Abstract
For the first time, relationships among maternal buprenorphine dose, meconium buprenorphine and metabolite concentrations, and neonatal outcomes are reported. Free and total buprenorphine and norbuprenorphine, nicotine, opiates, cocaine, benzodiazepines, and metabolites were quantified in meconium from 10 infants born to women who had received buprenorphine during pregnancy. Neither cumulative nor total third-trimester maternal buprenorphine dose predicted meconium concentrations or neonatal outcomes. Total buprenorphine meconium concentrations and buprenorphine/norbuprenorphine ratios were significantly related to neonatal abstinence syndrome (NAS ) scores >4. As free buprenorphine concentration and percentage free buprenorphine increased, head circumference decreased. Thrice-weekly urine tests for opiates, cocaine, and benzodiazepines and self-reported smoking data from the mother were compared with data from analysis of the meconium to estimate in utero exposure. Time of last drug use and frequency of use during the third trimester were important factors associated with drug-positive meconium specimens. The results suggest that buprenorphine and metabolite concentrations in the meconium may predict the onset and frequency of NAS.
Buprenorphine is the first prescription drug approved under the 2000 US Drug Addiction Treatment Act for office-based treatment of addiction to narcotics.1 Although methadone is the only recommended medication to treat opioid dependence during pregnancy, buprenorphine is being investigated for this purpose.2,3 Infants exposed in utero to methadone or buprenorphine may display neonatal abstinence syndrome (NAS) shortly after birth, although the quality and severity may differ.2 NAS is a generalized central nervous system disorder characterized by hyperirritability, gastrointestinal dysfunction, respiratory distress, and autonomic symptoms. 4 The Finnegan Scale assesses 21 of the most common signs of neonatal drug withdrawal syndrome5 and is scored on the basis of pathological significance and severity of the adverse symptoms, which sometimes require pharmacological treatment.6
Methadone and buprenorphine demonstrated equivalent safety and effectiveness for treatment of opiate-dependent pregnant women and their infants.2,3,7,8 In the first double-blind, double-dummy study that compared buprenorphine with methadone, similar NAS medication requirements and peak scores, and significantly fewer days in the hospital for buprenorphine-exposed infants were reported.2 Neonatal meconium specimens were also collected to monitor prenatal drug exposure. Meconium analysis detects more drug use than maternal or infant urine.9 Meconium begins forming at ~12 weeks of gestation and is excreted in the first bowel movements. Drugs in the meconium arise from ingestion of amniotic fluid and from bile.10 Meconium is not usually excreted in utero, and therefore meconium drug concentrations are thought to represent cumulative exposure from ~12 weeks onward during gestation.
Previous studies indicated that maternal buprenorphine dose does not predict the severity or duration of NAS, and therefore higher doses could be administered during pregnancy to control withdrawal symptoms and prevent relapse.3,8,11 Data continue to be collected on the fetal effects of in utero buprenorphine exposure as opioid treatment of pregnant women increases globally.
Evaluation of the disposition of illicit drugs in pregnant women and fetuses is difficult because, for ethical and safety reasons, controlled-administration studies using such drugs would never be performed. Daily administration of buprenorphine to pregnant opioid-dependent women participating in a research study comparing buprenorphine and methadone pharmacotherapies allowed the first opportunity to model the disposition of buprenorphine and its metabolites in meconium. The principal aims of this study were to characterize the relationships among maternal buprenorphine doses, meconium buprenorphine and metabolite concentrations, and neonatal outcomes. Self-reported tobacco use and thrice-weekly urine tests during the study allowed monitoring of the frequency and magnitude of heroin, cocaine, and nicotine use during gestation. The secondary aim of this study was to utilize urine test results to investigate the factors that influence the incorporation of these illicit drugs into the meconium. The frequency and timing of positive urine tests for drugs were compared between women whose infants had drug-positive meconium specimens and those whose infants had drug-negative meconium.
RESULTS
Participant demographics and dosing information
Of the nine women (eight African American, one Caucasian), who were buprenorphine-maintained during gestation, one gave birth to twins, making a total of 10 infants in this group. The mean ± SE of maternal age was 30.0 ± 1.1 years (range: 22–32 years), and the estimated gestational ages at admission were 18–26 weeks (mean: 22.8 ± 1.2). The participants were not employed outside their homes and had 10.3 ± 0.4 years (range: 8–12 years) of education. All participants were opioid dependent per the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition criteria, with cocaine use more than four times per day, heroin use more than four times per day, and nicotine use in the 30 days prior to admission being reported by 88.9, 55.6, and 77.8% of participants, respectively. Three women reported using alcohol for 2.3 ± 1.3 days (range: 1–5 days) in the preceding 30 days, and one was positive for hepatitis C. Table 1 summarizes the buprenorphine dosing data of the participants and study enrollment durations at delivery of the infant.
Table 1.
Maternal buprenorphine dosing and neonatal outcome measures
| subject | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | I | Mean ± SE | Range | ||
| Days in study at delivery | 87 | 106 | 120 | 92 | 118 | 152 | 111 | 151 | 98 | 115.0 ± 7.8 | 87–152 | |
| Buprenorphine dosing | ||||||||||||
| Dose at delivery (mg/day) | 20 | 16 | 24 | 18 | 24 | 16 | 18 | 18 | 14 | 18.7 ± 1.2 | 14–24 | |
| Mean daily dose (mg/day) | 15.6 | 14.4 | 18.3 | 15.9 | 18.3 | 11 | 15.9 | 12.3 | 13.6 | 15.0 ± 0.8 | 11.0–18.3 | |
| Average third trimester daily dose (mg/day) | 16.2 | 14.8 | 21.0 | 16.9 | 21.8 | 10.4 | 17.1 | 13.3 | 13.7 | 16.1 ± 1.2 | 10.4–21.8 | |
| Cumulative dose (mg) | 1,354 | 1,526 | 2,196 | 1,464 | 2,160 | 1,674 | 1,760 | 1,850 | 1,330 | 1,701.6 ± 107.2 | 1,330–2,196 | |
| Cumulative third trimester dose (mg) | 1,150 | 1,364 | 1,344 | 1,084 | 1,396 | 888 | 1,330 | 1,132 | 1,162 | 1,205.6 ± 55.5 | 888–1396 | |
| Neonatal outcome measures | Baby 1 | Baby 2 | ||||||||||
| Gender | F | F | M | M | M | F | F | F | M | F | ||
| 1-min Apgar score | 8 | 8 | 9 | 8 | 9 | 8 | 8 | 8 | 7 | 8 | 8.1 ± 0.2 | 7–9 |
| 5-min Apgar score | 9 | 8 | 8 | 9 | 9 | 9 | 9 | 9 | 9 | 8 | 8.7 ± 0.2 | 8–9 |
| Gestational age at delivery (weeks) | 38 | 41 | 37 | 37 | 37 | 37 | 40 | 39 | 40 | 40 | 38.6 ± 0.5 | 37–41 |
| Birth weight (g) | 3,185 | 4,340 | 3,170 | 2,740 | 2,730 | 2,890 | 2,985 | 3,525 | 3,355 | 3,560 | 3,248.0 ± 161.5 | 2,730–4,340 |
| Head circumference (cm) | 34 | 37 | 34 | 33 | 34 | 31 | 34 | 35 | 38 | 33 | 34.2 ± 0.6 | 31–38 |
| Length (cm) | 51 | 58 | 50 | 49 | 48 | 48 | 48 | 53 | 52 | 50 | 50.7 ± 1.0 | 48–58 |
| Length of hospital stay (days) | 9 | 11 | 4 | 5 | 5 | 8 | 4 | 4 | 4 | 4 | 5.8 ± 0.9 | 4–11 |
| Treated for NAS | Y | Y | N | N | N | N | N | N | N | N | ||
| Time to onset (h) | 56 | 89 | 18 | 34 | 19 | 29 | 56 | —a | 205 | 3 | 56.6 ± 19.4 | 3–205 |
| Peak NAS score | 13 | 12 | 7 | 6 | 8 | 9 | 7 | 3 | 5 | 7 | 7.7 ± 1.0 | 3–13 |
| Time-to-peak NAS score (h) | 128 | 118 | 82 | 34 | 122 | 167 | 56 | 78 | 205 | 45 | 97.9 ± 18.7 | 28–204 |
| Duration (h) | 149 | 68.5 | 66 | 110 | 149 | 208 | 150 | 0 | 6.5 | 110 | 113.1 ± 19.9 | 7–208 |
| % Of scores >4 | 36 | 27 | 37 | 14 | 24 | 29 | 12 | 0 | 3.6 | 58 | 24.1 ± 5.8 | 0–58 |
NAS, neonatal abstinence syndrome.
