Abstract
Combined lesions of retinal targets and ascending auditory pathways can induce, in developing animals, permanent retinal projections to auditory thalamic nuclei and to visual thalamic nuclei that normally receive little direct retinal input. Neurons in the auditory cortex of such animals have visual response properties that resemble those of neurons in the primary visual cortex of normal animals. Therefore, we investigated the behavioral function of the surgically induced retino-thalamo-cortical pathways. We showed that both surgically induced pathways can mediate visually guided behaviors whose normal substrate, the pathway from the retina to the primary visual cortex via the primary thalamic visual nucleus, is missing.
Brain lesions that occur during development can produce abnormal neural connectivity patterns. The superior colliculus (SC) is the principal midbrain target of the retina. Combined lesions of the SC and ascending auditory or somatosensory pathways to the thalamus induce growing retinal axons to form permanent, ectopic projections to auditory (medial geniculate, MG) (1–3) or somatosensory (ventrobasal) (2) thalamic nuclei, respectively. This rewiring occurs because of the combined effects of “pruning” retinal axons within their normal targets and of the increased availability of target tissue in the MG or ventrobasal nuclei (1). The SC lesions also induce robust retinal projections in the secondary visual (lateral posterior, LP) thalamic nucleus, which normally receives little direct retinal input but is the principal thalamic target of the SC (1–4). Retinal axons in the MG and ventrobasal thalamic nuclei are retinotopically organized (2) and form functional synapses on neurons in these nuclei (5–7). Neurons in the somatosensory (7, 8) and auditory§ cortices of animals with ectopic retinal projections have visual response properties that resemble those of neurons in the primary visual cortex (V1) of normal animals. Ferrets that have retino-MG projections but no visual cortex appear to perceive light stimuli as visual.¶ However, the contribution of the surgically induced pathways to visual behavior in neonatally operated (“rewired”) animals has not been assessed. Here, we show that surgically created retino-thalamo-cortical pathways involving the auditory or secondary visual thalamic nuclei can mediate visually guided behaviors whose normal substrate, the pathway from the retina to V1 via the primary thalamic visual (dorsal lateral geniculate, LGd) nucleus, is missing.
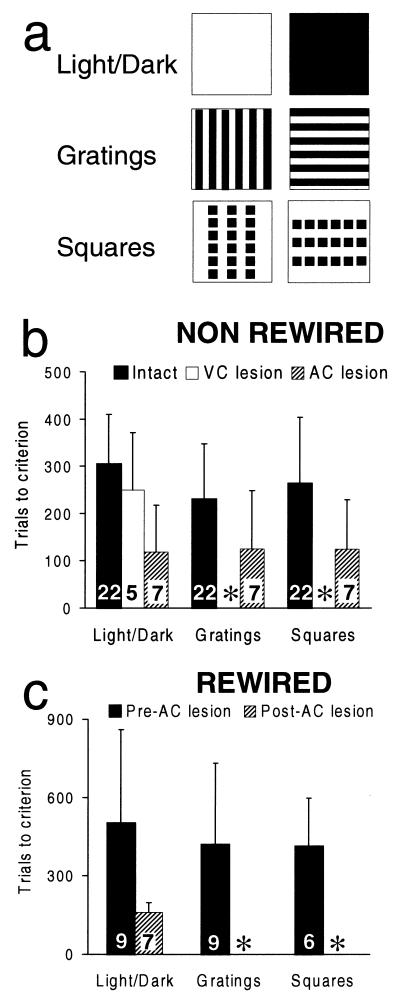
In hamsters, as in other mammals, multiple, parallel pathways mediate different aspects of visual processing (1, 11). In rodents, the ability to distinguish horizontal from vertical square wave gratings or horizontal from vertical rows of squares (see Fig. 2a) depends on the integrity of the retina-LGd-V1 pathway‖ (12). By contrast, discrimination of light from dark stimuli (see Fig. 2a) normally depends on the pretectal nuclei (20). Therefore, to study the efficacy of the surgically induced pathways at the behavioral level, we asked whether animals that lack the normal retina-LGd-V1 circuit but that have ectopic retino-thalamo-cortical circuits (see Fig. 5) can discriminate differently oriented gratings or rows of squares by using those pathways.
Figure 2.
