Abstract
Purpose:
Recent work has identified the presence of negative symptoms in a subset of temporal lobe epilepsy (TLE) patients. We hypothesized that negative symptoms in TLE are associated with disruption in the mesolimbic system.
Methods:
Basal ganglia and anterior cingulate region of interest volumes were compared between 22 TLE subjects with negative symptoms, 22 TLE group without negative symptoms, and 22 control subjects.
Results:
The negative symptom group showed significantly reduced volumes in the putamen and globus pallidus.
Discussion:
It appears these structures within the broader mesolimbic system contribute to the phenomenon of negative symptoms in TLE.
Keywords: temporal lobe epilepsy, quantitative MRI, negative symptoms, basal ganglia
Introduction
The concepts of negative and positive symptoms were first proposed by Hughlings Jackson (1931) to characterize different symptom complexes of schizophrenia. Negative symptoms include affective flattening, alogia and avolition, anergia, apathy, anhedonia, and loss of social drive. Unlike positive symptoms such as hallucinations and delusions, negative symptoms begin insidiously and patients typically report a long history of poor premorbid functioning {1}. Negative symptom patients tend to follow a chronic course with an adverse impact on quality of life, social functioning, and cognitive functioning {2}.
Recent interest has identified the presence negative symptoms in other neurological populations {3,4}. In the current study, we focus on the potential role of the basal ganglia and anterior cingulate in the expression of negative symptoms in temporal lobe epilepsy (TLE). Getz et al. {5} reported that 31% of a TLE sample demonstrated negative symptoms. There was no difference in the rate of positive symptoms or depression symptoms between the groups. Second, recent studies in TLE have demonstrated reduced D2/D3 dopamine receptor binding {6} and other volumetric abnormalities in basal ganglia nuclei {7, 8, 9}. Electrical stimulation and depth electrode studies indicate that these subcortical nuclei play a role in the behavioral manifestations, propagation, and possibly initiation of epileptic seizures {10, 11, 12}. MR volumetric abnormalities have been identified in several basal ganglia structures in TLE patients {7, 13}. It could be that in TLE, a distinct subset of patients have a propensity to propagate seizure activity beyond the temporal lobe and related structural damage to key subcortical structures within the broader mesolimbic system could be associated with negative symptoms.
In the discussion regarding a possible neural system supporting negative symptoms in schizophrenia, the mesolimbic dopamine system has received considerable attention. The mesolimbic frontal-subcortical network is comprised of many functionally related structures. The extent of direct and reciprocal connections within the network suggest that incoming cortical multimodal sensory and limbic input converge in the basal ganglia before influencing output to the anterior cingulate. After integration within the basal ganglia, these limbic, striatal, and corticocortical neural inputs combine to determine the emotional and motivational significance of incoming information and then guide output behavior accordingly. Non-human primate studies have demonstrated that selective lesioning of structures in the mesolimbic circuit results in behavior which mimic negative symptoms {14}. In first-episode neuroleptic naïve schizophrenia patients, reduced volumes of the basal ganglia have been observed compared to same age controls {15, 16}. Thus, disruption within the mesolimbic structures could result in negative symptoms including apathy, loss of motivation and diminished spontaneity, reduced verbal output, paucity of facial expression, diminished motor behavior, and increased response latency.
Examining other neurological disorders with documented structural and functional changes in key targets of basal ganglia and dopamine influence, one finds that negative symptoms often co-occur with both localized lesions {3, 17} and system-wide atrophy {18, 19, 20, 21}. In other neurological populations with prominent apathy, the basal ganglia have been targets of examination. Paul et al {4} found that increased ratings of apathy were negatively correlated with nucleus accumbens volumes in HIV+ patients. In Parkinson's disease, degeneration of the dopaminergic mesolimbic reward circuit has been offered as the putative neural substrate for apathy and anhedonia {22} and could also explain disruptions in other behaviors dependent on evaluating and reacting to incoming drive-related information. Geng et al. {23}, in a study comparing basal ganglia volumes in early and late stage Parkinson's disease patients to healthy controls, found that in the early stages of in Parkinson's disease, atrophy is observed in the putamen. In later stages, both putamen and globus pallidus volumes are significantly reduced compared to controls. However, the authors did not discuss psychiatric disorder in the patient groups.