NAS score never exceeded 4.
Distribution of drugs and metabolites in meconium
There was wide intersubject variation in the concentrations of drugs in meconium (Table 2), with one specimen (F) containing a total of only 24 ng/g BUP and free buprenorphine below limits of quantification (LOQ). This was despite the mother having received a total of 1,674 mg of buprenorphine during her pregnancy, of which 888 mg were received in the third trimester. Mean free and total norbuprenorphine concentrations exceeded mean free and total buprenorphine concentrations. A matched-pair t-test of total and free buprenorphine concentrations indicated a statistically significant higher concentration of total buprenorphine as compared to free buprenorphine (mean difference = 49 ± 10 ng/g, n = 9 pairs, t = 4.788, 8 degrees of freedom, P = 0.001). The total norbuprenorphine quantified in three of the specimens was less than the respective free concentrations, but the results were within ±20%. Another explanation could be the difficulty of completely homogenizing the meconium despite mixing for 10 min using mortar and pestle. Four of the 10 specimens had >99% free norbuprenorphine and the other 6 had 53–89% free norbuprenorphine. There were no statistically significant differences between total and free norbuprenorphine concentrations (mean difference = 143 ± 78 ng/g, t = 1.840, 9 degrees of freedom, P = 0.099), mean free buprenorphine/free-norbuprenorphine ratio (0.14 ± 0.02 ng/g), and mean total buprenorphine/total norbuprenorphine ratio (0.18 ± 0.03 ng/g) (P = 0.37).
Table 2.
Meconium buprenorphine and metabolite concentrations
| subject | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D (Baby 1) | D (Baby 2) | E | F | G | H | I | Mean ± sea | Median | Range | |
| Free BUP ng/g | 101.6 | 47.4 | 101.0 | 59.3 | 59.6 | 240.5 | NDb | 23.9 | 40.4 | 165.4 | 93.2 ± 22.1 | 59.6 | 23.9–240.5 |
| Total BUP ng/g | 123.8 | 95.9 | 131.3 | 96 | 169.6 | 296.8 | 24.3 | 32.1 | 91.6 | 245.9 | 130.7 ± 27.4 | 109.9 | 24.3–296.8 |
| % Free BUP | 82.0 | 49.4 | 76.9 | 61.7 | 35.1 | 81.0 | —c | 74.4 | 44.1 | 67.3 | 63.6 ± 5.7 | 67.3 | 35.1–82.0 |
| Free NBUP ng/g | 712.6 | 497.5 | 398.2 | 468.4 | 373.4 | 1228.6 | 331.1 | 730.6 | 505.4 | 858.4 | 610.4 ± 87.7 | 501.5 | 331.1–1228.5 |
| Total NBUP ng/g | 719.3 | 689.2 | 447.5 | 548.1 | 607.6 | 1880.2 | 323.9 | 629.9 | 955.6 | 737.1 | 753.8 ± 136.3 | 659.5 | 323.9–1880.2 |
| % Free NBUP | 99.1 | 72.2 | 89.0 | 85.5 | 61.5 | 65.3 | 102.2 | 116.0 | 52.9 | 116.5 | 86.0 ± 7.1 | 87.2 | 52.9–116.5 |
| Free BUP/Free NBUP | 0.14 | 0.10 | 0.25 | 0.13 | 0.16 | 0.20 | — | 0.03 | 0.08 | 0.19 | 0.14 ± 0.02 | 0.14 | 0.03–0.25 |
| Total BUP/Total NBUP | 0.17 | 0.14 | 0.29 | 0.18 | 0.28 | 0.16 | 0.08 | 0.05 | 0.10 | 0.33 | 0.18 ± 0.03 | 0.17 | 0.05–0.33 |
BUP, buprenorphine; NBUP, norbuprenorphine.
Mean ± SEM of positive specimens.
Not detected.
Cannot be calculated.
Nicotine and four of its metabolites were identified in 50% of the meconium specimens (Table 3), and two other specimens were positive for all analytes except norcotinine. One specimen with hydroxycotinine and nicotine levels higher than the method’s upper LOQ could not be reanalyzed because no additional specimen was available. For two of three self-reported nonsmokers, the meconium samples of the infants were negative for nicotine and metabolites. Participant F did not admit to having smoked tobacco, but hospital staff observed that she did; nicotine and all metabolites were identified in her infant’s meconium.
Table 3.
