Abstract
Two isoforms of HSP90, α and β, are abundantly expressed in the cytoplasm of cells, yet only HSP90α serves as a chaperone to potentiate epitope presentation in the context of MHC class I molecules. By contrast, the role of HSP90 isoforms in MHC class II presentation of exogenous and endogenous Ags remains less clear. Studies here using human B lymphoblasts demonstrate the importance of HSP90α and HSP90β isoforms in selectively regulating class II presentation of the diabetes autoantigen, glutamic acid decarboxylase (GAD). Inactivation of HSP90 function using geldanamycin (GA) or radicicol (RA) inhibited MHC class II presentation of exogenous and endogenous GAD, but did not perturb the presentation of several other intra- and extracellular Ags. Treatment of human B cells with GA and RA did not alter cellular MHC class II expression, but did induce a stress response in these APCs. Yet, cell stress alone failed to perturb MHC class II presentation of GAD. HSP90 was found to associate with select Ags such as GAD in cells and ex vivo. Knockdown of HSP90α or HSP90β expression using siRNA decreased the abundance of each isoform respectively, but did not affect MHC class II expression or induce a stress response. Notably, disruption of HSP90α or HSP90β expression specifically inhibited class II presentation of exogenous and endogenous GAD Ag. Pre-complexing HSP90 with GAD Ag enhanced exogenous GAD Ag presentation. These results demonstrate a requirement for HSP90α and HSP90β in regulating class II presentation of select Ags.
The following disclaimer is a requirement of The Journal of Immunology: “This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the United States National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.”
Keywords: B cells, Antigen Presentation/Processing, Antigens/Peptides/Epitopes, MHC, Autoimmunity, Human
APCs internalize and process exogenous Ags to yield antigenic peptides in endosomal/lysosomal compartments. MHC class II molecules transit these same compartments to selectively bind peptides. The resulting MHC class II:peptide complexes are then presented on the cell surface to circulating CD4+ T cells. Class II molecules also present peptides derived from cytoplasmic and nuclear Ags (1, 2). During studies to identify naturally processed T cell epitopes encoded in the diabetes autoantigen GAD, B cells were transduced to express endogenous, cytoplasmic human GAD (3, 4). These investigations revealed that B cells efficiently process and present both exogenous and endogenous sources of GAD via class II molecules (4). Exogenous GAD is presented following processing via the classical pathway requiring endosomal/lysosomal acidification and lysosomal proteases (4). Class II presentation of endogenous GAD is dependent upon cytoplasmic proteases, yet GAD epitopes are ultimately delivered into the endosomal network for final trimming by lysosomal proteases (4). Studies also demonstrated that both endogenous and exogenous GAD epitope presentation is modulated by intracellular HLA-DM (5). These studies have revealed key steps in the processing and presentation of autoantigens such as GAD which might be manipulated to regulate autoimmunity.
Research by our laboratory and others suggests that heat shock proteins (HSPs) such as HSC70 and HSP70 can act as chaperones to facilitate Ag processing for MHC class I and II presentation (6–16). Additionally, HSP90 has been implicated in regulating both direct and cross-presentation pathways for MHC class I molecules. HSP90 ligands include peptides which can bind MHC class I molecules, and inhibition of HSP90α expression disrupts cytoplasmic Ag presentation via class I molecules (13, 17). HSP90 also appears to regulate human dendritic cell (DC) functions including cell maturation, MHC class I and II expression, and MHC class II presentation (12). While MHC class II presentation pathways are regulated by the maturation state and differentiation of monocytes and DC, human B lymphoblastoid cells constitutively express high levels of HSPs, MHC, and co-stimulatory molecules to function as APCs. Thus, in the current study, B lymphoblasts were used to dissect the specific role of HSP90 isoforms in modulating Ag processing and presentation in the context of MHC class II.
B lymphocytes play an important role in the pathogenesis of type I diabetes, functioning as APCs in the autoimmune response to islet cell Ags such as GAD (18). However, it is still somewhat controversial whether resident as well as recruited MHC class II-positive cells function to present beta cell Ags in the islets of type I diabetes patients (19). Notably, in type I diabetes, beta cell stress results in an induction of HSP90 expression (20). An increase in select anti-HSP90 antibody isotypes has also been noted in type I diabetics and family members, suggesting cellular release of HSP90 (21). Thus, understanding the potential role of HSP90 isoforms in MHC class II presentation may yield mechanistic insights regarding the presentation of autoantigens such as GAD.
The current study reveals roles for HSP90 α and β isoforms in selectively modulating GAD Ag presentation. Pharmacological inhibition of HSP90 by GA and RA decreased MHC class II presentation of exogenous and endogenous GAD. Yet, class II presentation of several other Ags was unaffected by the pharmacological inhibition of HSP90. These inhibitor treatments did not affect MHC class II expression, but did induce a stress response in B cells. However, cell stress alone did not affect MHC class II presentation of GAD or other test Ags and peptides. Consistent with a direct role for HSP90 in facilitating class II presentation of select Ags, endogenous GAD Ag binding to intracellular HSP90 was detected in B cells. HSP90 association with another endogenous Ag, kappa, was not observed and presentation of this Ag was not reduced in cells with diminished HSP90 function. In vitro incubation of purified recombinant HSP90 and GAD also formed a complex, supporting a direct physical interaction between this chaperone and autoantigen. Complexing exogenous GAD with HSP90 also enhanced class II presentation of this Ag. To address the role of specific HSP90 isoforms in GAD presentation, HSP90α or HSP90β expression in B cells was disrupted using siRNA (small interfering RNA). Both HSP90 isoforms were found to be required for MHC class II presentation of GAD. These results implicate both HSP90α and HSP90β as potential regulators of autoantigen presentation by MHC class II molecules.
Materials and Methods
Cell Lines
The human B lymphoblastoid cell line Priess is homozygous for HLA-DR4 (DRA1*0101, DRB1*0401) expression. Retroviral transduction of Priess cells with GAD65 cDNA resulted in constitutive endogenous expression of GAD65 yielding PriessGAD cells (4). These B cells were maintained in Iscove’s MEM supplemented with 10% heat-inactivated calf serum, 100 µg/mL penicillin, and 100 µg/mL streptomycin. T cell hybridomas recognizing HLA-DR4 epitopes 33.1 (specific for GAD273–285), 1.21 (specific for κII145–159 epitope of Ig κ), 2.18 (specific for κI188–203 epitope of Ig κ), and 17.9 (specific for human serum albumin HSA64–76) were maintained in RPMI 1640 supplemented with 10% fetal calf serum, 50 µM β mercaptoethanol, 100 µg/mL penicillin, and 100 µg/mL streptomycin.
