Abstract
An activating point mutation in codon 12 of the HRAS gene was the first somatic point mutation identified in a human cancer and established the role of somatic mutations as the common driver of oncogenesis. Since then, there have been over 11,000 mutations in the three RAS (HRAS, KRAS and NRAS) genes in codons 12, 13 and 61 reported in the literature. We report here the identification of recurrent somatic missense mutations at alanine 146, a highly conserved residue in the guanine nucleotide binding domain. In two independent series of colorectal cancers from Hong Kong and the United States we detected KRAS A146 mutations in 7/126 and 2/94 cases, respectively, giving a combined frequency of 4%. We also detected KRAS A146 mutations in 2/40 (5%) colorectal cell lines, including the NCI-60 colorectal cancer line HCC2998. Codon 146 mutations thus are likely to make an equal or greater contribution to colorectal cancer than codon 61 mutations (4.2% in our combined series, 1% in the literature). Lung adenocarcinomas and large cell carcinomas did not show codon 146 mutations. We did, however, identify a KRAS A146 mutation in the ML-2 acute myeloid leukemia cell line and an NRAS A146 mutation in the NALM-6 B-cell acute lymphoblastic leukemia line, suggesting that the contribution of codon 146 mutations is not entirely restricted to colorectal cancers or to KRAS.
Keywords: KRAS, colorectal, cancer, mutation, A146
INTRODUCTION
Somatic mutations in critical target genes are the basic driving force in the development of cancer. The first somatic point mutation described in human cancer was an activating codon 12 glycine to valine mutation in the HRAS gene present in the EJ/T24 bladder carcinoma cell line.1,2 The further identification and characterisation of somatic mutations from the full spectrum of human cancers has been a major focus of cancer research in the ensuing 25 years.
To date, over 11,000 point mutations in codons 12, 13 and 61 have been reported in the HRAS, NRAS and KRAS genes combined (www.sanger.ac.uk/genetics/CGP/cosmic). The three RAS genes encode 21 kilodalton proteins with intrinsic GTPase activity which cycle between a GDP-bound “off” form and a GTP-bound “on” form. These alternate forms are regulated by GAPs (GTPase activating proteins) and GEFs (guanine nucleotide exchange factors), respectively. RAS proteins are involved in coupling signal transduction from cell surface receptors to cytoplasmic targets and mediating a variety of cellular responses including proliferation, cytoskeletal reorganisation and survival pathways. Activating mutations stabilise or otherwise promote a preponderance of the GTP-bound “on” form, thus inappropriately affecting downstream activities.
The KRAS gene is mutated in over 30% of colorectal cancers. There have been approximately 3000 KRAS point mutations in colorectal cancer reported in the literature (www.sanger.ac.uk/genetics/CGP/cosmic/). The majority (~82%) of reported mutations are in codon 12. Mutations at codons 13 and 61 contribute to a lesser degree, accounting for ~17% and ~1% respectively. We report here the identification of recurrent mutations at codon 146 in KRAS in colorectal cancers, indicating that mutations at this codon are making a heretofore unappreciated contribution to human neoplasia.
MATERIALS AND METHODS
The collection of all patient materials and their use in the current study were approved by appropriate local Institutional/ethical Review Board. Genomic DNA was extracted from tumour and paired normal tissues as previously described.3 The Johns Hopkins University colorectal cancer sample series was carried as early passage cell lines or xenografts as described previously.4 PCR amplification and direct sequencing of KRAS using fluorescent dideoxy sequencing on ABI-3730 sequencers was done as previously described5 using the following primers flanking all four coding exons of KRAS (isoform b, accession GI:34485723 NM_004985): exon 2:F-GTGTGACATGTTCTAATATAGTCA, R-GAATGGTCCTGCACCAGTAA; exon 3:F-TCAAGTCCTTTGCCCATTTT, R-TGCATGGCATTAGCAAAGAC exon 4: F-GAAACCAAAGCCAAAAGCAG, R-AGTAGAAGAAGGAAGGAAAATTTGG; exon 5: F-TGGGAATACTGGCACTTAGAGG, R-TTGACAAAACACCTATGCGG.
