above basal expressed as pmol·mg−1 · 15 min−1 in gastrocnemius muscle during stimulation with the β-AR agonist Iso (10−8, 10−6, and 10−4 M) (A) and stimulation with NaF and Fsk (B). Values are means + SE. * Significant difference (P < 0.05) vs. 6-mo-old rats. § Significant difference (P < 0.05) 18- vs. 28-mo-old rats.
DISCUSSION
We found an age-associated increase in basal arterial plasma norepinephrine levels in male F344xBN rats. Although an increase in basal plasma norepinephrine level is an indirect determinant of increased sympathetic nervous system activity, this finding is compatible with previous reports in humans and rodents suggesting that sympathetic nervous system activity increases with age (11, 16, 18). Despite the age-related increase in plasma norepinephrine, there was no observed age-associated increase in basal arterial epinephrine levels. The low concentrations of epinephrine document the basal (unstressed) conditions at the time of experiment across all age groups. As previously shown in other rat strains, in male F344xBN rats we have identified an age-associated decrease in β-AR density and β-AR-stimulated (Iso) AC activity in cardiac tissue. In contrast, β-AR density tended to be higher in skeletal muscle of older rats, and Iso-stimulated AC activity was preserved.
Skeletal muscle β-AR density and function in aging rodents have, to date, not been described. However, data from 6- vs. 24-mo-old F344 rats suggest that skeletal muscle adrenergic responsiveness is not diminished with age in response to exogenous epinephrine infusion (13). In our study of male F344xBN rats, we observed an age-associated increase in skeletal muscle β-AR density with no alteration in receptor-antagonist affinity. In addition, there was an increase in basal (unstimulated) AC activity with age. This increase in basal activity could be a result of an increase in AC protein concentration, AC activity, and/or constitutive activity of β-AR on AC activity, as suggested by the positive correlation between β-AR density and basal AC activity. An effect of constitutive activity of β-AR on AC activity has been previously described in transformed Chinese hamster fibroblasts transfected with human β-AR (2). Despite the increase in gastrocnemius β-AR number and basal AC activity, we observed no significant age-associated increase in AC activity (expressed as percent stimulation above basal) during stimulation with Iso, through postreceptor activation of Gs protein with NaF, or via direct stimulation of AC with Fsk. However, there was an age-associated increase in the absolute amount of cAMP produced above basal in all three mechanisms of AC stimulation. This suggests that function of the β-AR-AC effector system is intact in the gastrocnemius muscle of the 28-mo-old group.
An age-associated decrease in cardiac muscle β-AR density and/or function has been well characterized in several rat strains (1, 5–7, 20, 22, 23, 32, 33). We measured cardiac muscle β-AR density and function in the F344xBN rats to characterize β-AR density and function in this new rat strain and to use as a comparison in determining β-AR density and function in skeletal muscle. In our study, the cardiac β-AR density resembled data collected from male Wistar rats that declined with age from 62.9 fmol/mg protein in the 7-mo-old group to 46.4 fmol/mg protein in the 24-mo-old animals (7). Although several other studies have failed to observe consistent changes in cardiac β-AR density with age, these studies did find that β-AR function declines with age in the male or female F344 rat (5, 24, 32). It appears that the age-associated changes in cardiac β-AR density may differ in different rat strains but that cardiac β-AR function appears to decline with age in all rodent strains that have been studied.
The lack of a decline in skeletal muscle β-AR density and AC response with age was unexpected in light of the increase in circulating norepinephrine concentrations and the apparent decline observed in cardiac muscle. However, these findings are consistent with data from 6- vs. 24-mo-old F344 rats, which suggest that skeletal muscle adrenergic responsiveness is not diminished with age in response to exogenous epinephrine infusion (13). Several factors could explain the observed age-associated increase in gastrocnemius β-AR receptor density. These factors include differential sensitivity of β-AR subtypes to regulation by sympathetic nervous system-meditated mechanisms, the proportion of different skeletal muscle fiber types, and the proportion of contractile and connective tissue in muscle.
Two major subtypes of β-AR are found in muscle (27). β2-AR is the predominant β-AR receptor found in skeletal muscle (27). Although both β-AR subtypes may exhibit downregulation mediated by an increase in circulating norepinephrine, β1-AR may be more sensitive to regulation by norepinephrine than β2-AR. For example, when a norepinephrine-secreting tumor was implanted in rats for 3–4 wk, selective β1-AR, but not β2-AR, downregulation was observed in cardiac muscle membrane preparations (31). However, the apparent resistance of the β2-AR to downregulation by an increase in circulating norepinephrine cannot explain the increase in total skeletal muscle β-AR density observed in gastrocnemius muscle of the 28-mo-old group.
