Abstract
Immuno-receptor tyrosine based inhibitory motif (ITIM)-containing receptors play an essential role in modulating immune responses. Leukocyte associated inhibitory receptor (LAIR)-1, also known as CD305, is an ITIM-containing inhibitory receptor, expressed by all leukocytes, that binds collagens. In this report, we investigate the effect of a conservative R65K mutation on LAIR-1 ligand binding and function. Compared to LAIR-1 wild-type (wt) expressing cells, LAIR-1 R65K cells show markedly reduced binding to collagen, which correlates with a reduced level of LAIR-1 polarization to the site of interaction with collagens. Both LAIR-1 wt and R65K cells can generate intracellular signals when ligated by anti-LAIR-1 mAb, but only LAIR-1 wt cells respond to collagens or matrigel. In agreement, surface plasmon resonance (SPR) analyses showed that LAIR-1 R65K protein has markedly reduced avidity for collagen type I compared to LAIR-1 wt. Likewise, LAIR-1 R65K protein has decreased avidity for cells expressing transmembrane collagen XVII. Thus, a single residue, Arg 65, is critical for the interaction of LAIR-1 with collagens.
Keywords: Natural Killer Cells, Cell Surface Molecules, Signal Transduction, Cell Activation
Introduction
An adequate immune response is the result of a fine balance between activating and inhibitory signals that promotes elimination of the invading agent while suppressing hyper-responsiveness that could damage the host. Many mechanisms exist to accomplish this task, including the expression of both activating and inhibitory receptors by immune cells. A group of inhibitory receptors is characterized by the presence of one or more immuno-receptor tyrosine based inhibitory motifs (ITIM)3 in their cytoplasmic tail. After interaction with their ligands, the tyrosine residues in the ITIM motifs are phosphorylated by src family tyrosine kinases. These phosphorylated tyrosine residues serve as docking sites for phosphatases, such as Src homology 2 (SH2) domain-containing protein tyrosine phosphatase (SHP)-1, -2 and SH2 domain-containing inositol phosphatase (SHIP), which then become activated and initiate the propagation of the inhibitory signal (1, 2). The leukocyte associated inhibitory receptor (LAIR)-1, also known as CD305, possesses two ITIMs in its cytoplasmic tail that mediate its inhibitory capacity through interaction with SHP-1, SHP-2 and/or C-terminal Src kinase (Csk) (3-5). This receptor is expressed by all leukocytes, NK cells, T cells, B cells, dendritic cells (DC), monocytes, neutrophils, and hematopoietic stem cells (3, 6-9). The ligands for LAIR-1, and for the highly homologous, secreted protein LAIR-2, are collagens, the most abundant type of protein in the body (10, 11). The interaction between LAIR-1 and collagens is of high affinity and is dependent on the conserved glycine-proline-hydroxyproline (GPO) repeating sequence that is characteristic of all collagen molecules (10, 11). The broad expression pattern of this inhibitory receptor and the possibility that immune cells are able to interact with collagens at many places during their trafficking through the body suggests that LAIR-1 may be an important receptor for modulating immune responses. Multiple studies have shown that the ligation of LAIR-1 by mAb or collagens is able to inhibit activation signals (3, 6-8, 10, 12, 13).
Although the function of LAIR-1 in vivo is currently unknown, it is known that the engagement of LAIR-1 with specific mAb is able to inhibit proliferation of human myeloid leukemic cell lines by inducing programmed cell death independent of Fas/FasL interaction (14). Also, the engagement of LAIR-1 expressed on acute myeloid leukemia blasts, isolated from peripheral blood or bone marrow from patients, inhibits GM-CSF induced proliferation leading to apoptosis, possibly by a mechanism involving inhibition of GM-CSF induced AKT activation (15). Moreover, there is a correlation in patients with high risk B cell chronic lymphocytic leukemia with the absence of LAIR-1, suggesting that the absence of this receptor may be involved in the proliferation of leukemic cells (16). During the course of HIV infection, LAIR-1 expression is abnormally expressed both in naïve B cells (17) and in a unique tissue like memory B cell subset that is expanded in HIV infected patients (18). Finally, the levels of LAIR-2 have been shown to be elevated in the synovial fluid of patients with rheumatoid arthritis (11). Altogether, these data suggest that LAIR-1, and also LAIR-2, may be involved in the pathogenesis and natural history of several diseases.
As stated previously, LAIR-1 binds collagens with high avidity; however, nothing is known about the structural basis of this interaction. Here we show that a single residue in LAIR-1, Arg 65, is critical for the functional interaction of this receptor with several types of collagens.
Material and Methods
Constructs
In a manner analogous to previous studies (19), we generated human LAIR-1/CD3ζ wild-type (wt) and LAIR-1/CD3ζ R65K constructs. Briefly, RNA was isolated from human T cells using an RNAqueous-4PCR Kit (Ambion). cDNA was obtained by iScript cDNA Synthesis Kit (Bio-Rad). cDNAs corresponding to human LAIR-1 extracellular domain and CD3ζ transmembrane and cytoplasmic domains were then amplified using the following primers: LEC-F: GAATTCGCCGCCACCATGTCTCCCCACCCCACCG; LEC-R: GGATCCTCAGCTTTCAGGCCTTGGGAAG; CD3ZTM-F: GGATCCCAAACTCTGCTACCTG; and CD3ZTM-R: TCTAGAGCGTCTGCAGGTCTGGCC. The PCR products were digested and cloned into the pcDNA3.1(+) plasmid (Invitrogen). The same strategy was followed to make the KIR2DL2/CD3ζ construct except for using NK cells as a source of mRNA. The LAIR-1/Fc wt construct was a gift from Dr. Linde Meyaard (University Medical Center, Utrecht, the Netherlands). The LAIR-1-EGFP wt construct was made by cloning LAIR-1 in the pEGFP-N3 plasmid vector (Clontech). The LAIR-1/Fc R65K and LAIR-1-EGFP R65K mutants were made by using QuikChange Site-Directed Mutagenesis Kit (Stratagene). All constructs were sequenced to confirm their identities.
Cells and reagents
The LAIR-1/Fc wt and LAIR-1/Fc R65K fusion proteins were isolated from the culture supernatants of transiently transfected 293T cells using protein A Sepharose Fast Flow columns (Invitrogen). K562 cells were transiently transfected with LAIR-1-EGFP and LAIR-1-EGFP R65K constructs using the Amaxa nucleofection system (Amaxa). K562 cells were stably transfected by electroporation with the LAIR-1 wt or LAIR-1 R65K constructs, or a collagen XVII construct provided by Dr. Holger Notbohm (University of Lübeck, Germany). Detection of transmembrane collagen XVII on transfected K562 cells was done by flow cytometry with the NC16a-3 Ab kindly provided by Dr. Leena Bruckner-Tuderman (University of Freiburg, Germany).
