Abstract
Hypothalamic glutamate and gamma-aminobutyric acid (GABA) neurotransmission are involved in the ovarian hormone-induced GnRH-LH surge in rodents. We previously reported that middle-aged rats have significantly less glutamate release in the medial preoptic area than young rats on the day of the LH surge. The present study tested the hypothesis that the delayed and attenuated LH surge in ovariohysterectomized middle-aged rats primed with ovarian steroids results from reduced hypothalamic glutamate and increased GABAA neurotransmission. Microdialysis results show that middle-aged rats with attenuated LH surges had reduced extracellular glutamate and increased extracellular GABA levels in the medial preoptic area compared with young rats. Blocking GABAA receptors with bicuculline or inhibiting synaptic glutamate reuptake with l-trans-pyrrolidine-2,4-dicarboxylic acid increased extracellular Glu in the medial preoptic area and partially restored LH surge amplitude in middle-aged rats without altering LH surge onset. Complete recovery of LH surge amplitude was observed in middle-aged rats treated with the combination of bicuculline and l-trans-pyrrolidine-2,4-dicarboxylic acid. This treatment also restored the extracellular glutamate:GABA ratio in the medial preoptic area of middle-aged rats to the level of young rats. Immunoblot analysis revealed that estradiol and progesterone treatment reduced SLC32A1(formerly known as vesicular GABA transporter) levels and increased SLC17A6 (formerly known as vesicular glutamate transporter 2) levels in the anterior hypothalamus of ovariohysterectomized young but not middle-aged rats. These data suggest that both reduced availability of glutamate and increased activation of GABAA receptors under estrogen-positive feedback conditions contribute to the age-related delay in onset and attenuated amplitude of the LH surge..
Keywords: aging, estradiol, GABAA receptors, glutamate, gonadotropin-releasing hormone neurons, hypothalamus, luteinizing hormone
Age-related changes in the LH surge are caused at least in part by an imbalance in glutamatergic and GABAergic afferent inputs onto GnRH neurons on the day of the LH surge.
INTRODUCTION
Reproductive aging in many female mammals is characterized by dysregulated LH surges and increased follicle-stimulating hormone release [1, 2]. It has been postulated that the age-related decline in functional ovarian reserve [3, 4] leads to a hypoestrogenic and hypoprogestinemic hormonal milieu, which then triggers changes in gonadotropin release patterns that herald the transition into reproductive senescence [3–5]. Although it is clear that ovarian failure ultimately defines reproductive quiescence in most species, a growing body of literature implicates the neuroendocrine axis as an early contributor to the process of reproductive senescence in both primates and nonprimates [6–12].
In rodents, the onset of reproductive senescence is marked by a delayed onset and attenuated amplitude of the preovulatory LH surge [12, 13]. This change in LH release begins to occur when rodents are 8–12 mo old, before the onset of irregular estrous cycling and at an age equivalent to the early human perimenopause [14]. These early age-related changes in the LH surge correlate with evidence of hypothalamic dysfunction (for review, see Brann and Mahesh [15]). However, neither reduced basal GnRH peptide release [11, 16, 17], reduced capacity for activated GnRH neurons to release GnRH [16], nor reduced GnRH neuronal density [18, 19] account for the age-related changes in LH release.
Altered LH release in regularly cycling middle-aged females correlates with altered GnRH pulsatility [20] and release [21] and a 50% reduction in the number of GnRH neurons activated in response to estrogen-positive feedback [22], all of which result in reduced GnRH release on the day of proestrus [21]. The etiology of age-related changes in GnRH neuronal activation under estrogen-positive feedback conditions is poorly understood. Because GnRH neurons do not express ESR1 (formerly known as estrogen receptor-α) [23, 24], which mediates estrogen-positive feedback [25, 26], it is likely that age-related changes in the LH surge reflect a failure of GnRH neurons to receive or respond to excitatory and/or inhibitory afferent inputs that are normally regulated by gonadal steroids [6, 22].
Hypothalamic control of the LH surge involves estrogen-dependent shifts in the balance between excitatory and inhibitory neurotransmission in the hypothalamus [27–32]. The LH surge in young adult females is associated with increases and decreases in extracellular glutamate (Glu) and gamma-aminobutyric acid (GABA) levels, respectively, in the medial preoptic area (mPOA) [33–36]. In young adult, steroid-primed female rats, GABA inhibits [37, 38] and Glu stimulates GnRH release [39]. GABA agonists block the LH surge [37, 40], an effect mediated primarily by GABAA receptors [41, 42], and Glu agonists stimulate GnRH synthesis and LH release [39, 43]. In contrast, GABAA receptor antagonists advance [44] and Glu receptor antagonists block the LH surge [45]. We recently documented that reduced extracellular Glu levels in the mPOA accompany the delayed and attenuated LH surge of regularly cycling, middle-aged rats that were ovariohysterectomized and primed with ovarian steroids [46]. This suggests that middle-aged females do not exhibit an elevation of hypothalamic Glu release under estrogen-positive feedback conditions. The present study tests the hypothesis that prior to the appearance of irregular estrous cyclicity, middle-aged females also fail to respond to estrogen-positive feedback with decreased mPOA GABA levels on the day of the LH surge. In addition, we hypothesized that if decreased extracellular mPOA Glu and/or increased GABA levels are causally related to LH surge abnormalities in middle-aged rats, then increasing mPOA Glu levels and/or decreasing mPOA GABAA receptor activation should rescue the LH surge in these females. We also used semiquantitative immunoblotting to investigate the effects of age and hormone treatment on presynaptic Glu and GABA neuronal elements by examining vesicular proteins important for packaging of Glu and GABA officially called SLC17A6 and SLC32A1, respectively, but referred to as VGLUT2 and VGAT, respectively, in this manuscript [47, 48].
MATERIALS AND METHODS
Animals
Young (virgin, ages 2–3 mo) and middle-aged (retired breeder, ages 8–10 mo) female Sprague-Dawley rats were purchased from Taconic Farms (Germantown, NY). All rats had access to food and water ad libitum and were housed individually and maintained on a 14L:10D cycle with lights off at 2000 h. We are interested in identifying age-related changes in hypothalamic neurotransmitters under estrogen-positive feedback conditions that occur in the early stages of reproductive aging and that may underlie LH surge changes when middle-aged females still exhibit normal estrous cycles. Therefore, only rats that exhibited a minimum of two normal 4-day estrous cycles, as determined by daily vaginal smears, were used.
Drugs
Bicuculline, a GABAA receptor antagonist, and l-trans-pyrrolidine-2,4-dicarboxylic acid (TPDC), an inhibitor of Glu reuptake through the membrane transporter, were purchased from Tocris Bioscience (Ellisville, MO) and dissolved in artificial cerebrospinal fluid (ACSF; 124 mM sodium chloride, 5 mM potassium chloride, 1.2 mM monopotassium phosphate, 10 mM magnesium sulfate, 1.8 mM calcium chloride, 26 mM sodium bicarbonate, and 10 mM dextrose, pH 7.4) on the day of microdialysis. Components of the ACSF were purchased from Fisher Scientific (Pittsburgh, PA). Estradiol benzoate (E2B) and progesterone (P) were purchased from Steraloids Inc. (Newport, RI).
Stereotaxic Surgery and Jugular Vein Catheterization
Young and middle-aged rats that exhibited normal estrous cycles were anesthetized with intramuscular ketamine (80 mg/kg) and xylazine (4 mg/kg) for ovariohysterectomy (removal of both ovaries and uterine horns) [49] before intracerebral microdialysis guide cannula placement. Anesthetized rats were placed in a Kopf stereotaxic apparatus and secured with ear bars and a nose piece set at +5 mm. Using Bregma as a landmark and stereotaxic coordinates provided in the atlas of Pellegrino [50] (dorsal/ventral −8.6, anterior/posterior +2.0, and medial/lateral ±0.6), a unilateral 23-gauge guide cannula was implanted in the mPOA. Guide cannulae and concentric dialysis probes (2-mm dialysis surface with 340-μm outer diameter) were purchased from Bioanalytical Systems Inc. (West Lafayette, IN). On the seventh to eighth postoperative day, implanted rats were lightly anesthetized with ketamine/xylazine, and an internal jugular vein catheter was placed in the right atrium for serial blood sampling [51]. Catheters were kept patent with daily infusion of 0.2 ml heparinized saline (50 IU). All studies were carried out according to protocols approved by the Institutional Animal Care and Use Committee of Albert Einstein College of Medicine and according to National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Gonadal Steroid Priming
To insure that young and middle-aged females were exposed to equivalent E2 and P levels, ovariohysterectomized rats were primed with gonadal steroids for induction of the GnRH-LH surge [52]. At 0900 h on the day of catheterization (7 days after ovariohysterectomy), rats were injected subcutaneously (s.c.) with 2 μg E2B in 0.1 ml peanut oil; a second injection was given 24 h later. A single s.c. injection of 500 μg P was given at 0900 h, 48 h after the first E2B injection [53].
