Summary
Background
Low-dose aspirin is of definite and substantial net benefit for many people who already have occlusive vascular disease. We have assessed the benefits and risks in primary prevention.
Methods
We undertook meta-analyses of serious vascular events (myocardial infarction, stroke, or vascular death) and major bleeds in six primary prevention trials (95 000 individuals at low average risk, 660 000 person-years, 3554 serious vascular events) and 16 secondary prevention trials (17 000 individuals at high average risk, 43 000 person-years, 3306 serious vascular events) that compared long-term aspirin versus control. We report intention-to-treat analyses of first events during the scheduled treatment period.
Findings
In the primary prevention trials, aspirin allocation yielded a 12% proportional reduction in serious vascular events (0·51% aspirin vs 0·57% control per year, p=0·0001), due mainly to a reduction of about a fifth in non-fatal myocardial infarction (0·18% vs 0·23% per year, p<0·0001). The net effect on stroke was not significant (0·20% vs 0·21% per year, p=0·4: haemorrhagic stroke 0·04% vs 0·03%, p=0·05; other stroke 0·16% vs 0·18% per year, p=0·08). Vascular mortality did not differ significantly (0·19% vs 0·19% per year, p=0·7). Aspirin allocation increased major gastrointestinal and extracranial bleeds (0·10% vs 0·07% per year, p<0·0001), and the main risk factors for coronary disease were also risk factors for bleeding. In the secondary prevention trials, aspirin allocation yielded a greater absolute reduction in serious vascular events (6·7% vs 8·2% per year, p<0.0001), with a non-significant increase in haemorrhagic stroke but reductions of about a fifth in total stroke (2·08% vs 2·54% per year, p=0·002) and in coronary events (4·3% vs 5·3% per year, p<0·0001). In both primary and secondary prevention trials, the proportional reductions in the aggregate of all serious vascular events seemed similar for men and women.
Interpretation
In primary prevention without previous disease, aspirin is of uncertain net value as the reduction in occlusive events needs to be weighed against any increase in major bleeds. Further trials are in progress.
Funding
UK Medical Research Council, British Heart Foundation, Cancer Research UK, and the European Community Biomed Programme.
Introduction
In patients who are at high risk because they already have occlusive vascular disease, long-term antiplatelet therapy (eg, with aspirin) reduces the yearly risk of serious vascular events (non-fatal myocardial infarction, non-fatal stroke, or vascular death) by about a quarter.1,2 This decrease typically corresponds to an absolute reduction of about 10–20 per 1000 in the yearly incidence of non-fatal events, and to a smaller, but still definite, reduction in vascular death. Against this benefit, the absolute increase in major gastrointestinal or other major extracranial bleeds is an order of magnitude smaller. Hence, for secondary prevention, the benefits of antiplatelet therapy substantially exceed the risks.
For primary prevention, however, the balance is less clear because the risks without aspirin, and hence the absolute benefits of aspirin, are generally an order of magnitude lower than in secondary prevention. Previous meta-analyses of primary prevention trials were not based on individual participant data, so they could not compare reliably the benefits and risks of aspirin in prognostically important groups (such as older people and others at increased risk of coronary heart disease), and could not quantify reliably the extent to which people at increased risk of coronary heart disease might also be at increased risk of bleeding. Current guidelines largely ignore any differences in bleeding risk, and recommend that aspirin be used widely for primary prevention in those at moderately raised risk of coronary heart disease.3–5 It has also been suggested that, since age is a major determinant of the risk of coronary heart disease, daily aspirin should be started in all people above a specific age, either alone or in combination with other drugs.6–8
The alternative to primary prevention is deferral of the start of long-term aspirin until some evidence of occlusive vascular disease is noted. The main disadvantage of deferral is that the first manifestation of disease might be a disabling or fatal event, but the main advantage is that it could avoid decades of slightly increased risk of cerebral haemorrhage or major extracranial bleeding. In the primary prevention trials, most controls who had a non-fatal myocardial infarction or occlusive stroke while not on aspirin would probably then have started long-term aspirin to avoid recurrence, so the mortality results from those trials can help to decide between the policies of immediate versus deferred aspirin (ie, deferral of the start of long-term aspirin until there is evidence of disease).
In view of the limitations of the analyses underlying current guidelines, and the large populations affected by these guidelines, a collaborative meta-analysis of individual participant data was established involving the principal investigators of all large trials of primary prevention with aspirin. Meta-analyses of previously obtained individual participant data from 16 secondary prevention trials of aspirin were also undertaken to compare the proportional and absolute effects of aspirin in these two treatment settings.1,2
Methods
Trial eligibility
Primary or secondary prevention trials were eligible only if they involved a randomised comparison of aspirin versus no aspirin (with no other antiplatelet drug in either group). Primary prevention trials excluded individuals with any history of occlusive disease at entry. (Subsequent enquiry showed that 2% did in fact have some evidence of previous vascular disease, but they remain in all analyses apart from those estimating the absolute effects of aspirin.) Primary prevention trials were sought only if they recruited at least 1000 non-diabetic participants with at least 2 years of scheduled treatment. Individual participant data were provided from all six published trials.9–14 Unpublished trials were sought through electronic searches and discussions, but none was identified.
Secondary prevention trials were included in analyses if they involved individuals with previous myocardial infarction (six trials) or stroke or transient cerebral ischaemia (ten trials), and had contributed individual participant data to the 2002 Antithrombotic Trialists' (ATT) report (webappendix pp 11–23).1,2 Two further trials for which only tabular data were available are shown in the webappendix but do not contribute to analyses. Electronic searches established that no similar trials of aspirin had been reported since 2002.
Prespecified analyses
The comparisons were intention-to-treat analyses of first events during the scheduled treatment period in all participants allocated aspirin versus all those allocated control (irrespective of any other treatment allocated factorially). The main outcomes were serious vascular event, defined as myocardial infarction, stroke, or death from a vascular cause (including sudden death, pulmonary embolism, haemorrhage, and, for secondary prevention trials only, death from an unknown cause); major coronary event (myocardial infarction, coronary death, or sudden death); any stroke (haemorrhagic or probably ischaemic [ie, definitely ischaemic or of unknown type]); death from any cause; and major extracranial bleed (mainly gastrointestinal and usually defined as a bleed requiring transfusion or resulting in death). In the primary prevention trials, myocardial infarctions and strokes were classified as fatal or non-fatal in accordance with each trial's definitions. In the secondary prevention trials, as previously,2 these outcomes were regarded as non-fatal only if the patient was alive at the end of the trial or died of a non-vascular cause. Five of the primary prevention trials classified stroke subtypes on the basis of either clinical examination10 or CT imaging.9,11,13,14 In the sixth trial,12 imaging information was available only for strokes that had been confirmed as cerebral bleeds. In most secondary prevention trials little information about stroke causes was available (webappendix pp 15–18).1,2
Statistical analysis
The log-rank observed minus expected (o–e) statistics, one from each trial, and their variances (v), were summed to produce, respectively, a grand total observed minus expected (G) and its variance (V). The one-step estimate of the log of the event rate ratio is G/V. The χ2 test statistic (χ2n–1) for heterogeneity between n trials is S–(G2/V), where S is the sum over all the trials of (o–e)2/v. Heterogeneity of rate ratios among multiple subgroups defined by baseline characteristics was investigated by a global heterogeneity test, which helps to avoid misinterpreting false positive results arising from multiple comparisons. (For each characteristic [eg, age] a χ2 test for trend on 1 degree of freedom was calculated. If there are 11 different χ2 tests and none of the trends is real, then their sum has expectation 11 and has variance at least as great as that of χ211.)
