Abstract
Sensory maps in the nervous system often connect to each other in a topographic fashion. This is most strikingly seen in the visual system, where neighboring neurons in the retina project to neighboring neurons in the target structure, such as the superior colliculus. This article discusses the developmental mechanisms that are involved in the formation of topographic maps, with an emphasis on the role of theoretical models in helping us to understand these mechanisms. Recent experimental advances in studying the roles of guidance molecules and patterns of spontaneous activity mean that there are new challenges to be addressed by theoretical models. Key questions include understanding what instructional cues are present in the patterns of spontaneous activity, and how activity and guidance molecules might interact. Our discussion concludes by comparing development of visual maps with development of maps in the olfactory system, where the influence of neural activity seems to differ.
Our brain is organized to process data and respond to our perception of the world based on what we sense. All sensory pathways undergo a period of development when orderly connections which transfer information from the periphery to the central nervous system form and refine. These orderly connections can be represented in the form of a map, with input neurons projecting to a target area in the brain. The development of precise maps is especially interesting to study because many connections wire correctly before sensory mechanisms are fully functioning.
We want to understand the common themes that underlie map development and formation of orderly connections present in the adult nervous system. This will give us ideas about the self-organizing principles in the developing nervous system and their importance for preserving and continuing map development when sensory mechanisms become functional. Studying developmental processes may also give us insights into how neuronal networks might reorganize in response to injury and, hence, be of clinical importance.
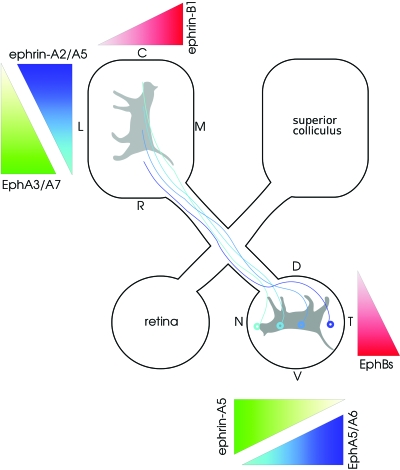
Figure 1 shows a typical schematic of a retinotopic map: neighboring neurons in the retina project to neighboring neurons in a target area, in this case, the superior colliculus (SC). Preserving neighboring relationships is important so that the visual image projected onto the retina is transferred in an orderly manner to the SC. If the projection were not topographic, the spatial representation of the image of the cat in Fig. 1 would be scrambled as it reaches the SC. Such topographic maps are found in other parts of the visual pathway, such as from retina to lateral geniculate nucleus (LGN), or from LGN to visual cortex, and also in other, but not all, sensory areas. However, most attention has been focused on development of visual maps, which is the key focus of this article.
Figure 1. Retinotopic projection of retina onto superior colliculus (SC).
This projection ensures that retinal neurons responding to neighboring parts of the visual world project to neighboring neurons in the SC. This map is established by molecular gradients: Ephs and ephrins. For example, the level of EphA5 receptor in a retinal neuron depends on whether it is located in nasal (N; low level) or temporal (T; high level) retina. The corresponding ephrin-A5 ligand is also differentially expressed: high in caudal (C) SC and low in rostral (R) SC. In this pathway, a retinal neuron with high levels of EphA5 preferentially targets a region of SC with low ephrin-A5 and vice-versa. The dorsoventral (D-V) axis of the retina maps onto the lateromedial (L-M) axis of the SC using the graded distribution of EphBs and ephrin-B1, but in this case, retinal neurons with a high level of EphB map onto the region of the SC with corresponding high level of ephrin-B1. Note also the presence of an EphA3/A7 gradient in the SC opposite to the ephrin-A gradients, which suppresses branching. Figure adapted from Luo (2006).
DEVELOPMENT OF RETINOTOPIC MAPS
The traditional view is that map formation is governed by two differing developmental processes: (a) Marker-based mechanisms postulate that pre- and postsynaptic neurons are given labels, and that presynaptic neurons will synapse onto postsynaptic neurons that have the matching label (Sperry, 1963). Given the large number of presynaptic neurons, having a distinct marker for each neuron is infeasible; instead, a few markers, in the form of receptors and ligands, are used. The amount of marker varies across a region, thus forming “molecular gradients.” For example, EphB receptors are found in low levels in neurons from dorsal retina and in high levels in ventral retina (Fig. 1). The corresponding ephrin-B ligands (which bind to the EphB receptors) are also distributed in an increasing lateral-to-medial gradient across the surface of the SC. A retinal neuron from ventral retina, with a high concentration of EphB receptors, will therefore contact a neuron in the medial SC where there is a high concentration of ephrin-B ligands. See Fig. 1 for further details on the distribution of other molecular gradients, and for recent reviews of these gradients, see McLaughlin and O’Leary (2005); Flanagan (2006).
(b) Activity-dependent mechanisms suggest that initially diffuse connections between pre- and postsynaptic neurons will strengthen if the two neurons tend to fire in synchrony; otherwise the connection between the two neurons will weaken (Hebb, 1949). The modern interpretation of this Hebbian hypothesis is encompassed by theories such as spike timing-dependent plasticity, as described in the section “Spike-based or burst-based rules?”
These two styles of mechanisms are sometimes referred to as activity-independent and activity-dependent mechanisms; however, this naming scheme seems somewhat over-inclusive as the two include all developmental mechanisms, since they either require activity or do not require it.
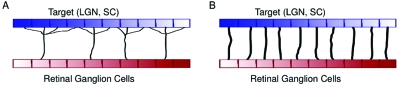
Although we focus here on visual maps, the developmental mechanisms described are quite general and are believed to govern map formation and refinement in other developing systems such as spinal cord, hippocampus, and neuromuscular junction, among others (O’Donovan, 1999). In Fig. 2 we illustrate the role of the two stages of formation and refinement of connections: first, marker-based mechanisms establish a coarse topography which is then refined by activity-dependent cues. Retinal ganglion cells (RGCs) initially sprout axons which overshoot their appropriate termination zones (defined by the matching or complementing of molecular gradients, outlined in Fig. 1) and innervate multiple target neurons. The target area could be the thalamus (which then projects to the cortex) or the SC for mammalian vertebrates (optic tectum for nonmammalian vertebrates). After molecular gradients establish the coarse map so that RGCs map roughly to their correct retinotopic locations making multiple connections with their target neurons [Fig. 2a], activity takes the lead and refines these connections so that each RGC maps to several target cells and the connections to these few target cells strengthen 50-fold [Fig. 2b] (Chen and Regehr, 2000; Jaubert-Miazza et al., 2005).
Figure 2. Development of connectivity between retina and LGN.