Antibodies, Antigens, Proteins, and Peptides
Abs used to detect human proteins include: anti-kappa pAb and anti-GAD65 pAb from Sigma (St. Louis, MO); anti-GAPDH mAb from Chemicon (Temecula, CA); anti-actin mAb, anti-HSP90α pAb, anti-HSP90β pAb from Neomarkers/Thermo Fisher (Fremont, CA); anti-HSP90 mAb, anti-HSP40 pAb, anti-HSP70 mAb, and anti-HSC70 mAb by Assay Designs (Ann Arbor, MI); anti-class II DRα (DA6.147) and anti-class II dimers (L243). The GAD6 Ab was obtained from the Developmental Studies Hybridoma Bank, Univ. Iowa (Iowa City, IA). Anti-rabbit IgG-HRP, anti-rat IgG-HRP, and anti-mouse IgG-HRP were obtained from Jackson Laboratories (West Grove, PA); anti-mouse IgG-PE was obtained from Dako (Carpinteria, CA). To detect OVA protein, rabbit anti-serum from Cappel Laboratories was used (Downington, PA).
Human GAD65 Ag was provided by J. Elliott (University of Alberta) or purchased from Diamyd (Pittsburgh, PA), and HSA Ag was purchased from Sigma-Aldrich (St. Louis, MO). Peptides GAD273–285 (IAFTSEHSHFSLK), kI 188–203 (KHKVYACEVTHQGLSS), κII145–159 (KVQWKVDNALQSGNS), and HSA64–76 (VKLVNEVTEFAKTK) were generated using FMOC technology, HPLC purified, and mass analyzed to confirm purity and structure. Purified native, human HSP90 protein was obtained from Assay Designs (Ann Arbor, MI). OVA was obtained from Sigma Aldrich (St. Louis, MO). Kappa Ag isolated from human Bence Jones protein was from Accurate Chemical and Scientific Corporation (Westbury, NY).
Pharmacological Inhibitors
The HSP90 inhibitors RA and GA were obtained from EMD Chemicals (Gibbstown, NJ). Solutions of RA and GA were freshly prepared every 1–2 months. The concentration and time of cell treatments with these agents were selected to minimize toxicity with cell viability typically ≥ 85%.
siRNA Nucleofection
HSP90α and HSP90β siRNA oligomers as well as RISC-free siRNA control were produced by Dharmacon (Lafayette, CO) (13). HSP90α, HSP90β, and RISC-free control siRNA were used at concentrations between 20–200 pMol. RISC-free siRNA lacks a signal for nuclear translocation and functions as a negative control siRNA for nucleofection. B cells were nuceleofected with siRNA and a GFP-expression plasmid in nucleofection solution V using program Y-01 prior to culture for 48 hours (Lonza; Gaithersburg, MD).
Co-Immunoprecipitation
For co-immunoprecipitation assays with B cells, cells were lysed in 1% N-octyl-β-glucopyranoside lysis buffer [10 mM Trizma base, 150 mM NaCl, 0.2 mM PMSF, 0.1 mM TLCK 1% N-octyl-β-glucopyranoside, pH = 7.4, and protease inhibitor cocktail from Sigma-Aldrich (St. Louis, MO)] for 15 minutes on ice. Lysates were first centrifuged at 1000 RPM for 5 minutes, then re-centrifuged at 14,000 RPM for 10 minutes. Pre-clearing of the lysates was performed to remove any non-specific binding. Normal rabbit serum (1:1,000 dilution) was added to the lysate and incubated at 4°C for 30 minutes while rocking. Protein G sepharose beads (Sigma-Aldrich, St. Louis, MO) were added and incubated for an additional 15 minutes at 4°C while rocking. The protein G sepharose beads were removed by centrifugation at 7000 RPM at 4°C and the cell supernatant was collected. Specific Abs or isotype control Abs were incubated with blocked PBS-washed protein G sepharose beads overnight at 4°C while rocking. After incubation, Ab-PGS complexes were washed with cold PBS and resuspended 1:1 with cold-PBS. Pre-cleared cell lysates were added to the Ab-PGS complexes and incubated at 4°C for 18 hours while rocking. The beads and captured Ag-Ab complexes were then washed three times with lysis buffer without protease inhibitors. Ag-Ab complexes were eluted by boiling in reducing sample buffer. Eluted proteins were resolved on 10–12% SDS-PAGE with Western immunoblotting performed as described below.
Western Immunoblotting
B cells were lysed at 4°C in buffer [10 mM Tris-HCL, pH 7.2, 150 mM NaCl, 1% Triton X-100, and protease inhibitor cocktail from Sigma-Aldrich (St. Louis, MO)]. Cellular protein concentrations were determined by Bio-RAD protein assay (Hercules, CA). Cell protein (50–100 µg) incubated in either reducing or non-reducing sample buffer was loaded on 10–12%-SDS-PAGE gels and transferred onto nitrocellulose membranes. Membranes were blocked overnight at 4°C and incubated with specific antibodies. Enhanced chemiluminescence (Pierce; Rockford, IL) was used to visualize antibodies on membranes. Densitometry was performed using the Quantity One program and electronic imaging (Bio-RAD).
Antigen Presentation Assays
To measure MHC class II presentation of endogenous Ags, treated B cells were washed and fixed in 0.5% paraformaldehyde (10 min, 25°C). For exogenous Ag or peptide presentation, B cells were pre-incubated with serially diluted Ags or peptides for 16 hours at 37°C. Variable numbers of B cells were added to peptide specific T cells and incubated for 24 hours at 37°C followed by IL-2 detection using a cytokine-dependent cell proliferation assay (4). Data is expressed as the average counts per minute (cpm) of triplicate samples.
Flow Cytometry
To detect cell surface expression of class II, B cells were incubated with L243 or an isotype control Ab (60 min, 4°C), washed, and incubated with PE-conjugated F(ab’)2 fragments of goat anti-mouse IgG prior to aldehyde fixation. For total class II expression, cells were fixed in 1% paraformaldehyde (10 min, 25°C) and permeabilized with PBS + 1% BSA + 0.1% NaN3 + 0.1% saponin (30 min) prior to Ab staining. Flow cytometry was performed on a FACScan™ and data analyzed using CELLQuest software.
Results
Pharmacological Inhibition of HSP90 Altered MHC Class II Presentation in an Antigen-Specific Manner
HSP90 is constitutively expressed by all cells including APCs. In order to explore the role of HSP90 in Ag processing and presentation in B cells, we utilized two HSP90 inhibitors, GA and RA. GA and RA have been shown to bind to HSP90 and at low concentrations inhibit its chaperone activity in cell lines and animals (22). PriessGAD cells were treated with GA and RA and analyzed for MHC class II presentation of endogenous, intracellular GAD. Both GA and RA treatment of PriessGAD cells resulted in a dose-dependent decrease in MHC class II presentation of endogenous GAD (Fig. 1A and B). Drug-treated PriessGAD cells were also analyzed for their capacity to present epitopes from another endogenous Ag, kappa. GA or RA treatment did not alter MHC class II presentation of endogenous kappa II or kappa I epitopes (Fig. 1C and data not shown). These results indicate that HSP90 inhibition by either GA or RA appears to perturb MHC class II presentation of select endogenous Ags such as GAD. These results point to a potential selectivity or specificity for HSP90 modulation of MHC class II presentation which is linked to Ag.
FIGURE 1.