(a) Test stimuli. The spatial resolution of normal hamsters, determined behaviorally, is 0.7 cycles/deg (9). The acuity of visually responsive neurons in AC of “rewired” hamsters is lower than that of neurons in V1 of normal hamsters,§ most likely because of the reduction in retinal ganglion cell density in the former animals (10). Therefore, the test stimuli were designed to fall within the resolution of the operated hamsters. The width of the stripes in the black and white gratings was 7 mm, which corresponds to a spatial frequency of 0.11 or 0.27 cycles/deg at distances of 9 or 22 cm, respectively, the distances over which the hamsters might have discriminated the stimuli. The size of the squares in each row was 10 × 10 mm (= 6.34 × 6.34 deg or 2.6 × 2.6 deg at distances of 9 or 22 cm, respectively); there were six squares in each of the three rows, separated by a distance of 3 mm, and each row was separated by 20 mm. Vertical and horizontal stimuli were identical except for their orientations. Each of the stimuli that constituted a pair to be discriminated was the rewarded stimulus for half of the hamsters in each group. (b) Histograms showing trials to criterion on each task, for “non-rewired” hamsters. Numbers within bars indicate the number of animals from which data were obtained (see text). Error bars indicate standard deviations. ∗, Means and standard deviations cannot be calculated because only one animal learned the grating discrimination and none learned the squares discrimination. (c) Histograms showing trials to criterion for “rewired” hamsters. Same conventions as b except that ∗ indicates that no animals with complete VC lesions learned the grating or squares discriminations when the AC was also ablated.
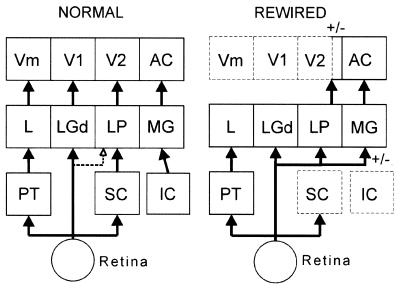
Figure 5.
Retino-thalamo-cortical pathways in normal and “rewired” hamsters (broken outlines indicate ablated structures). In normal hamsters, visual pattern discrimination is mediated by the retino-LGd-V1 pathway (dotted line from retina to LP indicates variability of projection; see text). In hamsters “rewired” as neonates, visual pattern discrimination can be mediated by the retino-LP-V2 pathway or by the retino-MG-AC pathway. +/−, Either of these surgically induced pathways can mediate visual pattern discrimination in the absence of the other (see text). Circuitry in normal animals based on refs. 11 and 19. IC, inferior colliculus; L, lateral nucleus of the thalamus; PT, pretectum.
Materials and Methods
Experimental Sequence and Surgery.
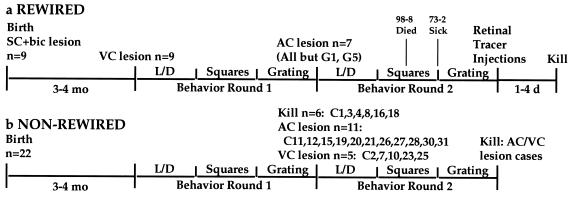
The sequence of experimental procedures is illustrated in Fig. 1. Newborn hamsters, anesthetized by hypothermia, received bilateral ablations of the SC by applying a hot pin to the surface of the overlying skull. We also transected the ascending auditory pathway to the thalamus, the brachium of the inferior colliculus, by inserting a razor knife into it through a small slit in the overlying skull. These procedures can induce the formation of permanent, ectopic retinal projections to the MG and robust retinal projections to the LP (see Figs. 4 and 5; see also refs. 1–4 and 14). At age 3–4 mo, under Nembutal anesthesia, the animals received large, bilateral aspiration lesions of the occipital cortex. The lesions, intended to ablate all of visual cortical (VC) areas V1, V2, and Vm (medial visual area) (15), varied with respect to the completeness of the V2 lesion (see below). After recovery (≥2 wk), these “rewired” animals were tested on a light/dark discrimination; those that succeeded were tested sequentially on the grating and rows of squares discriminations. As controls, we tested 22 normal adult hamsters on the same tasks. After testing, “rewired” animals that had learned the grating discrimination received bilateral ablations (by aspiration) of the auditory cortex [AC; areas 41 and 22 (18, 19)], and some normal animals were subjected to ablation of the visual cortex (n = 5 with histological analysis) or AC (n = 11; 7 with histological analysis). After recovery, the animals were retested sequentially on the three discriminations. At the end of behavior testing, “rewired” animals received binocular injections of tracers to label anterogradely their retinal projections. After an appropriate survival time, they were killed, and their brains were examined histologically.
Figure 1.
Experimental sequences for “rewired” (a) and “non-rewired” (b) hamsters. Five of nine “rewired” hamsters went through the full sequence in a. For G1 and G5, the second cortical lesion and second round of behavior testing were omitted. Subject 98-8 died during the second round of grating testing, and 73-2 became sick and in the second round was not tested on squares. Sixteen of twenty-two “non-rewired” cases went through the full sequence in b; 6/22 “non-rewired” hamsters were killed after the first round of behavior testing. Five received VC lesions. Eleven “non-rewired” cases received AC lesions, but only seven of these (C11, -19, -26, -27, -28, -30, and -31) were included in the histological/statistical analysis. bic, brachium of the inferior colliculus; VC = V1 + V2 + Vm (15).