Microdialysis Sampling and Serum Collection
Each animal was only used once and was dialyzed with a new probe to eliminate the risk of using a damaged probe. Additionally, to avoid nonspecific increases in neurotransmitter release caused by acute mechanical injury at the time of probe placement [54], probes were lowered and microdialysis initiated at a reduced flow rate (0.125 μl/min) at least 12 h prior to the start of each experiment. Microdialysis sample collection from unanesthetized, freely moving rats began 1 h before the P injection and continued at 30-min intervals for a total of 12–13 h. Probes were perfused at 1.25 μl/min with ACSF. In some animals, extracellular Glu levels in the mPOA were elevated by reverse microdialysis with 10 mM of the Glu transporter blocker TPDC starting 1 h before the P injection and continuing until the end of the experiment. Middle-aged rats dialyzed only with ACSF served as the control for middle-aged rats treated with TPDC. GABAA receptor antagonism was achieved by infusing 10 μM bicuculline in 1.5 μl ACSF over 1 min directly into the mPOA at 0900 h and 1300 h on the day of the P injection. Middle-aged rats dialyzed with ACSF and infused with 1.5 μl normal saline over 1 min directly into the mPOA at 0900 h and 1300 h on the day of the P injection served as the controls for middle-aged rats treated with bicuculline or bicuculline + TPDC. The mPOA infusions were delivered through a cannula attached to the dialysis probe. All microdialysis samples were acidified to 0.1 N perchloric acid, flash frozen, and stored at −70°C until analysis with high-performance liquid chromatography (HPLC). The recovery rate for Glu and GABA is approximately 15% and is quite consistent across individual probes, based on in vitro calibrations carried out with randomly selected probes. Blood sampling was initiated either 1 h before or at the time of the P injection and continued every 1–2 h for a total of 12 h. Approximately 200 μl blood was collected into Eppendorf tubes containing 100 μl ice-cold heparinized saline (10 IU), refrigerated overnight, and centrifuged at 10 000 × g for 20 min. Plasma was removed with a glass pipette and stored at −70°C until assayed for LH. An equal volume of warmed saline was infused to replace collected blood and to avoid hypovolemia. At the end of each experiment, animals were overdosed with ketamine, decapitated, and the brain rapidly frozen in 2-methylbutane. Frozen brains were stored at −70°C until cryostat sectioning for histological assessment of probe placement.
Histological Verification of Probe Placements
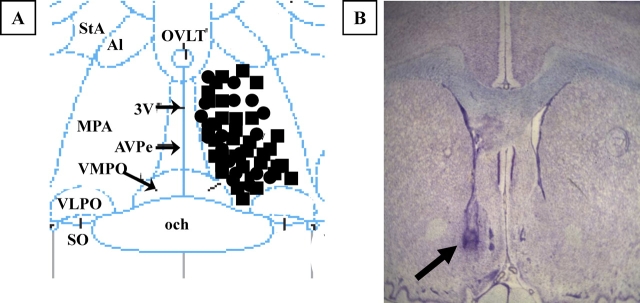
Frozen brains were sectioned in the transverse plane with a cryostat. Every third 40-μm section was saved throughout the extent of the dialysis probe track, stained with thionin, and the intracerebral microdialysis probe placement in the mPOA was evaluated (Fig. 1). Only rats with a confirmed GnRH-LH surge and appropriate probe placement in the mPOA were included in the data analysis. One to two rats from each age group were discarded due to inaccurate probe placement and/or clogging of the microdialysis probe during the experiment.
FIG. 1.
A) Illustration of microdialysis probes placement in the medial preoptic area. The diagram corresponds to a coronal section at approximately 0.0 mm relative to Bregma according to the atlas of Paxinos and Watson [55]. MPA, Medial preoptic area; 3V, third ventricle; och, optic chiasm; VMPO, ventromedial preoptic nucleus; VLPO, ventrolateral preoptic nucleus; AVPe, anteroventral periventricular nucleus; SO, supraoptic nucleus; Al, alar nucleus; StA, strial part preoptic nucleus; OVLT, organum vasculosum lamina terminalis. Black circles indicate young rats; black squares, middle-aged rats. B) Photomicrograph of thionin-stained coronal section showing the approximate location of a microdialysis probe. The arrow indicates the site of probe placement (original magnification ×4).
LH Assay
Plasma LH levels are reported as nanograms per milliliter. In Table 1 and Figure 2, the National Hormone and Peptide Program ( www.humc.edu/hormones ) determined LH levels using rat radioimmunoassay reagents. The lower limit of the assay was 0.1 ng/ml. Using rat LH radioimmunoassay reagents provided by the National Hormone and Peptide Program, the Hormone and Bioassay Core at Northwestern University determined plasma LH values in Table 2 and Figures 2 and 3. The lower limit of this LH assay was 0.2 ng/ml, and the intraassay and interassay coefficients of variation were 7.6% and 5.8%, respectively. Onset of the GnRH-LH surge was defined as plasma LH levels equal to or greater than 1.5 times baseline sustained for a minimum of two consecutive samples. Baseline LH levels were defined as the average LH value observed from samples collected 1 h before and at the time of the P injection. One to two animals from each age group were eliminated from data analysis because they failed to exhibit a GnRH-LH surge.
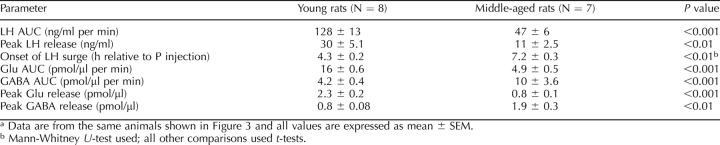
TABLE 1.
The age-related changes in LH surge onset and peak amplitude are associated with reduced extracellular Glu and GABA in the medial preoptic area.a
FIG. 2.
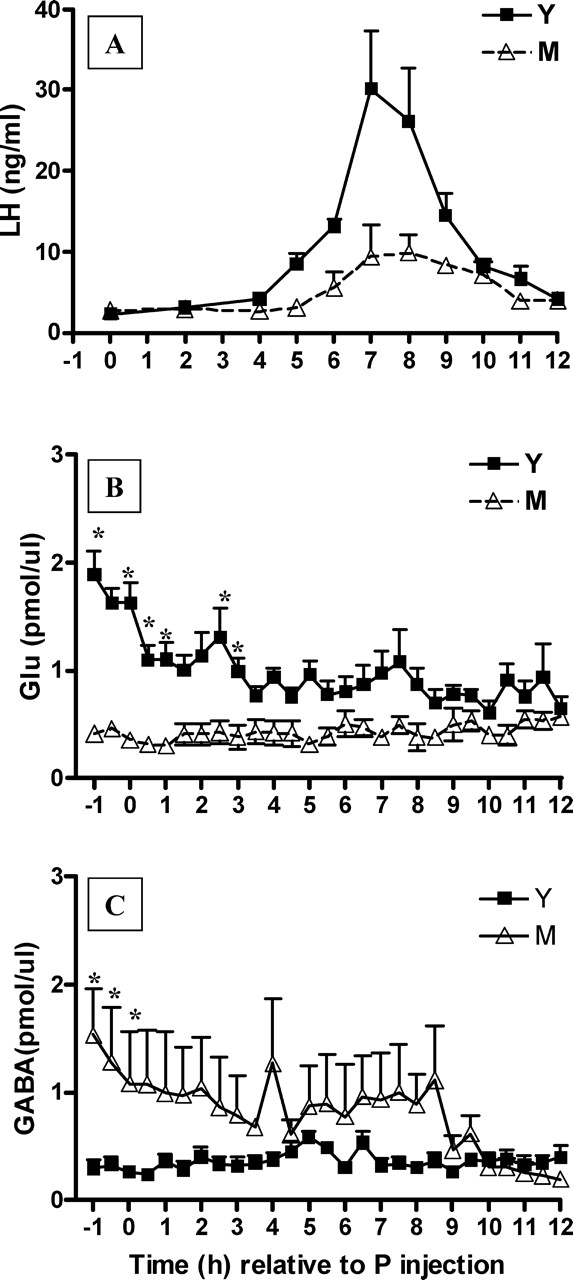
Middle-aged (M) rats exhibit a delayed and attenuated GnRH-LH surge that is accompanied by decreased extracellular Glu and increased extracellular GABA in the mPOA on the day of the GnRH-LH surge relative to young (Y) rats. Data are from GnRH-LH surges generated in Y (n = 8) and M (n = 7) rats primed with E2B and P as described in Materials and Methods. Time 0 represents the time of the P injection at 0900 h. LH (A), extracellular Glu (B), and extracellular GABA (C) levels in the mPOA. *P < 0.001. See Table 1 for additional quantitation and statistical comparisons. ul, Microliter.
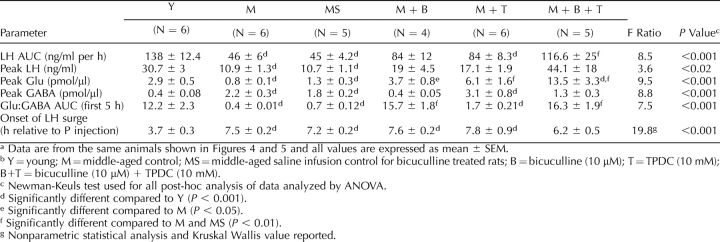
TABLE 2.
