Abstract
Paracellular transport through the tight junction shows selectivity for both ionic charge and solute size. It is known that charged residues on the extracellular loops of claudins control charge selectivity. It is also known that inducible expression of claudin-2, but not claudin-4, will selectively increase the permeability for PEG molecules which are <4Å in radius, but it is not known whether permeability is controlled by the same regions of claudins which control charge selectivity. Using inducible expression of chimeras of claudin-2 and claudin-4 in monolayers of MDCK II cells we show that the extracellular loops alone are responsible for controlling the permeability for noncharged PEGs as well as for charge selectivity. Further, the cytoplasmic C-terminal PDZ-binding motif is required for wild type claudin-2 to control permeability, suggesting a requirement for attachment to the PDZ scaffold in order to form pores. These observations support a model where the loops form pores controlling permeability for both charged and noncharged solutes which are smaller than 4Å. They leave unanswered why both claudin-2 and -4 can influence electrical properties while only -2 can selectively increase permeability for small PEGs.
Keywords: claudin, tight junction, permeability, paracellular, PDZ
Introduction
Epithelial tight junctions limit the paracellular flux of charged and non-charged solutes1,2. Many recent studies have demonstrated that alterations in the expression levels of individual claudins, members of a large family of integral membrane proteins of the tight junction, can influence paracellular ionic conductance and thus transmonolayer electrical resistance (TER)3,4. However, the role that claudins play in the regulation of permeability of nonionic compounds is less well understood. In fact, in many cases, changes in the levels of individual claudins, mediated by transfection or siRNA methods, results in increases or deceases in TER with little effect on paracellular flux of mannitol or fluorescent dextrans, the most commonly used markers to measure nonionic solute flux. However, mannitol and fluorescent dextrans have considerably larger hydrated radii (4.4 Å and 10Å or larger, respectively) than do Na+ and Cl− ions (less than 2Å,5), the relevant ions in conductance measurements. In fact, a number of studies have demonstrated that there is a disproportionately higher permeability for both ionic and nonionic compounds with radii less than 4Å when compared with the permeability of solutes with radii above 4Å6,7. These results suggested the possibility that small (<4Å) and larger (>4Å) solutes may travel through physically different paracellular pathways. This idea was supported by an elegant study published by Watson and coworkers8, who used a continuous series of PEG oligomers with increasing radii to demonstrate the presence of a high capacity paracellular pore pathway for compounds below 4Å and a lower capacity pathway for larger solutes.
It was previously shown that charged amino acid residues on the first extracellular domain of claudins influence the paracellular permeability for ions. Mutational studies performed on several different claudins all support the hypothesis that the first loop forms a selectivity filter lining the tight junction pores (reviewed in 4.) For example, increasing the expression of a claudin with low permeability for Na+ can increase TER9, and vice versa10. To determine the ability of claudins to influence the flux of noncharged solutes, we adapted the PEG assay described by Watson et al. 8 and in a previous study found that induction of claudin-2 but not claudin-4 could selectively increase permeability of PEGs with radii less than 4Å, while the permeability of larger PEGs was unaffected 11. In the present study, chimeric claudins were used to define the regions of claudin-2 that were responsible for the ability to increase PEG permeability; the results suggest that the extracellular domains are sufficient to mediate the increase in permeability, but also that the carboxy-terminal PDZ-binding motif is required. Taken together with the ability for the extracellular loops to control permeability for small ions, these results suggest that claudins form the pores controlling the permeability of all small solutes.
Materials and Methods
Cell culture, immunoblots, immunofluorescence microscopy and permeability measurements
Tet-off MDCK II cells (Clontech) expressing wild type claudin-2, claudin-4 and chimeras were cultured as described previously 12. Claudin-2 lacking the C-terminal PDZ binding motif (GYV) was constructed, transfected and expressed as described previously13. Protein induction, determination of transepithelial electrical resistance (TER), immunoblots and immunofluorescence microscopy were performed as described elsewhere 12; polyethylene glycol permeability measurements were also performed as previously described and reported as the apparent permeability (Papp) for each size11.