Reporter cell assay and binding assays
The reporter cell line BWZ.36 (20) was stably transfected by electroporation with the LAIR-1/CD3ζ wt or LAIR-1/CD3ζ R65K constructs. This strategy provides a facile means for detecting receptor ligation. In this system, if LAIR-1/CD3ζ expressed by the BWZ.36 cells interacts with a ligand, the ITAM motifs in the CD3ζ become phosphorylated and, as a consequence, cytoplasmic NFAT proteins translocate to the nucleus and bind tandem NFAT binding sites in the promoter of the LacZ gene. This induces ζ-galactosidase expression, whose level of induction can be quantitated through its enzyme activity. Specifically, BWZ.36 reporter cells (1 × 105) were added to 24 well plates coated with 5 μg of anti-LAIR-1 mAb clone DX26 (BD Pharmingen), anti-KIR2DL2 mAb (Beckman-Coulter) or isotype control Ig (Beckman Coulter); or to 20−50 μg matrigel (BD Biosciences), collagen type I, collagen type IV or laminin 24 well biocoat plates from BD Biosciences. After 24−48 h of incubation, the cells were harvested and LacZ activity was measured by β-galactosidase assay kits (CPRG) (Gene Therapy Systems). For cell binding assays, LAIR-1 wt or LAIR-1 R65K transfected K562 cells were labeled with 2 μg/ml of Cell Tracker Green CMFDA (Invitrogen) in RPMI containing 1% FCS for 30 min at 37°C. Then, cells were washed twice with PBS containing 5% FCS and resuspended at 1.5 × 106/ml. 100 μl of cells were added to 96 well plates (in triplicates), uncoated (for the input) or coated with collagen type I. After 2−3 h of incubation at 37°C, plates were flicked and washed four times with PBS containing 5% FCS and fluorescence was read with a plate reader. The binding percentage was calculated according to the following formula: [problem fluorescence – medium fluorescence / input fluorescence – medium fluorescence] × 100. The fusion protein binding assay was performed as previously described (21), except that the binding of the fusion protein was detected by FITC-conjugated mouse anti-human Fc (Jackson Immunoresearch). For conjugate formation, 1 × 106/ml untransfected K562 cells, LAIR-1 wt and LAIR-1 R65K transfected K562 cells were added to 24 well plates along with 100 μl of a solution that contained 1200 μl of RPMI medium with 12 μl of collagen I labeled microspheres, size 1.0 μm, yellow-green fluorescent (505/515) (Invitrogen). After 3 h of incubation at 37 °C cells are harvested and conjugate formation was analyzed in a FACScalibur cytometer (BD Biosciences).
Western blot and immunoprecipitations analysis
LAIR-1 wt or LAIR-1 R65K transfected K562 cells were treated with collagen type I at 1 μg/107cells/ml in medium without serum at 37 °C for 20 min. For pervanadate treatment, cells were incubated for 20 min with freshly prepared sodium pervanadate (0.1 mM sodium orthovanadate and 10 mM hydrogen peroxide from Sigma-Aldrich) in medium without serum at 37 °C. This treatment inhibits tyrosine phosphatases and thereby promotes detection of phosphorylated proteins. Cells were immediately lysed in cold lysis buffer (0.5% Triton X-100, 50 mM Tris HCl pH 7.4, 150 mM NaCl, 2mM EDTA, protease and phosphatase inhibitors) (Sigma-Aldrich) for 30 min on ice. After centrifugation for 30 min at 15000 rpm, supernatants were first precleared by mixing with protein G-Sepharose beads (Sigma-Aldrich) and then immunoprecipitated with anti-human LAIR-1 (clone DX26) prebound to protein G-Sepharose beads. After gentle rotation at 4°C for 12 h, the beads were washed five times with cold lysis buffer, and bound proteins were eluted with Laemmli buffer, resolved by SDS-PAGE on a 4−12% gradient NuPAGE polyacrylamide gel (Invitrogen) and transferred electrophoretically to nitrocellulose membranes (Invitrogen). Membranes were then blocked with 3% (w/v) BSA in TBS-T (20 mM Tris-HCl pH 8, 150 mM NaCl and 0.05% Tween 20) for 2 h and then probed with mouse anti-phosphotyrosine mAb (clone 4G10/Millipore). After extensive washing in TBS-T, the membranes were incubated with HRP-labeled goat anti-mouse Ig Ab (Amersham Biosciences), and immunoreactivity was visualized by using the ECL system (Amersham Biosciences). For loading controls, membranes were stripped and reprobed with mouse anti-human LAIR-1 mAb (clone 14/LAIR-1 from BD Transduction Laboratories).
Confocal microscopy
K562 transfected cells were deposited on glass coverslips coated with collagen type I (BD Biosciences), poly-lysine (Sigma), anti-LAIR-1 mAb or anti-KIR2DL2 mAb for 45−60 min at 37 °C. The cells attached to the coverslips were then fixed with 4% paraformaldehyde for 10 min at RT and washed with PBS before mounting on glass slides with mounting agent containing mowiol (EMD Biosciences). Images were acquired with a Leica SP2 AOBS confocal imaging system (Leica Microsystems Heidelberg GmbH). Images of approximately 0.3 μm thick sections of cells expressing LAIR-1-EGFP, LAIR-1-EGFP R65K or KIR2DL2-EGFP were acquired. The z stacks were analyzed for green pixel intensity. This was done using stack profile, an algorithm that is contained within the Leica LCS Lite software. The stack profile shows mean pixel intensity (MPI) from channel 1 (EGFP) plotted against the section number from the top to the bottom of the cell. To quantify the polarization of LAIR-1-EGFP wt or LAIR-1 EGFP R65K, the MPI of channel 1 (EGFP) inside a region of interest (ROI), drawn around the cell in each z-stack section was obtained and then, a ratio between MPI from the bottom section and middle sections was calculated.