Increasing medial preoptic area Glu and decreasing GABAA receptor activation restore LH surge amplitude and advances the LH surge onset in middle-aged rats.a,b
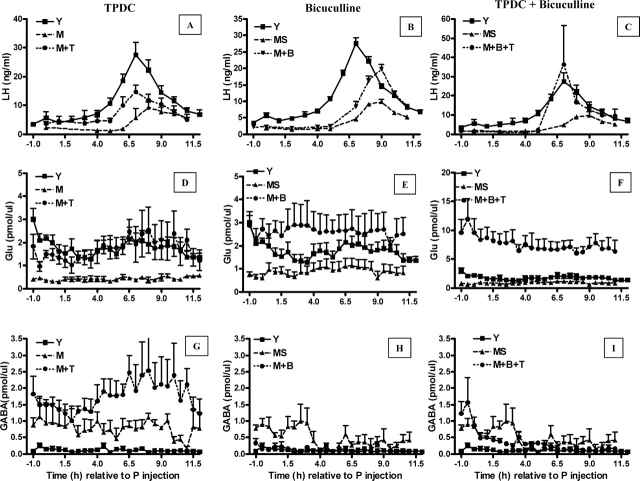
FIG. 3.
Elevation of extracellular Glu and inhibition of extracellular GABA in the mPOA can increase GnRH-LH surge amplitude in middle-aged females (A–I). Data are means ± SEM of young controls (Y; n = 7), middle-aged controls (M; n = 6), middle-aged controls with saline infusion at 0900 h and 1300 h (MS; n = 5), M infused at 0900 h and 1300 h with 10 μM bicuculline (M + B; n = 5), M reverse dialyzed with 10 mM TPDC (M + T; n = 6), and M treated with a combination of 10 μM bicuculline and 10 mM TPDC (M + B + T; n = 6). Time 0 is relative to the P injection at 0900 h. Each treatment is illustrated on a separate panel with the appropriate middle-aged control group (M) graphed for comparison. The same young control (Y) is depicted in each panel. See Figure 4 and Table 2 for additional quantitation and statistical comparisons.
HPLC Analysis of Glu and GABA
Amino acid content in the mPOA microdialysis samples was quantified by methods described previously [56]. Briefly, microdialysis samples were neutralized to pH 5 with 2.5 M potassium carbonate. After o-phthaldehyde derivatization, amino acids were separated by HPLC and detected with a Beckman 175 fluorometer. Derivatized samples were applied to a C-18 column (0.4 × 2.5-cm reverse phase; Beckman 5-μm Ultrasphere) at a flow rate of 0.75 ml/min. A programmed gradient elution was created with two solvent mixtures (Solvent A-0.1 M sodium acetate, pH 5.9, in 10% methanol; Solvent B-80% methanol). Amino acid identification and quantification were achieved by comparing peak retention times and heights of known standards (Sigma, St. Louis, MO) to unknown samples [57]. Amino acid content is reported as picomoles per microliters. The lower limit for detection of Glu and GABA was 0.08 pmol.
Hypothalamic Dissection and Synaptosome Preparation
Independent groups of ovariohysterectomized young and middle-aged rats were primed with peanut oil (control) or E2B + P as described above. Rats were killed 4 h after the P or final oil injection. A block of brain tissue bordered laterally by the hypothalamic sulci, rostrally 2 mm anterior to the optic chiasm, and caudally at the mammillary bodies was dissected. The hypothalamus was then transected just posterior to the optic chiasm. The anterior half of the hypothalamus contains GnRH cell bodies, and the posterior half contains the median eminence (site of GnRH nerve terminals) and the arcuate nucleus.
Synaptosomes were prepared from homogenized hypothalamic regions using a modification of a published method [58]. Briefly, hypothalamic fragments were homogenized with a glass-teflon homogenizer (12 strokes/900 rpm) in 5 ml chilled homogenizing buffer (0.32 M sucrose, 10 mM N-2 hydroxyethylpiperazine-N′-2-ethanesulfonic acid-sodium hydroxide (HEPES-NaOH), 1 mM PMSF, and protease inhibitor cocktail (Mini-complete; Roche, Indianapolis, IN) and centrifuged 10 min at 800 × g. The pellet was set aside and the supernatant centrifuged at 9200 × g for 15 min. The pellet was collected, washed in homgenization buffer, and lysed in buffer (50 mM HEPES, pH 7; 250 mM sodium chloride; 5 mM EDTA; 0.1% nonyl phenoxylpolyethoxyethanol containing protease inhibitor cocktail) and centrifuged at 20 000 × g for 30 min. The supernatant was collected, and the protein concentration was determined with the Bradford protein assay (Pierce, Rockford, IL). Samples were stored at −80°C until they were used for Western blots.
Western Blot
Western blots were analyzed to determine the effects of age and hormone treatment on anterior and posterior hypothalamic VGAT and VGLUT2 protein expression. Briefly, synaptosomal aliquots (5 μg protein) prepared from the anterior and posterior hypothalamic fragments were loaded onto 8% polyacrylamide gels and subjected to electrophoresis. Proteins were transferred to nitrocellulose membranes for immunoblotting with primary antibodies against VGAT (1:3000; Chemicon International, Temecula, CA), VGLUT2 (1:8000; Sigma), SYP (formerly known as synaptophysin, a synaptosome marker; 1:30 000; Sigma), and ACTB (formerly known as β-actin; loading control; 1:4000; Sigma) [59]. Immunoblots were first probed with anti-VGAT overnight at 4°C in TBS with 5% milk, washed, stripped, and subsequently reprobed with anti-VGLUT2, anti-SYP and, lastly, anti-ACTB. Protein bands were detected after 1.5 h of incubation with horseradish peroxidase-conjugated goat secondary antibody and chemiluminescence enhancement (1:4000; ECL reagent; Perkin-Elmer, Waltham, MA). To quantify protein levels, membranes were apposed to x-ray film and bands analyzed with a Scan Jet 4-C (Hewlett Packard) computing densitometer using National Institutes of Health image 1.61 software. Band densities for SYP were corrected for variations in loading and normalized to the corresponding band densities for total ACTB. All VGAT and VGLUT2 data were expressed relative to SYP abundance, the synaptosomal marker, in the same sample.
Statistical Analysis
The integrated area under the curve for total mPOA Glu and GABA levels and total serum LH release was calculated using Pharm/PCS version 4.2 ( PharmSoft.Net , division of MCS; Wynnewood, PA). All data were expressed as mean ± SEM. ANOVA or a Student t-test was used to evaluate age-related differences in peak and total serum LH, peak and total extracellular mPOA Glu and GABA release, and the onset time of the GnRH-LH surge relative to the time of P injection. A two-way ANOVA (age × hormone treatment) was used to determine differences in VGLUT2 and VGAT protein expression in the anterior and posterior hypothalamus on the day of the GnRH-LH surge. A P value of ≤0.05 was accepted as statistically significant. ANOVA with repeated measures on time were used to determine age-related differences in extracellular Glu and GABA, and plasma LH release over time on the day of the LH surge in young and middle-aged rats. When data were not normally distributed, a nonparametric test, Kruskal-Wallis or Mann-Whitney U, was used. Posthoc tests used for multiple comparisons were Newman-Keuls (NK) or Fisher protected least significant difference (PLSD) for ANOVA and two-way ANOVA and Bonferonni for repeated-measures ANOVA.
RESULTS
Middle-Aged Rats Exhibit a Delayed and Attenuated LH Surge
Equivalent percentages of young (14 of 16) and middle-aged (33 of 35) rats with verified probe placements (Fig. 1) exhibited a GnRH-LH surge with our E2B + P protocol. Baseline LH values at the time of P injection were equivalent in young and middle-aged rats (Fig. 2A). Middle-aged rats released significantly less LH on the day of the surge (t = 3.2, P < 0.001; Table 1), exhibited a GnRH-LH surge delayed by an average of 2.5 h (Mann-Whitney U, P < 0.001; Fig. 2A and Table 1), and had approximately two-thirds lower peak LH levels compared with young control rats (t = 3.2, P < 0.05; Table 1).
The Delayed and Attenuated GnRH-LH Surge in Middle-Aged Rats Is Associated with Lower Extracellular Glu and Higher Extracellular GABA in the mPOA
Consistent with our previous report [46], the hormone-induced GnRH-LH surge in middle-aged rats was associated with significantly less (t = 7.4, P < 0.001; Table 1) total extracellular Glu; mPOA Glu levels in middle-aged rats were more than two thirds less than those measured in young rats that exhibited a GnRH-LH surge (Fig. 2B and Table 1). In contrast with the pattern of Glu release, middle-aged rats had more than 2-fold higher total extracellular GABA levels in the mPOA than young rats (Fig. 2C and Table 1) on the day of the E2B + P-induced GnRH-LH surge. Two-way ANOVA with repeated measures on time indicated significant main effects of age (LH: F = 11.2(1,110), P < 0.01; Glu: F = 61(1,338), P < 0.001), with young rats releasing more LH and more Glu in the mPOA on the day of the LH surge. There was also an effect of time (LH: F = 11.8(10,110), P < 0.001; Glu: F = 2.1(26,338), P < 0.002; GABA: F = 3.28(26,338), P < 0.0001) and a significant interaction between age and time (LH: F = 11.8(10,110), P < 0.001; Glu: F = 2.7(26,338), P < 0.001; GABA: F = 3.60(26,338), P < 0.001) on all three measures (Fig. 2). Most of the difference between young and middle-aged Glu (3.8 ± 0.2 vs. 1.2 ± 0.2 pmol/μl per min; t = 11.7, P < 0.001; Fig. 2B) and GABA (1.6 ± 0.1 vs. 5.2 ± 1.7 pmol/μl per min; t = 2.2, P < 0.05; Fig. 2C) release in the mPOA occurred during the first 5 h of the experiment, before peak LH release (Fig. 2A). Middle-aged rats had peak extracellular mPOA Glu levels that were approximately two-thirds lower (t = 5.4, P < 0.001; Table 1) and peak extracellular GABA levels more than 2-fold greater (t = 3.9, P < 0.01; Table 1) than those observed in young controls on the day of the GnRH-LH surge.
