Abstract
In a case-control study with prevalence sampling, the authors explored the correlates for nocturia and their population-level impact. In 2003–2004, questionnaires were mailed to 6,000 subjects (aged 18–79 years) randomly identified from the Finnish Population Register (62.4% participated; 53.7% were female). Questionnaires contained items on medical conditions, medications, lifestyle, sociodemographic and reproductive factors, urinary symptoms, and snoring. Nocturia was defined as ≥2 voids/night. In age-adjusted analyses, factors associated with nocturia were entered into a multivariate model. Backward elimination was used to select variables for the final model, with adjustment for confounding. Although numerous correlates were identified, none affected ≥50% of nocturia cases of both sexes. The factors with the greatest impact at the population level were (urinary) urgency (attributable number/1,000 subjects (AN) = 24), benign prostatic hyperplasia (AN = 19), and snoring (AN = 16) for men and overweight and obesity (AN = 40), urgency (AN = 24), and snoring (AN = 17) for women. Moreover, correlates included prostate cancer and antidepressant use for men, coronary artery disease and diabetes for women, and restless legs syndrome and obesity for both sexes. Although several correlates were identified, none accounted for a substantial proportion of the population burden, highlighting the multifactorial etiology of nocturia.
Keywords: coronary artery disease; diabetes mellitus; life style; obesity; prostatic hyperplasia; prostatic neoplasms; sleep disorders; urinary bladder, overactive
Nocturia (waking at night to urinate) is a common cause of awakenings and may lead to sleep maintenance insomnia (1–3). Nocturia can be bothersome (4) and is associated with impaired mental and somatic health (5), impaired quality of life (4), and even increased mortality (6).
The etiology of nocturia is inadequately understood. Nocturia is frequently attributed to aging or childbirth in women and to benign prostatic hyperplasia (BPH) in men. Other related conditions include overactive bladder syndrome; nocturnal polyuria; obstructive sleep apnea; awakening for other reasons such as anxiety; primary sleep disorders; and use of diuretics, caffeine, or alcohol (7–9). Bedtime fluid intake correlates poorly with nocturia episodes (10).
Nocturia persists frequently following simple prostatectomy (11). In one study, 38% of men reported ≥2 voids/night 3 years after transurethral resection of the prostate (12). Several pharmacologic approaches have yielded limited nocturia reductions or significant side effects (7, 13–25).
To our knowledge, no earlier study comprehensively covered the possible associations of medical conditions; medications; lifestyle; and anthropometric, reproductive, and sociodemographic factors with nocturia. We explored correlates for nocturia and assessed their population-level impact in a large population-based study.
MATERIALS AND METHODS
Finnish National Nocturia and Overactive Bladder (FINNO) Study
In 2003–2004, questionnaires were mailed to 6,000 subjects aged 18–79 years randomly identified from the Population Register Centre. Age stratification was used with oversampling of younger age groups to ensure precise estimates even in age groups experiencing lower nocturia frequency (3, 26). More information on the FINNO Study (including characterization of nonrespondents) has been published previously (3, 26, 27).
Outcome
Nocturia cases were defined as subjects reporting 2 or more voids/night because this frequency involves clinically significant bother (4). All subjects without nocturia were considered controls in unconditional logistic regression analyses. Self-reported nocturia frequency was determined by using a previously described algorithm (27) combining responses to the Danish Prostatic Symptom Score (28) and the American Urological Association Symptom Index (29) (Web Table 1; this information is described in the first of 4 supplementary tables, each referred to as “Web table” in the text and posted on the Journal’s website (http://aje.oupjournals.org/)).
Correlate assessment
Self-reported information on physician-diagnosed conditions, prescribed medication, specific symptoms, and lifestyle factors was obtained by using questions modified from surveys conducted by the National Public Health Institute (30). Comorbidity indicators were formulated for 36 conditions deemed common or previously hypothesized as determinants of lower urinary tract symptoms (Web Tables 1 and 2). Medication use was classified into 27 groups by using the Anatomical Therapeutic Chemical Classification (31) (Web Tables 1 and 3). Lifestyle factors included body mass index, smoking, and alcohol and coffee consumption (Web Tables 1 and 4). The Danish Prostatic Symptom Score questions were used to evaluate (urinary) urgency and stress urinary incontinence (28) and the Basic Nordic Sleep Questionnaire to evaluate snoring (32) (Web Tables 1 and 4). These symptoms have been specifically shown to be risk factors for nocturia (26, 33, 34).
With the exception of the alcohol consumption question (response rate: men, 86%; women, 76%), data on potential correlates were highly available (men, 97%–100%; women, 95%–100%). Alcohol consumption was not associated with nocturia.
Potential confounders
Age, sociodemographic factors (marital status, education, employment, urbanization) (3), and female reproductive/gynecologic factors (parity, postpartum period, menopausal status, hormone therapy, hysterectomy, stress urinary incontinence surgery) (27) were treated as potential confounders. Data on urbanization, parity, and delivery date(s) were obtained from the population register. Information on each potential confounder was available for at least 99% of subjects.
Statistical analysis
Logistic regression was used for the analyses stratified by sex, with nocturia as the outcome. All potential correlates and confounders associated (P < 0.10) with nocturia in the age-adjusted analyses (basic analysis population) were entered into the multivariate model (Web Figure 1, also posted on the Journal’s website (http://aje.oupjournals.org/)). In these analyses, potential correlates and confounders numbered 16 and 2 for men and 18 and 6 for women, respectively. Backward elimination techniques were used to select variables for the final model, with likelihood ratio tests used to determine significance (P < 0.05). Finally, cofactors that were not actual confounders (they did not change any estimate by ≥10%) were eliminated. Confirmed confounders were age for men, menopausal status for women, and employment for both sexes. The final analysis included subjects with information on nocturia, correlates, and confounders (Web Figure 1). For the correlates identified, age-standardized sensitivity, positive predictive value, attributable fraction in the exposed, population attributable fraction, and attributable number were calculated (35).