Another explanation for the increase in β-AR density in skeletal muscle is an age-associated shift in skeletal muscle fiber type. There is evidence suggesting that skeletal muscle fiber type shifts to a more oxidative (type I) metabolic state in the aging rat (9). Type I fibers are associated with relatively higher β-AR density and function compared with either the fast-oxidative (type IIa) or fast-glycolytic (type IIb) (17). Previous data have indicated an age-associated increase in type I and a decrease in type II fibers in the gastrocnemius muscle of 26- compared with 6-mo-old male F344 rats (13). Thus an age-associated increase in slow-oxidative (type I) fibers could explain the observed increase in β-AR density in the skeletal muscle of the 28-mo-old animals in our study Quantitative analysis of fiber type in gastrocnemius muscle of the male F344xBN rat strain with aging would be required to test this hypothesis.
There is some evidence that the proportion of total protein represented by connective and contractile protein is altered in aging skeletal muscle (12, 19, 30, 34). An age-associated shift in the percentage of contractile protein per total protein in skeletal muscle could lead to error in interpretation of β-AR density and function data, which are generally normalized to total protein content of homogenate samples. However, the results from analysis of total protein and Western blot analysis of myosin content in skeletal muscle indicated no significant differences between the age groups with respect to either the percentage of contractile protein per total protein or total protein in our homogenate samples. Therefore, it is unlikely that the age-associated increase in gastrocnemius β-AR density is the result of a decrease in the concentration of skeletal muscle contractile or total protein content.
In conclusion, in the male F344xBN rat, there is an age-associated increase in plasma norepinephrine concentrations, suggesting an increase in sympathetic nervous system activity. There was no age-associated decrease in skeletal muscle β-stimulated AC activity, suggesting that function of the β-AR-AC effector system is intact in the gastrocnemius muscle of the 28-mo-old group. The increase in skeletal muscle β-AR density could be explained by an age-associated alteration in fiber-type proportions in the aged 28-mo-old group.
Acknowledgments
The authors thank Eric Leiendecker, Bahaa Qasawa, Jodi L. Kreuger, and Maria Smith for technical assistance.
This research was supported in part by National Institute on Aging Training Grant T32 AG-00114; by the Core Facility for Aged Rodents of the Claude Pepper Older Americans Independence Center (AG-00880) and Institute of Gerontology, University of Michigan; and by the Ann Arbor Geriatric Research, Education, and Clinical Center and the Medical Research Service of the Department of Veterans Affairs Medical Center.
REFERENCES
- 1.Abrass IB, Davis JL, Scarpace PJ. Isoproterenol responsiveness and myocardial β-adrenergic receptors in young and old rats. J. Gerontol. 1982;37:156–160. doi: 10.1093/geronj/37.2.156. [DOI] [PubMed] [Google Scholar]
- 2.Bouvier M, Hnatowich M, Collins S, Kobilka BK, Deblasi A, Lefkowitz RJ, Caron MG. Expression of a human cDNA encoding the β2-adrenergic receptor in Chinese hamster fibroblasts (CHW): functionality and regulation of the expressed receptors. Mol. Pharmacol. 1988;33:133–139. [PubMed] [Google Scholar]
- 3.Bronson R. Cross-sectional pathology of aging rodents. In: Harrison D, editor. Genetic Effects on Aging II. Caldwell, NJ: Telford; 1990. pp. 279–357. [Google Scholar]