Increasing mPOA Glu Levels Partially Restores the E2B + P-Induced GnRH-LH Surge
Reverse microdialysis with 10 mM TPDC, a Glu transporter blocker [60], into the mPOA of middle-aged rats had a significant main effect (F = 219.6(5,650), P < 0.0001) on Glu. On the day of the GnRH-LH surge, TPDC increased total extracellular mPOA Glu by 6-fold (Figs. 3D and 4B) and increased peak extracellular Glu levels by more than 3-fold compared with middle-aged control rats (Table 2). TPDC also increased total (Figs. 3G and 4C) and peak mPOA GABA levels (Table 2). Therefore, the mPOA Glu:GABA ratio of TPDC-treated, middle-aged females on the day of the GnRH-LH surge only increased by 2-fold and was still significantly less than the Glu:GABA ratio in young control rats (Table 2). TPDC did not recover total (Figs. 3A and 4A) or peak LH release (Table 2). TPDC-treated, middle-aged female LH values (area under the curve and peak LH) were not significantly different from those from either middle-aged or young control rats. Reverse microdialysis of TPDC did not change the onset of the GnRH-LH surge (Table 2).
FIG. 4.
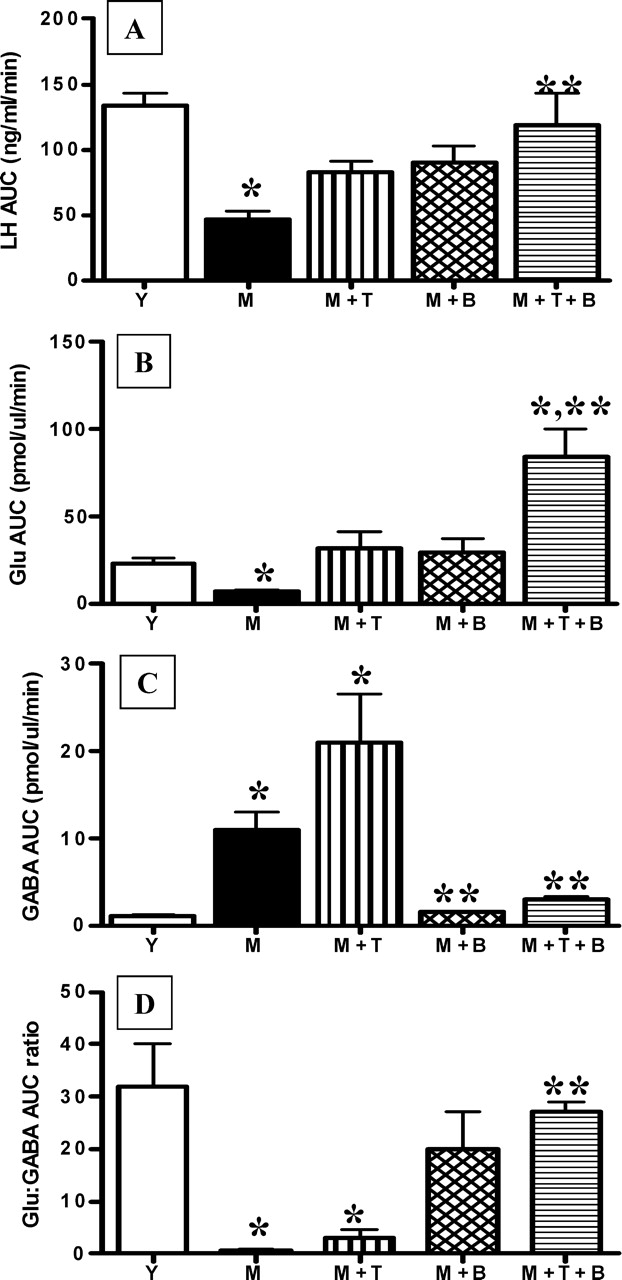
Increasing mPOA Glu release and decreasing mPOA GABAA receptor activation with TPDC plus bicuculline rescues total LH release. Because middle-aged controls (M) and middle-aged controls with saline infusion at 0900 h and 1300 h (MS) were not significantly different, data from M are illustrated. Total LH (A), Glu (B), GABA (C), and Glu:GABA ratio (D) during the GnRH-LH surge in young control (Y; n = 8), M (n = 6), M infused at 0900 h and 1300 h with 10 μM bicuculline (M + B; n = 5), M reverse dialyzed with 10 mM TPDC (M + T; n = 6) and M treated with a combination of 10 μM bicuculline and 10 mM TPDC (M + B + T; n = 6) rats are illustrated. *Significantly different from Y (P < 0.01). **Significantly different from M (P < 0.05). AUC, Area under the curve.
Medial POA GABAA Receptor Antagonism Partially Restores E2B + P-Induced LH Release
Infusion of 10 μM of the GABAA receptor antagonist bicuculline into the mPOA at the time of the P injection and 4 h later significantly affected extracellular GABA (F = 7.84(5,650), P < 0.001) in middle-aged rats. There was also a significant main effect of time on GABA release (F = 2.07(24,650), P < 0.003) in middle-aged rats (Figs. 3H and 4C). Bicuculline also significantly affected Glu release from middle-aged rats (F = 219(5,650), P < 0.001). Bicuculline treatment decreased total (Figs. 3H and 4C) and peak (Table 2) extracellular mPOA GABA levels by 4-fold and 5-fold, respectively, and increased total Glu (Figs. 3E and 4B) and peak (Table 2) extracellular Glu levels by more than 4-fold. This resulted in more than a 15-fold increase in the total Glu:GABA ratio (Table 2) relative to middle-aged controls, so that middle-aged females treated with bicuculline had total Glu:GABA ratios that were equivalent to those in young control rats (Table 2). However, although bicuclline increased peak and total LH release by almost 2-fold, these values were not significantly different from those in middle-aged controls (Figs. 3A and 4B and Table 2). Moreover, in contrast with reports in young adult females [61], bicuculline did not advance the onset of the GnRH-LH surge in middle-aged rats (Table 2).
Simultaneously Increasing Extracellular Glu and Blocking GABAA Receptors in the mPOA Restores GnRH-LH Surge Amplitude
The combination of 10 mM TPDC and 10 μM bicuculline administered as described above completely rescued total and peak LH release in E2B + P-primed, middle-aged rats to the level of young controls (Figs. 3C and 4A and Table 2). TPDC + bicuculline increased total extracellular Glu by more than 10-fold (Figs. 3F and 4B) and decreased total extracellular GABA by more than two thirds (Figs. 3I and 4C) relative to middle-aged control rats. Similarly, TPDC + bicuculline increased peak mPOA Glu levels by more than 10-fold (Table 2) relative to middle-aged controls and by 6-fold relative to young controls (Table 2). Peak GABA levels in TPDC + bicuculline-treated rats were not significantly different from those in young or middle-aged controls (Table 2). Additionally, a combination of bicuculline and TPDC increased the total Glu:GABA ratio to more than 20-fold greater than in middle-aged controls, so that it was equivalent to that in young controls (Fig. 4D). TPDC + bicuculline treatment did not significantly advance the onset of the GnRH-LH surge (Table 2); the GnRH-LH surge onset in middle-aged rats treated with both bicuculline and TPDC was not significantly different from that in young or middle-aged control rats.
