Abstract
This study investigates the role of caspase 2 in apoptotic signaling of nonhuman primate male germ cells triggered by mild testicular hyperthermia, testosterone (Te) implants, or by combined interventions. Mean incidence of germ cell apoptosis increased significantly by Day 3 in the heat (He) alone group and by Day 8 in the Te alone group but peaked at Day 3 in He + Te group. We found activation of caspase 2 in both germ cells and Sertoli cells after induction of apoptosis. Most notably, active caspase 2 immunoreactivity was detected only in those germ cells susceptible to apoptosis compared with controls, where little or no such staining is detected. To further explore the role of caspase 2 in regulating male germ cell death, we next evaluated the efficacy of caspase 2 inhibition in preventing or attenuating heat-induced germ cell apoptosis in rats. Caspase 2 inhibition significantly (P < 0.05) prevented such heat-induced germ cell apoptosis. The protection offered by the caspase 2 inhibitor occurred upstream of mitochondria, involving suppression of mitogen-activated protein kinase (MAPK) 14 activation and inducible nitric oxide synthase (NOS2) induction and, in turn, suppression of cytochrome c-mediated death pathway. Together, our results show that caspase 2 is activated in male germ cells undergoing apoptosis in nonhuman primates after heat stress, hormonal deprivation, or after combined interventions. Blockade of caspase 2 activation prevents heat-induced germ cell apoptosis in rats by suppressing the MAPK14- and NO-mediated intrinsic pathway signaling..
Keywords: apoptosis, caspase 2, male germ cells, MAPK14, monkey, NOS2, primate, rat, spermatogenesis
Caspase 2 promotes male germ cell apoptosis in rats through activation of MAPK14 and NO-mediated intrinsic pathway signaling.
INTRODUCTION
Germ cell apoptosis occurs spontaneously during spermatogenesis or can be induced in a stage- and cell-specific manner by a variety of apoptotic stimuli, such as mild testicular hyperthermia and deprivation of gonadotropins and reduction of intratesticular testosterone (Te) concentrations by administration of a gonadotropin-releasing hormone antagonist or by exogenous administration of Te [1–3]. We have previously reported that selective deprivation of gonadotropin and testicular Te is followed by a stage-specific apoptosis of germ cells involving preleptotene and pachytene spermatocytes and round and elongated spermatids at mid (VII and VIII) stages [1]. In subsequent studies, we have demonstrated that transient heat exposure also induces stage-specific activation of apoptosis but at different stages of the spermatogenic cycle [2, 3]. In striking contrast to the hormone deprivation model, a transient exposure of the testes to heat (He; 43°C for 15 min) induces germ cell apoptosis exclusively at early (I–IV) and late (XII–XIV) stages. Pachytene spermatocytes and early spermatids (steps one to four) at stages I–IV and pachytene, diplotene, and dividing spermatocytes at stages XII–XIV are most susceptible to heat.
We have further extended our experimental paradigm from rodents to monkeys [4] and demonstrated that, indeed, germ cell apoptosis plays an important role in the organized regression of spermatogenesis after Te implants alone or in combination with testicular hyperthermia (Te + He). Mean incidence of germ cell apoptosis increased significantly (P < 0.05) by Day 3 in the He alone group and by Day 8 in the Te alone group but peaked at Day 3 in the He + Te group. No attempts were made in that study to characterize the stage-specific activation of germ cell apoptosis triggered by these stimuli. However, similar to our previous studies in rodents, the predominant germ cells undergoing apoptosis were pachytene spermatocytes and round spermatids [4]. The upstream signaling pathways by which these hormonal and nonhormonal stimuli activate germ cell apoptosis are not well understood in the primate and are the topic of this publication. Understanding the specific molecular components of the apoptotic pathway in germ cells is an essential step toward the development of novel therapeutic regimens to control accelerated apoptosis leading to abnormal spermatogenesis and infertility [5, 6], as well as more targeted approaches to male contraception.
The downstream signaling events leading to apoptosis can be divided into two major pathways, involving either mitochondria (intrinsic) or death receptors (extrinsic). In earlier studies using murine models of testicular hyperthermia and hormone deprivation, we have demonstrated the involvement of the mitochondria-dependent (intrinsic) apoptotic pathway, characterized by cytochrome c and DIABLO release from mitochondria, and activation of the initiator caspase 9 and the executioner caspases 3, 6, and 7, and poly (ADP)-ribose polymerase 1 cleavage, in heat- as well as hormone deprivation-induced male germ cell apoptosis [7–9]. In concert with our previous works in murine models [7–9], in a recent study we have further demonstrated the involvement of the intrinsic pathway signaling in male germ cell apoptosis induced by mild testicular hyperthermia and/or deprivation of intratesticular Te in monkeys [10]. These studies indicate that the mitochondria-dependent intrinsic pathway signaling is the key apoptotic pathway for testicular germ cell apoptosis across species after these interventions. In additional studies using both rodent and primate models, we have further demonstrated that mitogen-activated protein kinase 14 (MAPK14) activation and inducible nitric oxide synthase (NOS2) induction represent essential upstream events in the activation of the intrinsic pathway signaling [8, 10, 11]. However, we do not know what triggers MAPK14- and NO-mediated intrinsic pathway signaling for male germ cell apoptosis.
Of all caspases discovered to date, caspase 2 is the most evolutionarily conserved and plays an important role in inducing apoptosis in various cell systems, including the oocyte [12–17]. Of importance, cellular stresses, including reactive oxygen species (ROS) and heat stress, can trigger caspase 2 activation and apoptosis in some cell lines [12, 15, 16] via stimulation of the cytochrome c-mediated death pathway, known to be the major contributor of male germ cell apoptosis [7–10] across species. Reactive oxygen species can also trigger MAPK14 [18–20] activation, which constitutes a critical component of the apoptotic signaling of male germ cells [8, 10, 11]. Caspase 2-mediated intrinsic pathway signaling has recently been implicated in the induction of germ cell apoptosis in mice during the first phase of spermatogenesis [21] as well as after ischemia-reperfusion [22]. However, the signaling events that link caspase 2 activation with MAPK14- and NO-mediated intrinsic pathway signaling and promote male germ cell death have not been previously identified.
The objectives of the present study were two-fold. The first was to characterize the role of caspase 2 in apoptotic signaling of nonhuman primate male germ cells triggered by mild testicular hyperthermia, hormonal deprivation, or combined interventions. Given that many aspects of the signal transduction pathways are not feasible to study directly in monkeys, our second objective was to determine, using our established rat model of germ cell death by testicular hyperthermia, whether a selective inhibitor of caspase 2 could prevent or attenuate heat-induced germ cell apoptosis. In the process of doing so, we also characterized the specific steps in the germ cell apoptotic signal transduction pathways that are affected by the inhibition of caspase 2.
MATERIALS AND METHODS
Primate Study Design and Tissue Collection
Testicular sections from monkeys used in our previous studies [4, 10] were also available for the present investigation. Briefly, a total of 32 male adult (ages 7–10 yr) Cynomolgus monkeys (Macaca fascicularis) were obtained and housed at the Guangxi Hongfeng Primate Research Center, Institute of Zoology (IOZ), Chinese Academy of Sciences (CAS). Animal handling, experimentation, and testicular tissue harvesting protocol were in accordance with the recommendation of the American Veterinary Medical Association and were approved by both the Animal Care and Use Review Committee of Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center and by the Chinese Academic Committees of the IOZ and the CAS. The monkeys were housed in a standard animal facility under controlled temperature (22°C) and photoperiod (12L:12D) with free access to water and monkey chow. Groups of eight adult cynomolgus male monkeys received one of the following treatments: 1) two empty silastic implants (C); 2) two 5.5-cm Te implants (Te); 3) daily exposure of testes to heat (43°C for 30 min) for 2 consecutive days (He); and 4) two Te implants plus exposure of the testes to heat for 2 consecutive days (Te + He). Testosterone implants of 5.5-cm length were prepared from polydimethylsilozane tubing (5.5-cm length, inner diameter 0.33 cm and outer diameter 0.46 cm; Dow Corning Corp., Midland, MI), packed with Te (Sigma, St. Louis, MO), and sealed with silastic medical adhesive A (Dow Corning Corp.), as described previously [4]. Sterilized Te-filled capsules were implanted subdermally along the dorsal surface under general anesthesia with ketamine (10 mg/kg) and atropine (0.05 mg/kg). Testicular warming in water bath was performed as described previously [4]. Briefly, under light sedation with ketamine (4 mg/kg), testicular hyperthermia was conducted by immersing the monkey scrota containing the testes into a thermostatically controlled water bath at 43°C for 30 min once daily for 2 consecutive days. After heat treatment, animals were dried, examined for any redness or injury to the scrota, then returned to their cages and allowed to recover from the effect of the anesthesia.