In summary, converging evidence suggests that damage at different locations within the mesolimbic circuit may result in a system-wide disruption in behavior manifested in the distinct behavioral phenomenon of negative symptoms. Given the evidence of volume abnormalities in these regions in TLE, we hypothesized that: 1) TLE subjects with negative symptoms would show more volume loss in the basal ganglia and anterior cingulate than a TLE group without negative symptoms, and 2) decreased volume in basal ganglia and anterior cingulate would be associated with increased number of negative symptoms.
Methods
Participants
A consecutive series of 100 patients with temporal lobe epilepsy and 92 healthy controls were available for inclusion in the current study. From this set, 22 patients with negative symptoms (NEG) were matched on age of epilepsy onset to 22 epilepsy patients without negative symptoms (NoNEG) and 22 healthy controls (C). Age and gender was also used in matching subjects.
Subjects were recruited from the University of Wisconsin Epilepsy Clinic as part of a larger project examining the neurodevelopmental features of TLE. Individuals were excluded due to mental retardation (IQ < 70) or additional neurological diagnoses. Initial selection criteria for the epilepsy patients included: a) a chronological age from 18 to 60 years, b) complex partial seizures. Diagnosis was conducted by a board certified neurologist with expertise in epileptology who reviewed patients' medical records. This review was blinded to all quantitative imaging data reported here. Healthy controls were primarily spouses or friends of the TLE participants. Controls were excluded if they were taking psychotropic medications or if there was evidence of a medical or psychiatric condition or current substance abuse that could affect cognitive functioning. Informed consent was obtained from all participants prior to the beginning of study procedures.
Assessment of Negative Symptoms
All participants underwent a comprehensive psychiatric assessment including the Scale for the Assessment of Negative Symptoms (SANS) {24} and b) Beck depression inventory. For the SANS, ratings were conducted by three trained investigators for negative symptoms on the SANS. These raters were blinded to information regarding the participants' cognitive performance or MRI volumes. The SANS contains four global domains including affective flattening or blunting, alogia, avolition-apathy, and anhedonia-asociality. The four domains consist of a total of eighteen individual items. Each subject was rated from absent (0) to severe (5) on each item by three independent raters who then reached a consensus on SANS ratings.
Quantitative MRI Volumetrics
MRIs were acquired at the University of Wisconsin-Madison and processed using semi-automated software package, i.e. Brain Research: Analysis of Images, Networks, and Systems (BRAINS2). University of Wisconsin staff was blinded to the clinical, sociodemographic and neuropsychological characteristics of the subjects. The T1 weighted images were spatially normalized so that the anterior-posterior axis of the brain was realigned parallel to the ACPC line and the interhemispheric fissure was aligned on the other two axes. A six-point linear transformation was used to warp the standard Talairach atlas space onto the resampled image. Images from the three pulse sequences were then co-registered using a local adaptation of automated image registration software. Following alignment of the image sets, the PD and T2 images were re-sampled into 1 mm cubic voxels following which an automated algorithm classified each voxel into gray matter, white matter, CSF, blood or “other.” The brains were then removed from the skull using a neural network application that had been trained on a set of manual traces. Manual inspection and correction of the output of the neural network tracing was conducted. A stereotaxic method based on the Talairach atlas yields measures of left and right frontal, temporal, parietal and occipital lobes and cerebellum. The BRAINS2 software and procedures have been shown to have high inter-rater reliability, intra-rater reliability and scan-rescan reproducibility {25}.
For this investigation, recently developed automated neural networks for the basal ganglia structures including the caudate, putamen, globus pallidus were manually edited based on guidelines published by the University of Iowa Imaging Center {26}. Manual traces of the nucleus accumbens and anterior cingulate were adapted from guidelines available in the scientific literature {27, 28}. All traces were conducted blinded to group membership. Inter-rater reliability was as follows for the ROIs: caudate nucleus (.97), putamen (.98), globus pallidus (.94), nucleus accumbens (.98), and anterior cingulate (.83). Figures 1 and 2 depict the regions under investigation.
Figure 1.
