Abstract
Rationale
In Syrian hamsters (Mesocricetus auratus), social defeat produces a prolonged change in subsequent agonistic behavior termed conditioned defeat. This stress-induced change in behavior is marked by increased submissive and defensive behavior toward a novel, nonaggressive opponent and a complete loss of normal territorial aggression. Corticotropin-releasing factor (CRF) has been shown to affect serotonergic neurons in the dorsal raphe nucleus (DRN) and to modulate learned helplessness via a CRF type-2 receptor (CRF-R2) mechanism.
Objectives
In this study, we tested the hypothesis that a nonselective CRF receptor antagonist (experiment 1: 250 or 500 ng d-Phe CRF in 200 nl saline), or a selective CRF-R2 antagonist (experiment 2: 500 ng anti-Svg-30 in 200 nl saline), injected into the DRN would reduce the acquisition of conditioned defeat in male hamsters. We also tested similar hypotheses for the expression of conditioned defeat (experiments 3 and 4).
Results
Infusion of d-Phe CRF into the DRN significantly reduced both the acquisition and expression of conditioned defeat compared to vehicle controls, whereas infusion of anti-Svg-30 into the DRN reduced expression but not acquisition. In particular, CRF antagonism in the DRN decreased fleeing from novel opponents but did not reinstate normal territorial aggression after social defeat.
Conclusions
Our results suggest that the increased flight associated with conditioned defeat is modulated by CRF-R2 activation within the DRN. Overall, social defeat is an ethologically relevant stressor that appears to activate at least some of the same neural substrates that have been implicated in the control of learned helplessness.
Keywords: Social defeat, Stress, Aggression, Anxiety, Fear, CRH, Defensive behavior
Introduction
Social conflict represents a potent stressor for humans and other animals. Social defeat, in particular, activates the hypothalamic–pituitary–adrenal (HPA) axis and produces stress-induced behavioral changes. A striking example of the consequences of social defeat occurs in Syrian hamsters. In the laboratory, hamsters are aggressive animals that readily defend their home cage against intruders (Albers et al. 2002). However, after experiencing a single defeat by a larger, more aggressive opponent, hamsters exhibit a loss of normal territorial aggression and instead submit without provocation even when tested with a smaller, nonaggressive opponent. We call this change in agonistic behavior conditioned defeat, and we use this model system to investigate the neurobiology of stress-induced changes in behavior (Huhman et al. 2003).
Corticotropin-releasing factor (CRF) is a neuropeptide that has been implicated in the regulation of neural, endocrine, and behavioral responses to stressful stimuli (Dunn and Berridge 1990; Owens and Nemeroff 1991). Anatomical studies indicate that CRF-immunoreactive cell bodies and receptors are distributed outside the HPA axis, suggesting that CRF does more than control the neuroen-docrine response to stress (Olschowka et al. 1982; Swanson et al. 1983; Chalmers et al. 1995); furthermore, hamsters appear to have a similar distribution of CRF-immunoreactive neurons and fibers as do rats (Delville et al. 1992). Several studies indicate that CRF modulates fear-related, anxiety-related, and defensive behavior. For instance, administration of CRF, or related peptides such as urocortins, has been shown to increase acoustic startle (Liang et al. 1992), anxiety-related behavior in open-field and elevated plus maze tests (Moreau et al. 1997; Jones et al. 1998), and defensive behavior shown in response to predators (Yang et al. 2006; Carvalho-Netto et al. 2007). Likewise, several studies have shown that CRF receptor antagonists reduce fear- and anxiety-related behavior (e.g., Schulz et al. 1996; Griebel et al. 1998; Deak et al. 1999). The actions of CRF and urocortins are mediated by CRF type-1 receptors (CRF-R1) and CRF type-2 receptors (CRF-R2). Each CRF receptor subtype has a unique distribution in the brain, suggesting potential functional differences between them (Chalmers et al. 1995; Bittencourt and Sawchenko 2000; van Pett et al. 2000). Data from pharmacological and receptor knockout studies indicate that activation of CRF-R1 increases a variety of stress-related behaviors (Heinrichs et al. 1997; Timpl et al. 1998; Contarino et al. 1999; Griebel et al. 2002). On the other hand, evidence from CRF-R2 has been mixed. Some studies have reported reduced anxiety-related behavior with CRF-R2 activation (Valdez et al. 2002) and increased depression-like and anxiety-like profiles for mice lacking the CRF-R2 gene (Bale et al. 2000; Coste et al. 2000; Kishimoto et al. 2000; Bale and Vale 2003). Other studies have reported reduced anxiety-related behavior with CRF-R2 antagonism (Radulovic et al. 1999; Ho et al. 2001; Bakshi et al. 2002; Risbrough et al. 2003). More recently, Risbrough et al. (2004) have proposed a combined role for CRF-R1 and CRF-R2 in modulating emotional behavior.
The DRN is innervated by CRF-immunoreactive fibers (Swanson et al. 1983; Sakanaka et al. 1987) and contains mRNA for CRF-R1 and CRF-R2 (Potter et al. 1994; Chalmers et al. 1995). The effect of CRF on the activity of DRN serotonin neurons is complex and is likely related to the heterogeneity of the DRN. Recognition of subregions within the DRN is important because these subregions have unique, topographically organized afferent inputs and efferent projections (van Bockstaele et al. 1993; Peyron et al. 1998). Data indicate that CRF primarily inhibits the firing of DRN serotonin neurons (Price et al. 1998; Kirby et al. 2000) and decreases extracellular levels of serotonin in the lateral septum and striatum (Price and Lucki 2001). In contrast, CRF has been shown to excite a subpopulation of serotonin neurons in the caudal DRN (Lowry et al. 2000). One possible explanation for these contrasting effects, as suggested by Lowry et al. (2000), is that Price and colleagues recorded primarily from neurons in rostral portions of the DRN while Lowry’s study focused on a small cluster of neurons in the caudal DRN. Also, it is possible that CRF-R1 might mediate inhibitory responses in the rostral DRN, whereas CRF-R2 might mediate excitation more caudally (Hammack et al. 2003a). More recently, however, it has been shown that CRF-R2 activation produces inhibitory, as well as excitatory, effects in DRN serotonin neurons (Pernar et al. 2004). CRF modulation of serotonin neurons in the DRN appears to contribute to the impaired escape behavior and increased fear conditioning associated with learned helplessness. Researchers have shown that injection of a CRF-R2 antagonist into the DRN reduces learned helplessness when given before inescapable shock (i.e., acquisition) but not when given before behavioral testing (i.e., expression) (Hammack et al. 2003b).