Pharmacological Disruption of HSP90 Function Specifically Inhibited MHC Class II Presentation of GAD Ag. PriessGAD cells were treated with increasing concentrations of HSP90 inhibitors GA (A) or RA (B–F) for 18 hours. Exogenous Ags or peptides were added to cells after 2 hrs of incubation with drugs. MHC class II presentation of endogenous GAD (A and B), endogenous kappa II (C), exogenous GAD Ag (D), exogenous GAD peptide (E), or exogenous HSA Ag (F) was analyzed by T cell assays. Results are representative of 3 separate experiments. The error bars indicate the standard deviation among triplicate samples in each experiment.
The question remained whether HSP90 is involved in the classical exogenous MHC class II pathway or functions only in the endogenous MHC class II presentation pathway. Thus, Priess cells were treated with GA or RA and pulsed with several Ags and peptides prior to testing for MHC class II presentation. GA or RA treatment inhibited MHC class II presentation of exogenous GAD Ag by Priess cells (Fig. 1D and data not shown). However, neither GA nor RA treatment of Priess cells affected MHC class II presentation of exogenous HSA Ag, HSA peptide, or the antigenic GAD peptide (Fig. 1 E, F, and data not shown). Taken together, these results suggest that disruption of HSP90 function by GA and RA selectively inhibits MHC class II presentation of GAD epitopes derived from both exogenous and endogenous sources of GAD Ag.
GA and RA Treatment of APCs Induced a Heat Shock Response
Prolonged exposure of cells to HSP90 inhibitors can induce a stress response, as monitored by increased cellular expression of HSP70, HSP90, HSC70, and HSP40 protein levels (23–25). To determine if RA and GA were inducing a stress response in B cells, levels of intracellular HSP90, HSP70, HSC70, and HSP40 protein were measured by Western immunoblotting. Results show that both RA and GA treatment induced HSP70 expression and to a lesser extent HSP40 (Fig. 2A and B). HSP90 expression was also slightly induced by drug treatment as cells attempt to compensate for loss of HSP90 function as previously reported (Fig. 2A and B) (23–25). These results indicate that inhibition of HSP90 function by RA and GA treatment induced a stress response in B cells.
FIGURE 2.
Stress Induction of HSPs Was Not Responsible for Reduced GAD Ag Presentation. (A) Cell treatment with GA or RA induced a stress response. Cell lysates from PriessGAD cells treated with DMSO as a control, GA, or RA for 18 hours were analyzed by Western immunoblotting for steady state HSP90, HSC70, HSP70, HSP40, and actin expression. In all panels, the intervening lanes were removed between samples. Results are representative of 2 separate experiments. The table (B) depicts the average relative expression of HSP/HSC as measured by densitometry using data from 2 separate experiments. (C) Heat stress induces HSP expression. PriessGAD cells were incubated for 20 min at 37, 40, or 42 °C followed by a 24 hr incubation at 37°C prior to Western immunoblotting for HSP expression. Results are displayed as the average relative expression of HSP/HSC as measured by densitometry of using data from 2 separate experiments. (D) Heat stress induction of HSPs failed to disrupt GAD Ag presentation. PriessGAD cells were incubated for 20 min at 37, 40, or 42 °C followed by a 24 hr incubation at 37°C with 5 µM of exogenous HSA Ag added after 8 hrs. Samples of B cells were divided and assayed for GAD or HSA Ag presentation. T cell assays were performed as previously described. Results are representative of 3 separate experiments. In panel D, the error bars indicate the standard deviation among triplicate samples in each experiment.
Studies have shown that cell stress such as heat shock can promote enhanced Ag processing and MHC class II presentation of some exogenous and endogenous Ags by B cells (26). It is possible that the altered Ag presentation seen in drug treated PriessGAD cells was due to the induction of a stress response rather than HSP90 inhibition. Experiments were performed to determine if cell stress by heat shock altered GAD Ag presentation. PriessGAD cells were incubated at 37, 40, or 42°C for 20 minutes followed by a 24 hour incubation at 37°C. Exposure of PriessGAD cells to 40 or 42°C resulted in increased HSP90, HSP70, and HSP40 expression confirming an induced stress response (Fig. 2C). This increase in HSP/HSC expression was maintained for up to 48 hours (data not shown). To determine the effect of this stress on Ag presentation, heat stressed PriessGAD cells were assayed for endogenous GAD, exogenous HSA Ag, and HSA peptide presentation. Heat stressing PriessGAD cells did not alter MHC class II presentation of endogenous GAD, exogenous HSA Ag, or exogenous HSA peptide (Fig. 2D and data not shown). These results indicate that while RA and GA each induced a stress response and upregulate overall HSP/HSC expression in B cells, cellular stress alone does not contribute to the observed decrease in MHC class II presentation of GAD Ag associated with pharmacological inhibition of HSP90.
GA and RA Treatment Does Not Alter MHC Class II Expression
Treatment of human DCs with GA resulted in decreases in MHC class I, II, and co-stimulatory molecule surface expression (12). In a study using macrophages, HSP90 overexpression was found to enhance MHC class II dimer stability while treatment with high concentrations of GA and RA decreased dimer stability (14). Both HSP90 overexpression and inhibition were reported not to alter surface and total MHC class II protein expression in these murine cells (14). To evaluate whether GA or RA inhibition of HSP90 altered MHC class II dimer levels in human B cells, PriessGAD cells were treated with either agent and then evaluated for total cellular MHC class II monomer and dimer expression as well as MHC class II surface expression. Neither GA nor RA significantly altered the total cellular content of MHC class II DRα monomers as detected by Western immunoblotting (Fig.3A). Moreover, steady state levels of MHC class II dimers were also unaffected by GA or RA treatment as detected by Western immunoblotting for MHC class II dimers (Fig. 3A). Flow cytometry of cells to detect total and surface levels of MHC class II also confirmed that GA and RA did not affect MHC class II expression (Fig. 3B). These results indicate that the decrease in MHC class II GAD Ag presentation observed with GA or RA treatment is not due to alterations in MHC class II expression or dimer stability in B cells. Studies were also carried out to measure B cell expression of HLA-DM and HLA-DO, MHC-encoded heterodimers that regulate epitope binding to MHC class II. Intracellular flow cytometric analysis revealed no change in DM or DO abundance in RA- or GA-treated B cells (data not shown).
FIGURE 3.
HSP90 inhibition by GA or RA Did Not Alter MHC Class II Expression. PriessGAD cells were treated with DMSO as a control, RA, or GA for 18 hours. (A) Following drug treatment, cells were lysed in 1% Triton-X 100 buffer. Cell lysates were analyzed by Western immunoblotting for expression of MHC class II DRα chain (left) or MHC class II DRαβ dimers (right) compared to actin control. In all panels, the intervening lanes were removed between samples. Results shown are representative of 3 separate experiments. The table depicts the average relative expression of MHC class II DRα and DRαβ dimers as measured by densitometry using data from 3 separate experiments. (B) Drug treated cells were also fixed in 1% paraformaldehyde and stained for surface MHC class II expression (left). For total MHC class II expression (right), cells were fixed, permeabilized, then stained for total MHC class II expression. MHC class II expression was detected by flow analysis. Thick black line indicates control cells, thin gray line indicates GA treated cells, and the dashed black line indicates RA treated cells. Results shown are representative of 3 separate experiments.