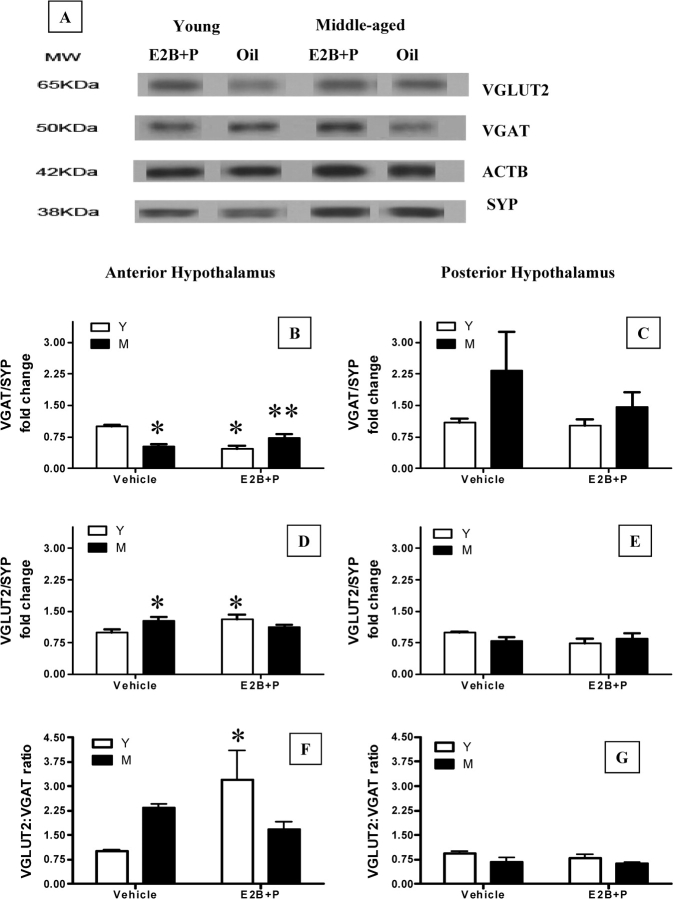
Hormone Treatment Increases VGLUT2 and Decreases VGAT Protein Expression in the Anterior Hypothalamus of Young Female Rats
The effect of age and hormone treatment on the abundance of VGAT and VGLUT2, markers for GABAergic and glutamatergic axonal terminals, respectively, were assessed by Western blot analysis (Fig. 5A). Two-way ANOVA found a main effect of hormone treatment on VGAT expression in the anterior hypothalamus (F = 6(1,19), P < 0.02), with higher levels observed in vehicle-treated young rats. There was also an interaction between age and hormone treatment (F = 28(1,19), P < 0.001); posthoc comparison indicated that VGAT expression was suppressed by gonadal steroid treatment in young but not middle-aged rats (NK P < 0.001; Fig. 5B). Middle-aged females treated with hormones also had higher VGAT than young females treated with hormones (NK P < 0.05; Fig. 5B). Although neither age nor hormone treatment alone significantly affected anterior hypothalamic VGLUT2 expression, there was an interaction between age and hormone treatment (F = 6.5(1,20), P < 0.02). Hormone treatment increased VGLUT2 expression in the anterior hypothalamus of young but not middle-aged rats (NK P < 0.05; Fig. 5D). In fact, middle-aged control females had elevated levels of VGLUT2 (P < 0.05, PLSD) compared with young controls, which were comparable to young, hormone-treated females. There was also an interaction between age and hormone treatment on the ratio of VGLUT2:VGAT expression in the anterior hypothalamus (F = 5.2(1,19), P < 0.05; Fig. 5F); hormone treatment significantly increased the ratio of VGLUT2:VGAT expression only in young rats (NK P < 0.05). There was neither a main effect of age or hormone treatment, nor an interaction between age and hormone treatment on VGAT, VGLUT2, or the ratio of VGLUT2:VGAT expression in the posterior hypothalamus (Fig. 5, C, E, and G).
FIG. 5.
Effects of steroid priming on VGAT and VGLUT2 abundance in the anterior and posterior hypothalamus. Representative Western blot gels showing abundance of synaptosomal VGAT and VGLUT2 in hypothalamic regions from vehicle and E2B + P-treated young and middle-aged rats are illustrated (A). SYP (formerly known as synaptophysin) was used as a marker for synaptosomes. ACTB (formerly known as β-actin), a cytoskeletal protein, was used as a loading control. The VGLUT2 and VGAT band densities were normalized to the SYP band density in the same sample. Normalized band density of young (Y) vehicle-treated rats (control) was assigned a value of 1 for comparison with all other groups (B–G). All values are mean ± SEM of five to six independent replications (*P < 0.05 vs. young vehicle; **P < 0.05 vs. young E2B + P). M, Middle-aged control group; KDa, kilodalton.
DISCUSSION
The present study provides evidence that in addition to reduced mPOA Glu release [46], the delayed onset and reduced amplitude of the GnRH-LH surge in middle-aged female rats is accompanied by increased GABA release in the mPOA. The total Glu:GABA ratio on the day of the surge was also significantly reduced in middle-aged compared with young rats, suggesting a net increase in inhibitory and a net decrease in excitatory neurotransmission. The higher levels of extracellular GABA in the mPOA on the day of the GnRH-LH surge in middle-aged rats also correlated with a failure of E2 and P to decrease VGAT and increase VGLUT2 levels in the anterior hypothalamus, where most GnRH neurons are located. Therefore, GnRH-LH surge abnormalities in middle-aged females may result in part from changes in the balance between extracellular Glu and GABA release in the mPOA under estrogen-positive feedback conditions. Moreover, increased availability of vesicular pools of GABA in the mPOA of middle-aged females may contribute to increased extracellular GABA and decreased Glu release on the day of the surge.
The opposing roles of GABAergic and glutamatergic neurotransmission in neuroendocrine regulation of reproduction [62], especially the importance of increased excitatory input by Glu [32, 33, 46] and decreased inhibitory input by GABA [63] acting on GABAA receptors [37, 64, 65] on the day of the steroid-induced LH surge, are well established in young rats. However, the role of these neurotransmitters and their receptors in the development of age-related reproductive failure is less clear [6, 11, 66–68]. It has been proposed that abnormal elevations and erratic production of gonadal steroids predispose middle-aged rats to irregular patterns of gonadotropin release [3]. To control for this possibility, we used ovariohysterectomized young and middle-aged rats that still exhibited normal estrous cycles and then provided equivalent and physiological serum levels of E2 and P [46]. Thus, in agreement with other reports [69], the abnormal GnRH-LH surge in middle-aged rats is independent of age-related differences in ovarian steroid production. Furthermore, the decreases in mPOA Glu and increases in GABA measured by microdialysis on the day of the GnRH-LH surge in middle-aged rats are not likely to result from inappropriate exposure of the hypothalamus to gonadal steroids.
If the observed amino acid neurotransmitter changes in the mPOA of middle-aged rats are responsible for the delayed and attenuated GnRH-LH surge, then we hypothesized that increasing extracellular Glu and/or decreasing GABAA neurotransmission should restore the GnRH-LH surge in middle-aged rats. To test this hypothesis, we manipulated local Glu and GABA neurotransmission in the mPOA on the day of the GnRH-LH surge by antagonizing GABAA receptor activity with bicuculline and by increasing extracellular Glu levels with TPDC. Either TPDC or bicuculline alone produced peak and total extracellular Glu levels that were equivalent to or greater than Glu levels in young controls; however, these treatments produced only partial restoration of the GnRH-LH surge. There are several possible explanations for the failure of bicuculline or TPDC alone to restore surge amplitude. Although TPDC treatment increased extracellular Glu by 3-fold, it also increased extracellular GABA by 2-fold; thus, the increase in excitatory neurotransmission may have been partly offset by the concomitant increase in GABA inhibition. Consistent with this hypothesis, the total Glu:GABA ratio of rats treated with TPDC alone was equivalent to that in middle-aged control rats and was significantly less than that in young controls. Bicuculline produced extracellular mPOA Glu release and Glu:GABA ratios that were similar to young controls, yet this drug produced only a partial recovery of peak and total LH release. It is possible that bilateral infusion of bicuculline into the mPOA would completely rescue LH surge amplitude. Alternatively, there is evidence that Glu receptor function in the hypothalamus of middle-aged females differs from that in young females [70], including reduced responsiveness of middle-aged females to the agonist N-methyl-d-aspartate (NMDA) [66]. Such postsynaptic changes in NMDA receptor function might also explain why extracellular Glu levels in the mPOA that were equivalent to those in young control rats failed to restore the GnRH-LH surge in TPDC- or bicuculline-treated, middle-aged rats.
The combination of TPDC and bicuculline completely restored GnRH-LH surge amplitude in middle-aged females, and it increased total mPOA Glu to levels that were more than 7-fold greater than those in middle-aged controls and 3-fold greater than those in young control rats. The combination of TPDC and bicuculline also decreased total GABA release in the mPOA and restored the Glu:GABA ratio to levels that were equivalent to those in young control rats. Notably, this was also the only treatment that completely rescued peak and total LH release in middle-aged rats. These findings suggest that GnRH neurons in middle-aged rats maintain some ability to respond to Glu. However, middle-aged rats may need much higher levels of extracellular Glu as well as reduced GABA to activate GnRH neurons and stimulate GnRH release. These observations support the hypothesis that middle-aged females have compromised Glu receptor activation and function in the hypothalamus under estrogen-positive feedback conditions [18, 66]. Taken together, these findings are consistent with the proposal of Wise and colleagues that age-related failure of the GnRH-LH surge reflects an altered balance of excitatory and inhibitory afferent input to GnRH neurons [6, 22], severely compromising GnRH neuron activation. Moreover, the data are in agreement with Carbone et al. [71] and Ouyang et al. [72], who showed that increased GABAergic neurotransmission attenuates Glu release, and with Yananli et al. [73], who showed that intracerebral bicuculline infusion increases Glu and decreases GABA release. Our observations are also consistent with other reports of age-related changes in the expression of Gad1 mRNA [6, 68] and increases in GAD1 activity [68]. Last, these data strongly imply that age-related changes in amino acid neurotransmission in the mPOA, and especially increased mPOA extracellular GABA levels in middle-aged rats, may inhibit Glu release, thereby reducing levels of Glu available for postsynaptic activation of GnRH neurons and/or interneurons that modulate the activity of GnRH neurons.