(A) Initially retinal axons innervate many target neurons with weak connections. (B) During development, connections from a subset of neighboring retinal neurons are enhanced, whilst the remaining connections from other retinal neurons weaken, or disappear entirely.
The contribution of theoretical modeling to understanding map formation
Computational modeling has been instructive in understanding the development of topographic mappings (see Swindale, 1996; Goodhill, 2007, for reviews). Most computational modeling work is based upon the pioneering work of Willshaw and von der Malsburg in the 1970s, where in two key papers they separately considered the role of neural activity (Willshaw and von der Malsburg, 1976) and markers (Willshaw and von der Malsburg, 1979) for the establishment of retinotopic maps. In these papers, the two models were able to generate retinotopic maps, but with slightly different mechanisms and implications. Computer models are useful for evaluating scientific hypotheses as they require an explicit description of the mechanisms being investigated. As it is rare that all the biological details are known, certain assumptions have to be made (and explicitly defined) within the models, which can also help further our understanding of the biological system.
Molecular gradients
Before the discovery of the Eph∕ephrin gradients (Cheng et al., 1995), the marker induction model (Willshaw and von der Malsburg, 1979) assumed that there were a small number of presynaptic markers that diffused from different retinal locations. Therefore each retinal neuron was assumed to have a certain amount of each marker inversely proportional to the distance of that neuron to the source of the marker. The combination of concentrations of each marker was, thus, unique to each retinal neuron. By contrast, postsynaptic neurons were not given markers initially; a key feature of this model was to propose that markers could be induced by the presynaptic neurons into the postsynaptic neurons. In a recent revision to this marker induction model, the model assumes that presynaptic labels are distributed similarly to those of the Eph gradients (Willshaw, 2006). An advantage of the marker induction hypothesis is that the postsynaptic gradients automatically adapt to postnatal growth of the target structures, although induction of markers has yet to be experimentally observed.
The changing nature of spontaneous activity
Another mechanism by which a postsynaptic neuron might identify whether two presynaptic neurons are neighbors is by examining their activity patterns; Lettvin, cited by Chung (1974), suggested that the firing activity of neighboring retinal axons is likely to be correlated. This suggestion that correlated presynaptic firing patterns could indicate neighbor relations was initially tested by the neural activity model (Willshaw and von der Malsburg, 1976).
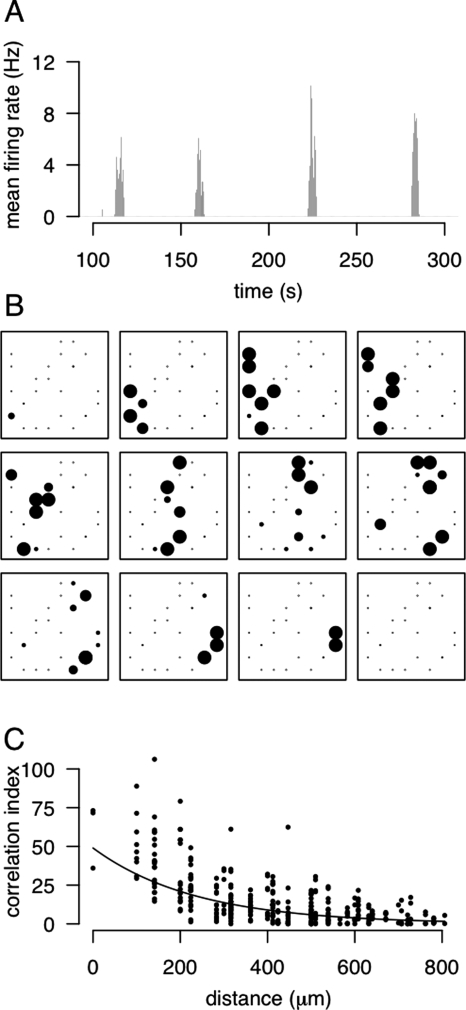
At the time the neural activity model was published, there was no direct evidence for correlated activity of retinal neurons when these maps develop. However, a potential concern was that the maps develop before the retina is visually responsive. Around ten years later, Shatz and Stryker (1988) showed that neural activity was required for refinement of the retinogeniculate pathway at a time before vision was possible. If the retina was not able to generate visually-mediated neural activity, then where was the activity coming from? Shortly afterwards, it was discovered that retinal ganglion cells are spontaneously active (Galli and Maffei, 1988) and that neighboring neurons have correlated activity (Maffei and Galli-Resta, 1990). Multi-electrode array recordings of this spontaneous activity revealed “waves” of activity spreading across the cat and ferret retina (Meister et al., 1991) (Fig. 3). These retinal waves provide the type of short-range correlated activity among neighboring retinal ganglion cells that was postulated by Willshaw and von der Malsburg (1976).
Figure 3. Dynamics of retinal waves.
Data shown here is taken from a P11 mouse retina (Demas et al., 2003). (A) Mean firing rate activity, averaged across the multi-electrode array. Periodic elevations in firing rates are surrounded by periods of quiescence. (B) Visualization of the activity patterns during one wave. Each electrode is represented by one dot, and the size of the dot is proportional to the firing rate during that 0.5 second interval. Separation between neighboring electrodes is 100 μm. (C) Estimation of correlation between pairs of neurons. Neighboring neurons are more correlated than pairs of neurons that are further apart on the retina. The correlation index is a measure of the degree of correlation between a pair of spike trains (using a time window of ±50 ms). Uncorrelated pairs of neurons will have a correlation index around one, whereas the more correlated the pair of neurons, the higher the index (Wong et al., 1993).
Since the initial discovery of retinal waves, they have subsequently been observed in a wide range of species. The mechanisms underlying their generation have been studied in detail, first by pharmacological means, and more recently using various mutants (Firth et al., 2005). Most recent work has been undertaken in mouse retina due to the genetic techniques available and, although developmental times will vary, the key properties of mouse retinal waves are thought to also exist in different species.
Retinal waves in mice are present from birth to around postnatal day 15–21 (P15–P21; Demas et al., 2003). During this period, the patterns and mechanisms underlying their generation seem to change quite rapidly. In the first week, the neurotransmitter acetylcholine (released by starburst amacrines in the retina) plays a key role in generation of spontaneous activity. However, in the second postnatal week, the waves become mediated mostly by glutamatergic connections. The source of the glutamate is as yet unknown, although it could be either bipolar cell terminals or axon collaterals from other retinal ganglion cells. Further, during the second postnatal week (peaking at P12), on- and off-center RGCs generate different firing patterns (Kerschensteiner and Wong, 2008). This changing nature of activity may help the stepwise refinement of neural connections in the LGN, as has been shown in the ferret. Early in development, ferret LGN neurons receive inputs from both on- and off-center RGCs; the emergence of differences between spontaneous firing patterns of on- and off-center RGCs matches the developmental period during which LGN neurons become purely on- or off-center responsive (Lee et al., 2002).