Results
Expression of claudin-2/claudin-4 chimeras reveals that the extracellular domains of claudin-2 increase permeability for small PEG species
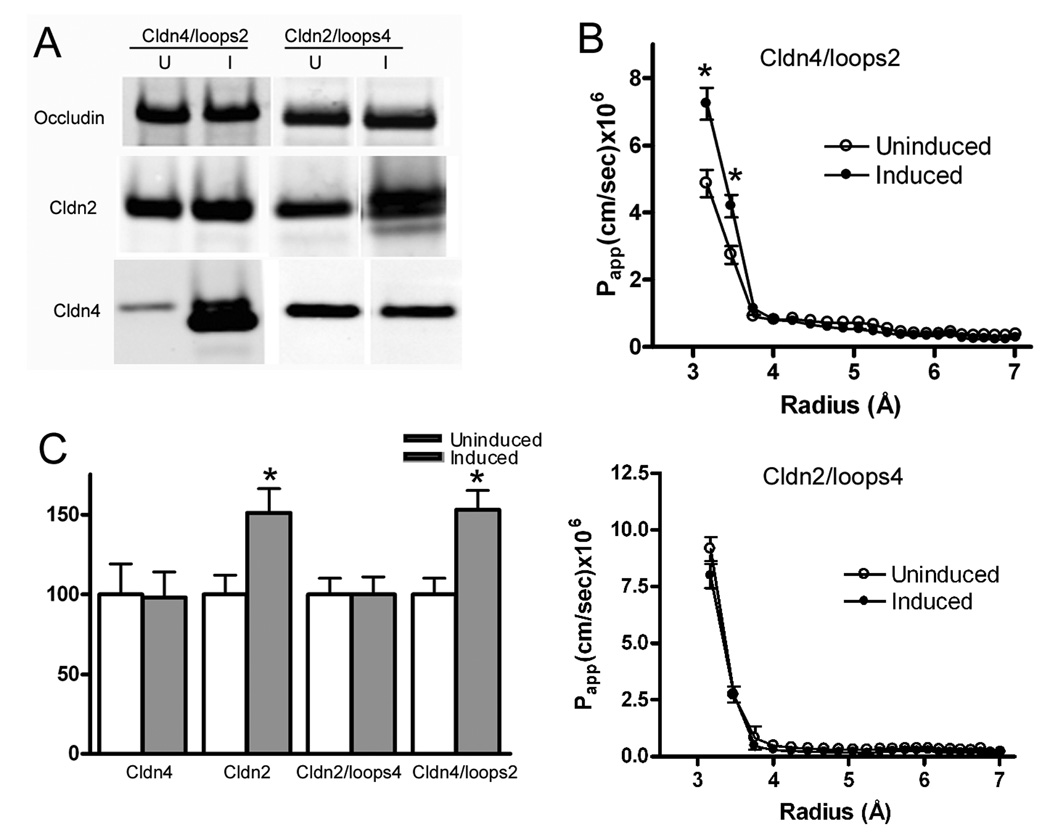
In a previous study 11, we demonstrated that inducing increased levels of claudin-2 in MDCK cell monolayers increased the permeability for PEGs which have radii of <4Å. The increase in permeability for small PEGs appeared to be specific, since there was little no significant effect on permeability for larger PEG species up to 7Å in radius, the largest size studied. In contrast, expression of claudin-4 had no effect on the permeability for PEGs either below or above 4Å. To define regions in claudin-2 that were responsible for the ability to increase permeability for the smaller PEGs, we examined PEG flux in monolayers which were induced to express chimeras of claudin-2 and -4. These had either the extracellular domains of claudin-2 on the transmembrane and cytosolic domains of claudin-4, or the converse, that is the extracellular domains of claudin-4 on a claudin-2 background; these constructs are diagrammed in Fig. 1. Successful induction of claudin chimeras is demonstrated in the immunoblots shown in Fig.2A. Expression of either the wild type or chimeric proteins had little effect on the levels of other claudins measured (not shown) or on levels of occludin, which is another type of transmembrane protein also found in tight junction strands, Fig 2A.