Conditioned defeat shares some behavioral characteristics with learned helplessness in the sense that both models involve stressors that cause animals to surrender to stimuli they would otherwise control. Researchers using the learned helplessness model have identified a neural circuit in the DRN where CRF modulates the acquisition but not expression of stress-induced changes in behavior. In the present study, we test the hypothesis that injection of a nonselective CRF receptor antagonist, and a selective CRF-R2 antagonist, into the DRN will reduce the acquisition but not expression of conditioned defeat.
Materials and methods
Subjects
We used male Syrian hamsters (Mesocricetus auratus) that weighed 120–140 g at the start of the study (3–4 months), and we individually housed them for 10–14 days before testing. Older hamsters (>6 months) that weighed 160–180 g were housed individually and used as resident aggressors. Younger hamsters (~2 months) that weighed 90–110 g were group-housed (five per cage) and used as nonaggressive opponents. All animals were housed in polycarbonate cages (20×40× 20 cm)with corncob bedding, cotton nesting materials, and wire mesh tops. Animal cages were not changed for at least 1 week before testing to allow individuals to scent mark their territory. Animals were housed in a temperature-controlled colony room (20±2°C) and were maintained on a 14:10-h light–dark cycle with food and water available ad libitum.
Stereotaxic surgery
Hamsters were anesthetized with sodium pentobarbital (90 mg/kg) and were stereotaxically implanted with a 4-mm, 26-gauge guide cannula aimed at the DRN. Lambda and bregma were leveled before guide cannula implantation. The angle of approach was 20° from vertical to avoid penetrating the fourth ventricle, and the stereotaxic coordinates were 4.5 mm posterior to bregma, 1.8 mm lateral to bregma, and 2.3 mm below dura. These coordinates aimed the guide cannula toward the midpoint of the rostral–caudal extent of the DRN. The guide cannula was aimed short of the DRN to avoid damaging the nucleus and a 33-gauge injection needle projected 3 mm below the guide cannula for a final depth of 5.3 mm below dura. After surgery, dummy stylets were placed in the guide cannula to help prevent clogging. All animals were given 10–14 days to recover from surgery before behavioral experiments. Hamsters were repeatedly handled after surgery by gently restraining them and removing and replacing the dummy stylet, which helped habituate themto the experimental procedure.
Conditioned defeat protocol
Our conditioned defeat protocol has been extensively described elsewhere (Potegal et al. 1993; Huhman et al. 2003) and is briefly described here. Social defeat training consisted of one 15-min encounter with a resident aggressor in the aggressor’s home cage. Resident aggressors reliably attacked and defeated the experimental hamsters. To equalize the duration of defeat, 15-min encounters began at the first attack by the resident aggressor, which usually occurred within the first 30 s. Any hamster bitten such that it bled was removed from the study and was examined by a veterinarian, and 1.9% of animals were excluded due to wounding. During social defeat, we recorded the total duration of aggression displayed by the resident aggressor, the number of attacks, and the total duration of submissive and defensive behavior displayed by the experimental subjects. No-defeat controls were given a 15-min exposure to a resident aggressor’s empty cage. We performed all training and subsequent testing under red light during the first 3 h of the dark phase of the light–dark cycle.
Behavioral testing occurred 24 h after training and consisted of one 5-min encounter with a novel, nonaggressive opponent in the experimental animal’s home cage. Testing sessions were later scored by a researcher blind to the experimental conditions using behavioral definitions adapted from the study of Albers et al. (2002). We recorded the total duration of four classes of behavior during the 5-min tests: (a) social (attend, approach, investigate, sniff, nose touch, and flank mark), (b) nonsocial (locomotion, exploration, self-groom, nest build, feed, and sleep), (c) submissive and defensive (flee, avoid, tail up, upright and side defense, full submissive posture, stretch-attend, head flag, and attempt to escape from cage), and (d) aggressive (upright and side offense, chase, and attack including bite). For a more detailed analysis of submissive and defensive behavior, we recorded the frequency of fleeing and stretch-attend. A second researcher separately scored a subset of testing sessions, and interrater reliability was 92% with r = 0.98.
Experiments 1 and 2: acquisition of conditioned defeat
We designed experiment 1 to test the hypothesis that injection of a nonselective CRF receptor antagonist (d-Phe CRF(12–41), Bachem) into the DRN would reduce the acquisition of conditioned defeat. We infused d-Phe CRF (250 or 500 ng in 200 nl saline) or vehicle (200 nl saline) into the DRN 10 min before social defeat. Likewise, for no-defeat controls, we infused d-Phe CRF (500 ng in 200 nl saline) or vehicle (200 nl saline) into the DRN 10 min before novel cage exposure. We performed infusions with a 1-µl Hamilton syringe connected to a 33-gauge needle via polyethylene tubing. The syringe was mounted onto a syringe pump (Harvard Apparatus PHD 2000, Natick, MA, USA), programmed to infuse 200 nl/min. The needle remained in place for an additional 1 min to allow diffusion of the solution. An air bubble separated the water in the tubing from the solution, and movement of the air bubble down the tubing indicated a successful injection. Hamsters that did not receive a successful injection were excluded from data analysis. Animals were tested for conditioned defeat 24 h later as described above.
We designed experiment 2 to test the hypothesis that injection of a selective CRF-R2 antagonist (anti-Svg-30 Amide, Polypeptide Laboratories) into the DRN would reduce the acquisition of conditioned defeat. We infused anti-Svg-30 (500 ng in 200 nl saline) or vehicle (200 nl saline) into the DRN 10 min before social defeat training.
Experiments 3 and 4: expression of conditioned defeat
We designed experiment 3 to test the hypothesis that injection of d-Phe CRF into the DRN would reduce the expression of conditioned defeat. At training, animals either experienced a 15-min social defeat or a novel cage exposure. The next day, d-Phe CRF (250 and 500 ng in 200 nl saline) or vehicle (200 nl saline) was infused into the DRN of previously defeated hamsters 10 min before behavioral testing. Similarly, we infused d-Phe CRF (500 ng in 200 nl saline) or vehicle (200 nl saline) into the DRN of no-defeat controls 10 min before testing.
We designed experiment 4 to test the hypothesis that injection of anti-Svg-30 into the DRN would reduce the expression of conditioned defeat. At training, animals experienced a 15-min social defeat. The next day, anti-Svg-30 (500 ng in 200 nl saline) or vehicle (200 nl saline) was infused into the DRN 10 min before behavioral testing.
Control experiments
The effect of CRF receptor antagonists on the acquisition of conditioned defeat could be due to their effect on conditioned defeat expression if they are biologically active 24 h later at behavioral testing. In a carryover control experiment, we investigated the hypothesis that injection of d-Phe CRF into the DRN 4 h after social defeat would reduce the expression of conditioned defeat. We performed injections 4 h after social defeat, a time that we supposed would be outside the consolidation time window for conditioned defeat. At training, animals experienced a 15-min social defeat. Four hours later, we infused d-Phe CRF (500 ng in 200 nl saline) or vehicle (200 nl saline) into the DRN, and the next day, animals were tested for conditioned defeat. Moreover, reduction in the acquisition and expression of conditioned defeat could be due to animals being trained and tested in different physiological states. In a state-dependency control experiment, we investigated the hypothesis that injection of d-Phe CRF into the DRN both before training and before testing would reinstate conditioned defeat. In this case, we infused d-Phe CRF (500 ng in 200 nl saline) or vehicle (200 nl saline) into the DRN 10 min before social defeat and again the next day 10 min before behavioral testing.