- 4.Evans MI, Halter JB, Porte D. Comparisons of double- and single-isotope enzymatic derivative methods for measuring catecholamines in human plasma. Clin. Chem. 1978;24:567–570. [PubMed] [Google Scholar]
- 5.Fan T-HM, Banerjee SP. Age-related reduction of beta-adrenoceptor sensitivity in rat heart occurs by multiple mechanisms. Gerontology. 1985;31:373–380. doi: 10.1159/000212726. [DOI] [PubMed] [Google Scholar]
- 6.Guarniere T, Filburn CR, Zitnik G, Roth GS, Lakatta EG. Contractile and biochemical correlates of β-adrenergic stimualtion of the aged heart. Am. J. Physiol. 1980;239:H501–H508. doi: 10.1152/ajpheart.1980.239.4.H501. (Heart Circ. Physiol. 8) [DOI] [PubMed] [Google Scholar]
- 7.Gudmundsdottir E, Benediktsdóttir E, Gudbjarnason S. Combined effects of age and dietary fat on β1-receptors and Ca2+ channels in rat hearts. Am. J. Physiol. 1991;260:H66–H72. doi: 10.1152/ajpheart.1991.260.1.H66. (Heart Circ. Physiol. 29) [DOI] [PubMed] [Google Scholar]
- 8.Hogikyan RV, Supiano MA. Arterial α-adrenergic responsiveness is decreased and SNS activity is increased in older humans. Am. J. Physiol. 1994;266:E717–E724. doi: 10.1152/ajpendo.1994.266.5.E717. (Endocrinol. Metab. 29) [DOI] [PubMed] [Google Scholar]
- 9.Holloszy JO, Chen M, Cartee GD, Young JC. Skeletal muscle atrophy in old rats: differential changes in the three fiber types. Mech. Ageing Dev. 1991;60:199–213. doi: 10.1016/0047-6374(91)90131-i. [DOI] [PubMed] [Google Scholar]
- 10.Iwase S, Mano T, Watanabe T, Saito M, Kobayashi F. Age-related changes of sympathetic outflow to muscles in humans. J. Gerontol. 1991;46:M1–M5. doi: 10.1093/geronj/46.1.m1. [DOI] [PubMed] [Google Scholar]
- 11.Kiristy-Roy JA, Halter JB, Smith MJ, Terry LC. Selective impairment of neuroendocrine and hemodynamic responses to a mu-opiod peptide in aged rats. J. Gerontol. 1992;47:B89–B97. doi: 10.1093/geronj/47.3.b89. [DOI] [PubMed] [Google Scholar]
- 12.Kovanen V, Suominen H, Peltonen L. Effects of aging and life-long physical training on collagen in slow and fast skeletal muscle in rats. A morphometric and immunohistochemical study. Cell Tissue Res. 1987;248:247–255. doi: 10.1007/BF00218191. [DOI] [PubMed] [Google Scholar]
- 13.Larkin L, Horwitz B, Eiffert K, McDonald R. Adrenergic stimulated skeletal muscle glycogenolysis in perfused hindlimbs of young and old male Fischer 344 rats. Am. J. Physiol. 1994;266:R749–R755. doi: 10.1152/ajpregu.1994.266.3.R749. (Regulatory Integrative Comp. Physiol. 35) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larkin LM, Horwitz BA, McDonald RB. Effect of cold on serum substrate and glycogen concentration in young and old Fischer 344 rats. Exp. Gerontol. 1992;27:179–190. doi: 10.1016/0531-5565(92)90042-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liggett SB, Shah SD, Cryer PE. Characterization of β-adrenergic receptors of human skeletal muscle obtained by needle biopsy. Am. J. Physiol. 1988;254:E795–E798. doi: 10.1152/ajpendo.1988.254.6.E795. (Endocrinol. Metab. 17) [DOI] [PubMed] [Google Scholar]
- 16.Linares OA, Halter JB. Sympathochromafin system activity in the elderly. J. Am. Geriatr. Soc. 1987;35:448–453. doi: 10.1111/j.1532-5415.1987.tb04667.x. [DOI] [PubMed] [Google Scholar]
- 17.Martin W, III, Murphree SS, Saffitz JE. Beta adrenergic receptor distribution among muscle fiber types and resistance arterioles of white, red, and intermediate skeletal muscle. Circ Res. 1989;64:1096–1105. doi: 10.1161/01.res.64.6.1096. [DOI] [PubMed] [Google Scholar]
- 18.Mazzeo RS, Grantham PA. Sympathetic response to exercise in various tissues with advancing age. J. Appl. Physiol. 1989;66:1506–1508. doi: 10.1152/jappl.1989.66.3.1506. [DOI] [PubMed] [Google Scholar]
