Abstract
We describe three families with X-linked mental retardation, two with a deletion of a single amino acid and one with a missense mutation in the proximal domain of the RSK2(RPS6KA3) (ribosomal protein S6 kinase, 90 kDa, polypeptide 3) protein similar to mutations found in Coffin–Lowry syndrome (CLS). In two families, the clinical diagnosis had been nonsyndromic X-linked mental retardation. In the third family, although CLS had been suspected, the clinical features were atypical and the degree of intellectual disability much less than expected. These families show that strict reliance on classical clinical criteria for mutation testing may result in a missed diagnosis. A less targeted screening approach to mutation testing is advocated.
Keywords: Coffin–Lowry syndrome, nonsyndromic mental retardation, RSK2 mutation
There are several consequences of discovering the molecular basis of a dysmorphic syndrome. In many instances, a redefinition of the diagnostic criteria occurs to include new features, with the removal of features previously believed to be necessary. One example of this is Borjeson–Forssman–Lehmann syndrome in which original descriptions suggested that all affected males have microcephaly. In a recent study of 25 affected males in nine families, with mutations demonstrated in PHF6, the majority had normal head circumferences, only one had microcephaly and three macrocephaly (1). Another consequence of mutation analysis is that families with the same mutations may show widely discrepant clinical features. This has been seen recently with the 24-base pair duplication in ARX. The first family found with the mutation had X-linked infantile spasms (West syndrome) with severe clinical manifestations. In the second family, affected males had a lesser degree of mental retardation and fewer seizures, but all had dystonic posturing of the hands (Partington syndrome) (2). Further families have since been described with a mixture of both clinical phenotypes (3, 4). In a particular family with four affected males in two generations, one had West syndrome, another the Partington syndrome (with autism) and two others had nonsyndromic X-linked mental retardation (NSXLMR) (3).
Coffin–Lowry syndrome (CLS) is well reported in the literature. Cardinal features include severe mental retardation, a characteristic facial appearance, digital tapering, short stature and progressive spinal deformity (5). The disorder is now recognized to be due to mutations in a serine threonine kinase RSK2(RPS6KA3) located at Xp22.2 (6).
We report two families with a 3-base pair deletion in the RSK2(RPS6KA3) gene and one with a missense mutation. In two families, the diagnosis had been NSXLMR and in the other, CLS had been suspected but the presentation was atypical and much milder than expected.
Methods
The families were seen during a number of outpatient and home visits. Blood samples for DNA were collected with appropriate consent from all available male members and their mothers, wherever possible. Samples from a proband in each family were sent for analysis to the GOLD (Genetics of Learning Disability) project based at the University of Cambridge and Sanger Institute, UK. UK Multicentre Research Ethics approval was obtained for this project.
Case histories
Family 1
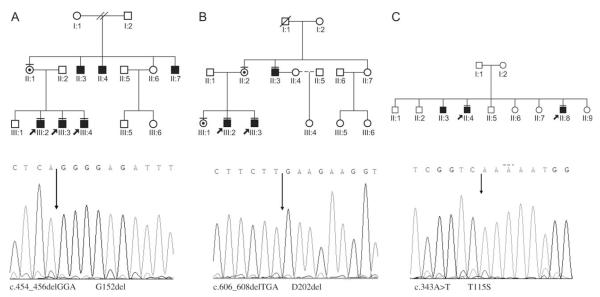
This family was referred because of developmental delay in two brothers. A maternal uncle was reported to have had mild learning difficulties and the children’s mother had had more educational difficulties than her sister, who had had a tertiary-level education. The pedigree is shown in Fig. 1.
Fig. 1.
Solid symbol indicates intellectually delayed male; carrier female indicated by bull’s-eye. Solid bar above symbol indicates subject examined.