Surface plasmon resonance (SPR) analysis
Binding of LAIR-1/Fc wt, LAIR-1/Fc R65K, or ICAM-1/Fc, as a negative control, to rat collagen type I was analyzed by surface plasmon resonance (SPR) using a BIAcore™ 2000. Collagen was covalently coupled to a CM-5 sensor chip by standard methods (22) to either 2600 resonance units (RU) or 575 RU, and samples were injected over the coupled surfaces in modified HBST which contains 10 mM HEPES pH 7.2, 125 mM NaCl, 2 mM CaCl2, and 0.005% Tween 20, without EDTA, at a flow rate of 50 μl/min. Washout was accomplished with the same buffer devoid of analyte. Data were collected at a rate of 5 sec−1 and were analyzed using BIAeval 3.2 using models for simultaneous kinetics of a 1:1 Langmuir binding reaction.
Molecular graphics comparisons
The amino acid sequence of human LAIR-1 was used to probe the protein database (23) and the two highest hits, LILRA5 (24), pdb designation 2D3V, and platelet glycoprotein VI (25) (pdb id: 2GI7) were used for further analysis. Structures were displayed and aligned in Pymol (http://www.pymol.org).
Results
Arg65 is essential for functional recognition of collagens by LAIR-1
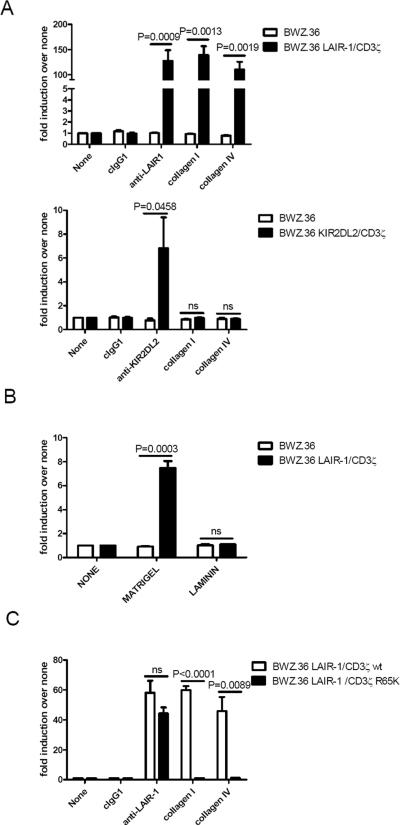
To study the functional interaction between LAIR-1 and its ligands, we used the BWZ.36 T cell mouse hybridoma reporter cell line transfected with a chimeric receptor consisting of the human LAIR-1 extracellular portion and human CD3ζ transmembrane and intracytoplasmic portions (BWZ.36 LAIR-1/CD3ζ cells). In Figure 1A, upper panel, we confirm previous results showing that LAIR-1 is able to functionally interact with collagens; specifically, collagen type I, a prototypic fibril-forming collagen that is present in non-cartilaginous connective tissues, and the network forming collagen type IV, which is an important component of basement membranes (26). As a specificity control, we used BWZ.36 KIR2DL2/CD3ζ cells that only are able to express β-galactosidase activity when KIR2DL2 is ligated (Figure 1A, lower panel). We next examined if LAIR-1 can recognize collagen in the context of other proteins that are expressed in the extracellular matrix. In Figure 1B, we show that LAIR-1 is able to functionally interact with matrigel, a commercial preparation of basement membranes that contains collagen type IV. As the epitope recognized by LAIR-1 is unique to collagens (26), it is unlikely that other components within matrigel, such as laminin (see Fig. 1B), heparan sulfate, proteoglycans or entactin, can bind to LAIR-1.
Figure 1.
LAIR-1 Arg 65 is a critical residue for collagen recognition leading to signal transmission. A) Relative levels of β-galactosidase activity generated by culture of BWZ.36 or BWZ.36 LAIR-1/CD3ζ cells (upper panel); or BWZ.36 KIR2DL2/CD3ζ cells (lower panel) on uncoated plates or coated with collagen type I, -type IV, anti-LAIR-1 mAb, anti-KIR2DL2 mAb or isotype control Ig. B) BWZ.36 or BWZ.36 LAIR-1/CD3ζ cells on uncoated plates or coated with matrigel or laminin. C) BWZ.36 LAIR-1/CD3ζ wt or BWZ.36 LAIR-1/CD3ζ R65K cells on uncoated plates or coated with collagen type I, -type IV, anti-LAIR-1 mAb or isotype control Ig. Each experiment was performed 3−5 independent times.
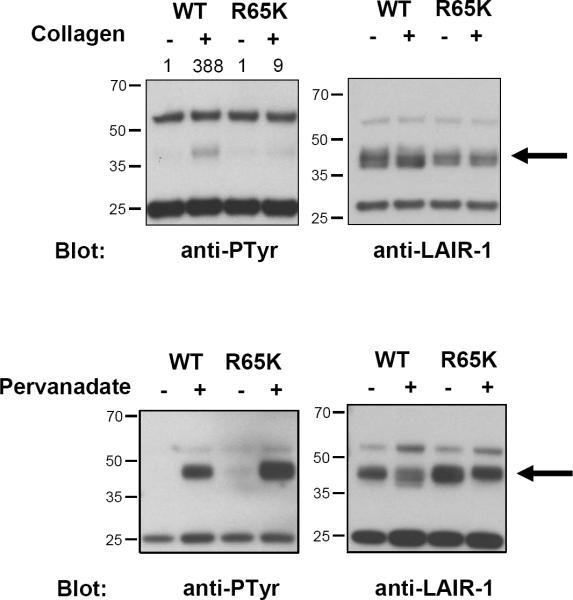
Although a simple triple helix (GPO)10 characteristic of collagens is sufficient for binding to LAIR-1 (10), it is unknown what residue(s) in LAIR-1 are important for the interaction with collagens. During the construction of LAIR-1/CD3ζ wt, we fortuitously generated the LAIR-1/CD3ζ R65K mutant construct and decided to investigate if this relatively conservative mutation significantly impacts LAIR-1 ligand recognition. We found that Arg65 is essential for the functional recognition of collagens by LAIR-1 expressed in the BWZ.36 reporter system (Figure 1C). As a positive control, we show that ligation of the LAIR-1/CD3ζ R65K expressed in BWZ.36 cells with anti-LAIR-1 mAb induces the same amount of β-galatosidase activity as LAIR-1/CD3ζ expressed in these cells. This indicates that LAIR-1/CD3ζ R65K is capable of signaling and that the binding sites for collagen and anti-LAIR-1 mAb to LAIR-1 are probably different. Next, we examined the effect of ligation on the phosphorylation status of LAIR-1 wt and LAIR-1 R65K. To do this experiment, we used K562 cells transfected with plasmids encoding LAIR-1 wt and LAIR-1 R65K. Figure 2 shows that after interaction with collagen type I LAIR-1 wt is markedly phosphorylated relative to LAIR-1 R65K (Figure 2, upper panel). As a control, we show that both LAIR-1 wt and LAIR-1 R65K are phosphorylated when cells are treated with pervanadate (Figure 2, lower panel). Altogether, these results indicate that Arg65 has an essential role in the functional interaction of LAIR-1 with collagens.