Histology
Hamsters were given a lethal dose of sodium pentobarbital and were infused with 200 nl of India ink to verify the placement of the injections. Brains were removed, frozen on dry ice, and stored at −80°C. Later, brains were sliced at 30 µm on a cryostat, and sections were stained with neutral red and were coverslipped with DPX mountant. Brain sections were examined under a light microscope for evidence of ink in the DRN. Only hamsters with ink injections 200 µm or less from the DRN were included in data analysis (see Fig. 1). Hamsters with ink injections further than 200 µm from the DRN were used as anatomical controls.
Fig. 1.
A representative photomicrograph for cannula site verification is shown. Histological maps of DRN injection placements are shown for experiments 1 and 3 (d-Phe CRF) and experiments 2 and 4 (anti-Svg-30). Closed circles represent acceptable injection placements, whereas open circles represent unacceptable sites. Circles indicate the center of one or more ink injections. Diagrams are adapted from the work of Morin and Wood (2001), and coordinates are reported from bregma. DRN Dorsal raphe nucleus, MLF medial longitudinal fasciculus, 4V fourth ventricle
Statistical analysis
Total durations (in seconds) of submissive and defensive, social, nonsocial, and aggressive behaviors were analyzed separately using independent sample t tests or one-way between-subjects ANOVAs. Likewise, frequencies of attack, flee, and stretch-attend were analyzed separately. Tukey tests were used for pairwise comparisons when necessary. All comparisons were two-tailed, and alpha was set at p<0.05.
Results
Experiments 1 and 2: acquisition of conditioned defeat
Hamsters injected with 500 ng of d-Phe CRF into the DRN before social defeat showed reduced conditioned defeat (Fig. 2). They showed less submissive and defensive behavior (F(4,41) = 9.36, p<0.05, Tukey, p<0.05) and more social behavior (F(4,41) = 6.41, p<0.05, Tukey, p<0.05) than did vehicle controls. They did not show a change in aggressive or nonsocial behavior compared to vehicle controls (p>0.05). Differences in the frequency of fleeing for individuals injected with vehicle (15.0, SE = 3.1), 250 ng (8.7, SE = 2.6), or 500 ng (6.4, SE = 1.8) of d-Phe CRF approached, but did not reach, statistical significance (F(2,27) = 3.15, p = 0.059). The frequency of stretch-attend posture in individuals injected with vehicle (2.8, SE = 0.38), 250 ng (1.9, SE = 0.51), or 500 ng (1.8, SE = 0.39) of d-Phe CRF was not significantly different (p>0.05). No-defeat animals differed from defeated animals in that they showed less submissive and defensive behavior (F(4,41) = 9.36, p<0.05), more aggressive behavior (F(4,41) = 6.74, p<0.05), and more social behavior (F(4,41) = 6.41, p<0.05) than did socially defeated animals. However, there was no effect of d-Phe CRF on the behavior of no-defeat controls (p>0.05).
Fig. 2.
The mean duration (s ± SE) of submissive and defensive, aggressive, social, and nonsocial behaviors is shown during a 5-min test with a novel, nonaggressive opponent. Social defeat animals received an injection of d-Phe CRF (250 ng/200 nl, N = 9; 500 ng/ 200 nl, N = 10) or vehicle (N = 11) into the dorsal raphe nucleus (DRN) 10 min before defeat training. Likewise, no-defeat controls received an injection of d-Phe CRF (500 ng/200 nl, N = 8) or vehicle (N = 8) into the DRN before novel cage exposure. These data demonstrate reduced submissive and defensive behavior and increased social behavior with increasing doses of d-Phe CRF (asterisk, p<0.05). Although no-defeat controls showed less submissive and defensive behavior, more aggressive behavior, and more social behavior than did socially defeated vehicle animals (caret, p<0.05), d-Phe CRF did not significantly alter the behavior of no-defeat controls (p>0.05)
Reduced conditioned defeat acquisition was not due to variation in the intensity of social defeat or the response of subjects during defeat. Resident aggressors directed 330.2 s (SE = 34.6), 299.5 s (SE = 35.8), and 339.9 s (SE = 29.7) of aggression toward subjects that received vehicle, 250 ng, or 500 ng of d-Phe CRF, respectively (p>0.05). Also, subjects did not significantly differ in the number of attacks they received during social defeat (vehicle: 14.8, SE = 2.0; 250 ng: 15.0, SE = 1.8; 500 ng: 15.7, SE = 1.3) (p>0.05). Furthermore, animals injected with vehicle, 250 ng, or 500 ng of d-Phe CRF displayed 535.6 s (SE = 48.8), 514.6 s (SE = 61.8), or 540.7 s (SE = 35.1) of submissive and defensive behavior during social defeat, respectively (p>0.05).
Anatomical controls did not show reduced acquisition of conditioned defeat since the duration of their submissive and defensive behavior did not significantly differ from vehicle controls (p>0.05). Moreover, they did not significantly differ from vehicle controls in the frequency of fleeing (p>0.05) or stretch-attend postures (p>0.05). Anatomical controls most often had injection placements inside the fourth ventricle, but some received an injection into the periaqueductal gray, tegmental nucleus, or trochlear nucleus.
Individuals injected with anti-Svg-30 into the DRN before social defeat training did not show a significant reduction in the duration of submissive and defensive behavior at testing (p>0.05; Fig. 3). However, they displayed more social behavior (t(19) = 2.14, p<0.05) and less nonsocial behavior than did vehicle controls (t(19) = 2.25, p<0.05). Similarly, individuals injected with anti-Svg-30 or vehicle did not significantly differ in the frequency of fleeing (9.8, SE = 2.9; 9.6, SE = 2.2; p>0.05, respectively) or stretch-attend postures (2.5, SE = .53; 2.6, SE = .50; p>0.05, respectively). Because conditioned defeat is defined in terms of agonistic behavior, anti-Svg-30 injected into the DRN did not reduce the acquisition of conditioned defeat.
Fig. 3.