Self-reported maternal tobacco smoking data, maternal urine test results during gestation, and infant nicotine, cocaine, opiate, and metabolites concentrations in meconium collected at birth
| subject | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D (Baby 1) |
D (Baby 2) |
E | F | G | H | I | Meana ± SE | Median | Range | |
| Nicotine | |||||||||||||
| Addiction severity interview: self-reported maternal tobacco smoking, last interview before delivery | |||||||||||||
| Days smoked cigarettes in past 30 days | 30 | 10 | 30 | 0 | 0 | 30 | 0 | 30 | 0 | 30 | 16.0 ± 6.1 | 20 | 0.0–30.0 |
| Number of cigarettes smoked per day | 10 | 2 | 10 | 0 | 0 | 3 | 0 | 20 | 0 | 10 | 5.5 ± 2.8 | 2.5 | 0.0 –20.0 |
| Nicotine biomarkers in meconium (ng/g)b | |||||||||||||
| Nicotine | 198.7 | 35.9 | 19.5 | —c | — | 79.1 | 403.8 | 46.6 | — | >ULOQd | 130.6 ± 60.6 | 62.9 | 19.5–403.8 |
| Nornicotine | 21.3 | 6.1 | 7.56 | — | — | 10.4 | 24.43 | 8.2 | — | 36.4 | 16.3 ± 4.3 | 10.4 | 6.1–36.4 |
| Cotinine | 241.0 | 55.0 | 48.0 | — | — | 97.5 | 259.2 | 80.9 | — | 420.6 | 171.7 ± 52.8 | 97.5 | 48.0–420.6 |
| Norcotinine | 4.3 | 1.8 | — | — | — | — | 5.9 | 1.6 | — | 6.6 | 4.0 ± 1.0 | 4.3 | 1.6–6.6 |
| OH-cotinine | 75.2 | 100.7 | 155.4 | — | — | 206.2 | 159.5 | 151.4 | — | >ULOQ | 141.4 ± 19.0 | 153.4 | 75.2–206.2 |
| Cocaine | |||||||||||||
| Maternal cocaine-positive urine tests | |||||||||||||
| % Positive tests, enrollment to birth | 41.2 | 2.2 | 0.0 | 32.5 | 32.5 | 35.3 | 0.0 | 2.1 | 23.8 | 0.0 | 17.3 ± 6.5 | 14.55 | 0.0–41.2 |
| % Positive tests, third trimester | 46.4 | 0.0 | 0.0 | 40.7 | 40.7 | 11.1 | 0.0 | 0.0 | 27.0 | 0.0 | 16.4 ± 8.8 | 5.55 | 0.0–46.4 |
| Days from last positive urine specimen to birth | 4 | 102 | N/Ae | 8 | 8 | 43 | N/A | 82 | 54 | N/A | 42.6 ± 14.6 | 43 | 3.0–101.0 |
| Cocaine biomarkers in meconium (ng/g)f | |||||||||||||
| Cocaine | 71 | — | — | — | 11 | — | — | — | — | — | 41.0 ± 30.0 | 41 | 11.0–71.0 |
| Cocaethylene | — | — | — | — | — | — | — | — | — | — | |||
| Benzoylecgonine | 211 | — | — | 5 | 10 | — | — | — | — | — | 75.3 ± 67.8 | 10 | 5.0–211.0 |
| m-OH-benzoylecgonine | 1,263 | — | — | 124 | 122 | — | — | — | — | 10 | 379.8 ± 295.6 | 123 | 10.0–1,263.0 |
| Cumulative cocaine and metabolites | 1,545 | – | – | 129 | 143 | – | – | – | – | 10 | 456.8 ± 364.0 | 136 | 10.0–1,545.0 |
| Opiate | |||||||||||||
| Maternal opiate positive urine tests | |||||||||||||
| % Positive tests, enrollment to birth | 26.5 | 5.4 | 3.9 | 75.0 | 75.0 | 45.1 | 4.7 | 8.5 | 17.5 | 5.0 | 26.7 ± 9.0 | 13 | 3.9–75.0 |
| % Positive tests, third trimester | 20.7 | 0.0 | 0.0 | 74.1 | 74.1 | 14.8 | 0.0 | 12.5 | 16.2 | 0.0 | 21.2 ± 11.8 | 13.65 | 0.0–74.1 |
| Days from last positive urine specimen to birth | 4 | 102 | 117 | 7 | 7 | 55 | 146 | 30 | 56 | 93 | 61.7 ± 16.0 | 55.5 | 4.0–146.0 |
| Opiate biomarkers in meconium (ng/g)f | |||||||||||||
| Codeine | — | — | — | — | — | — | — | — | — | — | |||
| Morphine | 891 | — | — | 1,163 | 1,185 | — | — | — | — | — | 1,079.7 ± 94.5 | 1,163 | 891.0–1,185.0 |
| Hydrocodone | — | — | — | — | — | — | — | — | — | – | |||
| Hydromorphone | — | — | — | — | — | — | — | – | – | – | |||
| Oxycodone | — | — | — | — | — | — | — | — | – | – | |||
LOQ, limit of quantification; N/A, not applicable; ULOQ, upper LOQ.
Mean of meconium specimen concentrations within the analytical range of the method. Zero values included in mean calculations for days cigarettes smoked in past 30 days, cigarettes smoked per day, % positive urine specimens and days from last positive urine specimen until birth.
LOQ in meconium 5 ng/g for nicotine and nornicotine, 1.25 ng/g for cotinine, norcotinine and 3-OH-trans-cotinine.
Less than LOQ.
Greater than the ULOQ (500 ng/g).
Not applicable.
LOQ in meconium 5 ng/g for all cocaine and opiate biomarkers.
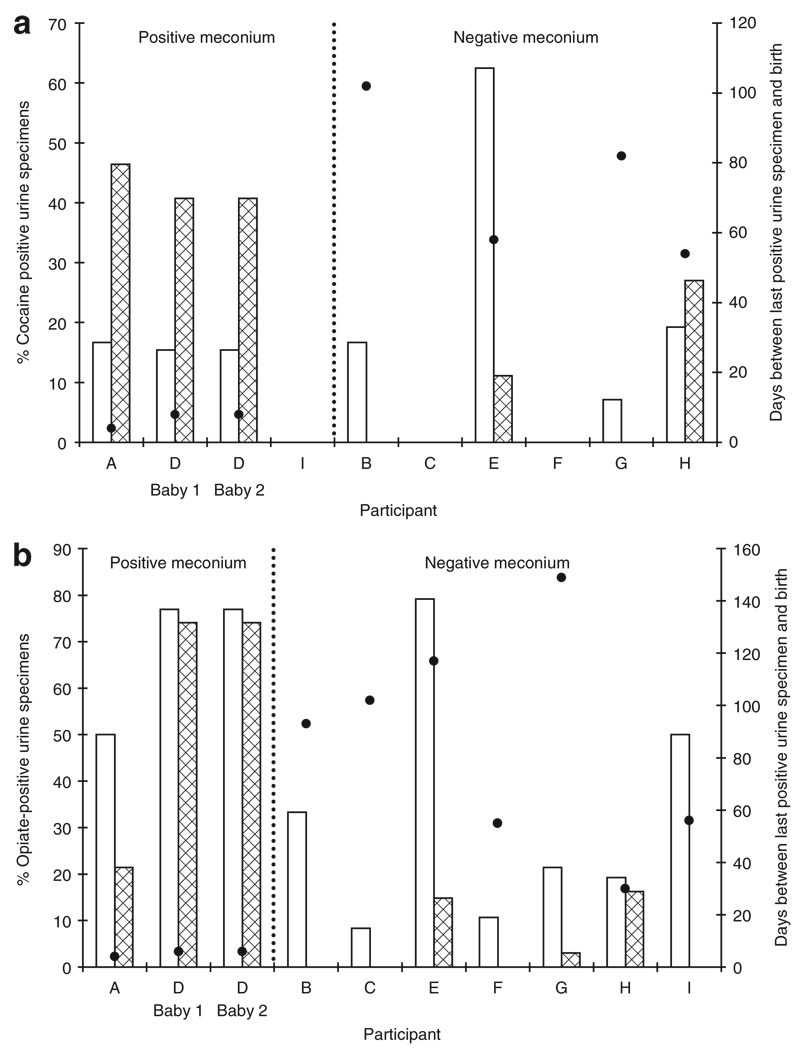
Cocaine and opiate concentrations in maternal urine samples and nicotine, cocaine, opiate, and metabolite concentrations in meconium samples are shown in Table 3. Participant E had eight benzodiazepine-positive urine specimens in the third trimester, the last urine specimen being 44 days before delivery. Her infant had a positive meconium specimen, with 69 ng/g oxazepam. For neonates B and G there was insufficient meconium for oxazepam quantification. The percentages of cocaine- and opiate-positive urine tests during the second and third trimesters, positive and negative cocaine and opiate tests in neonate meconium samples, and number of days between last positive maternal urine specimen and delivery are depicted in Figure 1. There were no statistically significant relationships between the meconium status of the neonates (positive or negative) and either the percentage of cocaine-positive maternal urine tests in the second or third trimester or the percentage of opiate-positive maternal urine tests in the second trimester. However, women with a higher percentage of opiate-positive urine specimens in the third trimester gave birth to infants with significantly higher rates of opiate-positive meconium specimens (P = 0.02). The time elapsed between the last positive maternal urine specimen and delivery was also analyzed. There was a statistically significant relationship between the time of last opiate-positive maternal urine test and opiate-positive meconium of the infant (P = 0.02). There was also a trend toward significance of association between time of the last cocaine-positive maternal urine test and meconium concentration of the drug (P = 0.06).
Figure 1.