Figure 2.
Tyrosine phosphorylation of LAIR-1 wt and LAIR-1 R65K in transfected K562 cells after interaction with collagen type I (upper panel) or with pervanadate treatment (lower panel). LAIR-1 wt and LAIR-1 R65K were immunoprecipitated after stimulation with clone DX26 antibody. The amount of immunoprecipitated LAIR-1 in each condition was detected by western blot using clone 14 antibody. Numbers on top of the anti-phosphotyrosine blot in the upper panel indicate the relative intensity of phosphorylated LAIR-1. Results shown here are representative of two experiments.
LAIR-1 R65K shows decreased ability for binding collagen type I
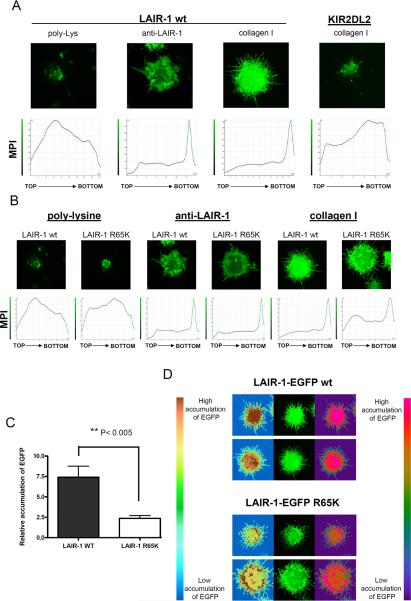
To further investigate the interaction of LAIR-1 wt and LAIR-1 R65K with collagens, we utilized the LAIR-1 negative K562 cell line transfected with LAIR-1-EGFP wt or LAIR-1-EGFP R65K to study binding to plate bound collagens. As shown in Figure 3A, there is extensive polarization of the LAIR-1-EGFP wt to the site of contact with collagen type I or anti-LAIR-1 mAb. LAIR-1-EGFP wt expressed in LAIR-1 positive Jurkat T cells showed similar polarization toward the site of contact with anti-LAIR-1 mAb and collagen type I (data not shown). As negative control, we observed a lack of polarization of LAIR-1-EGFP wt when the K562 cells are in contact with poly-L lysine. KIR2DL2-EGFP expressed in K562 cells failed to polarize to the site of contact with collagen (Figure 3A) or poly-L lysine (data not shown). These results indicate that polarization of LAIR-1 is specific to its interaction with ligand. Results in Figure 3B show that LAIR-1-EGFP R65K is still able to interact with collagen as shown by the polarization of the receptor towards the site of interaction with collagen type I. However, quantification of the polarization shows that LAIR-1 R65K polarizes less efficiently than LAIR-1 wt (Figure 3C). As expected, the extent of polarization of LAIR-1-EGFP R65K in response to ligation by anti-LAIR-1 mAb is virtually identical to that obtained with LAIR-1-EGFP wt (Figure 3B).
Figure 3.
LAIR-1-EGFP wt polarizes more efficiently than LAIR-1-EGFP R65K to sites of contact with collagen. A) K562 cells transfected with LAIR-1-EGFP wt or KIR2DL2-EGFP were deposited on glass coverslips coated with poly-lysine, anti-LAIR-1 mAb or collagen type I. B) K562 cells transfected with LAIR-1-EGFP wt or LAIR-1-EGFP R65K were deposited on glass coverslips coated with poly-lysine, anti-LAIR-1 mAb or collagen type I. For A) and B), the lower panels show the mean pixel intensity (MPI) of sections of the cell from the top (the distal part of the cell in relation to the glass coverslip) to the bottom (the part of cell in contact with the glass coverslip) and the upper panels show the fluorescence for the section of the cell interacting with the glass coverslip (bottom in lower panels). C) Relative accumulation of LAIR-1-EGFP wt and LAIR-1-EGFP R65K at the site of contact with collagen type I. MPI at the site of contact with collagen was divided by the average MPI of five sections from the middle part of the cell (N= 17). D) Pattern of LAIR-1-EGFP wt and LAIR-1-EGFP R65K polarization is shown in two different false color scales.
We also observed that the pattern of polarization of LAIR-1-EGFP R65K with collagen is different from that of LAIR-1-EGFP wt. As it is shown in Figure 3D, LAIR-1-EGFP wt accumulates with uniform distribution at the site of contact with collagen type I; on the other hand, LAIR-1-EGFP R65K accumulates in a ring-like pattern with an increased intensity in the periphery and less in the center of the contact site. Moreover, while cells expressing LAIR-1-EGFP wt tend to have straight protrusions (lamellapods) outside of the central body in the area in contact with collagen, cells that express LAIR-1- EGFP R65K have undulated protrusions. These observations indicate that, while both LAIR-1 receptors, wt and R65K, are able to polarize when interacting with collagen type I, the nature and dynamics of the polarization are quite different, probably reflecting the inability of LAIR-1 R65K to efficiently transmit intracellular signals.
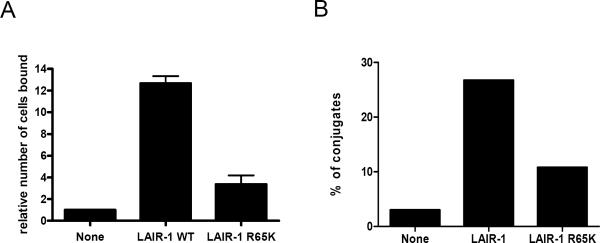
To rule out the possibility that the tagging of EGFP to LAIR-1 wt and LAIR-1 R65K causes the differences observed in the polarization of the receptor towards collagen, we performed cell binding assays with K562 cells transfected with LAIR-1 wt or LAIR-1 R65K. Results presented in Figure 4A clearly show that the binding by the LAIR-1 R65K K562 cells is dramatically diminished compared to LAIR-1 wt K562 cells. In a similar experiment, we show that LAIR-1 wt cells form more conjugates with FITC labeled collagen type I beads than LAIR-1 R65K K562 cells (Figure 4B).