The mean duration (s ± SE) of submissive and defensive (sub/def), aggressive, social, and nonsocial behaviors is shown during a 5-min test with a novel, nonaggressive opponents. Ten minutes before social defeat training, animals received an injection of anti-Svg-30 (500 ng/200 nl, N = 11) or vehicle (N = 10) into the dorsal raphe nucleus. Anti-Svg-30 did not significantly alter submissive and defensive behavior but did significantly increase social behavior and decrease nonsocial behavior compared to vehicle controls (asterisk, p<0.05)
Experiments 3 and 4: expression of conditioned defeat
Hamsters injected with 500 ng of d-Phe CRF into the DRN before testing showed reduced conditioned defeat (Fig. 4). They displayed less submissive and defensive behavior than did vehicle controls (F(4,42) = 6.79, p<0.05). They did not significantly differ from vehicle controls in the duration of aggressive, social, and nonsocial behaviors (p>0.05). Hamsters injected with vehicle, 250 ng, or 500 ng of d-Phe CRF displayed 13.6 (SE = 2.4), 7.4 (SE = 2.2), 6.2 (SE = 1.7) flees during testing, respectively. Statistical analysis showed that 500 ng of d-Phe CRF significantly reduced fleeing compared to vehicle controls (F(2,29) = 3.64, p<0.05, Tukey, p<0.05). In contrast, the frequency of stretch-attend postures did not significantly differ between vehicle (2.5, SE = 0.45), 250 ng (2.7, SE = 0.75), and 500 ng (2.2, SE = 0.54) groups (p>0.05). No-defeat controls injected with d-Phe CRF did not significantly differ from no-defeat vehicle controls in any behavioral class (p>0.05), although together, no-defeat controls often differed from defeated animals (Fig. 4). Specifically, no-defeat animals showed less submissive and defensive behavior (F(4,42) = 6.79, p<0.05), more aggressive behavior (F(4,42) = 4.13, p<0.05, Tukey, p<0.05), and more social behavior (F(4,42) = 3.52, p<0.05, Tukey, p<0.05) than did socially defeated animals.
Fig. 4.
The mean duration (s ± SE) of submissive and defensive, aggressive, social, and nonsocial behaviors is shown during a 5-min test with a novel, nonaggressive opponent. Social defeat animals received an injection of d-Phe CRF (250 ng/200 nl, N = 10; 500 ng/200 nl, N = 11) or vehicle (N = 11) into the dorsal raphe nucleus (DRN) 10 min before behavioral testing. Likewise, no-defeat controls received an injection of d-Phe CRF (500 ng/200 nl, N = 8) or vehicle (N = 7) into the DRN. These data demonstrate a significant reduction in submissive and defensive behavior with 500 ng of d-Phe CRF compared to vehicle controls (asterisk, p<0.05). No-defeat controls showed less submissive and defensive behavior, more aggressive behavior, and more social behavior than did socially defeated vehicle animals (caret, p<0.05). Nodefeat controls injected with d-Phe CRF or vehicle did not significantly differ in any behavioral class (p>0.05)
Anatomical controls did not show reduced expression of conditioned defeat, as evidenced by no change in their submissive and defensive behavior compared with vehicle controls (p>0.05). Also, they did not differ from vehicle controls in the fleeing (p>0.05) or stretch-attend (p>0.05). As in experiment 1, anatomical controls most often received an injection into the fourth ventricle but some received an injection into the periaqueductal gray, tegmental nucleus, or trochlear nucleus.
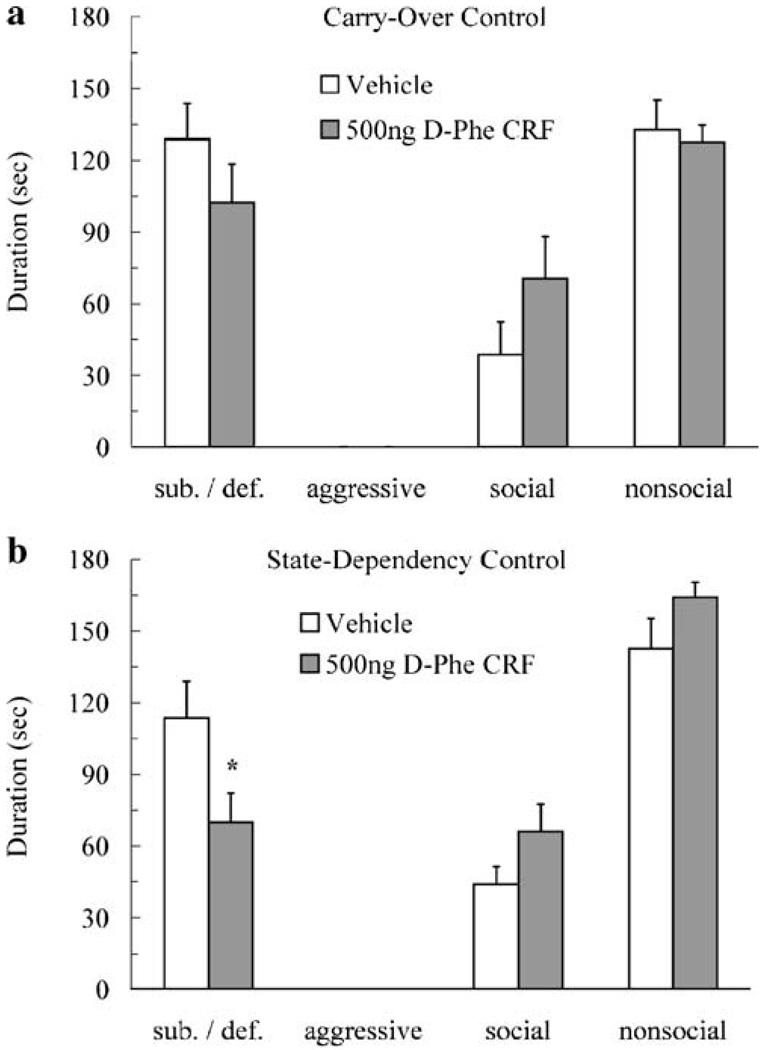
In the carryover control experiment, animals that received d-Phe CRF into the DRN 4 h after social defeat did not show reduced conditioned defeat compared to vehicle controls (p>0.05; Fig. 5a). Likewise, animals injected with d-Phe CRF or vehicle did not significantly differ in fleeing (p>0.05) or stretch-attend (p>0.05). Together, these results suggest that the effect of d-Phe CRF on the acquisition of conditioned defeat is not due to residual drug still present at testing. In the state-dependency control experiment, animals injected with d-Phe CRF into the DRN both before social defeat training and before testing showed reduced conditioned defeat compared to vehicle controls (Fig. 5b). They showed significantly less submissive and defensive behavior than did vehicle controls (t(18) = 2.24, p<0.05), and no significant change in social and nonsocial behavior (p>0.05) was observed. Furthermore, animals that received d-Phe CRF showed less fleeing (5.8, SE = 1.9) than did vehicle controls show (12.1, SE = 2.6) (t(18) = 2.43, p<0.05). Stretch-attend postures did not significantly differ (p>0.05). Thus, training and testing the animals in the same physiological state did not reinstate conditioned defeat.