Percentage of (a) cocaine-positive and (b) opiate-positive urine specimens in the second (open bars) and third (shaded bars) trimesters and days between last positive urine specimen and delivery (closed circles) among mothers of infants with positive or negative meconium specimens.
Infant birth characteristics and NAS
Table 1 contains birth and NAS characteristics of the infants. The infants (6 female, 4 male) were all delivered full-term and no infant was considered to be of low birth weight (<2,500 g).12 Two infants were treated for NAS: infant A for 20 days, with a total of 28 morphine sulfate drops; and infant B for 14 days, with 19 drops. No significant differences were detected in buprenorphine and metabolite concentrations and NAS across gender. Given that weight, length, and head circumference can be altered by twin status, correlation analyses were performed without data from the single set of twins in the group. There were no significant relationships between maternal buprenorphine dose and neonatal growth outcomes.
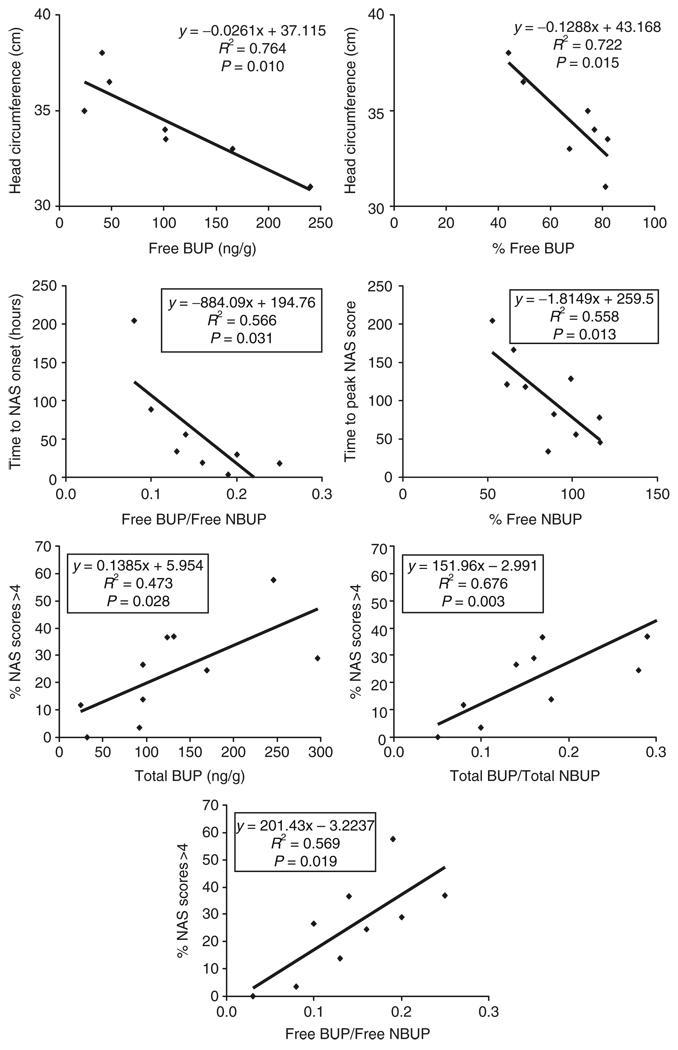
Statistically significant negative correlations existed between free and percentage of free buprenorphine concentrations in meconium and head circumference of the neonate (Figure 2). In addition, there was a negative correlation between free buprenorphine/free norbuprenorphine ratio and time to NAS onset and also between percentage of free norbuprenorphine and time to peak NAS score. Total buprenorphine, free buprenorphine/free norbuprenorphine ratio, and total buprenorphine/total norbuprenorphine ratio were all significantly correlated with the percentage of NAS scores >4.
Figure 2.
Significant correlations between buprenorphine and metabolite concentrations in meconium and neonatal outcome measures. BUP, buprenorphine; NAS, neonatal abstinence syndrome; NBUP, norbuprenorphine.
Because of the small specimen size (N = 10), trends in positive and negative correlations are also reported. Trends toward positive correlation included those between free buprenorphine concentrations and NAS duration (P = 0.06) and between total norbuprenorphine concentrations and time to peak NAS score (P = 0.05). Also, free buprenorphine concentrations tended to predict (P = 0.07) the percentage of NAS scores >4. A trend toward negative correlation between total buprenorphine/total norbuprenorphine ratio and NAS onset was also noted (P = 0.05). Finally, with the twins excluded, there were trends toward negative correlations between free norbuprenorphine concentration and head circumference (P = 0.8) and between free buprenorphine concentration and infant length (P = 0.06). No correlations or trends toward correlations were found when comparing nicotine and metabolite concentrations in meconium with neonatal outcomes.
DISCUSSION
These are the first data linking maternal buprenorphine dose, buprenorphine and metabolite concentrations in meconium, and neonatal outcomes in a cohort of women who received controlled dosing with buprenorphine. In addition, to our knowledge this is the first comparison of neonatal meconium with maternal urine data collected three times a week throughout gestation to estimate frequency and/or magnitude of illicit drug use. Evaluation of maternal buprenorphine dose and neonatal outcomes provided valuable information on buprenorphine safety in treating opioid dependence during pregnancy, and the inclusion of meconium analysis yielded the first data on the disposition of buprenorphine and metabolites disposition in this neonatal biological matrix. The ability of these meconium biomarker concentrations to predict many neonatal outcomes was also evaluated for the first time.