Figure 4.
Cells expressing LAIR-1 wt binds to collagens with higher avidity than cells expressing LAIR-1 R65K. A) Untransfected K562 cells and transfected with LAIR-1 wt or LAIR-1 R65K were incubated on collagen type I coated plates for 2 h, after extensive washing, the bound cells were quantified as detailed in Materials and Methods (N=4). B) Untransfected K562 cells and transfected with LAIR-1 wt or LAIR-1 R65K were incubated with beads coated with FITC labeled collagen type I and conjugate formation was measured by flow cytometric analyses. Results are representative of two experiments.
LAIR-1 R65K binds transmembrane collagen XVII with low efficiency
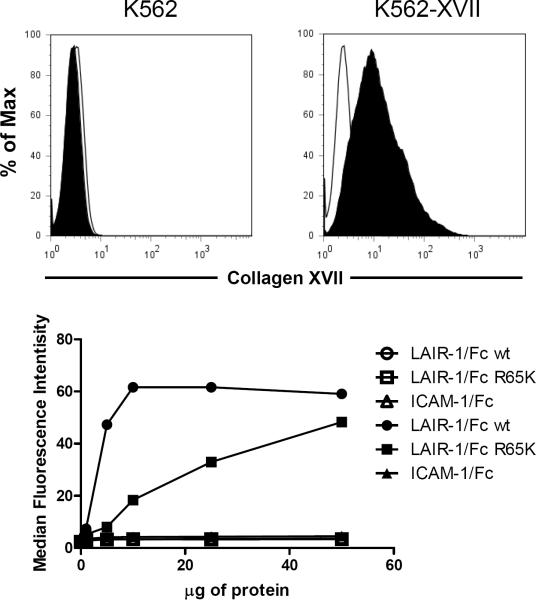
In the results presented above, the interaction of LAIR-1 wt and LAIR-1 R65K with collagens is in the context of the receptors expressed on cells. To confirm that the observed differences in collagen binding between LAIR-1 wt and LAIR-1 R65K were directly attributable to the R65K mutation and not to interactions with other molecules expressed on cell surfaces, we generated recombinant LAIR-1/Fc wt and LAIR-1/Fc R65K proteins for binding studies. First, we checked the binding of these proteins to K562 cells transfected with collagen XVII, a transmembrane form of collagen (27). Figure 5 shows that LAIR-1/Fc R65K protein binds with much lower efficiency to K562 cells transfected with collagen XVII cells than the LAIR-1/Fc wt protein. Negative controls show that neither protein binds to untransfected K562 cells nor does an irrelevant Fc fusion protein (ICAM-1/Fc) bind to K562 cells transfected with collagen XVII.
Figure 5.
Recombinant LAIR-1/Fc R65K binds to transmembrane collagen XVII with lower avidity than recombinant LAIR-1/Fc wt. Upper panels show staining with anti-collagen XVII mAb of untransfected (upper left) and collagen XVII transfected (upper right) K562 cells. Filled histograms represent the binding of anti-collagen XVII mAb and empty histograms the binding of isotype control Ig. The lower panel shows binding of the LAIR-1/Fc wt, LAIR-1/Fc R65K and ICAM-1/Fc fusion proteins to untransfected (empty symbols) and collagen XVII transfected (filled symbols) K562 cells. Results are representative of two independent experiments.
LAIR-1 R65K binds collagen with very low avidity
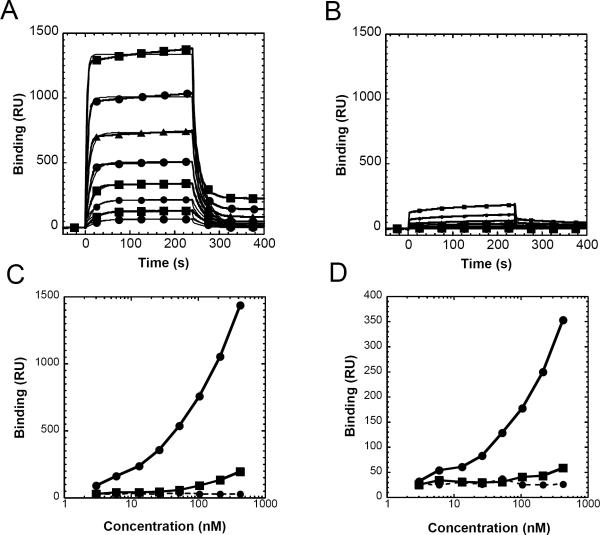
To examine further the quantitative difference in the binding of LAIR-1/Fc wt and of LAIR-1/Fc R65K to collagen, we tested the interaction by SPR (Figure 6). LAIR-1/Fc wt bound collagen with an apparent KD of 1.45 × 10−7 M (ka of 3.15 × 105 L/mole-sec; kd of 0.0456 sec−1) (Figure 6A). LAIR1/Fc R65K bound detectably, but to a much lower capacity, precluding reliable evaluation (Figure 6B). The profound differences in relative binding of LAIR-1/Fc wt and LAIR-1/Fc R65K to collagen are emphasized in the binding isotherms shown in Figure 6C,-D, which depict binding to a highly coupled (Figure 6C) and low density coupled (Figure 6D) surfaces.
Figure 6.
LAIR-1/Fc wt binds collagen type I with greater avidity than LAIR-1/Fc R65K. Rat collagen type I was covalently coupled to a biosensor surface as described in the Materials and Methods, and graded concentrations of LAIR-1/Fc wt (A) or LAIR-1/Fc R65K (B) in doubling dilutions from 410 nM to 3 nM were offered to the surface. Binding was initiated at t = 0 s and the washout (dissociation phase) was begun at t = 240 s. Experimental curves are indicated in the heavier lines marked with geometrical symbols and the curve fits to the data are indicated by the thinner solid lines. Binding isotherms for plateau values are plotted as a function of concentration;shown are a high density coupled (C) and low density coupled (D) surface respectively. Data for LAIR-1/Fc wt (solid circles), LAIR-1/Fc R65K (solid squares) and ICAM-1/Fc (solid circles with dashed line) are shown.
Discussion
LAIR-1 is a major ITIM containing receptor that is expressed on all hematopoietic cell types whose ligand is collagens. Elucidation of the mechanism of interaction of LAIR-1 with collagens is an essential step toward understanding the inhibitory signal mediated through this receptor. We show here that mutation of a single residue, Arg 65, dramatically decreases the binding of LAIR-1 to collagens. More importantly, we show that Arg 65 is a critical residue for the functional interaction of this receptor with its ligands.