Fig. 5.
The mean duration (s ± SE) of submissive and defensive (sub/def), aggressive, social, and nonsocial behaviors is shown during a 5-min test with a novel, nonaggressive opponent. In the carryover control experiment (a), animals received an injection of d-Phe CRF (500 ng/200 nl, N = 6) or vehicle (N = 6) into the dorsal raphe nucleus (DRN) 4 h after social defeat. d-Phe CRF did not significantly alter any behavioral class (p>0.05). In the state-dependency control experiment (b), animals received an injection of d-Phe CRF (500 ng/200 nl, N = 10) or vehicle (N = 10) into the DRN both 10 min before social defeat and 10 min before behavioral testing. DPhe CRF significantly reduced the duration of submissive and defensive behavior compared to vehicle (asterisk, p<0.05)
Individuals injected with anti-Svg-30 into the DRN before behavioral testing showed reduced conditioned defeat (Fig. 6). They displayed a lower duration of submissive and defensive behavior (t(20) = 2.81, p<0.05) and a greater duration of social behavior (t(20) = 2.25, p<0.05) than did vehicle controls. Similarly, anti-Svg-30 animals showed less fleeing (4.0, SE = 1.1) than did vehicle controls (10.1, SE = 2.2) (t(20) = 2.65, p<0.05). They also showed less stretch-attend (1.5, SE = 0.40) than did vehicle controls (2.8, SE = 0.39) (t(20) = 2.31, p<0.05). We did not have enough injections outside of the DRN for an analysis of anatomical controls.
Fig. 6.
The mean duration (s ± SE) of submissive and defensive (sub/def), aggressive, social, and nonsocial behaviors is shown during a 5- min test with a novel, nonaggressive opponent. Ten minutes before behavioral testing, animals received an injection of anti-Svg-30 (500 ng/200 nl, N = 12) or vehicle (N = 10) into the dorsal raphe nucleus. Anti-Svg-30 significantly reduced the duration of submissive and defensive behavior and increased the duration of social behavior compared to vehicle controls (asterisk, p<0.05)
Discussion
We found that blockade of CRF receptors in the DRN with a nonselective antagonist reduced the acquisition and expression of conditioned defeat. Data from anatomical controls suggest that d-Phe CRF did not leak into the ventricular system or diffuse into adjacent brain regions to reduce conditioned defeat. Also, a selective CRF-R2 antagonist reduced the expression but not acquisition of conditioned defeat. The effect of both d-Phe CRF and anti-Svg-30 on expression suggests that CRF-R2 activation of DRN neurons is part of the neural circuitry controlling the increased submissive and defensive behavior which marks conditioned defeat. Because injection of anti-Svg-30 into the DRN did not reduce the acquisition of conditioned defeat, it leaves open the possibility that d-Phe CRF could have acted through CRF-R1 to affect acquisition, although this possibility needs to be tested. Together, these data suggest similarities in the neurobiological mechanisms regulating conditioned defeat and learned helplessness.
CRF receptors in the DRN are part of the neural circuit modulating conditioned defeat in hamsters and learned helplessness in rats. The acquisition but not expression of the behavioral consequences of inescapable shock is blocked with an injection into the DRN of d-Phe CRF (Hammack et al. 2002) or anti-Svg-30 (Hammack et al. 2003b). In contrast, we found that blockade of CRF-R2 in the DRN reduced the expression of conditioned defeat, but that the effect of CRF antagonism was less clear-cut for the acquisition of conditioned defeat. The contrasting effects of CRF antagonism on the expression of learned helplessness and conditioned defeat may be due to differences in testing procedures. Learned helplessness testing involves escape training and fear conditioning, whereas conditioned defeat testing requires a behavioral response to a novel intruder. For learned helplessness, CRF activity in the DRN appears to sensitize DRN neurons during inescapable shock, but testing conditions apparently do not release CRF within the DRN (Maier and Watkins 2005). We may speculate that exposure to a novel intruder after social defeat leads to the release of CRF-related peptides within the DRN, which makes CRF receptors a necessary component of the neural circuit regulating the expression of conditioned defeat. The contrasting findings could also be related to species differences in the distribution and sensitivity of CRF receptors within the DRN. In any case, CRF or urocortin activity in the DRN appears to be an important component of the neural circuitry controlling conditioned defeat and learned helplessness, although the precise role of CRF-related peptides in the DRN likely differs.
Injection of anti-Svg-30 into the DRN produced a modest reduction in the expression of conditioned defeat. We interpret the modest effect of CRF antagonism to mean that CRF-related mechanisms modulate but do not mediate the neural circuitry underlying conditioned defeat. We have previously found that an intracerebroventricular injection of d-Phe CRF produces a similar 50% reduction in the submissive and defensive behavior (Jasnow et al. 1999). Likewise, we have shown that CRF antagonism in the BSNT produces a comparable reduction in submissive and defensive behavior (Jasnow et al. 2004b; Cooper and Huhman 2005). Thus, CRF-related mechanisms appear to modulate conditioned defeat at multiple brain sites, while non-CRF mechanisms likely mediate conditioned defeat. It seems unlikely that a higher dose of d-Phe CRF or anti-Svg-30 would produce a greater reduction in conditioned defeat because the highest doses used in this study are at the top end of the range of doses that produce behavioral effects in other models of stress-induced changes in behavior (Bakshi et al. 2002; Pelleymounter et al. 2002; Hammack et al. 2003b; Funk et al. 2006).
The increased submissive and defensive behavior associated with conditioned defeat includes behaviors which result in approach toward threatening stimuli, such as stretch-attend posture, and those which result in avoidance, such as flight. We found that CRF antagonism in the DRN reduced fleeing during conditioned defeat testing but had less consistent effects on stretch-attend posture. These data are consistent with research showing that infusion of CRF into the dorsal periaqueductal gray increases escape attempts in mice exposed to predator-stress situations (Carvalho-Netto et al. 2007). Furthermore, our results support McNaughton and Corr’s (2004) hypothesis that shows that separate neural mechanisms regulate defensive approach, which may indicate anxiety, and defensive avoidance, which may indicate fear. Moreover, although CRF antagonism reduced fleeing in our study, it did not restore normal territorial aggression. In fact, we have shown that several pharmacological treatments reduce submissive and defensive behavior after social defeat but fail to affect the suppression of aggression (Jasnow and Huhman 2001; Jasnow et al. 2004a; Cooper and Huhman 2005). Also, CRF antagonism did not alter the agonistic behavior of no-defeat controls. Peripheral administration of a CRF-R1 antagonist has been shown to reduce aggression in Syrian hamsters, suggesting a role for CRF in enhancing aggression (Farrokhi et al. 2004). However, CRF may not modulate aggression via its activity in the DRN. Although research indicates that serotonin acts within the anterior hypothalamus to inhibit hamster aggression and that DRN neurons account for at least part of the serotonergic innervation of the anterior hypothalamus (e.g., Ferris et al. 1999), our results suggest that CRF receptor blockade in the DRN is not sufficient to alter the serotonergic neural circuitry regulating aggression. Overall, it appears that submissive, defensive, and aggressive behaviors are regulated by separate, albeit interacting, neural circuits and that CRF antagonism in the DRN affects primarily a defensive avoidance circuit.