Sequence data was analysed using semi-automated analysis using Mutation Surveyor and in-house software coupled with manual inspection of potentially mutant traces followed by a manual rescoring of all sequence traces.
STATISTICAL METHODS
Differences in the relative incidences of KRAS mutations in codons 12, 13, 61 and 146, between tissue types, were assessed by first computing a chi-squared statistic based on the contingency table of mutation counts by codon and type. The significance of this statistic was evaluated by Monte Carlo simulation, using 10,000 simulations of the data under the null hypothesis of no difference in the relative incidence of codons, conditional on the number of mutations in each codon group and the total number of mutations of each type. This provides an exact statistic, necessary for contingency tables containing low counts.
RESULTS
Coding exons of the KRAS gene were resequenced in a series of 126 primary colorectal cancer cases (CRC) from Hong Kong. Recurrent point mutations resulting in amino acid substitutions of alanine 146 were identified in seven cases (Table 1 and Fig. 1). Further screening in an additional series of 94 colorectal cancer samples from the United States yielded two further codon 146 mutations. All of these codon 146 mutations from cancers were demonstrated to be somatic by analysis of normal DNA from the same individuals. Two additional codon 146 mutations were detected in 40 colorectal cancer cell lines (Tables 1 and 2). However, normal DNAs from these individuals were not available to confirm their somatic origin. Combining colorectal cancer primary tumor, xenograft and cell line data, 11 mutations (Table 1) were identified: 8 c.436G>A p.A146T, 2 c.437C>T p.A146V and 1 c.436G>C p.A146P. A set of 94 sporadic colorectal adenomas were screened in addition and no instances of A146 mutation were identified. Full frequency and distribution data for KRAS mutations in the colorectal screens are shown in Table 3. In addition, a series of 63 adenomas from familial adenomatous polyposis patients and 34 hyperplastic colorectal polyps where both KRAS and BRAF have previously6 been shown to play a role were also screened but no A146 mutations were identified (data not shown). Further screening of all three RAS genes in series of ~700 cancer cell lines revealed two additional A146 mutations: a KRAS c.436G>A p.A146T in the ML-2 acute myeloid leukemia cell line and a c.436G>A p.A146T in NRAS in the NALM-6 B-cell acute lymphoblastic leukemia cell line (Table 1).
Table 1. Codon A146 mutations detected.
| Colorectal Cancers | ||
|---|---|---|
| Gene | Sample | Mutation |
| KRAS | PD2032a | c.436G>A p.A146T |
| KRAS | PD2048a | c.436G>A p.A146T |
| KRAS | PD2070a | c.436G>A p.A146T |
| KRAS | PD2019a | c.436G>A p.A146T |
| KRAS | PD1978a | c.436G>C p.A146P |
| KRAS | PD2068a | c.437C>T p.A146V |
| KRAS | PD1532a | c.437C>T p.A146V |
| KRAS | JHU-1 | c.436G>A p.A146T |
| KRAS | JHU-2 | c.436G>A p.A146T |
| Cancer Cell Lines | ||
|---|---|---|
| Gene | Sample/Cancer Type | Mutation |
| KRAS | HCC2998, CRC | c.436G>A p.A146T |
| KRAS | LS1034, CRC | c.436G>A p.A146T |
| KRAS | ML-2, AML | c.436G>A p.A146T |
| NRAS | NALM-6, B-ALL | c.436G>A p.A146T |
CRC, colorectal cancer; AML, acute myeloid leukemia; B-ALL- B-cell acute lymphoblastic leukemia.
Figure 1.