Collagens are the most abundant protein in mammals, and they are important not only for the biomechanical properties of tissues and organs, but also they are involved in many cell functions such as cell adhesion and migration, morphogenesis, differentiation, etc (28). The hallmark of all collagen molecules is a triple helix made up of repeating sequences of glycine-x-y repeats, where x and y, in many cases, are proline and hydroxyproline (26). There are several mammalian receptors known to bind collagens, including α1β1, α2β1, α10β1 and α11β1 integrins, discoidin domain receptor (DDR) 1 and DDR2, glycoprotein VI (GPVI), LAIR-1 and members of the mannose receptor family (28). Each receptor binds different types of collagens; for example, integrin α1β1 preferentially binds collagens type IV and VI, as well as fibril-forming collagens, while GPVI binds fibril-forming collagen and the synthetic collagen related peptide (GPO)10 (28). The α1β1 integrin and DDR2 have been shown to bind a distinct sites within collagen type I and type II, respectively, and not (GPO)10 (28). LAIR-1 has been shown to bind fibril-forming collagens and transmembrane collagens (10). In addition, we show here that LAIR-1 also interacts with collagen type IV, including within the context of the extracellular matrix. Similar to GPVI (29), LAIR-1 binds (GPO)10, but not (GPP)10.
The structure of LAIR-1, alone or in complex with collagen, is unknown. Moreover, there are no studies that have described what residues in the LAIR-1 receptor are important for binding collagen. To gain insight into the structural location of the R65 in the LAIR-1 molecule, and its possible relationship to its capacity to bind collagen, we searched the protein structure database (23) for proteins showing homology to LAIR-1. Several molecules with significant amino acid sequence homology were identified, including the human monocyte-activating receptor, designated LILRA5/LIR9/ILT11 or CD85f (24) (42% identity over 96 residues), and the human platelet GPVI (25) (42% identity over 91 residues). LAIR-1 R65 aligns with S42 and S44 of LILRA5 and GPVI, respectively (Supplementary Figure S1A). Ribbon diagrams (Supplementary Figure S1B and S1C) show the position of S42 and S44 of the mature LILRA5 and GPVI, respectively. The collagen-related peptide (GPO)n binding site of GPVI has been identified by molecular docking algorithms (25). Examination of surface representations of GPVI reveals that S44 lies within a region (Supplementary Figure S1D) that contributes to the collagen-related peptide binding site as illustrated in Supplementary Figure S1E.
The gene encoding GPVI is located in the leukocyte receptor cluster (LRC) on human chromosome 19 along with other genes encoding receptors with one, two or more immunoglobulin-like domains, such as KIR, LIR/ILT, as well as LAIR-1 and LAIR-2 themselves (30). LAIR-2 is a soluble receptor that binds collagen with high affinity and it is able to antagonize the LAIR-1/collagen inhibitory interaction (11). The sequence homology of LAIR-1 and LAIR-2 is 84% (see Supplementary Figure 1) and, very importantly, LAIR-2 also has an Arg in position 65, in agreement with our data showing the necessity of this residue for collagen binding. The GPVI D1 domain has a 12 residue deletion when compared with other members of the LRC. This deletion promotes the creation of a shallow hydrophobic groove, which has been shown by computational algorithms to accommodate the collagen related peptide (GPO)n (25) (Supplementary Figure S1D-E). However, LAIR-1 lacks the 12 residue deletion (Supplementary Figure S1A) which raises the possibility that LAIR-1 and GPVI bind collagen differently. This is supported by the fact that LAIR-1 binds more types of collagens that GPVI does (10).
Another important question is how binding of collagen to LAIR-1 leads to intracellular signaling. It is known that multiple GPVI molecules can bind a single (GPO)n triple helix (25). This is consistent with the observation that collagen binding induces clustering of GPVI, resulting in a signaling cascade via the FcRγ chain adaptor protein (25). Although it is likely, it is unknown if more than one LAIR-1 molecule can bind to a single collagen triple helix, and it is also not known if LAIR-1 clustering is required for signaling transmission. Assuming that clustering occurs and it is involved in signal transmission, the role that R65 plays in this process will need to be determined. Another non exclusive possibility is that R65K mutation abolishes the ability of collagen to induce conformational changes in the LAIR-1 molecule that result in signaling transmission.
We have shown that a very conservative substitution of Lys for Arg at position 65 is enough to dramatically affect the ability of LAIR-1 to bind several types of collagen. Even though LAIR-1 R65K can still bind collagens, SPR analysis indicates that the inherent avidity of the interaction of the mutant with collagen is considerably reduced compared to the wild type molecule. The facts that LAIR-1/CD3ζ R65K expressing cells fail to transmit signals and that LAIR-1 R65K in expressing cells fails to become phosphorylated upon exposure to collagen indicate that the R65K mutation affects LAIR-1 function. Solving the crystal structure of LAIR-1 complexed with collagen, along with additional mutagenesis studies, will clarify the role of Arg65, and other residues, in the interaction of LAIR-1 with its ligands.
Supplementary Material
Supplemental Figure Legend:
Supplemental Figure S1. Location of R65 of LAIR-1 is consistent with a collagen docking site previously identified on GPVI. Excluding LAIR-2, the two best matches of LAIR-1 amino acid sequence to proteins in the rcsb structural database (23) as determined by a BLASTP query were LILRA5 (pdb id: 2D3V (24) and GPVI (pdb id: 2GI7 (25)). A) Alignment of D1 domains of GPVI and LILRA5 with the putative Ig extracellular domain of LAIR-1, numbered according to LAIR-1. Alignment with the LAIR-2 sequence is also shown. Identical and conserved residues for the four receptors (red) or for three of them (blue) are shown. LAIR-1 R65, GPVI S44 and LILRA5 S42 are circled. B) and C) The LILRA5 structure (magenta) was aligned with chain A of the GPVI dimer (cyan) with PyMOL (http://www.pymol.org) and displayed in PyMOL as well. The residues of LILRA5 (S42) and GPVI (S44) that align with R65 of LAIR-1 are shown as magenta (S42) and cyan (S44) spheres in (B) and (C). Panels B and C are approximate 90° rotations from each other as indicated. The amino terminal domain 1 (D1) and domain 2 (D2) are labelled. D) The D1 domain of GPVI is colored according to electrostatic calculation with blue basic, and red acidic, as performed in Pymol. E) Surface representation of the collagen-related peptide (GPO)n binding site of D1 domain of GPVI as determined by Horii et al. (2006) is indicated, and the surface of S44 on GPVI, at the base of the putative (GPO)n binding site is shown in yellow.