- 19.Mohan S, Radha E. Age-related changes in rat muscle collagen. Gerontology. 1980;26:61–67. doi: 10.1159/000212396. [DOI] [PubMed] [Google Scholar]
- 20.O'Connor SW, Scarpace PJ, Abrass IB. Age-associated decrease of adenylate cyclase activity in rat myocardium. Mech. Ageing Dev. 1981;16:91–95. doi: 10.1016/0047-6374(81)90036-1. [DOI] [PubMed] [Google Scholar]
- 21.Perrie WT, Bumford SJ. Electrophoretic separation of myosin isoenzymes. Implications for the histochemical demonstration of fibre types in biopsy specimens of human skeletal muscle. J. Neurosci. 1986;73:89–96. doi: 10.1016/0022-510x(86)90067-5. [DOI] [PubMed] [Google Scholar]
- 22.Sakai M, Danziger RS, Xiao RP, Spurgeon HA, Lakatta EG. Contractile responses of individual cardiac myocytes to norepinephrine declines with senescence. Am. J. Physiol. 1992;262:H184–H189. doi: 10.1152/ajpheart.1992.262.1.H184. (Heart Circ. Physiol. 31) [DOI] [PubMed] [Google Scholar]
- 23.Scarpace PJ. Forskolin activation of adenylate cyclase in rat myocardium with age: effects of guanine nucleotide analogs. Mech. Ageing Dev. 1990;52:169–178. doi: 10.1016/0047-6374(90)90122-v. [DOI] [PubMed] [Google Scholar]
- 24.Scarpace PJ, Abrass IB. Beta-adrenergic agonist-mediated desensitization in senescent rats. Mech. Ageing Dev. 1986;35:255–264. doi: 10.1016/0047-6374(86)90128-4. [DOI] [PubMed] [Google Scholar]
- 25.Scarpace PJ, Mooradian AD, Morley JE. Age-associated decrease in beta-aderenergic receptors and adenylate cyclase activity in rat brown adipose tissue. J. Gerontol. 1988;43:B65–B70. doi: 10.1093/geronj/43.3.b65. [DOI] [PubMed] [Google Scholar]
- 26.Scarpace PJ, Yu BP. Diet restriction retards the age-related loss of beta-adrenergic receptors and adenylate cyclase activity in rat lung. J. Gerontol. 1987;42:442–446. doi: 10.1093/geronj/42.4.442. [DOI] [PubMed] [Google Scholar]
- 27.Strosberg AD. Biotechnology of β-adrenergic receptors. Mol. Neurobiol. 1992;4:211–250. doi: 10.1007/BF02780342. [DOI] [PubMed] [Google Scholar]
- 28.Supiano MA, Linares OA, Halter JB, Reno KM, Rosen SG. Functional uncoupling of the platelet alpha2-adrenergic receptor-adenylate cyclase complex in the elderly. J. Clin. Endocrinol. Metab. 1987;64:1160–1164. doi: 10.1210/jcem-64-6-1160. [DOI] [PubMed] [Google Scholar]
- 29.Supiano MA, Linares OA, Smith MJ, Halter JB. Age-related differences in norepinephrine kinetics: effect of posture and sodium-restricted diet. Am. J. Physiol. 1990;259:E422–E431. doi: 10.1152/ajpendo.1990.259.3.E422. (Endocrinol. Metab. 22) [DOI] [PubMed] [Google Scholar]
- 30.Thomas DP, McCormick RJ, Zimmerman SD, Vadlamudi RK, Gosselin LE. Aging- and training-induced alterations in collagen characteristics of rat left ventricle and papillary muscle. Am. J. Physiol. 1992;263:H778–H783. doi: 10.1152/ajpheart.1992.263.3.H778. (Heart Circ. Physiol. 32) [DOI] [PubMed] [Google Scholar]
- 31.Tsujimoto G, Manger WM, Hoffman BB. Desensitization of β-adrenergic receptors by pheochromocytoma. Endocrinology. 1984;114:1272–1278. doi: 10.1210/endo-114-4-1272. [DOI] [PubMed] [Google Scholar]
- 32.Turner N, Houck WT, Roberts J. Effect of age on upregulation of the cardiac adrenergic beta receptors. J. Gerontol. 1990;45:B48–B51. doi: 10.1093/geronj/45.2.b48. [DOI] [PubMed] [Google Scholar]
- 33.Urasawa K, Murakami T, Yasuda H. Age-related alterations in adenylyl cyclase system of rat hearts. Jpn. Cire. J. 1991;55:676–684. doi: 10.1253/jcj.55.676. [DOI] [PubMed] [Google Scholar]
- 34.Zimmerman SD, McCormick RJ, Vadlamudi RK, Thomas DP. Age and training alter collagen characteristics in fast- and slow-twitch rat limb muscle. J. Appl. Physiol. 1993;75:1670–1674. doi: 10.1152/jappl.1993.75.4.1670. [DOI] [PubMed] [Google Scholar]