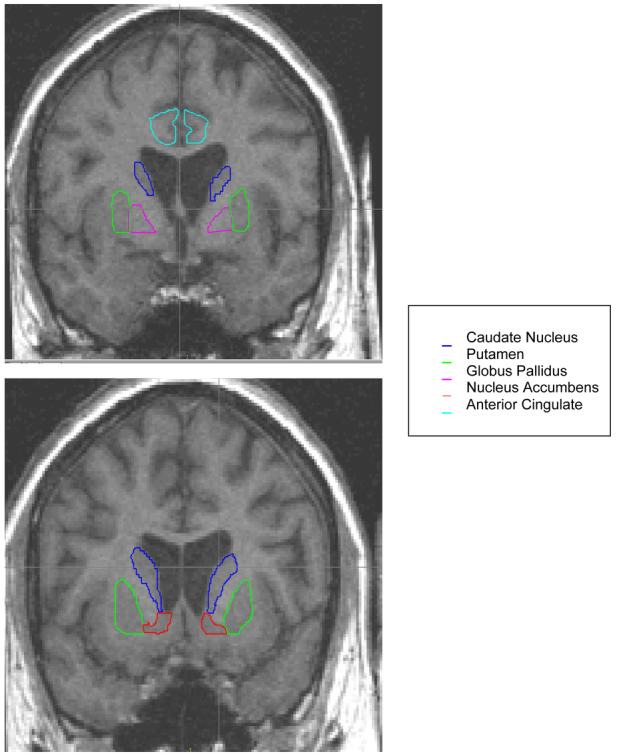
Basal Ganglia and Anterior Cingulate Regions of Interest
Figure 2.
Anterior Cingulate Region of Interest
Data Analyses
MRI Volumetric Statistical Analyses
MRI volumes were standardized in two ways relative to the value of the total sample of controls (n = 92): 1) using a regression based z-score transformation controlling for the effects of intracranial volume (ICV), and 2) using a regression based z-score transformation controlling for the effects of height. Beta weights, regression coefficient, and the standard residuals from these analyses were then used to calculate z-scores for the two epilepsy groups (n = 44) and the subsample of controls included in the current study (n = 22). Analyses were run using both ICV and height z-score transformations with no difference on the direction of the findings or statistical significance. For the reported analyses, height was chosen as a covariate as it had fewer problems with collinearity with the ROIs compared to intracranial volume.
Results
Table 1 details the demographic and clinical features of the epilepsy and control groups. The two epilepsy groups showed no significant differences on basic clinical seizure characteristics including duration, age of onset, number of antiepileptic medications, number of secondarily generalized seizures, or frequency of complex-parital seizures.
Table 1.
Demographic Characteristics of Temporal Epilepsy Patients and Comparison Subjects
| Control (N=22) |
No Negative (N=22) |
Negative (N=22) |
||||
|---|---|---|---|---|---|---|
| Mean | sd | Mean | sd | Mean | sd | |
| Age (yrs) | 35.9 | 11.2 | 36.6 | 12.0 | 37.9 | 11.0 |
| Height | 66.7 | 3.5 | 66.5 | 3.5 | 67.1 | 4.6 |
| Frequency of Complex Partial Seizures | 2.2 | 1.4 | 2.5 | 1.1 | ||
| Frequency of Secondarily Generalized Seizures |
0.6 | 1.3 | 1.9 | 2.1 | ||
| Duration (yrs) | 21.2 | 9.7 | 25.1 | 13.9 | ||
| Age of Onset (yrs) | 15.4 | 9.04 | 12.9 | 8.9 | ||
| Antiepileptic Medication (AED) | 1.7 | .9 | 2.0 | .8 | ||
| BDI * | 5.1 | 3.9 | 8.2 | 8.8 | 9.8 | 8.3 |
| Gender (M/F) | 7/15 | 6/16 | 8/14 | |||
| Number Mono/Poly AED Therapy | 10/12 | 6/16 | ||||
| Range | 1-26 | |||||
| Number of Positive Symptoms | 0 | 0 | 0 | 0 | 0.5 | 0.8 |
| Number of Negative Symptoms | 0 | 0 | 0 | 0 | 8.8 | 6.6 |
significant between Negative versus Controls
Negative Symptoms
The NEG group had an average of 8.8 negative symptoms (SD =6.6, range = 1-26) compared to NoNEG and control groups. Affective flattening was the most common negative symptom (59.0%) followed by alogia (45.5%), avolition (24.0%), and anhedonia (18.2%). The control and NEG groups differed on total number of depression symptoms as assessed by the BDI t (43) =−2.24, p=.03. However, there was no significant difference between the two epilepsy groups on BDI score t (38) = −.613, p=.54, or a significant correlation between number of negative symptoms and total BDI score (r =.12, p=.47), or BDI and any of the ROI volumes examined (all p's >.05).
