Abstract
After nerve-repair grafting of medial gastrocnemius muscle, there is incomplete recovery of specific force and sustainable power, perhaps due to overcompensation by synergistic muscles. We hypothesized that increased workload due to synergist ablation would enhance graft recovery. Contractile and metabolic properties of control and nerve-repair grafted muscles, with and without synergist ablation, were determined after 120 days recovery. Specific force (N/cm2) and normalized power (W/kg) were less in the experimental groups compared with controls. Sustained power (W/kg) in the synergist-ablated nerve-repair grafted muscle was higher than nerve-repair grafted muscle, returning to control values. GLUT-4 protein was higher and glycogen content was diminished in both synergist-ablated groups. In summary, synergist ablation did not enhance the recovery of specific force or normalized power, but sustained power did recover, suggesting that metabolic and not mechanical parameters were responsible for this recovery. The enhanced endurance after synergist ablation was accompanied by increased GLUT-4 protein, suggesting a role for increased uptake of circulating glucose during contraction.
Keywords: force, sustained power, reinnervation
Skeletal muscle dysfunction may arise from inherited or acquired diseases or from denervation resulting from direct trauma (19, 20). Neurovascular grafting of whole skeletal muscles is used clinically to correct dysfunction or repair injuries to skeletal muscle. Under appropriate conditions, many of the morphological and physiological characteristics return to control values after standard (7, 11), standard with nerve implant (26), nerve-repair (18), or nerve-intact (7, 11) models for grafting of whole skeletal muscles in rats. However, recovery of grafts in the presence of synergist muscles is generally associated with deficits in the development of specific force (18, 30) and with increased fatigability (18). Clinically, muscle transfer procedures involve replacement of a whole muscle group with one muscle in the absence of synergist muscles (19).
Therefore, it is possible that the synergistic muscles overcompensate for or “stress shield” the recovering grafted muscle, thereby reducing the need for full recovery. If this is true, eliminating synergist muscles may lead to improved recovery of mechanical function in grafted muscle. In nongrafted and revascularized grafted muscle, overload due to synergist muscle ablation leads to muscle hypertrophy accompanied by increases in muscle mass and cross-sectional area (8, 24, 25). This increase in muscle mass was accompanied by increases in maximum twitch force, maximum tetanic force, and resistance to fatigue (10, 25). However, the specific force (force normalized for mass) of synergist ablated soleus and medial gastrocnemius (MG) muscle was not significantly different from control muscle (8, 25). Thus findings in grafted muscle after synergist ablation might be similar to effects of muscle hypertrophy: a shift toward a more aerobic muscle metabolism, potentially increasing the muscle’s ability to resist fatigue (17), and shifts in muscle fiber types toward more type I and IIa (more oxidative) fibers (25). If similar metabolic alterations occur in grafted muscle after synergist ablation, we would likely observe an increase in endurance capacity.
Although previous studies have been done to assess the affects of nerve-intact and nerve-repair grafting on muscle function in rats (8, 29), in these studies the blood flow was severed and left to spontaneously revascularize. This disruption and reorganization of blood flow can adversely affect contractile function, especially sustained power for which delivery of substrate to the muscle is critical. Our study is unique in that we focused only on the impact of reinnervation only on recovery from grafting with and without synergist muscle ablation.
To study the effect of nerve-repair grafting on metabolic and contractile function in skeletal muscle, we needed to find a muscle that is large enough to collect adequate samples for determining several assays of metabolic function and that has an accessible muscle with a fiber-type composition comparable to that of humans. The mass of the MG muscle in a Fischer 344 rat is adequate to supply enough tissue to perform several metabolic measures. The MG muscle is innervated by only one nerve (a branch of the tibial nerve), which makes microsurgical denervation and repair and subsequent monitoring of contractile properties feasible.
In this study, we investigated the effect of synergist muscle ablation on the recovery of contractile and metabolic function in nerve-repair MG muscle. We hypothesize that overload hypertrophy of the recovering stabilized grafts of MG muscles after synergist muscle ablation may lead to full recovery of force and power capacity in the grafted muscle and would be associated with an enhanced glucose transporter content, glycogen storage capacity, or oxidative capacity.
METHODS
Animals and animal care
Eighty male Fischer 344 rats, 2 mo of age, were obtained from the National Institute on Aging’s animal colony maintained by Harlan Sprague Dawley Laboratory (Indianapolis, IN). Rats were acclimated to our colony conditions, i.e., light cycle and temperature, for 1 wk before surgical procedure. Rats were housed individually in hanging plastic cages (28 × 56 cm) and kept on a 12:12 light-dark cycle at a temperature of 20–22°C. The rats were fed Purina rodent chow, 5001 laboratory chow, and water ad libitum. All surgical procedures were performed in an aseptic environment in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals (DHEW Publication No. 85-23).
Experimental design
In a two-by-two balanced design, 80 animals were randomly divided into four experimental groups of 20 animals. One group of rats underwent the nerve-repair grafting procedure using the left MG muscle. Because of the inability to obtain viable control contractile data from the contralateral leg, a second group of animals were used as sham operated controls (sham controls). Another group underwent both the nerve-repair grafting of the left MG muscle and synergist muscle ablation. The fourth group was used as controls for the synergist-ablated nerve-repair grafted group and underwent ablation of synergist muscles to the left MG muscles (synergist-ablated control). At 120 days after surgery, each of the four surgical groups was further divided into two groups: one subgroup (n = 10) to study muscle metabolic parameters and a second subgroup (n = 10) to study muscle contractile function. Several animals did not recovery fully from the various surgical procedures and were excluded from the study. Thus the number of animals in each of the various experimental subgroups ranged from 6 to 10. Criteria for exclusion were on the basis of excessive weight loss and/or nonresponse of MG muscle with stimulation of sciatic nerve during contractile measurements.