The older brother (III:2) was born at term, after a normal pregnancy and delivery with a birth weight of 2.5 kg (<1st centile). He had some early motor and speech delay associated with hypotonia. A strabismus was corrected surgically. Developmental assessment in early childhood placed him in the borderline to mildly delayed range. At age 7, he was noted to have facial hypotonia, a tent-shaped mouth and a high-arched palate. Neuromuscular disorders including myotonic dystrophy were considered, however, DNA testing for this proved negative. At 13 years of age, he had reduced muscle tone and bulk with some winging of the scapulae but no scoliosis. His hands and feet were long, and there was dental crowding. A diagnosis of NSXLMR was made.
He was reassessed at 17 years of age after research testing had identified a small deletion in the RSK2(RPS6KA3) gene. He had a mild to moderate intellectual disability. His height was 168 cm (10th centile), his weight 46 kg (3rd centile) and head circumference 56.5 cm (50–90th centile). He had normal pubertal development. There was a significant kyphoscoliosis. He had clamminess and some redundancy of his palmar skin, and horizontal creases were noted on his hypothenar eminences. There had been mild coarsening of his facial features with increasing age (Fig. 2).
Fig. 2.
Family 1. (a) III:2 (aged 7 years). Note long face with hypotonic appearance. (b) Left to right: III:3, III:1 and III:2 (aged 16, 19 and 18 years, respectively). Note fullness and eversion of lower lip, prominent forehead and mild coarsening of facial features in males. (c) II:3 (age 40 years).
The younger brother (III:3) was born at term after a normal pregnancy and delivery. His birth weight was 3.4 kg (50th centile). Developmental delay and hypotonia were similar to his brother. His most recent review after identification of a mutation in the RSK2(RPS6KA3) gene was at age 16 years. His height was 171 cm (25–50th centile), weight 58 kg (25th centile) and head circumference was 58 cm (98th centile). There was a mild scoliosis. He had a prominent forehead, broad nasal tip, with thickened columella and full lower lip. He had a small chin with dental malocclusion. His digits were not tapered, but he had some redundancy of his palmar skin and creases on his hypothenar eminences. He wore glasses for myopia.
The boy’s sister (III:1) had a prominent forehead, broad nasal tip and myopia. Her hands were long, with the same soft texture of her palmar skin. She had a mild learning disability and required educational support.
Their uncle (II:3) was first assessed at age 40 after the confirmation of a molecular diagnosis in his nephews. He had a mild scoliosis detected at age 4, which was treated conservatively and did not progress. He was married, lived independently with his wife and drove a motor car. He was able to read and write and worked in a packaging department of a disability workshop. His weight was 101 kg (>97th centile), height 167 cm (3–10th centile) and head circumference 59 cm (>98th centile). He had a prominent forehead and broad nasal tip, without facial coarsening (Fig. 2). He had normally spaced dentition and a cross bite. He had small horizontal creases on his hypothenar eminence. He has a mild scoliosis. Recent spinal X rays because of chronic back pain showed no vertebral end-plate change often seen in males with CLS.
The facial features of the children and their uncle are shown in Fig. 2, including photographs of (III:2) taken during his initial assessment. Figure 3 illustrates the lack of digital tapering in the three siblings.
Fig. 3.

Family 1. Note the absence of digital tapering in III:1, III:2 and III:3.
Mutation analysis of the RSK2(RPS6KA3) gene revealed an in-frame 3-base pair deletion c.454_456delGGA (reference sequence NM_004586). This results in a deletion of a glycine residue at codon 152. This was identified in all the affected males and the boy’s mother and sister.
Family 2
The pedigree of family 2 is shown in Fig. 1. Affected males in generation III were examined on two occasions. There was a clinical suspicion of CLS, based on the facial features and scoliosis in one of the males examined, however, their degree of intellectual impairment and the absence of significant scoliosis or digital changes in many of the affected males were very atypical for this diagnosis. The second assessment occurred after the identification of a mutation in the RSK2 (RPS6KA3) gene.