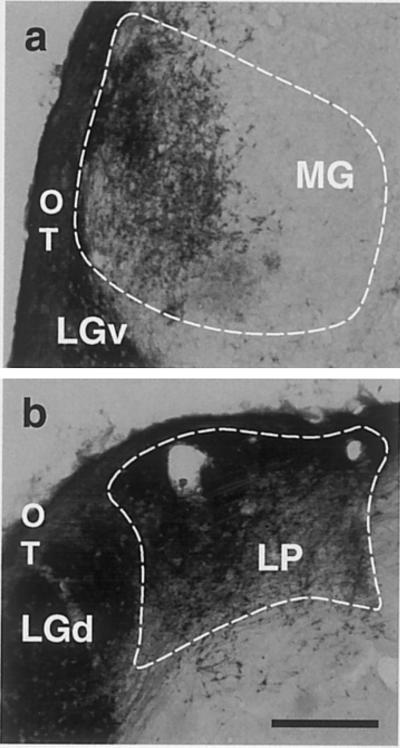
Figure 4.
Retinothalamic projections in “rewired” adult hamsters. Video micrographs showing (a) retino-MG projections (case G1) and (b) retino-LP projections (case 98-2). LGv, ventral lateral geniculate nucleus; OT, optic tract.
Behavior Testing.
All hamsters were maintained on a regular light cycle (14 h light/10 h dark). Hamsters had free access to water but were food deprived for 22 h before behavior testing during the later part of the inactive (light) phase of their diurnal cycle. Hamsters were tested on the three visual discrimination tasks in an open Y-maze illuminated by an overhead light bulb (40 or 25 W = 10.97 or 4.93 footcandles, respectively).
Each arm of the maze was 9 cm long and 10 cm wide. The arms of the maze were separated by a partition 18 cm high; a wall 18 cm high ran along the edges of the maze. A removable barrier separated a waiting chamber on the stem of the maze from the stimuli at the ends of the maze arms, 22 cm away. Stimulus cards were mounted on two hinged doors. In back of each door was a small chamber in which the hamsters received a food reward if they selected the correct stimulus. Hamsters were initially adapted to the apparatus, then trained on the light/dark discrimination. In this and the pattern discrimination tasks, after the hamster was placed on the waiting platform, the barrier was removed, permitting the animal to approach the stimuli. The left-right position of the correct stimulus was varied according to a preset pseudorandom (Gellerman) series. On each trial, the door bearing the negative stimulus was locked, whereas the door bearing the positive stimulus was unlocked, permitting the hamster to pass through it to receive food. To ensure that animals used visual cues at a distance of at least 9 cm, a trial was terminated and an error recorded if both front paws of the hamster crossed into the maze arm with the incorrect stimulus. A correction procedure was used to break the hamsters of position habits: after an erroneous choice, the hamsters were retested with the stimuli in the same positions until they chose the correct stimulus. Ten trials were presented daily until a criterion of 85% correct (90% correct on one and 80% correct on the other of two consecutive days) was attained or 500 trials were completed unsuccessfully. Testing was continued beyond 500 trials (with or without a change in ambient illumination) if and only if one of two conditions was met: (i) a hamster's performance improved significantly over the first 500 trials, as determined by linear regression analysis (P < 0.05); or (ii) a hamster had a run of sessions close to criterion (≥7/10) in the last few blocks of 10 trials that completed the first 500. Improvements in performance were reevaluated every 100–150 trials until the animals either reached criterion or stopped improving.
Pathway Tracing, Histology, and Lesion Analysis.
“Rewired” hamsters received bilateral intraocular injections (under Nembutal anesthesia) of horseradish peroxidase (HRP, 40% in H2O) or cholera toxin B (CTB, List Biological Laboratories, Campbell, CA; 1% in H2O/2% DMSO). After an appropriate survival time (24 h for HRP, 4 days for CTB), the hamsters were killed by an overdose of Nembutal and perfused (2% glutaraldehyde/1% paraformaldehyde for HRP, 4% paraformaldehyde for CTB). The brains were cryoprotected and sectioned frozen in the coronal plane. A regularly spaced series of sections were processed using standard techniques to reveal anterograde labeling of retinal projections by HRP (16) or CTB (17), and the same or adjacent sections were stained with cresyl violet. The outlines of cortical areas and thalamic nuclei were traced on the basis of standard cytoarchitectonic criteria (18, 19) using a computer-assisted microscopy system.
Results
Visual Behavior in “Rewired” Hamsters.
Complete behavioral data and histological analysis confirming the total ablation of V1 were obtained from nine “rewired” hamsters (Fig. 2, Table 1). All “rewired” hamsters were able to learn all three discriminations (Table 1, Fig. 2c) after visual cortex lesions (e.g., case G1, Fig. 3). Importantly, for all three tasks, there was no significant difference between “rewired” hamsters and intact control hamsters with respect to the number of trials it took them to learn each task (cf. Tables 1 and 2; Fig. 2 b and c; Mann–Whitney U test, 2-tailed; P > 0.05 on all three tasks).
Table 1.