RESULTS
Of the 6,000 subjects approached, 3,727 (62.4%) participated; 23 were unavailable, and 130 were excluded because of pregnancy, puerperium, or urinary tract infection (Web Figure 1). (The response rate was approximately 32% after the first round, 50% after the second round, and 62.4% after the final, third round.) Of the 3,597 subjects included, 98% provided nocturia information (basic analysis population). The 3,307 subjects (92%) who responded to all nocturia, correlate, and confounder questions formed the final analysis population. Prevalence of nocturia was 12.5% (95% confidence interval: 10.7, 14.3) among men and 12.9% (95% confidence interval: 11.0, 14.9) among women (age standardized to match Finland's age structure (36)). Excluding subjects with missing information on any correlate or confounder (final analysis population) did not change these estimates. For more detail, refer to Table 1 and to Web Tables 2–4. Correlates for nocturia included (urinary) urgency, snoring, restless legs syndrome, and obesity for both sexes; BPH, antidepressant use, and prostate cancer for men; and overweight, diabetes, and coronary artery disease for women (Table 1).
Table 1.
Prevalences (%) and Odds Ratios of Correlates for Nocturia in Multivariate Analysesa in the Population-based Finnish National Nocturia and Overactive Bladder Study, Finland, 2003–2004
| Prevalenceb | 95% CI | OR | 95% CI | |
| Men | ||||
| Urinary urgency | 7.5 | 6.1, 9.0 | 7.39 | 4.46, 12.23 |
| Prostate cancer | 1.2 | 0.7, 1.8 | 5.45 | 1.74, 17.08 |
| Antidepressant use | 2.5 | 1.7, 3.3 | 3.16 | 1.29, 7.73 |
| Restless legs syndrome | 3.0 | 2.1, 4.0 | 2.91 | 1.30, 6.52 |
| Benign prostatic hyperplasia | 7.8 | 6.3, 9.3 | 2.18 | 1.31, 3.65 |
| Obesityc | 13.2 | 11.2, 15.1 | 2.07 | 1.17, 3.67 |
| Snoring | 35.1 | 31.9, 38.2 | 1.49 | 1.00, 2.22 |
| Women | ||||
| Urinary urgency | 9.9 | 8.2, 11.6 | 4.92 | 3.15, 7.67 |
| Coronary artery disease | 4.5 | 3.1, 5.8 | 3.13 | 1.48, 6.64 |
| Restless legs syndrome | 3.6 | 2.5, 4.8 | 2.86 | 1.41, 5.83 |
| Diabetes | 4.7 | 3.4, 5.9 | 2.68 | 1.38, 5.20 |
| Obesityc | 13.3 | 11.4, 15.3 | 2.18 | 1.30, 3.66 |
| Overweightc | 32.3 | 29.2, 35.4 | 1.90 | 1.25, 2.88 |
| Snoring | 18.4 | 16.1, 20.7 | 1.76 | 1.17, 2.64 |
Abbreviations: CI, confidence interval; OR, odds ratio.
Also adjusted for identified confounders (age and employment for men; employment and menopausal status for women).
Age standardization using the age structure of Finland (36).
Normal-weight (body mass index < 25 kg/m2) subjects were considered the reference (37).
At the population level (Table 2), urgency and snoring (both sexes), BPH (men only), and overweight/obesity (women only) accounted for the largest proportion of nocturia. At the individual level, the strongest correlate for both sexes was urgency, although odds ratio differences between correlates were mainly statistically nonsignificant (Table 1).
Table 2.
Fraction of Nocturia Attributable to Identified Correlates in the Population-based Finnish National Nocturia and Overactive Bladder Study, Finland, 2003–2004a
| Attributable Fraction in the Exposed, %bc | Population Attributable Fraction, %cd | Attributable No./1,000 Subjectsce | |
| Men | |||
| Urinary urgency | 77.2 | 24.0 | 24 |
| Benign prostatic hyperplasia | 69.1 | 13.1 | 19 |
| Snoring | 30.3 | 14.4 | 16 |
| Obesityf | 29.1 | 5.9 | 6 |
| Antidepressant use | 65.6 | 4.3 | 6 |
| Restless legs syndrome | 53.1 | 4.7 | 4 |
| Prostate cancer | 65.8 | 3.9 | 3 |
| Women | |||
| Overweight/obesityf | 51.5 | 35.4 | 40 |
| Urinary urgency | 71.0 | 21.3 | 24 |
| Snoring | 46.8 | 16.4 | 17 |
| Diabetes | 63.3 | 8.6 | 9 |
| Restless legs syndrome | 63.4 | 7.4 | 7 |
| Coronary artery disease | 44.9 | 7.4 | 4 |
Age standardization using the age structure of Finland (36).
Attributable fraction in the exposed refers to the proportion by which prevalence of the condition (nocturia) among exposed persons (with the correlate) would be reduced if the exposure (correlate) were eliminated (35).
Regarding formulae of attributable fractions and attributable number, it is assumed that causes other than the one under investigation have similar effects on the exposed and unexposed groups (35).
Population attributable fraction refers to the proportion by which prevalence of the condition (nocturia) in the entire population would be reduced if the exposure (correlate) were eliminated (35).
Attributable number refers to the number of prevalent cases of the condition (nocturia) attributable to the exposure (correlate) (35).
Body mass index (BMI) was classified as a dichotomous variable when calculating attributable fractions because of the dichotomous nature of these measures. Hence, the reference groups were not obese (BMI < 30 kg/m2) for men and normal weight (BMI < 25 kg/m2) for women because overweight (BMI 25–30 kg/m2) was associated with nocturia in women only.