Murine Study Design and Tissue Collection
To further explore the role of caspase 2 in regulating germ cell apoptosis, we examined whether a selective inhibitor of caspase 2 (Z-VDAVDK-fmk; Axxora LLC, San Diego, CA) could prevent or attenuate heat-induced germ cell apoptosis. Adult (90-day-old) male Sprague-Dawley rats were purchased from Charles River Laboratories (Wilmington, MA). Rats were housed in a standard animal facility under controlled temperature (22°C) and photoperiod (12L:12D) with liberal access to food and water. Groups of three to five adult Sprague-Dawley rats pretreated with intratesticular injection of 50 μl vehicle (dimethyl sulfoxide) or of 50 or 100 μg caspase 2 inhibitor (Z-VDAVDK-fmk) were exposed once to local testicular heating (43°C for 15 min) and killed 0.5 and 6 h later. The inhibitor or vehicle was administered 1 h prior to local testicular heating. Additional groups of three rats received an intratesticular injection of vehicle or of 50 or 100 μg Z-VDAVDK-fmk and served as controls. Animal handling and experimentation were in accordance with recommendation of the American Veterinary Medical Association and were approved by the Los Angeles Biomedical Research Institute Animal Care and Use Review Committee.
Both control and experimental animals were injected with heparin (130 IU/100 g body weight, i.p.) 15 min before a lethal injection of sodium pentobarbital (100 mg/kg body weight, i.p.) to facilitate testicular perfusion using a whole-body perfusion technique [23]. After perfusion with saline, one testis was removed, decapsulated, weighed, snap frozen in liquid N2, and stored at −70°C for subcellular fractionation and Western blotting. The contralateral testis was then fixed by vascular perfusion with 5% glutaraldehyde in 0.05 M cacodylate buffer (pH 7.4) or Bouin solution and was processed for routine paraffin embedding for either in situ detection of apoptosis or immunocytochemistry.
Assessment of Apoptosis
In situ detection of cells with DNA strand breaks was performed in glutaraldehyde-fixed, paraffin-embedded testicular sections by the terminal deoxynucleotidyl transferase (TdT)-mediated deoxy-UTP nick-end labeling (TUNEL) technique [1–4, 7, 8] using an ApopTag-peroxidase kit (Chemicon International Inc., Temecula, CA). Enumeration of the nonapoptotic Sertoli cell nuclei with distinct nucleoli and apoptotic germ cell population was carried out at stages XII–XIV of the seminiferous epithelial cycle using an Olympus BH-2 microscope (New Hyde Park, NY) with a 100× oil immersion objective. We applied the same strategy that was used to determine the efficacy of a pan caspase inhibitor or minocycline, a second-generation tetracycline that effectively inhibits cytochrome c release and caspase activation, in protecting germ cells from heat-induced apoptosis in mice [24]. The analysis was carried out 6 h after heating, the earliest time point associated with a stage-specific activation of germ cells [7, 25]. At least 10 tubules were used for each rat. Stages were identified according to the criteria proposed by Russell et al. [26] for paraffin sections. The rate of germ cell apoptosis (apoptotic index) was expressed as the number of apoptotic germ cells per 100 Sertoli cells [3, 8].
Immunohistochemical and Immunofluorescence Analyses
Bouin-fixed, paraffin-embedded testicular sections from both monkeys and rats were immunostained as described previously [7–10, 24, 25]. Briefly, testicular sections were deparaffinized, hydrated by successive series of ethanols, rinsed in phosphate-buffered saline (PBS), and then incubated in 2% H2O2 in PBS for 10 min to quench endogenous peroxidase. For the primate study, primary antibodies included rabbit polyclonal X-chromosome-linked inhibitor of apoptosis protein (XIAP; 1:25) and cleaved caspase 2 (1:25; Santa Cruz Biotechnology, Santa Cruz, CA). Immunoreactivity was detected using biotinylated goat anti-rabbit IgG secondary antibody followed by avidin-biotinylated horseradish peroxidase complex visualized with diaminobenzidine tetrahydrochloride (DAB) per the manufacturer's instructions (rabbit Unitect Immunohistochemistry Detection System; Oncogene Science). Slides were counterstained with hematoxylin. Negative and positive controls were run for every assay. The negative controls were processed in an identical manner, except the primary antibody was substituted with the rabbit IgG. Rat testicular sections exposed to heat [7] or hormone deprivation [8] were used as positive controls.
Caspase 2 activation in germ cells undergoing apoptosis in monkeys was further detected by confocal microscopy using double immunostaining as previously described [7, 8]. In situ detection of cells with DNA strand breaks was performed in Bouin-fixed, paraffin-embedded testicular sections using an ApopTag-fluorescein kit (Chemicon International). In brief, after deparaffinization and rehydration, tissue sections were incubated with proteinase K for 15 min at room temperature, washed in PBS, and then incubated with a mixture containing digoxigenin-conjugated nucleotide and TdT in a humidified chamber at 37°C for 1 h and subsequently treated with antidigoxigenin-fluorescein for 30 min in the dark. After fluorescein staining, slides were washed in PBS and incubated with blocking serum for 20 min to reduce nonspecific antibody binding. Slides then were incubated in a humidified chamber for overnight at 4°C with a rabbit polyclonal active caspase 2 antibody followed by goat-anti-rabbit Texas Red-labeled secondary antibody for 45 min at room temperature, washed, and mounted in Vectashield mounting medium (Vector Laboratories Inc., Burlingame, CA).
Colocalization of XIAP and the active caspase 2 in the monkey testis was also detected by confocal microscopy using double immunostaining as described above. In brief, after deparaffinization and rehydration, tissue sections were incubated with blocking serum for 20 min at room temperature. After washing the slides, sections were incubated with the active caspase 2 (1:25) at 4°C overnight, followed by donkey anti-rabbit fluorescein isothiocyanate secondary antibody for 45 min at room temperature. The sections were then incubated with a rabbit polyclonal XIAP (1:25) at 4°C overnight, followed by goat anti-rabbit Texas Red-labeled secondary antibody for 45 min at room temperature. For negative controls, sections were treated only with secondary antibody, and no signals were detected. Confocal imaging was performed using a Leica TCS-SP-MP confocal microscope equipped with a 488-nm argon laser for excitation of green fluorophores and a 543-nm helium-neon laser for excitation of red flurophores.
For rat studies, primary antibodies included rabbit polyclonal cleaved caspase 2 (1:25), cleaved caspase 9 (1:25), and cleaved caspase 3 (1:50; Santa Cruz Biotechnology) antibodies and a rabbit monoclonal phospho-MAPK14 antibody (1:50; Cell Signaling Technology, Beverly, MA), which detects MAPK14 only when dually phosphorylated at threonine 180 and tyrosine 182. Immunoreactivity was visualized with DAB per the manufacturer's instructions (rabbit Unitect Immunohistochemistry Detection System; Oncogene Science). Slides were counterstained with hematoxylin.
Enumeration of the Sertoli cell nuclei with distinct nucleoli and caspase 9- or caspase 3-positive cells was carried out at stages XII–XIV of the seminiferous epithelial cycle 6 h after heating in rats pretreated with 100 μg caspase 2 inhibitor. Cell count was obtained using an Olympus BH-2 microscope with a 100× oil immersion objective. In light of the fact that the same level of protection against heat-induced germ cell apoptosis was noted between low (50 μg) and high (100 μg) doses of Z-VDAVDK, we used only the high-dose group. At least 10 tubules were used for each rat. Cell counts were finally expressed as the number of caspase 9- or caspase 3-positive cells per 100 Sertoli cells [24].