Acknowledgements
We thank Dr. L. Meyaard for the LAIR-1 Fc fusion construct, Dr. E.O. Long and Dr. M. March for the ICAM-1/Fc fusion protein, Dr. E.O. Long and Dr. D.N. Burshtyn for the KIR2DL2-EGFP construct, Dr. H. Notbohm the collagen XVII construct, Dr. L. Bruckner-Tuderman for the anti-collagen XVII mAb, Dr. N. Shastri for the BWZ.36 cells and Dr. E.O. Long for the 293T cells. We also thank Dr. David Garboczi for critical review of the manuscript.
Footnotes
This work was supported by the intramural program of NIAID.
References
- 1.Kabat J, Borrego F, Brooks A, Coligan JE. Role that each NKG2A immunoreceptor tyrosine-based inhibitory motif plays in mediating the human CD94/NKG2A inhibitory signal. J Immunol. 2002;169:1948–1958. doi: 10.4049/jimmunol.169.4.1948. [DOI] [PubMed] [Google Scholar]
- 2.Ravetch JV, Lanier LL. Immune inhibitory receptors. Science. 2000;290:84–89. doi: 10.1126/science.290.5489.84. [DOI] [PubMed] [Google Scholar]
- 3.Meyaard L, Adema GJ, Chang C, Woollatt E, Sutherland GR, Lanier LL, Phillips JH. LAIR-1, a novel inhibitory receptor expressed on human mononuclear leukocytes. Immunity. 1997;7:283–290. doi: 10.1016/s1074-7613(00)80530-0. [DOI] [PubMed] [Google Scholar]
- 4.Verbrugge A, Rijkers ES, de Ruiter T, Meyaard L. Leukocyte-associated Ig-like receptor-1 has SH2 domain-containing phosphatase-independent function and recruits C-terminal Src kinase. Eur J Immunol. 2006;36:190–198. doi: 10.1002/eji.200535226. [DOI] [PubMed] [Google Scholar]
- 5.Verbrugge A, Ruiter Td T, Clevers H, Meyaard L. Differential contribution of the immunoreceptor tyrosine-based inhibitory motifs of human leukocyte-associated Ig-like receptor-1 to inhibitory function and phosphatase recruitment. Int Immunol. 2003;15:1349–1358. doi: 10.1093/intimm/dxg134. [DOI] [PubMed] [Google Scholar]
- 6.Lebbink RJ, de Ruiter T, Kaptijn GJ, Bihan DG, Jansen CA, Lenting PJ, Meyaard L. Mouse leukocyte-associated Ig-like receptor-1 (mLAIR-1) functions as an inhibitory collagen-binding receptor on immune cells. Int Immunol. 2007;19:1011–1019. doi: 10.1093/intimm/dxm071. [DOI] [PubMed] [Google Scholar]
- 7.Maasho K, Masilamani M, Valas R, Basu S, Coligan JE, Borrego F. The inhibitory leukocyte-associated Ig-like receptor-1 (LAIR-1) is expressed at high levels by human naive T cells and inhibits TCR mediated activation. Mol Immunol. 2005;42:1521–1530. doi: 10.1016/j.molimm.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 8.van der Vuurst de Vries AR, Clevers H, Logtenberg T, Meyaard L. Leukocyte-associated immunoglobulin-like receptor-1 (LAIR-1) is differentially expressed during human B cell differentiation and inhibits B cell receptor-mediated signaling. Eur J Immunol. 1999;29:3160–3167. doi: 10.1002/(SICI)1521-4141(199910)29:10<3160::AID-IMMU3160>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 9.Verbrugge A, de Ruiter T, Geest C, Coffer PJ, Meyaard L. Differential expression of leukocyte-associated Ig-like receptor-1 during neutrophil differentiation and activation. J Leukoc Biol. 2006;79:828–836. doi: 10.1189/jlb.0705370. [DOI] [PubMed] [Google Scholar]
- 10.Lebbink RJ, de Ruiter T, Adelmeijer J, Brenkman AB, van Helvoort JM, Koch M, Farndale RW, Lisman T, Sonnenberg A, Lenting PJ, Meyaard L. Collagens are functional, high affinity ligands for the inhibitory immune receptor LAIR-1. J Exp Med. 2006;203:1419–1425. doi: 10.1084/jem.20052554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lebbink RJ, van den Berg MC, de Ruiter T, Raynal N, van Roon JA, Lenting PJ, Jin B, Meyaard L. The soluble leukocyte-associated Ig-like receptor (LAIR)-2 antagonizes the collagen/LAIR-1 inhibitory immune interaction. J Immunol. 2008;180:1662–1669. doi: 10.4049/jimmunol.180.3.1662. [DOI] [PubMed] [Google Scholar]
- 12.Jansen CA, Cruijsen CW, de Ruiter T, Nanlohy N, Willems N, Janssens-Korpela PL, Meyaard L. Regulated expression of the inhibitory receptor LAIR-1 on human peripheral T cells during T cell activation and differentiation. Eur J Immunol. 2007;37:914–924. doi: 10.1002/eji.200636678. [DOI] [PubMed] [Google Scholar]
- 13.Meyaard L, Hurenkamp J, Clevers H, Lanier LL, Phillips JH. Leukocyte-associated Ig-like receptor-1 functions as an inhibitory receptor on cytotoxic T cells. J Immunol. 1999;162:5800–5804. [PubMed] [Google Scholar]
- 14.Poggi A, Pellegatta F, Leone BE, Moretta L, Zocchi MR. Engagement of the leukocyte-associated Ig-like receptor-1 induces programmed cell death and prevents NF-kappaB nuclear translocation in human myeloid leukemias. Eur J Immunol. 2000;30:2751–2758. doi: 10.1002/1521-4141(200010)30:10<2751::AID-IMMU2751>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 15.Zocchi MR, Pellegatta F, Pierri I, Gobbi M, Poggi A. Leukocyte-associated Ig-like receptor-1 prevents granulocyte-monocyte colony stimulating factor-dependent proliferation and Akt1/PKB alpha activation in primary acute myeloid leukemia cells. Eur J Immunol. 2001;31:3667–3675. doi: 10.1002/1521-4141(200112)31:12<3667::aid-immu3667>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 16.Poggi A, Catellani S, Bruzzone A, Caligaris-Cappio F, Gobbi M, Zocchi MR. Lack of the leukocyte-associated Ig-like receptor-1 expression in high-risk chronic lymphocytic leukaemia results in the absence of a negative signal regulating kinase activation and cell division. Leukemia. 2008;22:980–988. doi: 10.1038/leu.2008.21. [DOI] [PubMed] [Google Scholar]