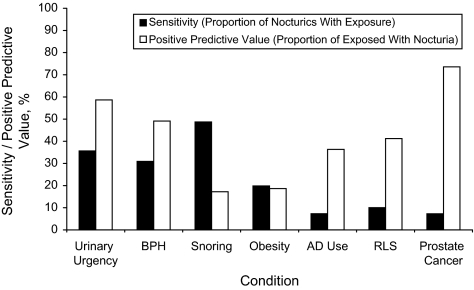
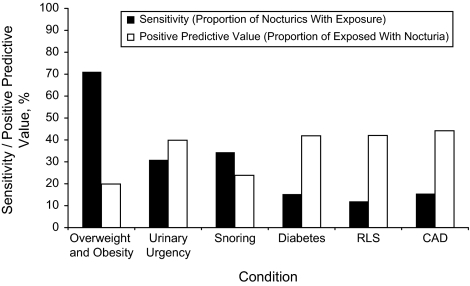
No correlate affected 50% or more of men with nocturia (Figure 1): BPH, urgency, and snoring had the highest sensitivity for nocturia (31%–49%). Of the women with nocturia, 71% were overweight or obese; other correlates were reported by 50% or more of women with nocturia (Figure 2).
Figure 1.
Sensitivity and positive predictive value for correlates of nocturia among men (in order: the correlate with the greatest impact at the population level is given first) in the population-based Finnish National Nocturia and Overactive Bladder Study, Finland, 2003–2004. AD, antidepressant; BPH, benign prostatic hyperplasia; RLS, restless legs syndrome. Body mass index (BMI) was classified as a dichotomous variable when calculating sensitivity and positive predictive value because of the dichotomous nature of these measures. Hence, the reference groups were not obese (BMI < 30 kg/m2) for men and normal weight (BMI < 25 kg/m2) for women because overweight (BMI 25–30 kg/m2) was associated with nocturia in women only.
Figure 2.
Sensitivity and positive predictive value for correlates of nocturia among women (in order: the correlate with the greatest impact at the population level is given first) in the population-based Finnish National Nocturia and Overactive Bladder Study, Finland, 2003–2004. CAD, coronary artery disease; RLS, restless legs syndrome. Body mass index (BMI) was classified as a dichotomous variable when calculating sensitivity and positive predictive value because of the dichotomous nature of these measures. Hence, the reference groups were not obese (BMI < 30 kg/m2) for men and normal weight (BMI < 25 kg/m2) for women because overweight (BMI 25–30 kg/m2) was associated with nocturia in women only.
A majority of men with prostate cancer or urgency reported nocturia, yielding positive predictive values of 74% and 59%, respectively. Half of the men with BPH, and a minority of men with other correlates, reported nocturia (Figure 1). Among women, no correlates were associated with a 50% or greater probability of nocturia (Figure 2).
Generally, questionnaire mailing round did not affect correlate prevalence. However, 4 exceptions emerged (age adjusted). First-round responders reported more nocturia and urgency than responders in subsequent rounds (P for trend = 0.01 for both nocturia and urgency; sexes combined). Moreover, first-round male responders reported more antidepressant use (P for trend = 0.04), and first-round female responders were slightly less obese than those in subsequent rounds (P for trend = 0.05). However, the odds ratio estimates for these factors were similar for each round, suggesting absence of systematic error.
DISCUSSION
In this large, population-based study, numerous factors associated with nocturia were identified. However, no single correlate accounted for more than half of the cases of nocturia, highlighting its multifactorial etiology. At the population level, urgency, BPH, and snoring for men and overweight/obesity, urgency, and snoring for women accounted for the largest proportion of nocturia.
Our FINNO Study population is representative of Finnish adults in terms of sociodemographic, anthropometric, and female reproductive factors (3, 27, 37). The frequencies of comorbidities, medications, and lifestyle factors were similar to those in large-scale population surveys conducted by the National Public Health Institute (38–40).
Concordant with earlier reports (26, 41–44), urinary urgency was strongly associated with nocturia in both sexes, yet only 1 in 3 with nocturia reported urgency. Among men, BPH had the second highest population impact, but only a third with nocturia reported BPH. Lower urinary tract symptoms suggestive of benign prostatic obstruction constitute a well-recognized risk factor for nocturia (45). However, the impact of BPH may be overestimated. In Japanese studies, nocturia was the lower urinary tract symptom least related to prostatic obstruction, and treatment to relieve obstruction had less of an effect on nocturia than on other symptoms (15, 46). In a lower urinary tract symptoms/BPH study, patients receiving doxazosin experienced very modest reductions in nocturia, whereas finasteride had no effect (23). In the current study, prostate cancer was associated with nocturia. More than 70% of men with prostate cancer reported at least 2 voids/night, yet only 7% of men with nocturia reported prostate cancer.
Concurring with earlier findings (37, 47–49), nocturia was associated with obesity in both sexes and, among women, also with overweight. Indeed, overweight/obesity had the greatest population impact among women.
Snoring had a strong population impact because of its high prevalence, yet the strength of association was relatively weak (odds ratio = 1.5–1.8). Snoring has been associated with nocturia (34). The severity of obstructive sleep apnea predicted nocturia frequency, and continuous positive airway pressure treatment decreased nocturia (50). In a home sleep study, the prevalence of obstructive sleep apnea was double among urogynecology patients with nocturia compared with those without (51). In our study, reported obstructive sleep apnea was not associated with nocturia after adjustment, which may be due to correlation with snoring (three-quarters of subjects with obstructive sleep apnea reported snoring, and snoring was 10 times more prevalent than obstructive sleep apnea).