Knowledge of the mechanisms generating the activity is important, as this then allows us to perturb the circuit in some fashion, and see if or how the changes in activity cause changes in development of connections. For example, increasing the frequency of retinal waves in one eye resulted in that eye solely innervating more of the target structure (Stellwagen and Shatz, 2002). A key concern, however, with experiments that evaluate the roles of activity by manipulating the level of activity is that it is unclear whether the patterns of activity are important or if activity simply needs to be present (Crair, 1999). A recent mutant knockout mouse, the β2 mutant, has been useful in this regard, since the retina is still spontaneously active but cholinergic-mediated waves are absent. In the β2 mutant, retinotopy is perturbed, suggesting that correlated patterns of activity are required for correct map formation (McLaughlin et al., 2003; Grubb et al., 2003). (See the section “Instructional cues present in spontaneous activity” for counter arguments regarding the β2 mutant.)
Spontaneous neural activity in development is not restricted to the visual system; it is found in many other systems, including hippocampus and spinal cord (O’Donovan, 1999), as well as in cultures of cortical neurons (Maeda et al., 1995). Furthermore, rather than being implicated only in the development of neural connections, its role in development is quite varied, influencing many different aspects of neural development via the regulation of intracellular calcium (see Moody and Bosma, 2005, for review). For example, diverse developmental events in cortex such as neuronal migration, neuronal survival, and dendritic arborization are all thought to rely on calcium influx, mediated by spontaneous neuronal activity (Moody and Bosma, 2005).
THEORETICAL PRINCIPLES UNDERLYING MAP FORMATION DRIVEN BY SPONTANEOUS ACTIVITY
In this section, we list some of the principles that are embodied in computational models of map formation. We have focused here on the traditional view that molecular gradients set up a coarse map which activity-dependent mechanisms then refine; see Goodhil and Xu (2005) for a recent review of gradient-based models. This limitation to one style of mechanism was chosen for simplicity; as noted in the section “Future Directions,” both activity and molecular gradients may work together in interesting ways to form retinotopic maps.
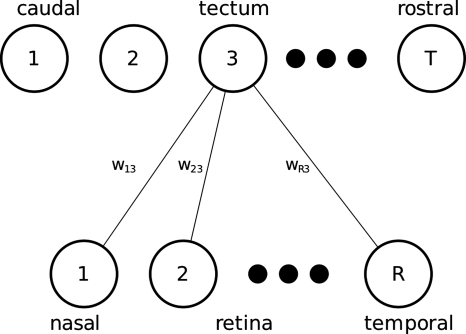
We first introduce a typical mathematical architecture and conventions, so that terms and equations can be provided alongside the relevant principles. We assume that we are modeling a one-dimensional strip of presynaptic retinal neurons projecting onto a one-dimensional arrangement of postsynaptic tectal neurons (Fig. 4). The simplification of studying just one-dimensional projections is for convenience (e.g., so that the synaptic connections between retina and tectum can be shown as a matrix, as in Fig. 5), and most models in practice simulate two-dimensional retinas and tecta. The strength of connection between retinal neuron r and tectal neuron c is given by wrc. The firing rate at any time of the rth retinal neuron and cth tectal neuron is given by xr and yc, respectively.
Figure 4. Example of one-dimensional layout used in modeling retinotopic maps.
The synapses from all retinal neurons to just one tectal neuron are shown here for simplicity; in most theoretical frameworks, synapses exist between all presynaptic and all postsynaptic neurons.
Figure 5. Typical synaptic weight matrices representing one-dimensional mappings during development.
(Maps are illustrative, rather than the output from computer simulations.) Each synapse is represented by a square, with the area of the square proportional to the strength of the synapse. (A) Typical initial conditions, where synapses are initialized to have small values chosen at random. To ensure that temporal retina avoids connecting to caudal tectum and that nasal retina avoids rostral tectum, synapses in these regions are reduced. This creates a bias along the diagonal of the matrix, and represents the rough ordering that might be imposed by molecular gradients. (B) Typical synaptic profile after synapses have been modified according to the Hebbian principles. Note that each tectal neuron receives inputs predominantly from a few neighboring retinal neurons, and that neighboring tectal neurons respond to similar parts of the retina. Together with the initial bias in connectivity, this creates a globally ordered map. (C) A typical map that might develop if no bias is provided in the initial synapses. Although each tectal neuron receives inputs from a few neighboring neurons, neighboring tectal neurons do not always respond to neighboring parts of visual space, resulting in a map with only local order.
How does a postsynaptic neuron decide which inputs to strengthen, and which to weaken?
-
1.
Neighboring presynaptic neurons fire in synchrony. Two neighboring retinal neurons [e.g., within 50–200 μm of each other; see Fig. 3c] are likely to fire together and thus be more correlated than two retinal neurons that are further apart (more than 200–300 μm apart). Hence, the correlation in firing of two retinal neurons, xi and xj, is inversely correlated with the distance separating neurons i and j [see Fig. 3c].
-
2.Inferring postsynaptic activity. In mathematical models, the firing rate of a postsynaptic neuron is often assumed to be of the form
where the function f(⋅) is usually a sigmoidal-like function to ensure that the firing rate of a tectal neuron does not exceed some upper bound. More realistic models are typically based on the integrate-and-fire formalism. -
3.Cells that fire together wire together (Hebb, 1949). Given some predefined presynaptic activity and using the above rule to estimate the postsynaptic activity, Hebbian-based rules can be used to modify connection strengths. A key limitation of the original Hebbian hypothesis is that it specifies only how connections should increase in strength; in this case, all connections would grow over time. A more thorough formulation for specifying how weights should change might be a covariance-based rule (Sejnowski, 1977)
where α and β are thresholds for presynaptic and postsynaptic activity, and ϵ is a small constant to ensure that connections change slowly with respect to neuronal firing during development. As long as both presynaptic and postsynaptic neurons are above threshold, the connection increases; else if one neuron is above threshold and the other below threshold, the presynaptic and postsynaptic neurons are firing asynchronously and so the connection weakens. A drawback of this rule is that when pre- and postsynaptic activity are both below threshold, connections will grow; this can be prevented, for example, by allowing β to change during development according to the recent activity of the postsynaptic neuron (Bienenstock et al., 1982).The value of β is often set such that several presynaptic neurons need to be coactive for yj to exceed threshold. Since neighboring presynaptic neurons are likely to be coactive, together they can generate sufficient activity to make a postsynaptic neuron fire, and, hence, the postsynaptic neuron increases the strength to all of the neighboring active presynaptic neurons. Rules such as this are termed rate-based as neural activity is summarized by firing rates; more recently, modification rules based on timing of individual action potentials have been proposed, as described in the section, “Spike-based or burst-based rules?”