Volumetric Region of Interest Findings
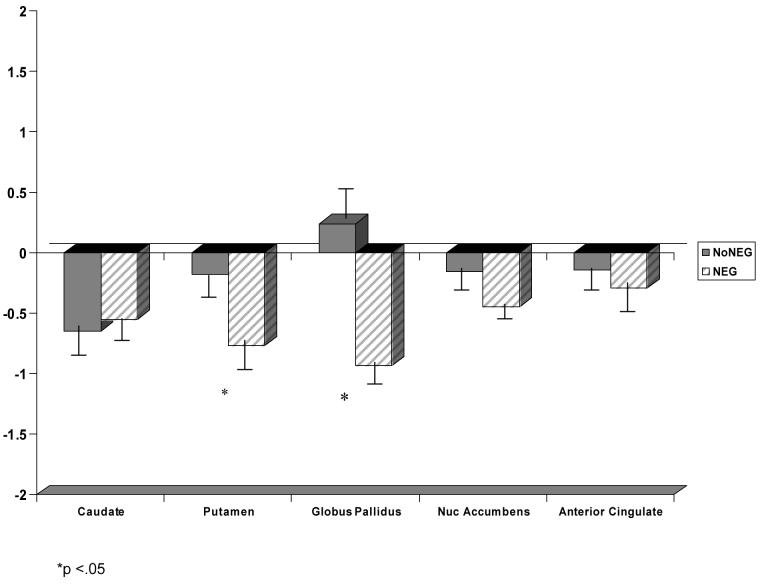
Using Wilks' criterion, a multivariate analysis of variance including all five ROIs (i.e., average left/right, adjusted for height) yielded a significant difference between groups F (10, 114) = 2.74, eta squared = .19, p= .01. Univariate analyses determined that the three groups differed significantly on volumes of the caudate nucleus F (2, 61) = 3.13, eta squared =.09, p= .05, the putamen F (2, 61) = 3.69, eta squared =.11, p= .03, and the globus pallidus F (2, 61) = 9.65, eta squared =.24, p= .001.
Of particular interest are regions in which the NEG TLE group shows significant volume differences from both the control and the NoNEG TLE group, and regions which also showed no differences between the NoNEG and control groups. Figure 3 displays these results. Significant differences were found between the control and NEG group for putamen, t (43) = 2.43, eta squared= .12, p= .02 and the globus pallidus, t (43) = 3.59, eta squared = .23, p=.001. Critically, the NEG group also had significantly smaller putamen t (39) = 2.21, eta squared= .11, p=.03 and globus pallidus t (39) = 3.85, eta squared = .28, p=.00 volumes compared to the NoNEG group. There were no significant differences observed between the control and NoNEG epilepsy groups on putamen t (40) = .39, eta squared = .00, p=.70 or globus pallidus t (40) = −.51, eta squared = .00, p=.62. No significant difference was found between the three groups for the volumes of the nucleus accumbens or anterior cingulate. The epilepsy groups did have smaller caudate volumes than the control group, but there was no difference between the epilepsy groups on the volumes of the caudate. Moreover, there was no significant difference between the two epilepsy groups on right t (35) = −1.02, p = .34 or left t (35) = −.76, p = .45 temporal lobe volume or volumes of the right hippocampus t (34) = −.03, p = .98 or left hippocampus t (34) = 1.02, p = .31. There was no significant relationship between antiepileptic medication and brain volumes.
Figure 3.
Average Z-Score MR Volumes of NO NEG and NEG TLE Groups
To further examine the relationship between MRI volumes and negative symptoms, we conducted Pearson product-moment correlation coefficients. The negative symptom of alogia was correlated with both the volume of the globus pallidus (r = −.30, p = .02) and the putamen (r = −.29, p = .02). Affective flattening was significantly correlated with the globus pallidus (r = −.32, p = .01). Thus, a higher number of negative symptoms on these dimensions were associated with lower volumes in these regions of interest. There was no relationship between volumes and the negative symptoms of anhedonia-asociality and avolition-apathy.