To our knowledge, no association has been reported between nocturia and restless legs syndrome. Increased nocturia among patients with restless legs syndrome may reflect sleep disturbance (52). Moreover, such patients use more medications (particularly antidepressants) than controls do (53). Nocturia has previously been linked to (untreated) depression (54, 55) and use of selective serotonin-reuptake inhibitors (54). In our study, only for men was nocturia associated with antidepressant use; depression itself was not associated with nocturia.
Diabetes and coronary artery disease were associated with nocturia in the age-adjusted analyses for both sexes but for only women in multivariate analysis. An association between diabetes and nocturia has been reported sometimes (43, 47, 49, 50, 56–60), but not always (10, 42, 45). In the BACH Survey (47), nocturia was associated with increasing body mass index, diabetes, and cardiac disease, whereas among Danes aged 60–80 years (49), increasing body mass index, diabetes, urinary incontinence, and recurrent cystitis were associated with 2 voids/night. In both surveys (47, 49), sex was used as a covariate, but results were not reported by gender. Some earlier reports (47, 59, 61), but not all (42, 43, 45, 49, 58), found cardiac/coronary disease a correlate for nocturia.
Coffee or alcohol consumption (10, 33, 41, 43, 49, 62, 63) and smoking (33, 49, 61, 63) have been shown elsewhere not to be associated with nocturia. Our findings were the same.
Differences in our results from previous findings may be explained by differences in study procedures and samples (64). Several factors explored here were not assessed in earlier studies (10, 41–43, 45–47, 49–51, 55, 56, 58, 59, 61–63). In addition, several previous studies were not population based (41, 43, 46, 50, 51, 59, 61). The association of numerous factors with nocturia in age-adjusted analyses, partly differing by gender, highlights the importance of appropriate analysis, including controlling for confounders. Given the multiple possible determinants (7–10, 34, 41–43, 45–51, 54, 56–63), we assessed numerous candidates. Because of inconsistencies in the literature, using existing evidence to choose the potential confounders was not justified. Hence, because of the exploratory nature of this analysis, we used stepwise methods for model building (65).
By our methodology, we avoided selection bias due to treatment seeking (reflecting both severity and health care service use). Our study's strengths include 1) a representative sample of both sexes and all adult ages, 2) a high participation rate and completeness of questionnaire responses, 3) a large number of relevant factors, 4) systematic control for confounding, and 5) assessment of nocturia and related symptoms with validated instruments. Furthermore, determinant prevalences were largely similar by response round, indicating absence of selection bias; any trend in the risk estimates by response round was also lacking.
This study has some limitations. First, the validity of self-report has not been established for all characteristics we considered. Second, alcohol consumption reporting was incomplete, yet nocturia prevalence did not vary by alcohol consumption among those reporting this information. In addition, reported alcohol consumption was comparable with the national statistics (66). Third, we had no information on physical activity, although physical activity has not previously been related to nocturia (41). Finally, these results from the Finnish population may not be directly generalizable to other ethnicities because impact measures generally are context specific. There may be ethnic differences in the prevalence of nocturia. Socioeconomic status attenuated, but did not entirely remove, the effect of race/ethnicity on nocturia (67).
Nocturia has been classified as a symptom caused by 1) nocturnal polyuria, 2) low nocturnal bladder capacity, 3) diminished global bladder capacity, 4) a combination of nocturnal polyuria and low bladder capacity, 5) global polyuria, and/or 6) sleep disorders (7). We found several risk factors that may well cause these pathways and, finally, nocturia. However, the pathways are probably complex, and there may also be numerous other underlying causes for the associations, such as autonomic nervous system hyperactivity and/or metabolic syndrome (68, 69). At the population level, urgency, BPH, and snoring for men and overweight and obesity, urgency, and snoring for women explained the largest proportion of nocturia, whereas obesity, antidepressant use, and prostate cancer in men; diabetes and coronary artery disease in women; and restless legs syndrome in both sexes had less of an impact. Even though numerous correlates for nocturia were identified, none was associated with nocturia in more than half of the affected subjects of both sexes, highlighting the multifactorial etiology.
Supplementary Material
Acknowledgments
Author affiliations: Department of Urology, Helsinki University Central Hospital, Helsinki, Finland (Kari A. O. Tikkinen); Clinical Research Institute HUCH Ltd., Helsinki, Finland (Kari A. O. Tikkinen); Department of Urology, Tampere University Hospital, Tampere, Finland (Kari A. O. Tikkinen, Teuvo L. J. Tammela); Medical School, University of Tampere, Tampere, Finland (Kari A. O. Tikkinen, Teuvo L. J. Tammela); School of Public Health, University of Tampere, Tampere, Finland (Anssi Auvinen); Birmingham/Atlanta VA Geriatric Research, Education, and Clinical Center, Decatur, Georgia (Theodore M. Johnson); Division of Geriatric Medicine and Gerontology, Emory University School of Medicine, Atlanta, Georgia (Theodore M. Johnson); Department of Urology, SUNY Downstate Medical School, Brooklyn, New York (Jeffrey P. Weiss); Division of Neurology and Rehabilitation, Tampere University Hospital, Tampere, Finland (Tapani Keränen); Department of Obstetrics and Gynecology, Helsinki University Central Hospital, Helsinki, Finland (Aila Tiitinen); Department of Pulmonary Diseases, Tampere University Hospital, Tampere, Finland (Olli Polo); Department of Neurology, University of Helsinki, Helsinki, Finland (Markku Partinen); and Vitalmed Research Center, Helsinki, Finland (Markku Partinen).
This work was funded by unrestricted grants from the Competitive Research Funding of the Pirkanmaa Hospital District (Tampere, Finland) and Pfizer Inc. (New York, New York). The work of the corresponding author was funded by unrestricted grants from the Emil Aaltonen Foundation and the Pirkanmaa Regional Fund of the Finnish Cultural Foundation. The funding sources had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
The authors thank Dr. Aila Rissanen for her valuable comments on earlier versions of the manuscript and Virginia Mattila for language revision.