MG muscle neurovascular grafting and synergist muscle ablation procedures
As previously described by Larkin et al. (18), the tendon and nerve of the left MG muscle were severed, and the muscle was grafted orthotopically with repair of the nerve and tendon. Briefly, animals were anesthetized with pentobarbital sodium (65 mg/kg). The left MG muscle was isolated from surrounding muscle and connective tissue, the distal and proximal tendons were severed, and the muscle was removed from its bed. The muscle was then placed back into its original position, and the tendons were repaired by using 8-0 Ethilon suture. The branch of the tibial nerve innervating the MG was isolated, severed, and then repaired by using epineurial sutures of 11-0 Ethilon suture. Care was taken to leave the blood supply intact. The incision was closed in layers by using 4-0 Ethilon suture. The animals were allowed to recover for 120 days before measurements of contractile and metabolic properties. Previous work has demonstrated that this recovery period is sufficient to allow stabilization of whole muscle maximal tetanic tension of neurovascular muscle grafts (14).
In the two synergist muscle ablation groups, all of the major synergistic muscles for the MG muscle were removed from both hindlimbs. These muscles included the lateral gastrocnemius, the plantaris, and the soleus muscles. The animals were allowed to recover for 120 days before measurements of contractile and metabolic properties.
Surgical preparation for contractile measurements
After the 120-day recovery period, the animals were again anesthetized with pentobarbital sodium (65 mg/kg). Supplemental doses of the anesthesia were administered as required to maintain an adequate depth of anesthesia. The MG muscle was isolated from surrounding muscle and connective tissue. A 0-0 silk suture was tied around the distal tendon, and then the tendon was severed. The animal was then placed on a Plexiglas platform, and the animal’s body temperature was maintained with a heating pad. The hindlimb was secured by pinning the femur near the origin of the MG muscle and clamping the foot to the platform. The distal tendon of the MG muscle was then tied to a lever arm of a servomotor (model 6650, Cambridge Technology). The servomotor was used to measure force and also served to shorten the muscle during power measurements. Once the muscle was secured to the platform, the tibial nerve was exposed and stimulated with a bipolar electrode (Harvard Apparatus) connected to a Grass model S88 stimulator. Data were viewed on an oscilloscope and analyzed by using ASYST computer software (Keithley Instruments).
Contractile measurements
The distal tendon of the MG muscle was tied to the servomotor lever arm and the muscle length was adjusted to the optimum length for development of maximum isometric force (Lo) (2). The Lo was used to calculate the optimum fiber length (Lf) by using the Lf-to-Lo ratio of 0.312. Maximum isometric tetanic force was determined by subjecting the muscle to periods of stimulation at increasing frequencies.
All power measures were made during isovelocity shortening contractions through 10% of Lf, as previously described by Brooks and Faulkner (3). Briefly, contractions were initiated at 105% of Lf, and muscle was shortened to 95% of Lf. Stimulation of the nerve and initiation of the shortening ramp occurred simultaneously, and stimulation was terminated at the end of the shortening ramp. The integrated area under the force curve during only the shortening ramp was used to determine the average force developed during a contraction, and the power was calculated as the product of the average force during the shortening and the velocity of shortening (3). Increasing the shortening velocity increased the power until maximum was reached. The shortening velocity that resulted in maximum power was defined as the optimal shortening velocity (Lf/s).
Maximum sustained power was determined by using repeated isovelocity shortening contractions and gradually increasing the number of contractions per unit time (train rate) at a constant train duration. Duty cycle is defined as the product of the train rate (number of contractions per unit time in Hz) and train duration (in s). A stimulation frequency (100 Hz) and train duration (67 ms) that produced ~75% of maximum power in both the graft and control muscle was used for all tests of sustained power. Because the train duration was held constant at 67 ms for both the graft and control muscles at each duty cycle, the train rate (number of contractions per unit time in Hz) was also identical for graft and control muscles at each duty cycle. Sustained power was calculated as the product of the shortening velocity, the average force developed during shortening contractions at a given duty cycle, and the duty cycle. The train rate, and therefore the duty cycle, was increased every 5 min until the maximum sustained power was reached.
Tissue preparation and analytic procedures
After collection of contractile property data, muscles were removed, weighed, frozen in a mixture of isopentane and dry ice, covered with Tissue Freezing Medium (Triangle Biomedical Sciences, Durham, NC) and stored at −80°C until used for histology measures. The functional muscle fiber cross-sectional area (CSAf) was calculated (13) as the volume of the muscle divided by Lf (in mm), where muscle volume is the product of muscle mass (mg) and 1.06 (mg/mm3), which is the density of mammalian skeletal muscle (22).
In a second group of animals used for metabolic studies, after pentobarbital sodium anesthesia (65 mg/kg), control and grafted MG muscles were dissected, weighed, frozen between liquid nitrogen-cooled aluminum clamps, and stored at −80°C until analyzed. Frozen muscles (100 mg) were powdered in a mortar and pestle cooled in liquid N2. Powdered tissues were used for analysis of metabolic parameters. One aliquot of tissue was analyzed for glycogen content (µmol/g muscle) via the method of Hassid and Abraham (16). Muscle homogenates (from a 700-g supernatant) were used to determine citrate synthase activity (µmol·µg protein−1-min−1) by using the method of Serre (27). Hexokinase activity (U/mg protein) was measured by using the method of Easterby and Qadri (9). Protein content (mg/g tissue) was determined by using a Bradford protein assay kit from Bio-Rad (Richmond, CA).
