Abstract
Objectives
Active smoking has a well-documented role in the etiology of inflammatory bowel disease (IBD), but the role of passive smoking has been unclear. This meta-analysis examined the relationship between prenatal smoke exposure and childhood passive smoke exposure and the development of IBD.
Methods
We searched the MEDLINE and EMBASE databases to identify observational studies regarding the relationship between prenatal and/or childhood passive smoke exposure and the development of Crohn’s disease (CD) and/or ulcerative colitis (UC). Pooled odds ratios were calculated for each relationship.
Results
A total of 534 and 699 potential studies were identified from MEDLINE and EMBASE, respectively, of which 13 met all of our inclusion criteria. Overall we did not observe a positive relationship between childhood passive smoke exposure and CD (OR, 1.10; 95% CI, 0.92–1.30) or UC (OR, 1.01; 95% CI, 0.85–1.20). Likewise, we did not observe an association between prenatal smoke exposure and CD (OR, 1.10; 95% CI, 0.67–1.80), or prenatal smoke exposure and UC (OR, 1.11; 95% CI, 0.63–1.97).
Conclusions
Our meta-analysis suggests that there is not a strong association between childhood passive smoke exposure and the development of CD. We found no evidence that childhood passive smoke exposure exerts a protective effect against UC, as is the case in active smoke exposure. Heterogeneity among the small number of studies limited the ability to draw conclusions about prenatal smoke exposure.
Keywords: Inflammatory bowel disease, Crohns disease, colitis, passive smoking, environmental exposure
Introduction
The dramatic increase in the incidence of inflammatory bowel disease (IBD), especially Crohn’s disease (CD) in developed countries over the second half of the 20th century highlights the important role that environmental factors play in the pathogenesis of IBD (1). Cigarette smoking is one of the most well documented environmental risk factors for IBD. Active smoking increases the risk of developing CD and is protective against the development of ulcerative colitis (UC) (2).
In 1982, Harries et al were the first to suggest that a relationship might also exist between passive smoke exposure and development of IBD, although their study did not focus on children (3). Since then, several case-control studies have addressed that possibility in regards to two specific categories of passive smoke exposure; prenatal exposure due to maternal smoking during pregnancy and passive smoke exposure during childhood (4–16). Conclusions drawn from these studies have been mixed. As both of these exposures are relatively common in the US, with 9–34% of pregnant women smoking and with 35% of children exposed to passive smoke at home, it is important to clarify any relationship that might exist between them and the development of IBD (17, 18).
Meta-analysis is a method of pooling data from multiple studies in order to draw more definitive conclusions from a body of research. In 2000, The Meta-analysis Of Observational Studies in Epidemiology Group published guidelines for the reporting of meta-analyses of observational studies. Using these guidelines as a framework, we performed a meta-analysis of existing observational studies that explored the association between prenatal smoke exposure and/or passive smoke exposure during childhood and the subsequent development of IBD. Based on the well-established and divergent effects of active smoking on risk for IBD, we hypothesized that passive smoke exposure in utero and during childhood would similarly, although perhaps to a lesser degree, increase risk for CD development while decreasing risk for development of UC.
Methods
Study Selection
We performed a search of the MEDLINE database for articles published between January 1950 and September 2007, using Ovid as a search engine, and restricting our search to English language publications involving humans. The following key words were used: ulcerative colitis, Crohn’s disease, inflammatory bowel disease, maternal smoking, passive smoking, smoking, tobacco smoke, environmental risk factors, perinatal risk factors, fetal risk factors, maternal perinatal behaviors. These key words were linked by Boolean operators (and, or) to refine the search. We subsequently repeated this search using identical search criteria in the EMBASE database. If a study could not be included/excluded based on title and/or abstract, the full text article was reviewed. Also, the reference lists of any studies meeting inclusion criteria as well as from pertinent review articles (4–16, 19–23) were reviewed manually to identify additional relevant publications.
Inclusion/Exclusion Criteria
Abstracts and full text articles identified using the search algorithm were retrieved and evaluated for the presence of the following inclusion criteria:
Observational studies that evaluate the association between prenatal and/or passive smoke exposure during childhood and the development of inflammatory bowel disease later in life
Studies include a control group (composed of community controls, hospital controls, clinic controls, or some combination thereof)
-
Studies explicitly define prenatal/childhood passive smoke with the following:
passive smoke exposure during childhood defined by age criteria OR by exposure in parental home
prenatal smoke exposure defined by maternal smoking during some part of pregnancy OR by maternal smoking at birth
Studies are published as a full article in English language
One author (D.J.) performed the initial MEDLINE search, the results of which were scrutinized by 2 independent reviewers (D.J. and M.B.) to identify studies for inclusion. One author (D.J.) performed the duplicate EMBASE search. Any discrepancies between the two reviewers were resolved by consultation with the senior author (J.L.).
Contact with Authors
When studies were identified with pertinent data not included in the publication (for example, if an estimate of effect was not included because the identified relationship was not statistically significant), attempts were made to contact the authors to procure the missing data. Authors of 4 of the included studies were contacted (6–9). The investigators from the studies published by Baron et al in 2005, Bernstein et al in 2006, and Feeney at el in 2002 supplied their data regarding prenatal and/or childhood passive smoke exposure and the development of CD and/or UC that had not been reported in the publication. The most adjusted estimates of effect provided by these investigators were utilized in our pooled analysis. When only the raw data were provided, we used these data to calculate unadjusted estimates of effect for inclusion in our meta-analysis. We were unable to obtain the results for two of our subgroup analyses (subgroup of data limited to IBD diagnosed during childhood and subgroup of data limited to maternal source of passive smoke exposure) from the study performed by Eliakim et al in 2000, however this study was included in other analyses where data were available.
Data Abstraction
Data were abstracted by 2 independent investigators (D.J and M.O.) using a standardized data collection form. Discrepancies between the two abstractors were resolved by discussion, and if necessary by re-evaluation by the senior author (J.L.). There was no blinding in the collection of data.