Selective Association of Ag with HSP90
HSP90 can bind to both proteins and peptides to potentiate intra- and extracellular transport, folding, and proteolysis although specific recognition motifs for this HSP have not been well defined (27–29). HSP90 binding to peptides promotes direct and indirect MHC class I presentation (17). Yet whether HSP90 selectively regulates MHC class II presentation via direct association with native Ag has not been addressed. The intracellular association of HSP90 with endogenous Ag such as GAD was assessed using the human B cell line PriessGAD. In B cells, complexes of GAD and HSP90 were detected via co-immunoprecipitation, consistent with a direct role for this HSP in regulating GAD Ag presentation (Fig. 4A). Inhibition of HSP90 function in cells did not reduce class II presentation of another endogenous Ag, kappa (Fig. 1). Consistent with this, association of kappa Ag and HSP90 was not detected in B cells (Fig. 4B). These results demonstrate selective intracellular association of HSP90 with GAD Ag, but not kappa Ag in B cells co-expressing these proteins.
FIGURE 4.
HSP90 Association with Select Ags. (A and B) Cell lysates from PriessGAD were incubated overnight at 4°C with Abs to GAD, kappa, or the isotype control Ab bound to protein-G sepharose beads. Immunoprecipitated proteins eluted with 2X reducing sample buffer were subjected to 10–12% SDS-PAGE and Western immunoblotting with anti-HSP90 Ab. Whole cell lysate (WCL) was run as a positive control. (C, D, and E) Purified GAD, kappa, or OVA was pre-incubated with HSP90 at a 3:1 molar ratio overnight at 37°C prior to immunoprecipitation with antibodies to HSP90, GAD, kappa, OVA, or isotype control Ab bound to protein-G sepharose beads. Immunoprecipitated proteins eluted with 2X reducing sample buffer were subjected to 10–12% SDS-PAGE and Western immunoblotting with anti-HSP90 Ab. WCL was run as a control for immunoblotting. Gamma settings were adjusted in panel B. Results are representative of 3 separate experiments. In all panels, the intervening lanes were removed between the immunoprecipitated samples and WCL.
Intracellularly, HSP90 has been shown to work in concert with other HSPs including HSC70, HSP70, and HSP40 (30). The association of HSP90 with GAD could be due to a direct interaction of these two molecules or indirectly via binding through an additional HSP. In order to determine if HSP90 and GAD can directly interact, purified HSP90 protein was incubated with either purified GAD Ag or kappa Ag, followed by immunoprecipitation with Abs to GAD, kappa Ag, or HSP90 as well as an isotype control Ab. Immunoprecipitated proteins were Western immunoblotted and the membranes probed with an HSP90 Ab to detect GAD or kappa association with this HSP (Fig. 4C and D). HSP90 was detected co-precipitating with GAD, indicating that HSP90 can bind directly to GAD. Kappa Ag was not found to co-immunoprecipitate with HSP90. These results suggest the association of HSP90 and GAD seen in PriessGAD cell lysates is likely due to a direct association of this Ag and HSP.
HSP90 was found to not only associate with N-terminal extended OVA peptides, but also play a role in both direct and indirect class I presentation (17). An additional study found HSP90 inhibition by GA to decrease MHC class II presentation of exogenous OVA Ag in murine APCs (14). The authors suggested that the effect of HSP90 inhibition on OVA Ag presentation was due to alterations in overall MHC class II expression upon exposure of cells to relatively high concentrations of GA. Whether HSP90 can bind to OVA Ag and selectively chaperone OVA Ag for MHC class II presentation was not addressed. Here, purified HSP90 was incubated with OVA Ag, followed by immunoprecipitation with HSP90, OVA, or isotype control Abs. HSP90 readily associated with OVA Ag, suggesting that this HSP and OVA Ag can directly interact in the absence of other HSPs (Fig. 4E). These results support a selective and direct interaction between HSP90 and Ags which may influence Ag uptake and presentation by class II molecules.
Complexing HSP90 and GAD Ag Enhanced MHC Class II Presentation of this Ag at Low Concentrations
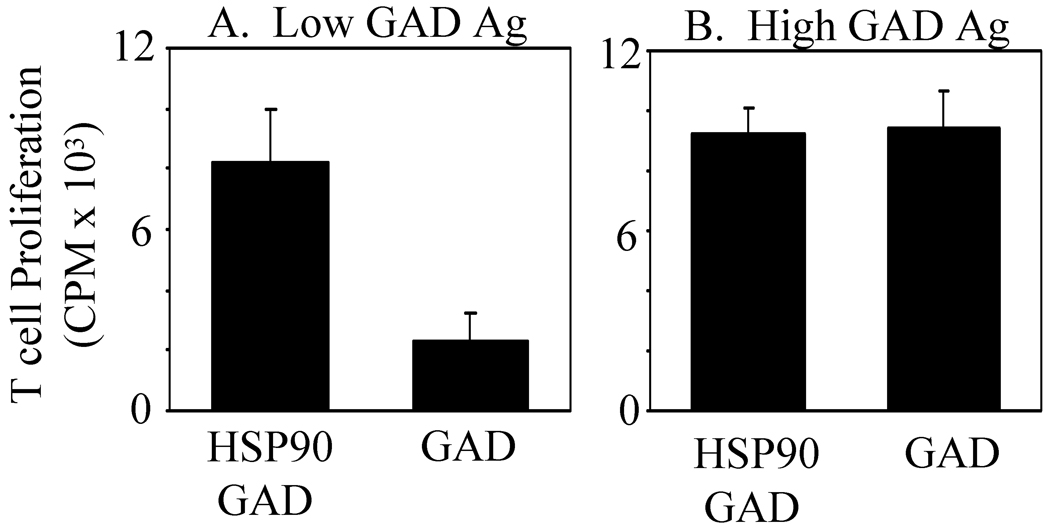
Experiments were performed to determine whether the addition of exogenous HSP90 complexed to GAD Ag could enhance the presentation of this Ag. Purified human GAD Ag was pre-complexed with HSP90 at a 3:1 molar ratio prior to the addition to Priess cells. MHC class II presentation of GAD Ag using HSP90/GAD pre-complexes was compared to presentation of GAD in the absence of exogenous HSP90. Pre-complexing HSP90 with GAD prior to addition to cells enhanced GAD presentation only at low concentrations of GAD Ag (Fig. 5A). At higher concentrations, the presence of HSP90 did not enhance GAD Ag presentation (Fig. 5B). These results suggest that the direct binding of HSP90 to GAD Ag can enhance GAD Ag presentation by MHC class II molecules.
FIGURE 5.
Exogenous HSP90 Enhanced Class II Presentation of Low Concentrations of GAD Ag. Purified HSP90 and GAD Ag (3:1 molar ratio), purified GAD Ag in PBS, or HSP90 in PBS were pre-incubated for 24 hours. Priess cells were then cultured with variable concentrations of HSP90/GAD Ag, GAD Ag alone, or HSP90 alone. The ability of these Priess cultures to activate GAD-specific T cells was tested as in Fig. 1. (A) Results from Priess cells incubated with low concentrations of HSP90/GAD (0.00192µM/ 0.00064 µM), GAD Ag (0.00064 µM), or HSP90 (0.00192 µM). (B) Results from Priess cells treated with high concentrations of HSP90/GAD Ag (0.0192 µM/0.0064 µM), GAD Ag (0.0064 µM), or HSP90 (0.0192 µM). Results are displayed as presentation over background. Results are representative of 3 experiments and standard deviation from triplicate samples is shown.