For trials that randomised unequally (ie, 2:1), we multiplied the control group by two when displaying adjusted control totals and describing the total amount of information available, but not in other calculations. For the purposes of discussion, we calculated what the absolute effects of aspirin allocation would be on outcome at 5 years (only two trials9,14 had much longer follow-up) if the yearly event rates were constant and the proportional effects of aspirin were independent of age, sex, and other risk factors.
To identify risk factors for various outcomes in people in the primary prevention trials without any known history of vascular disease, we used Poisson regression, stratified by trial, to estimate the common linear dependence of the log of the event rate on age, sex, diabetes, current cigarette smoking, total cholesterol, mean (of systolic and diastolic) blood pressure, body-mass index, and allocation to aspirin or control (webappendix p 24). Additionally, the results of this model for major coronary events in control participants only, together with the absolute event rates in the controls of each trial, were used to classify the baseline risks of all participants (including those allocated aspirin) as very low (predicted 5-year risk of coronary heart disease without aspirin <2·5%), low (2·5–5%), moderate (5–10%), or high (≥10%).
Further details of the trials and of the analyses are available in the webappendix.
Role of the funding sources
The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The secretariat had full access to all the data in the study and the writing committee had final responsibility for the decision to submit for publication.
Results
Six primary prevention trials were available (95 000 individuals, 3554 serious vascular events; table 1, figure 1).9–14 One12 recruited people with hypertension, and two9,13 recruited people with coronary risk factors (although without overt disease). The results were contrasted with those from the 16 secondary prevention trials (17 000 individuals, 3306 vascular events).2
Table 1.
Design and eligibility criteria of primary prevention trials
| Dates of recruitment | Participating countries | Year of main publication | Number of participants | Mean duration of follow-up (years) | Target population | Eligible age range (years) at entry | Aspirin regimen | Randomised factorial comparison | Placebo control | |
|---|---|---|---|---|---|---|---|---|---|---|
| British Doctors' Study10 | Nov 1978–Nov 1979 | UK | 1988 | 5139 | 5·6 | Male doctors | 19–90 | 500 mg daily | None | No |
| US Physicians' Health Study11 | Aug 1981–Apr 1984 | USA | 1988 | 22071 | 5·0 | Male doctors | 45–73 | 325 mg alternate days | β carotene vs placebo | Yes |
| Thrombosis Prevention Trial9 | Feb 1989–May 1994 | UK | 1998 | 5085 | 6·7 | Men with risk factors for CHD | 45–69 | 75 mg daily | Warfarin vs placebo | Yes |
| Hypertension Optimal Treatment Trial12 | Oct 1992–May 1994 | Europe, North and South America, Asia | 1998 | 18790 | 3·8 | Men and women with DBP 100–115 mm Hg | 50–80 | 75 mg daily | Three blood pressure regimens | Yes |
| Primary Prevention Project13 | June 1993–Apr 1998 | Italy | 2001 | 4495 | 3·7 | Men and women with one or more risk factors for CHD | 45–94 | 100 mg daily | Vitamin E vs open control | No |
| Women's Health Study14 | Sep 1992–May 1995 | USA | 2005 | 39876 | 10·0 | Female health professionals | ≥45 | 100 mg alternate days | Vitamin E vs placebo | Yes |
CHD=coronary heart disease. DBP=diastolic blood pressure.
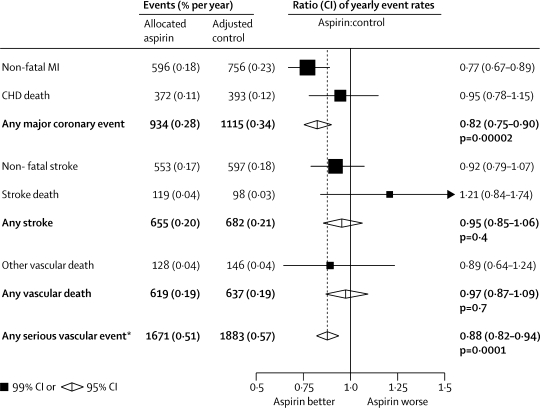
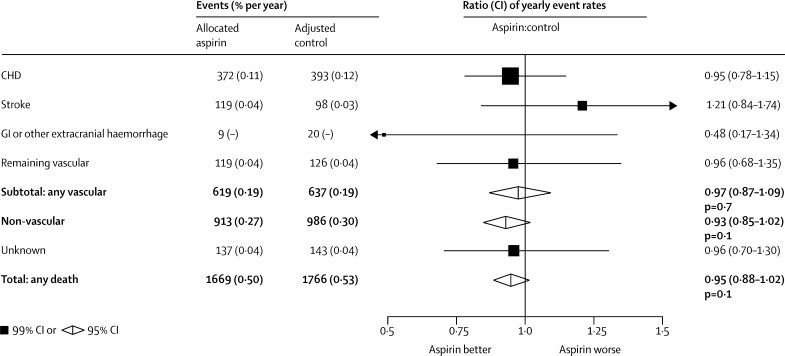
Figure 1.
Serious vascular events in primary prevention trials—proportional effects of aspirin allocation
Actual numbers for aspirin-allocated trial participants, and adjusted numbers for control-allocated trial participants, are presented, together with the corresponding mean yearly event rate (in parentheses). Participants can contribute only once to the total of serious vascular events. Rate ratios (RRs) for all trials are indicated by squares and their 99% CIs by horizontal lines. Subtotals and their 95% CIs are represented by diamonds. Squares or diamonds to the left of the solid line indicate benefit. MI=myocardial infarction. CHD=coronary heart disease. *Myocardial infarction, stroke, or vascular death. Vascular death is coronary heart disease death, stroke death, or other vascular death (which includes sudden death, death from pulmonary embolism, and death from any haemorrhage, but in the primary prevention trials excludes death from an unknown cause).