Discussion
In the current study, we hypothesized that negative symptoms in TLE result from a system-wide disruption in an integrated neural network including the caudate, putamen, globus pallidus, nucleus accumbens, and anterior cingulate. Consistent with this hypothesis, the overall omnibus contrast indicated a significant difference between groups on volumes of these regions. The NEG group had less volume in all five regions of interest and showed significantly reduced volumes compared to both control and the epilepsy NoNEG symptom groups in two regions of interest within the basal ganglia network, the putamen and globus pallidus. In addition, increased negative symptoms were associated with decreased volume in these two structures. We suggest that these two subcortical structures may play an important role in the expression of a system-wide disruption in a network that is associated with negative symptoms.
To our knowledge, this is the first study to determine that basal ganglia involvement is related to the phenomenon of negative symptoms in TLE. In that context, it is noteworthy that the two epilepsy groups did not differ from each other in temporal lobe and hippocampal volumes, but both groups significantly differed from controls. In addition, the TLE groups did not differ on an objective measure of depression, and negative symptoms were not related to depression (i.e., BDI) symptoms or basal ganglia volumes.
Consistent with our findings, negative symptoms have been observed in patients with lesions localized to both the putamen and the globus pallidus. Troyer et al. {29} found that unilateral left putamen ischemic infarction resulted in alogia and abulia. Strub {30} described a male with bilateral hemorrhages in the globus pallidus who evidenced apathy, social withdrawal, flat affect, and alogia. In our sample, globus pallidus and the putamen volume were correlated with alogia; globus pallidus volume correlated with affective flattening. In schizophrenia patients, the volume of the globus pallidus has also been shown to be correlated with severity of negative symptoms {31}.
Volume reductions in the globus pallidus and putamen have also been observed in schizophrenia and Parkinson's disease. Kreczmanski et al. {32} conducted a post-mortem study of subcortical structures in thirteen male schizophrenia patients and thirteen age matched controls. While the authors did not discuss the relationship between neuronal changes to positive or negative symptoms, they found reductions in the volume and total number of neurons in the putamen of the schizophrenia patients. Moreover, volumes of both the putamen and globus pallidus, but not the caudate nucleus, have recently been shown to be reduced in early and late stage Parkinson's disease {23}.
Accumulating evidence indicates that subcortical structures play a role in the behavioral manifestations, propagation, and possibly initiation of epileptic seizures {10, 11, 33}. The ventral amygdalofugal pathway projects directly from the amygdala to the ventral striatum with some of its densest connections to the putamen and globus pallidus {33}. In TLE, seizures that originate in the amygdala have been shown to have a high propensity to propagate within the mesial temporal lobe and to extratemporal areas {12, 34}. It is possible that the amygdalofugal propagation pathway differentially impacts the globus pallidus and putamen in the NEG sample. Behavioral changes, including negative symptoms, have been observed in patients exhibiting amygadalar dysfunction {35, 36}.
Study Limitations & Future Directions
This is a community based sample of epilepsy patients who were recruited as part of a larger investigation of the neurobehavioral features of TLE. As most of these participants were not surgical candidates and did not undergo video-EEG monitoring, definitive information on the laterality of seizure focus was not available. In that context, we only examined average volume in the basal ganglia areas of interest.
We have speculated on the importance of the amygdala and its interconnectivity with the basal ganglia in the phenomenon of negative symptoms. Amydalar damage has been observed in TLE {37}. While there is support for this possibility in both animal and human clinical literature, we did not conduct a direct volumetric trace of the amygdala. Future research focusing on the role of the amygdala and negative symptoms in TLE appears warranted.
In our study, we examined volumetric reductions in a sample of TLE patients with and without negative symptoms compared to age and gender matched controls. We found volume reductions in our TLE patients relative to controls in three out of the five ROIs. Further, we suggest that the volume reductions in the putamen and globus pallidus observed between the two TLE groups is related to a system-wide disruption associated with negative symptoms. However, future studies should examine these critical areas using other epilepsy groups such as extratemporal or non-focal generalized epilepsy types. If such groups demonstrate negative symptoms, but not volumetric reductions in the two ROIs, it would further strengthen our contention that the volume reductions observed in these two ROIs are specific to negative symptoms in TLE.
In our sample, the epilepsy patients were matched on duration of epilepsy. However, as a cross-sectional study, it is unclear when negative symptoms emerged in the NEG patients. In retrospective studies in schizophrenia patients, negative symptoms have been reported years prior to the onset of the first psychotic episode {38}. Recent attention has been devoted to determining whether the severity of negative symptoms in neuroleptic-naïve schizophrenia relates to volumetric changes. In a cross-sectional study involving schizophrenia patients, the volume of the globus pallidus have been shown to be correlated with severity of negative symptoms {31}. In volumetric studies of first episode of schizophrenia patients, Glenthoj et al. {15} found evidence of basal ganglia pathology prior to initiating treatment with neuroleptics.