K. A. O. T. had full access to all the data used in the study and takes responsibility for the integrity and the accuracy of the data. K. A. O. T., A. A., and T. L. J. T. designed the study. K. A. O. T. collected the data. K. A. O. T. and A. A. performed statistical analysis of the data. All authors contributed to the interpretation of the findings. K. A. O. T. drafted the manuscript. All authors contributed to revising the manuscript.
These results were presented, in part, at the 103rd Annual Meeting of the American Urological Association, Orlando, Florida, May 17–22, 2008; the 2nd North Eastern European Meeting of the European Association of Urology, Vilnius, Lithuania, September 12–13, 2008; and the 24th Annual Meeting of the European Association of Urology, Stockholm, Sweden, March 17–21, 2009.
K. A. O. T., A. A., T. K., and A. T. declare no conflicts of interest. K. A. O. T. served as a speaker at a Pfizer-sponsored overactive bladder symposium, but his honorarium was donated to the Public Library of Science. T. M. J. is a consultant for Ferring (Saint-Prex, Switzerland), Johnson & Johnson (New Brunswick, New Jersey), and Pfizer. J. P. W. is a consultant for Ferring, Pfizer, and Watson Pharma (Salt Lake City, Utah). O. P. is a consultant for Actelion (Allschwil, Switzerland), AstraZeneca (London, United Kingdom), Boehringer-Ingelheim (Ingelheim, Germany), Cephalon (Frazer, Pennsylvania), Eli Lilly (Indianapolis, Indiana), GlaxoSmithKline (London, United Kingdom), Lundbeck (Copenhagen, Denmark), Merck Sharp & Dohme (Readington Township, New Jersey), Organon (Oss, The Netherlands), Orion Pharma (Espoo, Finland), Pfizer, ResMed (Espoo, Finland), Respironics (Murrysville, Pennsylvania), Sanofi-Aventis (Paris, France), and Servier (Neuilly sur Seine, France). M. P. is a consultant for Actelion, Boehringer-Ingelheim, GlaxoSmithKline, IST Technology (Helsinki, Finland), Leiras (Helsinki, Finland), Organon, Orion Pharma, ResMed, Sanofi-Aventis, Servier, Somnomedics GmbH (Randersacker, Germany), and UCB (Brussels, Belgium) and received honoraria from GlaxoSmithKline, Leiras, Sanofi-Aventis, and UCB. T. L. J. T. is a consultant for AstraZeneca, GlaxoSmithKline, Orion Pharma, and Pfizer and received honoraria from Astellas Pharma (Tokyo, Japan), GlaxoSmithKline, Leiras, and Pfizer.
Glossary
Abbreviations
- BPH
benign prostatic hyperplasia
- FINNO
Finnish National Nocturia and Overactive Bladder
References
- 1.Middelkoop HA, Smilde-van den Doel DA, Neven AK, et al. Subjective sleep characteristics of 1,485 males and females aged 50–93: effects of sex and age, and factors related to self-evaluated quality of sleep. J Gerontol A Biol Sci Med Sci. 1996;51(3):M108–M115. doi: 10.1093/gerona/51a.3.m108. [DOI] [PubMed] [Google Scholar]
- 2.van Kerrebroeck P, Abrams P, Chaikin D, et al. The standardisation of terminology in nocturia: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21(2):179–183. doi: 10.1002/nau.10053. [DOI] [PubMed] [Google Scholar]
- 3.Tikkinen KA, Tammela TL, Huhtala H, et al. Is nocturia equally common among men and women? A population based study in Finland. J Urol. 2006;175(2):596–600. doi: 10.1016/S0022-5347(05)00245-4. [DOI] [PubMed] [Google Scholar]
- 4.Tikkinen KA, Johnson TM, 2nd, Tammela TL, et al. Nocturia frequency, bother and quality of life: how often is too often? A population-based study in Finland. Eur Urol. doi: 10.1016/j.eururo.2009.03.080. Advance Access: April 3, 2009. (DOI: 10.1016/j.eururo.2009.03.080) [DOI] [PubMed] [Google Scholar]
- 5.Asplund R, Marnetoft SU, Selander J, et al. Nocturia in relation to somatic health, mental health and pain in adult men and women. BJU Int. 2005;95(6):816–819. doi: 10.1111/j.1464-410X.2005.05407.x. [DOI] [PubMed] [Google Scholar]
- 6.Asplund R. Mortality in the elderly in relation to nocturnal micturition. BJU Int. 1999;84(3):297–301. doi: 10.1046/j.1464-410x.1999.00157.x. [DOI] [PubMed] [Google Scholar]
- 7.Weiss JP, Blaivas JG. Nocturia. J Urol. 2000;163(1):5–12. [PubMed] [Google Scholar]
- 8.Lose G, Alling-Møller L, Jennum P. Nocturia in women. Am J Obstet Gynecol. 2001;185(2):514–521. doi: 10.1067/mob.2001.116091. [DOI] [PubMed] [Google Scholar]
- 9.Hunskaar S. Epidemiology of nocturia. BJU Int. 2005;96(suppl 1):4–7. doi: 10.1111/j.1464-410X.2005.05650.x. [DOI] [PubMed] [Google Scholar]
- 10.Johnson TM, 2nd, Sattin RW, Parmelee P, et al. Evaluating potentially modifiable risk factors for prevalent and incident nocturia in older adults. J Am Geriatr Soc. 2005;53(6):1011–1016. doi: 10.1111/j.1532-5415.2005.53321.x. [DOI] [PubMed] [Google Scholar]
- 11.Abrams PH, Farrar DJ, Turner-Warwick RT, et al. The results of prostatectomy: a symptomatic and urodynamic analysis of 152 patients. J Urol. 1979;121(5):640–642. doi: 10.1016/s0022-5347(17)56918-9. [DOI] [PubMed] [Google Scholar]