Rules for choosing among several estimates of effect
Many of the studies meeting inclusion criteria for this meta-analysis reported more than one estimate of effect in the results. For this reason, we developed a priori criteria for choosing among several estimates of effect. In all cases, the most adjusted estimate of effect was used in the pooling of data. If it was unclear which was most adjusted, the estimate of effect presented in the abstract was used. If separate estimates of effect were calculated for males and females as well as for both genders combined, the gender-combined estimate of effect was used. For childhood passive smoke exposure, only estimates of effect based on exposure during childhood at home were used (estimates of effect based on exposures at other locations were not used). If separate estimates of effect were calculated for individual sources of passive smoke exposure (mother, father, other) as well as for overall passive smoke exposure, the most inclusive estimate of effect was used. If separate estimates of effect were calculated for separate control groups (community vs. hospital controls) the estimate of effect based on comparison with the community controls was used, as we felt this group of controls would be more representative of the general population. If estimates of effect were presented stratified by active smoking status, the estimate of effect calculated for non-smokers was used. However, if an overall estimate of effect for all categories of active smoking status combined was presented, this estimate was used in the pooling of data. For prenatal smoke exposure, only estimates of effect calculated for maternal smoking during pregnancy or maternal smoking at birth were used. Estimates of effect based on other sources of passive smoke exposure (paternal, other household member) during pregnancy or at birth were not used. Any dose response data or estimates of effect calculated separately for groups with a longer period of time of smoke exposure were recorded separately, but not included in the pooling of data.
Rating study quality
Studies were required to meet all 3 of the following predetermined criteria to be defined as “high quality.”
Controls are community-based controls selected randomly by investigators, and not by cases.
Adequate definition of cases: CD/UC diagnosis confirmed by investigator/medical chart and not based solely on self report or some other subjective method of report.
Source of exposure data: information regarding classification of maternal smoking during pregnancy/childhood passive smoke exposure collected from parents or other older relative of study subject, or collected from medical charts/records from the time of exposure.
Statistical Analyses
Meta-analysis was performed according to the Meta-analysis Of Observational Studies in Epidemiology guidelines (24). The pooled OR and its 95% confidence interval were calculated using the DerSimonian and Laird method, which is based on a random effects model (25). This model does not assume homogeneity between studies in terms of methodological or clinical characteristics, and is overall a more conservative approach than the fixed effects model.
We tested for heterogeneity among studies using the chi-squared test as well as the I2 test. The I2 test describes the percentage of the variability in the estimates of effect that is due to heterogeneity rather than chance, with a value greater than 50% being considered substantial heterogeneity (26, 27). We also performed Begg and Egger tests to evaluate for the presence of publication bias (28, 29). All analyses were conducted using StataIC 10 (Stata Corp, College Station, TX).
Subgroup Analyses
Subgroup analyses were performed to evaluate the robustness of the observed associations and to identify sources of heterogeneity. Because so few studies met all three of our predetermined quality criteria, we expanded the inclusion criteria for this subgroup analysis in a second definition to include studies that met at least 2 of the 3 quality criteria. Because several previous studies from Israel have not found the typical relationship between active smoking and CD, one subgroup analysis excluded studies performed in Israeli populations (8, 30, 31). Another analysis was limited to studies where all participants were diagnosed with IBD during childhood. Also, because it is well documented that the mother is the most important source for passive smoke exposure during childhood, we performed a subgroup analysis including only estimates of effect based on maternal smoking, and another analysis including only estimates not based exclusively on maternal smoking (32–36). In addition, we performed subgroup analyses based on the source of the case and control populations and stratified on whether the study was performed in U.S. populations or non-U.S. populations.
Results
Search Results
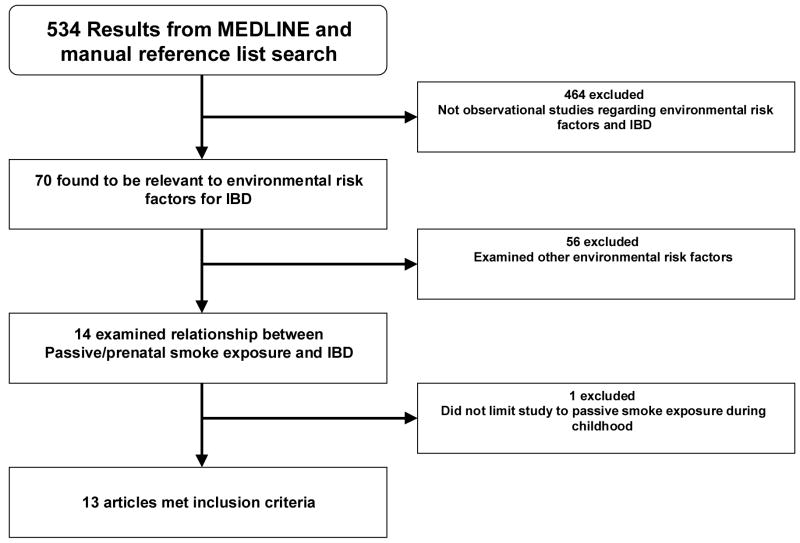
Our search strategy in MEDLINE yielded a total of 534 studies, of which 70 were relevant to our general topic (Figure 1). Of those, 56 were excluded because they did not examine passive smoke exposure as an environmental risk factor for CD and/or UC. Finally, one of the remaining 14 studies was excluded because, although passive smoke exposure was studied as a risk factor, this exposure was not limited to that during childhood. The remaining 13 studies met all of the inclusion criteria (4–16). Our duplicate search of the EMBASE database yielded a total of 699 studies (including the13 studies identified by our original search strategy and without any additional studies for inclusion). All of the studies utilized a case-control design. Some, but not all studies limited case populations to individuals diagnosed with CD or UC during childhood.
Figure 1.
Selection of Studies for Inclusion
Childhood passive smoke exposure and CD
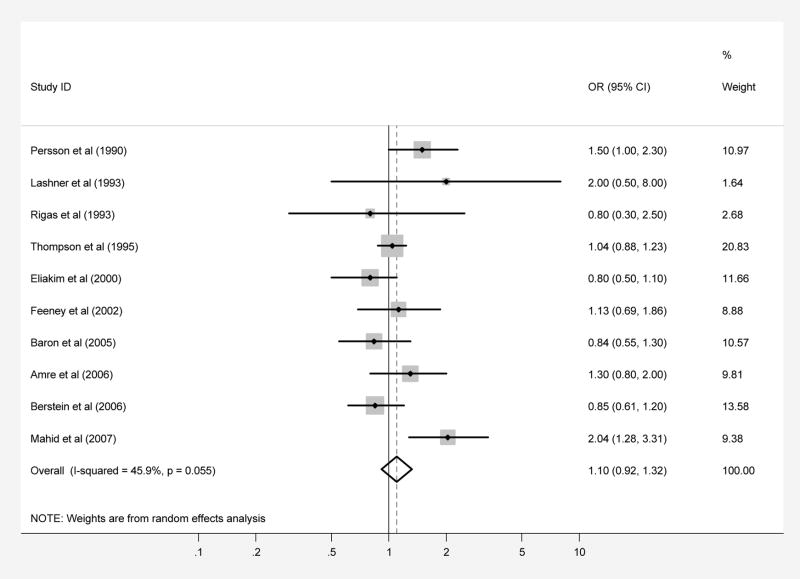
Ten case-control studies regarding exposure to passive smoke during childhood included a total of 3,337 patients with CD and 3,955 controls (Tables 1 and 2). When the results from all of the studies were combined, passive smoke exposure was not associated with CD (pooled OR 1.10, 95% CI 0.92–1.30; test of heterogeneity p=0.06, I2 =45.9%) (Figure 2A). There was no evidence of publication bias in this overall analysis (Begg p-value=0.28, Egger p-value=0.50). The results of subgroup analyses yielded OR’s similar in magnitude and direction to the overall analysis (Table 3). Only the subgroup analysis based on estimates effect calculated for “mother” as the only passive smoke exposure source yielded a statistically significant association, with OR 1.31 (95% CI, 1.07–1.60; test of heterogeneity p=0.82, I2 =0.0%).