Manipulating HSP90 α and β Isoform Expression in APC
In humans, two closely related isoforms, HSP90α and HSP90β, are constitutively expressed (27). Assays using pharmacological inhibitors such as RA and GA fail to distinguish between the function of these two isoforms, with both proteins inactivated by these compounds (28). While both isoforms bind similarly to their intracellular co-chaperones, in some cases HSP90α and HSP90β were found to behave differently with respect to substrate interactions (29). Research has implicated HSP90α, but not HSP90β in regulating class I presentation (13).
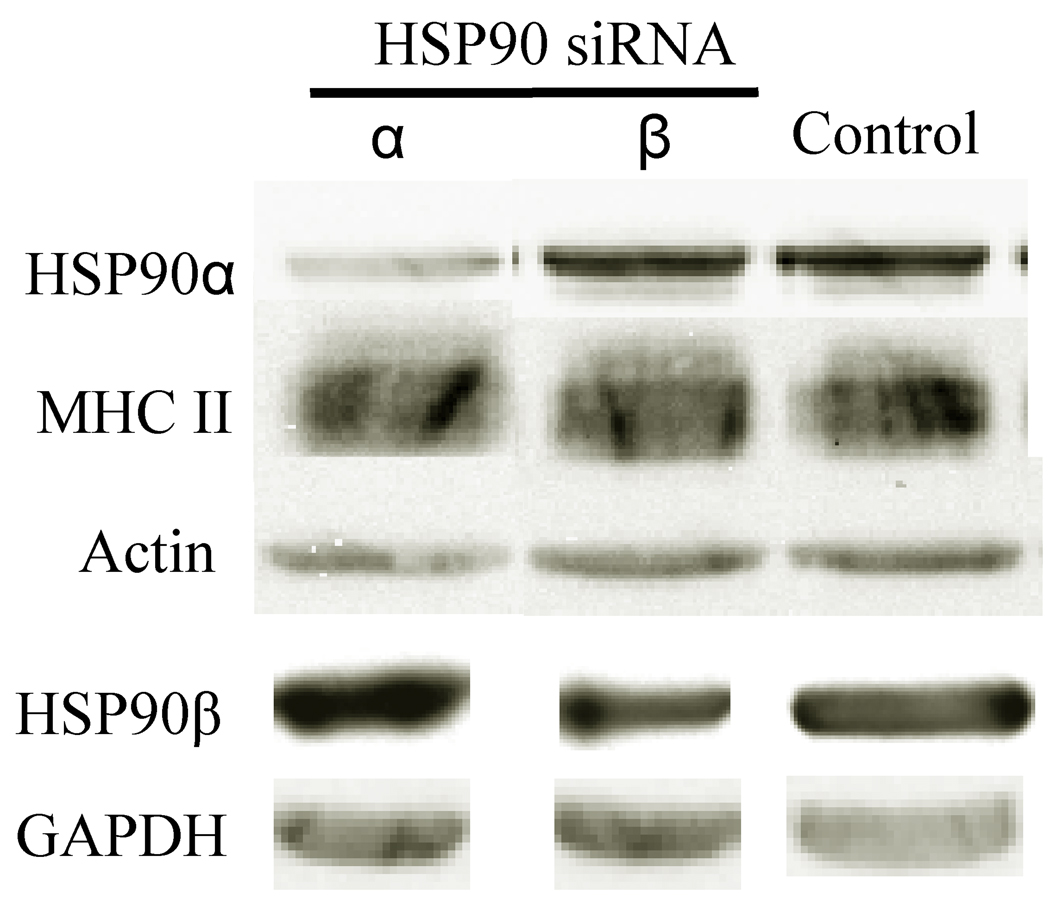
To explore the function of HSP90 isoforms in MHC class II presentation, PriessGAD B cells were assayed to confirm the expression of both HSP90α and HSP90β isoforms. Western immunoblotting of PriessGAD cell lysates as well as other human B cell lines revealed expression of both HSP90 isoforms (data not shown). To further evaluate the biological function of these HSP90 isoforms, siRNA specifically designed to disrupt HSP90α and HSP90β mRNA expression were utilized (13). PriessGAD cells were treated with HSP90α or HSP90β siRNA as well as controls such as a RISC-free siRNA. Total HSP90α and HSP90β protein expression as well as the relative abundance of other HSPs and actin were determined by Western immunoblotting. Treatment of B cells with HSP90α siRNA specifically knocked down HSP90α expression, but did not affect HSP90β levels (Fig. 6). HSP90β siRNA treatment of B cells decreased HSP90β protein expression, but did not affect HSP90α protein abundance (Fig. 6). In contrast to HSP90 inhibition by RA and GA, neither HSP90α nor HSP90β siRNA treatment altered HSP70, HSC70, or HSP40 protein expression levels in cells (data not shown). This may be due to low levels of residual HSP90α or HSP90β in siRNA treated cells. Moreover, total cellular MHC class II monomer and dimer protein abundance were unaffected by siRNA treatment as confirmed by Western immunoblotting (data not shown). Flow cytometry of permeabilized and non permeabilized cells indicated no affect of these siRNAs on MHC class II expression (data not shown). Together, these results suggest that HSP90α or HSP90β siRNA treatment specifically knocks down the expression of discrete HSP90 isoforms, respectively, without altering the expression of MHC class II and other HSPs.
FIGURE 6.
Manipulation of HSP90 α and β Expression Using siRNA. PriessGAD cells were treated with siRNA and a GFP-containing plasmid as described in the methods. Flow cytometry to detect GFP-positive cells indicated the DNA transfection efficiency ranged from 45–55%. Cell lysates were analyzed for HSP90α, HSP90β, MHC class II DRα chain, and actin expression by Western immunoblotting. Densitometry confirmed specific disruption of each HSP90 isoform expression by its respective siRNA. HSP90α siRNA treatment reduced HSP90α protein expression by 66% compared to HSP90β and controls. HSP90β siRNA treatment reduced HSP90β protein expression by 30% compared to HSP90α and controls. Results are representative of 3 separate experiments.
Disruption of HSP90α or HSP90β Specifically Inhibits MHC Class II Presentation of GAD
To determine the role of HSP90α and HSP90β in MHC class II presentation, PriessGAD cells were treated with siRNA for HSP90α, HSP90β, or controls such as RISC-free siRNA followed by analysis of MHC class II presentation for several Ags and epitopes. Treatment of PriessGAD cells with HSP90α or HSP90β siRNA resulted in decreased MHC class II presentation of endogenous GAD as well as exogenous GAD Ag (Fig. 7). MHC class II presentation of epitopes from exogenous GAD peptide, exogenous HSA protein, exogenous HSA peptide, endogenous kappa II, and endogenous kappa I was not affected by HSP90α or HSP90β siRNA treatment of these APCs (Fig 7 and data not shown). These results indicate that both HSP90α and HSP90β isoforms can regulate MHC class II presentation of GAD Ag. Thus, these studies establish that in contrast to MHC class I Ag presentation, both HSP90α and HSP90β can regulate the MHC class II pathway. Furthermore, HSP90 appears to selectively promote MHC class II presentation of the diabetes autoantigen GAD.