GLUT-1 and GLUT-4 Western blotting
Total membranes were isolated from 100 mg of frozen MG muscle as follows. Frozen tissues (200 mg) were powdered in a mortar and pestle cooled in liquid N2. Powdered tissues were suspended in 5.0 ml of ice-cold SSS buffer (10 mM NaHCO3, 0.25 M sucrose, and 5.0 mM NaN3) and homogenized twice for 10 s by using a Brinkman polytron (setting 7). Homogenates were centrifuged at 1,200 g for 10 min, at 4°C, and pellets were resuspended in 5 ml of ice-cold SSS buffer and centrifuged at 1,200 g for 10 min at 4°C. Supernatants were combined and centrifuged at 190,000 g for 66 min at 4°C. Pellets were then resuspended in 500 µl ice-cold SSS buffer and stored at −70°C.
Western blot analysis of GLUT-1 and GLUT-4 glucose transporters was performed by using vertical slab polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate on molecular weight markers (Amersham, Arlington Heights, IL) and aliquots of muscle membranes each containing 30 (µg protein. An internal control sample was run on all gels, which consisted of an aliquot (30 µg protein) of a muscle membrane pool obtained from combined muscle homogenates. Duplicate samples from each animal were separated on a 10% polyacrylamide resolving gel and then eletrophoretically transferred onto Immobilon polyvinyldifluoride membrane (Millipore, Milford, MA). Immobilon polyvinyldifluoride membranes were then blocked for 60 min with Tris-buffered saline with Tween 20 (100 mM Tris, 0.9% NaCl, 2.0 ml Tween 20, pH 7.5) and 5% Carnation nonfat dry milk. Immunoblotting was performed by using a rabbit polyclonal antibody against rat brain glucose transporter (GLUT-1) or insulin-regulatable glucose transporter (GLUT-4) protein (East Acres Biologicals, Southbridge, MA), followed by 125I-labeled anti-rabbit immunoglobulin (Amersham, Arlington Heights, IL) and exposure of the transfer membrane to Kodak X-OMAT film at −80°C for 24 h. 125I-labeled transfer membranes were then cut and counted on a gamma counter (Tm Analytic, Elk Village, IL). Total counts per minute were corrected for background, and then specific counts were normalized for the internal standard. There was a single band for both GLUT-1 and GLUT-4 protein at a molecular mass of 45 kDa.
Histology
In a subgroup of animals from each experimental group (n = 4), muscle biopsies were taken from the middle of the MG muscles from each of the animals used to measure contractile properties. Ten-micrometer-thick sections were cut in a cryostat and incubated at varying pH conditions to measure myofibrillar ATPase activity. Muscle fibers were classified as type I and type II on the basis of myofibrillar ATPase activity at pH 9.4, as previously described by Brooke and Kaiser (1). Type II fibers were further subdivided into type IIa and IIb on the basis of myofibrillar ATPase activity at pH 4.45. The entire cross-sectional area of each muscle was digitized, ~7,700 fibers were counted and type classified, and fiber was area calculated from each MG muscle by using a computerized Bioquant imaging system (R and M Biometrics, Nashville, TN).
Statistics
Values are presented as means ± SE. Statistical analysis was performed by using Statview 4.01 (Abacus Concepts, Berkeley, CA). A two-way ANOVA was used to compare differences between grafted and synergist ablation groups. When a significant main effect was found, the Fisher’s least significant difference post hoc test was used to determine differences between groups. A repeated-measures ANOVA was used to determine the effect grafting and synergist muscle ablation on sustained power. Differences were considered significant at P ≤ 0.05.
RESULTS
Contractile properties
There was a significant effect of grafting and ablation on muscle mass (g) and CSAf (mm2). Muscle mass and CSAf of the synergist-ablated groups were increased compared with those of sham controls (Table 1). Grafting, in combination with ablation, led to significantly less increase in muscle mass and cross-sectional area in the synergist-ablated nerve-repair grafted group compared with synergist-ablation alone. There was a significant effect of grafting and ablation on power normalized for muscle mass (W/kg). Both of these measures were significantly lower in all three experimental groups compared with sham controls. There was a significant interaction between grafting and ablation: grafting in combination with ablation led to lower normalized power than either grafting or ablation alone.
Table 1.