There was no relationship between the total or cumulative third trimester dose of buprenorphine, or dose at delivery, and free or total buprenorphine or norbuprenorphine concentrations in the meconium of the neonate. This is the first study to examine the relationship between maternal buprenorphine dose and buprenorphine and metabolite concentrations in the meconium of the neonate; previous research has evaluated this relationship for methadone and cocaine. A positive relationship was reported between maternal methadone dose and methadone concentrations in the meconium.13 Ostrea et al. reported a statistically significant relationship between maternal cocaine dose and cocaine concentrations in meconium in rats.14
Buprenorphine, norbuprenorphine, and glucuronide conjugates were identified in meconium specimens, indicating passage of analytes across the placenta and/or creation through placental or fetal metabolism. Factors affecting the distribution of buprenorphine and its metabolites to the fetus include maternal plasma concentration, placental transfer and metabolism, and fetal metabolism. Nanovskaya et al. reported <10% buprenorphine transfer to the fetus, and ~5% metabolized to norbuprenorphine by the placenta.15 Low placental permeability to glucuronidated compounds was documented.16 There are no data available on phase II placental buprenorphine metabolism; however, enzymes capable of buprenorphine glucuronidation were identified in first trimester as well as full-term placentas.17–19
Variability in fetal metabolism may also influence buprenorphine and metabolite concentrations in meconium. CYP3A4 and CYP2C8 account for 95% of the metabolism of buprenorphine to norbuprenorphine, although CYP3A5, CYP3A7, CYP2C18, and CYP2C19 also contribute to the process.20,21 CYP3A7 is the principal fetal hepatic enzyme, with CYP3A7 and CYP3A5 expression becoming evident as early as 42 days after fertilization.22 CYP3A7 expression decreases during gestation, and hepatic metabolism shifts primarily to CYP3A4 within days of birth.22,23 There are also large interindividual variations in CYP expression, and the data available on fetal hepatic uridine diphospho–glucuronosyltransferase expression and activity are limited. Significant hepatic morphine glucuronidation was shown to occur in fetal baboon liver, but the glucuronidation of other xenobiotics was limited.23,24 Sulfation also appears to be more prevalent in fetal liver.23
Buprenorphine and norbuprenorphine concentrations in meconium taken from 16 infants whose mothers had been exposed to 1–16 mg/day buprenorphine during gestation have been popiureviously reported.25 Data were obtained from buprenorphine assays for urine, serum, or plasma, with no details relating to analysis of meconium and no mention of whether the data represented free or total concentrations. After maternal doses of 1–16 mg/day for variable time periods during pregnancy, buprenorphine and norbuprenorphine concentrations in a single infant were 107 and 295 ng/g, respectively,25 and mean concentrations for 15 others were 122 and 176 ng/g, respectively. 26 These data relating to buprenorphine concentrations in meconium are similar to those in this study, whereas the norbuprenorphine concentrations reported were much lower. In this study, meconium was analyzed with and without hydrolysis, allowing estimation of the extent of conjugation. Glusulase hydrolysis frees both glucuronide and sulfate conjugates.27
Drug analysis in meconium samples is an alternative to maternal self-report and maternal urine testing for monitoring the use of illicit drugs during pregnancy. Maternal self-report underestimated in utero drug exposure by up to 44% when compared with data from meconium analyses.28,29 Two previous studies reported higher sensitivity for meconium data than for maternal urine data for identifying in utero cocaine exposure; one investigation showed equal sensitivity.30–32 In this study, the thrice-weekly analysis of maternal urine specimens followed by analysis of infant meconium permitted a comparison of efficacies in documenting cocaine, benzodiazepine, and opiate use during pregnancy. These are the first data on timing and frequency of illicit drug use, as monitored by urine testing in the mothers and detection in neonate meconium. Meconium begins to form at ~12–13 weeks of gestation, and this would suggest that meconium drug concentrations are cumulative until birth. Our data suggest that drug use during the third trimester is more likely to be reflected in meconium than is second-trimester use. Meconium testing failed to identify four infants born to women who used cocaine in the second trimester; three of the women had >15% cocaine-positive urine specimens. All participants had opiate-positive urine specimens in the second trimester because morphine was administered at enrollment to transfer the participants onto treatment medication; however, only three infants had opiate-positive meconium specimens. These findings are supported by maternal methamphetamine self-report data,33 where detection rates were 68% when women reported third-trimester use, and ≤10% with only first or second-trimester exposure. Our data show that time of last drug use, as documented by a positive urine test, is another important factor influencing incorporation of the drug in meconium. It is possible that meconium can be contaminated by infant urine, although only cocaine or opiate use within ~72 hours of birth would be reflected.
Our research results support previous findings that there is no relationship between maternal mean daily buprenorphine dose, dose in mg/kg, or total dose and NAS intensity or total number of infants requiring treatment for NAS.11 In a second investigation comparing methadone with buprenorphine, no association was observed between mean dose of medication at delivery and NAS intensity.3 The largest study to date, of 159 women treated with 0.4–24 mg/day of buprenorphine, showed no significant interaction of dose with NAS intensity.8 Together, these data suggest that up to 24 mg/day of buprenorphine can be safely administered to the mother without increasing the risk of NAS in the infant.
These data are the first from a prospective clinical study to evaluate the value of meconium buprenorphine and metabolite concentrations in predicting neonatal outcomes. Although there is no apparent relationship between maternal buprenorphine dose and neonatal NAS, meconium concentrations reflect infant exposure, taking into account differences in total metabolism and placental transfer, and may therefore provide a better neonatal outcome predictor than the maternal drug dose. The research was limited by its small sample size, but preliminary data suggest that several relationships should be further investigated. Higher levels of buprenorphine concentration and percentage of free buprenorphine in meconium were associated with lower head circumference in the infant. In our study, women received prenatal care and a wide variety of support measures that helped control for socioeconomic and psychosocial factors that negatively affect fetal growth. However, 78% of the women smoked tobacco and 67% used cocaine during pregnancy; both of these are associated with lower head circumference in infants.34–36 Therefore, additional research is needed in a larger sample to confirm the effect of maternal exposure to buprenorphine on the infant head circumference.
The retrospective study by Marquet et al., discussed earlier, compared buprenorphine and norbuprenorphine concentrations in the meconium of infants who experienced withdrawal (n = 9) with those in infants who did not experience withdrawal (n = 6).26 The meconium of infants who experienced withdrawal tended to have higher buprenorphine concentrations than those who did not (P = 0.53), but no relationship was noted for norbuprenorphine concentrations (P = 0.13). In this study, a negative correlation was observed between the percentage of free norbuprenorphine in meconium and the time-to-peak NAS score. In addition, as the free buprenorphine/free norbuprenorphine ratio increased, time to NAS onset decreased. These data suggest that increased prenatal drug exposure may exceed placental and fetal phase I and phase II metabolism capacity. There was a strong relationship between total buprenorphine concentrations and buprenorphine/norbuprenorphine ratios (free and total) and the percentage of NAS scores >4. Increased buprenorphine exposure may increase the frequency of occurrence of NAS symptoms as a result of the fetus’ inability to effectively metabolize buprenorphine to less active compounds. Measurement of free buprenorphine and free norbuprenorphine may allow clinicians to predict the onset and severity of NAS.
The labor-intensive and costly hydrolysis step required for quantifying total drug did not improve predictive value. Although one infant’s buprenorphine concentrations in meconium were below the LOQ, an analysis of free norbuprenorphine documented buprenorphine exposure. The measurement of free drug concentration alone enables laboratories to report results more rapidly, with savings in cost.
Only limited data are available on the effects of maternal exposure to nicotine, either alone or in combination with other drugs, on NAS in infants. Infants with no NAS or only mild NAS were born to women who reported smoking fewer cigarettes, whereas the mothers of infants with moderate NAS reported higher levels of tobacco use.11 Infants of opioid-dependent women receiving methadone had significantly lower peak NAS scores (P = 0.014) and significantly shorter (P = 0.016) time-to-peak scores when mothers smoked ≤10 cigarettes/day (n = 16) as compared to ≥20 cigarettes/day.37 The protocol of this study provided for analysis of nicotine concentrations in the meconium of infants in relation to self-reported cigarette use in the mothers. No relationship was found between nicotine concentrations in meconium and neonatal outcomes. The application of a 10 ng/g cutoff for nicotine, cotinine, or OH-cotinine in meconium to define maternal tobacco smoking was able to identify the self-reported smokers. It also identified one participant who was observed smoking, although she denied use.
There are several limitations to this study. The small sample size and large number of statistical comparisons limit the robustness of the results; however, these data are among the first to examine the associations among maternal urine test results throughout gestation, drug concentrations in neonatal meconium, and neonatal outcomes to identify the important factors responsible for producing positive meconium test results. Also, although there was a 10-week variability in the duration of study enrollment, all of the women participated throughout their third trimesters. Similar results were obtained for evaluations of total cumulative dose and third-trimester dose. Also, there was no relationship between duration of study enrollment and drug concentrations in meconium or neonatal outcomes. Concomitant drug use is a confounder that must be considered when interpreting outcome measures.
Despite its limitations, this comprehensive treatment program allowed close monitoring of study compliance and illicit drug use. Thereby, accurate and precise information on buprenorphine doses received throughout gestation and in the third trimester and consistent evaluation of NAS for 10–14 days after birth were available. The lack of correlation between maternal buprenorphine dose and neonatal outcomes highlights the safety of buprenorphine pharmacotherapy during pregnancy. If buprenorphine and metabolite concentrations in meconium prove to accurately predict the onset and frequency of occurrence of NAS symptoms, clinicians could better identify and treat neonates with negative sequelae. Comparison with maternal urine tests suggests that meconium may not accurately identify infants exposed to drugs in utero in the second trimester, a finding with implications for prenatal drug exposure diagnosis and research. Moreover, these data suggest that measurement of free buprenorphine concentrations alone should be sufficient, thereby eliminating the need for costly and labor-intensive analysis of total drug concentrations in meconium.