Table 1.
Childhood Passive Smoke Exposure Study Characteristics
| Author, year of publication, location | Case population | Sample size |
Source of Controls | Controls identified by | Study limited to IBD dx during childhood? | IBD dx confirmed by investigator or medical chart? | Source of smoke exposure information | Definition of Childhood Passive Smoke Exposure | |
|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | ||||||||
| Persson et al, 1990, Stockholm | CD and UC | 184 CD 181 UC |
390 | community | investigators | no | yes | study subjects | exposure to “regular” smokers in the household from ages 0–15 |
| Sandler et al, 1992, North Carolina | UC only | 172 UC | 131 | community | cases | no | yes | study subjects | mother or father smoked in the home on a regular basis ages 0–15 |
| Lashner et al, 1993, Chicago | CD and UC | 39 CD 33 UC |
72 | community | cases | yes | yes | parents of study subjects | smoking of atleast 5 cigs/day by parent or sibling in the house at time of symptom onset (<18 years of age) |
| Rigas et al, 1993, New York | CD and UC | 68 CD 39 UC |
202 | clinic | investigators | yes | yes | medical records | maternal smoking during childhood |
| Thompson et al, 1995, United Kingdom | CD and UC | 1489 CD 790 UC |
1489 CD 790 UC |
community | cases | no | no description | study subjects | regular smoking by either parent during childhood |
| Eliakim et al, 2000, Israel | CD and UC | 261 CD 273 UC |
430 (478) | community (and clinic) | cases | no | yes | study subjects | passive smoking in parental home |
| Feeney et al, 2002, United Kingdom | CD and UC | 139 CD 137 UC |
139 CD 137 UC |
clinic | investigators | no | yes | study subjects | one or both parents regular smoker (at least 1 cigarette per day for at least 3 months) during childhood |
| Baron et al, 2005, Northern France | CD and UC | 222 CD 60 UC |
222 CD 60 UC |
community | investigators | yes | yes | study subjects and study subject mothers | smoking at home (or by nurse during infancy) between birth and IBD symptom onset before age 17 |
| Amre et al, 2006, Montreal | CD only | 194 CD | 194 | clinic | investigators | yes | yes | study subject mothers | maternal smoking during childhood (<20 years of age) |
| Bernstein et al, 2006, Manitoba | CD and UC | 364 CD 217 UC |
433 | community | investigators | no | yes | study subjects | family member smoking at leat 1 cigarette/day during childhood |
| Mahid et al, 2007, Kentucky | CD and UC | 377 CD 253 UC |
384 | clinic | investigators | no | yes | study subjects | mother, father, or other household member smoking |
Table 2.
Childhood Passive Smoke Exposure Study Data
| Author, year of publication, location | controls | cases | case-control matching variables | adjustment variables | OR (95% CI) | ||
|---|---|---|---|---|---|---|---|
| exposed | unexposed | exposed | unexposed | ||||
| Persson et al, 1990, Stockholm | 179 | 122 | 105 CD 84 UC |
44 CD 59 UC |
age, sex | age, smoking, sex | CD 1.5(1.0–2.3) UC 0.98(0.6–1.5) |
| Sandler et al, 1992, North Carolina | 53 | 19 | 53 UC | 38 UC | age, sex, race | sex, education, age at symptom onset, year of symptom onset | UC 0.5 (0.25–1.0) |
| Lashner et al, 1993, Chicago | 26 | 46 | 17 CD 17 UC |
22 CD 16 UC |
age, sex | adjustment variables not reported | CD 2.0 (0.5–8.0) UC 1.71(0.67–4.35) |
| Rigas et al, 1993, New York | 15 | 59 | 11 CD 5 UC |
34 CD 14 UC |
none | breast-feeding, maternal age at birth, birthdate seasonality, sex, age at diagnosis, sibship size, race, birthplace | CD 0.8 (0.3–2.5) UC 1.4(0.4–5.1) |
| Thompson et al, 1995, United Kingdom | 1013 CD 537 UC |
476 CD 253 UC |
1093 CD 605 UC |
491 CD 259 UC |
age, sex, ethnic origin | none | CD 1.04(0.88–1.23) UC 1.1(0.86–1.41) |
| Eliakim et al, 2000, Israel | 106 CD 115 UC |
84 CD 82 UC |
111 CD 118 UC |
112 CD 111 UC |
age, sex, Jewish community group, education status | none | CD 0.8(0.5–1.1) UC 0.7(0.5–1.1) |
| Feeney et al, 2002, United Kingdom | 91 | 58 | 96 CD 98 UC |
54 CD 45 UC |
age, sex | none | CD 1.13(0.69–1.86) UC 1.39(0.83–2.32) |
| Baron et al, 2005, Northern France | 156 CD 37 UC |
66 CD 23 UC |
144 CD 44 UC |
78 CD 16 UC |
age, sex, geographic location | CD: none UC: socio-educational level of mother and father |
CD 0.84(0.55–1.30) UC 0.69(0.3–1.58) |
| Amre et al, 2006, Montreal | 48 | 146 | 58 CD | 136 CD | calendar time of diagnosis, place | none | CD 1.3(0.8–2.0) |
| Bernstein et al, 2006, Manitoba | 184 | 235 | 180 CD 104 UC |
170 CD 113 UC |
age, sex | none | CD 0.85(0.61–1.20) UC 1.05(0.70–1.59) |
| Mahid et al, 2007, Kentucky | 183 | 59 | 217 CD 135 UC |
36 CD 42 UC |
none | CD: age UC: none |
CD 2.04(1.28–3.31) UC 1.04(0.66–1.64) |
Figure 2.