The cellular mechanisms responsible for altered extracellular levels of Glu and GABA on the day of the GnRH-LH surge in the mPOA of middle-aged rats are still unknown. Ottem et al. [74] recently showed that in response to E2 priming, neuronal elements immunoreactive for VGAT and VGLUT2 decreased and increased, respectively, in the anteroventral periventricular region of the anterior hypothalamus of young female rats. Because these cells innervate GnRH neurons, the findings suggest that E2 alters the balance of afferent input onto GnRH neurons toward excitation on the day of the GnRH-LH surge. Therefore, we hypothesized that age-related changes in Glu and GABA might result from the failure of neuronal elements located in the anterior hypothalamus of middle-aged rats to upregulate VGLUT2 and downregulate VGAT expression. Using immunoblots, we observed that hormone treatments that generate GnRH-LH surges reduced anterior hypothalamic VGAT expression and increased the VGLUT2:VGAT ratio in the anterior hypothalamus of young females. In contrast, ovarian hormones increased anterior hypothalamic VGAT expression and did not change the ratio of anterior hypothalamic VGLUT2:VGAT expression in middle-aged females. This could explain why middle-aged rats have increased extracellular GABA levels and reduced extracellular Glu in the absence of significantly different VGLUT2:VGAT ratios relative to young rats. It is quite possible that middle-aged rats have equivalent or even higher levels of presynaptic stores of Glu than young rats; however, increased vesicular stores and release of GABA attenuates Glu release on the day of the LH surge. Thus, we propose that if elevated VGAT is associated with increased vesicular stores of GABA, then increased extracellular GABA levels in the mPOA could account, at least in part, for reduced Glu release [71], decreased GnRH neuronal activation [22], decreased GnRH release [21] and, consequently, attenuated LH release [12].
Our interpretation of the VGAT and VGLUT2 data is limited in that immunoblotting does not allow us to identify which populations of hypothalamic neurons manifest age-related changes in VGAT and VGLUT2 expression. The most likely neuronal populations to exhibit age-related changes in VGAT and VGLUT2 in response to gonadal steroid feedback are those located in the anteroventral periventricular region [74]. Future studies will attempt to identify the precise anatomical location and phenotype of the neurons that account for the age-related changes in VGAT and VGLUT2 expression.
Although the present study focused on hypothalamic Glu and GABA, it is clear that many different neurotransmitter systems regulate the GnRH-LH surge [75]. Therefore, our findings do not imply that Glu and GABA are the only possible neurotransmitter systems involved in LH surge failure. Of note, age-related decreases in hypothalamic norepinephrine are associated with LH surge abnormalities in middle-aged rats [8, 69, 76, 77]. Because GABAA agonists can attenuate norepinenphrine-induced LH release [37, 42], and bicuculline treatment can increase the sensitivity to norepinenphrine [78], it is quite possible that bicuculline treatment also modulates the noradrenergic system. Future studies are needed to determine whether the restoration of the GnRH-LH surge involves norepinephrine and/or other neurotransmitters and neuropeptides that are important for generation of the surge.
In addition to age-related changes in the amount of LH released on the day of the GnRH-LH surge, middle-aged rats also exhibit a delayed surge onset. GABAA receptor antagonism alone did not advance the onset of the GnRH-LH surge in middle-aged rats, which contrasts with the effect of this manipulation in young rats [61]. Indeed, none of our treatments completely restored the timing of the GnRH-LH surge in middle-aged rats. In rodents, neural signals that regulate the GnRH-LH surge and maintain estrous cyclicity are critically linked to the generation of circadian rhythms by the suprachiasmatic nucleus (SCN). A deterioration of the “biological clock” and a dyssynchrony in the rhythmicity of several key neurotransmitters communicating with GnRH neurons or their afferents have been proposed to occur in reproductively aged rats [69]. GABA turnover rates increase in the SCN of senescent, constant estrous rats [79]. It is possible that middle-aged rats also have increased GABAergic activity in the SCN on the day of the steroid-induced GnRH-LH surge and that this contributes to the delayed onset of the surge.
We have demonstrated that the attenuated and delayed LH surge in middle-aged rats is associated with an increase in GABA and a decrease in Glu in the mPOA. However, microdialysis methods cannot discern whether the increased extracellular GABA detected in middle-aged rats results in direct excitation [80] or inhibition [80] of GnRH neurons. Indeed, it is possible that the GABA we measured acts primarily on interneurons or presynaptic afferents (e.g., Glu terminals from the anteroventral periventricular region) that synapse with GnRH neurons. Electrophysiological experiments that assess the responsiveness of GnRH neurons to Glu and GABA in middle-aged rats exhibiting abnormal LH surges are needed to address this matter. Nevertheless, our data are consistent with the hypothesis that increased mPOA GABA in middle-aged rats may suppress Glu release and thereby reduce direct and/or indirect excitatory input to GnRH neurons and, as a consequence, adversely affect the generation of the LH surge.
In summary, these studies are the first to combine in vivo microdialysis with serial blood sampling to test the hypothesis that the inability of ovarian steroids to produce a normal LH surge in reproductively senescing female rats reflects altered hypothalamic Glu and GABA responsiveness to estrogen-positive feedback. Thus, we propose that a shift in the glutamatergic and GABAergic neurotransmitter environment in the mPOA on the day of the GnRH-LH surge produces a net reduction in excitatory neurotransmission mediated by Glu. Decreased mPOA Glu may be related, at least in part, to increased GABA release. Consistent with this hypothesis, simultaneously increasing mPOA Glu levels and decreasing GABA levels and GABAA receptor activation completely restores peak and total LH release in middle-aged, hormone-replaced females. Thus, GnRH neurons in middle-aged females maintain some ability to respond to a glutamatergic stimulus. However, they may require much higher levels of synaptic Glu than young rats to release equivalent total GnRH and LH. Thus, there may also be changes in the expression and/or binding affinity of Glu receptors in the mPOA of middle-aged females. These observations strongly support the hypothesis that the aging hypothalamus is less responsive to the positive feedback actions of gonadal steroids, and that altered levels of extracellular Glu and GABA in the mPOA may contribute to the delayed and attenuated GnRH-LH surge.
Acknowledgments
We are thankful to Drs. Nanette Santoro, Diane Lebesgue, Andrea Reyna, and Brigitte Todd for critical review and thoughtful comments. We would like to thank Zewei Jiang for technical support with intracerebral microdialysis, Arthur Parlow and Brigitte Mann for their assistance with LH assays, and Brittany Law for her contribution to early studies of hormonal regulation of VGAT and VGLUT2. We also acknowledge and thank Laura Bernard, who assisted with HPLC determination of Glu and GABA.
Footnotes
1Supported by National Institutes of Health grants T32 HD40135 and RO1 HD29856, the Robert Wood Johnson Foundation, and the American Federation for Aging Research.
REFERENCES
- Burger HG, Dudley EC, Robertson DM, Dennerstein L.Hormonal changes in the menopause transition. Recent Prog Horm Res 2002; 57: 257–275.. [DOI] [PubMed] [Google Scholar]
- Park SJ, Goldsmith LT, Weiss G.Age-related changes in the regulation of luteinizing hormone secretion by estrogen in women. Exp Biol Med 2002; 227: 455–464.. [DOI] [PubMed] [Google Scholar]
- Finch CE, Felicio LS, Mobbs CV, Nelson JF.Ovarian and steroidal influences on neuroendocrine aging processes in female rodents. Endocr Rev 1984; 5: 467–497.. [DOI] [PubMed] [Google Scholar]
- Faddy MJ, Gosden RG, Gougeon A, Richardson SJ, Nelson JF.Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum Reprod 1992; 7: 1342–1346.. [DOI] [PubMed] [Google Scholar]
- Burger HG, Dudley EC, Hopper JL, Shelley JM, Green A, Smith A, Dennerstein L, Morse C.The endocrinology of the menopausal transition: a cross-sectional study of a population-based sample. J Clin Endocrinol Metab 1995; 80: 3537–3545.. [DOI] [PubMed] [Google Scholar]
- Cashion AB, Smith MJ, Wise PM.Glutamic acid decarboxylase 67 (GAD67) gene expression in discrete regions of the rostral preoptic area change during the oestrous cycle and with age. J Neuroendocrinol 2004; 16: 711–716.. [DOI] [PubMed] [Google Scholar]