FIGURE 7.
Disruption of HSP90α or HSP90β Expression Using siRNA Selectively Inhibited MHC Class II Presentation of GAD. PriessGAD or Priess cells were treated with siRNA to reduce HSP90α or HSP90β expression or controls such as RISC-free siRNA prior to incubation for 48 hr at 37°C. Exogenous Ag was added for the last 16 hours. These APCs were then cultured with epitope specific T cells for GAD, HSA, or kappa Ag. T cell responses were monitored as a measure of MHC class II presentation of endogenous GAD, exogenous GAD (10 µg/ml), exogenous HSA Ag (5 µM), or endogenous kappa Ag. In each panel, black bars represent control cells, light gray bars represent HSP90α siRNA-treated cells, and dark gray bars represent HSP90β siRNA-treated cells. Results are representative of 3 separate experiments. The error bars indicate the standard deviation among triplicate samples in each experiment.
Discussion
HSP90 has been shown to play a key role in chaperoning client proteins in a variety of cellular processes including cell proliferation, differentiation, and apoptosis (28). Manipulation of HSP90 activity has been used to modulate protein folding and to induce the proteolysis of misfolded or mutant proteins in a variety of disease conditions including a wide range of malignancies and neurological disorders including Alzheimer’s disease, Parkinson’s disease, autoimmune encephalomyelitis, and polyglutamine diseases (23, 31–33). HSP90 inhibitors have also been tested in human clinical trials to promote tumor regression (31). Recent studies have implicated HSP90 as a regulator of both class I and II Ag processing and presentation (12, 14). Studies of the MHC class I pathway suggest HSP90 may guide Ag processing or select epitopes for presentation (13). Yet, whether HSP90 broadly controls MHC class II function or modulates instead the display of select antigenic epitopes has not been dissected.
In our studies, inhibition of HSP90 in B cells by either pharmacological agents such as GA and RA or siRNA specifically decreased MHC class II presentation of both exogenous and endogenous GAD (Fig. 1 and Fig. 7). MHC class II presentation of other endogenous and exogenous Ags was unaffected by HSP90 inhibition (Fig. 1 and Fig. 7). Furthermore, disruption of HSP90 function or expression failed to alter exogenous peptide presentation by these human APCs. Thus, HSP90 inhibition appears to selectively affect MHC class II presentation in an Ag specific manner. HSP90 exhibits some substrate specificity, although clear motifs recognized by this HSP have yet to be defined (27–29). HSP90 may also recognize target proteins based on conformation or folding. The autoantigen GAD is well known for its hydrophobic nature and association with lipid membranes via its N-terminus (34, 35). Whether these properties contribute to HSP90 association with GAD remains unclear. However, we found HSP90 interacts with both full length GAD Ag as well as an N-terminal truncated form of GAD Ag (Fig. 4 and data not shown). Moreover, HSP90 inhibition decreased MHC class II presentation of both exogenous full length GAD and N-terminal truncated GAD (Fig. 1 and data not shown).
The physical association of HSP90 with GAD, but not kappa Ag in cells co-expressing these molecules was demonstrated (Fig. 4). Moreover, in vitro incubation of HSP90 with either GAD, kappa, or OVA Ag resulted in the specific association of HSP90 with GAD and OVA Ag, but not kappa Ag. Functional studies using OVA had suggested a role for this HSP in class I and II presentation (14, 17). Taken together, these results suggest that HSP90 may modulate class II Ag presentation by directly interacting with select Ags. HSP90 has been shown in some cases to work in concert with additional HSPs as part of a multi-protein chaperone complex. Incubating purified HSP90 with purified GAD Ag resulted in the two proteins co-immunoprecipitating, indicating that HSP90 and GAD Ag can bind directly. Yet, this does not rule out the association of GAD-HSP90 complexes with other intracellular HSPs. Potentially, complex formation between HSP90 and GAD may influence GAD processing by APCs. Pre-complexing purified HSP90 with exogenous GAD in vitro prior to addition to Priess cells enhanced GAD presentation, in support of this hypothesis (Fig. 5). This is also supported by studies which indicated manipulating cellular HSP90 function failed to influence exogenous GAD peptide presentation (Fig. 1E). Thus, HSP90 appears to selectively influence the class II presentation of native Ag in B cells.
While the individual functions of HSP90 α and β isoforms have been dissected in terms of MHC class I presentation, the current work marks a first step in addressing the role of these HSP90 isoforms in MHC class II presentation. HSP90α alone was found to modulate Ag processing for MHC class I presentation (13). Using HSP90 α and β specific siRNA to modulate HSP90 protein abundance revealed both isoforms were involved in MHC class II presentation of exogenous and endogenous GAD (Fig. 7). These two isoforms may have distinct rather than redundant roles in the Ag processing and presentation pathway.
It is interesting that inhibition of HSP90 affects both endogenous and exogenous presentation of GAD Ag. Analysis of MHC class II presentation of short synthetic GAD peptides failed to reveal a role for HSP90 in the cell surface loading of MHC class II molecules or B-T cell interactions. Thus, HSP90 likely exerts its effect on intracellular GAD Ag processing or via unfolding GAD to facilitate class II binding to antigenic epitopes. The pathways of exogenous and endogenous MHC class II presentation for GAD Ag are distinct yet convergent with shared processing in endosomal and lysosomal compartments. This leads to speculation as to exactly where HSP90 modulates GAD processing and presentation. While HSP90 is located primarily in the cytoplasm of cells, a study in B cells detected HSP90 in exosomes, and heat stress induced the enrichment of HSPs in those exosomes (36). HSP70, which can associate with HSP90, was secreted by tumor cells through a pathway involving lysosomal endosomes (37). Notably, another intracellular chaperone, HSC70 is found both in the cytoplasm and endosomes/lysosomes with studies suggesting it translocates into the endosomal network in an ATP-dependent process (38). Whether HSP90 uses a similar pathway to access the endosomal network is unknown. Several studies have suggested peptide-HSP90 complexes released by tumors can promote MHC class I presentation (15–17). Islet cell stress including exposure to cytokines such as IL-1β is known to induce HSP90 expression prior to cell death (20). Western immunoblotting of concentrated conditioned media from PriessGAD cells failed to detect HSP90 released from these cells (data not shown). Yet, we cannot rule out the retention of HSP90 in endosomes and lysosomes of these cells where it may intersect exogenous GAD.