Subcellular Fractionation and Western Blotting
Cytosolic and mitochondrial fractions were prepared as described previously [7–10]. Briefly, testes were homogenized using a dounce homogenizer in 3 ml buffer A (0.25 M sucrose, 50 mM HEPES, 10 mM NaCl, 10 mM EDTA, 2 mM dithiothreitol) supplemented with protease inhibitors (Complete Protease Inhibitors; Roche). The crude homogenates were centrifuged at 1000 × g for 10 min at 4°C and the resultant supernatant centrifuged at 10 000 × g for 15 min at 4°C to sediment the low-speed fraction containing mainly mitochondria. The mitochondria were washed two times in buffer A and pelleted. The cytosolic and high-speed fractions were isolated following centrifugation of the 10 000 × g supernatant fraction at 100 000 × g for 60 min at 4°C. The resulting supernatant was the cytosolic fraction. The purity of the cytosolic and mitochondrial fractions was assessed by Western blotting using antibodies to actin (1:2000; Sigma-Aldrich) and cytochrome c oxidase subunit IV (COX IV; 1:500; Molecular Probes, Eugene, OR), respectively.
Western blotting was performed using rat testicular lysates and subcellular fractions as described previously [7–10]. In brief, proteins (50–80 μg) were separated on a 4%–12% SDS-polyacrylamide gel with 2–4-morpholino-ethane-sulfonic acid or morpholine-propane-sulfonic acid buffer purchased from Invitrogen (Carlsbad, CA) at 200 V. Gel was transferred on an Immuno-blot PVDF Membrane (Bio-Rad, Hercules, CA) overnight at 4°C. Membranes were blocked in blocking solution (0.3% Tween 20 in Tris-buffered saline and 10% nonfat dry milk) for 1 h at room temperature and then probed using a mouse monoclonal NOS2 (1:1000; BD Transduction Laboratories, San Diego, CA), rabbit polyclonal cleaved caspase 2 (1:500), and cytochrome c (1:2000; Santa Cruz Biotechnology) for 1 h at room temperature or overnight at 4°C with constant shaking. Following 3 × 10-min washes in TBS-T (0.3% Tween 20 in Tris-buffered saline) buffer, membranes were then incubated in anti-rabbit (Amersham Biosciences, Piscataway, NJ), anti-goat, or anti-mouse IgG-horseradish peroxidase (Santa Cruz Biotechnology) secondary antibodies at a 1:2000 dilution. All antibodies were diluted in blocking buffer. For immunodetection, membranes were washed three times in TBS-T wash buffer, incubated with ECL solutions per the manufacturer's specifications (Amersham Biosciences), and exposed to Hyper film ECL. The membranes were stripped and reprobed with a goat polyclonal actin (1:2000) or a rabbit polyclonal COX IV (1:500) for normalization of the loading. Band intensities were determined using Quantity One software from Bio-Rad.
Measurements of MAPK14
Activation of MAPK14 was measured using an assay kit (Cell Signaling Technology) as described previously [8, 10]. In brief, a monoclonal phospho-specific antibody to MAPK14 (Thr180/Tyr182) was used to selectively immunoprecipitate active MAPK14 from testis lysates. The resulting immunoprecipitate was then incubated with activating transcription factor 2 (ATF2) fusion proteins in the presence of ATF and kinase buffer, which allows immunoprecipitated active MAPK14 to phosphorylate ATF2. Phosphorylation of ATF2 at Thr71 was measured by Western blotting as described before using a rabbit polyclonal phospho-ATF2 (Thr71) antibody [8, 10].
Activation of MAPK14 was further confirmed by an Enzyme Immunometric Assay (EIA) Kit (Assay Designs Inc., Ann Arbor, MI). The kit uses a monoclonal antibody to MAPK14 immobilized on a microtiter plate to bind the active phospho-MAPK14 in the standards or tissue lysates. After a short incubation, the excess sample or standard is washed out and rabbit polyclonal antibody to phospho-MAPK14 is added, which then binds to the phospho-MAPK14 captured on the plate. A goat anti-rabbit IgG conjugated to horseradish peroxidase is added; this generates a signal for chromogenic substrate. The color generated is read at 450 nm. The measured optical density is directly proportional to the concentrations of phospho-MAPK14 in either standards or samples.
Statistical Analyses
Statistical analyses were performed using the SigmaStat 2.0 Program (Jandel Cooperation, San Rafael, CA). The Student-Newman-Keuls test after one-way repeated-measures ANOVA was used for statistical significance for Western blot analyses. Differences were considered significant if P < 0.05.
RESULTS
Hormone Deprivation Alone or in Combination with Testicular Hyperthermia Results in Activation of Caspase 2
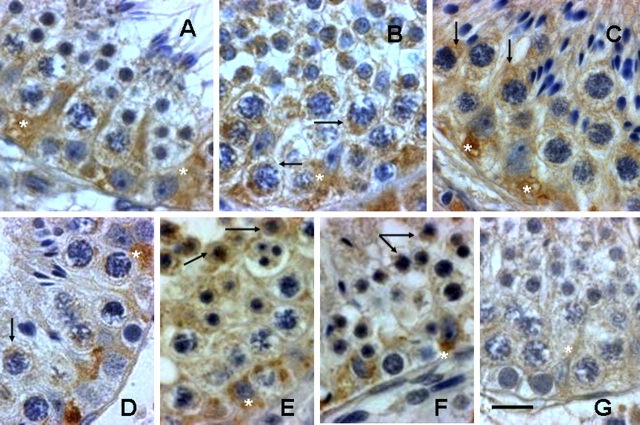
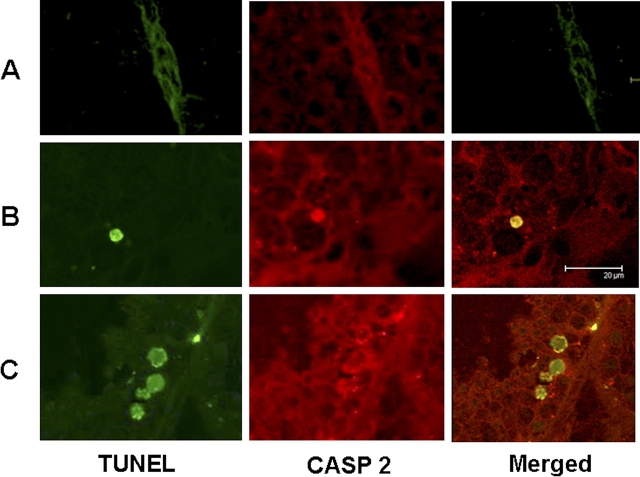
We recently analyzed the temporal changes in the incidence of male germ cell apoptosis in monkeys triggered by deprivation of the gonadotropic support, testicular hyperthermia, or both [4]. To determine whether caspase 2 plays a role in germ cell apoptosis in monkeys after these treatments, in this study we examined the activation of caspase 2 in testicular sections from the same group of animals (Fig. 1). We found activation of caspase 2, as evidenced by an increase in immunoreactivity for active caspase 2, in both germ cells and Sertoli cells at 3 days in the He (Fig. 1B) and He + Te (Fig. 1C) groups and in all treatment groups by 8 days after treatment (Fig. 1, D–F). Most notably, active caspase 2 immunoreactivity was detected only in those susceptible germ cells involving pachytene spermatocytes and round spermatids after induction of apoptosis compared with controls, where little or no such staining was detected (Fig. 1G). In control testes, we also detected a modest immunostaining for active caspase 2 in Sertoli cells. Costaining for TUNEL and active caspase 2 in testis sections 8 days after exposure to Te or He further confirmed activation of caspase 2 only in those germ cells undergoing apoptosis (Fig. 2).
FIG. 1.