Summary of data from “rewired” hamsters
| Animal | First
round behavior
|
Second round behavior
|
Retinal projections | Cortex lesion | ||||
|---|---|---|---|---|---|---|---|---|
| Light/ dark | Grating | Squares | Light/ dark | Grating | Squares | |||
| 73-2 | 680 | 1010 | 340 | 140 | 800# at 25W; then 400 at 40W | Sick | LP: (R+L); MG: R++, L++ | VC remnants: V2 (+; D; R&L); AC: lesion complete (R&L) |
| 76-4 | 650 | 840 | 240 | 160 | 500# | — | LP: (R+L) MG: R+, L− | VC: Lesion complete (R&L); AC: lesion complete (R&L) |
| 78-2 | 1280 | 50 | 180 | 190 | 500# | — | LP: (R+L) MG: R−, L− | VC: Lesion complete (R&L); AC: lesion complete (R&L) |
| 98-2 | 480 | 240 | 560 | 140 | 500# | — | LP: (R+L) MG: R+, L+ | VC: Lesion complete (R&L); AC: lesion complete (R&L) |
| 98-3 | 600 | 280 | 610 | 100 | 500 | 500# | LP: (R+L) MG: R+, L+ | VC remnants: V2 (+; D; R&L); AC: lesion complete (R&L) |
| 98-5 | 260 | 400 | 550 | 170 | 500# | — | LP: (R+L) MG: R+, L+ | VC: Lesion complete (R+L); AC: lesion complete (R+L) |
| 98-8 | 190 | 390 | 1030# | 220 | 220# (DIED) | — | LP: (R+L) MG: R++, L+ | VC remnants: V2 (+/−; D; R) AC: lesion complete (R&L) |
| G1 | 160 | 160 | 840# | * | — | — | LP: (R+L) MG: R+++, L− | VC remnants: V2? (+/−; D; R&L); AC: no lesion |
| G5 | 220 | 400 | 500# | * | — | — | **LP: (R+L) MG: L+, R− | VC remnants: V2 (+/−; D; R&L); AC: no lesion |
| MEAN | 502 | 419 | 413 | 160 | ||||
| SD | 357 | 313 | 184 | 39 | ||||
| MEDIAN | 480 | 390 | 445 | 160 | ||||
| n | 9 | 9 | 6 | 7 | ||||
Mean, SD, and median of number of trials to criterion and number of cases (n) are shown. “Sick” indicates that case 73-2 had to be euthanized prior to post-AC lesion testing on the rows of squares discrimination because it became sick. *, Two cases that did not receive an AC lesion after the first round of behavior testing. **, The extent of the retino-MG and retino-LP projections is likely underestimated in case G5 because the labeling of the retinal projections was incomplete, as demonstrated by partial labeling of the LGd. For retino-MG projections, +, ++, and +++ indicate the relative extent; − indicates no projection. #, Behavior testing was discontinued because hamsters did not reach criterion by the number of trials indicated and their performance was not improving (see text). Missing entries indicate that a hamster was not tested on the corresponding task. For cortical remnants, relative size is indicated by +/−. −, Very small, barely detectable; +, small. D, disrupted cytoarchitecture. L, left; R, right.
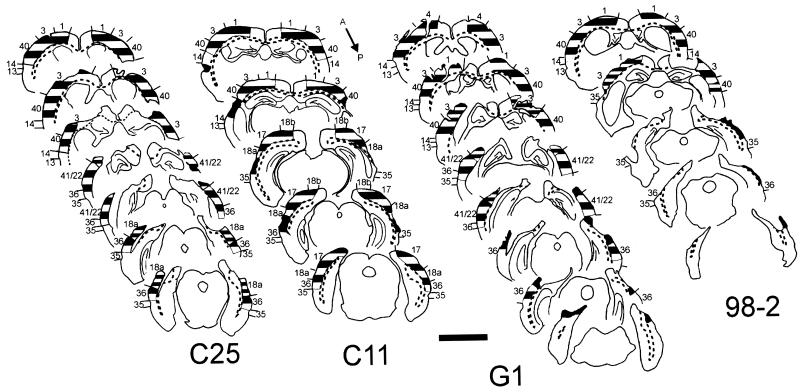
Figure 3.
Histological analysis of cortical lesions in two “rewired” (G1 and 98-2) and two “non-rewired” (C25 and C11) cases. Numbers indicate cytoarchitectonic areas. Broken lines indicate borders of cortical white matter. Before an initial round of behavior testing, both “rewired” cases received a VC lesion. No further lesions were made in the brain of case G1. In case 98-2, the first round of behavior testing was followed by an additional lesion of the AC, then further behavior testing (Fig. 1a). Cases C25 and C11 were tested behaviorally while intact, then subjected to lesions of the VC or AC, respectively, followed by a second round of behavior testing (Fig. 1b). In G1 and some other cases there were small regions at the edges of the cortical lesions in which cytoarchitectonic disruption prevented unambiguous assignment to a particular cortical area. Crosshatching: wide stripes, cortical cytoarchitecture maintained; narrow stripes, regions of disrupted architecture containing healthy neurons; black, severely damaged, gliotic cortical remnants with few if any neurons. Section spacing: G1, 720 μm; 98-2, C25, and C11, 960 μm.