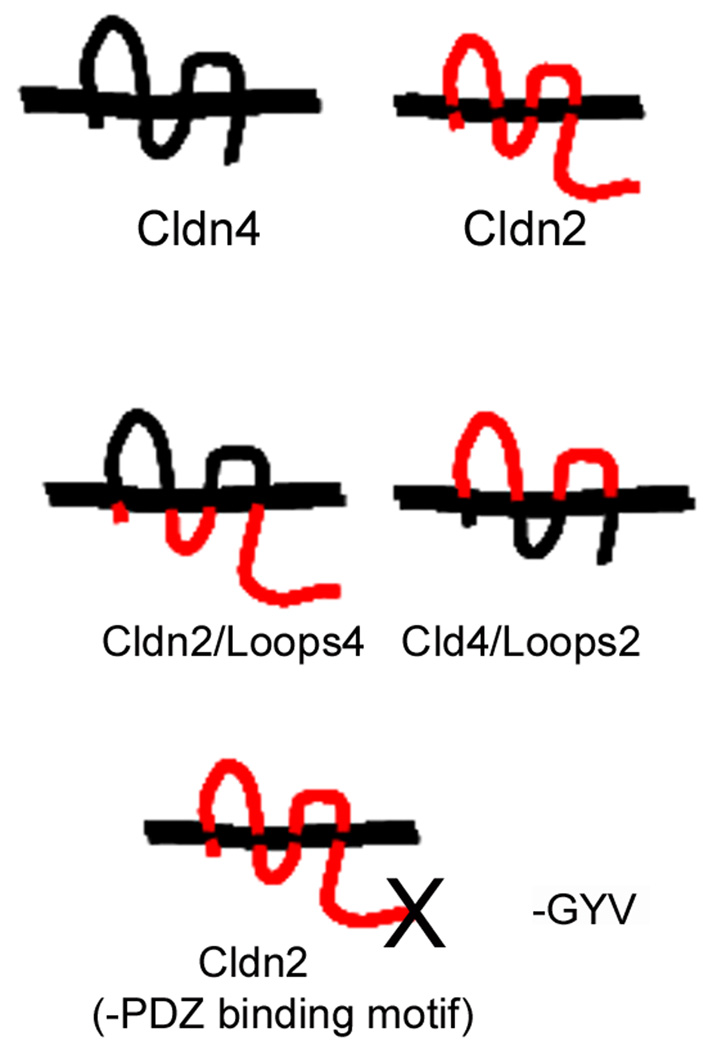
Fig. 1.
Diagram of claudin loop chimeras. Constructions of chimeras has been reported elsewhere 12,13; briefly the extracellular domains were exchanged by creating unique restriction sites by mutagenesis at the ends of the extracellular domains which allow for their exchange between claudin-4 (black) and claudin 2 (red); where necessary, inadvertent amino acid changes created during the construction were changed back to parental sequence by mutagenesis. Removal of the PDZ binding motif was also carried out by mutagenesis, with replacement of the last three amino acids with a stop codon. Endogenous epitopes used for antibody detection are in the normal claudin carboxyl terminal domain (internal to the PDZ binding domain) such that the chimera Cldn2/Loops4 is detected with an anti-claudin-2 antibody and Cln4/Loops2 with an anti-claudin-4 antibody; these can be distinguished from wild type by the inducibility of the constructs.
Fig. 2.