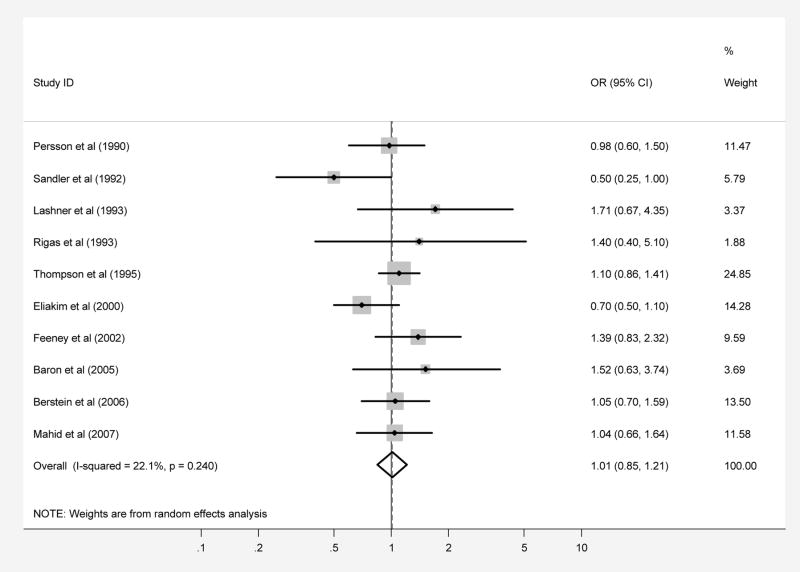
Patients with CD (part A) and UC (part B) were compared with controls regarding exposure to passive smoke during childhood. Each point on the figure represents an OR. The diamond represents the pooled estimate of effect, as calculated according to the random effects model. The error bars display the 95% CI for each estimate of effect. The contributing weight of each estimate of effect to the pooled analysis is represented by the size of the grey box around the point estimate of effect. Estimates of effect are considered significant if the 95% CI does not include 1.0.
Table 3.
Prenatal Smoke Exposure Study Characteristics
| Author, year of publication, location | Case population | Sample size |
Source of Controls | Controls identified by | Study limited to IBD dx during childhood? | IBD dx confirmed by investigator or medical chart? | Source of smoke exposure information | Definition of Prenatal Smoke Exposure | |
|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | ||||||||
| Lashner et al, 1993, Chicago | CD and UC | 39 CD 33 UC |
72 | community | cases | yes | yes | parents of study subjects | maternal smoking at birth |
| Gruber et al, 1996, New York | CD only | 54 CD | 90 | community | cases | yes | not described | study subject mothers | maternal smoking during pregnancy |
| Baron et al, 2005, Northern France | CD and UC | 222 CD 60 UC |
222 CD 60 UC |
community | investigators | yes | yes | study subjects and study subject mothers | maternal smoking during pregnancy |
| Aspberg et al, 2006, Sweden | CD and UC | 172 CD 307 UC |
1434829 | community | investigators | yes | yes | medical records | maternal smoking of any amount of cigarettes during 1st trimester |
| Mahid et al, 2007, Kentucky | CD and UC | 377 CD 253 UC |
384 | clinic | investigators | no | yes | study subjects | maternal smoking during pregnancy |
Childhood passive smoke exposure and UC
Ten case-control studies regarding passive smoke exposure during childhood included a total of 2,155 patients with UC and 3,029 controls (Tables 1 and 2). When the results from all of the studies were combined, no association was observed between childhood passive smoke exposure and ulcerative colitis, with a pooled OR of 1.01 (95% CI, 0.85–1.20; test of heterogeneity p=0.24, I2 =22.1%), with no evidence of publication bias (Begg p-value=0.59, Egger p-value=0.76) (Figure 2B). These results suggest that, in contrast to the inverse association between active smoking and UC development, there is no significant association between childhood passive smoke exposure and development of ulcerative colitis. Results of all subgroup analyses yielded OR’s similar in magnitude and direction to the overall analysis with 95% CI overlapping 1.0, while none of the analyses showed evidence of significant statistical heterogeneity (Table 4).
Table 4.
Prenatal Smoke Exposure Study Data
| Author, year of publication, location | controls | cases | case-control matching variables | adjustment variables | OR (95% CI) | ||
|---|---|---|---|---|---|---|---|
| exposed | unexposed | exposed | unexposed | ||||
| Lashner et al, 1993, Chicago | 34 | 38 | 27 CD 21 UC |
12 CD 12 UC |
age, sex | not given | CD 2.76(0.88–8.66) UC 1.71(0.67–4.35) |
| Gruber et al, 1996, New York | 33 | 57 | 14 CD | 40 CD | age | none | CD 0.604(0.29–1.3) |
| Baron et al, 2005, Northern France | 21 CD 5 UC |
201 CD 55 UC |
22 CD 5 UC |
200 CD 55 UC |
age, sex, geographic location | socio-educational level of mother and father | CD 0.93(0.49–1.78) UC 0.79(0.19–3.26) |
| Aspberg et al, 2006, Sweden | 274390 | 1069229 | 30 CD 50 UC |
106 CD 214 UC |
none | maternal age, education, parity | CD 0.73(0.58–0.94) UC 0.70(0.56–0.86) |
| Mahid et al, 2007, Kentucky | 44 | 175 | 64 CD 39 UC |
146 CD 107 UC |
none | CD: age UC: none |
CD 1.72(1.1–2.71) UC 1.53(0.93–2.49) |
Prenatal smoke exposure and CD
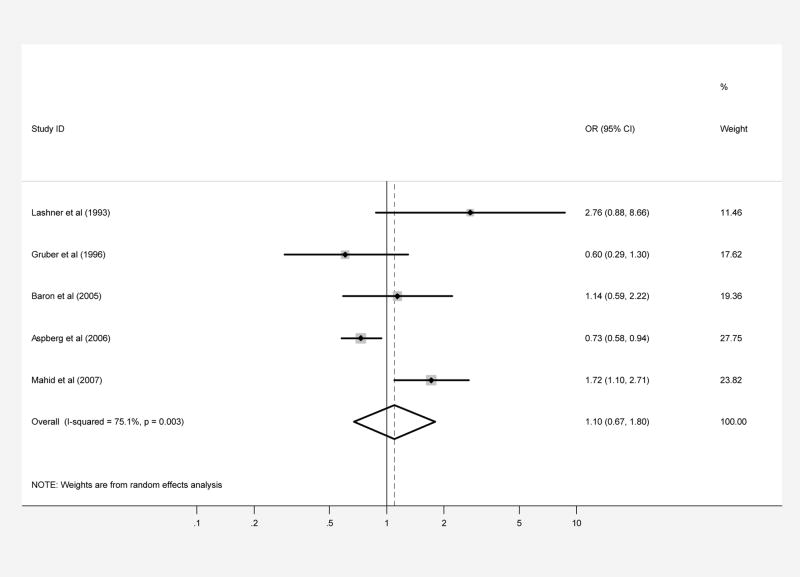
Five case-control studies regarding exposure to maternal smoking during pregnancy included a total of 864 patients with CD and 1,344, 387 controls (1,343,619 were from a single study) (Tables 5 and 6). The pooled OR for this analysis was 1.10 (95% CI, 0.67–1.80) but with significant heterogeneity (test of heterogeneity p=0.003, I2 =75.1%). There was no evidence of publication bias (Begg p-value=0.81, Egger p-value=0.33) (Figure 3A).