Table 2.
Summary of data from hamsters that were not “rewired”
| Animal | First
round behavior
|
Posttest surgery | Second round
behavior
|
Cortex lesions | ||||
|---|---|---|---|---|---|---|---|---|
| Light/dark | Grating | Squares | Light/dark | Grating | Squares | |||
| C-1 | 200 | 60 | 350 | — | — | — | — | — |
| C-3 | 210 | 140 | 220 | — | — | — | — | — |
| C-4 | 320 | 70 | 160 | — | — | — | — | — |
| C-8 | 150 | 350 | 80 | — | — | — | — | — |
| C-16 | 420 | 280 | 320 | — | — | — | — | — |
| C-18 | 330 | 400 | 440 | — | — | — | — | — |
| C-2 | 180 | 290 | 70 | VC | 370 | 500# | — | VC remnants: V2 (+; D; R&L), V3 (+; D; R) V1 (+; R; layers IV–VI only) |
| C-7 | 110 | 200 | 230 | VC | 130 | 500# | — | VC remnants: V2 (+; R&L) V3 (+; R&L) V1 (+; R) |
| C-10 | 320 | 130 | 110 | VC | 390 | 500# | — | VC remnants: V2 (+; D; L&R), V3 (+; R&L) |
| C-23 | 390 | 260 | 160 | VC | 160 | 70 | 500# | VC remnants: V2 (+; D; R&L) |
| C-25 | 360 | 530 | 160 | VC | 200 | 500# | — | VC remnants: V2 (+; D; R&L) |
| C-11 | 310 | 250 | 260 | AC | 280 | 130 | 130 | AC remnants: (+/−; D; L) |
| C-12 | 380 | 410 | 490 | AC | 230 | 230 | 290 | Not analyzed |
| C-15 | 240 | 260 | 260 | AC | 220 | 60 | 180 | Not analyzed |
| C-19 | 470 | 190 | 180 | AC | 230 | 430 | 200 | AC remnants: (+/−; D; L) |
| C-20 | 320 | 230 | 440 | AC | 90 | 150 | 390 | Not analyzed |
| C-21 | 300 | 210 | 400 | AC | 290 | 280 | 150 | Not analyzed |
| C-26 | 310 | 110 | 540 | AC | 60 | 40 | 20 | AC lesion: complete (R&L) |
| C-27 | 240 | 240 | 400 | AC | 70 | 60 | 40 | AC lesion: complete (R&L); Also damage to WM & V2 (R) |
| C-28 | 310 | 240 | 190 | AC | 20 | 100 | 120 | AC remnants (+; L) + damage to V1,V2 (L) |
| C-30 | 400 | 150 | 260 | AC | 60 | 40 | 140 | AC lesion: complete (R&L) |
| C-31 | 430 | 60 | 80 | AC | 110 | 80 | 220 | AC lesion: complete (R&L) |
| Normal mean | 305 | 230 | 264 | VC mean | 250 | |||
| Normal SD | 95 | 119 | 140 | VC SD | 121 | |||
| Normal median | 315 | 235 | 245 | VC median | 200 | |||
| Normal n | 22 | 22 | 22 | VCn | 5 | |||
| AC mean | 119 | 126 | 124 | |||||
| AC SD | 98 | 138 | 74 | |||||
| AC median | 70 | 80 | 130 | |||||
| ACn | 7 | 7 | 7 | |||||
Normal, data collected from intact adult hamsters. After the initial round of behavior testing, some animals were subjected to ablation of the VC (n = 5 with histological analysis) or AC (n = 11, 7 with histological analysis) and retested. Statistical analyses and pre-/postoperative comparisons used only data from animals with histologically verified lesions. WM, white matter. Other conventions and abbreviations as in text and other legends.
Visual Behavior in “Rewired” Hamsters After Lesions of “Rewired” Neural Circuits.
After behavior testing, 7/9 “rewired” hamsters received bilateral lesions of the AC (e.g., Fig. 3, case 98-2). The two remaining “rewired” animals were not subjected to further lesions or behavior testing so that the extent of the original cortical lesions could be assessed without enlargement by a second lesion (e.g., Fig. 3, case G1). After recovery from the AC lesion, all seven hamsters relearned the light/dark discrimination, and their prior experience with this task significantly reduced the number of trials they required to reach criterion, i.e., there was “postoperative savings” (Fig. 2c, Table 1; Wilcoxon signed rank test; P = 0.028). Two hamsters of seven (cases 98-3 and 73-2) relearned the grating discrimination; only one of these two (98-3) could be tested subsequently on the rows of squares discrimination (Table 1 legend), which it did not relearn (Table 1). These hamsters, like 2/5 of the other hamsters that received AC lesions and 2/2 of the hamsters that did not, had a small, cytoarchitecturally disrupted remnant of visual area V2, unilaterally or bilaterally (Table 1). Retention of the ability to perform the light/dark discrimination after combined AC and VC lesions demonstrates that the lesions do not cause a nonspecific deficit in the relearning of all visual discriminations.