Prior studies had suggested more global effects of HSP90 in regulating DC maturation and MHC class II presentation by murine monocytes and B cells (12, 14). Changes in class II expression and dimer formation during studies to manipulate HSP90 function in these cells suggest roles for HSP90 in the maturation or differentiation of some APCs. In testing several B-lymphoblastoid cell lines, we found that disrupting HSP90 expression or function failed to alter class II expression or dimer stability. Also, in the current study, nearly 30 fold lower concentrations of GA were used to disrupt HSP90 function compared with the analysis in murine B cells and monocytes where more global disruption of class II function was observed (14). In these latter investigations, changes in HSP90 function lead to alterations in the presentation of synthetic peptides as well as native Ags. Whether pharmacological inhibition of HSP90 leads to cell stress was also not addressed in these studies with murine APCs (14). In the current analysis, induction of stress responses in human B cells failed to alter class Ag or peptide presentation. Testing a panel of protein Ags and peptides revealed a selective role for HSP90 isoforms in class II presentation by B cells.
In conclusion, the current study supports a role for HSP90 in regulating MHC class II presentation of both exogenous and endogenous GAD Ag by human B cells. Both pharmacological and siRNA targeted disruption of HSP90 specifically inhibited GAD presentation by class II molecules, yet failed to perturb MHC class II presentation of other Ags unaffected. Both HSP90 α and β isoforms were found to be involved in GAD Ag processing and presentation. Together, these observations reveal a novel function for HSP90 isoforms and suggest these HSPs may regulate the development of T cell responses to self and potentially foreign Ags. Further understanding of the role of HSP90 isoforms in GAD Ag processing and presentation by MHC class II molecules may also prove useful in developing new therapeutics to prevent and treat autoimmune diseases.
Acknowledgments
This work was supported by National Institutes of Health (NIH) Grants AI49589.
Abbreviations used in this paper
- GAD
Glutamic acid decarboxylase
- GA
geldanamycin
- RA
radicicol
- HSP
heat shock protein
- HSC
heat shock constitutive protein
- B-LCL
B lymphoblastoid cell line
- HSA
Human serum albumin
References
- 1.Mukherjee P, Dani A, Bhatia S, Singh N, Rudensky AY, George A, Bal V, Mayor S, Rath S. Efficient presentation of both cytosolic and endogenous transmembrane protein antigens on MHC class II is dependent on cytoplasmic proteolysis. J Immunol. 2001;167:2632–2641. doi: 10.4049/jimmunol.167.5.2632. [DOI] [PubMed] [Google Scholar]
- 2.Chicz RM, Urban RG, Gorga JC, Vignali DA, Lane WS, Strominger JL. Specificity and promiscuity among naturally processed peptides bound to HLA-DR alleles. J Exp Med. 1993;178:27–47. doi: 10.1084/jem.178.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wicker LS, Chen SL, Nepom GT, Elliott JF, Freed DC, Bansal A, Zheng S, Herman A, Lernmark A, Zaller DM, Peterson LB, Rothbard JB, Cummings R, Whiteley PJ. Naturally processed T cell epitopes from human glutamic acid decarboxylase identified using mice transgenic for the type 1 diabetes-associated human MHC class II allele, DRB1*0401. J Clin Invest. 1996;98:2597–2603. doi: 10.1172/JCI119079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lich JD, Elliott JF, Blum JS. Cytoplasmic processing is a prerequisite for presentation of an endogenous antigen by major histocompatibility complex class II proteins. J Exp Med. 2000;191:1513–1524. doi: 10.1084/jem.191.9.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lich JD, Jayne JA, Zhou D, Elliott JF, Blum JS. Editing of an immunodominant epitope of glutamate decarboxylase by HLA-DM. J Immunol. 2003;171:853–859. doi: 10.4049/jimmunol.171.2.853. [DOI] [PubMed] [Google Scholar]
- 6.Mycko MP, Cwiklinska H, Szymanski J, Szymanska B, Kudla G, Kilianek L, Odyniec A, Brosnan CF, Selmaj KW. Inducible heat shock protein 70 promotes myelin autoantigen presentation by the HLA class II. J Immunol. 2004;172:202–213. doi: 10.4049/jimmunol.172.1.202. [DOI] [PubMed] [Google Scholar]
- 7.Tobian AA, Canaday DH, Boom WH, Harding CV. Bacterial heat shock proteins promote CD91-dependent class I MHC cross-presentation of chaperoned peptide to CD8+ T cells by cytosolic mechanisms in dendritic cells versus vacuolar mechanisms in macrophages. J Immunol. 2004;172:5277–5286. doi: 10.4049/jimmunol.172.9.5277. [DOI] [PubMed] [Google Scholar]
- 8.Haug M, Schepp CP, Kalbacher H, Dannecker GE, Holzer U. 70-kDa heat shock proteins: specific interactions with HLA-DR molecules and their peptide fragments. Eur J Immunol. 2007;37:1053–1063. doi: 10.1002/eji.200636811. [DOI] [PubMed] [Google Scholar]
- 9.Tobian AA, Canaday DH, Harding CV. Bacterial heat shock proteins enhance class II MHC antigen processing and presentation of chaperoned peptides to CD4+ T cells. J Immunol. 2004;173:5130–5137. doi: 10.4049/jimmunol.173.8.5130. [DOI] [PubMed] [Google Scholar]
- 10.Li Z, Menoret A, Srivastava P. Roles of heat-shock proteins in antigen presentation and cross-presentation. Curr Opin Immunol. 2002;14:45–51. doi: 10.1016/s0952-7915(01)00297-7. [DOI] [PubMed] [Google Scholar]
- 11.Zhou D, Li P, Lin Y, Lott JM, Hislop AD, Canaday DH, Brutkiewicz RR, Blum JS. Lamp-2a facilitates MHC class II presentation of cytoplasmic antigens. Immunity. 2005;22:571–581. doi: 10.1016/j.immuni.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Bae J, Mitsiades C, Tai YT, Bertheau R, Shammas M, Batchu RB, Li C, Catley L, Prabhala R, Anderson KC, Munshi NC. Phenotypic and functional effects of heat shock protein 90 inhibition on dendritic cell. J Immunol. 2007;178:7730–7737. doi: 10.4049/jimmunol.178.12.7730. [DOI] [PubMed] [Google Scholar]
- 13.Kunisawa J, Shastri N. Hsp90alpha chaperones large C-terminally extended proteolytic intermediates in the MHC class I antigen processing pathway. Immunity. 2006;24:523–534. doi: 10.1016/j.immuni.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 14.Rajagopal D, Bal V, Mayor S, George A, Rath S. A role for the Hsp90 molecular chaperone family in antigen presentation to T lymphocytes via major histocompatibility complex class II molecules. Eur J Immunol. 2006;36:828–841. doi: 10.1002/eji.200535326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurotaki T, Tamura Y, Ueda G, Oura J, Kutomi G, Hirohashi Y, Sahara H, Torigoe T, Hiratsuka H, Sunakawa H, Hirata K, Sato N. Efficient cross-presentation by heat shock protein 90-peptide complex-loaded dendritic cells via an endosomal pathway. J Immunol. 2007;179:1803–1813. doi: 10.4049/jimmunol.179.3.1803. [DOI] [PubMed] [Google Scholar]