- 17.De Milito A, Nilsson A, Titanji K, Thorstensson R, Reizenstein E, Narita M, Grutzmeier S, Sonnerborg A, Chiodi F. Mechanisms of hypergammaglobulinemia and impaired antigen-specific humoral immunity in HIV-1 infection. Blood. 2004;103:2180–2186. doi: 10.1182/blood-2003-07-2375. [DOI] [PubMed] [Google Scholar]
- 18.Moir S, Ho J, Malaspina A, Wang W, Dipoto AC, O'Shea MA, Roby G, Kottilil S, Arthos J, Proschan MA, Chun TW, Fauci AS. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med. 2008 doi: 10.1084/jem.20072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iizuka K, Naidenko OV, Plougastel BF, Fremont DH, Yokoyama WM. Genetically linked C-type lectin-related ligands for the NKRP1 family of natural killer cell receptors. Nat Immunol. 2003;4:801–807. doi: 10.1038/ni954. [DOI] [PubMed] [Google Scholar]
- 20.Sanderson S, Shastri N. LacZ inducible, antigen/MHC-specific T cell hybrids. Int Immunol. 1994;6:369–376. doi: 10.1093/intimm/6.3.369. [DOI] [PubMed] [Google Scholar]
- 21.Lebbink RJ, de Ruiter T, Verbrugge A, Bril WS, Meyaard L. The mouse homologue of the leukocyte-associated Ig-like receptor-1 is an inhibitory receptor that recruits Src homology region 2-containing protein tyrosine phosphatase (SHP)-2, but not SHP-1. J Immunol. 2004;172:5535–5543. doi: 10.4049/jimmunol.172.9.5535. [DOI] [PubMed] [Google Scholar]
- 22.Khilko SN, Corr M, Boyd LF, Lees A, Inman JK, Margulies DH. Direct detection of major histocompatibility complex class I binding to antigenic peptides using surface plasmon resonance. Peptide immobilization and characterization of binding specificity. J Biol Chem. 1993;268:15425–15434. [PubMed] [Google Scholar]
- 23.Berman HM, Bhat TN, Bourne PE, Feng Z, Gilliland G, Weissig H, Westbrook J. The Protein Data Bank and the challenge of structural genomics. Nat Struct Biol. 2000;7(Suppl):957–959. doi: 10.1038/80734. [DOI] [PubMed] [Google Scholar]
- 24.Shiroishi M, Kajikawa M, Kuroki K, Ose T, Kohda D, Maenaka K. Crystal structure of the human monocyte-activating receptor, “Group 2” leukocyte Ig-like receptor A5 (LILRA5/LIR9/ILT11). J Biol Chem. 2006;281:19536–19544. doi: 10.1074/jbc.M603076200. [DOI] [PubMed] [Google Scholar]
- 25.Horii K, Kahn ML, Herr AB. Structural basis for platelet collagen responses by the immune-type receptor glycoprotein VI. Blood. 2006;108:936–942. doi: 10.1182/blood-2006-01-010215. [DOI] [PubMed] [Google Scholar]
- 26.Kadler KE, Baldock C, Bella J, Boot-Handford RP. Collagens at a glance. J Cell Sci. 2007;120:1955–1958. doi: 10.1242/jcs.03453. [DOI] [PubMed] [Google Scholar]
- 27.Franzke CW, Tasanen K, Schumann H, Bruckner-Tuderman L. Collagenous transmembrane proteins: collagen XVII as a prototype. Matrix Biol. 2003;22:299–309. doi: 10.1016/s0945-053x(03)00051-9. [DOI] [PubMed] [Google Scholar]
- 28.Leitinger B, Hohenester E. Mammalian collagen receptors. Matrix Biol. 2007;26:146–155. doi: 10.1016/j.matbio.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Knight CG, Morton LF, Onley DJ, Peachey AR, Ichinohe T, Okuma M, Farndale RW, Barnes MJ. Collagen-platelet interaction: Gly-Pro-Hyp is uniquely specific for platelet Gp VI and mediates platelet activation by collagen. Cardiovasc Res. 1999;41:450–457. doi: 10.1016/s0008-6363(98)00306-x. [DOI] [PubMed] [Google Scholar]
- 30.Barrow AD, Trowsdale J. The extended human leukocyte receptor complex: diverse ways of modulating immune responses. Immunol Rev. 2008;224:98–123. doi: 10.1111/j.1600-065X.2008.00653.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure Legend:
Supplemental Figure S1. Location of R65 of LAIR-1 is consistent with a collagen docking site previously identified on GPVI. Excluding LAIR-2, the two best matches of LAIR-1 amino acid sequence to proteins in the rcsb structural database (23) as determined by a BLASTP query were LILRA5 (pdb id: 2D3V (24) and GPVI (pdb id: 2GI7 (25)). A) Alignment of D1 domains of GPVI and LILRA5 with the putative Ig extracellular domain of LAIR-1, numbered according to LAIR-1. Alignment with the LAIR-2 sequence is also shown. Identical and conserved residues for the four receptors (red) or for three of them (blue) are shown. LAIR-1 R65, GPVI S44 and LILRA5 S42 are circled. B) and C) The LILRA5 structure (magenta) was aligned with chain A of the GPVI dimer (cyan) with PyMOL (http://www.pymol.org) and displayed in PyMOL as well. The residues of LILRA5 (S42) and GPVI (S44) that align with R65 of LAIR-1 are shown as magenta (S42) and cyan (S44) spheres in (B) and (C). Panels B and C are approximate 90° rotations from each other as indicated. The amino terminal domain 1 (D1) and domain 2 (D2) are labelled. D) The D1 domain of GPVI is colored according to electrostatic calculation with blue basic, and red acidic, as performed in Pymol. E) Surface representation of the collagen-related peptide (GPO)n binding site of D1 domain of GPVI as determined by Horii et al. (2006) is indicated, and the surface of S44 on GPVI, at the base of the putative (GPO)n binding site is shown in yellow.