METHODS
Participants
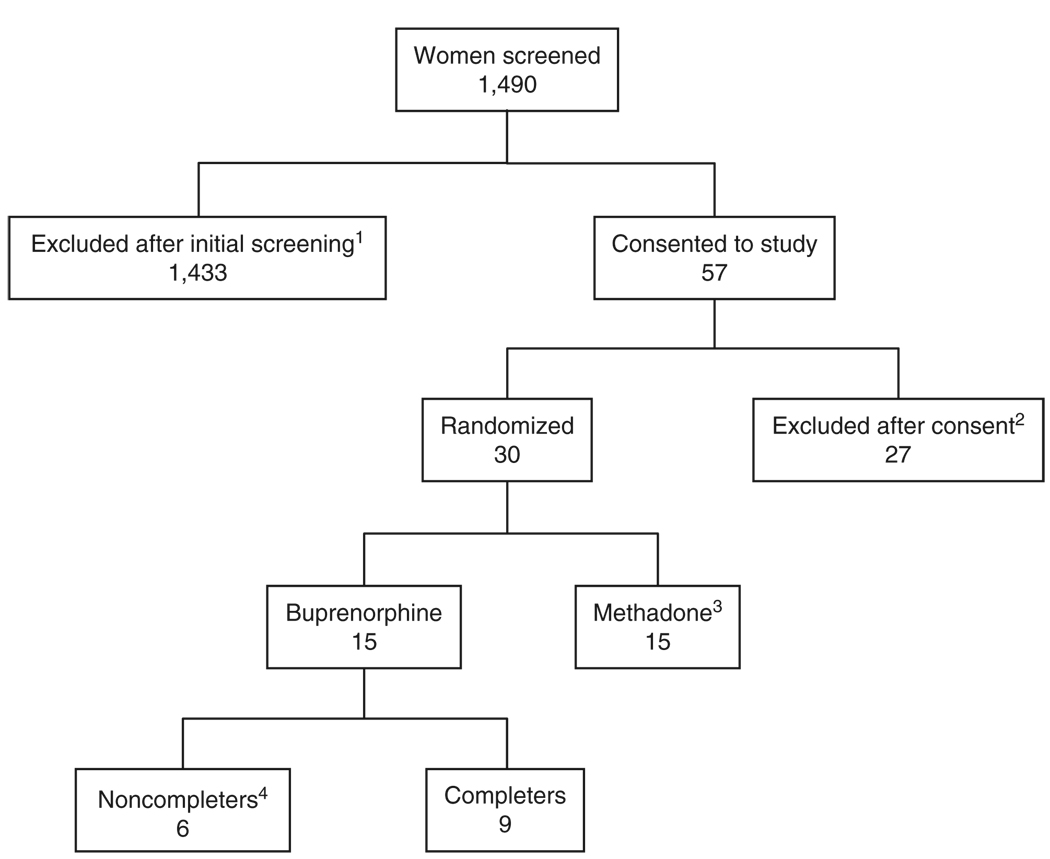
The Center for Addiction and Pregnancy at the Johns Hopkins Bayview Medical Center in Baltimore, MD, recruited participants for a double-blind, double-dummy, flexible, randomized, stratified, parallel-group controlled study comparing methadone and buprenorphine for opiate addiction treatment during pregnancy. Of 1,490 pregnant women evaluated, 57 qualified for initial screening and provided written consent to participate.2 The inclusion criteria were: 21–40 years old, estimated 16–30 weeks of gestational age of the fetus by sonogram, Diagnostic and Statistical Manual of Mental Disorders, 4th Edition diagnosis of current opioid dependence, maintenance pharmacotherapy request, recent self-reported opioid use of more than 4 days in the past 7, and an opiate-positive urine specimen. Exclusion criteria were undocumented methadone-positive urine, current Diagnostic and Statistical Manual of Mental Disorders, 4th Edition alcohol abuse or dependence, self-reported benzodiazepine use more frequent than seven times monthly or once weekly, currently taking another Axis I disorder medication, serious concurrent illness, previous diagnosis of preterm labor, evidence of fetal malformation, and human immunodeficiency virus or sickle-cell trait positive tests. The flow of participants through the study is shown in Figure 3.
Figure 3.
Flow of participants through the study from initial contact through completion or discharge. 1Reasons for exclusion: outside gestational age (641), did not qualify for methadone maintenance (167), chose detoxification (71), did not show for intake (245), medical reasons (98), other drug use (88), other (123); 2reasons for not excluding after consent: chose detoxification (6), medical/psychological reasons (4), AMA during screening (4), outside gestational age (3), other reasons (10); 311 completers, 4 noncompleters; results not included in this study; 4reasons for noncompletion: discharged for medical condition (1), missed consecutive dosing days (4), and elected to withdraw (1).
Once they were enrolled in the study, the participants were stratified by cocaine use (yes/no), estimated gestational age of the fetus (16–23 weeks or 24–30 weeks), and opioid use (<4 times a day or ≥5 times a day). After stratification, the women were randomly assigned to methadone or buprenorphine treatment groups. One buprenorphine-maintained woman delivered twins, yielding 10 in utero exposed infants.
The outcome measures relating to infants exposed to methadone and to buprenorphine have been compared earlier.2 This study focuses only on women maintained on buprenorphine during pregnancy and their infants. Given their distinct physiochemical characteristics, the results relating to methadone and buprenorphine are analyzed separately. Methadone data are not yet available.
Dosing and meconium collection
All of the women received oral methadone for 3–5 days during screening. The women were randomized and transitioned to the study medication through immediate-release morphine in divided daily doses. Buprenorphine-maintained women received sublingual buprenorphine HCl (2 mg each, maximum 24 mg/day; Reckitt-Benckiser Pharmaceuticals, Richmond, VA) and placebo tablets totaling 12 tablets/day, along with 40 ml liquid placebo, whereas the methadone-group women received 12 daily placebo sublingual tablets and 40 ml of active methadone medication to maintain double-blind dosing conditions. The total buprenorphine dose from the time of enrollment until the delivery of the infant and the total third-trimester dose (calculated from gestational age at the time of delivery) were calculated from the time point of entering the study until the time point of delivery. The infants’ diapers were collected for 72 h after birth and the meconium was pooled and stored at −20 °C until analysis.
Meconium analysis
Meconium specimens were analyzed using the first validated liquid chromatography–tandem mass spectrometry method for buprenorphine, norbuprenorphine, buprenorphine-glucuronide, and norbuprenorphine-glucuronide.38 Briefly, analytes were extracted in buffer from 0.25 ± 0.01 g homogenized meconium, isolated, and concentrated by solid-phase extraction and quantified on an LCQ Deca XPPlus Ion-Trap Mass Spectrometer (ThermoScientific, San Jose, CA) with atmospheric pressure chemical ionization in selected reaction-monitoring mode. LOQ were 20 ng/g for buprenorphine and norbuprenorphine with linearity to 2,000 ng/g. Total and free analyte concentrations were determined by analysis with and without enzyme hydrolysis. The accuracy of the method was at least 85.7%, with intra-assay and interassay imprecision <14%. Buffer extraction followed by solid-phase extraction yielded recoveries of ≥85.0%.