- 16.Binder RJ, Blachere NE, Srivastava PK. Heat shock protein-chaperoned peptides but not free peptides introduced into the cytosol are presented efficiently by major histocompatibility complex I molecules. J Biol Chem. 2001;276:17163–17171. doi: 10.1074/jbc.M011547200. [DOI] [PubMed] [Google Scholar]
- 17.Callahan MK, Garg M, Srivastava PK. Heat-shock protein 90 associates with N-terminal extended peptides and is required for direct and indirect antigen presentation. Proc Natl Acad Sci U S A. 2008;105:1662–1667. doi: 10.1073/pnas.0711365105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falcone M, Lee J, Patstone G, Yeung B, Sarvetnick N. B lymphocytes are crucial antigen-presenting cells in the pathogenic autoimmune response to GAD65 antigen in nonobese diabetic mice. J Immunol. 1998;161:1163–1168. [PubMed] [Google Scholar]
- 19.Harris KM, Spirito P, Maron MS, Zenovich AG, Formisano F, Lesser JR, Mackey-Bojack S, Manning WJ, Udelson JE, Maron BJ. Prevalence, clinical profile, and significance of left ventricular remodeling in the end-stage phase of hypertrophic cardiomyopathy. Circulation. 2006;114:216–225. doi: 10.1161/CIRCULATIONAHA.105.583500. [DOI] [PubMed] [Google Scholar]
- 20.Helqvist S, Polla BS, Johannesen J, Nerup J. Heat shock protein induction in rat pancreatic islets by recombinant human interleukin 1 beta. Diabetologia. 1991;34:150–156. doi: 10.1007/BF00418268. [DOI] [PubMed] [Google Scholar]
- 21.Qin HY, Mahon JL, Atkinson MA, Chaturvedi P, Lee-Chan E, Singh B. Type 1 diabetes alters anti-hsp90 autoantibody isotype. J Autoimmun. 2003;20:237–245. doi: 10.1016/s0896-8411(03)00035-0. [DOI] [PubMed] [Google Scholar]
- 22.Roe SM, Prodromou C, O'Brien R, Ladbury JE, Piper PW, Pearl LH. Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J Med Chem. 1999;42:260–266. doi: 10.1021/jm980403y. [DOI] [PubMed] [Google Scholar]
- 23.Waza M, Adachi H, Katsuno M, Minamiyama M, Sang C, Tanaka F, Inukai A, Doyu M, Sobue G. 17-AAG, an Hsp90 inhibitor, ameliorates polyglutamine-mediated motor neuron degeneration. Nat Med. 2005;11:1088–1095. doi: 10.1038/nm1298. [DOI] [PubMed] [Google Scholar]
- 24.Yorgin PD, Hartson SD, Fellah AM, Scroggins BT, Huang W, Katsanis E, Couchman JM, Matts RL, Whitesell L. Effects of geldanamycin, a heat-shock protein 90-binding agent, on T cell function and T cell nonreceptor protein tyrosine kinases. J Immunol. 2000;164:2915–2923. doi: 10.4049/jimmunol.164.6.2915. [DOI] [PubMed] [Google Scholar]
- 25.Ryhanen T, Mannermaa E, Oksala N, Viiri J, Paimela T, Salminen A, Atalay M, Kaarniranta K. Radicicol but not geldanamycin evokes oxidative stress response and efflux protein inhibition in ARPE-19 human retinal pigment epithelial cells. Eur J Pharmacol. 2008;584:229–236. doi: 10.1016/j.ejphar.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 26.Cristau B, Schafer PH, Pierce SK. Heat shock enhances antigen processing and accelerates the formation of compact class II alpha beta dimers. J Immunol. 1994;152:1546–1556. [PubMed] [Google Scholar]
- 27.Csermely P, Schnaider T, Soti C, Prohaszka Z, Nardai G. The 90-kDa molecular chaperone family: structure, function, and clinical applications. A comprehensive review. Pharmacol Ther. 1998;79:129–168. doi: 10.1016/s0163-7258(98)00013-8. [DOI] [PubMed] [Google Scholar]
- 28.Sreedhar AS, Kalmar E, Csermely P, Shen YF. Hsp90 isoforms: functions, expression and clinical importance. FEBS Lett. 2004;562:11–15. doi: 10.1016/s0014-5793(04)00229-7. [DOI] [PubMed] [Google Scholar]
- 29.Taherian A, Krone PH, Ovsenek N. A comparison of Hsp90alpha and Hsp90beta interactions with cochaperones and substrates. Biochem Cell Biol. 2008;86:37–45. doi: 10.1139/o07-154. [DOI] [PubMed] [Google Scholar]
- 30.Murphy PJ, Kanelakis KC, Galigniana MD, Morishima Y, Pratt WB. Stoichiometry, abundance, and functional significance of the hsp90/hsp70-based multiprotein chaperone machinery in reticulocyte lysate. J Biol Chem. 2001;276:30092–30098. doi: 10.1074/jbc.M103773200. [DOI] [PubMed] [Google Scholar]
- 31.Sharp S, Workman P. Inhibitors of the HSP90 molecular chaperone: current status. Adv Cancer Res. 2006;95:323–348. doi: 10.1016/S0065-230X(06)95009-X. [DOI] [PubMed] [Google Scholar]
- 32.Waza M, Adachi H, Katsuno M, Minamiyama M, Tanaka F, Sobue G. Alleviating neurodegeneration by an anticancer agent: an Hsp90 inhibitor (17-AAG) Ann N Y Acad Sci. 2006;1086:21–34. doi: 10.1196/annals.1377.012. [DOI] [PubMed] [Google Scholar]
- 33.Dello Russo C, Polak PE, Mercado PR, Spagnolo A, Sharp A, Murphy P, Kamal A, Burrows FJ, Fritz LC, Feinstein DL. The heat-shock protein 90 inhibitor 17-allylamino-17-demethoxygeldanamycin suppresses glial inflammatory responses and ameliorates experimental autoimmune encephalomyelitis. J Neurochem. 2006;99:1351–1362. doi: 10.1111/j.1471-4159.2006.04221.x. [DOI] [PubMed] [Google Scholar]
- 34.Lernmark A. Glutamic acid decarboxylase--gene to antigen to disease. J Intern Med. 1996;240:259–277. doi: 10.1046/j.1365-2796.1996.27859000.x. [DOI] [PubMed] [Google Scholar]
- 35.Jun HS, Khil LY, Yoon JW. Role of glutamic acid decarboxylase in the pathogenesis of type 1 diabetes. Cell Mol Life Sci. 2002;59:1892–1901. doi: 10.1007/PL00012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clayton A, Turkes A, Navabi H, Mason MD, Tabi Z. Induction of heat shock proteins in B-cell exosomes. J Cell Sci. 2005;118:3631–3638. doi: 10.1242/jcs.02494. [DOI] [PubMed] [Google Scholar]
- 37.Mambula SS, Calderwood SK. Heat shock protein 70 is secreted from tumor cells by a nonclassical pathway involving lysosomal endosomes. J Immunol. 2006;177:7849–7857. doi: 10.4049/jimmunol.177.11.7849. [DOI] [PubMed] [Google Scholar]
- 38.Agarraberes FA, Terlecky SR, Dice JF. An intralysosomal hsp70 is required for a selective pathway of lysosomal protein degradation. J Cell Biol. 1997;137:825–834. doi: 10.1083/jcb.137.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]