- 12.Bruskewitz RC, Larsen EH, Madsen PO, et al. 3-year followup of urinary symptoms after transurethral resection of the prostate. J Urol. 1986;136(3):613–615. doi: 10.1016/s0022-5347(17)44991-3. [DOI] [PubMed] [Google Scholar]
- 13.Cardozo L, Rekers H, Tapp A, et al. Oestriol in the treatment of postmenopausal urgency: a multicentre study. Maturitas. 1993;18(1):47–53. doi: 10.1016/0378-5122(93)90028-g. [DOI] [PubMed] [Google Scholar]
- 14.Reynard JM, Cannon A, Yang Q, et al. A novel therapy for nocturnal polyuria: a double-blind randomized trial of frusemide against placebo. Br J Urol. 1998;81(2):215–218. doi: 10.1046/j.1464-410x.1998.00511.x. [DOI] [PubMed] [Google Scholar]
- 15.Homma Y, Yamaguchi T, Kondo Y, et al. Significance of nocturia in the International Prostate Symptom Score for benign prostatic hyperplasia. J Urol. 2002;167(1):172–176. [PubMed] [Google Scholar]
- 16.Mattiasson A, Abrams P, Van Kerrebroeck P, et al. Efficacy of desmopressin in the treatment of nocturia: a double-blind placebo-controlled study in men. BJU Int. 2002;89(9):855–862. doi: 10.1046/j.1464-410x.2002.02791.x. [DOI] [PubMed] [Google Scholar]
- 17.Johnson TM, II, Jones K, Williford WO, et al. Changes in nocturia from medical treatment of benign prostatic hyperplasia: secondary analysis of the Department of Veterans Affairs Cooperative Study Trial. J Urol. 2003;170(1):145–148. doi: 10.1097/01.ju.0000069827.09120.79. [DOI] [PubMed] [Google Scholar]
- 18.Lose G, Lalos O, Freeman RM, et al. Efficacy of desmopressin (Minirin) in the treatment of nocturia: a double-blind placebo-controlled study in women. Am J Obstet Gynecol. 2003;189(4):1106–1113. doi: 10.1067/s0002-9378(03)00593-3. [DOI] [PubMed] [Google Scholar]
- 19.Araki T, Yokoyama T, Kumon H. Effectiveness of a nonsteroidal anti-inflammatory drug for nocturia on patients with benign prostatic hyperplasia: a prospective non-randomized study of loxoprofen sodium 60 mg once daily before sleeping. Acta Med Okayama. 2004;58(1):45–49. doi: 10.18926/AMO/32115. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan SA, Roehrborn CG, Rovner ES, et al. Tolterodine and tamsulosin for treatment of men with lower urinary tract symptoms and overactive bladder: a randomized controlled trial. JAMA. 2006;296(19):2319–2328. doi: 10.1001/jama.296.19.2319. [DOI] [PubMed] [Google Scholar]
- 21.Addla SK, Adeyoju AB, Neilson D, et al. Diclofenac for treatment of nocturia caused by nocturnal polyuria: a prospective, randomised, double-blind, placebo-controlled crossover study. Eur Urol. 2006;49(4):720–725. doi: 10.1016/j.eururo.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 22.Rackley R, Weiss JP, Rovner ES, et al. Nighttime dosing with tolterodine reduces overactive bladder-related nocturnal micturitions in patients with overactive bladder and nocturia. Urology. 2006;67(4):731–736. doi: 10.1016/j.urology.2005.10.061. discussion 736. [DOI] [PubMed] [Google Scholar]
- 23.Johnson TM, II, Burrows PK, Kusek JW, et al. The effect of doxazosin, finasteride and combination therapy on nocturia in men with benign prostatic hyperplasia. J Urol. 2007;178(5):2045–2050. doi: 10.1016/j.juro.2007.07.013. discussion 2050–2051. [DOI] [PubMed] [Google Scholar]
- 24.Robinson D, Cardozo L, Terpstra G, et al. A randomized double-blind placebo-controlled multicentre study to explore the efficacy and safety of tamsulosin and tolterodine in women with overactive bladder syndrome. BJU Int. 2007;100(4):840–845. doi: 10.1111/j.1464-410X.2007.07162.x. [DOI] [PubMed] [Google Scholar]
- 25.Brubaker L, Fitzgerald MP. Nocturnal polyuria and nocturia relief in patients treated with solifenacin for overactive bladder symptoms. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(7):737–741. doi: 10.1007/s00192-006-0239-y. [DOI] [PubMed] [Google Scholar]
- 26.Tikkinen KA, Tammela TL, Rissanen AM, et al. Is the prevalence of overactive bladder overestimated? A population-based study in Finland [electronic article] PLoS ONE. 2007;2(2) doi: 10.1371/journal.pone.0000195. e195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tikkinen KA, Auvinen A, Tiitinen A, et al. Reproductive factors associated with nocturia and urinary urgency in women—a population-based study in Finland [electronic article] Am J Obstet Gynecol. 2008;199(2):153.e1–153.e12. doi: 10.1016/j.ajog.2008.03.054. [DOI] [PubMed] [Google Scholar]
- 28.Schou J, Poulsen AL, Nordling J. The value of a new symptom score (DAN-PSS) in diagnosing uro-dynamic infravesical obstruction in BPH. Scand J Urol Nephrol. 1993;27(4):489–492. doi: 10.3109/00365599309182282. [DOI] [PubMed] [Google Scholar]
- 29.Barry MJ, Fowler FJ, Jr, O'Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148(5):1549–1557. doi: 10.1016/s0022-5347(17)36966-5. discussion 1564. [DOI] [PubMed] [Google Scholar]