Properties of nerve-repair grafted and control medial gastrocnemius muscle with or without synergistic muscles intact in 6-mo-old male Fischer 344 rats
| Experimental Group | P Value | ||||||
|---|---|---|---|---|---|---|---|
| Sham control |
Nerve-repair grafted |
Synergist-ablated control |
Synergist-ablated nerve-repair grafted |
Effect of grafting |
Effect of ablation |
Effect of grafting × ablation |
|
| Muscle mass, g | 716 ± 14 (10) |
720 ± 23 (7) |
1,141 ± 42c (9) |
872 ± 24b,d,e (10) |
< 0.0001 | < 0.0001 | < 0.0001 |
| CSAf, mm2 | 55 ± 1 (10) |
56 ± 2 (7) |
84± 3c (9) |
65 ± 2b,d,e (10) |
< 0.0001 | < 0.0001 | < 0.0001 |
| Maximum tetanic tension, N | 1,367 ± 23 (10) |
1,060 ±52a(7) | 1,776 ± 45c (8) |
1,335 ± 23b,d (10) |
< 0.0001 | < 0.0001 | 0.057 |
| Specific force, N/cm2 | 25 ± 0.2 (10) |
19 ± 0.7a (7) |
21 ± 0.8c (8) |
21 ± 0.8e (10) |
< 0.0001 | 0.073 | < 0.0001 |
| Optimal velocity, Lf/s | 1.67 ±0.06 (10) |
1.75 ± 0.09 (6) |
1.44 ± 0.6c (9) |
1.3 ± 0.05d,e (10) |
NS | < 0.0001 | 0.077 |
| Normalized power, W/Kg | 182 ± 3 (10) |
111± 7a (6) |
107 ± 4c (9) |
88 ± 4b,d,e (10) |
< 0.0001 | < 0.0001 | < 0.0001 |
| Maximum normalized sustained power, W/kg |
4.8 ± 0.1 (9) |
3.7± 0.3a (10) |
4.5 ± 0.2 (10) |
5.1 ± 0.2b,d (10) |
NS | 0.021 | 0.0002 |
Values are means ± SE; nos. in parentheses is no. of rats for each group. CSAf, functional muscle fiber cross-sectional area; NS, not significant.
Nerve-repair grafted is significantly different from sham control group, P ≤ 0.05.
Synergist-ablated nerve-repair grafted is significantly different from synergist-ablated control group, P ≤ 0.05.
Synergist-ablated control is significantly different from sham control group, P ≤ 0.05.
Synergist-ablated nerve-repair grafted is significantly different from nerve-repair grafted group, P ≤ 0.05.
Synergist-ablated nerve-repair grafted is significantly different from sham control group, P ≤ 0.05.
There was an effect of both grafting and ablation on maximum tetanic tension: maximum tetanic tension was greater in the two synergist-ablated groups compared with their respective control groups and was lower in both grafted groups compared with respective control groups. Specific force (N/cm2) was lower in all three experimental groups compared with the sham control animals. Overall, the effect of ablation on specific force was not significant, but there was a significant effect of grafting, primarily in the animals not treated with synergist-ablation. Optimal shortening velocity (Lf/s) was lower in the synergist ablation groups compared with sham control (Table 1). There was no effect of grafting on optimal shortening velocity. The interaction of grafting and ablation was not significant, with the synergist-ablated nerve-repair muscle having slightly lower optimal shortening velocity than the synergist-ablated control muscle.
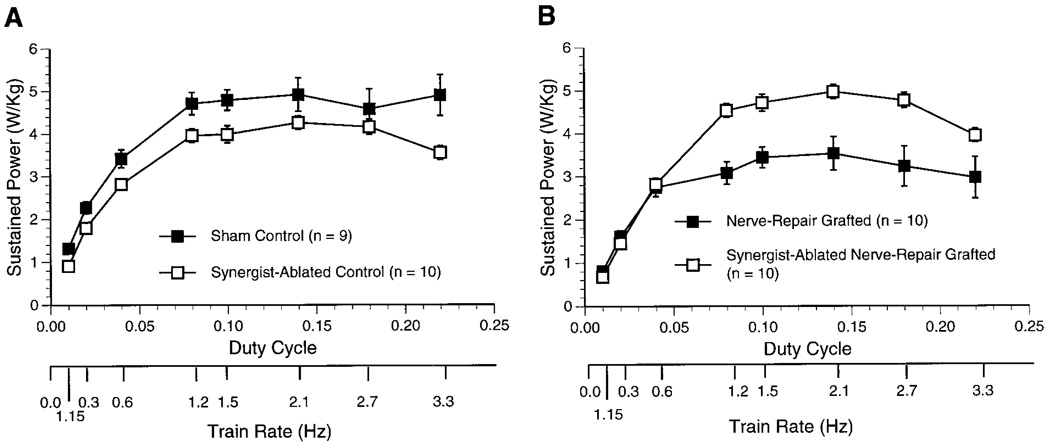
Peak or maximum normalized sustained power (W/ kg) was diminished by grafting alone but was enhanced by synergist muscle ablation (Table 1). In fact, the maximum normalized sustained power of the synergist-ablated nerve-repair grafted muscle was significantly greater compared with values for both nerve-repair grafted and sham control muscles. At each duty cycle, normalized sustained power (W/kg) of the synergist-ablated control muscle was slightly, but significantly less, compared with values for sham control muscles (Fig. 1). In contrast, the normalized sustained power of the synergist-ablated nerve-repair grafted muscle was significantly greater compared with values for nerve-repair grafted muscle. There was no significant difference in the ability to sustain power in the synergist-ablated nerve-repair grafted muscle compared with the sham control muscle.
Fig. 1.
Sustained power developed at increasing duty cycles in control (A) and grafted (B) medial gastrocnemius muscles in 6-mo-old male Fischer 344 rats. A stimulation frequency (100 Hz) and train duration (67 ms) that produced ~75% of maximum power in both the control and grafted muscles was used for all tests of sustained power. Values are means ± SE; n, no. of rats. A: at each duty cycle, normalized sustained power of the synergist-ablated control muscle was slightly, but significantly, less compared with values for sham control muscles (P = 0.0133; repeated-measures ANOVA). B: normalized sustained power of the synergist-ablated nerve-repair grafted muscle was significantly greater compared with values for nerve-repair grafted muscle (P = 0.0038; repeated-measures ANOVA). There was no significant difference in the ability to sustain power in the synergist-ablated nerve-repair grafted muscle compared with the sham control muscle.