III:2 was delivered at 38 weeks. His birth weight was 2.64 kg (10th centile), with a head circumference and length close to the 50th centile. There was a congenital cataract in his left eye, which did not require surgical treatment. He had failure to thrive over his first year of life. He was treated with growth hormone at age 4 and showed apparent response; treatment was continued until he was 14 years of age. His early milestones were all significantly delayed. He walked independently at 3 years of age and said his first word at 2 years of age. He was educated in a support class. He was able to look after his own basic needs with supervision and was moderately intellectually delayed.
At age 17, his height was 167 cm (3–10th centile), weight 65 kg (25–50th centile) and head circumference 56 cm (50th centile). He had a prominent forehead and a broad nasal tip with thickening of his alae nares. His lips were full and everted, and his palate narrow and high anteriorly with dental malocclusion. He had reduced muscle bulk and decreased muscle tone, with normal strength. His spine was straight, but he had some winging of his scapulae. He had some ulnar deviation of the tip of his middle finger on the right hand and some mild tapering of this digit.
Individual III:3 was born at term with a birth weight of 3.32 kg (10–50th centile). He grew normally in childhood. He had a moderate intellectual disability. He had a rapidly evolving scoliosis, which was treated in a brace at age 4 and then surgically at age 13. At age 16, his height was 162 cm (3rd centile), his weight 69 kg (50th centile) and head circumference 57.5 cm (98th centile). His oral and facial features were similar to that of his brother. He had a prominent midline lingual furrow. His digits were normally shaped.
Individual III:4 is a 10-year-old boy, who was delivered at term, with a birth weight of 3.49 kg (50th centile). Turricephaly was present at birth. He had a craniectomy in infancy and had secondary complications with raised intracranial pressure requiring ventriculoperitoneal shunting. He was educated in a support class. At age 10, his height was 134 cm (25th centile), his weight 40 kg (90th centile) and head circumference 54 cm (50–90th centile). He had a prominent forehead, telecanthus, down-sloping palpebral fissures and an everted lower lip. His dentition and palate were normal. His palmar skin was doughy, and he had some mild tapering of his middle digits. He had winging of his scapulae and a prominent lumbar lordosis. A recent photograph of the brothers is shown in Fig. 4. Access to the maternal uncles was denied, but a family photograph of all three indicated that they had facial features similar to those of their affected nephews.
Fig. 4.

Family 2. Left to right: III:2, III:4 and III:3 (aged 17, 10 and 16 years, respectively). Note prominent forehead, full lower lip and broad nasal tip and thickening alae nares in older males.
Mutation testing of the RSK2(RPS6KA3) gene identified a 3-base pair deletion c.606_608delTGA in the three boys in generation II and their mother. The mutation results in the deletion of aspartic acid at codon 202, within the proximal kinase domain of the protein.
Family 3
This family was referred to clinical genetics services in the North of England. The diagnosis of NSXLMR was made on the basis of mild developmental delay without distinguishing features in three affected male siblings. The pedigree is shown in Fig. 1. A male cousin through a maternal aunt was reported to have learning difficulties, though the degree of his difficulty was unclear and no clinical contact was made with this individual. The features in the males examined included heights on the 3rd centile, hypertelorism and a slightly full lower lip. While a diagnosis of CLS was considered given the X-linked history and examination findings, the clinical features in the boys were considered far too subtle for this diagnosis. The family failed to attend subsequent consultations and was lost to follow-up. Clinical photographs were reviewed after the identification of a missense mutation (c.343A>T T115S) in two of the affected boys and the features were not believed to be typical of CLS. No further details are available.
No further pathological mutations were detected in RSK2(RPS6KA3) in any of the remaining 300 families screened except in one family where the clinical diagnosis of CLS was suspected and a classical splice site mutation was identified. Furthermore, in these three families, no other pathological mutations were identified in the remaining ~50 X-linked genes associated with X-linked mental retardation (XLMR), which were screened suggesting that these mutations are indeed the cause of disease in these three families.