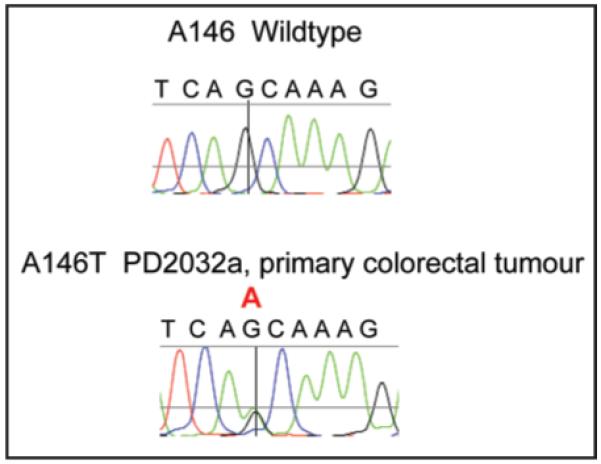
Representative sequencing chromatogram of c.436G>A p.A146T KRAS mutation from primary colorectal tumour PD2032a. The postion of the G>A substitution is indicated by the crosshairs and red A above the trace.
Table 2. KRAS mutations in CRC cell lines.
| CRC Cell Line | KRAS mutation | Zygosity |
|---|---|---|
| C2BBe1 | ND | - |
| Car-1 | ND | - |
| CoCM-1 | ND | - |
| COLO-205 | ND | - |
| COLO-320-HSR | ND | - |
| COLO-678 | c.35G>A p.G12D | Het |
| COLO-741 | ND | - |
| CW-2 | ND | - |
| DLD-1-JCRB | c.38G>A p.G13D | Het |
| ECC4 | c.A182A>G p.Q61R | Hom |
| Gp2D | c.35G>A p.G12D | Het |
| GP5d | c.35G>A p.G12D | Het |
| HCC2998 | c.436G>A p.A146T | Het |
| HCT-116 | c.38G>A, p.G13D | Het |
| HCT-15 | c.38G>A, p.G13D | Het |
| HT-29 | ND | - |
| HT55 | ND | - |
| KM12 | ND | - |
| LoVo | c.38G>A p.G13D | Het |
| LS1034 | c.436G>A p.A146T | Hom |
| LS-123 | c.34G>A p.G12S | Het |
| LS-174T | c.35G>A p.G12D | Het |
| LS-411N | ND | |
| LS-513 | c.35G>A p.G12D | Het |
| NCI-H508 | ND | - |
| NCI-H630 | ND | - |
| NCI-H716 | ND | - |
| NCI-H747 | c.38G>A p.G13D | Het |
| RCM-1 | c.35G>T p.G12V | Hom |
| RKO | ND | - |
| SK-CO1 | c.35G>T p.G12V | Hom |
| SNU-C1 | ND | - |
| SNU-C2B | c.35G>A p.G12D | Hom |
| SW1116 | c.G35G>C p.G12A | Het |
| SW1417 | ND | - |
| SW1463 | c.35G>A p.G12D | Hom |
| SW403 | c.35G>T p.G12V | Hom |
| SW48 | ND | - |
| SW480 | c.35G>T p.G12V | Hom |
| SW620 | c.35G>T p.G12V | Hom |
| SW837 | c.G34G>T p.G12C | Het |
| SW948 | c.A182A>T p.Q61L | Het |
| T84 | c.38G>A p.G13D | Het |
Het, heterozygous; Hom, homozygous; ND, none detected
Table 3. KRAS mutation distribution in study sets.
| HK_CGP CRC | JHU CRC | ||
|---|---|---|---|
| WT | 76 | WT | 29 |
| Codon 12 MUT | 24 | Codon 12 MUT | 46 |
| Codon 13 MUT | 18 | Codon 13 MUT | 15 |
| Codon 61 MUT | 1 | Codon 61 MUT | 2 |
| Codon 146 MUT | 7 | Codon 146 MUT | 2 |
| Total | 126 | Total | 94 |
| CRC Cell Lines | HK_CGP sporadic adenoma | ||
|---|---|---|---|
| WT | 19 | WT | 68 |
| Codon 12 MUT | 14 | Codon 12 MUT | 18 |
| Codon 13 MUT | 3 | Codon 13 MUT | 6 |
| Codon 61 MUT | 2 | Codon 61 MUT | 2 |
| Codon 146 MUT | 2 | Codon 146 MUT | 0 |
| Total | 40 | Total | 94 |
HK_CGP, Hong Kong, Cancer Genome Project; JHU, Johns Hopkins University; CRC, colorectal cancer.