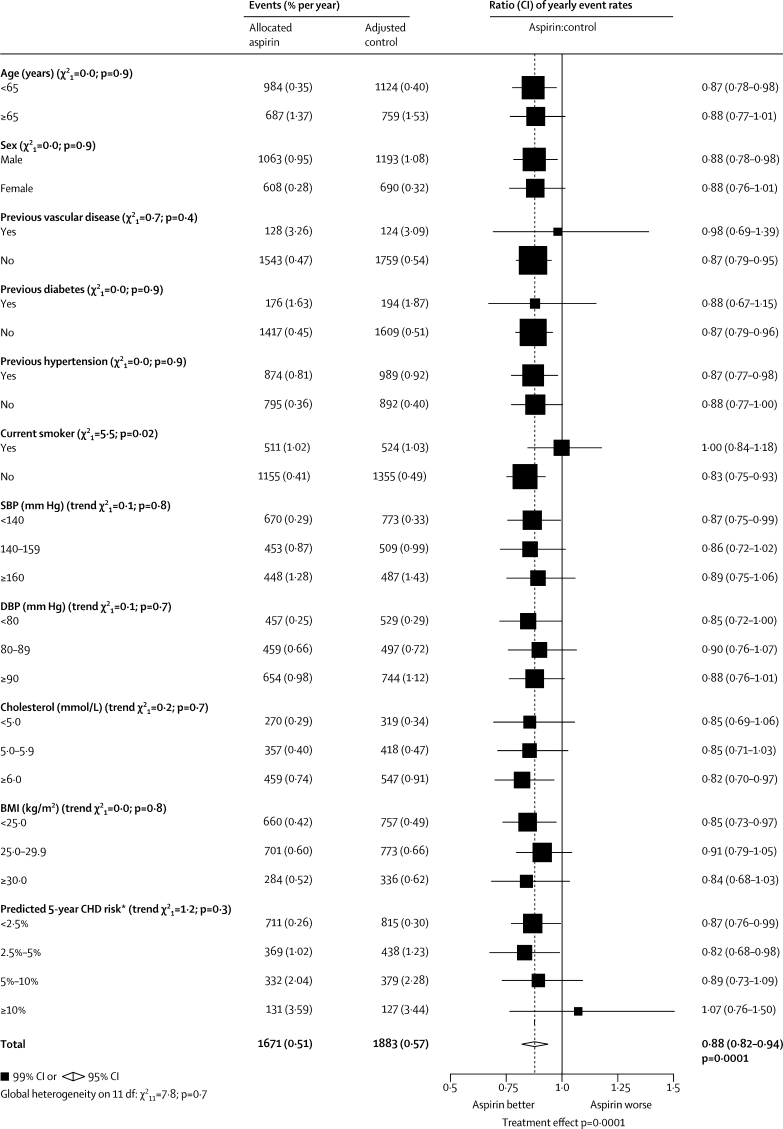
In the primary prevention trials, 1671 serious vascular events occurred during 330 000 person-years (0·51% per year) in people allocated aspirin compared with an adjusted total of 1883 events during 330 000 person-years (0·57% per year) in those allocated control. This small absolute reduction (only 0·07% per year) represented a 12% proportional reduction (rate ratio [RR] 0·88 [95% CI 0·82–0·94], p=0·0001; figure 1), with no significant heterogeneity between the prespecified subgroups (global test for heterogeneity p=0·7; figure 2). The proportional reduction seemed similar (p=0·9) in men and women, and did not differ significantly (p trend=0·3) between those with predicted 5-year risk of coronary heart disease less than 2·5%, 2·5–5%, 5–10%, or 10% or more (figure 2). The apparently unpromising result in the 2% of all participants in the primary prevention trials who were in the highest risk group was statistically unreliable because it involved small numbers (131 [3·59% per year] vs 127 [3·44% per year] vascular events; RR 1·07 [99% CI 0·76–1·50], p=0·6; figure 2). The most important predictor of risk was age; the mean age at entry for those in the highest risk group was 69 years (SD 6) (so their mean age during the trials was over 70 years). The proportional reduction in risk of any serious vascular event did not differ significantly between primary and secondary prevention trials, but the absolute risk reduction was much smaller in primary than in secondary prevention (table 2).
Figure 2.
Serious vascular events in primary prevention trials—subgroup analyses
Actual numbers for aspirin-allocated trial participants, and adjusted numbers for control-allocated trial participants, are presented, together with the corresponding mean yearly event rates (in parentheses). Rate ratios (RRs) for all trials are indicated by squares and their 99% CIs by horizontal lines. Subtotals and their 95% CIs are represented by diamonds. Squares or diamonds to the left of the solid line indicate benefit. A global test for heterogeneity (χ2 on 11 degrees of freedom) is provided. Unknown values are not plotted. SBP=systolic blood pressure. DBP=diastolic blood pressure. BMI=body-mass index. CHD=coronary heart disease. *Excluding patients with a history of vascular disease.
Table 2.
Comparison of proportional and absolute effects of aspirin in primary and secondary prevention trials
|
Number of events (aspirin vs control) |
Rate ratio (95% CI) (aspirin vs control) |
Yearly absolute difference (% per year) |
||||||
|---|---|---|---|---|---|---|---|---|
| Primary prevention (660 000 person-years) | Secondary prevention (43 000 person-years) | Primary prevention | Secondary prevention | p value for heterogeneity | Primary prevention | Secondary prevention | ||
| Major coronary event | 934 vs 1115 | 995 vs 1214 | 0·82 (0·75–0·90) | 0·80 (0·73–0·88) | 0·7 | −0·06 | −1·00* | |
| Non-fatal MI | 596 vs 756 | 357 vs 505 | 0·77 (0·69–0·86) | 0·69 (0·60–0·80) | 0·5 | −0·05 | −0·66 | |
| CHD mortality | 372 vs 393 | 614 vs 696 | 0·95 (0·82–1·10) | 0·87 (0·78–0·98) | 0·4 | −0·01 | −0·34 | |
| Stroke | 655 vs 682 | 480 vs 580 | 0·95 (0·85–1·06) | 0·81 (0·71–0·92) | 0·1 | −0·01 | −0·46* | |
| Haemorrhagic | 116 vs 89 | 36 vs 19 | 1·32 (1·00–1·75) | 1·67 (0·97–2·90) | 0·4 | 0·01 | ..† | |
| Ischaemic | 317 vs 367 | 140 vs 176 | 0·86 (0·74–1·00) | 0·78 (0·61–0·99) | 0·5 | −0·02 | ..† | |
| Unknown cause | 222 vs 226 | 304 vs 385 | 0·97 (0·80–1·18) | 0·77 (0·66–0·91) | 0·1 | −0·001 | ..† | |
| Vascular death | 619 vs 637 | 825 vs 896 | 0·97 (0·87–1·09) | 0·91 (0·82–1·00) | 0·4 | −0·01 | −0·29 | |
| Any serious vascular event | 1671 vs 1883 (0·51% vs 0·57% per year) | 1505 vs 1801 (6·69% vs 8·19% per year) | 0·88 (0·82–0·94) | 0·81 (0·75–0·87) | 0·1 | −0·07 | −1·49* | |
| Major extracranial bleed | 335 vs 219 | 23 vs 6 | 1·54 (1·30–1·82) | 2·69 (1·25–5·76) | 0·2 | 0·03 | ..† | |
MI=myocardial infarction. CHD=coronary heart disease. Non-fatal MI definitions vary; see methods.