Conclusions
Results of this study implicate specific nodes (i.e., globus pallidus and putamen) within the broader mesolimbic system in the phenomenon of negative symptoms in TLE. We suggest that these two subcortical structures may play an important role in the expression of a system-wide disruption in a neural network that is associated with negative symptoms.
Acknowledgements
The first author received a dissertation fellowship from the Epilepsy Foundation of America to support her work on this project. This work was also supported by 2RO1 NINDS 37738 and MO1 RR 03186 (GCRC). The authors would like to thank Drs. Jana Jones and Brian Bell for their help on this project. Additional thanks goes to Dalin Pulsipher, M.S, Jared Morton, M.S. and Leslie Guidotti, B.A. for their participation in the reliability analyses.
References
- 1.Andreasen N. Positive versus negative symptoms: a critical evaluation. Schizophr Bull. 1985;11(3):380–389. doi: 10.1093/schbul/11.3.380. [DOI] [PubMed] [Google Scholar]
- 2.Selten J, Wiersma D, vanden Bosch R. Distress attributed to negative symptoms in schizophrenia. Schizophr Bull. 2000;26(3):737–744. doi: 10.1093/oxfordjournals.schbul.a033490. [DOI] [PubMed] [Google Scholar]
- 3.Levy M, Cummings J, Fairbanks L, Masterman D, Miller B, Craig A, Paulsen J, Litvan I. Apathy is not depression. Journal Neuropsychiatry Clin Neurosciences. 1998;10:314–319. doi: 10.1176/jnp.10.3.314. [DOI] [PubMed] [Google Scholar]
- 4.Paul R, Brickman A, Navia B, Hinkin C, Malloy P, Jefferson A, Cohen R, Tate D, Flanigan T. Apathy is associated with volume of the nucleus accumbens in patients infected with HIV. J Neuropsychiatry Clin Neuroscience. 2005;17(2):167–171. doi: 10.1176/appi.neuropsych.17.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Getz K, Hermann B, Seidenberg M, Bell B, Dow C, Jones J, Woodard A, Rutecki P, Sheth R, O'Leary D, Magnotta V. Negative symptoms in temporal lobe epilepsy. American Journal of Psychiatry. 2002;159(4):644–651. doi: 10.1176/appi.ajp.159.4.644. [DOI] [PubMed] [Google Scholar]
- 6.Werhahn K, Landvogt C, Klimpe S, Buchholz H, Yakushev I, Siessmeier T, Muller-Forell W, Piel M, Rosch F, Glaser M, Schreckenberger M, Bartenstein P. Decreased dopamine D2/D3-receptor binding in temporal lobe epilepsy: an [18F]fallypride PET study. Epilepsia. 2006;47(8):1392–1396. doi: 10.1111/j.1528-1167.2006.00561.x. [DOI] [PubMed] [Google Scholar]
- 7.Dreifuss S, Vingerhoets F, Lazeyras F, Andino S, Spinelli L, Delavelle J, Seeck M. Volumetric measurements of subcortical nuclei in patients with temporal lobe epilepsy. Neurology. 2001;57(9):1636–1641. doi: 10.1212/wnl.57.9.1636. [DOI] [PubMed] [Google Scholar]
- 8.Parashos I, Oxley S, Boyko O. In vivo quantification of basal ganglia and thalamic degenerative changes in two temporal lobectomy patients with affective disorder. J Neuropsychiatry Clin Neurosci. 1993;5(3):337–341. doi: 10.1176/jnp.5.3.337. [DOI] [PubMed] [Google Scholar]
- 9.Szabo C, Lancaster J, Lee S, Xiong J, Cook C, Mayes B, Fox P. MR imaging volumetry of subcortical structures and cerebellar hemispheres in temporal lobe epilepsy. AJNR Am J Neuroradiol. 2006;27(10):2155–2160. [PMC free article] [PubMed] [Google Scholar]
- 10.Dematteis M, Kahane P, Vercueik L, Depaulis A. MRI evidence for the involvement of basal ganglia in epileptic seizures: an hypothesis. Epileptic Disord. 2001;5(3):161–164. [PubMed] [Google Scholar]
- 11.Norden A, Blumenfeld H. The role of subcortical structures in human epilepsy. Epilepsy and Behavior. 2002;3(3):219–231. doi: 10.1016/s1525-5050(02)00029-x. [DOI] [PubMed] [Google Scholar]