Visual Behavior in “Non-Rewired” Hamsters.
The preceding results contrasted with the consequences of cortex lesions in “non-rewired” hamsters. After initially learning all three tasks, five animals (e.g., case C25, Fig. 3) received visual cortex lesions, as for “rewired” hamsters. After a minimum recovery period of 2 wk, 5/5 animals relearned the light/dark discrimination (Table 2, Fig. 2b), although their prior experience with this task did not result in “postoperative savings” (Wilcoxon signed rank test; P > 0.89). One of the five animals that relearned the light/dark task was able subsequently to relearn the grating discrimination but it did not relearn the rows of squares discrimination (Table 2). This animal had a remnant of the lateral part of V2 bilaterally, although other animals with V2 remnants did not relearn the task (Table 2). Most probably, in the exceptional hamster, the area V2 remnant received input from the part of the LP nucleus that receives the normally small and variable retino-LP projection (1, 14). In the other cases the V2 remnants either did not get their input from the retinorecipient region of LP or for some other reason were unable to mediate visual pattern discrimination. Notably [and consistent with data of others (12)], neither case with small V1 remnants relearned the pattern discrimination tasks. Performance of the light/dark discrimination by hamsters with large VC lesions excludes a nonspecific deficit in the relearning of all visual discriminations.
In “non-rewired” hamsters, lesions of the AC similar in size to those made in the visual cortex (e.g., case C11, Fig. 3) do not prevent relearning of any of the three tasks (n = 11; Fig. 2b, Table 2). “Non-rewired” hamsters subjected to AC lesions showed significant postoperative savings in relearning the light/dark discrimination (Fig. 2b, Table 2; Wilcoxon signed rank test; P = 0.018) and no significant pre/postoperative difference in the rate at which they learned the grating and rows-of-squares discriminations. Thus, in “non-rewired” hamsters, the effects of cortical lesions on visual pattern discrimination performance are dependent on the locus of the lesion.
Neural Pathways Mediating Visual Behavior in “Rewired” Hamsters.
These data establish that surgically created retino-thalamo-cortical circuits (Fig. 5) can mediate visual pattern discriminations that normally depend on the integrity of the missing retino-LGd-V1 pathway. To what extent is the residual behavioral capacity mediated by the retino-MG-AC or the retino-LP-V2 pathways, respectively? Postmortem histological analysis (Fig. 4) showed that all “rewired” hamsters had robust retino-LP projections. All but one “rewired” hamster had retino-MG projections of variable size. Small V2 remnants were present in some cases (Table 1). Correlation of the histological and behavioral data of individual cases suggests that both the retino-MG-AC pathway and the retino-LP-V2 pathway can contribute to visual pattern discrimination in “rewired” hamsters.
First, we consider some issues in lesion evaluation. (i) In some instances, the cortex at the edge of a lesion is disrupted, making its definitive cytoarchitectonic identification impossible. Such disruption can pose analytic difficulties in the region of the border between adjacent cortical areas V2 and AC. In these instances, our interpretation is conservative; to set the most stringent criteria for data supporting a role for AC in visual pattern discrimination behavior, we assume that any cytoarchitecturally ambiguous cortical remnant near the V2/AC border is part of V2 and can potentially mediate that behavior. (ii) The brains of “rewired” hamsters are smaller than normal and distorted in shape to varying degrees. Thus, it is impossible to assess quantitatively the relative fraction of any damaged cortical area that is contained in a remnant near the edge of the lesion. Comparison by inspection with brains of normal animals clearly indicates that, in all instances, cortical remnants contain only a small fraction of the cytoarchitectonic areas indicated.
Case 78-2 demonstrates the contribution of the retino-LP-V2 pathway to visual pattern discrimination. This hamster had no retino-MG projection, and the combined lesions of the VC and AC were complete. Therefore, the residual pattern discrimination capability of this hamster after the visual cortex lesion but before the AC lesion must have been due to a remnant of area V2 that received its input from the retinorecipient region of LP. This conclusion is supported by the data of cases 73-2 and 98-3, that relearned the grating discrimination after ablation of AC. Both hamsters had bilateral retino-MG and retino-LP projections. Both animals also had complete AC lesions, but both had small remnants of area V2. Thus, their pattern discrimination abilities after the AC lesion resulted from a small remnant of the retino-LP-V2 pathway, although the retino-MG-AC pathway probably also contributed to their behavioral performance before the AC lesion.