Major coronary event rates (percent per year, aspirin vs control) 6·0 vs 7·4 in post-MI trials and 2·4 vs 3·0 in post-cerebral vascular disease trials; corresponding rates of stroke (mainly of unknown cause) 0·6 vs 0·8 in post-MI trials and 3·9 vs 4·7 in post-cerebral vascular disease trials (webappendix pp 14–18).
Stroke causes, and extracranial bleeds, very incompletely reported.
Since major coronary events and strokes accounted for a large proportion of serious vascular events, the effects of aspirin on each outcome were assessed separately. In the primary prevention trials, allocation to aspirin yielded an 18% proportional reduction in major coronary events, but only a small absolute reduction (0·28% vs 0·34% per year; RR 0·82 [95% CI 0·75–0·90], p<0·0001; figure 1). Most of this decrease derived from a 23% proportional reduction in non-fatal myocardial infarction (0·18% vs 0·23% per year; RR 0·77 [99% CI 0·67–0·89], p<0·0001; figure 1), with no clear reduction in mortality from coronary heart disease (0·11% vs 0·12% per year; RR 0·95 [99% CI 0·78–1·15], p=0·5), although the CI for the proportional reduction in mortality from coronary heart disease is wide. The proportional reduction in major coronary events seemed to be similar in primary and secondary prevention (RR 0·82 [95% CI 0·75–0·90] primary and RR 0·80 [0·73–0·88] secondary), but the absolute benefit differed by an order of magnitude (absolute benefits 0·06% per year primary and 1·00% per year secondary) (table 2).
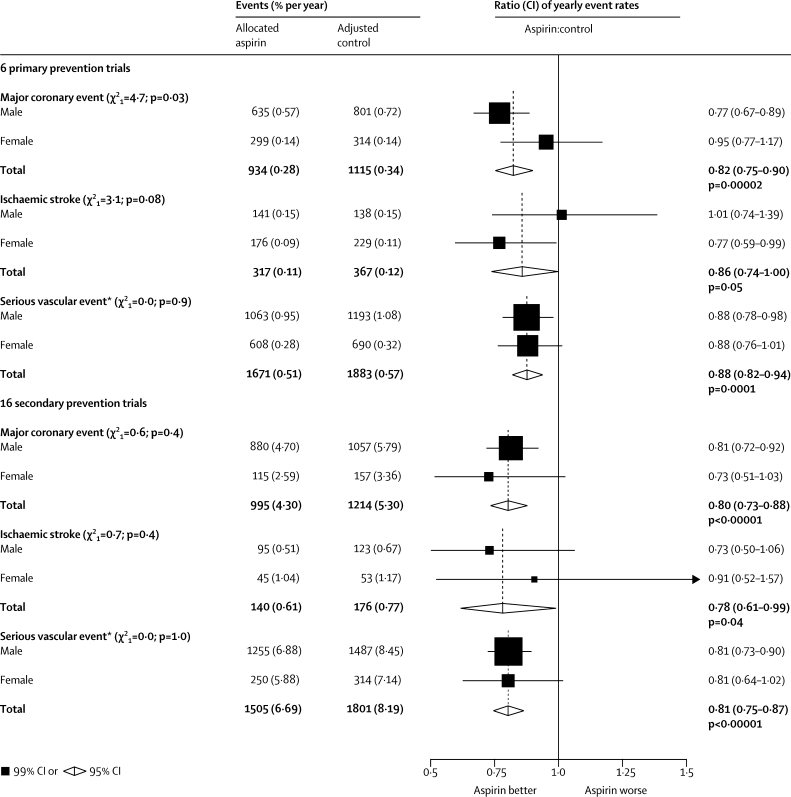
For major coronary events in the primary prevention trials, the 11 separate tests for trend or heterogeneity of effect yielded only one, between men and women, that was marginally significant (p=0·03; figure 3 and webappendix p 4) if considered in isolation, but it was no longer significant if multiplied by 11 to allow for multiple comparisons (p=0·33). Furthermore, the 16 secondary prevention trials also suggested no heterogeneity of the effect on major coronary events between men and women (figure 3). Conversely, for ischaemic stroke, the six primary prevention trials suggested a greater proportional risk reduction in women than in men (p=0·08 for heterogeneity of effect between men and women if considered in isolation, but p=0·88 [not significant] if multiplied by 11 to allow for multiple comparisons); again, however, the 16 secondary prevention trials suggested no such heterogeneity of effect. For the aggregate of all serious vascular events, gender was of no apparent relevance in either type of trial to the proportional reduction produced by allocation to aspirin (figure 3).
Figure 3.
Selected outcomes in primary and secondary prevention trials of aspirin, by sex
Actual numbers for aspirin-allocated trial participants, and adjusted numbers for control-allocated trial participants, are presented together with the corresponding mean yearly event rate (in parentheses). Rate ratios (RRs) for all trials are indicated by squares and their 99% CIs by horizontal lines. Subtotals and their 95% CIs are represented by diamonds. Squares or diamonds to the left of the solid line indicate benefit. *Myocardial infarction, stroke (haemorrhagic or other), or vascular death.
Aspirin seemed to increase the incidence of haemorrhagic stroke both in the primary and in the secondary prevention trials (p=0·05 and p=0·07, respectively; p=0·01 when analysed together). Conversely, aspirin seemed to reduce the incidence of ischaemic stroke in both types of trial (p=0·05 and p=0·04, respectively; p=0·005 when analysed together). The proportion of strokes of known cause that were haemorrhagic was greater in the primary than in the secondary prevention trials (23% vs 15%; table 2); this was probably also true for strokes of unknown cause. For, at least 84% (893/1060: webappendix p 18) of the strokes in the secondary prevention trials were in patients with a previous history of ischaemic stroke or transient cerebral ischaemia, who would be at high risk of recurrence.
In the primary prevention trials, aspirin had no net effect on strokes of known cause (haemorrhagic plus ischaemic), on strokes of unknown cause, or on the aggregate of all strokes (table 2). In the secondary prevention trials, however (in which a smaller proportion of the strokes of known cause were haemorrhagic), aspirin significantly reduced the aggregate of all strokes.