Table 5.
Subgroup Analyses for Childhood Passive Smoke Exposure and CD
| Subgroup | # Studies included (references) | Pooled OR (95% CI) | Test for heterogeneity | I2 |
|---|---|---|---|---|
| All studies | 10 (4, 6–9, 11–14, 16) | 1.10(0.92–1.32) | p=0.06 | 45.9% |
| at least 2/3 quality criteria | 6 (4,6,7,11,13,14) | 1.07(0.83–1.38) | p=0.20 | 31.2% |
| Israeli study excluded | 9 (4, 6–7, 9, 11–14, 16) | 1.15 (0.94–1.39) | p=0.07 | 44.7% |
| US population | 3 (11, 12, 14) | 1.66 (0.96–2.86) | p=0.28 | 20.9% |
| non-US population | 7 (4, 6–9, 13, 16) | 1.02 (0.88–1.19) | p=0.24 | 24.4% |
| IBD dx in childhood | 4 (4, 6, 11, 14) | 1.04 (0.78–1.40) | p=0.40 | 0.0% |
| Community Controls | 6 (6–8, 11, 13, 16) | 1.00 (0.83–1.20) | p=0.18 | 33.8% |
| Clinic controls | 4 (4, 9, 12, 14) | 1.17 (0.85–1.62) | p=0.70 | 0.0% |
| Controls identified by investigators | 7 (4, 6, 7, 9, 12–14) | 1.17 (0.90–1.52) | p=0.04 | 53.9% |
| mother smoker | 4 (4, 7, 12, 14) | 1.31(1.07–1.60) | p=0.82 | 0.0% |
Table 6.
Subgroup Analyses for Childhood Passive Smoke Exposure and UC
| Subgroup | # Studies included (references) | Pooled OR (95% CI) | Test for heterogeneity | I2 |
|---|---|---|---|---|
| All studies | 10 (6–9, 11–16) | 1.01 (0.85–1.20) | p=0.24 | 22.1% |
| at least 2/3 quality criteria | 5 (6, 7, 11, 13, 14) | 1.12 (0.85–1.47) | p=0.78 | 0.0% |
| US population | 3 (11, 12, 14) | 0.97 (0.58–1.61) | p=0.15 | 46.3% |
| non-US population | 7 (6–9, 13–15) | 1.03 (0.86–1.24) | p=0.30 | 17.4% |
| IBD dx in childhood | 3 (6, 11, 14) | 1.56 (0.88–2.78) | p=0.97 | 0.0% |
| Community Controls | 7 (6–8, 11, 13, 15, 16) | 0.96 (0.76–1.21) | p=0.14 | 37.9% |
| Clinic controls | 3 (9, 12, 14) | 1.19 (0.86–1.66) | p=0.69 | 0.0% |
| Controls identified by investigators | 6 (6, 7, 9, 12–14) | 1.12 (0.90–1.39) | p=0.88 | 0.0% |
| mother smoker | 3 (7, 12,14) | 1.26(0.97–1.62) | p=0.829 | 0.0% |
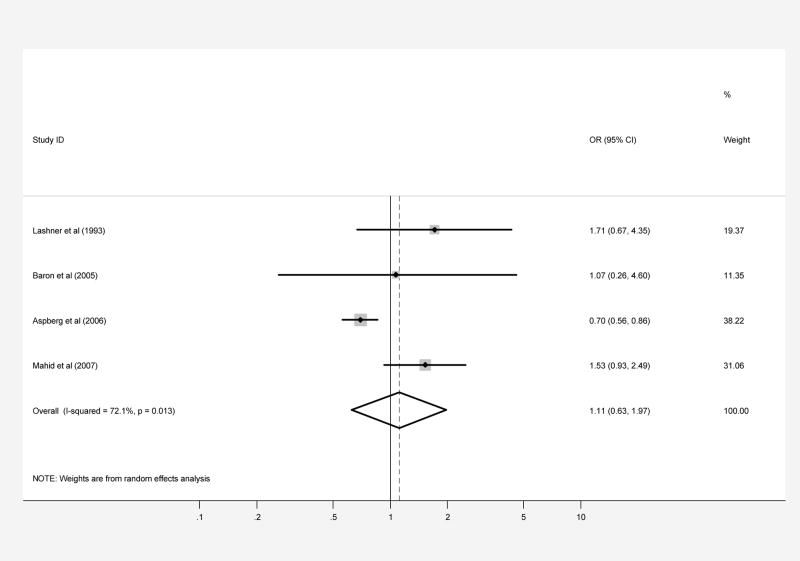
Figure 3.
Patients with CD (part A) and UC (part B) were compared with controls regarding prenatal smoke exposure secondary to maternal smoking during pregnancy. Each point on the figure represents an OR. The diamond represents the pooled estimate of effect, as calculated according to the random effects model. The error bars display the 95% CI for each estimate of effect. The contributing weight of each estimate of effect to the pooled analysis is represented by the size of the grey box around the point estimate of effect. Estimates of effect are considered significant if the 95% CI does not include 1.0.
In subgroup analyses, results were similar in magnitude and direction to the overall analysis, and most showed evidence of significant heterogeneity between studies, suggesting that grouping of these studies for a pooled analysis may not be appropriate (Table 7).
Table 7.
Subgroup Analyses for Prenatal Smoke Exposure and CD
| Subgroup | # Studies included (references) | Pooled OR (95% CI) | Test for heterogeneity | I2 |
|---|---|---|---|---|
| All studies | 5 (5, 6, 10–12) | 1.10 (0.67–1.80) | p=0.003 | 75.1% |
| at least 2/3 quality criteria | 3 (5, 6, 11) | 1.09 (0.58–2.05) | p=0.05 | 67.5% |
| US population | 3 (10–12) | 1.35 (0.61–3.02) | p=0.03 | 71.3% |
| non-US population | 2 (5, 6) | 0.82 (0.56–1.19) | p=0.22 | 34.8% |
| IBD dx in childhood | 4 (5, 6, 10, 11) | 0.92 (0.58–1.46) | p=0.08 | 55.1% |
| community controls | 4 (5, 6, 10, 11) | 0.92 (0.58–1.46) | p=0.08 | 55.1% |
| Controls identified by investigators | 3 (5, 6, 12) | 1.10 (0.61–1.99) | p=0.004 | 82.3% |
Prenatal smoke exposure and UC
Four case-control studies regarding exposure to maternal smoking during pregnancy included a total of 653 patients with UC and 1,344, 225 controls (1,343,619 were from a single study) (Table 5 and 6). When the results from all of the studies were combined, the pooled OR was 1.11 (95% CI, 0.63–1.97; test of heterogeneity p=0.01, I2 =72.1%), suggesting that there is no significant overall association between prenatal smoke exposure and the development of UC, but again with significant heterogeneity between studies (Figure 3B). There was no evidence of publication bias (Begg p-value=1.0, Egger p-value=0.26).