The strongest support for the contribution of the retino-MG-AC pathway to visual pattern discrimination comes from case G1. This “rewired” hamster received a visual cortical lesion that was virtually complete bilaterally. Because this animal had a large retinal projection to the left MG and its AC was intact, its performance was mediated by the retino-MG-AC pathway.
Discussion
This study demonstrates that distinct, surgically created projections can mediate some of the behavioral functions of an ablated neural pathway. Our data are consistent with several other findings. (i) Lesions sustained during development can cause changes in neural connectivity or synaptic efficacy that produce striking neurophysiological alterations (reviews in refs. 21 and 22). (ii) In patients who become blind early in life, the visual cortex appears to contribute to Braille reading (23). (iii) In deaf and hearing subjects who learn American sign language early, watching signing activates cortical regions that process language, whereas similar activation does not occur in non-signing, hearing subjects (24).
It is theoretically possible that a decrease in the total amount of cortical tissue remaining between the first and second rounds of behavior testing, rather than the location of the second lesion in the AC, accounts for the pattern discrimination performance decrement of “rewired” hamsters in the second round (“mass effect”). Several considerations render this explanation of our results highly improbable. (i) Neuroanatomical and neurophysiological data obtained in hamsters (cited above) demonstrate the existence of a functional retino-thalamo-cortical pathway through the LP/MG to V2/AC. (ii) Single unit recording and neuroanatomical pathway tracing demonstrate in normal mammals of all species examined that each cytoarchitectonic area has unique patterns of connectivity and electrical activation. (iii) Functional imaging studies demonstrate the recruitment of unique subsets of cortical areas in the performance of different behavioral tasks. Control experiments to test for a “mass effect” might be performed by behavioral testing after sequential visual and non-auditory cortex lesions in “rewired” hamsters, but such experiments would be problematic because of the paucity of “association” cortices in rodents. The secondary lesions would necessarily include cortical areas important for cognition and required for performing discriminative behaviors, or somatic sensorimotor cortices critical for locomotion.
One cannot say from our data whether “rewired” hamsters perceive a visual stimulus as auditory or visual when it activates the AC. Early blind patients appear to perceive Braille characters as somatosensory stimuli despite the contribution of their visual cortex to Braille reading (23, 25). Ferrets that have a surgically induced retino-MG-AC pathway, but no visual cortex, distinguish visual from auditory stimuli.¶ Thus, “rewired” hamsters with retino-MG projections may perceive light stimuli as visual, even in the absence of the visual cortex.
Our behavioral data have both fundamental biological and clinical significance. Together with our neurophysiological findings§ (7, 8), they suggest two hypotheses concerning the functional organization of mature sensory systems and the development of those systems. The “systems theoretic” hypothesis states that the ability of the auditory and somatosensory systems to process visual information derives from the fact that their respective primary thalamic and cortical areas analyze their inputs in ways similar to those of the primary visual thalamus and cortex, respectively (8). The “developmental” hypothesis states that, at the ontogenetic stages when “rewired” animals receive their subcortical lesions, the anatomical and biochemical substrates of sensory processing in the auditory, somatosensory, and visual thalamic nuclei or cortices are equipotential; these substrates differentiate under the influence of their afferents (8). The two hypotheses are not mutually exclusive: some features of the different mature sensory systems may be shared, whereas others may develop in an input-dependent fashion. Indeed, the relative importance of shared and activity-dependent features may differ between the novel circuits involving V2 and AC.
Our results raise the possibility that surgically created neural circuits may one day be used therapeutically. However, it should be emphasized that the visual capacities of our “rewired” hamsters are not normal. For example, visual acuity in these animals is reduced (Fig. 2 legend). The extent to which surgically created neural circuits can sustain vision must be elucidated in other species that have more highly differentiated visual systems and more elaborate visual behaviors than rodents. Furthermore, it remains to be demonstrated whether neurophysiological and behavioral results similar to those we have obtained in neonatally operated animals can be reproduced in subjects that receive lesions and reparative surgery as adults.
Acknowledgments
We thank P. Fishman, E. Kandel, B. Kolb, F. Margolis, V. Mountcastle, L. Richards, M. Schwartz, and M. Stryker for helpful comments and discussions of the manuscript. This work was supported by grants from the National Institute of Mental Health (R01-MH49568) and from the Medical Research Council of Canada (MT-13359).
Abbreviations
- AC
auditory cortex
- CTB
cholera toxin B
- HRP
horseradish peroxidase
- LGd
dorsal lateral geniculate nucleus
- LP
lateral posterior nucleus
- MG
medial geniculate nucleus
- OT
optic tract
- SC
superior colliculus
- VC
visual cortex
- V1
primary visual cortex
- V2
second visual area
Note
While this paper was under review, the data of Carman et al.¶ were published (13).
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Giguère, J.-F., Desautels, A., Ptito, M., Frost, D. O. & Casanova, C. (1998) Neurosci. Abstr. 24, 306 (abstr.).
Carman, L. S., Pallas, S. L. & Sur, M. (1992) Neurosci. Abstr. 18, 593 (abstr.).