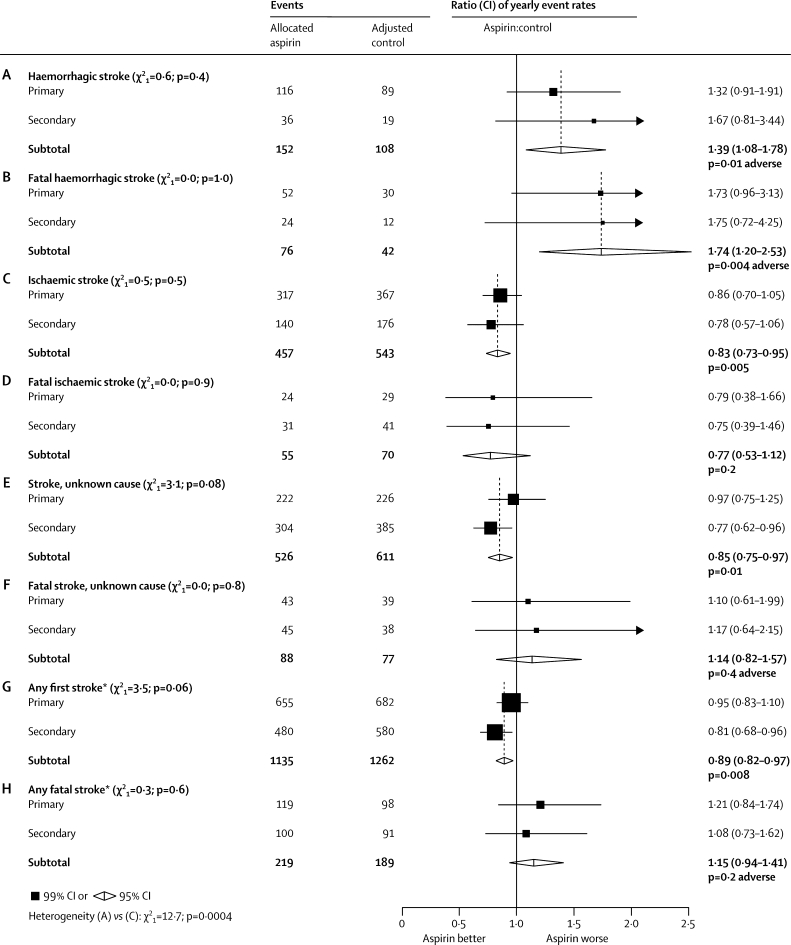
As most strokes do not cause death, and haemorrhagic strokes may be more dangerous than ischaemic strokes, the proportional effects of aspirin on overall stroke mortality and on non-fatal stroke could differ. In figure 4 the results are therefore subdivided not only by cause but also by outcome (any outcome, or fatal). For strokes with any outcome (figures 4A, 4C, 4E, and 4G) the results are those from table 2. The subtotals in figure 4 combine the primary and secondary trial results. Taking both types of trial together, there was evidence of an adverse effect on haemorrhagic stroke (RR 1·39 [95% CI 1·08–1·78], p=0·01; figure 4A) but a protective effect on ischaemic stroke (RR 0·83 [0·73–0·95], p=0·005; figure 4C).
Figure 4.
Stroke subtypes in primary and secondary prevention trials
Actual numbers for aspirin-allocated trial participants, and adjusted numbers for control-allocated trial participants, are presented. Rate ratios (RRs) for all trials are indicated by squares and their 99% CIs by horizontal lines. Subtotals and their 95% CIs are represented by diamonds. Squares or diamonds to the left of the solid line indicate benefit. *Haemorrhagic, ischaemic, or unknown cause.
If attention is restricted to the fatal strokes just in the primary prevention trials, then fatal haemorrhagic strokes outnumber fatal ischaemic strokes (82 vs 53) and there is, if anything, an adverse effect on overall stroke mortality (119 vs 98 fatal strokes, p=0·18 [non-significant]; figure 4H). For, there was a significant excess of fatal haemorrhagic strokes in participants allocated aspirin (52 vs 30; RR 1·73 [99% CI 0·96–3·13], p=0·02; figure 4B) plus similar numbers of other fatal strokes (24 vs 29 ischaemic plus 43 vs 39 unknown cause; figures 4D and 4F).
Since allocation to aspirin had no significant effect on fatal stroke, fatal coronary heart disease or other vascular causes of death, there was no significant reduction in overall vascular mortality in the primary prevention trials (RR 0·97 [95% CI 0·87–1·09], p=0·7; figure 1). Since there was also no significant effect on non-vascular mortality (RR 0·93 [95% CI 0·85–1·02], p=0·1) or on mortality from unknown causes (RR 0·96 [99% CI 0·70–1·30], p=0·7), there was no significant effect on total mortality (RR 0·95 [95% CI 0·88–1·02], p=0·1; figure 5). By contrast, in the secondary prevention trials, aspirin seemed to reduce vascular mortality (RR 0·91 [0·82–1·00], p=0·06) and had no significant effect on other mortality (RR 0·85 [0·66–1·08], p=0·2), yielding a 10% reduction in total mortality (RR 0·90 [0·82–0·99], p=0·02).
Figure 5.
Mortality by cause in primary prevention trials
Actual numbers for aspirin-allocated trial participants, and adjusted numbers for control-allocated trial participants, are presented together with the corresponding mean yearly event rate (in parentheses). Rate ratios (RRs) for all trials are indicated by squares and their 99% CIs by horizontal lines. Subtotals and their 95% CIs are represented by diamonds. Squares or diamonds to the left of the solid line indicate benefit. CHD=coronary heart disease. GI=gastointestinal.
The main hazard of aspirin is haemorrhage and, apart from any effect on intracerebral haemorrhage, aspirin increased major gastrointestinal and other extracranial bleeds by about half in the primary prevention trials (0·10% vs 0·07% per year; RR 1·54 [1·30–1·82], p<0·0001; table 2 and webappendix pp 9, 10). This increase was non-significantly greater in participants with high cholesterol (p=0·02 for trend if considered in isolation, but p=0·22 if multiplied by 11; webappendix p 10). The excess risk was chiefly of non-fatal bleeds; perhaps by chance, there were actually fewer fatal bleeds in participants allocated aspirin than in controls (nine vs 20; figure 5). Major bleeds were recorded in only five of the 16 secondary prevention trials, so a meta-analysis might be unreliable. There was again, however, a significant excess of major bleeds among those allocated aspirin (RR 2·69 [1·25–5·76], p=0·01; table 2 and webappendix p 20), with no significant heterogeneity between the relative risks in the six primary and these five secondary prevention trials (p=0·2; table 2).