Activation of caspase 2, as detected by immunohistochemistry using an antibody that detects cleaved caspase 2, in monkey testicular sections 3 days (A–C) or 8 days (D–F) after exposure to Te (A, D), He (B, E), or He + Te (C, F). Compared with control (G), where a modest expression of active caspase 2 is detected in Sertoli cells (asterisks) but not in germ cells, a marked increase in active caspase 2 immunoreactivity is noted in Sertoli cells after testosterone treatment and both in Sertoli cells and germ cells (arrows) after He or He + Te treatment. Bar = 25 μm.
FIG. 2.
Activation of caspase 2 (CASP 2) only in those germ cells undergoing apoptosis. Confocal images from control (A) and 8 days after exposure to Te (B) or He (C) show TUNEL (green), active caspase 2 (red), and colocalization of TUNEL and active caspase 2 (yellow) in germ cells only after Te or He treatment. Bar = 20 μm.
Mild Testicular Hyperthermia and Hormone Deprivation Alone or in Combination Also Concurrently Upregulates XIAP in Sertoli Cells
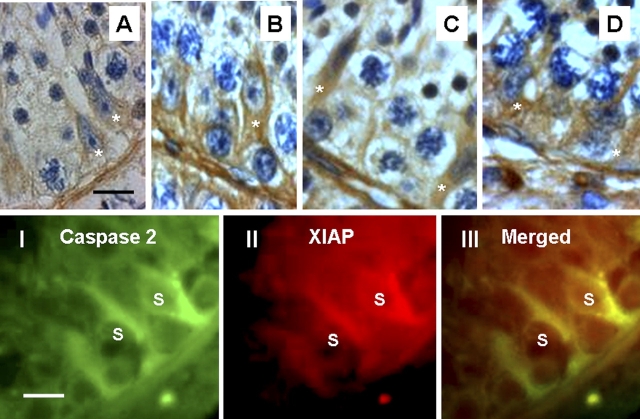
To find out why germ cells are undergoing apoptosis and Sertoli cells continue to survive in spite of caspase 2 activation in response to these apoptotic stimuli, we studied the potential role of XIAP in preventing Sertoli cell apoptosis. Testicular sections from all treatment groups were immunostained for XIAP. As shown in Figure 3, A–D, after apoptosis induction we detected an intense immunostaining of XIAP in Sertoli cells with little or no expression in germ cells. Costaining for XIAP and active caspase 2 showed colocalization of XIAP with active caspase 2 in the Sertoli cells (Fig. 3, panels I–III).
FIG. 3.
Immunohistochemical analysis of changes in XIAP expression in monkey testes (A–D). In control testis (A), a weak expression of XIAP is detected in Sertoli cells (asterisks) with little or no expression in germ cells. Testicular sections 8 days after exposure to Te (B), He (C), or Te + He (D) show an increase in XIAP immunoreactivity in Sertoli cells (asterisks). Bar = 25 μm. I–III) Confocal images show active caspase 2 (green), XIAP (red), and colocalization of active caspase 2 and XIAP (yellow) in Sertoli cells (S). Bar = 15 μm.
Caspase 2 Inhibitor Markedly Inhibits Caspase 2 Activation, Suppresses Cytochrome c Release from Mitochondria, and Attenuates Heat-Induced Germ Cell Apoptosis in Rats
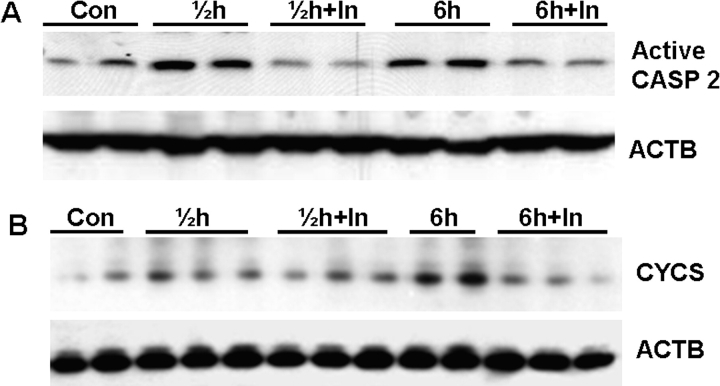
In the above-mentioned studies, we have provided evidence for caspase 2 activation in apoptotic signaling of male germ cells in monkeys after heat stress, hormonal deprivation, or combined interventions. To further explore the role of caspase 2 in male germ cell apoptosis, in this study, using our established rat model of germ cell death by testicular hyperthermia [7], we sought to determine whether a specific inhibitor of caspase 2 could prevent or attenuate heat-induced male germ cell apoptosis. Activation of caspase 2, as evidenced by immunoblotting for active caspase 2, was detected both at 0.5 and 6 h after heat treatment, which can be effectively suppressed by Z-VDAVDK pretreatment (Fig. 4A).
FIG. 4.
A) Caspase 2 inhibitor (In) effectively suppresses heat-induced caspase 2 activation. Activation of caspase 2, as evidenced by immunoblotting of active caspase 2 (Active CASP 2; 12 kDa), is detected at 0.5 (labeled ½) and 6 h after heat treatment. Pretreatment with 50 μg caspase 2 inhibitor (In) 1 h before local testicular heating effectively suppresses caspase 2 activation at both time points. The gels are representative of two animals in each treatment group from one of three separate experiments. Actin (ACTB) in the immunoblot is shown as a loading control. B) Pretreatment with 50 μg caspase 2 inhibitor inhibits the release of cytochrome c (CYCS; 15 kDa) from mitochondria during heat-induced male germ cell apoptosis. Western blots of cytosolic fractions of testicular lysates from control (Con), heat (0.5 h or 6 h), and caspase 2 inhibitor + heat (0.5 h + In or 6 h + In) show pretreatment with Z-VDAVDK inhibits cytochrome c release from mitochondria into the cytosol clearly at 6 h after heat treatment. ACTB in the immunoblot is shown as a loading control.
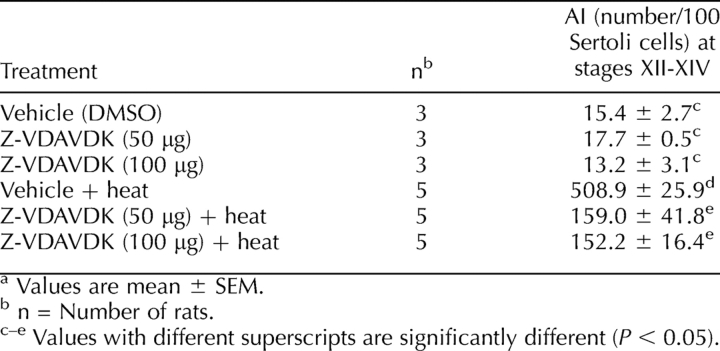
As summarized in Table 1, the apoptotic index (expressed as number per 100 Sertoli cells) measured at stages XII–XIV was very low in controls (15.4 ± 2.7). No significant changes in the apoptotic index were noted between control and mice treated with 50 or 100 μg Z-VDAVDK alone. Testicular hyperthermia within 6 h resulted in a marked activation of germ cell apoptosis, which can be significantly (P < 0.05) prevented by pretreatment with Z-VDAVDK at both dose levels. No significant differences in the degree of protection against heat-induced germ cell apoptosis were noted between low (50 μg) and high (100 μg) doses of Z-VDAVDK.
TABLE 1.
Z-VDAVDK prevents heat-induced germ cell apoptosis in rats.a
We next investigated the mechanism by which Z-VDAVDK protects heat-induced germ cell apoptosis. Because of the involvement of the mitochondria-dependent apoptotic pathway in heat-induced germ cell apoptosis [7, 9], we examined the cytochrome c release from mitochondria. As shown in Figure 4B, cytosolic translocation of cytochrome c was readily detected at 0.5 h and became more pronounced at 6 h after heat treatment. Z-VDAVDK effectively prevented such heat-induced release of cytochrome c 6 h after heat treatment.