The distribution of KRAS mutant alleles was different between CRC from Hong Kong and United States (p = 0.0306). This was primarily due to a difference in relative mutation prevalence between codons 12 and 13 which has been noted previously.7 However, a higher proportion of A146 mutations in Hong Kong compared to US CRC also contributed to the difference between the two series and larger genetic epidemiological studies are warranted to determine if this A146 trend is reproducible. Overall, KRAS A146 mutations were detected in 4% (11/260) of CRC and accounted for 8% (11/135) of all observed KRAS mutations. These data suggest that A146 mutations make a larger contribution to colorectal cancer than Q61 mutations, which accounted for 2% (5/260) of cases in this study and 1% in the literature (www.sanger.ac.uk/genetics/CGP/cosmic/). Screening for mutations in BRAF revealed no cases with both A146 and BRAF mutations (data not shown). One primary tumor (PD1532) had both a heterozygous p.A146V mutation as well as a heterozygous p.G12V mutation in KRAS. Whether this reflects intratumoral heterogeneity or truly coincident, presumably biallelic, KRAS activation in the same clone is not known. As this case is a primary tumour, single-cell cloning experiments are not possible to differentiate the two possibilities. Further work to determine if biallelic RAS mutations have greater transforming potential would be of interest.
The contribution of A146 mutations to another cancer type that is driven in large part by KRAS mutations was investigated by analysing a series of non-small-cell lung cancers (NSCLC) comprised of adenocarcinomas, bronchioalveolar and large cell undifferentiated carcinomas in which KRAS mutations are known to be prevalent. In total, 99 primary tumor and 66 cell lines were sequenced for KRAS. While KRAS codon 12, 13 and 61 mutations were detected at the expected combined frequency of 33% (55/165), no instances of A146 mutations were detected (p = 0.0305 compared to CRC).
DISCUSSION
We have identified A146 missense substitutions as a new class of recurrent somatic mutation in the KRAS gene in colorectal cancers. Data from independent series indicate that A146 mutations are involved in approximately 4% of colorectal cancers. Extrapolating from a worldwide CRC incidence of approximately 950,000 cases/year,8 these data suggest there are approximately 30,000 cases/year of CRC that are KRAS A146 mutant. Additional A146 (both KRAS and NRAS) mutations were detected in leukaemia cell lines, suggesting a role for A146 alleles in other tumour types. However, we did not detect A146 mutations in a series of NSCLC where KRAS mutations are also prevlaent. It is possible that the sequence context of codon 146 (tca GCA146 aag) renders the guanine at codon 146 less susceptible to adduction by tobacco smoke carcinogens than guanines at codons 12 and 13 (gct GGT12 GGC13 gta). Alternatively, it may be that substitution of serine for alanine at codon 146, which would require a G>T transversion typical of cigarette smoke polycyclic aromatic hydrocarbon mutagenesis,9 may not confer transforming activity on KRAS.
To our knowledge there has been no report of recurrent A146 mutations to date and only two reports of single A146 mutations in human cancer in the literature from over 15 years ago.10-12 It is remarkable that, despite ~70,000 cancer samples analysed for mutations in RAS genes over the last 25 years (www.sanger.ac.uk/genetics/CGP/cosmic/), the role of codon 146 mutations essentially has been overlooked. This reflects a persistent bias in mutation screening which has been almost exclusively directed at the first two coding exons of the RAS genes (encoding codons 12,13 and 61). Mutation screening should be extended to include A146 for all three RAS genes in future.