The absolute yearly incidence of vascular events and of major extracranial bleeds varied substantially among participants in the primary prevention trials. Poisson regression in 93 918 individuals without known vascular disease at entry within the primary prevention trials indicated that age (per decade), male sex, diabetes, current smoking, and mean blood pressure (per 20 mm Hg) were each associated with about a two-fold increased risk of major coronary events, whereas total cholesterol (per 1 mmol/L) and body-mass index (per 5 kg/m2) were more weakly associated with such events (table 3). Measurements of cholesterol fractions were not sought, and body-mass index acts mainly as a determinant of other cardiac risk factors15 so is of little independent relevance in these multivariate analyses. The main risk factors for coronary events in table 3 were also associated with haemorrhagic events, although for most the associations were slightly weaker for bleeding than for occlusive events.
Table 3.
Rate ratios (95% CI) associated with risk factors for selected outcomes in people with no known vascular disease in primary prevention trials
| Major coronary event | Probably ischaemic stroke | Haemorrhagic stroke | Major extracranial bleed | |
|---|---|---|---|---|
| Age (per decade) | 1·84 (1·74–1·95) | 2·46 (2·27–2·65) | 1·59 (1·33–1·90) | 2·15 (1·93–2·39) |
| Male sex* | 2·43 (1·94–3·04) | 1·44 (1·14–1·82) | 1·11 (0·52–2·34) | 1·99 (1·45–2·73) |
| Diabetes mellitus | 2·66 (2·28–3·12) | 2·06 (1·67–2·54) | 1·74 (0·95–3·17) | 1·55 (1·13–2·14) |
| Current smoker | 2·05 (1·85–2·28) | 2·00 (1·72–2·31) | 2·18 (1·57–3·02) | 1·56 (1·25–1·94) |
| Mean blood pressure (per 20 mm Hg)† | 1·73 (1·59–1·89) | 2·00 (1·77–2·26) | 2·18 (1·65–2·87) | 1·32 (1·09–1·58) |
| Cholesterol (per 1 mmol/L) | 1·18 (1·12–1·24) | 1·02 (0·95–1·09) | 0·90 (0·77–1·07) | 0·99 (0·90–1·08) |
| Body-mass index (per 5 kg/m2) | 1·09 (1·03–1·15) | 1·06 (0·98–1·14) | 0·85 (0·71–1·02) | 1·24 (1·13–1·35) |
Analyses are stratified by trial. The relevance of male sex can therefore be assessed only in the two trials that included both men and women, so the 95% CIs for it are wide, particularly for stroke.
Mean of systolic and diastolic blood pressure. Associations with measured values are not corrected for the effects of regression dilution.
Discussion
Previous meta-analyses have shown that aspirin is of substantial net benefit in secondary prevention,1,2 but the balance of beneficial effects and bleeding hazards in primary prevention was less clear. The availability of individual participant data for the present meta-analysis has allowed more reliable comparison of the benefits and hazards of aspirin in apparently healthy people. All four of the proportional reductions in major coronary events and in ischaemic stroke in the primary and in the secondary prevention trials were similar to each other (figure 3). Vascular mortality was not significantly reduced in the primary prevention trials, although a proportional reduction comparable with that in the secondary prevention trials could not be excluded. Whether or not the proportional benefits are similar, however, the absolute benefits of aspirin are an order of magnitude smaller in the primary than in the secondary prevention trials (table 2). (We have ignored the hypothesis16,17 of an eventual reduction in cancer mortality, since it would be expected to have little effect on our analyses of mortality during the scheduled treatment period.)
In the primary prevention trials, the proportional reduction in serious vascular events did not depend significantly on age or sex (and the suggestion, on the basis of the primary prevention trials,18 that the proportional reductions in particular vascular outcomes might differ between men and women was not supported by the secondary prevention trials; figure 3). Nor did it depend significantly on smoking history, blood pressure, total cholesterol, body-mass index, history of diabetes, or predicted risk of coronary heart disease. In particular, there was no significant trend in the proportional effects of aspirin in people at very low, low, moderate, and high estimated risk of coronary heart disease. If the proportional risk reductions in these different subgroups really are similar, then the absolute risk reductions will depend chiefly on an individual's absolute risk without treatment.
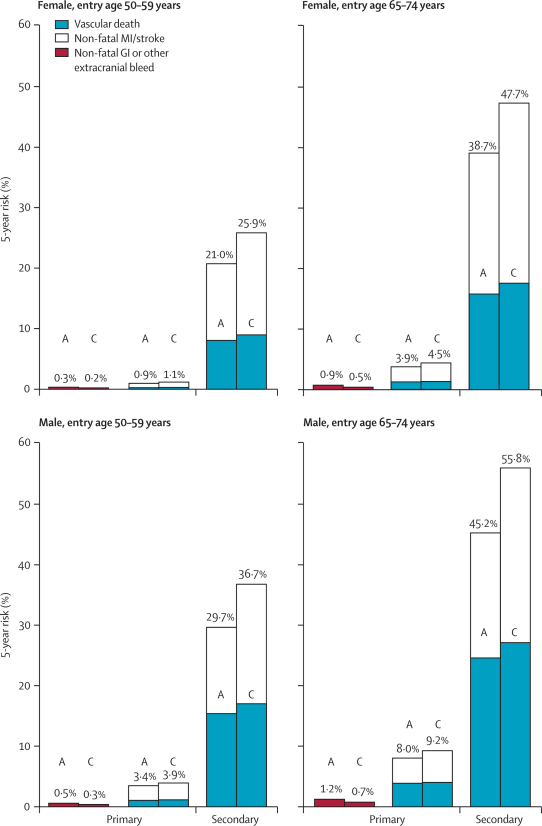
Figures 6 and 7 provide hypothetical calculations of what the absolute effects of aspirin allocation on 5-year outcome would be (in the absence of non-vascular causes of death) if the yearly event rates and the proportional effects of aspirin were as in the primary and as in the secondary prevention trials, and if these proportional effects were independent of age, sex, and other risk factors. Long-term low-dose aspirin had significant effects on both fatal and non-fatal events in people who already had occlusive vascular disease (table 2). Figure 6 suggests that, in this secondary prevention setting, aspirin would be of substantial net benefit (irrespective of age or sex), that it would reduce non-fatal vascular events by much more than it would increase major extracranial bleeds, and that—despite any adverse effects on cerebral haemorrhage—it would reduce overall vascular mortality (a result that is strongly reinforced by meta-analyses of all of the trials of any antiplatelet regimen in secondary prevention1,2). Nowadays, however, many patients with a history of occlusive stroke or myocardial infarction would have their risks of recurrence reduced substantially by statins, other modern drugs, and, when appropriate, vascular procedures. For occlusive vascular events, the relative risks produced by these other interventions and by aspirin might well be approximately multiplicative. If so, and if the other interventions approximately halve the recurrence risk, then the absolute benefit of adding aspirin to these other methods might be only about half as great as that of giving aspirin alone. Still, figure 6 suggests that, for secondary prevention, the net benefits of adding aspirin would substantially exceed the bleeding hazards, irrespective of age or sex.