Z-VDAVDK Inhibits Caspase Activation
Given that caspase 9 and caspase 3 are activated during heat-induced apoptosis, we next examined whether Z-VDAVDK inhibits such caspase activation. In vehicle-treated rats, immunostaining of active caspase 9 in heat-susceptible germ cells other than a few spermatocytes late in meiosis were rarely detected at stages XII–XIV (Fig. 5A). After induction of apoptosis, the initiator caspase 9 was activated in a large majority of heat-susceptible late spermatocytes (Fig. 5B). Rats pretreated with Z-VDAVDK had markedly fewer active caspase 9-positive germ cells (Fig. 5C) compared with the heat alone group (Fig. 5B). We found almost an identical suppression in the number of caspase 3-positive cells at stages XII–XIV 6 h after heating in rats pretreated with caspase 2 inhibitor compared with heat alone (Fig. 5, D–F). Morphometric analysis further confirmed the histological findings and revealed a significant (P < 0.05) decrease in the number of caspase 9- and caspase 3-positive germ cells by 62.9% and 57.7%, respectively, 6 h after heating in rats pretreated with 100 μg caspase 2 inhibitor (Fig. 5G).
FIG. 5.
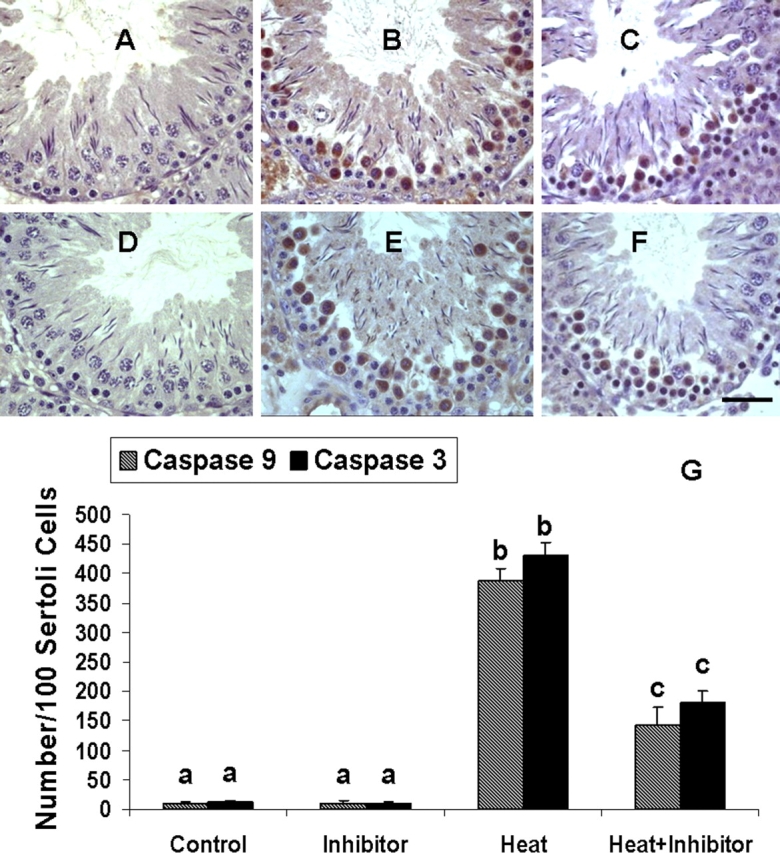
Reduction in the number of caspase 9- and caspase 3-positive germ cells after testicular hyperthermia in rats pretreated with 100 μg Z-VDAVDK (A–F). Portion of a stage XII tubule from a control rat shows no active caspase 9 immunoreactivity in germ cells (A). In contrast, the same stage tubule from a heat-treated rat shows a marked increase in the number of active caspase 9-positive germ cells 6 h after heat treatment (B). Rats pretreated with caspase 2 inhibitor 1 h before induction of hyperthermia exhibit a reduction in the number of active caspase 9-positive germ cells 6 h after heat treatment (C) compared with the heat alone group (B). A portion of stage XII tubules from control (D) and heat-treated (E) rats show a marked increase in the number of active caspase 3-positive germ cells 6 h after heat treatment compared with controls, in which no such staining was detected. Rats pretreated with caspase 2 inhibitor 1 h before induction of hyperthermia exhibit a reduction in the number of active caspase 3-positive germ cells 6 h after heat treatment (F) when compared to the heat alone group (E). Bar = 50 μm. G) Quantitation of caspase 9- and caspase 3-positive germ cells at late-stage (XII–XIV) tubules in heat-treated rats with or without Z-VDAVDK treatment. Note a significant reduction in the number of caspase 9- or caspase 3-positive germ cells in heat + ZVDAVDK-treated rats compared with heat alone. Values are means ± SEM. Means with different letters differ significantly (P < 0.05).
Inhibition of Caspase 2 Downregulates MAPK14 and NOS2
We next investigated the mechanism by which inhibition of caspase 2 suppresses mitochondria-dependent apoptotic pathway and, in turn, heat-induced germ cell apoptosis. Given that MAPK14 and NO-mediated intrinsic pathway signaling constitutes a critical component of apoptotic signaling in male germ cells in rats after hormone deprivation [8] or testicular hyperthermia [11], we examined the effects of Z-VDAVDK on MAPK14 activation and NOS2 induction. Activation of MAPK14, as evidenced by an increase in phospho-ATF2 in testis lysates, was detected at both 0.5 and 6 h after heat treatment, and that could be effectively suppressed by Z-VDAVDK (Fig. 6A). Preventive effects of Z-VDAVDK on heat-induced activation of MAPK14 in susceptible germ cells were also ascertained by immunocytochemistry using a phospho-specific antibody, which detects MAPK14 only when dually phosphorylated at threonine 180 and tyrosine 182. Compared with controls, where no staining was detected, strong phospho-MAPK14 immunoreactivity was noted in susceptible germ cells at stages XII–XIV 6 h after heat treatment. Rats pretreated with Z-VDAVDK had markedly fewer phospho-MAPK14-positive germ cells compared with the heat alone group (Fig. 6B). An effect of caspase 2 inhibition on heat-induced activation of MAPK14 was further substantiated by an EIA assay. As shown in Figure 6C, upon exposure of the testes to mild heat, the concentrations of phospho-MAPK14 increased significantly (P < 0.05) by more than 3.2-fold (over the values measured in controls) at 0.5 and 6 h after treatment. Pretreatment with Z-VDAVDK significantly (P < 0.05) suppressed such heat-induced activation of MAPK14 at both time points.
FIG. 6.