The extracellular domains of claudin-2 are responsible for the increase in permeability of small PEGs seen after expression of wild-type claudin-2. A. Chimeric claudins are inducible in MDCK II cells. Left: immunoblots of cells uninduced (U) or induced (I) to express cldn4/loops2 demonstrate induction of the chimera (as detected by the claudin-4 antibody; see legend for Fig 1) and relatively little effect on either occludin or endogenous claudin-2. Right: similarly, induction of cldn2/loops4 chimera (as detected with the claudin-2 antibody) has little effect on occludin expression, but does result in a small decrease in endogenous claudin-4 expression, as has been previously demonstrated. B. PEG profiling demonstrates that (top) expression of cldn4/loops2 results in increased Papp for PEGs smaller than 4Å, with little effect on larger PEGs; induction of cldn2/loops4 (bottom panel) has no effect on PEG permeability. C. When Papp for PEG 3.5Å is compared in cell lines induced to express claudin-2, -4 or chimeric claudins, only expression of claudins containing the extracellular domains of claudin-2 result in significant increases in PEG permeability. (N=3 or greater for each construct; *P<0.05).
Induction of claudin-4 and the chimera consisting of the extracellular loops of claudin-4 on the claudin-2 background have both previously been demonstrated to alter paracellular charge selectivity and increase TER 12; this was verified in the present study (not shown). Conversely, as previously shown, neither induction of claudin-2 nor a chimera with the extracellular domains of claudin-2 on the background of claudin-4 had much effect on either charge selectivity or TER when compared with uninduced MDCK II cells. Our conclusions from these previous studies were that the extracellular domains of claudin-4, rather than transmembrane segments or cytosolic domains, contained the necessary information for altering charge selectivity and TER. In an analogous fashion, we asked if the extracellular domains of claudin-2 were the determinants responsible for the observed increase in paracellular flux for small PEGs seen after induction of wild-type claudin-2. As can be seen in Figures 2B and C, expression of a chimera with both extracellular loops of claudin-2 on the claudin-4 background results in a similar increase in flux to that seen with wild-type claudin-2 expression. This increase in flux is specific for small PEGs (below 4Å in radius, Fig. 2B). Neither claudin-4 nor a chimera with the extracellular domains of claudin-4 on a claudin-2 background alters paracellular flux for PEGs of any size, Figures 2B and C.
PDZ-dependent interactions are required for claudin-2 to increase PEG permeability
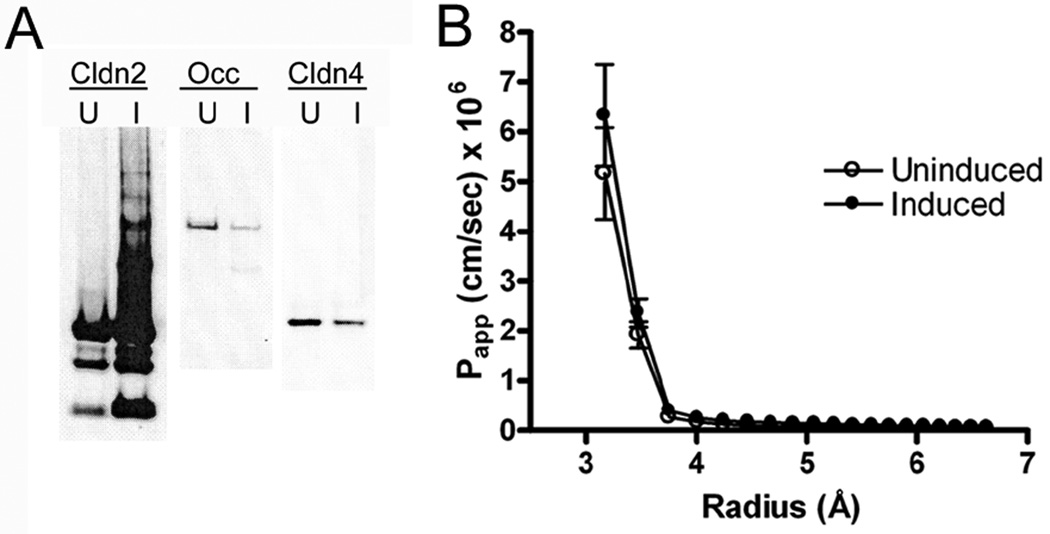
Claudins have been demonstrated to interact with ZO-1, -2 and other PDZ-containing tight junction proteins via a carboxy- terminal PDZ-binding motif; there are some data indicating that this interaction may be required for proper localization and function of claudins 14. To test whether the ability of claudin-2 to alter paracellular flux requires interaction with PDZ- containing proteins, PCR-based mutagenesis was used to remove the last three amino acids, which are required for PDZ-binding, from the cytoplasmic tail of claudin-2. When expressed in MDCK II cells, as shown, this construct is inducible, Fig. 3A, and expression results in decreased expression of occludin and