Injection of d-Phe CRF reduced the acquisition of conditioned defeat, and carryover and state-dependency control experiments indicate that the effect of d-Phe CRF is due to pharmacological blockade of CRF receptors during social defeat. However, the effect of CRF antagonism in the DRN was less consistent for acquisition than it was for expression. Although d-Phe CRF infusion into the DRN before social defeat reduced the duration of submissive and defensive behavior at testing, the effect on the frequency of fleeing approached but did not reach statistical significance. Also, the effect of d-Phe CRF was not reproduced with a selective CRF-R2 antagonist. It is possible that CRF-R1 activation in the DRN, or a combination of R1 and R2 activation, contributes to the acquisition of conditioned defeat. For instance, CRF or urocortin activation of DRN neurons might desensitize 5-HT1a autoreceptors, as has been suggested for learned helplessness (Greenwood et al. 2003; Maier and Watkins 2005). The possibility that CRF-related neural plasticity in the DRN contributes to conditioned defeat, however, does not preclude a critical role for neural plasticity in other brain areas. In fact, the data showing that d-Phe CRF in the DRN reduced, but did not completely block, the acquisition of conditioned defeat suggests that other neural mechanisms are involved. For example, the basolateral amygdala (BLA) has been implicated as a site of neural plasticity controlling conditioned defeat. Phosphorylation of cyclic AMP response element-binding protein (CREB) has been shown to be critical in learning and memory for stressful events, and we have demonstrated that overexpression of CREB in the BLA enhances the acquisition but not expression of conditioned defeat (Jasnow et al. 2005).
In conclusion, our results indicate that antagonism of CRF-R2 in the DRN reduces the expression of conditioned defeat. CRF-R2 antagonism in the DRN mainly reduced defensive avoidance, which is consistent with the hypothesis that the DRN modulates conditioned defeat via serotonergic projections to fear circuits in the extended amygdala (Lowry 2002; Amat et al. 2004). In sum, social defeat is a psychosocial stressor that appears to activate at least some of the same neural substrates that have been implicated in the control of learned helplessness.
Acknowledgments
This research was supported by the National Institutes of Health grants MH62044 to K.L.H. and F32 MH72085 to M.A.C. and in part by The Center for Behavioral Neuroscience, a National Science Foundation Science and Technology Center program under agreement no. IBN-9876754. We are grateful to Lauren Zelinski and Alisa Norvelle for their technical assistance. All procedures were approved by the Georgia State University Animal Care and Use Committee and comply with US law.
Contributor Information
Matthew A. Cooper, Department of Psychology, Austin Peay Building, University of Tennessee, Knoxville, TN 37996-0900, USA, e-mail: mcooper@utk.edu
Kim L. Huhman, Department of Psychology, Center for Behavioral Neuroscience, Georgia State University, Atlanta, GA, USA
References
- Albers HE, Huhman KL, Meisel RL. Hormonal basis of social conflict and communication. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, brain and behavior. San Diego: Academic; 2002. pp. 393–433. [Google Scholar]
- Amat J, Tamblyn JP, Paul ED, Bland ST, Amat P, Foster AC, Watkins LR, Maier SF. Microinjection of urocortin 2 into the dorsal raphe nucleus activates serotonergic neurons and increases extracellular serotonin in the basolateral amygdala. Neuroscience. 2004;129:509–519. doi: 10.1016/j.neuroscience.2004.07.052. [DOI] [PubMed] [Google Scholar]
- Bakshi VP, Smith-Roe S, Newman SM, Grigoriadis DE, Kalin NH. Reduction of stress-induced behavior by antagonism of corticotropin-releasing hormone 2 (CRH2) receptors in lateral septum or CRH1 receptors in amygdala. J Neurosci. 2002;22:2926–2935. doi: 10.1523/JNEUROSCI.22-07-02926.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Vale WW. Increased depression-like behaviors in corticotropin-releasing factor receptor-2-deficient mice: sexually dichotomous responses. J Neurosci. 2003;23:5295–5301. doi: 10.1523/JNEUROSCI.23-12-05295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, Koob GF, Vale WW, Lee KF. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet. 2000;24:410–414. doi: 10.1038/74263. [DOI] [PubMed] [Google Scholar]
- Bittencourt JC, Sawchenko PE. Do centrally administered neuropeptides access cognate receptors?: an analysis in the central corticotropin-releasing factor system. J. Neurosci. 2000;20:1142–1156. doi: 10.1523/JNEUROSCI.20-03-01142.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho-Netto EF, Litvin Y, Nunes-de-Souza RL, Blanchard DC, Blanchard RJ. Effects of intra-PAG infusion of ovine CRF on defensive behaviors in Swiss–Webster mice. Behav Brain Res. 2007;176:222–229. doi: 10.1016/j.bbr.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF 2)mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contarino A, Dellu F, Koob GF, Smith GW, Lee KF, Vale W, Gold LH. Reduced anxiety-like and cognitive performance in mice lacking the corticotropin-releasing factor receptor-1. Brain Res. 1999;835:1–9. doi: 10.1016/s0006-8993(98)01158-5. [DOI] [PubMed] [Google Scholar]
- Cooper MA, Huhman KL. Corticotropin-releasing factor type II (CRF2) receptors in the bed nucleus of the stria terminalis modulate conditioned defeat in Syrian hamsters (Mesocricetus auratus) Behav Neurosci. 2005;119:1042–1051. doi: 10.1037/0735-7044.119.4.1042. [DOI] [PubMed] [Google Scholar]
- Coste SC, Kesterson RA, Heldwein KA, Stevens SL, Heard AD, Hollis JH, Murray SE, Hill JK, Pantely GA, Hohimer AR, Hatton DC, Phillips TJ, Finn DA, Low MJ, Rittenberg MB, Stenzel P, Stenzel-Poore MP. Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropinreleasing hormone receptor-2. Nat Genet. 2000;24:403–409. doi: 10.1038/74255. [DOI] [PubMed] [Google Scholar]
- Deak T, Nguyen KT, Ehrlich AL, Watkins LR, Spencer RL, Maier SF, Licinio J, Wong ML, Chrousos GP, Webster E, Gold PW. The impact of the nonpeptide corticotropin-releasing hormone antagonist antalarmin on behavioral and endocrine responses to stress. Endocrinology. 1999;140:79–86. doi: 10.1210/endo.140.1.6415. [DOI] [PubMed] [Google Scholar]