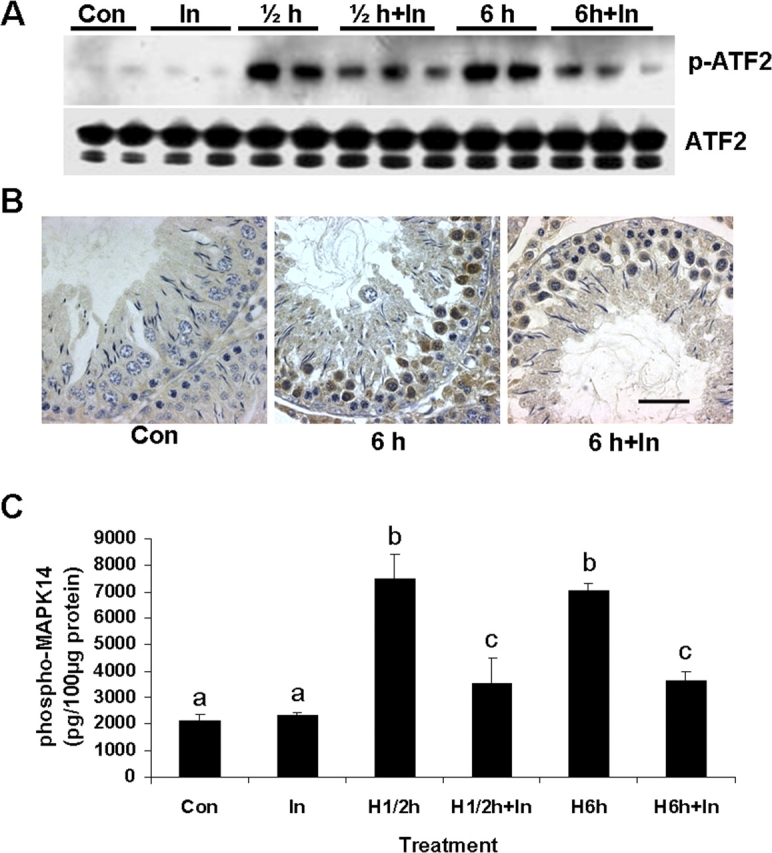
Pretreatment with 50 μg caspase 2 inhibitor prevents heat-induced activation of MAPK14. A) Analysis of MAPK14 activation by Western blotting using phospho-ATF2 (p-ATF2; 40 kDa) antibody in testicular lysates after treatments. A monoclonal phospho-specific antibody to MAPK14 was used to selectively immunoprecipitate active MAPK14 from testis lysates. Resulting immunoprecipitate was then incubated with ATF2 (38 kDa) fusion protein in the presence of ATF and kinase buffer, which allows immunoprecipitated active MAPK14 to phosphorylate ATF2. The gels are representative of two to three animals at each time point from one of three separate experiments. ATF2 is used as a loading control. B) Immunocytochemical analysis of in vivo changes of phosphorylated MAPK14 at late-stage tubules in rats after no treatment (Con), testicular hyperthermia (6 h), and testicular hyperthermia + caspase 2 inhibitor (6 h + In). Note reduction in the number of phospho-MAPK14-positive germ cells after testicular hyperthermia in rats pretreated with caspase 2 inhibitor. Bar = 50 μm. C) Effect of caspase 2 inhibition on heat-induced (H) activation of MAPK14 detected by an EIA assay. Upon exposure of the testes to heat, the concentrations of phospho-MAPK14 rose by more than 3.2-fold (over the values measured in controls) at 0.5 (labeled ½) and 6 h after treatment, which can be significantly (P < 0.05) prevented by pretreatment with caspase 2 inhibitor. Values are means ± SEM of three to four rats at each group. Means with different letters are significantly different.
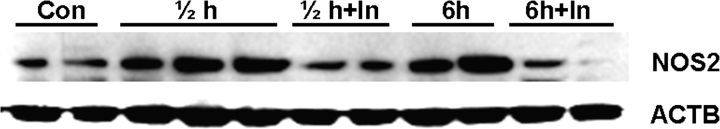
We then examined the effects of caspase 2 inhibition on NOS2 induction during heat-induced germ cell apoptosis by Western blotting. As shown in Figure 7, induction of NOS2 was readily detected both at 0.5 and 6 h after heat treatment, and that could be effectively suppressed by Z-VDAVDK treatment. Together, these results indicate that caspase 2 is an upstream activator of MAPK14- and NO-mediated signaling during heat-induced germ cell apoptosis in rats.
FIG. 7.
Western blot analysis of effects of caspase 2 inhibitor (In) on heat-induced induction of monoclonal NOS2 in testicular lysates. Upregulation of NOS2 (130 kDa) is detected both at 0.5 (labeled ½) and 6 h of heating, which can be effectively suppressed by pretreatment with 50 μg caspase 2 inhibitor (In). ACTB in the immunoblot is shown as a loading control.
DISCUSSION
This investigation was performed to address the following questions. Does caspase 2 play a role in apoptotic signaling of nonhuman primate male germ cells triggered by mild testicular hyperthermia, hormonal deprivation, or both? Does caspase 2 inhibition prevent testicular germ cell apoptosis? Where does caspase 2 fit in the apoptotic signaling pathway for male germ cells?
Role of Caspase 2 in Apoptotic Signaling of Male Germ Cells in Monkeys
Consistent with the role of caspase 2 in apoptotic signaling in various cell types [12–17], including murine testicular germ cells [21, 22], in this study, we show activation of caspase 2 in both germ cells and Sertoli cells at 3 days in the He and He + Te groups and at 8 days in all treatment groups (Te, He, and He + Te). Temporal activation of caspase 2 coincided with the increased incidence of germ cell apoptosis in response to these stimuli [4]. Most notably, active caspase 2 immunoreactivity was detected only in those susceptible germ cells after induction of apoptosis, compared with controls, where little or no such staining was detected. Costaining for TUNEL and active caspase 2 further confirmed activation of caspase 2 only in those germ cells undergoing apoptosis. These data indicated that caspase 2 may play a role in apoptotic germ cell death in monkeys.
A growing body of evidence indicates that cellular stresses, including heat stress, withdrawal of growth factor, and ROS, can trigger caspase 2 activation [12–17, 22], which through stimulation of the mitochondria-dependent pathway promotes apoptosis in various cell systems [12–17, 27]. Indeed, in our earlier studies, we have shown the involvement of mitochondria-dependent pathway in male germ cell death in monkeys induced by testicular hyperthermia and/or deprivation of intratesticular Te [10]. Thus, the signal for activation of caspase 2 most likely emanates from the stress generated by the dramatic loss of intratesticular Te and/or testicular hyperthermia.
Interestingly, activated caspase 2 was also found in Sertoli cells, even though these cells did not undergo apoptosis. Most notably, after induction of apoptosis, these cells consistently displayed increased expression of XIAP. No such immunostaining was detected in the apoptotic germ cells. Costaining for XIAP and active caspase 2 further revealed colocalization of XIAP with active caspase 2 in Sertoli cells. XIAP has regions that are known to bind specifically to caspase 2 and, in turn, abrogate its apoptotic activity [28]. Thus, one could assume that although activation of caspase 2 in germ cells initiates their demise through activation of the cytochrome c-mediated death pathway, upregulation of XIAP in Sertoli cells could bind and inhibit caspase 2 activation and, in turn, promote their survival.
Functional Role of Caspase 2 in Heat-Induced Testicular Germ Cell Apoptosis in Rats
In above studies, we have provided evidence for a role of caspase 2 in apoptotic signaling of male germ cells in monkeys after heat stress, hormonal deprivation, or combined interventions. To further explore the role of caspase 2, in this study we sought to determine whether a specific inhibitor of caspase 2 could prevent or attenuate heat-induced male germ cell apoptosis. Pretreatment with 50 and 100 μg Z-VDAVDK significantly (P < 0.05) prevented heat-induced germ cell apoptosis by 68.8% and 70.1%, respectively. The protection offered by Z-VDAVDK involved suppression of cytochrome c release from mitochondria and subsequent inhibition of caspase 9 and caspase 3. This is consistent with an earlier study that caspase 2, through stimulation of the mitochondria-dependent death pathway, contributes to germ cell apoptosis during the first phase of spermatogenesis [21]. Earlier studies involving non-gonadal cell systems have also indicated that caspase 2 promotes apoptosis through activation of the cytochrome c-mediated death pathway [12–17]. In this context, it is important to note that we found an almost identical level of protection (by 67.0%) of testicular germ cells from heat-induced apoptosis in mice pretreated with a Quinoline-Val-asp (Ome)-CH2-O-Ph (Q-VD-OPH), a broad-spectrum pan-caspase inhibitor [24]. However, compared with Z-VDAVDK, the protection offered by Q-VD-OPH was independent of mitochondrial cytochrome c release and occurred downstream of mitochondria by inhibiting caspase activation [24].
In recent studies, we have shown the involvement of MAPK14 and NOS2 that, through activation of the intrinsic pathway signaling, promotes male germ cell apoptosis in rats after hormone deprivation [8] or heat stress [11]. The signaling events that couple caspase 2 activation with stimulation MAPK14- and NO-mediated intrinsic pathway signaling have not been previously identified. Our data constitute the first demonstration that Z-VDAVDK significantly suppressed both MAPK14 activation and NOS2 induction, indicating that caspase 2 is an upstream activator of MAPK14 and NOS2 during heat-induced germ cell apoptosis. Consistent with these observations, we found similar protection from germ cell apoptosis triggered by hormone deprivation [29] or heat stress [24] by minocycline, which effectively suppresses MAPK14 activation NOS2 induction, cytochrome c release and, as a consequence, downstream caspase activation [30–32]. Thus, the protection offered by caspase 2 inhibition occurred upstream of the mitochondria.