Rothblat, L. A. & Schwartz, M. L. (1978) Neurosci. Abstr. 4, 643 (abstr.).
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.190179997.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.190179997
References
- 1.Schneider G E. Brain Behav Evol. 1973;8:73–109. doi: 10.1159/000124348. [DOI] [PubMed] [Google Scholar]
- 2.Frost D O. J Comp Neurol. 1981;203:227–256. doi: 10.1002/cne.902030206. [DOI] [PubMed] [Google Scholar]
- 3.Sur M, Garraghty P E, Roe A W. Science. 1988;242:1437–1441. doi: 10.1126/science.2462279. [DOI] [PubMed] [Google Scholar]
- 4.Crain B J, Hall W C. J Comp Neurol. 1980;193:383–401. doi: 10.1002/cne.901930206. [DOI] [PubMed] [Google Scholar]
- 5.Kalil R E, Schneider G E. Brain Res. 1975;100:690–698. doi: 10.1016/0006-8993(75)90171-7. [DOI] [PubMed] [Google Scholar]
- 6.Campbell G, Frost D O. J Comp Neurol. 1988;272:383–408. doi: 10.1002/cne.902720308. [DOI] [PubMed] [Google Scholar]
- 7.Frost D O, Métin C. Nature (London) 1985;317:162–164. doi: 10.1038/317162a0. [DOI] [PubMed] [Google Scholar]
- 8.Métin C, Frost D O. Proc Natl Acad Sci USA. 1989;86:357–361. doi: 10.1073/pnas.86.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emerson V F. Exp Brain Res. 1980;38:43–52. doi: 10.1007/BF00237929. [DOI] [PubMed] [Google Scholar]
- 10.Métin C, Irons W A, Frost D O. J Comp Neurol. 1995;353:179–199. doi: 10.1002/cne.903530203. [DOI] [PubMed] [Google Scholar]
- 11.Schneider G E. Neurosci Res Prog Bull. 1975;13:255–257. [PubMed] [Google Scholar]
- 12.Schneider G E. Science. 1969;163:895–902. doi: 10.1126/science.163.3870.895. [DOI] [PubMed] [Google Scholar]
- 13.Von Melchner L, Pallas S L, Sur M. Nature (London) 2000;404:871–876. doi: 10.1038/35009102. [DOI] [PubMed] [Google Scholar]
- 14.Crain B J, Hall W C. J Comp Neurol. 1980;193:351–370. doi: 10.1002/cne.901930204. [DOI] [PubMed] [Google Scholar]
- 15.Tiao Y C, Blakemore C. J Comp Neurol. 1976;168:459–482. doi: 10.1002/cne.901680403. [DOI] [PubMed] [Google Scholar]
- 16.Mesulam M-M. J Histochem Cytochem. 1978;26:106–117. doi: 10.1177/26.2.24068. [DOI] [PubMed] [Google Scholar]
- 17.Angelucci A, Clascá F, Sur M. J Neurosci Methods. 1996;65:101–112. doi: 10.1016/0165-0270(95)00155-7. [DOI] [PubMed] [Google Scholar]
- 18.Caviness V S., Jr J Comp Neurol. 1975;164:247–264. doi: 10.1002/cne.901640207. [DOI] [PubMed] [Google Scholar]
- 19.Caviness V S, Jr, Frost D O. J Comp Neurol. 1980;194:335–367. doi: 10.1002/cne.901940205. [DOI] [PubMed] [Google Scholar]
- 20.Blochert P K, Ferrier R J, Cooper R M. Brain Res. 1976;104:121–128. doi: 10.1016/0006-8993(76)90651-x. [DOI] [PubMed] [Google Scholar]
- 21.Frost D O. In: Advances in Neural and Behavioral Development. Casagrande V A, Shinkman P, editors. Norwood, N. J.: Ablex; 1994. pp. 123–147. [Google Scholar]
- 22.Rauschecker J P. Trends Neurosci. 1995;18:36–43. doi: 10.1016/0166-2236(95)93948-w. [DOI] [PubMed] [Google Scholar]
- 23.Cohen L G, Celnik P, Pascual-Leone A, Corwell B, Falz L, Dambrosia J, Honda M, Sadato N, Gerloff C, Catalá M D, Hallett M. Nature (London) 1997;389:180–183. doi: 10.1038/38278. [DOI] [PubMed] [Google Scholar]
- 24.Neville H J, Bavelier D, Corina D, Ruschecker J, Karni A, Lalwani A, Braun A, Clark V, Jezzard P, Turner R. Proc Natl Acad Sci USA. 1998;95:922–929. doi: 10.1073/pnas.95.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sadato N, Pascual-Leone A, Grafman J, Deiber M-P, Ibáñez V, Hallett M. Brain. 1998;121:1213–1229. doi: 10.1093/brain/121.7.1213. [DOI] [PubMed] [Google Scholar]