- Felicio LS, Nelson JF, Gosden RG, Finch CE.Restoration of ovulatory cycles by young ovarian grafts in aging mice: potentiation by long-term ovariectomy decreases with age. Proc Natl Acad Sci U S A 1983; 80: 6076–6080.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MohanKumar SM, MohanKumar PS.Aging alters norepinephrine release in the medial preoptic area in response to steroid priming in ovariectomized rats. Brain Res 2004; 1023: 24–30.. [DOI] [PubMed] [Google Scholar]
- Scarbrough K, Wise PM.Age-related changes in pulsatile luteinizing hormone release precede the transition to estrous acyclicity and depend upon estrous cycle history. Endocrinology 1990; 126: 884–890.. [DOI] [PubMed] [Google Scholar]
- Weiss G, Skurnick JH, Goldsmith LT, Santoro NF, Park SJ.Menopause and hypothalamic-pituitary sensitivity to estrogen. JAMA 2004; 292: 2991–2996.. [DOI] [PubMed] [Google Scholar]
- Zuo Z, Mahesh V, Zamorano P, Brann D.Decreased gonadotropin-releasing hormone neurosecretory response to glutamate agonists in middle-aged female rats on proestrus afternoon: a possible role in reproductive aging? Endocrinology 1996; 137: 2334–2338.. [DOI] [PubMed] [Google Scholar]
- Cooper RL, Conn PM, Walker RF.Characterization of the LH surge in middle-aged female rats. Biol Reprod 1980; 23: 611–615.. [DOI] [PubMed] [Google Scholar]
- Wise PM.Alterations in the proestrous pattern of median eminence LHRH, serum LH, FSH, estradiol and progesterone concentrations in middle-aged rats. Life Sci 1982; 31: 165–173.. [DOI] [PubMed] [Google Scholar]
- Soules MR, Sherman S, Parrott E, Rebar R, Santoro N, Utian W, Woods N.Executive summary: Stages of Reproductive Aging Workshop (STRAW). Fertil Steril 2001; 76: 874–878.. [DOI] [PubMed] [Google Scholar]
- Brann DW, Mahesh VB.The aging reproductive neuroendocrine axis. Steroids 2005; 70: 273–283.. [DOI] [PubMed] [Google Scholar]
- Rubin BS.Isolated hypothalami from aging female rats do not exhibit reduced basal or potassium-stimulated secretion of luteinizing hormone-releasing hormone. Biol Reprod 1992; 47: 254–261.. [DOI] [PubMed] [Google Scholar]
- Chakraborty TR, Gore AC.Aging-related changes in ovarian hormones, their receptors, and neuroendocrine function. Exp Biol Med (Maywood) 2004; 229: 977–987.. [DOI] [PubMed] [Google Scholar]
- Miller BH, Gore AC.N-Methyl-D-aspartate receptor subunit expression in GnRH neurons changes during reproductive senescence in the female rat. Endocrinology 2002; 143: 3568–3574.. [DOI] [PubMed] [Google Scholar]
- Krajnak K, Rosewell KL, Wise PM.Fos-induction in gonadotropin-releasing hormone neurons receiving vasoactive intestinal polypeptide innervation is reduced in middle-aged female rats. Biol Reprod 2001; 64: 1160–1164.. [DOI] [PubMed] [Google Scholar]
- Rubin BS.Hypothalamic alterations and reproductive aging in female rats: evidence of altered luteinizing hormone-releasing hormone neuronal function. Biol Reprod 2000; 63: 968–976.. [DOI] [PubMed] [Google Scholar]
- Rubin BS, Bridges RS.Alterations in luteinizing hormone-releasing hormone release from the mediobasal hypothalamus of ovariectomized, steroid-primed middle-aged rats as measured by push-pull perfusion. Neuroendocrinology 1989; 49: 225–232.. [DOI] [PubMed] [Google Scholar]
- Le WW, Wise PM, Murphy AZ, Coolen LM, Hoffman GE.Parallel declines in Fos activation of the medial anteroventral periventricular nucleus and LHRH neurons in middle-aged rats. Endocrinology 2001; 142: 4976–4982.. [DOI] [PubMed] [Google Scholar]
- Herbison AE, Horvath TL, Naftolin F, Leranth C.Distribution of estrogen receptor-immunoreactive cells in monkey hypothalamus: relationship to neurones containing luteinizing hormone-releasing hormone and tyrosine hydroxylase. Neuroendocrinology 1995; 61: 1–10.. [DOI] [PubMed] [Google Scholar]
- Legan SJ, Tsai HW.Oestrogen receptor-alpha and -beta immunoreactivity in gonadotropin-releasing hormone neurones after ovariectomy and chronic exposure to oestradiol. J Neuroendocrinol 2003; 15: 1164–1170.. [DOI] [PubMed] [Google Scholar]
- Hewitt SC, Korach KS.Oestrogen receptor knockout mice: roles for oestrogen receptors alpha and beta in reproductive tissues. Reproduction 2003; 125: 143–149.. [DOI] [PubMed] [Google Scholar]
- Rissman EF, Wersinger SR, Taylor JA, Lubahn DB.Estrogen receptor function as revealed by knockout studies: neuroendocrine and behavioral aspects. Horm Behav 1997; 31: 232–243.. [DOI] [PubMed] [Google Scholar]
- Carbone S, Szwarcfarb B, Losada M, Moguilevsky JA.Effect of ovarian hormones on the hypothalamic excitatory amino acids system during sexual maturation in female rats. Neuroendocrinology 1995; 61: 235–242.. [DOI] [PubMed] [Google Scholar]
- Goroll D, Arias P, Wuttke W.Preoptic release of amino acid neurotransmitters evaluated in peripubertal and young adult female rats by push-pull perfusion. Neuroendocrinology 1993; 58: 11–15.. [DOI] [PubMed] [Google Scholar]
- Han SK, Abraham IM, Herbison AE.Effect of GABA on GnRH neurons switches from depolarization to hyperpolarization at puberty in the female mouse. Endocrinology 2002; 143: 1459–1466.. [DOI] [PubMed] [Google Scholar]
- Kasuya E, Nyberg CL, Mogi K, Terasawa E.A role of gamma-amino butyric acid (GABA) and glutamate in control of puberty in female rhesus monkeys: effect of an antisense oligodeoxynucleotide for GAD67 messenger ribonucleic acid and MK801 on luteinizing hormone-releasing hormone release. Endocrinology 1999; 140: 705–712.. [DOI] [PubMed] [Google Scholar]
- Keen KL, Burich AJ, Mitsushima D, Kasuya E, Terasawa E.Effects of pulsatile infusion of the GABA(A) receptor blocker bicuculline on the onset of puberty in female rhesus monkeys. Endocrinology 1999; 140: 5257–5266.. [DOI] [PubMed] [Google Scholar]
- Jarry H, Hirsch B, Leonhardt S, Wuttke W.Amino acid neurotransmitter release in the preoptic area of rats during the positive feedback actions of estradiol on LH release. Neuroendocrinology 1992; 56: 133–140.. [DOI] [PubMed] [Google Scholar]
- Ping L, Mahesh VB, Wiedmeier VT, Brann DW.Release of glutamate and aspartate from the preoptic area during the progesterone-induced LH surge: in vivo microdialysis studies. Neuroendocrinology 1994; 59: 318–324.. [DOI] [PubMed] [Google Scholar]
- Jarry H, Leonhardt S, Schwarze T, Wuttke W.Preoptic rather than mediobasal hypothalamic amino acid neurotransmitter release regulates GnRH secretion during the estrogen-induced LH surge in the ovariectomized rat. Neuroendocrinology 1995; 62: 479–486.. [DOI] [PubMed] [Google Scholar]
- Neal-Perry G, Adel T, Aubuchon M, Santoro N.Evidence for a hypothalamic basis for female reproductive senescence in the human. Program & Abstracts of 87th Annual Meeting of The Endocrine Society, June 4-7, 2005, San Diego, California. The Endocrine Society2005; Abstract P2-620:513–514..
- Terasawa E, Luchansky LL, Kasuya E, Nyberg CL.An increase in glutamate release follows a decrease in gamma aminobutyric acid and the pubertal increase in luteinizing hormone releasing hormone release in the female rhesus monkeys. J Neuroendocrinol 1999; 11: 275–282.. [DOI] [PubMed] [Google Scholar]
- Adler BA, Crowley WR.Evidence for gamma-aminobutyric acid modulation of ovarian hormonal effects on luteinizing hormone secretion and hypothalamic catecholamine activity in the female rat. Endocrinology 1986; 118: 91–97.. [DOI] [PubMed] [Google Scholar]
- Seltzer AM, Donoso AO.Restraining action of GABA on estradiol-induced LH surge in the rat: GABA activity in brain nuclei and effects of GABA mimetics in the medial preoptic nucleus. Neuroendocrinology 1992; 55: 28–34.. [DOI] [PubMed] [Google Scholar]
- Brann DW, Mahesh VB.Excitatory amino acid regulation of gonadotropin secretion: modulation by steroid hormones. J Steroid Biochem Mol Biol 1992; 41: 847–850.. [DOI] [PubMed] [Google Scholar]
- Donoso AO, Banzan AM.Effects of increase of brain GABA levels on the hypothalamic-pituitary-luteinizing hormone axis in rats. Acta Endocrinol (Copenh) 1984; 106: 298–304.. [DOI] [PubMed] [Google Scholar]
- Roozendaal MM, De Kruijf HF, Reuling RJ, Threels A, Swarts JJ, Wiegant VM, Mattheij JA.Inhibition of the LH surge by restraint stress in cyclic rats: studies on the role of GABAA and GABAB receptors. Stress 1997; 1: 241–248.. [DOI] [PubMed] [Google Scholar]
- Akema T, Chiba A, Kimura F.On the relationship between noradrenergic stimulatory and GABAergic inhibitory systems in the control of luteinizing hormone secretion in female rats. Neuroendocrinology 1990; 52: 566–572.. [DOI] [PubMed] [Google Scholar]
- Carbone S, Szwarcfarb B, Otero Losada ME, Moguilevsky JA.Effects of ovarian steroids on the gonadotropin response to N-methyl-D-aspartate and on hypothalamic excitatory amino acid levels during sexual maturation in female rats. Endocrinology 1992; 130: 1365–1370.. [DOI] [PubMed] [Google Scholar]