The obvious question raised by these results is what triggers caspase 2 activation? One possibility is that this could be attributed in part to increased oxidative stress [15, 22] generated by testicular hyperthermia. Indeed, there have been in vitro studies employing caspase 2−/− cells, indicating caspase 2 as a proximal mediator of heat shock-induced apoptosis [16]. It is also pertinent to note here that caspase 2 may also be activated by a decline in cellular metabolism. For example, Nutt and colleagues [13] have demonstrated that depletion of stores of glucose-6-phosphate, an intermediate of glucose metabolism, can activate caspase 2 and promote oocyte death. Conversely, stimulation of pentose phosphate pathway leading to NADPH generation can restrain caspase 2 and promote oocyte survival [13]. Thus, it is possible that heat stress could promote caspase 2 activation and male germ cell death through a defective cellular metabolism. These possibilities merit further investigations.
The reasons for our inability to achieve complete protection of germ cells triggered by heat stress remain unknown. One obvious possibility is that the dose of Z-VDAVDK is not optimum. Consistent with this is the demonstration that Z-VDAVDK even at a high (100 μg) dose level caused the number of caspase 9- and caspase 3-positive cells to decline to 62.9% and 57.7%, respectively, of the values measured in heat-treated groups. Given that Z-VDAVDK inhibits caspase 2 activation, it remains possible that other caspases, such as 1, 11, and 12, are involved in heat-induced germ cell apoptosis. Indeed, there have been studies indicating the role of these caspases in inducing apoptosis in various cell systems other than spermatogenesis [33–35]. The possibility that a caspase-independent mechanism is involved in heat-induced germ cell apoptosis also cannot be excluded on the basis of the data presently available. Nevertheless, the present study clearly demonstrates a protective role of Z-VDAVDK in heat-induced testicular germ cell apoptosis in rats.
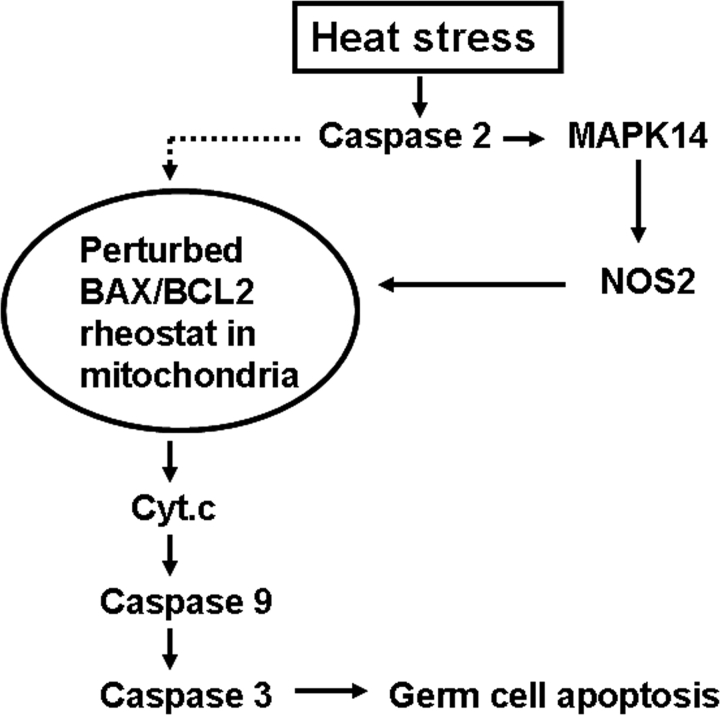
In summary, we have demonstrated a new signal transduction pathway involving caspase 2 that, through activation of the MAPK14 and NO-mediated intrinsic pathway signaling, promotes germ cell apoptosis in rats after testicular hyperthermia (Fig. 8). Targeting caspase 2 activation and interrupting MAPK14 activation and NO production may have protective role in acute testicular injury associated with increased germ cell apoptosis. Future efforts toward improved fertility control and clinical management of infertility associated with reduced sperm production in men are hampered by incomplete understanding of the processes responsible for normal germ cell homeostasis. Elucidation of the mechanisms by which various environmental stresses regulates germ cell death will fill a major gap in our knowledge of this fundamental biological process.
FIG. 8.
Summary of caspase 2-mediated signaling cascades in heat-induced germ cell apoptosis. Activation of caspase 2 after heat stress results in activation of MAPK14 and NOS2 induction. It is likely that activation of such signaling cascade perturbs the BAX/BCL2 rheostat in the mitochondria [8, 10, 11] and triggers the cytochrome c (Cyt.c)-mediated death pathway. Caspase 2-mediated signaling could also activate heat-induced germ cell apoptosis by targeting mitochondria directly, possibly through a decline in cellular metabolism (dotted line).
Footnotes
1Supported by grants from the Mellon Reproductive Biology Center (R.S.S., C.W., Y.H.L., and A.P.S.H.), National Institutes of Health (RO1 HD39293 to A.P.S.H., R.S.S., and C.W.), Major Research Plan Project (2006 CBOF 1001), 973 project (2006 CB 504001), Chinese Academy of Sciences Chuangxi program (KSCA2-YW-R-55), and National Nature Science Foundation of China (30230190). C.J. is supported through the Initiative for Minority Student Development Program (IMSD) from the National Institutes of Health (R25 GM560902).
REFERENCES
- Sinha Hikim AP, Rajavashisth TB, Sinha Hikim I, Lue Y, Bonavera JJ, Leung A, Wang C, Swerdloff RS.Significance of apoptosis in the temporal and stage-specific loss of germ cells in the adult rat after gonadotropin deprivation. Biol Reprod 1997; 57: 1193–1201.. [DOI] [PubMed] [Google Scholar]
- Lue YH, Sinha Hikim AP, Swerdloff RS, Im P, Taing KS, Bui T, Leung A, Wang C.Single exposure to heat induces stage-specific germ cell apoptosis in rats: role of intratesticular testosterone (T) on stage specificity. Endocrinology 1999; 140: 1709–1717.. [DOI] [PubMed] [Google Scholar]
- Lue YH, Sinha Hikim AP, Wang C, Im M, Leung A, Swerdloff RS.Testicular heat exposure enhances the suppression of spermatogenesis by testosterone in rats: the “two-hit” approach to male contraceptive development. Endocrinology 2000; 141: 1414–1424.. [DOI] [PubMed] [Google Scholar]
- Lue YH, Wang C, Liu YX, Zhang XS, Sinha Hikim AP, Zhang XS, Ng CM, Hu ZY, Li YC, Leung A, Swerdloff RS.Transient testicular warming enhances the suppressive effect of testosterone on spermatogenesis in adult cynomolgus monkeys (Macaca fascicularis). J Clin Endocrinol Metab 2006; 91: 539–545.. [DOI] [PubMed] [Google Scholar]
- Dunkel L, Taskinen S, Hovatta O, Tilly JL, Wikstrom S.Germ cell apoptosis after treatment of cryptorchidism with human chorionic gonadotropin is associated with impaired reproductive function in the adult. J Clin Invest 1997; 100: 2341–2346.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha Hikim AP, Swerdloff RS.Hormonal and genetic control of germ cell apoptosis in the testis. Rev Reprod 1999; 4: 38–47. [DOI] [PubMed] [Google Scholar]
- Sinha Hikim AP, Lue Y, Yamamoto CM, Vera Y, Rodriguez S, Yen PH, Soeng K, Wang C, Swerdloff RS.Key apoptotic pathways for heat-induced programmed germ cell death in the testis. Endocrinology 2003; 144: 3167–3175.. [DOI] [PubMed] [Google Scholar]
- Vera Y, Erkkila K, Wang C, Nunez C, Kyttanen S, Lue Y, Dunkel L, Swerdloff RS, Sinha Hikim AP.Involvement of p38 mitogen-activated protein kinase and inducible nitric oxide synthase in apoptotic signaling of murine and human male germ cells after hormone deprivation. Mol Endocrinol 2006; 20: 1597–1609.. [DOI] [PubMed] [Google Scholar]
- Vera Y, Diaz-Romero M, Rodriguez S, Lue Y, Wang C, Swerdloff RS, Sinha Hikim AP.Mitochondria-dependent pathway is involved in heat-induced male germ cell death: lessons from mutant mice. Biol Reprod 2004; 70: 1534–1540.. [DOI] [PubMed] [Google Scholar]