Separate meconium aliquots (0.50 ± 0.01 g) were sonicated with acidic methanol for 1 h followed by solid-phase extraction. The quantification of nicotine and metabolites was performed using a MDS Sciex API 3200 QTrap mass spectrometer (Applied Biosystems, Foster City, CA) with atmospheric pressure chemical ionization and multiple reaction monitoring.39 LOQ were 1.25 ng/g for cotinine, 3-hydroxycotinine, and norcotinine; and 5 ng/g for nornicotine and nicotine, with all analytes’ linearity to 500 ng/g. Cocaine, opiates, and oxazepam were tested for, using gas chromatography–mass spectrometry (United States Drug Testing Laboratories, Des Plaines, IL) with LOQ of 5 ng/g for cocaine, benzoylecgonine, and m-OH-benzoylecgonine; 50 ng/g for morphine, codeine, hydrocodone, hydromorphone, and oxycodone; and 16 ng/g for oxazepam.
Drug screening of urine and self-reported smoking
Urine specimens were assayed three times a week for cocaine, opiates, cannabis, and benzodiazepines using immunoassay (Dade Behring Diagnostics, Deerfield, IL) with cutoffs of 300 ng/ml for cocaine and opiates, 200 ng/ml for benzodiazepines, and 100 ng/ml for cannabis. Self-reported smoking data were extracted from the last Addiction Severity Interview before the delivery of the infant.
NAS assessment
The participants resided on the premises of a research unit prior to the estimated delivery date, and the infants were hospitalized for at least 4 days after birth per standard hospital procedure. NAS signs were evaluated using the 19-item (excessive crying, sleep habits, reflex, undisturbed tremors, disturbed tremors, muscle tone, excoriation, generalized seizure, fever, frequent yawning, sweating, nasal stuffiness, sneezing, tachypnea, poor feeding, vomiting, loose stools, failure to thrive, and excessive irritability) modified Finnegan Scale2,15 six to eight times a day. Item presence, severity, and frequency were scored for a total of 0–42. Oral morphine sulfate (0.02 mg morphine/drop) was administered to the infants every 3–5 h depending upon the total score. Infants treated for NAS were discharged 24 h after the last medication. After being discharged, the infants stayed with their respective mothers in the research unit through day 10 of life, with NAS evaluation being carried out twice a day.
Neonatal outcome measures
For each infant, gestational age at delivery, birth weight (g), head circumference (cm), length (cm), and hospital stay duration (days) from birth until discharge to the research unit were obtained from medical records. NAS onset (h) was defined as the time from birth until the first score >4. The score of 4 was selected as the cutoff on the basis of clinical experience and preliminary blinded–condition comparison of data from drug-exposed and nondrug-exposed neonates. Peak NAS score was defined as the highest score obtained and time-to-peak (h) was calculated from birth to NAS peak time. The NAS duration (h) was defined as the time from first score >4 to the time after which the scores remained <5.
Statistical analysis
A matched-pair t-test showed significant differences between hydrolyzed and nonhydrolyzed meconium concentrations. Mann–Whitney U-tests were used for comparing NAS outcomes between genders, NAS for infants born to smokers and nonsmokers, percentage of positive maternal urine specimens and cocaine- and opiate-positive meconium specimens. Linear regression was used for examining relationships among maternal buprenorphine dose, meconium drug concentrations, and neonatal outcomes. Twins were excluded from weight, length, and head circumference analyses, given that their twin status might impact these measurements. Mean values are reported as mean value ± SE. All analyses were performed using SPSS 13.0 for Windows. P values < 0.05 were taken to be statistically significant.
ACKNOWLEDGMENTS
We thank the women who participated in this study. We also thank Paul Wakim for statistical guidance and Rosemarie Rios from the United States Drug Testing Laboratory for analyzing meconium samples for cocaine, opiates, and benzodiazepines. We thank the research and clinical staff of the Center for Addiction and Pregnancy. This study was supported by National Institute on Drug Abuse grants R01 12220 and R01 DA015764, and M01RR-02719 from the General Clinical Research Centers Program, National Center of Research Resources, National Institutes of Health.
Footnotes
CONFLICT OF INTEREST
R.E.J. is employed at Reckitt-Benckiser Pharmaceuticals, the manufacturer and distributor of buprenorphine. He was Associate Professor, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, when the study was initiated. The other authors declared no conflict of interest.
References
- 1.Drug Addiction Treatment Act of 2000. 2000 Page 111 STAT. 1101. [Google Scholar]
- 2.Jones HE, et al. Buprenorphine versus methadone in the treatment of pregnant opioid-dependent patients: effects on the neonatal abstinence syndrome. Drug Alcohol Depend. 2005;79:1–10. doi: 10.1016/j.drugalcdep.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 3.Fischer G, et al. Methadone versus buprenorphine in pregnant addicts: a double-blind, double-dummy comparison study. Addiction. 2006;101:275–281. doi: 10.1111/j.1360-0443.2006.01321.x. [DOI] [PubMed] [Google Scholar]
- 4.Finnegan LP, Kaltenbach K. Neonatal abstinence syndrome. In: Hoekelman RA, Friedman SB, Nelson NM, Seidel HM, editors. Primary Pediatric Care. 2nd edn. St. Louis: Mosby Year Book; 1992. pp. 1367–1378. [Google Scholar]
- 5.Sarkar S, Donn SM. Management of neonatal abstinence syndrome in neonatal intensive care units: a national survey. J. Perinatol. 2006;26:15–17. doi: 10.1038/sj.jp.7211427. [DOI] [PubMed] [Google Scholar]
- 6.Kaltenbach K, Finnegan LP. Neonatal abstinence syndrome, pharmacotherapy and developmental outcome. Neurobehav. Toxicol. Teratol. 1986;8:353–355. [PubMed] [Google Scholar]
- 7.Rohrmeister K, Bernert G, Langer M, Fischer G, Weninger M, Pollak A. Opiate addiction in gravidity—consequences for the newborn. Results of an interdisciplinary treatment concept. Z. Geburtshilfe Neonatol. 2001;205:224–230. doi: 10.1055/s-2001-19054. [DOI] [PubMed] [Google Scholar]
- 8.Lejeune C, Simmat-Durand L, Gourarier L, Aubisson S Groupe d’Etudes Grossesse et Addictions (GEGA) Prospective multicenter observational study of 260 infants born to 259 opiate-dependent mothers on methadone or highdose buprenorphine substitution. Drug Alcohol Depend. 2006;82:250–257. doi: 10.1016/j.drugalcdep.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Ostrea EM., Jr Understanding drug testing in the neonate and the role of meconium analysis. J. Perinat. Neonatal. Nurs. 2001;14:61–82. doi: 10.1097/00005237-200103000-00006. quiz 105–106. [DOI] [PubMed] [Google Scholar]
- 10.Kwong TC, Ryan RM. Detection of intrauterine illicit drug exposure by newborn drug testing. Clin. Chem. 1997;43:235–242. [PubMed] [Google Scholar]