The three mutations we report are likely to be pathological as they cosegregate with disease in the families and are not present in a control population. Furthermore, all three residues are located within a highly conserved sequence block within the serine/threonine protein kinase domain of the protein, suggesting they are critical for normal protein function, and all three mutant residues are normally highly conserved across species including Gallus, Xenopus, Mus, Canis, Rattus, Danio and Tetraodon (Fig. 5).
Fig. 5.
Amino acid conservation across species.
Discussion
This report shows that some patients with an RSK2(RPS6KA3) mutation present with a very mild CLS phenotype. In the three families described, the clinical features in the affected males were atypical. In the first and third family, the diagnosis was not considered on clinical grounds. In family 1, mild facial coarsening and fullness and protuberance of the lower lip became apparent in the brothers with increasing age. The boy’s uncle, who has the same mutation, is not dysmorphic. Their early presentation was with hypotonia and nonspecific physical features. If samples from these families had not been sent for mutation screening, these diagnoses may never have been identified.
Two other families with RSK2(RPS6KA3) mutations and a mild or nonspecific phenotype have been described (7, 8). Manouvrier-Hanu et al. (7) described a pair of brothers. The older, at age 10 years, had hypertelorism, anteverted nares with thickened columella, full lower lip, irregular teeth and some digital tapering. His height was close to the 3rd centile; he was only mildly intellectually delayed. His brother had similar growth, facial and digital features. A pathogenic RSK2(RPS6KA3) missense mutation was identified in both boys, affecting the proximal kinase domain of the protein. The report by Merienne et al. (8) is of a family initially reported to have NSXLMR-MRX19 (9). The linkage interval in this family prompted the analysis of the RSK2 (RPS6KA3) gene, and a missense mutation was identified affecting a protein phosphorylation site (8). Two adult males in this family were reviewed by a geneticist and no facial, digital, or skeletal features characteristic of CLS were found (8). They only had a mild intellectual disability.
The clinical features in our families are compared with those in the literature in Table 1. The main differences in our families were a lesser degree of intellectual handicap, absent or minimal coarsening of the facial appearance, a height within the normal range, and a lower frequency of digital changes and scoliosis. Some atypical features were also observed including cataracts, which have previously been reported though not at such a young age (10), premature coronal suture fusion and early growth failure, which responded to growth hormone therapy.
Table 1.
| Features | Males in family 1 |
Males in family 2 |
Manouvrier- Hanu et al. |
Summary of literature from Hanauer and Young |
|---|---|---|---|---|
| Coarsening/prominent forehead | 2/3 | 2/3 | 2/2 | 37/37 |
| Hypertelorism | 0/3 | 0/3 | 1/2 | 42/42 |
| Height >3rd centile | 3/3 | 2/3 | 1/2 | 2/39 |
| Scoliosis | 3/3 | 1/3 | 0/2 | 30/38 |
| Winging scapulae | 2/3 | 2/3 | N/A | N/A |
| Delay, severe | 0/3 | 0/3 | 2/2 | 42/42 |
| Delay, mild to moderate | 2/3 | 3/3 | 2/2 | 0/41 |
| Full everted lower lips | 2/3 | 3/3 | 2/2 | 40/42 |
| Hypodontia | 0/3 | 0/3 | 0/2 | 19/22 |
| Irregular dentition | 2/3 | 2/3 | 2/2 | N/A |
| Digital tapering | 0/3 | 2/3a | 2/2 | 42/42 |
| Hypothena creases | 3/3 | 0/3 | 0/2 | 20/22 |
N/A, information not specifically commented on.
Minimal digital tapering.