claudin-4, as has been previously noted 12. Claudin-2 lacking the PDZ-binding motif localizes to the tight junction in a similar fashion as wild-type claudin-2 at the immunofluorescent light microscopic level (not shown). However, unlike wild-type claudin-2, when MDCK II monolayers are induced to express the truncated construct, there is no alteration in paracellular PEG permeability, Fig, 3C. This finding points to a functional requirement for attachment to the tight junction scaffolding proteins through this PDZ-binding motif.
Fig 3.
Claudin-2 lacking the PDZ-binding motif does not increase permeability for small PEGs. A. Immunoblot of MDCK II tet-off cells uninduced (U) or induced to express claudin-2 without the last three amino acids. Left panel, probed for claudin-2, middle panel occludin and right panel for claudin-4. Overexpression of claudin-2(-3) results in decrease levels of occludin and claudin-4 expression, as has been described elsewhere accompanying wild-type claudin-2 induction (Colegio et al. 2003). B. Papp using PEG permeability profiling indicates no change following induction of claudin-2(-3).
Discussion
The results presented here define two critical regions required for the ability of claudin-2 to increase permeability for small uncharged solutes (PEGs): namely, the extracellular domains and the cytoplasmic PDZ-binding motif. Our previous results demonstrated that chimeras expressing the first extracellular domains of claudins in MDCK tet-off cells were sufficient to cause the changes in paracellular charge selectivity observed after expression of the wild-type claudin 12. In addition, we 12 and others 15,16,17 have also shown that paracellular charge selectivity can be modified by mutating selected positively or negatively charged amino acids in the first extracellular domains of several claudins; together, the results suggest that the extracellular domains of claudins line paracellular space. However, how the claudins are organized in the paracellular space is unclear. The findings in the present study demonstrate that the extracellular domains of claudin-2 were sufficient to mediate the increase in non-charged solute permeability observed with the wild-type claudin. This suggests that the extracellular domains may be key structural components of the paracellular pore, since the increase in permeability is specific for small PEGs and does not alter overall paracellular permeability for larger solutes. However, it is worth noting that claudin-2 is unusual, since it is the only claudin that we have found to date that can increase permeability for small PEGs 11; claudins 4, 11 and 18 [11] and 6 (unpublished) can not..
It is informative that like wild type claudin-4, expression of the chimera with the extracellular domains of claudin-4 and the transmembrane domains and cytosolic domains of claudin-2 did not change permeability. Previous studies have shown that the transmembrane and cytosolic domains are responsible both for the characteristic subcellular localizations detected by immunofluorescent light microscopy and the intramembrane strand architecture seen using freeze-fracture electron microscopy12. The present results are consistent with the idea that interactions between the extracellular domains across cells are critical in determining the physiologic characteristics of the tight junction, while other domains likely serve organizational and regulatory functions.