Figure 6.
Predicted 5-year absolute effects of allocation to aspirin in different categories of age and sex in the primary and secondary prevention trials (ignoring non-vascular mortality)
Results are generally for otherwise untreated individuals; other risk reduction measures might approximately halve the vascular event rates in both aspirin (A) and control (C) groups. Three outcomes were analysed: non-fatal gastrointestinal (GI) (or other non-cerebral) bleeds in the primary prevention trials only; non-fatal vascular events in the primary trials and in the secondary trials; and vascular mortality (including any fatal bleeds) in the primary trials and in the secondary trials. For every outcome, the overall risk ratio (aspirin vs control in all participants, irrespective of age or sex) was combined with the absolute yearly risk among the controls in these four categories of sex and age. The risk ratios are those resulting from allocation to daily aspirin, so they underestimate the effects of actually taking aspirin for the whole 5-year period. MI=myocardial infarction.
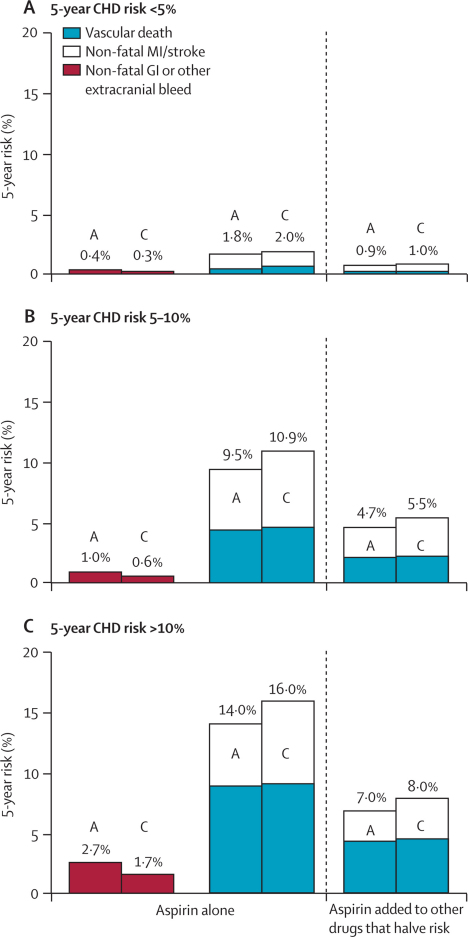
Figure 7.
Predicted 5-year absolute effects of allocation to aspirin in the primary prevention trials in different categories of 5-year risk (if untreated) of coronary heart disease (CHD) (ignoring non-vascular mortality)
Three outcomes were analysed in aspirin (A) and control (C) groups: non-fatal gastrointestinal (GI) (or other non-cerebral) bleeds when aspirin is given alone; non-fatal vascular events when aspirin is given alone and when aspirin is added to other drugs that halve risk; and vascular mortality (including any fatal bleeds) when aspirin is given alone and when aspirin is added to other drugs that halve risk. For every outcome, the overall risk ratio, irrespective of risk of coronary heart disease, was combined with the absolute yearly risk among the controls in three categories of predicted 5-year risk of a major coronary event (<5%, 5–10%, >10%). Absolute effects are estimated both directly from the data (middle column) and in the hypothetical situation in which risk is halved by statins and other primary prevention measures (right-hand column).
In the primary prevention trials, however, the absolute risk of a serious vascular event among people of a given age and sex was an order of magnitude less than in the secondary prevention trials. Figure 6 suggests that (irrespective of age or sex) the absolute reduction in occlusive events would be only about twice as large as the absolute increase in bleeding. Moreover, these trials of aspirin were mainly in people who were not taking statin therapy, which would have reduced both myocardial infarction and ischaemic stroke with little hazard.19,20 Generic statins are now widely available at low cost and, because of their efficacy and safety, primary prevention by a statin could well be preferred to primary prevention only by aspirin. If so, then one of the main questions for aspirin in primary prevention nowadays is whether it is worthwhile to add it to a statin (or to some statin-based combination of measures). If the risk of occlusive vascular disease is already approximately halved by statins or other measures, then the further absolute benefit of adding aspirin could well be only about half as large as was suggested by these primary prevention trials, but the main bleeding hazards could well remain. In that case, the benefits and hazards of adding long-term aspirin in people without pre-existing disease might be of approximately similar magnitude.
There is, of course, still the possibility that the primary prevention trials have, by chance, somewhat underestimated the main effects on mortality in the populations they studied (as is shown by the CIs in figure 5). There is also the possibility that some particular category of individuals will eventually be identified in which primary prevention with aspirin is of definite net benefit. One particularly important such category might be adults with diabetes but no known vascular disease, for whom aspirin is at present recommended.21 Although the evidence from the six primary prevention trials reviewed here is consistent with some net benefit in such patients (figure 2), the evidence from three other primary prevention trials in diabetes has been unpromising.22–24 Two much larger trials are, however, now recruiting only patients with diabetes.25,26 More generally only 9% of participants in the six primary prevention trials had predicted coronary heart disease incidence rates above 1% per year, so the present results among them are not, on their own, reliable (figure 2). Two major new trials in such moderately high-risk individuals are, however, now being undertaken,27,28 which will eventually yield more reliable evidence.
To maximise the excess of benefit over hazard in primary prevention, most current guidelines3–5 recommend that aspirin be given to those with risk of coronary heart disease exceeding a particular threshold. These guidelines implicitly assume, however, either that the absolute risk of bleeding remains approximately constant irrespective of risk of coronary heart disease,4,5 or that it depends solely on age,3 whereas the present analyses showed that other risk factors for this disease are also risk factors for bleeding (table 3). As a result, even for people at moderately increased risk of coronary heart disease, the major absolute benefits and hazards of adding aspirin to a statin-based primary prevention regimen could still be approximately evenly balanced, as is suggested by the calculations in figure 7.
A non-fatal stroke or heart attack is more likely to result in long-term disability than is a non-fatal gastrointestinal (or other extracranial) bleed, but in primary prevention the net absolute reduction in disabling or fatal occlusive events is likely to be small, and at least partially offset by a small increase in serious intracranial and extracranial bleeds. Thus, although it might cost little to add aspirin to any other drugs that are being used for the primary prevention of vascular disease, the additional effectiveness against fatal or disabling outcomes has not been reliably demonstrated for men or women of any age who do not yet have any relevant disease (and, if effectiveness is uncertain then detailed estimates of cost-effectiveness29 are of limited relevance). Moreover, drug safety (like vaccine safety) is of particular importance in public health recommendations for large, apparently disease-free populations; there should be good evidence that benefits exceed risks by an appropriate margin. Hence, although the currently available trial results could well help inform personally appropriate judgments by individuals about their own use of long-term aspirin, they do not seem to justify general guidelines advocating the routine use of aspirin in all apparently healthy individuals above a moderate level of risk of coronary heart disease.3–8
Acknowledgments
Acknowledgments
We thank the trial participants and investigators. Sources of funding of each individual trial are described in its publications. The CTSU is supported by a core grant from the UK MRC, the BHF, and Cancer Research UK, and has previously received funding from the European Community Biomed Programme. C Baigent is supported by the MRC, R Collins by a BHF Personal Chair, and J Emberson by a BHF Intermediate Research Fellowship. This paper is dedicated to the memory of Richard Doll (1912–2005), in collaboration with whom the first primary prevention trial10 was undertaken.