The mutations identified in our families were small in frame deletions and a missense mutation involving the proximal kinase domain. A range of mutations has previously been described in CLS patients including both missense and truncating mutations. The mutations are spread along the length of the gene, although most are located in one of the two kinase domains (11). Truncating mutations have been found in about 80% of families with an identified mutation. In general, these have been associated with more severe physical and intellectual impairment. The families reported by Manouvrier-Hanu et al. (7) and Merienne et al. (8) both had missense mutations. Another case reported briefly in the literature details a male who was mildly delayed and worked independently as a janitor. He also had a proximally placed missense mutation (11). The mutation in this man and the brothers reported by Manouvrier-Hanu et al. were located in the proximal kinase domain of the protein, which corresponds to the location of the mutation in our cases. Interestingly, the deletion of aspartic acid at codon 202 seen in family 2 has recently been identified as a de novo deletion in a male referred for CLS mutation testing (12). It is unclear from the report whether this patient had mild or classical CLS features.
Functional assays in the patients reported by Manouvrier-Hanu et al. (7) and Merienne et al. were able to demonstrate significant residual enzymatic activity (10–20% residual activity), which seems to be related to the relatively mild phenotype (8, 11). The level of residual enzymatic activity is also linked to cognitive performance, with higher levels being associated with a higher level of intellectual function (13). Typically, those individuals without skeletal features have a much milder degree of intellectual disability (14). There appears to be a direct correlation between the amount of residual enzymatic activity and the presence of skeletal features. The RSK2(RPS6KA3) gene encodes an intracellular signalling kinase protein. Targets for the kinase include the cAMP-responsive binding protein (14) and the ATF4 transcription factor (15). The skeletal features of the syndrome are believed to be a result of impaired osteoblast differentiation due to loss of RSK2(RPS6KA3)-mediated ATF4 phosphorylation (15). It seems that the presence of a small amount of residual enzymatic activity may be sufficient to maintain normal osteoblast differentiation and ameliorate the skeletal phenotype associated with CLS. The mild skeletal features and mild to moderate intellectual disability in our cases seem to be in accordance with these observations.
Screening for mutations in RSK2(RPS6KA3) in 300 families with undiagnosed suspected XLMR identified only three pathogenic mutations. This suggests that RSK2(RPS6KA3) mutations not producing the classical phenotype are a rare, but not insignificant, cause of NSXLMR, given that five families have now been documented. It should be noted that the rate of de novo mutations in males with CLS appears high (67% in the largest series) (12), implying that similar mutations may account for some cases of isolated intellectual disability. We recommend that clinicians consider this diagnosis in males with coarsening of their facial feature, a broad thickened nasal tip and columella, eversion of the lower lip and dental malocclusion, without more characteristic skeletal or digital findings. The presence of hypothenar creases and a redundancy of the palmar skin are also useful diagnostic features.
These cases illustrate that strict reliance on characteristic dysmorphic features may result in a missed diagnosis. Molecular diagnostic technology is constantly redefining the range of disability and physical features associated with mutations in specific genes. A family history suggestive of X linkage with or without other phenotypic features warrants a less targeted screening approach, searching for mutations in a panel of genes, which may soon be feasible with current developments in technology. The array of genes causing XLMR and the small number of families identified with mutations in each of these genes still make this a daunting task (16).
Acknowledgements
This work was supported by grants from the Wellcome Trust, UK, and the NSW Department of Health. The group wish to thank Prof M Partington for editorial assistance.