-
4.
Neighboring postsynaptic neurons should develop similar connections. With the above rules, each postsynaptic neuron acts independently of each other. Yet, to produce a retinotopic map, the receptive fields of neighboring postsynaptic neurons should be similar. This is achieved by coupling the postsynaptic neurons in some manner. For example, the activity of two neighboring postsynaptic neurons, yj and yj+1, could be coupled by introducing fixed excitatory lateral connections between neighboring neurons (Willshaw and von der Malsburg, 1976).
-
5.
Constraints on synaptic growth. Even if the weight adaptation rule can specify decrements to connections, connections can often grow arbitrarily large unless otherwise constrained. Such constraints are imposed by normalization terms, e.g., ensuring that for each postsynaptic neuron j, , where K is some constant. Such normalization terms are often required for computational reasons rather than explicitly to model particular biological behavior, although it seems intuitive that individual connections are presumably constrained from growing too large.
Starting from an initial set of random connection strengths with a slight bias [Fig. 5a], the above steps are run repeatedly until the model converges upon a set of mature connection strengths [Fig. 5b]. To obtain a global ordering, the initial connection strengths need to biased [as in Fig. 5a] so that, e.g., nasal retina neurons preferentially connect to caudal tectal neurons. By contrast, without any bias in the initial connection strengths, although neighboring postsynaptic neurons respond to neighboring presynaptic neurons, there is no global order [Fig. 5c].
FUTURE DIRECTIONS
In this section we outline several open questions of interest, some of which may be amenable by further theoretical study.
Instructional cues present in spontaneous activity
Although computational models have previously been built to study how correlated neural activity might influence map formation, they can often be criticized for making highly-simplifying assumptions regarding the correlational structure in the input activity (Wilshaw and von der Malsburg, 1976; Eglen, 1999). One key exception to this, however, is the modeling of segregation of on- and off-center RGCs in ferrets, where it has been possible to use experimental recordings of spontaneous activity as the input to the model (Lee et al., 2002). Experimental data collected over the last decade consistently show that the nature of the spontaneous activity changes in development, from early cholinergic-mediated patterns to late glutamatergic-mediated patterns (Sernagor et al., 2000; Torborg and Feller, 2005). However, it is still unclear whether the changes in retinal wave patterns (e.g., switching from cholinergic to glutamatergic activity) convey instructional information relevant to different aspects of retinotopic map formation. Such a question could be investigated theoretically by varying the structure of retinal wave patterns generated in computational models (Feller et al., 1997; Godfrey and Swindale, 2007).
Furthermore, until relatively recently, it was claimed that the β2 mouse was an ideal mutant to investigate the effects of disrupted correlated neural activity, as this mutant was reported to exhibit spontaneous, but uncorrelated, activity in developing retina (McLaughin et al., 2003). These results have now been called into question by recent re-analysis of spontaneous activity in the β2 mutants (Sun et al., 2008). Instead of finding uncorrelated activity, this study reported that neighboring neurons were highly correlated during development, even over longer distances than reported for wild type. Increasing the distance over which RGCs are correlated would presumably lead to topographic errors being made during development, but it is unclear whether this could account for earlier findings on how retinotopic maps are perturbed in β2 mutants (McLaughlin et al., 2003; Grubb et al., 2003). The recent detailed models of retinal wave activity would be useful to help investigate this question.
Spike-based or burst-based rules?
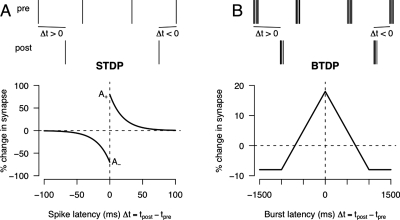
Further to the question of what information is contained in spontaneous activity, as well as considering issues such as spatial correlations, it is also important to consider the temporal precision of neural activity. This question was first addressed by using information-theoretic approaches (Butts and Rokhsar, 2001) suggesting that information content was mostly preserved when using broad time scales (on the order of seconds, rather than milliseconds). This led to the proposal that information was transmitted at the level of bursts of action potentials, rather than individual action potentials (Butts and Rokhsar, 2001; Butts et al., 2007). To use this information, burst time-dependent plasticity (BTDP) has been proposed, based upon recordings in developing retinogeniculate pathway, mirroring that of spike time-dependent plasticity (STDP) (Butts et al., 2007). These two styles of rule are compared in Fig. 6.
Figure 6. Spike time-dependent plasticity (STDP) and burst time-dependent plasticity (BTDP) rules (Zhang et al., 1998; Butts et al., 2007).
In each case, a set of presynaptic and postsynaptic spike trains is shown above and the learning rule below. (A) In STDP, if the presynaptic spike occurs shortly before the postsynaptic spike (Δt>0), then the synapse is potentiated. Otherwise, if the presynaptic occurs shortly after the postsynaptic spike (Δt<0), the synapse is depressed. (B) By contrast, the BTDP rule is symmetric, so that as long as the presynaptic and postsynaptic burst occur within some short time of each other, the synapse is potentiated. Note also that the rule operates at the level of the start of each burst, not the time of each individual spike.
STDP rules have been proposed to account for experimental work showing that long-term synaptic modification is based upon the relative timing of individual pre- and postsynaptic action potentials (Zhang et al., 1998). The exact nature (shape and temporal width) of the STDP curves has varied in different experimental preparations (Feldman, 2000), but the underlying theory is broadly similar. If a presynaptic neuron fires shortly (e.g., about 20 ms) before a postsynaptic neuron, the synapse connecting the two neurons is potentiated. By contrast, if the postsynaptic neuron fires first, then the presynaptic neuron could not have contributed to the postsynaptic firing and, hence, the synapse is depressed. Naive interpretations of the STDP rules suggest that each presynaptic and postsynaptic pair of action potentials is treated separately, although there is accumulating evidence against this assumption of independence (Froemke and Dan, 2002; Wang et al., 2005). By contrast, the BTDP rule proposes that the relative timing of bursts of action potential is the relevant variable controlling potentiation or depression. In addition to the longer temporal scales, the BTDP rule is symmetric, so that it is not important whether the presynaptic or postsynaptic burst occurred first, but whether the two bursts overlap in time or not (Butts et al., 2007). It is an open question as to whether STDP or BTDP is more relevant for the formation of retinotopic maps. By again using detailed models of retinal wave activity, theoretical models should be able to compare the implications of STDP and BTDP for retinotopic map formation.