- Delville Y, Stires C, Ferris CF. Distribution of corticotropinreleasing hormone immunoreactivity in golden hamster brain. Brain Res Bull. 1992;29:681–684. doi: 10.1016/0361-9230(92)90138-n. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Rev. 1990;15:71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- Farrokhi C, Blanchard DC, Griebel G, Yang M, Gonzales C, Markham C, Blanchard RJ. Effects of the CRF1 antagonist SSR125543A on aggressive behaviors in hamsters. Pharmacol Biochem Behav. 2004;77:465–469. doi: 10.1016/j.pbb.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Stolberg T, Delville Y. Serotonin regulation of aggressive behavior in male golden hamster (Mesocricetus auratus) Behav Neurosci. 1999;113:804–815. doi: 10.1037//0735-7044.113.4.804. [DOI] [PubMed] [Google Scholar]
- Funk CK, O’Dell LE, Crawford EF, Koob GF. Corticotropinreleasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Day HE, Campisi J, Hammack SH, Campeau S, Maier SF, Fleshner M. Freewheel running prevents learned helplessness/behavioral depression: role of dorsal raphe serotonergic neurons. J Neurosci. 2003;23:2889–2898. doi: 10.1523/JNEUROSCI.23-07-02889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G, Perrault G, Sanger DJ. Characterization of the behavioral profile of the non-peptide CRF receptor antagonist CP-154526 in anxiety models in rodents. Comparison with diazepam and buspirone. Psychopharmacology. 1998;138:55–66. doi: 10.1007/s002130050645. [DOI] [PubMed] [Google Scholar]
- Griebel G, Simiand J, Steinberg R, Jung M, Gully D, Roger P, Geslin M, Scatton B, Maffrand JP, Soubrie P. 4-(2-Chloro-4-methoxy-5-methylphenyl)-N-[(1S)-2-cyclopropyl-1-(3-fluoro-4-methylphenyl) ethyl]5-methyl-N-(2-propynyl)-1, 3-thiazol-2-amine hydrochloride (SSR125543A), a potent and selective corticotrophin-releasing factor(1) receptor antagonist. II. Characterization in rodent models of stress-related disorders. J Pharmacol Exp Ther. 2002;301:333–345. doi: 10.1124/jpet.301.1.333. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Richey KJ, Schmid MJ, LoPresti ML, Watkins LR, Maier SF. The role of corticotropin-releasing hormone in the dorsal raphe nucleus in mediating the behavioral consequences of uncontrollable stress. J Neurosci. 2002;22:1020–1026. doi: 10.1523/JNEUROSCI.22-03-01020.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Pepin JL, DesMarteau JS, Watkins LR, Maier SF. Low doses of corticotropin-releasing hormone injected into the dorsal raphe nucleus block the behavioral consequences of uncontrollable stress. Behav Brain Res. 2003a;147:55–64. doi: 10.1016/s0166-4328(03)00133-5. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Schmid MJ, LoPresti ML, Der-Avakian A, Pellymounter MA, Foster AC, Watkins LR, Maier SF. Corticotropin releasing hormone type 2 receptors in the dorsal raphe nucleus mediate the behavioral consequences of uncontrollable stress. J Neurosci. 2003b;23:1019–1025. doi: 10.1523/JNEUROSCI.23-03-01019.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs SC, Lapsansky J, Lovenberg TW, De Souza EB, Chalmers DT. Corticotropin-releasing factor CRF1, but not CRF2, receptors mediate anxiogenic-like behavior. Regul Pept. 1997;71:15–21. doi: 10.1016/s0167-0115(97)01005-7. [DOI] [PubMed] [Google Scholar]
- Ho SP, Takahashi LK, Livanov V, Spencer K, Lesher T, Maciag C, Smith MA, Rohrbach KW, Hartig PR, Arneric SP. Attenuation of fear conditioning by antisense inhibition of brain corticotropin releasing factor-2 receptor. Brain Res Mol Brain Res. 2001;89:29–40. doi: 10.1016/s0169-328x(01)00050-x. [DOI] [PubMed] [Google Scholar]
- Huhman KL, Solomon MB, Janicki M, Harmon AC, Lin SM, Israel JE, Jasnow AM. Conditioned defeat in male and female Syrian hamsters. Horm Behav. 2003;44:293–299. doi: 10.1016/j.yhbeh.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Huhman KL. Activation of GABA(A) receptors in the amygdala blocks the acquisition and expression of conditioned defeat in Syrian hamsters. Brain Res. 2001;920:142–150. doi: 10.1016/s0006-8993(01)03054-2. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Banks MC, Owens EC, Huhman KL. Differential effects of two corticotropin-releasing factor antagonists on conditioned defeat in male Syrian hamsters (Mesocricetus auratus) Brain Res. 1999;846:122–128. doi: 10.1016/s0006-8993(99)02007-7. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Cooper MA, Huhman KL. N-Methyl-D-aspartate receptors in the amygdala are necessary for the acquisition and expression of conditioned defeat. Neuroscience. 2004a;123:625–634. doi: 10.1016/j.neuroscience.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Davis M, Huhman KL. Involvement of central amygdalar and bed nucleus of the stria terminalis corticotropinreleasing factor in behavioral responses to social defeat. Behav Neurosci. 2004b;118:1052–1061. doi: 10.1037/0735-7044.118.5.1052. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Shi C, Israel JE, Davis M, Huhman KL. Memory of social defeat is facilitated by cAMP response element-binding protein overexpression in the amygdala. Behav Neurosci. 2005;119:1125–1130. doi: 10.1037/0735-7044.119.4.1125. [DOI] [PubMed] [Google Scholar]
- Jones DN, Kortekaas R, Slade PD, Middlemiss DN, Hagan JJ. The behavioural effects of corticotropin-releasing factor-related peptides in rats. Psychopharmacology. 1998;138:124–132. doi: 10.1007/s002130050654. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Rice KC, Valentino RJ. Effects of corticotropinreleasing factor on neuronal activity in the serotonergic dorsal raphe nucleus. Neuropsychopharmacology. 2000;22:148–162. doi: 10.1016/S0893-133X(99)00093-7. [DOI] [PubMed] [Google Scholar]
- Kishimoto T, Radulovic J, Radulovic M, Lin CR, Schrick C, Hooshmand F, Hermanson O, Rosenfeld MG, Spiess J. Deletion of crhr2 reveals an anxiolytic role for corticotropinreleasing hormone receptor-2. Nat Genet. 2000;24:415–419. doi: 10.1038/74271. [DOI] [PubMed] [Google Scholar]