The requirement for the PDZ-binding motif in the ability of claudin-2 to alter permeability demonstrates the necessity of an interaction with tight junction scaffolding proteins (possibly ZO-1, -2, -3 or MUPP-1). Although the PDZ-binding motif is not required for strand formation 18 or adhesive cell-to-cell interactions, it is likely required for localization to authentic apical tight junction strands 14. Lack of proper localization may be the reason for the lack of effect on PEG permeability, although an additional possibility is that interaction with the cytoplasmic scaffold may be required for inducing an effective extracellular pore conformation.
The unusual ability of claudin-2 to influence permeability may have implications for pathology. There is considerable evidence that intestinal inflammation is accompanied by a relatively unique induction in the levels of claudin-2 compared to other claudins. This is accompanied by an increase in paracellular ion permeability and decrease in epithelial resistance 19,20,21 which has been proposed to contribute to so-called, ”back leak diarrhea”, for example in Inflammatory Bowel Disease 22. As noted, claudin-2 also appears to be unusual among claudins in its ability to increase overall permeability for small ions and noncharged solutes 11. More comprehensive understanding of normal and pathologically disrupted paracellular permeability will depend on a better understanding of how claudins and other proteins organize to form the paracellular barrier and specifically how claudin-2 controls the small tight junction pores.
Acknowledgements
We thank Alan Fanning for helpful discussions. This work was supported by NIH grants DK45134 (to J.M.A.) and P30 DK 034987.
References
- 1.Berry CA, Boulpaep EL. Nonelectrolyte permeability of the paracellular pathway in Necturus proximal tubule. Am. J. Physiol. 1975;228:581–595. doi: 10.1152/ajplegacy.1975.228.2.581. [DOI] [PubMed] [Google Scholar]
- 2.Diamond JM. Channels in epithelial cell membranes and junctions. Fed. Proc. 1978;37:2639–2643. [PubMed] [Google Scholar]
- 3.Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annu. Rev. Physiol. 2006;68:403–429. doi: 10.1146/annurev.physiol.68.040104.131404. [DOI] [PubMed] [Google Scholar]
- 4.Angelow S, Ahlstrom R, Yu AS. Biology of Claudins. Am. J. Physiol Renal Physiol. 2008 doi: 10.1152/ajprenal.90264.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiriukhin MY, Collins KD. Dynamic hydration numbers for biologically important ions. Biophys. Chem. 2002;99:155–168. doi: 10.1016/s0301-4622(02)00153-9. [DOI] [PubMed] [Google Scholar]
- 6.Knipp GT, Ho NF, Barsuhn CL, Borchardt RT. Paracellular diffusion in Caco-2 cell monolayers: effect of perturbation on the transport of hydrophilic compounds that vary in charge and size. J. Pharm. Sci. 1997;86:1105–1110. doi: 10.1021/js9700309. [DOI] [PubMed] [Google Scholar]
- 7.Tavelin S, Taipalensuu J, Soderberg L, Morrison R, Chong S, Artursson P. Prediction of the oral absorption of low-permeability drugs using small intestine-like 2/4/A1 cell monolayers. Pharm. Res. 2003;20:397–405. doi: 10.1023/a:1022699920043. [DOI] [PubMed] [Google Scholar]
- 8.Watson CJ, Rowland M, Warhurst G. Functional modeling of tight junctions in intestinal cell monolayers using polyethylene glycol oligomers. Am. J. Physiol Cell Physiol. 2001;281:C388–C397. doi: 10.1152/ajpcell.2001.281.2.C388. [DOI] [PubMed] [Google Scholar]