References
- 1.Turner G, Lower KM, White SM, et al. The clinical picture of the Borjeson-Forssman Lehmann syndrome in males and heterozygous females with PHF6 mutations. Clin Genet. 2004;65:226–232. doi: 10.1111/j.0009-9163.2004.00215.x. [DOI] [PubMed] [Google Scholar]
- 2.Stromme P, Mangelsdorf ME, Shaw MA, et al. Mutations in the human ortholog of Aristaless cause X-linked mental retardation and epilepsy. Nat Genet. 2002;30:441–445. doi: 10.1038/ng862. [DOI] [PubMed] [Google Scholar]
- 3.Turner G, Partington M, Kerr B, et al. Variable expression of mental retardation, autism, seizures, and dystonic hand movements in two families with an identical ARX gene mutation. Am J Med Genet. 2002;112:405–411. doi: 10.1002/ajmg.10714. [DOI] [PubMed] [Google Scholar]
- 4.Partington MW, Turner G, Boyle J, et al. Three new families with X-linked mental retardation caused by the 428-451dup(24bp) mutation in ARX. Clin Genet. 2004;66:39–45. doi: 10.1111/j.0009-9163.2004.00268.x. [DOI] [PubMed] [Google Scholar]
- 5.Hanauer A, Young ID. Coffin-Lowry syndrome: clinical and molecular features. J Med Genet. 2002;39:705–713. doi: 10.1136/jmg.39.10.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trivier E, De Cesare D, Jacquot S, et al. Mutations in the kinase Rsk-2 associated with Coffin-Lowry syndrome. Nature. 1996;384:567–570. doi: 10.1038/384567a0. [DOI] [PubMed] [Google Scholar]
- 7.Manouvrier-Hanu S, Amiel J, Jacquot S, et al. Unreported RSK2 missense mutation in two male sibs with an unusually mild form of Coffin-Lowry syndrome. J Med Genet. 1999;26:775–778. doi: 10.1136/jmg.36.10.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merienne K, Jacquot S, Pannetier S, et al. A missense mutation in RPS6KA3 (RSK2) responsible for non-specific mental retardation. Nat Genet. 1999;22:13–14. doi: 10.1038/8719. [DOI] [PubMed] [Google Scholar]
- 9.Donnelly AJ, Choo KH, Kozman HM, et al. Regional localisation of a non-specific X-linked mental retardation gene (MRX19) to Xp22. Am J Med Genet. 1994;51:581–585. doi: 10.1002/ajmg.1320510457. [DOI] [PubMed] [Google Scholar]
- 10.Hunter AGW, Partington MW, Evans JA. The Coffin-Lowry syndrome. Experience from four centres. Clin Genet. 1982;21:321–335. doi: 10.1111/j.1399-0004.1982.tb01379.x. [DOI] [PubMed] [Google Scholar]
- 11.Delauney JP, Abidi F, Zeniou M, et al. Mutations in the X-linked RSK2 gene (RPS6KA3) in patients with Coffin-Lowry syndrome. Hum Mutat. 2001;17:103–116. doi: 10.1002/1098-1004(200102)17:2<103::AID-HUMU2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 12.Delauney JP, Dubos A, Marques Pereira P, et al. Identification of novel mutations in the RSK2 gene (RPSKA3) in patients with Coffin-Lowry syndrome. Clin Genet. 2006;70:161–166. doi: 10.1111/j.1399-0004.2006.00660.x. [DOI] [PubMed] [Google Scholar]
- 13.Harum KH, Alemi L, Johnston MV. Cognitive impairment in Coffin–Lowry syndrome correlates with reduced RSK2 activation. Neurology. 2001;56:207–214. doi: 10.1212/wnl.56.2.207. [DOI] [PubMed] [Google Scholar]
- 14.De Cesare D, Jacquot S, Hanauer A, et al. Rsk-2 activity is necessary for epidermal growth factor-induced phosphorylation of CREB protein and transcription of c-fos gene. Proc Natl Acad Sci USA. 1998;95:12202–12207. doi: 10.1073/pnas.95.21.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang X, Matsuda K, Bialek P, et al. ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology. Implication for Coffin-Lowry Syndrome. Cell. 2004;117:387–398. doi: 10.1016/s0092-8674(04)00344-7. [DOI] [PubMed] [Google Scholar]
- 16.Ropers HH, Hamel BCJ. X linked mental retardation. Nat Rev Genet. 2005;6:46–57. doi: 10.1038/nrg1501. [DOI] [PubMed] [Google Scholar]