RAS mutations at alanine 146 have been identified and characterised in experimental systems twice in the literature. A transforming Kras p.A146T allele was detected in a thymic lymphoma induced by exposure of mice to an acute whole-body dose of neutron radiation.13 NIH 3T3 focus-forming assays with tumour DNA from the lymphoma-derived nude mouse tumours demonstrated transforming capability of the A146T allele, albeit at lower efficiency than Kras codon 12 and 13 alleles. In an in vitro mutagenesis screen for mutations that increased guanine nucleotide exchange rates, GTPase activity and transforming potential in HRAS, a p.A146V mutation was found to have partial transforming activity which was attributable to >1000-fold increase in the GDP → GTP exchange rate without affecting the GTPase activity.14 Alanine 146 is within the highly conserved G-5 (aa144-146) region of the protein (Fig. 2) and forms a hydrogen bond with the guanine ring of GTP.15 Mutations of this residue presumably alter the local structure such that the GTP-bound state is much favoured over GDP-bound form. Whether this is accomplished by increased GEF binding or through some other mechanism is unknown. It is likely however that increased exchange is accounting, at least in part, for the oncogenic properties of A146 mutant alleles.
Figure 2.

Conservation of alanine 146 in RAS genes. Accession number of sequence and species of origin/gene are given the left and right of the alignment, respectively. The position of A146 is underlined.
Various studies in colorectal cancer have suggested that KRAS codon 12 and 13 mutations are generally predicitve of a poorer prognosis, with evidence being presented for mutation-specific prognostic as well as histopathology correlates.16-19 The influence of A146 alleles on clinical outcome and tumour characteristics therefore requires investigation. As well, the extent to which colorectal cancers harbouring this class of KRAS mutation are more or less responsive to current therapies needs to be investigated. It has recently been shown that KRAS mutations are predictive of resistance to treatment with cetuximab in colorectal cancer.20 This study analysed only mutations in codons 12 and 13 and it is therefore plausible that a further subset of patients are resistant due to A146 mutations. It will be also be interesting to assess response of the A146 mutant cancer cell lines detailed here (one of which, HCC2998 is a component of the NCI-60 series) to various anticancer agents and compare these responses to cancer cells that have other RAS and BRAF mutations, as exemplified by the recently described work on MEK inhibitors.21
In summary, we have demonstrated that KRAS A146 mutations are recurrent in human colorectal cancers, are more prevalent than Q61 mutations in this cancer type and are thus making a substantial contribution to colorectal cancer in the population. These findings will further empower the molecular pathology and clinical investigation of this common tumor type.
Acknowledgements
The authors wish to thank the sequencing, analysis and informatics groups of the Cancer Genome Project for their efforts that contributed to this study. The authors would like to acknowledge the financial support of the Wellcome Trust (MRS, PAF), the Research Grant Council of the Hong Kong Special Administrative Region HKU7622/05M (T.L.C., S.Y.L. and S.T.Y.) and the Pew Charitable Trusts and NIH grants CA121113 and CA062924 (V.E.V.).