Metabolic properties
There was an overall effect of grafting on body weight (g) and protein content (mg protein/g tissue) in which the two synergist muscle ablated groups were significantly lower compared with their respective nonablated group (Table 2). Grafting in combination with ablation led to a smaller loss in muscle protein than with synergist-ablation alone. Glycogen content (µmol/g muscle) was significantly lower in the two nerve-repair grafted groups compared with the sham control group. Synergist muscle ablation significantly decreased glycogen content by over 50% compared with sham control and nerve-repair grafted muscle. Grafting in combination with ablation blunted the graft-related decline in glycogen content. Citrate synthase activity (µmol·min−1·mg protein−1) was significantly diminished in the two nerve-repair grafted groups compared with the two control groups. Hexokinase activity (U/mg protein) was significantly greater in all three experimental groups compared with the sham control group, although this increase was less in the two synergist muscle ablation groups. GLUT-4 protein content (%sham control) was significantly increased in the two synergist muscle ablated groups compared with the other two groups. There was an overall graft-associated increase in GLUT-1 protein content in the two nerve-repair grafted groups compared with their respective controls by ANOVA. However, the differences between the means of each of the two grafted groups and their respective controls did not reach statistical significance (Table 2).
Table 2.
Metabolic characteristics of nerve-repair grafted and control medial gastrocnemius muscle with or without synergistic muscles intact in 6-mo-old male Fischer 344 rats
| Experimental Group | P Value | ||||||
|---|---|---|---|---|---|---|---|
| Sham control |
Nerve-repair grafted |
Synergist-ablated control |
Synergist-ablated nerve-repair grafted |
Effect of grafting |
Effect of ablation |
Effect of grafting × ablation |
|
| Body weight, g | 371 ± 9 (10) |
380 ± 5 (10) |
346 ± 5c (10) |
356 ± 5d (10) |
NS | 0.003 | NS |
| Muscle protein content, mg protein/g tissue |
232 ± 12 (8) |
205 ± 11 (7) |
153 ± 10c (9) |
173 ± 7d,e (10) |
NS | < 0.001 | 0.024 |
| Glycogen content, µmol/g muscle |
36 ± 2 (9) |
30 ± 1a (7) |
12 ± 1c (10) |
14 ± 1b,d,e (10) |
NS | < 0.0001 | 0.0002 |
| Citrate synthase, µmol · µg−1 · min−1 |
176 ± 18 (6) |
139 ± 3a (5) |
175 ± 4 (9) |
149 ± 8b,e (10) |
0.002 | NS | NS |
| Hexokinase, U/mg protein | 25 ± 6 (8) |
58 ± 5a (7) |
47 ± 2c (10) |
48 ± 1d,e (10) |
< 0.0001 | NS | < 0.0001 |
| GLUT-4 protein, %sham control |
100 ± 4 (10) |
101 ± 5 (10) |
170 ± 15c (8) |
175 ± 12d,e (8) |
NS | < 0.0001 | NS |
| GLUT-1 protein, %sham control |
100 ± 9 (8) |
143 ± 19 (9) |
85 ± 11 (5) |
137 ± 24 (5) |
0.012 | NS | NS |
Values are means ± SE; nos. in parentheses is no. of rats for each group.
Nerve-repair grafted is significantly different from sham control group, P ≤ 0.05.
Synergist-ablated nerve-repair grafted is significantly different from synergist-ablated control group, P ≤ 0.05.
Synergist-ablated control is significantly different from sham control group, P < 0.05.
Synergist-ablated nerve-repair grafted is significantly different from nerve-repair grafted group, P ≤ 0.05.
Synergist-ablated nerve-repair grafted is significantly different from sham control group, P ≤ 0.05.
Histochemistry
The percentage of the three predominant fiber types did not differ significantly among the four experimental groups (Table 3). The predominant fiber type was type IIb, representing on average 80.9 ± 2.0% of the total fiber population. The remaining population consisted of type IIa and type I fibers, representing 7.9 ± 1.2 and 11.2 ± 1.1% of the total fiber population, respectively. There was an overall effect of ablation on the average cross-sectional area of each fiber type among the experimental groups. Overall, the average cross-sectional area of all three fiber types was significantly increased in the two synergist-ablated groups compared with the nonablated muscles. The average cross-sectional area of type IIb fibers was lower in the grafted group. There was no interaction between grafting and ablation in any of the histological parameters measured.
Table 3.
Histological properties of nerve-repair grafted and control medial gastrocnemius muscle with or without synergistic muscles intact in 6-mo-old male Fischer 344 rats
| Experimental Group | P Value | ||||||
|---|---|---|---|---|---|---|---|
| Sham control |
Nerve-repair grafted |
Synergist-ablated conrol |
Synergist-ablated nerve-repair grafted |
Effect of grafting |
Effect of ablation |
Effect of grafting × ablation |
|
| n | 4 | 4 | 4 | 4 | |||
| %Type I | 10 ± 2 | 13 ± 1 | 12 ± 2 | 10 ± 3 | NS | NS | NS |
| %Type IIa | 9 ± 3 | 7 ± 3 | 11 ± 2 | 5 ± 2 | NS | NS | NS |
| %Type IIb | 81 ± 5 | 80 ± 4 | 77 ± 4 | 85 ± 4 | NS | NS | NS |
| Area type I, µm2 | 2,075 ± 140 | 1,485 ± 88 | 4,000 ± 977 | 3,465 ±567d | NS | 0.009 | NS |
| Area type IIa, µm2 | 2,563 ± 136 | 2,175 ± 58 | 4,030 ± 775 | 4,037 ± 471d | NS | 0.006 | NS |
| Area type IIb µm2 | 3,616 ± 178 | 2,496 ± 105 | 6,309 ± 848c | 4,748 ± 704d | 0.049 | 0.002 | NS |
Values are average ± SE; n, no. of rats.