Nature of molecular gradients
Most theoretical models of map formation involving molecular gradients assume that the gradients have already been established. Key questions still to be addressed include how are the gradients established, and how do they change over time, e.g., in response to continual growth? The marker induction model (Willshaw, 2006), for instance, proposes that presynaptic markers drive the expression of corresponding markers in postsynaptic tissue, but this awaits experimental validation.
A different question concerns the interaction of various multiple gradients (e.g., EphA7 and ephrin-A gradients in the tectum) to help map formation. Here we have described topographic mapping in terms of labeling along two axes of orthogonal molecular gradients. This is indeed the case for animals with lateral positioning of the eyes (such as mice; Fig. 1) where each retina projects contralaterally to the target, but is not the case for binocular species. As Sperry suggested, in binocular species the nasotemporal retinal axis should not express a uniform but a central-to-peripheral gradient, such that the temporal projection of one eye and the nasal projection of the other eye will map to the same target position (Sperry, 1963). Lambot et al. (2005) reported such expression patterns of ephrins and Ephs in developing human retina, confirming Sperry’s proposal. This finding emphasizes that, although the mouse is a good model system, it is clearly not sufficient for explaining human development.
Linking molecular gradients and neural activity
Despite the hypothesis that activity and molecular cues act as two independent mechanisms to drive precise map formation, recently experiments were performed with altered ephrin∕Eph expression patterns and disrupted activity. This work identifies the more general interaction between activity and molecular cues and the implications it has for normal functional properties.
Pfeiffenberger et al. (2005 a ) focused on abnormalities in eye-specific segregation in triple knockout mice lacking ephrin-A2∕A3∕A5 in combination with pharmacologically blocked correlated retinal activity. Triple knockout mice with normal activity exhibited severe defects in the location of the eye-specific layers in the LGN but segregation still occurred. However, blocking activity in addition affected wave structure and prevented eye-specific segregation as in animals with normal ephrin expression (Huberman et al., 2002; Rossi et al., 2001). A similar pattern was observed by Pfeiffenberger et al. (2005b) who analyzed abnormalities in retinogeniculate and retinocollicular mapping in combined ephrin-A2∕A3∕A5 and β2 mutants. These studies suggest an independent action of molecular cues and activity in map formation where ephrin∕Eph gradients are required for establishing the coarse map topography and activity is needed to refine axonal arbors.
As a result of these experiments, most research has focused on analyzing the role of molecular guidance cues and activity as two separate mechanisms operating independently of each other and sequentially to establish precise retinotopic maps. New experiments show that two mechanisms are likely to be closely interlinked, with each regulating the expression of the other. Electrical stimulation of Xenopus spinal neurons affected axon growth patterns initially set up by attractive and repelling cues (Ming et al., 2001). Hanson and Landmesser (2004) demonstrated that blocking or slowing down spontaneous neural activity at different stages of mouse spinal cord development affected accurate axonal positioning direction. Recently, Nicol et al. (2007) reported that ephrin-A responses are modulated by spontaneous activity: ephrin-A5 normally acts as a repellant cue, but when neural activity was blocked using tetrodotoxin, it lost its repellant activity. This loss could be rescued by oscillations in cAMP suggesting that activity regulated ephrin-A expression is mediated via a cAMP-dependent pathway (Nicol et al., 2007). These experimental results suggest that separate modeling of developmental processes involving molecular gradients and neural activity can ignore potential key sources of interaction that generate dynamics important for the formation of retinotopic maps. Combined models of neural activity and molecular gradients are already underway (Tsignkov and Koulakov, 2006), and are going to be more prominent in the coming years.
Comparison with other sensory systems: audition and olfaction
The visual system is often described as a model system for investigating the developmental principles underlying topographic map formation; how might the principles learned from the visual system apply to other sensory systems? In the auditory system, the representation of sound frequency is often tonotopic, such that neighboring neurons in a target area respond to similar frequencies. These maps emerge during development (Lippe and Rubel, 1985; Pienkowski and Harrison, 2005) and may rely on similar principles to the developing visual pathway: both synchronous spontaneous activity and Eph∕ephrin gradients are present in development (Lippe, 1994; Cramer, 2005). Compared to the visual system, there have been relatively few studies investigating the role of molecular gradients and neural activity upon tonotopic map formation. However, a recent study reported that overexpression of EphA receptors resulted in altered tonotopic formation in the auditory brainstem of chicks (Huffman and Cramer, 2007).
Unlike the visual and auditory systems which map sensory information to higher-order processing areas in the brain topographically, the olfactory system exhibits a remarkable wiring specificity on a more discrete, single synapse level. Olfactory neurons specify an orderly map not by their position in the receptor area preserving neighboring relations, but by the type of receptor they express (Fig. 7). Each olfactory neuron (ORN) in the olfactory neuroepithelium expresses a single olfactory receptor (OR) from many receptor genes [Buck and Axel (1991), ∼1,000 in mammals and ∼60 in insects]. ORNs expressing the same OR converge their axons to mirror-image target glomeruli (one medial and one lateral) in each mammalian olfactory bulb (or antennal lobe for insects), which serves as a relay station from the nose towards higher-order processing areas in the brain (Ressler et al., 1994; Vassar et al., 1994). In the olfactory bulb (or antennal lobe), second order neurons known as mitral cells (or projection neurons) project to the glomeruli where they synapse with ORN axons targeting these glomeruli. These second order neurons then project their axons to higher-order olfactory brain centers. A similarity with the relay of visual information from the retina through the thalamus to the visual cortex, where ORNs expressing the same OR correspond to neighboring retinal cells and the olfactory bulb to the thalamus, suggests the need for pre-processing and spatial segregation of afferent inputs to ensure accurate representation of sensory information from the environment to the brain.
Figure 7. Continuous and discrete maps.
In each case, a layer of presynaptic neurons connects to a layer of postsynaptic neurons. (A) In the visual map, neighboring retinal neurons project to neighboring patches of the target area, e.g., LGN. (B) In the olfactory map, input neurons expressing the same olfactory receptor are randomly dispersed throughout the olfactory epithelium. Hence, in this case, neighboring olfactory bulb neurons do not respond to neighboring parts of the olfactory epithelium.
While older work suggested that same type ORNs are organized into to several (4 in mammals) topographically distinct zones in the olfactory epithelium (Ressler et al., 1993; Vassar et al., 1993), more recent work has found that glomerulus organization along the dorso-ventral axis of the olfactory bulb matches a continuous distribution of ORN position in the olfactory epithelium following molecular guidance cues, while the antero-posterior axis is independent of ORN location (Iwema et al., 2004; Miyamichi et al., 2005). Multiple molecules set up this topography as in the visual system [reviewed in Komiyama and Luo (2006)], with ORs being primary candidates (Mombaerts et al., 1996; Wang et al., 1988), along with OR-specific adhesion molecules, Kirrels 2 and 3 (Serizawa et al., 2006), and Ephs∕ephrins (Cutforth et al., 2003). Despite the discovery of these guidance molecules, it is still unknown whether ORNs are autonomous and induce molecular gradients in the target [as Willshaw (2006) proposed in the marker-induction model], or if the olfactory bulb is pre-patterned.