- 30.Vartiainen E, Jousilahti P, Juolevi A, et al. Helsinki, Finland: National Public Health Institute; 1998. The National FINRISK Study 1997 [in Finnish] [Google Scholar]
- 31.ATC Index With DDDs. Oslo, Norway: WHO Collaborating Centre for Drug Statistics Methodology; 2004. [Google Scholar]
- 32.Partinen M, Gislason T. Basic Nordic Sleep Questionnaire (BNSQ): a quantitated measure of subjective sleep complaints. J Sleep Res. 1995;4(suppl 1):150–155. doi: 10.1111/j.1365-2869.1995.tb00205.x. [DOI] [PubMed] [Google Scholar]
- 33.Samuelsson E, Victor A, Tibblin G. A population study of urinary incontinence and nocturia among women aged 20–59 years. Prevalence, well-being and wish for treatment. Acta Obstet Gynecol Scand. 1997;76(1):74–80. doi: 10.3109/00016349709047789. [DOI] [PubMed] [Google Scholar]
- 34.Kinn AC, Harlid R. Snoring as a cause of nocturia in men with lower urinary tract symptoms. Eur Urol. 2003;43(6):696–701. doi: 10.1016/s0302-2838(03)00141-6. [DOI] [PubMed] [Google Scholar]
- 35.Last JM. A Dictionary of Epidemiology. 4th ed. New York, NY: Oxford University Press; 2001. [Google Scholar]
- 36.Population Register Centre. Pocket information. [in Finnish and Swedish]. Helsinki, Finland: Population Register Centre; 2004. ( http://www.vrk.fi/vrk/files.nsf/files/5EC4A1734BB7E9B8C2256ECC0039B42C/$file/Taskutieto+2004.pdf) (Accessed October 20, 2008) [Google Scholar]
- 37.Tikkinen KA, Auvinen A, Huhtala H, et al. Nocturia and obesity: a population-based study in Finland. Am J Epidemiol. 2006;163(11):1003–1011. doi: 10.1093/aje/kwj139. [DOI] [PubMed] [Google Scholar]
- 38.Helakorpi S, Patja K, Prättälä R, et al. Health Behaviour and Health Among the Finnish Adult Population, Spring 2004. [in Finnish with English summary]. Helsinki, Finland: National Public Health Institute; 2005. ( http://www.ktl.fi/attachments/suomi/julkaisut/julkaisusarja_b/2004b13.pdf) (Accessed October 20, 2008) [Google Scholar]
- 39.Aromaa A, Koskinen S. Health and Functional Capacity in Finland. Baseline Results of the Health 2000 Examination Survey. Helsinki, Finland: National Public Health Institute; 2004. ( http://www.ktl.fi/attachments/suomi/julkaisut/julkaisusarja_b/2004b12.pdf) (Accessed October 20, 2008) [Google Scholar]
- 40.Männistö S, Ovaskainen M, Valsta L. The National FINDIET 2002 Study. [in Finnish with English summary]. Helsinki, Finland: National Public Health Institute; 2003. ( http://www.ktl.fi/portal/suomi/osastot/eteo/yksikot/ravitsemusyksikko/julkaisut/finravinto_2002_-tutkimuksen_raportti/) (Accessed October 20, 2008) [Google Scholar]
- 41.Schatzl G, Temml C, Schmidbauer J, et al. Cross-sectional study of nocturia in both sexes: analysis of a voluntary health screening project. Urology. 2000;56(1):71–75. doi: 10.1016/s0090-4295(00)00603-8. [DOI] [PubMed] [Google Scholar]
- 42.Rembratt A, Norgaard JP, Andersson KE. Nocturia and associated morbidity in a community-dwelling elderly population. BJU Int. 2003;92(7):726–730. doi: 10.1046/j.1464-410x.2003.04467.x. [DOI] [PubMed] [Google Scholar]
- 43.Yoshimura K, Terada N, Matsui Y, et al. Prevalence of and risk factors for nocturia: analysis of a health screening program. Int J Urol. 2004;11(5):282–287. doi: 10.1111/j.1442-2042.2004.00791.x. [DOI] [PubMed] [Google Scholar]
- 44.Weiss JP, Blaivas JG, Jones M, et al. Age related pathogenesis of nocturia in patients with overactive bladder. J Urol. 2007;178(2):548–551. doi: 10.1016/j.juro.2007.03.117. discussion 551. [DOI] [PubMed] [Google Scholar]
- 45.Blanker MH, Bohnen AM, Groeneveld FP, et al. Normal voiding patterns and determinants of increased diurnal and nocturnal voiding frequency in elderly men. J Urol. 2000;164(4):1201–1205. [PubMed] [Google Scholar]
- 46.Yoshimura K, Ohara H, Ichioka K, et al. Nocturia and benign prostatic hyperplasia. Urology. 2003;61(4):786–790. doi: 10.1016/s0090-4295(02)02444-5. [DOI] [PubMed] [Google Scholar]
- 47.Fitzgerald MP, Litman HJ, Link CL, et al. The association of nocturia with cardiac disease, diabetes, body mass index, age and diuretic use: results from the BACH survey. J Urol. 2007;177(4):1385–1389. doi: 10.1016/j.juro.2006.11.057. [DOI] [PubMed] [Google Scholar]
- 48.Laven BA, Orsini N, Andersson SO, et al. Birth weight, abdominal obesity and the risk of lower urinary tract symptoms in a population based study of Swedish men. J Urol. 2008;179(5):1891–1895. doi: 10.1016/j.juro.2008.01.029. discussion 1895–1896. [DOI] [PubMed] [Google Scholar]
- 49.Bing MH, Moller LA, Jennum P, et al. Nocturia and associated morbidity in a Danish population of men and women aged 60–80 years. BJU Int. 2008;102(7):808–814. doi: 10.1111/j.1464-410X.2008.07813.x. discussion 814–815. [DOI] [PubMed] [Google Scholar]