References
- 1.Tabin C, Bradley S, Bargmann C, Weinberg R, Papageorge A, Scolnick E, Dhar R, Lowy D, Chang E. Mechanism of activation of a human oncogene. Nature. 1982;300:143–9. doi: 10.1038/300143a0. [DOI] [PubMed] [Google Scholar]
- 2.Reddy E, Reynolds R, Santos E, Barbacid M. A point mutation is responsible for the acquisition of transforming properties by the T24 human bladder carcinoma oncogene. Nature. 1982;300:149–52. doi: 10.1038/300149a0. [DOI] [PubMed] [Google Scholar]
- 3.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JWC, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 4.Bardelli A, Parsons DW, Silliman N, Ptak J, Szabo S, Saha S, Markowitz S, Willson JKV, Parmigiani G, Kinzler KW, Vogelstein B, Velculescu VE. Mutational analysis of the tyrosine kinome in colorectal cancers. Science. 2003;300:949. doi: 10.1126/science.1082596. [DOI] [PubMed] [Google Scholar]
- 5.Davies H, Hunter C, Smith R, Stephens P, Greenman C, Bignell G, Teague J, Butler A, Edkins S, Stevens C, Parker A, O’Meara S, Avis T, Barthorpe S, Brackenbury L, Buck G, Clements J, Cole J, Dicks E, Edwards K, Forbes S, Gorton M, Gray K, Halliday K, Harrison R, Hills K, Hinton J, Jones D, Kosmidou V, Laman R, Lugg R, Menzies A, Perry J, Petty R, Raine K, Shepherd R, Small A, Solomon H, Stephens Y, Tofts C, Varian J, Webb A, West S, Widaa S, Yates A, Brasseur F, Cooper CS, Flanagan AM, Green A, Knowles M, Leung SY, Looijenga LHJ, Malkowicz B, Pierotti MA, Teh BT, Yuen ST, Lakhani SR, Easton DF, Weber BL, Goldstraw P, Nicholson AG, Wooster R, Stratton MR, Futreal PA. Somatic mutations of the protein kinase gene family in human lung cancer. Cancer Res. 2005;65:7591–5. doi: 10.1158/0008-5472.CAN-05-1855. [DOI] [PubMed] [Google Scholar]
- 6.Yuen ST, Davies H, Chan TL, Ho JW, Bignell GR, Cox C, Stephens P, Edkins S, Tsui WW, Chan AS, Futreal PA, Stratton MR, Wooster R, Leung SY. Similarity of the phenotypic patterns associated with BRAF and KRAS mutations in colorectal neoplasia. Cancer Res. 2002;62:6451–5. [PubMed] [Google Scholar]
- 7.Naoko H, Sachiko S, Isao I, Shoji N, Michio O, Yusuke N. Ethnic difference in the pattern of K-ras oncogene mutations in human colorectal cancers. Human Mutation. 1996;8:258–61. doi: 10.1002/(SICI)1098-1004(1996)8:3<258::AID-HUMU9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 8.Parkin DM, Bray F, Ferlay J, Pisani P. Global Cancer Statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 9.DeMarini DM. Genotoxicity of tobacco smoke and tobacco smoke condensate: A review. Mutation Research/Reviews in Mutation Research. 2004;567:447–74. doi: 10.1016/j.mrrev.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Kakunaga T. Neoplastic transformation of human diploid fibroblast cells by chemical carcinogens. PNAS. 1978;75:1334–8. doi: 10.1073/pnas.75.3.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCormick J, Yang D, Maher V, Farber R, Neuman W, Peterson W, Pollack M. The HuT series of ‘carcinogen-transformed’ human fibroblast cell lines are derived from the human fibrosarcoma cell line 8387. Carcinogenesis. 1988;9:2073–9. doi: 10.1093/carcin/9.11.2073. [DOI] [PubMed] [Google Scholar]
- 12.Orita S, Higashi T, Kawasaki Y, Harada A, Igarashi H, Monden T, Morimoto H, Shimano T, Mori T, Miyoshi J. A novel point mutation at codon 146 of the K-ras gene in a human colorectal cancer identified by the polymerase chain reaction. Virus Genes. 1991;5:75–9. doi: 10.1007/BF00571733. [DOI] [PubMed] [Google Scholar]