In subgroup analyses, the analysis limited to studies performed with US populations yielded a positive significant association (without evidence significant heterogeneity between studies) while that limited to non-US populations yielded a significant negative association (without evidence of significant heterogeneity between studies). None of the remaining subgroup analyses yielded a significant association (Table 8).
Table 8.
Subgroup Analyses for Prenatal Smoke Exposure and UC
| Subgroup | # Studies included (references) | Pooled OR (95% CI) | Test for heterogeneity | I2 |
|---|---|---|---|---|
| All studies | 4 (5, 6, 11, 12) | 1.11 (0.63–1.97) | p=0.01 | 72.1% |
| at least 2/3 quality criteria | 3 (5, 6, 11) | 0.93 (0.51–1.67) | p=0.17 | 44.3% |
| US population | 2 (11, 12) | 1.57 (1.01–2.42) | p=0.84 | 0.0% |
| non-US population | 2 (5, 6) | 0.71 (0.57–0.87) | p=0.57 | 0.0% |
| IBD dx in childhood | 3 (5, 6, 11) | 0.93 (0.51–1.67) | p=0.17 | 44.3% |
| community controls | 3 (5, 6, 11) | 0.93 (0.51–1.67) | p=0.17 | 44.3% |
| Controls identified by investigators | 3 (5, 6, 12) | 1.00 (0.53–1.90) | p=0.02 | 75.9% |
Discussion
The role of active smoking in the pathogenesis of IBD is well-documented (3, 13, 37–42). A recent meta-analysis demonstrated significant positive associations with CD for current smoking (OR 1.76, 95% CI 1.40–2.22) and ever smoking (OR 1.61, 95% CI 1.27–2.03), while current smoking had a protective effect on the development of UC (OR 0.58, 95% CI 0.45–0.75). As expected, former smoking increased the risk for UC (OR 1.79, 95% CI 1.37–2.34) (2).
We hypothesized that the roles of prenatal smoke exposure and childhood passive smoke exposure in the development of IBD would mirror that of active smoke exposure in promoting the development of CD while protecting against UC. Alternatively, prenatal and childhood passive smoke exposure may increase the risk for UC, if individuals exposed to smoke early in life in effect become “former smokers” when they are removed from the source of passive smoke. The overall pooled OR for the studies examining childhood passive smoke exposure suggest that there is no significant association between this exposure and the development of CD. For studies examining the relationship between childhood passive smoke exposure and the development of UC, the overall pooled OR convincingly demonstrated no significant association (pooled OR 1.01). The small number of studies and high degree of heterogeneity among studies precludes drawing any strong conclusions regarding prenatal smoke exposure and the risk of subsequently developing IBD.
The studies included in this meta-analysis have individually drawn conflicting conclusions regarding the relationship between childhood passive smoke exposure and development of IBD, particularly CD, which was confirmed by the finding of statistical heterogeneity in our overall analysis. There are several possible explanations for the heterogeneity in results: differences in study quality, lack of uniformity in the ethnicity of study populations, differences in methods of case and control identification and selection, differences in control population characteristics (clinic vs. community controls), differences in smoke exposure definitions, and variation in the latency period between the exposure to passive smoke in utero or during childhood and the diagnosis of IBD (with some studies limited to diagnosis during childhood while others examined those diagnosed into their 60s and 70s). .
When we performed subgroup analyses, we were able to isolate subgroups of studies based on predetermined study characteristics that demonstrated less evidence of heterogeneity. The relationship between childhood passive smoke exposure and CD remained consistent in magnitude and direction across these analyses (all with OR>1.0), and in some cases the relationship was strengthened. Of note, we observed a statistically significant positive association between childhood passive smoke exposure and CD when we limited our analysis to data regarding passive smoke exposure from a mother smoking (as opposed to analyses that included other household members as sources of passive smoke). However, the small number of studies and the pooled OR of only 1.31 preclude drawing strong conclusions based on this subgroup analysis.
A dose-response relationship could explain our finding for passive smoke exposure (i.e. ORs greater than unity but smaller than that observed in Mahid’s meta-analysis of active smoking and CD (2)). A dose-response relationship between smoke exposure and risk for CD has been described for active smoking (13, 42). Perhaps passive smoke exposure during childhood is akin to a very low level of active smoke exposure, thus yielding a weaker positive association. Further support of this hypothesis are the limited data that has been published regarding a possible dose-response relationship between passive smoke exposure and development of CD (7, 11). Bernstein et al found a small, but significant positive association between development of CD and childhood passive smoke exposure when the analysis was adjusted for “living longer years with a smoker.” Similarly, Lashner et al found a positive (but not statistically significant) association between development of IBD (analysis included both CD and UC) and childhood passive smoke exposure when the analysis was adjusted for packs smoked/day.
As noted previously, we found a statistically significant positive association between childhood passive smoke exposure and CD when we limited our analysis to data regarding passive smoke exposure from a mother smoking. Importantly, mothers are the primary source for childhood passive smoke exposure in the home (32–36, 43, 44), and maternal smoking accounts for a greater percentage of the variation in urinary cotinine (metabolite of nicotine) levels in children than all other sources combined (43). As such, it is possible that this subgroup analysis represents an analysis based on the highest levels of passive smoke exposure. Our finding of a significant positive association between childhood passive smoke exposure and development of CD in this subgroup provides further support of a dose-response relationship. However, as already described, this result must be interpreted with caution.
We performed similar subgroup analyses for the data regarding childhood passive smoke exposure and the development of UC. All of the analyses demonstrated an effect similar in direction to the overall analysis, with 95% CI overlapping 1.0. Neither the overall pooled analysis nor any of the subgroups showed significant heterogeneity. Thus, it is unlikely that childhood passive smoke exposure significantly alters the risk of development of UC. The dose-response relationship is again a possible explanation for the failure of this meta-analysis to show a protective effect of childhood passive smoke exposure on UC, as a negative dose-response relationship has been documented for active smoking (42, 45). It is possible that the level of childhood passive smoke exposure does not reach a threshold level that is required for the protective effect that has been well documented in active smokers.