- Funabashi T, Jinnai K, Kimura F.Bicuculline infusion advances the timing of Fos expression in LHRH neurons in the preoptic area of proestrous rats. Neuroreport 1997; 8: 771–774.. [DOI] [PubMed] [Google Scholar]
- Gore AC.Gonadotropin-releasing hormone neurons, NMDA receptors, and their regulation by steroid hormones across the reproductive life cycle. Brain Res Brain Res Rev 2001; 37: 235–248.. [DOI] [PubMed] [Google Scholar]
- Neal-Perry GS, Zeevalk GD, Santoro NF, Etgen AM.Attenuation of preoptic area glutamate release correlates with reduced luteinizing hormone secretion in middle-aged female rats. Endocrinology 2005; 146: 4331–4339.. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Voglmaier S, Seal RP, Edwards RH.VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci 2004; 27: 98–103.. [DOI] [PubMed] [Google Scholar]
- Henny P, Jones BE.Vesicular glutamate (VGlut), GABA (VGAT), and acetylcholine (VACht) transporters in basal forebrain axon terminals innervating the lateral hypothalamus. J Comp Neurol 2006; 496: 453–467.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkanias GB, Ansonoff MA, Etgen AM.Estradiol regulation of alpha 1b-adrenoceptor mRNA in female rat hypothalamus-preoptic area. J Neuroendocrinol 1996; 8: 449–455.. [DOI] [PubMed] [Google Scholar]
- Pellegrino LJ. New York:: Plenum Press;; 1979. A Stereotaxic Atlas of the Rat Brain. [Google Scholar]
- Harms PG, Ojeda SR.A rapid and simple procedure for chronic cannulation of the rat jugular vein. J Appl Physiol 1974; 36: 391–392.. [DOI] [PubMed] [Google Scholar]
- Quesada A, Etgen AM.Functional interactions between estrogen and insulin-like growth factor-I in the regulation of alpha 1B-adrenoceptors and female reproductive function. J Neurosci 2002; 22: 2401–2408.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman MC, Dupke KC, Croteau CM.Extinction of the estrogen-induced daily signal for LH release in the rat: a role for the proestrous surge of progesterone. Endocrinology 1976; 99: 223–229.. [DOI] [PubMed] [Google Scholar]
- Herbison AE, Chapman C, Dyer RG.Role of medial preoptic GABA neurones in regulating luteinising hormone secretion in the ovariectomised rat. Exp Brain Res 1991; 87: 345–352.. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Burlington, VT:: Academic Press of Elsevier;; 2007. [Google Scholar]
- Zeevalk GD, Hyndman AG, Nicklas WJ.Excitatory amino acid-induced toxicity in chick retina: amino acid release, histology, and effects of chloride channel blockers. J Neurochem 1989; 53: 1610–1619.. [DOI] [PubMed] [Google Scholar]
- Nicklas WJ, Browning ET.Glutamate uptake and metabolism in C-6 glioma cells: alterations by potassium ion and dibutyryl cAMP. J Neurochem 1983; 41: 179–187.. [DOI] [PubMed] [Google Scholar]
- Varoqui H, Schafer MK, Zhu H, Weihe E, Erickson JD.Identification of the differentiation-associated Na+/PI transporter as a novel vesicular glutamate transporter expressed in a distinct set of glutamatergic synapses. J Neurosci 2002; 22: 142–155.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller NR, Jover T, Cohen HW, Zukin RS, Etgen AM.Estrogen can act via estrogen receptor alpha and beta to protect hippocampal neurons against global ischemia-induced cell death. Endocrinology 2005; 146: 3070–3079.. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Hediger MA.The glutamate and neutral amino acid transporter family: physiological and pharmacological implications. Eur J Pharmacol 2003; 479: 237–247.. [DOI] [PubMed] [Google Scholar]
- Kimura F, Jinnai K.Bicuculline infusions advance the timing of luteinizing hormone surge in proestrous rats: comparisons with naloxone effects. Horm Behav 1994; 28: 424–430.. [DOI] [PubMed] [Google Scholar]
- Bourguignon JP, Gerard A, Purnelle G, Czajkowski V, Yamanaka C, Lemaitre M, Rigo JM, Moonen G, Franchimont P.Duality of glutamatergic and GABAergic control of pulsatile GnRH secretion by rat hypothalamic explants: I. Effects of antisense oligodeoxynucleotides using explants including or excluding the preoptic area. J Neuroendocrinol 1997; 9: 183–191.. [DOI] [PubMed] [Google Scholar]
- Mitsushima D, Shwe TT, Funabashi T, Shinohara K, Kimura F.GABA release in the medial preoptic area of cyclic female rats. Neuroscience 2002; 113: 109–114.. [DOI] [PubMed] [Google Scholar]
- Leonhardt S, Seong JY, Kim K, Thorun Y, Wuttke W, Jarry H.Activation of central GABAA-but not of GABAB-receptors rapidly reduces pituitary LH release and GnRH gene expression in the preoptic/anterior hypothalamic area of ovariectomized rats. Neuroendocrinology 1995; 61: 655–662.. [DOI] [PubMed] [Google Scholar]
- Mitsushima D, Jinnai K, Kimura F.Possible role of the gamma-aminobutyric acid-A receptor system in the timing of the proestrous luteinizing hormone surge in rats. Endocrinology 1997; 138: 1944–1948.. [DOI] [PubMed] [Google Scholar]
- Arias P, Carbone S, Szwarcfarb B, Feleder C, Rodriguez M, Scacchi P, Moguilevsky JA.Effects of aging on N-methyl-D-aspartate (NMDA)-induced GnRH and LH release in female rats. Brain Res 1996; 740: 234–238.. [DOI] [PubMed] [Google Scholar]
- Gore AC, Yeung G, Morrison JH, Oung T.Neuroendocrine Aging in the Female Rat: The Changing Relationship of Hypothalamic Gonadotropin-Releasing Hormone Neurons and N-Methyl-D-Aspartate Receptors. Endocrinology 2000; 141: 4757–4767.. [DOI] [PubMed] [Google Scholar]
- Grove-Strawser D, Jimenez-Linan M, Rubin BS.Middle-aged female rats lack the dynamic changes in GAD(67) mRNA levels observed in young females on the day of a luteinising hormone surge. J Neuroendocrinol 2007; 19: 708–716.. [DOI] [PubMed] [Google Scholar]
- Wise PM, Smith MJ, Dubal DB, Wilson ME, Rau SW, Cashion AB, Bottner M, Rosewell KL.Neuroendocrine modulation and repercussions of female reproductive aging. Recent Prog Horm Res 2002; 57: 235–256.. [DOI] [PubMed] [Google Scholar]
- Gore AC, Oung T, Woller MJ.Age-related changes in hypothalamic gonadotropin-releasing hormone and N-methyl-d-aspartate receptor gene expression, and their regulation by oestrogen, in the female rat. J Neuroendocrinol 2002; 14: 300–309.. [DOI] [PubMed] [Google Scholar]
- Carbone S, Ponzo O, Szwarcfarb B, Rondina D, Reynoso R, Scacchi P, Alberto Moguilevsky J.Ontogenic modifications in the effect of the GABAergic system on the hypothalamic excitatory amino acids: its relationship with GABAergic control of gonadotrophin secretion during sexual maturation in female rats. Brain Res Dev Brain Res 2002; 133: 13–18.. [DOI] [PubMed] [Google Scholar]
- Ouyang C, Guo L, Lu Q, Xu X, Wang H.Enhanced activity of GABA receptors inhibits glutamate release induced by focal cerebral ischemia in rat striatum. Neurosci Lett 2007; 420: 174–178.. [DOI] [PubMed] [Google Scholar]
- Yananli HR, Terzioglu B, Goren MZ, Aker RG, Aypak C, Onat FY.Extracellular hypothalamic gamma-aminobutyric acid (GABA) and L-glutamic acid concentrations in response to bicuculline in a genetic absence epilepsy rat model. J Pharmacol Sci 2008; 106: 301–309.. [DOI] [PubMed] [Google Scholar]
- Ottem EN, Godwin JG, Krishnan S, Petersen SL.Dual-phenotype GABA/glutamate neurons in adult preoptic area: sexual dimorphism and function. J Neurosci 2004; 24: 8097–8105.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda SR, Lomniczi A, Mastronardi C, Heger S, Roth C, Parent AS, Matagne V, Mungenast AE.Minireview: the neuroendocrine regulation of puberty: is the time ripe for a systems biology approach? Endocrinology 2006; 147: 1166–1174.. [DOI] [PubMed] [Google Scholar]
- Wise PM.Norepinephrine and dopamine activity in microdissected brain areas of the middle-aged and young rat on proestrus. Biol Reprod 1982; 27: 562–574.. [DOI] [PubMed] [Google Scholar]
- Mohankumar PS, Thyagarajan S, Quadri SK.Correlations of catecholamine release in the medial preoptic area with proestrous surges of luteinizing hormone and prolactin: effects of aging. Endocrinology 1994; 135: 119–126.. [DOI] [PubMed] [Google Scholar]
- Hartman RD, He JR, Barraclough CA.Gamma-aminobutyric acid-A and -B receptor antagonists increase luteinizing hormone-releasing hormone neuronal responsiveness to intracerebroventricular norepinephrine in ovariectomized estrogen-treated rats. Endocrinology 1990; 127: 1336–1345.. [DOI] [PubMed] [Google Scholar]
- Jarry H, Wise PM, Leonhardt S, Wuttke W.Effects of age on GABA turnover rates in specific hypothalamic areas in female rats. Exp Clin Endocrinol Diabetes 1999; 107: 59–62.. [DOI] [PubMed] [Google Scholar]
- Moenter SM, DeFazio RA.Endogenous gamma-aminobutyric acid can excite gonadotropin-releasing hormone neurons. Endocrinology 2005; 146: 5374–5379.. [DOI] [PubMed] [Google Scholar]
- Han SK, Todman MG, Herbison AE.Endogenous GABA release inhibits the firing of adult gonadotropin-releasing hormone neurons. Endocrinology 2004; 145: 495–499.. [DOI] [PubMed] [Google Scholar]