- Liang KC, Melia KR, Miserendino MJ, Falls WA, Campeau S, Davis M. Corticotropin-releasing factor: long-lasting facilitation of the acoustic startle reflex. J Neurosci. 1992;12:2303–2312. doi: 10.1523/JNEUROSCI.12-06-02303.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry CA. Functional subsets of serotonergic neurones: implications for control of the hypothalamic-pituitary–adrenal axis. J Neuroendocrinol. 2002;14:911–923. doi: 10.1046/j.1365-2826.2002.00861.x. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Rodda JE, Lightman SL, Ingram CD. Corticotropin- releasing factor increases in vitro firing rates of serotonergic neurons in the rat dorsal raphe nucleus: evidence for activation of a topographically organized mesolimbocortical serotonergic system. J Neurosci. 2000;20:7728–7736. doi: 10.1523/JNEUROSCI.20-20-07728.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- McNaughton N, Corr PJ. A two-dimensional neuropsychology of defense: fear/anxiety and defensive distance. Neurosci Biobehav Rev. 2004;28:285–305. doi: 10.1016/j.neubiorev.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Moreau JL, Kilpatrick G, Jenck F. Urocortin, a novel neuropeptide with anxiogenic-like properties. Neuroreport. 1997;8:1697–1701. doi: 10.1097/00001756-199705060-00027. [DOI] [PubMed] [Google Scholar]
- Morin LP, Wood RI. A stereotaxic atlas of the golden hamster brain. New York: Academic; 2001. [Google Scholar]
- Olschowka JA, O’Donohue TL, Mueller GP, Jacobowitz DM. Hypothalamic and extrahypothalamic distribution of CRF-like immunoreactive neurons in the rat brain. Neuroendocrinology. 1982;35:305–308. doi: 10.1159/000123398. [DOI] [PubMed] [Google Scholar]
- Owens MJ, Nemeroff CB. Physiology and pharmacology of corticotropin-releasing factor. Pharmacol Rev. 1991;43:425–473. [PubMed] [Google Scholar]
- Pelleymounter MA, Joppa M, Ling N, Foster AC. Pharmacological evidence supporting a role for central corticotropin-releasing factor (2) receptors in behavioral, but not endocrine, response to environmental stress. J Pharmacol Exp Ther. 2002;302:145–152. doi: 10.1124/jpet.302.1.145. [DOI] [PubMed] [Google Scholar]
- Pernar L, Curtis AL, Vale WW, Rivier JE, Valentino RJ. Selective activation of corticotropin-releasing factor-2 receptors on neurochemically identified neurons in the rat dorsal raphe nucleus reveals dual actions. J Neurosci. 2004;24:1305–1311. doi: 10.1523/JNEUROSCI.2885-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience. 1998;82:443–468. doi: 10.1016/s0306-4522(97)00268-6. [DOI] [PubMed] [Google Scholar]
- Potegal M, Huhman K, Moore T, Meyerhoff J. Conditioned defeat in the Syrian golden hamster (Mesocricetus auratus) Behav Neural Biol. 1993;60:93–102. doi: 10.1016/0163-1047(93)90159-f. [DOI] [PubMed] [Google Scholar]
- Potter E, Sutton S, Donaldson C, Chen R, Perrin M, Lewis K, Sawchenko PE, Vale W. Distribution of corticotropin-releasing factor receptor mRNA expression in the rat brain and pituitary. Proc Natl Acad Sci U S A. 1994;91:8777–8781. doi: 10.1073/pnas.91.19.8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price ML, Lucki I. Regulation of serotonin release in the lateral septum and striatum by corticotropin-releasing factor. J Neurosci. 2001;21:2833–2841. doi: 10.1523/JNEUROSCI.21-08-02833.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price ML, Curtis AL, Kirby LG, Valentino RJ, Lucki I. Effects of corticotropin-releasing factor on brain serotonergic activity. Neuropsychopharmacology. 1998;18:492–502. doi: 10.1016/S0893-133X(97)00197-8. [DOI] [PubMed] [Google Scholar]
- Radulovic J, Ruhmann A, Liepold T, Spiess J. Modulation of learning and anxiety by corticotropin-releasing factor (CRF) and stress: differential roles of CRF receptors 1 and 2. J Neurosci. 1999;19:5016–5025. doi: 10.1523/JNEUROSCI.19-12-05016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbrough VB, Hauger RL, Pelleymounter MA, Geyer MA. Role of corticotropin releasing factor (CRF) receptors 1 and 2 in CRF-potentiated acoustic startle in mice. Psychopharmacology. 2003;170:178–187. doi: 10.1007/s00213-003-1535-6. [DOI] [PubMed] [Google Scholar]
- Risbrough VB, Hauger RL, Roberts AL, Vale WW, Geyer MA. Corticotropin-releasing factor receptors CRF1 and CRF2 exert both additive and opposing influences on defensive startle behavior. J Neurosci. 2004;24:6545–6552. doi: 10.1523/JNEUROSCI.5760-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakanaka M, Shibasaki T, Lederis K. Corticotropin releasing factor-like immunoreactivity in the rat brain as revealed by a modified cobalt-glucose oxidase-diaminobenzidine method. J Comp Neurol. 1987;260:256–298. doi: 10.1002/cne.902600209. [DOI] [PubMed] [Google Scholar]
- Schulz DW, Mansbach RS, Sprouse J, Braselton JP, Collins J, Corman M, Dunaiskis A, Faraci S, Schmidt AW, Seeger T, Seymour P, Tingley FD, III, Winston EN, Chen YL, Heym J. CP- 154526: a potent and selective nonpeptide antagonist of corticotropin releasing factor receptors. Proc Natl Acad Sci U S A. 1996;93:10477–10482. doi: 10.1073/pnas.93.19.10477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Timpl P, Spanagel R, Sillaber I, Kresse A, Reul JM, Stalla GK, Blanquet V, Steckler T, Holsboer F, Wurst W. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nat Genet. 1998;19:162–166. doi: 10.1038/520. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Inoue K, Koob GF, Rivier J, Vale W, Zorrilla EP. Human urocortin II: mild locomotor suppressive and delayed anxiolytic-like effects of a novel corticotropin-releasing factor related peptide. Brain Res. 2002;943:142–150. doi: 10.1016/s0006-8993(02)02707-5. [DOI] [PubMed] [Google Scholar]
- van Bockstaele EJ, Biswas A, Pickel VM. Topography of serotonin neurons in the dorsal raphe nucleus that send axon collaterals to the rat prefrontal cortex and nucleus accumbens. Brain Res. 1993;624:188–198. doi: 10.1016/0006-8993(93)90077-z. [DOI] [PubMed] [Google Scholar]
- van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Yang M, Farrokhi C, Vasconcellos A, Blanchard RJ, Blanchard DC. Central infusion of ovine CRF (oCRF) potentiates defensive behaviors in CD-1 mice in the Mouse Defense Test Battery (MDTB) Behav Brain Res. 2006;171:1–8. doi: 10.1016/j.bbr.2006.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]