Nerve-repair grafted is significantly different from sham control group, P ≤ 0.05.
Synergist-ablated nerve-repair grafted is significantly different from synergist-ablated control group, P ≤ 0.05.
Synergist-ablated control is significantly different from sham control group, P ≤ 0.05.
Synergist-ablated nerve-repair grafted is significantly different from nerve-repair grafted group, P < 0.05.
DISCUSSION
In accordance with previous studies in the rat (4, 7, 11, 30), the present study on stabilized muscle grafts demonstrated a 25% decrease in specific force and a 23% decrease in maximum normalized sustained power compared with control muscle. In agreement with previous studies on synergist muscle ablation in control and revascularized grafted muscle (8, 10, 24, 25), we also observed increases muscle mass, muscle cross-sectional area, and maximum tetanic force (N) when synergist muscles were ablated. The present study extends these previous observations by assessing contractile and metabolic function nerve-repair grafting in conjunction with overload hypertrophy due to synergist muscle ablation. Although maximum tetanic force of the synergist-ablated nerve-repair grafted and synergist-ablated muscles recovered to levels equal to, or greater than, that of sham controls, specific force did not increase. In fact, specific force after synergist muscle ablation was comparable to nerve-repair grafted muscle in that both synergist ablated groups showed a 16% decrease in specific force.
Therefore, the removal of the “stress shield” provided by intact synergist muscles did not enhance recovery of specific force by nerve-repair grafted muscle in this study. Such deficits after synergist ablation in nerve-repair revascularized and vascular-intact grafts have been previously observed in muscle transfers (8, 24). This deficit in specific force is apparent in nerve-repair grafts, the mass of which is > 20% of the mass of their original muscle group (23). In contrast, if 80% of the muscle mass in a muscle group is removed, the workload on the remaining 20% is great enough to elicit full recovery of the muscle (24). The MG muscle alone represents 36% of the mass of the total plantar flexor groups. Removal of its synergist would require the MG muscle to assume the workload of the missing 64% of the muscle mass. In this study, the increased workload imposed on the synergist-ablated nerve-repair grafted muscle was not sufficient to elicit enhanced recovery of specific force or normalized power compared with nerve-repair grafting alone.
Normalized power was lower after nerve-repair grafted synergist ablation than in nerve-repair grafting alone. Normalized power depends on both specific force production during shortening and the velocity of shortening. The optimal velocity of shortening decreased 13% after synergist ablation alone and decreased an additional 9% after synergist-ablation nerve-repair grafting compared with sham controls. This decrease in the optimal velocity of shortening may, in part, explain the diminished power output after ablation. Decreases in the optimal velocity of shortening are generally explained by decreases in the percentage of fast-glycolytic (IIb) or fast-oxidative (IIa) fibers (5). The percentages of fiber types did not change. Therefore, changes in fiber-type composition cannot explain the decrease in optimal shortening velocity observed after synergist-ablation. However, we did not test for the presence of hybrid fibers. It is possible that uncharacterized slow isoforms or other fast isoforms such as IIx/d could have an impact on velocity of shortening. Further studies need to be done to investigate the alterations in the populations of hybrid fiber after synergist muscle ablation.
In contrast to the specific force and normalized power data, no deficit in MG muscle contractile function was observed with sustained power. The ability to sustain power recovered fully in the synergist-ablated nerve-repair grafted group compared with controls. GLUT-4 glucose transporter content and hexokinase activity were significantly increased by the synergist ablation procedure, suggesting that increased glucose uptake or metabolism may have played a role in the recovery of the ability of grafted muscle to sustain power. The observed increase in GLUT-1 glucose transporter in both of the grafted groups is compatible with the altered expression of glucose transporter isoforms observed in populations of denervated fibers. Skeletal muscle GLUT-1 protein and mRNA content are increased 3 days postdenervation (6, 15). Increased GLUT-1 protein content may improve the ability of muscle to recover after an injury by increasing basal glucose transport and increasing available energy for tissue repair.
Previous studies have shown that the recovery of muscle function in grafted muscle is associated with the degree of reinnervation and revascularization of the muscle after the grafting procedure (12). Incomplete revascularization cannot explain any of the observed alterations in the grafted groups in the present study, because the vasculature was left intact during the surgical procedure. On the other hand, the nerve to the MG muscle was severed and repaired during the grafting procedure, so denervation could be a contributing factor. However, in the synergist-ablated nerve-repair grafted group, there was no decline in the MG muscle’s ability to sustain power compared with that of control muscles, suggesting that if there is a population of denervated muscle fibers, they do hinder the ability of the synergist-ablated nerve-repair grafted MG muscle to sustain power.