The role of activity in olfactory map development has only recently been investigated, showing only minor ORN-glomeruli map defects after disrupting activity (Zheng et al., 2000; Lin et al., 2000). Additionally, Zou et al. (2004) reported the effects of naris closure postnatally: (1) activity instructs glomerular maturation; (2) there exists a critical period when activity influences this glomerular maturation the most [as found in visual system development (Hooks and Chen, 2006)]; (3) while initially ORNs expressing the same ORs may project to two or more glomeruli, this number reduces to one during development; and (4) similarly, numbers of ORN axons targeting a single glomerulus reduce during development, but through a process of cell death rather than competition. Finally, although disrupting activity resulted in minor map defects, manipulating expression levels of Kirrels and ephrins∕Ephs led to larger defects (Serizawa et al., 2006). These first studies suggest that the role of activity might be quite different in the olfactory system in contrast to the visual system.
Compared to the visual system, theoretical models of the development of the olfactory system are in their infancy. While several types of models have addressed the processing of information in adult olfactory system (Cleland and Linster, 2005), no theoretical models have addressed the development of connections [although see Gill and Pearce (2003)]. While similar questions arise as for development of the visual system, the modeling approaches to olfactory map formation could be very different, especially with the different reliance on neural activity. By studying the mechanisms for the development of different sensory maps, we hope to discover the major themes underlying self-organization in the nervous system.
SUMMARY
In this review we have outlined the key mechanisms thought to be involved in the formation of topographic maps in the nervous system, with particular emphasis on activity-dependent processes. Computational modeling has played an important role in understanding how such topographic maps form. There is still much to be discovered, however, as recent experimental evidence suggests that there may be significant interactions between mechanisms based on molecular gradients and activity-dependent processes. We have outlined some open areas that we think are of interest in this field, including the issue of whether developmental mechanisms are similar or vary in different sensory modalities. We hope that there will be a continuing role for computational models in the understanding of processes of self-organization in the nervous system.
ACKNOWLEDGMENTS
This work is supported by a Wellcome Trust Programme grant, awarded to David Willshaw, Uwe Drescher, Ian Thomspon, and SJE. JG is supported by an Overseas Research Scholarship and Trinity College Internal Graduate Studentship.
References
- Bienenstock, E L, Cooper, L N, and Munro, P W (1982). “Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex.” J. Neurosci. 2, 32–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck, L, and Axel, R (1991). “A novel multigene family may encode odorant receptors: a molecular basis for odor recognition.” Cell 65, 175–187. [DOI] [PubMed] [Google Scholar]
- Butts, D A, Kanold, P O, and Shatz, C J (2007). “A burst-based “hebbian” learning rule at retinogeniculate synapses links retinal waves to activity-dependent refinement.” PLoS Biol. 5, e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts, D A, and Rokhsar, D S (2001). “The information content of spontaneous retinal waves.” J. Neurosci. 21, 961–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C, and Regehr, W G (2000). “Developmental remodeling of the retinogeniculate synapse.” Neuron 28, 955–966. [DOI] [PubMed] [Google Scholar]
- Cheng, H-J, Nakamoto, M, Bergemann, A D, and Flanagan, J G (1995). “Complementary gradients in expression and binding of ELF-1 and Mek4 in development of the topographic retinotectal projection map.” Cell 82, 371–381. [DOI] [PubMed] [Google Scholar]
- Chung, S H (1974). “In search of the rules for nerve connections.” Cell 3, 201–205. [DOI] [PubMed] [Google Scholar]
- Cleland, T A, and Linster, C (2005). “Computation in the olfactory system.” Chem. Senses 30, 801–813. [DOI] [PubMed] [Google Scholar]
- Crair, M C (1999). “Neuronal activity during development: permissive or instructive?” Curr. Opin. Neurobiol. 10.1016/S0959-4388(99)80011-7 9, 88–93. [DOI] [PubMed] [Google Scholar]
- Cramer, K S (2005). “Eph proteins and the assembly of auditory circuits.” Hear. Res. 206, 42–51. [DOI] [PubMed] [Google Scholar]
- Cutforth, T, Moring, L, Mendelsohn, M, Nemes, A, Shah, N M, Kim, M M, Frisén, J, and Axel, R (2003). “Axonal Ephrin-As and odorant receptors coordinate determination of the olfactory sensory map.” Cell 114, 311–322. [DOI] [PubMed] [Google Scholar]
- Demas, J A, Eglen, S J, and Wong, R OL (2003). “Developmental loss of synchronous spontaneous activity in the mouse retina is independent of visual experience.” J. Neurosci. 23, 2851–2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eglen, S J (1999). “The role of retinal waves and synaptic normalization in retinogeniculate development.” Philos. Trans. R. Soc. London, Ser. B 354, 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman, D E (2000). “Timing-based LTP and LTD at vertical inputs to layer II/III pyramidal cells in rat barrel cortex.” Neuron 10.1016/S0896-6273(00)00008-8 27, 45–56. [DOI] [PubMed] [Google Scholar]
- Feller, M B, Butts, D A, Aaron, H L, Rokhsar, D S, and Shatz, C J (1997). “Dynamic processes shape spatiotemporal properties of retinal waves.” Neuron 19, 293–306. [DOI] [PubMed] [Google Scholar]
- Firth, S I, Wang, C T, and Feller, M B (2005). “Retinal waves: mechanisms and function in visual system development.” Cell Calcium 10.1016/j.ceca.2005.01.010 37, 425–432. [DOI] [PubMed] [Google Scholar]
- Flanagan, J G (2006). “Neural map specification by gradients.” Curr. Opin. Neurobiol. 16, 59–66. [DOI] [PubMed] [Google Scholar]
- Froemke, R C, and Dan, Y (2002). “Spike timing-dependent synaptic modification induced by natural spike trains.” Nature 10.1038/416433a 416, 433–438. [DOI] [PubMed] [Google Scholar]