- 50.Fitzgerald MP, Mulligan M, Parthasarathy S. Nocturic frequency is related to severity of obstructive sleep apnea, improves with continuous positive airways treatment. Am J Obstet Gynecol. 2006;194(5):1399–1403. doi: 10.1016/j.ajog.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 51.Lowenstein L, Kenton K, Brubaker L, et al. The relationship between obstructive sleep apnea, nocturia, and daytime overactive bladder syndrome in women [electronic article] Am J Obstet Gynecol. 2008;198(5):598.e1–598.e5. doi: 10.1016/j.ajog.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 52.Hornyak M, Kopasz M, Berger M, et al. Impact of sleep-related complaints on depressive symptoms in patients with restless legs syndrome. J Clin Psychiatry. 2005;66(9):1139–1145. doi: 10.4088/jcp.v66n0909. [DOI] [PubMed] [Google Scholar]
- 53.Pearson VE, Gamaldo CE, Allen RP, et al. Medication use in patients with restless legs syndrome compared with a control population. Eur J Neurol. 2008;15(1):16–21. doi: 10.1111/j.1468-1331.2007.01991.x. [DOI] [PubMed] [Google Scholar]
- 54.Asplund R, Johansson S, Henriksson S, et al. Nocturia, depression and antidepressant medication. BJU Int. 2005;95(6):820–823. doi: 10.1111/j.1464-410X.2005.05408.x. [DOI] [PubMed] [Google Scholar]
- 55.Häkkinen JT, Shiri R, Koskimäki J, et al. Depressive symptoms increase the incidence of nocturia: Tampere Aging Male Urologic Study (TAMUS) J Urol. 2008;179(5):1897–1901. doi: 10.1016/j.juro.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 56.Asplund R. Nocturia in relation to sleep, somatic diseases and medical treatment in the elderly. BJU Int. 2002;90(6):533–536. doi: 10.1046/j.1464-410x.2002.02975.x. [DOI] [PubMed] [Google Scholar]
- 57.Lee WC, Wu HP, Tai TY, et al. Effects of diabetes on female voiding behavior. J Urol. 2004;172(3):989–992. doi: 10.1097/01.ju.0000136255.83054.0c. [DOI] [PubMed] [Google Scholar]
- 58.Yu HJ, Chen TH, Chie WC, et al. Prevalence and associated factors of nocturia among adult residents of the Matsu area of Taiwan. J Formos Med Assoc. 2005;104(6):444–447. [PubMed] [Google Scholar]
- 59.Gourova LW, van de Beek C, Spigt MG, et al. Predictive factors for nocturia in elderly men: a cross-sectional study in 21 general practices. BJU Int. 2006;97(3):528–532. doi: 10.1111/j.1464-410X.2006.06029.x. [DOI] [PubMed] [Google Scholar]
- 60.Sarma AV, Burke JP, Jacobson DJ, et al. Associations between diabetes and clinical markers of benign prostatic hyperplasia among community-dwelling Black and White men. Diabetes Care. 2008;31(3):476–482. doi: 10.2337/dc07-1148. [DOI] [PubMed] [Google Scholar]
- 61.Bursztyn M, Jacob J, Stessman J. Usefulness of nocturia as a mortality risk factor for coronary heart disease among persons born in 1920 or 1921. Am J Cardiol. 2006;98(10):1311–1315. doi: 10.1016/j.amjcard.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 62.Asplund R, Aberg HE. Nocturia in relation to body mass index, smoking and some other life-style factors in women. Climacteric. 2004;7(3):267–273. doi: 10.1080/13697130400001398. [DOI] [PubMed] [Google Scholar]
- 63.Hsieh CH, Chen HY, Hsu CS, et al. Risk factors for nocturia in Taiwanese women aged 20–59 years. Taiwan J Obstet Gynecol. 2007;46(2):166–170. doi: 10.1016/S1028-4559(07)60012-6. [DOI] [PubMed] [Google Scholar]
- 64.Ioannidis JP. Why most published research findings are false [electronic article] PLoS Med. 2005;2(8):e124. doi: 10.1371/journal.pmed.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun GW, Shook TL, Kay GL. Inappropriate use of bivariable analysis to screen risk factors for use in multivariable analysis. J Clin Epidemiol. 1996;49(8):907–916. doi: 10.1016/0895-4356(96)00025-x. [DOI] [PubMed] [Google Scholar]
- 66.Laatikainen T, Tapanainen H, Alfthan G, et al. The National FINRISK Study 2002. [in Finnish with English summary]. Helsinki, Finland: National Public Health Institute; 2003. ( http://www.ktl.fi/publications/2003/b72.pdf) (Accessed October 20, 2008) [Google Scholar]
- 67.Kupelian V, Link CL, Hall SA, et al. Are racial/ethnic disparities in the prevalence of nocturia due to socioeconomic status? Results of the BACH Survey. J Urol. 2009;181(4):1756–1763. doi: 10.1016/j.juro.2008.11.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McVary KT, Rademaker A, Lloyd GL, et al. Autonomic nervous system overactivity in men with lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol. 2005;174(4 pt 1):1327–1433. doi: 10.1097/01.ju.0000173072.73702.64. [DOI] [PubMed] [Google Scholar]
- 69.Rohrmann S, Smit E, Giovannucci E, et al. Association between markers of the metabolic syndrome and lower urinary tract symptoms in the Third National Health and Nutrition Examination Survey (NHANES III) Int J Obes (Lond) 2005;29(3):310–316. doi: 10.1038/sj.ijo.0802881. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.