In physiological conditions in which low oxidative capacity exists, skeletal muscle will utilize glycogen stores as the major fuel for energy (21). However, glycogen stores become rapidly depleted and are thus unsuitable for sustained contractile activity. Chronic stimulation of untrained skeletal muscle leads to a transient increase of glucose uptake and glycolysis to compensate for the rapid depletion of available glycogen stores. To compensate for the increased need for glycolysis from free glucose, the muscle transiently increases the content and activity of hexokinase (28). In this study, glycogen content was significantly decreased and hexokinase activity was increased 1.9-fold in the synergist-ablated muscle compared with sham controls, findings compatible with chronic stimulation. In addition, oxidative capacity was decreased and GLUT-4 glucose transporter protein content was significantly higher after synergist-ablated nerve-repair grafting. These data suggest that the stabilized synergist-ablated nerve-repair grafted muscle is in a state of chronic stimulation with a shift toward glycolytic metabolism and is likely relying on glucose uptake from the circulation as the major source of energy.
MG muscle mass was increased 60% in the synergist-ablated control group and 20% in the synergist-ablated nerve-repair grafted group. In contrast, protein content was 25% less in both synergist-ablated groups. These findings indicate that the increase in muscle size may, in part, be explained by an increase in water content rather than an increase in muscle contractile proteins. This increase in muscle mass without an increase in contractile proteins may, in part, explain why specific force was lower, not higher in the synergist-ablated groups because specific force is normalized for changes in muscle mass. However, further studies need to be done to ascertain the mechanism for the increase of muscle mass after ablation of synergist muscles.
In conclusion, the increased workload after ablation of synergist muscles did not enhance the recovery of specific force or normalized power in synergist-ablated nerve-repair grafted muscle. In fact, muscle hypertrophy after synergist muscle ablation alone was associated with diminished specific force and normalized power, whereas the ability to sustain power was enhanced, suggesting that metabolic and not mechanical parameters are responsible for this recovery of sustained power. Although the maximum tetanic force produced by the synergist-ablated nerve-repair grafted muscle was not significantly different from the sham control muscles, the synergist-ablated MG muscle functions as the entire plantar flexor group and in this sense the total functional force for the plantar-flexor group is greatly diminished. Furthermore, the enhanced endurance of the synergist-ablated nerve-repair grafted muscle was observed despite a decrease in energy available from glycogen stores and a likely decrease in oxidative capacity. Therefore, we conclude that recovery of grafted muscle endurance after synergist muscle ablation may be explained in part by an increase in utilization of circulating glucose during sustained power contractions. In addition, the increase in sustained power may be explained by an increase in capillary density and/or blood flow in the overloaded grafts. However, further studies need to be done to address this issue.
Acknowledgments
We thank Christina Espinoza, Stephen Fortino, Cheryl Hassett, Geoff Rezvani, and Jonathan Williams for technical assistance.
This research was supported in part by the Core Facility for Aged Rodents of the Claude Pepper Older Americans Independence Center at the University of Michigan (National Institutes on Aging Grants AG-08808, AG-00710, and AG-10821) and by the Geriatric Research, Education, and Clinical Center, Ann Arbor Department of Veterans Affairs Medical Center.
REFERENCES
- 1.Brooke MH, Kaiser KK. Muscle fiber types: how many and what kind? Arch Neurol. 1970;23:369–379. doi: 10.1001/archneur.1970.00480280083010. [DOI] [PubMed] [Google Scholar]
- 2.Brooks SV, Faulkner JA. Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol (Lond) 1988;404:71–82. doi: 10.1113/jphysiol.1988.sp017279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brooks SV, Faulkner JA. Maximum and sustained power of extensor digitorum longus muscles from young, adult, and old mice. J Gerontol. 1991;46:B28–B33. doi: 10.1093/geronj/46.1.b28. [DOI] [PubMed] [Google Scholar]
- 4.Ciske PE, Faulkner JA. Chronic electrical stimulation of nongrafted and grafted skeletal muscles in rats. J Appl Physiol. 1985;59:1434–1439. doi: 10.1152/jappl.1985.59.5.1434. [DOI] [PubMed] [Google Scholar]
- 5.Claflin DR, Faulkner JA. Shortening velocity extrapolated to zero load and unloaded shortening velocity of whole rat skeletal muscle. J Physiol (Lond) 1985;359:357–363. doi: 10.1113/jphysiol.1985.sp015589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coderre L, Monfar MM, Chen KS, Heydrick SJ, Kurowski TG, Ruderman NB, Pilch PF. Alteration in the expression of GLUT-1 and GLUT-4 protein and messenger RNA levels in denervated rat muscles. Endocrinology. 1992;131:1821–1825. doi: 10.1210/endo.131.4.1396328. [DOI] [PubMed] [Google Scholar]