- Galli, L, and Maffei, L (1988). “Spontaneous impulse activity of rat retinal ganglion cells in prenatal life.” Science 242, 90–91. [DOI] [PubMed] [Google Scholar]
- Gill, D S, and Pearce, T C (2003). “Wiring the olfactory bulb—activity-dependent models of axonal targeting in the developing olfactory pathway.” Rev. Neurosci. 14, 63–72. [DOI] [PubMed] [Google Scholar]
- Godfrey, K B, and Swindale, N V (2007). “Retinal wave behavior through activity-dependent refractory periods.” PLOS Comput. Biol. 3, e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodhill, G J (2007). “Contributions of theoretical modeling to the understanding of neural map development.” Neuron 56, 301–311. [DOI] [PubMed] [Google Scholar]
- Goodhill, G J, and Xu, J (2005). “The development of retinotectal maps: a review of models based on molecular gradients.” Network 16, 5–34. [DOI] [PubMed] [Google Scholar]
- Grubb, M S, Rossi, F M, Changeux, J P, and Thompson, I D (2003). “Abnormal functional organization in the dorsal lateral geniculate nucleus of mice lacking the beta 2 subunit of the nicotinic acetylcholine receptor.” Neuron 40, 1161–1172. [DOI] [PubMed] [Google Scholar]
- Hanson, M G, and Landmesser, L T (2004). “Normal patterns of spontaneous activity are required for correct motor axon guidance and the expression of specific guidance molecules.” Neuron 43, 687–701. [DOI] [PubMed] [Google Scholar]
- Hebb, D (1949). The Organization of Behavior, Wiley, New York. [DOI] [PubMed] [Google Scholar]
- Hooks, B M, and Chen, C (2006). “Distinct roles for spontaneous and visual activity in remodeling of the retino-geniculate synapse.” Neuron 52, 281–291. [DOI] [PubMed] [Google Scholar]
- Huberman, A D, Stellwagen, D, and Chapman, B (2002). “Decoupling eye-specific segregation from lamination in the lateral geniculate nucleus.” J. Neurosci. 22, 9419–9429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman, K J, and Cramer, K S (2007). “EphA4 misexpression alters tonotopic projections in the auditory brainstem.” Dev. Neurobiol. 67, 1655–1668. [DOI] [PubMed] [Google Scholar]
- Iwema, C L, Fang, H, Kurtz, D B, Youngentob, S L, and Schwob, J E (2004). “Odorant receptor expression patterns are restored in lesion-recovered rat olfactory epithelium.” J. Neurosci. 24, 356–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaubert-Miazza, L, Green, E, Lo, F, Bui, K, Mills, J, and Guido, W (2005). “Structural and functional composition of the developing retinogeniculate pathway in the mouse.” Visual Neurosci. 22, 661–676. [DOI] [PubMed] [Google Scholar]
- Kerschensteiner, D, and Wong, R OL (2008). “A precisely timed asynchronous pattern of on and off retinal ganglion cell activity during propagation of retinal waves.” Neuron 58, 851–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama, T, and Luo, L (2006). “Development of wiring specifity in the olfactory system.” Curr. Opin. Neurobiol. 16, 67–73. [DOI] [PubMed] [Google Scholar]
- Lambot, M, Depasse, F, Noel, J, and Vanderhaeghen, P (2005). “Mapping labels in the human developing visual system and the evolution of binocular vision.” J. Neurosci. 25, 7232–7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, C W, Eglen, S J, and Wong, R OL (2002). “Segregation of on and off retinogeniculate connectivity directed by patterned spontaneous activity.” J. Neurophysiol. 88, 2311–2321. [DOI] [PubMed] [Google Scholar]
- Lin, D M, Wang, F, Lowe, G, Gold, G H, Axel, R, Ngai, J, and Brunet, L (2000). “Formation of precise connections in the olfactory bulb occurs in the absence of odorant-evoked neuronal activity.” Neuron 26, 69–80. [DOI] [PubMed] [Google Scholar]
- Lippe, W, and Rubel, E W (1985). “Ontogeny of tonotopic organization of brain stem auditory nuclei in the chicken: implications for development of the place principle.” J. Comp. Neurol. 10.1002/cne.902370211 237, 273–289. [DOI] [PubMed] [Google Scholar]
- Lippe, W R (1994). “Rhythmic spontaneous activity in the developing avian auditory system.” J. Neurosci. 14, 1486–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, L (2006). “Two gradients are better than one.” Nature 439, 23–24. [DOI] [PubMed] [Google Scholar]
- Maeda, E, Robinson, H P, and Kawana, A (1995). “The mechanisms of generation and propagation of synchronized bursting in developing networks of cortical neurons.” J. Neurosci. 15, 6834–6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei, L, and Galli-Resta, L (1990). “Correlation in the discharges of neighboring rat retinal ganglion cells during prenatal life.” Proc. Natl. Acad. Sci. U.S.A. 87, 2861–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin, T, and O’Leary, D DM (2005). “Molecular gradients and development of retinotopic maps.” Annu. Rev. Neurosci. 28, 325–355. [DOI] [PubMed] [Google Scholar]
- McLaughlin, T, Torborg, C L, Feller, M F, and O’Leary, D DM (2003). “Retinotopic map refinement requires spontaneous retinal waves during a critical period of development.” Neuron 40, 1147–1160. [DOI] [PubMed] [Google Scholar]
- Meister, M, Wong, R OL, Baylor, D A, and Shatz, C J (1991). “Synchronous bursts of action potentials in ganglion cells of the developing mammalian retina.” Science 252, 939–943. [DOI] [PubMed] [Google Scholar]
- Ming, G, Henley, J, Tessier-Lavigne, M, Songdouble, H, and Poo, M (2001). “Electrical activity modulates growth cone guidance by diffusible factors.” Neuron 29, 441–452. [DOI] [PubMed] [Google Scholar]
- Miyamichi, K, Serizawa, S, Kimura, H M, and Sakano, H (2005). “Continuous and overlapping expression domains of odorant receptor genes in the olfactory epithelium determine the dorsal/ventral positioning of glomeruli in the olfactory bulb.” J. Neurosci. 25, 3586–3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts, P, Wang, F, Dulac, C, Chao, S K, Nemes, A, Mendelsohn, M, Edmondson, I, and Axel, R (1996). “Visualizing an olfactory sensory map.” Cell 87, 675–686. [DOI] [PubMed] [Google Scholar]
- Moody, W J, and Bosma, M M (2005). “Ion channel development, spontaneous activity and activity-dependent development in nerve and muscle cells.” Physiol. Rev. 85, 883–941. [DOI] [PubMed] [Google Scholar]
- Nicol, X, Voyatzis, S, Muzêrelle, A, Narboux-NĔme, N, Südhof, T C, Miles, R, and Gaspar, P (2007). “cAMP oscillations and retinal activity are permissive for ephrin signaling during the establishment of the retinotopic map.” Nat. Neurosci. 10, 340–347. [DOI] [PubMed] [Google Scholar]