For prenatal smoke exposure, the overall analyses for both CD and UC revealed no significant association, but with significant heterogeneity between studies for both diseases. This degree of heterogeneity suggests to us that this small group of studies regarding prenatal smoke exposure and the development of IBD cannot be combined to come up with a definitive conclusion. Studies with larger numbers of participants, and more detailed quantification of the amount of maternal smoking during pregnancy are needed before definitive conclusions can be drawn.
When studies regarding prenatal smoke exposure were divided into two groups: one that included a US study population and the other group including all studies with non-US populations, the results were remarkably different. For UC, division of the studies based on this characteristic led to the observation of a statistically significant positive association between prenatal smoke exposure and development of UC for US populations and a statistically significant negative association for non-US populations. We attribute these surprising results to chance, given the small number of studies available for meta-analysis.
Limitations of this meta-analysis include the relatively small number of studies available for pooling, especially in the case of prenatal smoke exposure. Also, our pooled analyses were hindered by significant heterogeneity between studies, both in study design and in study population characteristics. In some cases we were able to address this heterogeneity by performing subgroup analyses, but in other cases, significant heterogeneity remained even in subgroup analyses, indicating that pooling may have been inappropriate for those studies. In addition, all of the studies were retrospective and therefore possibly subject to recall bias. Finally, none of the studies were limited to offspring of parents with IBD, a population who may be at particularly high risk to develop IBD. A study examining the risk of IBD associated with passive smoke exposure in this already at-risk population may be more likely to find an association.
In conclusion, our findings do not support a strong association between childhood passive smoke exposure and CD. In contrast to the inverse association that has been documented to exist between active smoking and UC, we found no association between childhood passive smoke exposure and UC. It is possible that this can be explained by childhood passive smoke exposure not generating sufficient levels of smoke exposure to execute protective effects against UC. Likewise, we found no evidence of a relationship between prenatal smoke exposure and either CD or UC. However, due to the small number of studies addressing this question and to the significant heterogeneity between studies, the effect of prenatal smoke exposure on the risk of IBD remains unclear. In the future, it would be informative to perform studies that use urinary cotinine levels to quantify levels of passive smoke exposure to further examine these relationships and to conduct studies limited to offspring of parents with IBD.
Acknowledgments
We thank the authors from the studies published by Baron et al in 2005, Bernstein et al in 2006, and Feeney et al in 2002 for providing us with missing data.
Study Support
This study was supported in part by K24-DK078228 and T32 DK007740.
Footnotes
Conflict of Interest: Dr. Lewis reports having served as an expert on behalf of Roche in legal proceedings. None of the other authors have any potential conflicts of interest to declare.
Author Contributions
Guarantor of the submission: James D. Lewis, MD, MSCE
Dr. Lewis, Ms. Jones, and Dr. Osterman designed the study. All authors participated in data collection. Ms. Jones conducted the statistical analyses and created the first draft of the manuscript. All authors participated in editing the manuscript.
References
- 1.Calkins BM, Lilienfeld AM, Garland CF, et al. Trends in incidence rates of ulcerative colitis and Crohn’s disease. Dig Dis Sci. 1984;29:913–20. doi: 10.1007/BF01312480. [DOI] [PubMed] [Google Scholar]
- 2.Mahid SS, Minor KS, Soto RE, et al. Smoking and inflammatory bowel disease: a meta-analysis. Mayo Clin Proc. 2006;81:1462–71. doi: 10.4065/81.11.1462. [DOI] [PubMed] [Google Scholar]
- 3.Harries AD, Baird A, Rhodes J. Non-smoking: a feature of ulcerative colitis. Br Med J (Clin Res Ed) 1982;284:706. doi: 10.1136/bmj.284.6317.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amre DK, Lambrette P, Law L, et al. Investigating the hygiene hypothesis as a risk factor in pediatric onset Crohn’s disease: a case-control study. Am J Gastroenterol. 2006;101:1005–11. doi: 10.1111/j.1572-0241.2006.00526.x. [DOI] [PubMed] [Google Scholar]
- 5.Aspberg S, Dahlquist G, Kahan T, et al. Fetal and perinatal risk factors for inflammatory bowel disease. Acta Paediatr. 2006;95:1001–4. doi: 10.1080/08035250600573151. [DOI] [PubMed] [Google Scholar]
- 6.Baron S, Turck D, Leplat C, et al. Environmental risk factors in paediatric inflammatory bowel diseases: a population based case control study. Gut. 2005;54:357–63. doi: 10.1136/gut.2004.054353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernstein CN, Rawsthorne P, Cheang M, et al. A population-based case control study of potential risk factors for IBD. Am J Gastroenterol. 2006;101:993–1002. doi: 10.1111/j.1572-0241.2006.00381.x. [DOI] [PubMed] [Google Scholar]
- 8.Eliakim R, Reif S, Lavy A, et al. Passive smoking in patients with inflammatory bowel disease: an Israeli multicentre case-control study. Eur J Gastroenterol Hepatol. 2000;12:975–9. doi: 10.1097/00042737-200012090-00002. [DOI] [PubMed] [Google Scholar]
- 9.Feeney MA, Murphy F, Clegg AJ, et al. A case-control study of childhood environmental risk factors for the development of inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2002;14:529–34. doi: 10.1097/00042737-200205000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Gruber M, Marshall JR, Zielezny M, et al. A case-control study to examine the influence of maternal perinatal behaviors on the incidence of Crohn’s disease. Gastroenterol Nurs. 1996;19:53–9. doi: 10.1097/00001610-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Lashner BA, Shaheen NJ, Hanauer SB, et al. Passive smoking is associated with an increased risk of developing inflammatory bowel disease in children. Am J Gastroenterol. 1993;88:356–9. [PubMed] [Google Scholar]