- 12.Wennberg R, Arruda F, Quesney L, Olivier A. Preeminence of extrahippocampal structures in the generation of mesial temporal seizures: Evidence from human depth electrode recordings. Epilepsia. 2002;42(7):716–726. doi: 10.1046/j.1528-1157.2002.31101.x. [DOI] [PubMed] [Google Scholar]
- 13.Pulsipher D, Seidenberg M, Morton J, Geary E, Parrish J, Hermann B. MRI volume loss of subcortical structures in unilateral temporal lobe epilepsy. Epilepsy & Behavior. 2007;11(3):442–449. doi: 10.1016/j.yebeh.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldman P, Rosvold H. The effects of selective caudate lesions in infant and juvenile rhesus monkeys. Brain Research. 1972;43:53–66. doi: 10.1016/0006-8993(72)90274-0. [DOI] [PubMed] [Google Scholar]
- 15.Glenthoj A, Glenthoj B, Mackeprang T, Pagsberg A, Hemmingsen R, Jernigan T, Baare W. Basal ganglia volumes in drug-naive first-episode schizophrenia patients before and after short-term treatment with either a typical or an atypical antipsychotic drug. Psychiatry Research. 2007;154(3):199–208. doi: 10.1016/j.pscychresns.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Gunduz H, Wu H, Ashtari M, Bogerts B, Crandall D, Robinson D, Alvir J, Lieberman J, Kane J, Bilder R. Basal ganglia volumes in first-episode schizophrenia and healthy comparison subjects. Biological Psychiatry. 2002;52:801–808. doi: 10.1016/s0006-3223(01)01345-2. [DOI] [PubMed] [Google Scholar]
- 17.Galynker I, Levinson I, Miner C. Negative symptoms in patients with basal ganglia strokes. Neuropsychiatry, Neuropsychol Behav Neurology. 1995;8(2):113–117. [Google Scholar]
- 18.Aarsland D, Litvan I, Larsen J. Neuropsychiatric symptoms of patients with progressive supranuclear palsy and parkinson's disease. J Neuropsychiatry Clin Neurosci. 2001;13(1):42–49. doi: 10.1176/jnp.13.1.42. [DOI] [PubMed] [Google Scholar]
- 19.Buchsbaum M. The frontal lobes, basal ganglia, and temporal lobes three sites for schizophrenia. Schizophr Bull. 1990;16(3):379–389. doi: 10.1093/schbul/16.3.379. [DOI] [PubMed] [Google Scholar]
- 20.Reichman W, Negron A. Negative symptoms in the elderly patient with dementia. Int Journal of Geriatric Psychology. 2001;16(Suppl 1):S7–S11. doi: 10.1002/1099-1166(200112)16:1+<::aid-gps566>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 21.Shenton M, Dickey C, Frumin M, McCarley R. A review of MRI findings in schizophrenia. Schizophr Bull. 1997;23:483–501. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isella V, Iurlaro S, Piolti R, Ferrarese C, Frattola L, Appollonio I. Physical anhedonia in parkinson's disease. J Neurol Neurosurg Psychiatry. 2003;74:1308–1311. doi: 10.1136/jnnp.74.9.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geng D, Li Y, Zee C. Magnetic resonance imaging-based volumetric analysis of basal ganglia nuclei and substantia nigra in patients with Parkinson's disease. Neurosurgery. 2006;58(2):256–262. doi: 10.1227/01.NEU.0000194845.19462.7B. [DOI] [PubMed] [Google Scholar]
- 24.Andreasen N. Scale for the Assessment of Negative Symptoms (SANS) University of Iowa; Iowa City, Iowa: 1983. [Google Scholar]
- 25.Magnotta VA, Bockholt HJ, et al. Subcortical, cerebellar, and magnetic resonance based consistent brain image registration. Neuroimage. 2003;19:233–245. doi: 10.1016/s1053-8119(03)00100-9. [DOI] [PubMed] [Google Scholar]
- 26.University of Iowa, Mental Health Clinical Research Center Imaging Processing Online www.psychiatry.uiowa.edu/mhcrc/IPLpages/manual_tracing.htm.