- 7.Cote C, Faulkner JA. Motor unit function in skeletal muscle autografts of rats. Exp Neurol. 1984;84:292–305. doi: 10.1016/0014-4886(84)90226-7. [DOI] [PubMed] [Google Scholar]
- 8.Donovan CM, Faulkner JA. Muscle grafts overloaded by ablation of synergistic muscles. J Appl Physiol. 1986;61:288–292. doi: 10.1152/jappl.1986.61.1.288. [DOI] [PubMed] [Google Scholar]
- 9.Easterby JS, Qadri SS. Hexokinase type II from rat skeletal muscle. Methods Enzymol. 1982;90:11–15. doi: 10.1016/s0076-6879(82)90098-2. [DOI] [PubMed] [Google Scholar]
- 10.Egginton S, Hudlicka O, Brown MD, Walter H, Weiss JB, Bate A. Capillary growth in relation to blood flow and performance in overload rat skeletal muscle. J Appl Physiol. 1998;85:2025–2032. doi: 10.1152/jappl.1998.85.6.2025. [DOI] [PubMed] [Google Scholar]
- 11.Faulkner JA, Carlson BM. Contractile properties of standard and nerve-intact muscle grafts in the rat. Muscle Nerve. 1985;8:413–418. doi: 10.1002/mus.880080511. [DOI] [PubMed] [Google Scholar]
- 12.Fu SY, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: prolonged denervation. J Neurosci. 1995;15:3886–3895. doi: 10.1523/JNEUROSCI.15-05-03886.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gans C. Fiber architecture and muscle function. Exerc Sport Sci Rev. 1982;10:160–207. [PubMed] [Google Scholar]
- 14.Guelinckx PJ, Faulkner JA, Essig DA. Neurovascular-anastomosed muscle grafts in rabbits: functional deficits result from tendon repair. Muscle Nerve. 1988;11:745–751. doi: 10.1002/mus.880110710. [DOI] [PubMed] [Google Scholar]
- 15.Handberg A, Megeney LA, McCullagh KJA, Kayser L, Han XX, Bonen A. Reciprocal GLUT-1 and GLUT-4 expression and glucose transport in denervated muscles. Am J Physiol Endocrinol Metab. 1996;271:E50–E57. doi: 10.1152/ajpendo.1996.271.1.E50. [DOI] [PubMed] [Google Scholar]
- 16.Hassid WZ, Abraham S. Chemical procedures of analysis of polysaccharides. Methods Enzymol. 1957;1:34–50. [Google Scholar]
- 17.Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol. 1984;56:831–838. doi: 10.1152/jappl.1984.56.4.831. [DOI] [PubMed] [Google Scholar]
- 18.Larkin LM, Faulkner JA, Hinkle RT, Hassett CA, Supiano MA, Halter JB. Functional deficits in medial gastrocnemius grafts in rats: relation to muscle metabolism and β-AR regulation. J Appl Physiol. 1997;83:67–73. doi: 10.1152/jappl.1997.83.1.67. [DOI] [PubMed] [Google Scholar]
- 19.Manktelow RT, Zuker RM. In: Extremity Reconstruction With Functioning Muscle Transplantation—Factors Affecting Functional Return. Freilinger G, Dutinger M, editors. Vienna, Austria: Blackwell-MZV; 1992. pp. 234–243. (Third Vienna Muscle Symp.) [Google Scholar]
- 20.Markley JM, Faulkner JA, Niemeyer JH, White TP. Functional properties of palmaris longus muscles of rhesus monkeys transplanted as index finger flexors. Plast Reconstr Surg. 1985;76:574–577. doi: 10.1097/00006534-198510000-00017. [DOI] [PubMed] [Google Scholar]
- 21.Max SR. Disuse atrophy of skeletal muscle: loss of functional activity of mitochondria. Biochem Biophys Res Commun. 1972;36:1394–1398. doi: 10.1016/s0006-291x(72)80130-x. [DOI] [PubMed] [Google Scholar]
- 22.Mendez J, Keys A. Density and composition of mammalian muscle. Metabolism. 1960;9:184–188. [Google Scholar]
- 23.Miller SW, Hassett CA, Faulkner JA. Recovery of muscle transfers replacing the total plantar flexor muscle group in rats. J Appl Physiol. 1998;84:1865–1871. doi: 10.1152/jappl.1998.84.6.1865. [DOI] [PubMed] [Google Scholar]
- 24.Miller SW, Opiteck JA, White TP, Faulkner JA. Functional evaluation at the medial gastrocnemius donor site in rats. J Reconstr Microsurg. 1996;12:143–147. doi: 10.1055/s-2007-1006467. [DOI] [PubMed] [Google Scholar]
- 25.Roy RR, Baldwin KM, Martin TP, Chimarusti SP, Edgerton VR. Biochemical and physiological changes in overload rat fast- and slow-twitch ankle extensors. J Appl Physiol. 1985;59:639–646. doi: 10.1152/jappl.1985.59.2.639. [DOI] [PubMed] [Google Scholar]
- 26.Segal SS, White TP, Faulkner JA. Architecture, composition, and contractile properties of rat soleus muscle grafts. Am J Physiol Cell Physiol. 1986;250:C474–C479. doi: 10.1152/ajpcell.1986.250.3.C474. [DOI] [PubMed] [Google Scholar]
- 27.Serre PA. Citrate synthase. In: Lowenstein JM, editor. Methods in Enzymology. vol. 13. New York: Academic; 1969. pp. 3–11. [Google Scholar]
- 28.Weber FE, Pette D. Changes in free and bound forms and total amount of hexokinase isozyme II of rat muscle in response to contractile activity. Eur J Biochem. 1990;191:85–90. doi: 10.1111/j.1432-1033.1990.tb19096.x. [DOI] [PubMed] [Google Scholar]
- 29.White TP, Villanacci JF, Morales PG, Segal SS, Essig DA. Exercise-induced adaptations of rat soleus muscle grafts. J Appl Physiol. 1984;56:1325–1334. doi: 10.1152/jappl.1984.56.5.1325. [DOI] [PubMed] [Google Scholar]
- 30.Yoshimura K, Asato H, Cederna PS, Urbanchek MG, Kuzon JWM. The effect of reinnervation on force production and power output in skeletal muscle. J Surg Res. 1999;81:201–208. doi: 10.1006/jsre.1998.5498. [DOI] [PubMed] [Google Scholar]