- 12.Mahid SS, Minor KS, Stromberg AJ, et al. Active and passive smoking in childhood is related to the development of inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:431–8. doi: 10.1002/ibd.20070. [DOI] [PubMed] [Google Scholar]
- 13.Persson PG, Ahlbom A, Hellers G. Inflammatory bowel disease and tobacco smoke--a case-control study. Gut. 1990;31:1377–81. doi: 10.1136/gut.31.12.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rigas A, Rigas B, Glassman M, et al. Breast-feeding and maternal smoking in the etiology of Crohn’s disease and ulcerative colitis in childhood. Ann Epidemiol. 1993;3:387–92. doi: 10.1016/1047-2797(93)90066-d. [DOI] [PubMed] [Google Scholar]
- 15.Sandler RS, Sandler DP, McDonnell CW, et al. Childhood exposure to environmental tobacco smoke and the risk of ulcerative colitis. Am J Epidemiol. 1992;135:603–8. doi: 10.1093/oxfordjournals.aje.a116339. [DOI] [PubMed] [Google Scholar]
- 16.Thompson NP, Pounder RE, Wakefield AJ. Perinatal and childhood risk factors for inflammatory bowel disease: a case-control study. Eur J Gastroenterol Hepatol. 1995;7:385–90. [PubMed] [Google Scholar]
- 17.Ebrahim SH, Floyd RL, Merritt RK, 2nd, et al. Trends in pregnancy-related smoking rates in the United States, 1987–1996. JAMA. 2000;283:361–6. doi: 10.1001/jama.283.3.361. [DOI] [PubMed] [Google Scholar]
- 18.Schuster MA, Franke T, Pham CB. Smoking patterns of household members and visitors in homes with children in the United States. Arch Pediatr Adolesc Med. 2002;156:1094–100. doi: 10.1001/archpedi.156.11.1094. [DOI] [PubMed] [Google Scholar]
- 19.Krishnan A, Korzenik JR. Inflammatory bowel disease and environmental influences. Gastroenterol Clin North Am. 2002;31:21–39. doi: 10.1016/s0889-8553(01)00003-6. [DOI] [PubMed] [Google Scholar]
- 20.Danese S, Sans M, Fiocchi C. Inflammatory bowel disease: the role of environmental factors. Autoimmun Rev. 2004;3:394–400. doi: 10.1016/j.autrev.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Birrenbach T, Bocker U. Inflammatory bowel disease and smoking: a review of epidemiology, pathophysiology, and therapeutic implications. Inflamm Bowel Dis. 2004;10:848–59. doi: 10.1097/00054725-200411000-00019. [DOI] [PubMed] [Google Scholar]
- 22.Cosnes J. Tobacco and IBD: relevance in the understanding of disease mechanisms and clinical practice. Best Pract Res Clin Gastroenterol. 2004;18:481–96. doi: 10.1016/j.bpg.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Thomas GA, Rhodes J, Green JT. Inflammatory bowel disease and smoking--a review. Am J Gastroenterol. 1998;93:144–9. doi: 10.1111/j.1572-0241.1998.00144.x. [DOI] [PubMed] [Google Scholar]
- 24.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 25.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 26.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 27.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta- analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 30.Reif S, Lavy A, Keter D, et al. Lack of association between smoking and Crohn’s disease but the usual association with ulcerative colitis in Jewish patients in Israel: a multicenter study. Am J Gastroenterol. 2000;95:474–8. doi: 10.1111/j.1572-0241.2000.01771.x. [DOI] [PubMed] [Google Scholar]
- 31.Reif S, Klein I, Arber N, et al. Lack of association between smoking and inflammatory bowel disease in Jewish patients in Israel. Gastroenterology. 1995;108:1683–7. doi: 10.1016/0016-5085(95)90129-9. [DOI] [PubMed] [Google Scholar]
- 32.Cook DG, Whincup PH, Jarvis MJ, et al. Passive exposure to tobacco smoke in children aged 5–7 years: individual, family, and community factors. BMJ. 1994;308:384–9. doi: 10.1136/bmj.308.6925.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greenberg RA, Bauman KE, Glover LH, et al. Ecology of passive smoking by young infants. J Pediatr. 1989;114:774–80. doi: 10.1016/s0022-3476(89)80135-0. [DOI] [PubMed] [Google Scholar]
- 34.Henschen M, Frischer T, Pracht T, et al. The internal dose of passive smoking at home depends on the size of the dwelling. Environ Res. 1997;72:65–71. doi: 10.1006/enrs.1996.3688. [DOI] [PubMed] [Google Scholar]
- 35.Wong GC, Berman BA, Hoang T, et al. Children’s exposure to environmental tobacco smoke in the home: comparison of urine cotinine and parental reports. Arch Environ Health. 2002;57:584–90. doi: 10.1080/00039890209602092. [DOI] [PubMed] [Google Scholar]
- 36.Thaqi A, Franke K, Merkel G, et al. Biomarkers of exposure to passive smoking of school children: frequency and determinants. Indoor Air. 2005;15:302–10. doi: 10.1111/j.1600-0668.2005.00361.x. [DOI] [PubMed] [Google Scholar]
- 37.Somerville KW, Logan RF, Edmond M, et al. Smoking and Crohn’s disease. Br Med J (Clin Res Ed) 1984;289:954–6. doi: 10.1136/bmj.289.6450.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abraham N, Selby W, Lazarus R, et al. Is smoking an indirect risk factor for the development of ulcerative colitis? An age- and sex-matched case-control study. J Gastroenterol Hepatol. 2003;18:139–46. doi: 10.1046/j.1440-1746.2003.02953.x. [DOI] [PubMed] [Google Scholar]
- 39.Boyko EJ, Koepsell TD, Perera DR, et al. Risk of ulcerative colitis among former and current cigarette smokers. New England Journal of Medicine. 1987;316:707–10. doi: 10.1056/NEJM198703193161202. [DOI] [PubMed] [Google Scholar]
- 40.Logan RF, Edmond M, Somerville KW, et al. Smoking and ulcerative colitis. British Medical Journal Clinical Research Ed. 1984;288:751–3. doi: 10.1136/bmj.288.6419.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Silverstein MD, Lashner BA, Hanauer SB. Cigarette smoking and ulcerative colitis: a case-control study. Mayo Clinic Proceedings. 1994;69:425–9. doi: 10.1016/s0025-6196(12)61637-1. [DOI] [PubMed] [Google Scholar]
- 42.Franceschi S, Panza E, La Vecchia C, et al. Nonspecific inflammatory bowel disease and smoking. Am J Epidemiol. 1987;125:445–52. doi: 10.1093/oxfordjournals.aje.a114550. [DOI] [PubMed] [Google Scholar]
- 43.Jordaan ER, Ehrlich RI, Potter P. Environmental tobacco smoke exposure in children: household and community determinants. Arch Environ Health. 1999;54:319–27. doi: 10.1080/00039899909602494. [DOI] [PubMed] [Google Scholar]
- 44.Oddoze C, Dubus JC, Badier M, et al. Urinary cotinine and exposure to parental smoking in a population of children with asthma. Clin Chem. 1999;45:505–9. [PubMed] [Google Scholar]
- 45.Lindberg E, Tysk C, Andersson K, et al. Smoking and inflammatory bowel disease. A case control study Gut. 1988;29:352–7. doi: 10.1136/gut.29.3.352. [DOI] [PMC free article] [PubMed] [Google Scholar]