- 13.Sloan SR, Newcomb EW, Pellicer A. Neutron radiation can activate K-ras via a point mutation in codon 146 and induces a different spectrum of ras mutations than does gamma radiation. Mol Cell Biol. 1990;10:405–8. doi: 10.1128/mcb.10.1.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feig LA, Cooper GM. Relationship among guanine nucleotide exchange, GTP hydrolysis, and transforming potential of mutated ras proteins. Mol Cell Biol. 1988;8:2472–8. doi: 10.1128/mcb.8.6.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vetter IR, Wittinghofer A. The guanine nucleotide-binding switch in three dimensions. Science. 2001;294:1299–304. doi: 10.1126/science.1062023. [DOI] [PubMed] [Google Scholar]
- 16.Andreyev HJ, Norman AR, Cunningham D, Oates J, Dix BR, Iacopetta BJ, Young J, Walsh T, Ward R, Hawkins N, Beranek M, Jandik P, Benamouzig R, Jullian E, Laurent-Puig P, Olschwang S, Muller O, Hoffmann I, Rabes HM, Zietz C, Troungos C, Valavanis C, Yuen ST, Ho JW, Croke CT, O’Donoghue DP, Giaretti W, Rapallo A, Russo A, Bazan V, Tanaka M, Omura K, Azuma T, Ohkusa T, Fujimori T, Ono Y, Pauly M, Faber C, Glaesener R, de Goeij AF, Arends JW, Andersen SN, Lovig T, Breivik J, Gaudernack G, Clausen OP, De Angelis PD, Meling GI, Rognum TO, Smith R, Goh HS, Font A, Rosell R, Sun XF, Zhang H, Benhattar J, Losi L, Lee JQ, Wang ST, Clarke PA, Bell S, Quirke P, Bubb VJ, Piris J, Cruickshank NR, Morton D, Fox JC, Al-Mulla F, Lees N, Hall CN, Snary D, Wilkinson K, Dillon D, Costa J, Pricolo VE, Finkelstein SD, Thebo JS, Senagore AJ, Halter SA, Wadler S, Malik S, Krtolica K, Urosevic N. Kirsten ras mutations in patients with colorectal cancer: The ‘RASCAL II’ study. British Journal of Cancer. 2001;85:692–6. doi: 10.1054/bjoc.2001.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bazan V, Migliavacca M, Zanna I, Tubiolo C, Grassi N, Latteri MA, La Farina M, Albanese I, Dardanoni G, Salerno S, Tomasino RM, Labianca R, Gebbia N, Russo A. Specific codon 13 K-ras mutations are predictive of clinical outcome in colorectal cancer patients, whereas codon 12 K-ras mutations are associated with mucinous histotype. Ann Oncol. 2002;13:1438–46. doi: 10.1093/annonc/mdf226. [DOI] [PubMed] [Google Scholar]
- 18.Conlin A, Smith G, Carey FA, Wolf CR, Steele RJC. The prognostic significance of K-ras, p53, and APC mutations in colorectal carcinoma 0.1136/gut.2005.066514. Gut. 2005;54:1283–6. doi: 10.1136/gut.2005.066514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esteller M, Gonzalez S, Risques RA, Marcuello E, Mangues R, Germa JR, Herman JG, Capella G, Peinado MA. K-ras and p16 aberrations confer poor prognosis in human colorectal cancer. J Clin Oncol. 2001;19:299–304. doi: 10.1200/JCO.2001.19.2.299. [DOI] [PubMed] [Google Scholar]
- 20.Lievre A, Bachet JB, Le Corre D, Boige V, Landi B, Emile JF, Cote JF, Tomasic G, Penna C, Ducreux M, Rougier P, Penault-Llorca F, Laurent-Puig P. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66:3992–5. doi: 10.1158/0008-5472.CAN-06-0191. [DOI] [PubMed] [Google Scholar]
- 21.Solit DB, Garraway LA, Pratilas CA, Sawai A, Getz G, Basso A, Ye Q, Lobo JM, She Y, Osman I, Golub TR, Sebolt-Leopold J, Sellers WR, Rosen N. BRAF mutation predicts sensitivity to MEK inhibition. Nature. 2006;439:358–62. doi: 10.1038/nature04304. [DOI] [PMC free article] [PubMed] [Google Scholar]