- 9.Van Itallie C, Rahner C, Anderson JM. Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability. J. Clin. Invest. 2001;107:1319–1327. doi: 10.1172/JCI12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amasheh S, Meiri N, Gitter AH, Schoneberg T, Mankertz J, Schulzke JD, Fromm M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J. Cell Sci. 2002;115:4969–4976. doi: 10.1242/jcs.00165. [DOI] [PubMed] [Google Scholar]
- 11.Van Itallie CM, Holmes J, Bridges A, Gookin JL, Coccaro MR, Proctor W, Colegio OR, Anderson JM. The density of small tight junction pores varies among cell types and is increased by expression of claudin-2. J. Cell Sci. 2008;121:298–305. doi: 10.1242/jcs.021485. [DOI] [PubMed] [Google Scholar]
- 12.Colegio OR, Van Itallie C, Rahner C, Anderson JM. Claudin extracellular domains determine paracellular charge selectivity and resistance but not tight junction fibril architecture. Am. J. Physiol Cell Physiol. 2003;284:C1346–C1354. doi: 10.1152/ajpcell.00547.2002. [DOI] [PubMed] [Google Scholar]
- 13.Van Itallie CM, Colegio OR, Anderson JM. The cytoplasmic tails of claudins can influence tight junction barrier properties through effects on protein stability. J. Membr. Biol. 2004;199:29–38. doi: 10.1007/s00232-004-0673-z. [DOI] [PubMed] [Google Scholar]
- 14.Mccarthy KM, Francis SA, Mccormack JM, Lai J, Rogers RA, Skare IB, Lynch RD, Schneeberger EE. Inducible expression of claudin-1-myc but not occludin-VSV-G results in aberrant tight junction strand formation in MDCK cells. J. Cell Sci. 2000;113 Pt 19:3387–3398. doi: 10.1242/jcs.113.19.3387. [DOI] [PubMed] [Google Scholar]
- 15.Hou J, Paul DL, Goodenough DA. Paracellin-1 and the modulation of ion selectivity of tight junctions. J. Cell Sci. 2005;118:5109–5118. doi: 10.1242/jcs.02631. [DOI] [PubMed] [Google Scholar]
- 16.Alexandre MD, Jeansonne BG, Renegar RH, Tatum R, Chen YH. The first extracellular domain of claudin-7 affects paracellular Cl-permeability. Biochem. Biophys. Res. Commun. 2007;357:87–91. doi: 10.1016/j.bbrc.2007.03.078. [DOI] [PubMed] [Google Scholar]
- 17.Hou J, Renigunta A, Konrad M, Gomes AS, Schneeberger EE, Paul DL, Waldegger S, Goodenough DA. Claudin-16 and claudin-19 interact and form a cation-selective tight junction complex. J. Clin. Invest. 2008;118:619–628. doi: 10.1172/JCI33970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furuse M, Sasaki H, Tsukita S. Manner of interaction of heterogeneous claudin species within and between tight junction strands. J. Cell Biol. 1999;147:891–903. doi: 10.1083/jcb.147.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fries W, Muja C, Crisafulli C, Cuzzocrea S, Mazzon E. Dynamics of enterocyte tight junctions: effect of experimental colitis and two different anti-TNF strategies. Am. J. Physiol Gastrointest. Liver Physiol. 2008;294:G938–G947. doi: 10.1152/ajpgi.00469.2007. [DOI] [PubMed] [Google Scholar]
- 20.Ridyard AE, Brown JK, Rhind SM, Else RW, Simpson JW, Miller HR. Apical junction complex protein expression in the canine colon: differential expression of claudin-2 in the colonic mucosa in dogs with idiopathic colitis. J. Histochem. Cytochem. 2007;55:1049–1058. doi: 10.1369/jhc.7A7211.2007. [DOI] [PubMed] [Google Scholar]
- 21.Zeissig S, Burgel N, Gunzel D, Richter J, Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn's disease. Gut. 2007;56:61–72. doi: 10.1136/gut.2006.094375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mankertz J, Schulzke JD. Altered permeability in inflammatory bowel disease: pathophysiology and clinical implications. Curr. Opin. Gastroenterol. 2007;23:379–383. doi: 10.1097/MOG.0b013e32816aa392. [DOI] [PubMed] [Google Scholar]