- 27.Crespo-Facorro B, Kim J, Andreasen N, O'Leary D, Wiser A, Bailey J, Harris G, Magnotta V. Human frontal cortex: an MRI-based parcellation method. Neuroimage. 1999;10(5):500–519. doi: 10.1006/nimg.1999.0489. [DOI] [PubMed] [Google Scholar]
- 28.Sullivan E, Deshmukh A, De Rosa E, Rosenbloom M, Pfefferbaum A. Striatal and forebrain nuclei volumes: contribution to motor function and working memory deficits in alcoholism. Biol Psychiatry. 2005;57(7):768–776. doi: 10.1016/j.biopsych.2004.12.012. 2005. [DOI] [PubMed] [Google Scholar]
- 29.Troyer A, Black S, Armilio M, Moscovitch M. Cognitive and motor functioning in a patient with selective infarction of the left basal ganglia: evidence for decreased non-routine response selection and performance. Neuropsychologia. 2004;42(7):902–911. doi: 10.1016/j.neuropsychologia.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 30.Strub R. Frontal lobe syndrome in patient with bilateral globus pallidus lesions. Arch Neurology. 1986;46:1024–1027. doi: 10.1001/archneur.1989.00520450096027. [DOI] [PubMed] [Google Scholar]
- 31.Spinks R, Nopoulos P, Ward J, Fuller R, Magnotta V, Andreasen N. Globus pallidus volume is related to symptom severity in neuroleptic naïve patients with schizophrenia. Schizophr Res. 2005;73(23):229–233. doi: 10.1016/j.schres.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 32.Kreczmanski P, Heinsen H, Mantua V, Woltersdorf F, Masson T, Ulfig N, Schmidt-Kastner R, Korr H, Steinbusch H, Hof P, Schmitz C. Volume, neuron density and total neuron number in five subcortical regions in schizophrenia. Brain. 2007;130(Pt 3):678–692. doi: 10.1093/brain/awl386. [DOI] [PubMed] [Google Scholar]
- 33.Lothman E, Bertram E, Stringer J. Functional anatomy of hippocampal seizures. Prog Neurobiol. 1991;37:1–82. doi: 10.1016/0301-0082(91)90011-o. [DOI] [PubMed] [Google Scholar]
- 33.Fudge J, Kunishio K, Walsh P, Richard C, Haber S. Amygdaloid projections to ventromedial striatal subterritories in the primate. Neuroscience. 2002;110(2):257–275. doi: 10.1016/s0306-4522(01)00546-2. [DOI] [PubMed] [Google Scholar]
- 34.Bartolomei F, Wendling F, Bellanger J, Regis J, Chauvel P. Neural networks involving the medial temporal structures in temporal lobe. Clin Neurophysiol. 2001;112(9):1746–1760. doi: 10.1016/s1388-2457(01)00591-0. [DOI] [PubMed] [Google Scholar]
- 35.Bowers D, Miller K, Mikos A, Kirsch-Darrow L, Springer U, Fernandez H, Foote K, Okun M. Startling facts about emotion in Parkinson's disease: blunted reactivity to aversive stimuli. Brain. 2006;129:3356–3365. doi: 10.1093/brain/awl301. [DOI] [PubMed] [Google Scholar]
- 36.Butman J, Allegri R, Thomson A, Fontela E, Abel C, Viaggio B, Drake M, Serrano C, Lon L. Behavioral flexibility impairment with negative feedback in refractory temporal lobe epileptic patients with unilateral amygdala and hippocampal resection. Actas Esp Psiquiatr. 2007;35(1):8–14. [PubMed] [Google Scholar]
- 37.Bernasconi N, Natsume J, Bernasconi A. Progression in temporal lobe epilespy: differential atrophy in mesial temporal structures. Neurology. 2005;65(2):223–228. doi: 10.1212/01.wnl.0000169066.46912.fa. [DOI] [PubMed] [Google Scholar]
- 38.Malaspina D, Goetz R, Yale S, Berman A, Friedman J, Tremeau F, Printz D, Amador X, Johnson J, Brown A, Gorman J. Relation of familial schizophrenia to negative symptoms but not to the deficit syndrome. Am J Psychiatry. 2000;157(6):994–1003. doi: 10.1176/appi.ajp.157.6.994. [DOI] [PubMed] [Google Scholar]