- O’Donovan, M J (1999). “The origin of spontaneous activity in developing networks of the vertebrate nervous system.” Curr. Opin. Neurobiol. 10.1016/S0959-4388(99)80012-9 9, 94–104. [DOI] [PubMed] [Google Scholar]
- Pfeiffenberger, C, Cutforth, T, Woods, G, Yamada, J, Renteri’a, R C, Copenhagen, D R, Flanagan, J G, and Feldheim, D A (2005a). “Ephrin-As and neural activity are required for eye-specific patterning during retinogeniculate mapping.” Nat. Neurosci. 8, 1022–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffenberger, C, Yamada, J, and Feldheim, D A (2005b). “Ephrin-As and patterned retinal activity act together in the development of topographic maps in the primary visual system.” J. Neurosci. 8, 1022–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pienkowski, M, and Harrison, R V (2005). “Tone frequency maps and receptive fields in the developing chinchilla auditory cortex.” J. Neurophysiol. 93, 454–466. [DOI] [PubMed] [Google Scholar]
- Ressler, K J, Sullivan, S L, and Buck, L B (1993). “A zonal organization of odorant receptor gene expression in the olfactory epithelium.” Cell 73, 597–609. [DOI] [PubMed] [Google Scholar]
- Ressler, K J, Sullivan, S L, and Buck, L B (1994). “Information coding in the olfactory system: evidence for a stereotyped and highly organized epitope map in the olfactory bulb.” Cell 79, 1245–1255. [DOI] [PubMed] [Google Scholar]
- Rossi, F M, Pizzorusso, T, Porciatti, V, Marubio, L M, Maffei, L, and Changeux, J P (2001). “Requirement of the nicotinic acetylcholine receptor beta 2 subunit for the anatomical and functional development of the visual system.” Proc. Natl. Acad. Sci. U.S.A. 98, 6453–6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sejnowski, T J (1977). “Storing covariance with nonlinearly interacting neurons.” J. Math. Biol. 10.1007/BF00275079 4, 303–321. [DOI] [PubMed] [Google Scholar]
- Serizawa, S, Miyamichi, K, Takeuchi, H, Yamagishi, Y, Suzuki, M, and Sakano, H (2006). “A neuronal identity code for the odorant receptor-specific and activity-dependent axon sorting.” Cell 127, 1057–1069. [DOI] [PubMed] [Google Scholar]
- Sernagor, E, Eglen, S J, and O’Donovan, M J (2000). “Differential effects of acetylcholine and glutamate blockade on the spatiotemporal dynamics of retinal waves.” J. Neurosci. 20, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz, C J, and Stryker, M P (1988). “Prenatal tetrodotoxin infusion blocks segregation of retinogeniculate afferents.” Science 242, 87–89. [DOI] [PubMed] [Google Scholar]
- Sperry, R W (1963). “Chemoaffinity in the orderly growth of nerve fibre patterns and connections.” Proc. Natl. Acad. Sci. U.S.A. 50, 703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen, D, and Shatz, C J (2002). “An instructive role for retinal waves in the development of retinogeniculate connectivity.” Neuron 33, 357–367. [DOI] [PubMed] [Google Scholar]
- Sun, C, Warland, D K, Ballesteros, J M, van der List, D, and Chalupa, L M (2008). “Retinal waves in mice lacking the β2 subunit of the nicotinic acetylcholine receptor.” Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.0807178105 105, 13638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindale, N V (1996). “The development of topography in the visual cortex: a review of models.” Network 7, 161–247. [DOI] [PubMed] [Google Scholar]
- Torborg, C L, and Feller, M B (2005). “Spontaneous patterned retinal activity and the refinement of retinal projections.” Prog. Neurobiol. 76, 213–235. [DOI] [PubMed] [Google Scholar]
- Tsigankov, D N, and Koulakov, A A (2006). “A unifying model for activity-dependent and activity-independent mechanisms predicts complete structure of topographic maps in ephrin-A deficient mice.” J. Comput. Neurosci. 21, 101–114. [DOI] [PubMed] [Google Scholar]
- Vassar, R, Chao, S K, Sitcheran, R, Nunez, J M, Vosshall, L B, and Axel, R (1994). “Topographic organization of sensory projections to the olfactory bulb.” Cell 79, 981–991. [DOI] [PubMed] [Google Scholar]
- Vassar, R, Ngai, J, and Axel, R (1993). “Spatial segregation of odorant receptor expression in the mammalian olfactory epithelium.” Cell 74, 309–18. [DOI] [PubMed] [Google Scholar]
- Wang, F, Nemes, A, Mendelsohn, M, and Axel, R (1998). “Odorant receptors govern the formation of a precise topographic map.” Cell 93, 47–60. [DOI] [PubMed] [Google Scholar]
- Wang, H X, Gerkin, R C, Nauen, D W, and Bi, G Q (2005). “Coactivation and timing-dependent integration of synaptic potentiation and depression.” Nat. Neurosci. 10.1038/nn1387 8, 187–193. [DOI] [PubMed] [Google Scholar]
- Willshaw, D J (2006). “Analysis of mouse EphA knockins and knockouts suggests that retinal axons programme target cells to form ordered retinotopic maps.” Development 133, 2705–2717. [DOI] [PubMed] [Google Scholar]
- Willshaw, D J, and von der Malsburg, C (1976). “How patterned neural connections can be set up by self-organization.” Proc. R. Soc. London, Ser. B 194, 431–445. [DOI] [PubMed] [Google Scholar]
- Willshaw, D J, and von der Malsburg, C (1979). “A marker induction mechanism for the establishment of ordered neural mappings: its application to the retinotectal problem.” Philos. Trans. R. Soc. London, Ser. B 287, 203–243. [DOI] [PubMed] [Google Scholar]
- Wong, R OL, Meister, M, and Shatz, C J (1993). “Transient period of correlated bursting activity during development of the mammalian retina.” Neuron 10.1016/0896-6273(93)90122-8 11, 923–938. [DOI] [PubMed] [Google Scholar]
- Zhang, T I, Tao, H W, Holt, C E, Harris, W A, and Poo, M (1998). “A critical window for cooperation and competition among developing retinotectal synapses.” Nature 10.1038/25665 395, 37–44. [DOI] [PubMed] [Google Scholar]
- Zheng, C, Feinstein, P, Bozza, T, Rodriguez, I, and Mombaerts, P (2000). “Peripheral olfactory projections are differentially affected in mice deficient in a cyclic nucleotide-gated channel subunit.” Neuron 26, 81–91. [DOI] [PubMed] [Google Scholar]
- Zou, D J, Feinstein, P, Rivers, A L, Mathews, G A, Kim, A, Greer, C A, Mombaerts, P, and Firestein, S (2004). “Postnatal refinement of peripheral olfactory projections.” Science 304, 1976–1979. [DOI] [PubMed] [Google Scholar]