- Jia Y, Sinha Hikim AP, Swerdloff RS, Lue YH, Vera Y, Zhang XS, Hu ZY, Li YC, Liu YX, Wang C.Signaling pathways for germ cell death in adult Cynomolgus monkeys (Macaca fascicularis) induced by mild testicular hyperthermia and exogenous testosterone treatment. Biol Reprod 2007; 77: 83–92.. [DOI] [PubMed] [Google Scholar]
- Castellanos J. Characterization of signaling pathways that culminate in activation of the intrinsic pathway signaling for male germ cell death triggered by hormone deprivation or by testicular hyperthermia. Dominguez Hills: California State University;; 2007. Master of Science Thesis. [Google Scholar]
- Lassus P, Opitz-Araya X, Lazebnik Y.Requirement for caspase-2 in stress-induced apoptosis before mitochondrial permeabilization. Science 2002; 297: 1352–1354.. [DOI] [PubMed] [Google Scholar]
- Nutt LK, Margolis SS, Jensen M, Herman CE, Dunphy WG, Rathmell JC, Kornbluth S.Metabolic regulation of oocyte cell death through CaMKII-mediated phosphorylation of caspase 2. Cell 2005; 123: 89–103.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonzon C, Bouchier-Hayes L, Pagliari LJ, Green DR, Newmeyer DD.Caspase 2-induced apoptosis requires Bid cleavage: a physiological role for Bid in heat-shock-induced death. Mol Biol Cell 2006; 17: 2150–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad V, Chandele A, Jagtap JC, Kumar S, Shastry P.ROS-triggered caspase 2 activation and feedback amplification loop in β-carotene-induced apoptosis. Free Radic Biol Med 2006; 41: 431–442.. [DOI] [PubMed] [Google Scholar]
- Tu S, McStay GP, Boucher LM, Mak T, Beere HM, Green DR.In situ trapping of activated initiator caspases reveals a role for caspase 2 in heat shock-induced apoptosis. Nat Cell Biol 2006; 8: 72–77.. [DOI] [PubMed] [Google Scholar]
- Mhaidat NM, Wang Y, Kiejda KA, Zhang XD, Hersey P.Docetaxel-induced apoptosis in melanoma cells is dependent on activation of caspase-2. Mol Cancer Ther 2007; 6: 752–761.. [DOI] [PubMed] [Google Scholar]
- Jeohn GH, Cooper CL, Wilson B, Chang RCC, Jang KJ, Kim HC, Liu B, Hong JS.p38 MAP kinase is involved in lipopolysaccharide-induced dopaminergic neuronal cell death in rat mesencephalic neuron-glia cultures. Ann N Y Acad Sci 2002; 962: 332–346.. [DOI] [PubMed] [Google Scholar]
- Nolan Y, Verker E, Lynch AM, Lynch MA.Evidence that lipopolysaccharide-induced cell death is mediated by accumulation of reactive oxygen species and activation of p38 in rat cortex and hippocampus. Exp Neurol 2003; 184: 794–804.. [DOI] [PubMed] [Google Scholar]
- Tamagno E, Robino G, Obbili A, Bardini P, Aragno M, Parola M, Danni O.H2O2 and 4-hydroxynonenal mediate amyloid β-induced neuronal apoptosis by activation JNKs and p38 MAPK. Exp Neurol 2003; 180: 144–155.. [DOI] [PubMed] [Google Scholar]
- Zheng S, Turner TT, Lysiak JJ.Caspase 2 activity contributes to the initial wave of germ cell apoptosis during the first round of spermatogenesis. Biol Reprod 2006; 74: 1026–1033.. [DOI] [PubMed] [Google Scholar]
- Lysiak JJ, Zheng S, Woodson R, Turner TT.Caspase-9-dependent pathway to murine germ cell apoptosis: mediation by oxidative stress, BAX, and caspase 2. Cell Tissue Res 2007; 328: 411–419.. [DOI] [PubMed] [Google Scholar]
- Sinha Hikim AP, Swerdloff RS.Temporal and stage-specific changes in spermatogenesis of rat after gonadotropin deprivation by a potent gonadotropin-releasing hormone antagonist treatment. Endocrinology 1993; 133: 2161–2170.. [DOI] [PubMed] [Google Scholar]
- Vera Y, Rodriguez A, Castanares M, Lue Y, Atienza V, Wang C, Swerdloff RS, Sinha Hikim AP.Functional role of caspases in heat-induced testicular germ cell apoptosis. Biol Reprod 2005; 72: 516–522.. [DOI] [PubMed] [Google Scholar]
- Yamamoto CM, Sinha Hikim AP, Huynh PN, Shapiro B, Lue Y, Salameh WA, Wang C, Swerdloff RS.Redistribution of Bax is an early step in an apoptotic pathway leading to germ cell death in rats, triggered by mild testicular hyperthermia. Biol Reprod 2000; 63: 1683–1690.. [DOI] [PubMed] [Google Scholar]
- Russell LD, Ettlin RA, Sinha Hikim AP, Clegg ED.Histological and Histopathological Evaluation of the Testis Clearwater, FL:Cache River Press;1990: 62–118.. [Google Scholar]
- Robertson JD, Enoksson M, Suomela M, Zhivotovsky B, Orrenius S.Caspase-2 acts upstream of mitochondria to promote cytochrome c release during etopside-induced apoptosis. J Bio Chem 2002; 277: 29803–29809.. [DOI] [PubMed] [Google Scholar]
- Cheung HH, Lynn Kelly N, Liston P, Korneluk RG.Involvement of caspase-2 and caspase-9 in the endoplasmic stress-induced apoptosis: a role for the IAPs. Exp Cell Res 2006; 312: 2347–2357.. [DOI] [PubMed] [Google Scholar]
- Castanares M, Vera Y, Erkkila K, Kyttanen S, Lue Y, Dunkel L, Wang C, Swerdloff RS, Sinha Hikim AP.Minocycline up-regulates BCL-2 levels in mitochondria and attenuates male germ cell apoptosis. Biochem Biophys Res Commun 2005; 337: 663–669. [DOI] [PubMed] [Google Scholar]
- Zhu S, Stavrovskaya IG, Drozda M, Kim BYS, Ona V, Li M, Sarang S, Liu AS, Hartely DM, Wu DC, Gullans S, Ferrante RJ, et al. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature 2002; 417: 74–78.. [DOI] [PubMed] [Google Scholar]
- Teng YD, Choi H, Onario RC, Zhu S, Desilets FC, Lan S, Woodard EJ, Snyder EY, Eichler ME, Friedlander RM.Minocycline inhibits contusion-triggered mitochondrial cytochrome c release and mitigates functional deficits after spinal cord injury. Proc Natl Acad Sci U S A 2004; 101: 3071–3076.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Zhao L, Liu J, Dodel RC, Farlow MR, Du Y.Minocycline prevents gentamicin-induced cytotoxicity by inhibiting p38 MAP kinase phosphorylation and caspase 3 activation, Neuroscience 2005; 131: 513–521.. [DOI] [PubMed] [Google Scholar]
- Kang SJ, Wang S, Hara H, Peterson EP, Namura S, Amin-Hanjani S, Huang Z, Srinivasan A, Tomaselli KJ, Thornberry NA, Moskowitz MA, Yuan J.Dual role of caspase-11 in mediating activation caspase-1 and caspase-3 under pathological conditions. J Cell Biol 2000; 149: 613–622.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata M, Hisahara S, Hara H, yamawaki T, Fukuuchi Y, Yuan J, Okano H, Miura M.Caspases determine the vulnerability of oligodendrocytes in the ischemic brain. J Clin Invest 2000; 106: 643–653.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J.Caspase-12 mediated endoplasmic reticulum-specific apoptosis and cytotoxicity by amyloid β. Nature 2000; 403: 98–103.. [DOI] [PubMed] [Google Scholar]