- 11.Fischer G, et al. Treatment of opioid-dependent pregnant women with buprenorphine. Addiction. 2000;95:239–244. doi: 10.1046/j.1360-0443.2000.95223910.x. [DOI] [PubMed] [Google Scholar]
- 12.Behrman RE, Kliegman RM, Arvin AM. The high-risk infant. In: Behrman RE, Kliegman RM, Arvin AM, editors. Textbook of Pediatrics. Ch. 82. Philadelphia: W. B. Saunders Company; 1996. pp. 451–463. [Google Scholar]
- 13.Tagliaro F, DeBattisti Z, Lubli G, Neri C, Manetto G, Marigo M. Integrated use of hair analysis to investigate the physical fitness to obtain the driving licence: a casework study. Forensic Sci. Int. 1997;84:129–135. doi: 10.1016/s0379-0738(96)02055-5. [DOI] [PubMed] [Google Scholar]
- 14.Ostrea EM, Romero A, Knapp K, Ostrea AR, Lucena JE, Utarnachitt RB. Postmortem drug analysis of meconium in early-gestation human fetuses exposed to cocaine: clinical implications. J. Pediatr. 1994;124:477–479. doi: 10.1016/s0022-3476(94)70379-5. [DOI] [PubMed] [Google Scholar]
- 15.Nanovskaya T, Deshmukh S, Brooks M, Ahmed MS. Transplacental transfer and metabolism of buprenorphine. J. Pharmacol. Exp. Ther. 2002;300:26–33. doi: 10.1124/jpet.300.1.26. [DOI] [PubMed] [Google Scholar]
- 16.Myllynen P, Pasanen M, Vahakangas K. The fate and effects of xenobiotics in human placenta. Expert Opin. Drug Metab. Toxicol. 2007;3:331–346. doi: 10.1517/17425255.3.3.331. [DOI] [PubMed] [Google Scholar]
- 17.De Leon J. Glucuronidation enzymes, genes and psychiatry. Int. J. Neuropsychopharmacol. 2003;6:57–72. doi: 10.1017/S1461145703003249. [DOI] [PubMed] [Google Scholar]
- 18.Collier AC, Tingle MD, Paxton JW, Mitchell MD, Keelan JA. Metabolizing enzyme localization and activities in the first trimester human placenta: the effect of maternal and gestational age, smoking and alcohol consumption. Hum. Reprod. 2002;17:2564–2572. doi: 10.1093/humrep/17.10.2564. [DOI] [PubMed] [Google Scholar]
- 19.Collier AC, et al. UDP-glucuronosyltransferase activity, expression and cellular localization in human placenta at term. Biochem. Pharmacol. 2002;63:409–419. doi: 10.1016/s0006-2952(01)00890-5. [DOI] [PubMed] [Google Scholar]
- 20.Picard N, Cresteil T, Djebli N, Marquet P. In vitro metabolism study of buprenorphine: evidence for new metabolic pathways. Drug Metab. Dispos. 2005;33:689–695. doi: 10.1124/dmd.105.003681. [DOI] [PubMed] [Google Scholar]
- 21.Moody DE, Slawson MH, Strain EC, Laycock JD, Spanbauer AC, Foltz RL. A liquid chromatographic-electrospray ionization-tandem mass spectrometric method for determination of buprenorphine, its metabolite, norbuprenorphine, and a coformulant, naloxone, that is suitable for in vivo and in vitro metabolism studies. Anal. Biochem. 2002;306:31–39. doi: 10.1006/abio.2002.5673. [DOI] [PubMed] [Google Scholar]
- 22.Oesterheld JR. A review of developmental aspects of cytochrome P450. J. Child Adolesc. Psychopharmacol. 1998;8:161–174. doi: 10.1089/cap.1998.8.161. [DOI] [PubMed] [Google Scholar]
- 23.Ring JA, Ghabrial H, Ching MS, Smallwood RA, Morgan DJ. Fetal hepatic drug elimination. Pharmacol. Ther. 1999;84:429–445. doi: 10.1016/s0163-7258(99)00046-7. [DOI] [PubMed] [Google Scholar]
- 24.Garland M, et al. Fetal morphine metabolism and clearance are constant during late gestation. Drug Metab. Dispos. 2006;34:636–646. doi: 10.1124/dmd.105.007567. [DOI] [PubMed] [Google Scholar]
- 25.Marquet P, Chevrel J, Lavignasse P, Merle L, Lachatre G. Burprenorphine withdrawal syndrome in a newborn. Clin. Pharmacol. Ther. 1997;62:569–571. doi: 10.1016/S0009-9236(97)90053-9. [DOI] [PubMed] [Google Scholar]
- 26.Marquet P, Lavignasse P, Gaulier J, Lachatre G. Case study of neonates born to mothers undergoing buprenorphine maintenance treatment. In: Kintz P, Marquent P, editors. Buprenorphine Therapy of Opiate Addiction. Totowana, NJ: Humana; 2002. pp. 125–135. [Google Scholar]
- 27.Glusulase® (contains both β-Glucuronidase and β-Glucuronide Sulfatase), 10 mL—Certificate of Analysis (PC2882-0504) Boston, MA: Perkin Elmer; 2007. [Google Scholar]
- 28.Ostrea EM, Jr, Brady M, Gause S, Raymundo AL, Stevens M. Drug screening of newborns by meconium analysis: a large-scale, prospective, epidemiologic study. Pediatrics. 1992;89:107–113. [PubMed] [Google Scholar]
- 29.Lester BM, et al. The maternal lifestyle study: drug use by meconium toxicology and maternal self-report. Pediatrics. 2001;107:309–317. doi: 10.1542/peds.107.2.309. [DOI] [PubMed] [Google Scholar]
- 30.Wingert WE, Feldman MS, Kim MH, Noble L, Hand I, Yoon JJ. A comparison of meconium, maternal urine and neonatal urine for detection of maternal drug use during pregnancy. J. Forensic Sci. 1994;39:150–158. [PubMed] [Google Scholar]
- 31.Bibb KW, Stewart DL, Walker JR, Cook VD, Wagener RE. Drug screening in newborns and mothers using meconium samples, paired urine samples, and interviews. J. Perinatol. 1995;15:199–202. [PubMed] [Google Scholar]
- 32.Ryan RM, et al. Meconium analysis for improved identification of infants exposed to cocaine in utero. J. Pediatr. 1994;125:435–440. doi: 10.1016/s0022-3476(05)83291-3. [DOI] [PubMed] [Google Scholar]
- 33.Zhao Z, et al. Methamphetamine and amphetamine concentrations in meconium of neonates of women enrolled in the IDEAL study of in utero methamphetamine exposure; 2004 FBI/SOFT/TIAFT Meeting; 2004. [Google Scholar]
- 34.Schempf AH. Illicit drug use and neonatal outcomes: a critical review. Obstet. Gynecol. Surv. 2007;62:749–757. doi: 10.1097/01.ogx.0000286562.31774.76. [DOI] [PubMed] [Google Scholar]
- 35.Roza SJ, et al. Effects of maternal smoking in pregnancy on prenatal brain development. The Generation R Study. Eur. J. Neurosci. 2007;25:611–617. doi: 10.1111/j.1460-9568.2007.05393.x. [DOI] [PubMed] [Google Scholar]
- 36.Shankaran S, et al. Association between patterns of maternal substance use and infant birth weight, length, and head circumference. Pediatrics. 2004;114:e226–e234. doi: 10.1542/peds.114.2.e226. [DOI] [PubMed] [Google Scholar]
- 37.Choo RE, Huestis MA, Schroeder JR, Shin AS, Jones HE. Neonatal abstinence syndrome in methadone-exposed infants is altered by level of prenatal tobacco exposure. Drug Alcohol Depend. 2004;75:253–260. doi: 10.1016/j.drugalcdep.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 38.Kacinko SL, Shakleya DM, Huestis MA. Validation and application of a method for the determination of buprenorphine, norbuprenorphine, and their glucuronide conjugates in human meconium. Anal. Chem. 2008;80:246–252. doi: 10.1021/ac701627q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gray TR, Shakleya DM, Huestis MA. Quantification of nicotine, cotinine, trans-3′-hydroxycotinine, nornicotine and norcotinine in human meconium by liquid chromatography tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life. Sci. 2008;863:107–114. doi: 10.1016/j.jchromb.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]