Contributors
All members of the writing committee contributed to the collection or analysis of the data, or both, to the interpretation of the results, and to the preparation of the report.
Primary prevention working group of the ATT Collaboration
British Doctors Study—Rory Collins, Richard Peto, Charles Hennekens, Richard Doll (deceased). US Physicians Study—Vadim Bubes, Julie Buring, Rimma Dushkesas, Michael Gaziano, Charles Hennekens. Thrombosis Prevention Trial—Patrick Brennan, Tom Meade, Alicja Rudnicka. Hypertension Optimal Treatment Study—Lennart Hansson (deceased), Ingrid Warnold (AstraZeneca), Alberto Zanchetti. Primary Prevention Project—Fausto Avanzini, Maria Carla Roncaglioni, Gianni Tognoni. Women's Health Study—Julie Buring, Marilyn Chown, Michael Gaziano, Charles Hennekens. Secretariat (CTSU)—Colin Baigent, Ian Barton, Alex Baxter, Neeraj Bhala, Lisa Blackwell, Jill Boreham, Louise Bowman, Georgina Buck, Rory Collins, Jonathan Emberson, Jon Godwin, Heather Halls, Lisa Holland, Patricia Kearney, Richard Peto, Christina Reith, Kate Wilson. Writing Committee—Colin Baigent, Lisa Blackwell, Rory Collins, Jonathan Emberson, Jon Godwin, Richard Peto (CTSU, Oxford University, Oxford, UK); Julie Buring (Brigham and Women's Hospital, Harvard University, Boston, MA, USA); Charles Hennekens (Charles E Schmidt College of Biomedical Science and Center of Excellence, Florida Atlantic University, Boca Raton, FL, USA); Patricia Kearney (Department of Medical Gerontology, Trinity College Dublin, Dublin, Ireland); Tom Meade (London School of Hygiene and Tropical Medicine, London University, London, UK); Carlo Patrono (Catholic University School of Medicine, Rome, Italy); Maria Carla Roncaglioni (Mario Negri Institute, Milan, Italy); and Alberto Zanchetti (Istituto Auxologico Italiano, University of Milan, Milan, Italy).
Conflicts of interest
The Clinical Trial Service Unit and Epidemiological Studies Unit (CTSU), where the ATT secretariat is located, has a policy of staff not accepting fees, honoraria, or paid consultancies. The CTSU continues, however, to be involved in clinical trials of cholesterol modification therapy and of antiplatelet therapy with funding from the Medical Research Council (MRC), British Heart Foundation (BHF), and/or various companies (Bayer, Merck, Merck Schering Plough, Solvay and, for the 1978 study of aspirin in British Doctors, the Aspirin Foundation) as research grants to (and administered by) Oxford University. J Buring received grant support in the form of pills and packaging, as well as one speaker's honorarium from Bayer. T Meade and C Patrono received consultancy or speaker fees, grant support, or both, from Bayer. C Hennekens receives investigator-initiated research grant support from Bayer and serves as an independent scientist on the Data and Safety Monitoring Board for the ARRIVE trial.
Web Extra Material
References
- 1.Antiplatelet Trialists' Collaboration Collaborative overview of randomised trials of antiplatelet therapy—I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ. 1994;308:81–106. [PMC free article] [PubMed] [Google Scholar]
- 2.Antithrombotic Trialists' Collaboration Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.US Preventive Services Task Force Aspirin for the prevention of cardiovascular disease: US Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2009;150:396–404. doi: 10.7326/0003-4819-150-6-200903170-00008. [DOI] [PubMed] [Google Scholar]
- 4.Pearson TA, Blair SN, Daniels SR. AHA Guidelines for primary prevention of cardiovascular disease and stroke: 2002 update. Circulation. 2002;106:388–391. doi: 10.1161/01.cir.0000020190.45892.75. [DOI] [PubMed] [Google Scholar]
- 5.JBS 2: Joint British Societies' guidelines on prevention of cardiovascular disease in clinical practice. Heart. 2005;91(suppl 5):v1–v52. doi: 10.1136/hrt.2005.079988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elwood P, Morgan G, Brown G, Pickering J. Aspirin for everyone older than 50? For. BMJ. 2005;330:1440–1441. doi: 10.1136/bmj.330.7505.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bulugahapitiya U, Siyambalapitiya S, Sithole J, Fernando DJ, Idris I. Age threshold for vascular prophylaxis by aspirin in patients without diabetes. Heart. 2008;94:1429–1432. doi: 10.1136/hrt.2008.150698. [DOI] [PubMed] [Google Scholar]
- 8.Wald NJ, Law MR. A strategy to reduce cardiovascular disease by more than 80% BMJ. 2003;326:1419. doi: 10.1136/bmj.326.7404.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The Medical Research Council's General Practice Research Framework Thrombosis prevention trial: randomised trial of low-intensity oral anticoagulation with warfarin and low-dose aspirin in the primary prevention of ischaemic heart disease in men at increased risk. Lancet. 1998;351:233–241. [PubMed] [Google Scholar]
- 10.Peto R, Gray R, Collins R. Randomised trial of prophylactic daily aspirin in British male doctors. BMJ. 1988;296:313–316. doi: 10.1136/bmj.296.6618.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steering Committee of the Physicians' Health Study Research Group Final report on the aspirin component of the ongoing Physicians' Health Study. N Engl J Med. 1989;321:129–135. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- 12.Hansson L, Zanchetti A, Carruthers SG. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. Lancet. 1998;351:1755–1762. doi: 10.1016/s0140-6736(98)04311-6. [DOI] [PubMed] [Google Scholar]
- 13.Collaborative Group of the Primary Prevention Project Low-dose aspirin and vitamin E in people at cardiovascular risk: a randomised trial in general practice. Lancet. 2001;357:89–95. doi: 10.1016/s0140-6736(00)03539-x. [DOI] [PubMed] [Google Scholar]
- 14.Ridker PM, Cook NR, Lee IM. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 15.Prospective Studies Collaboration Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flossmann E, Rothwell P, on behalf of the British Doctors Aspirin Trial and the UK-TIA Aspirin Trial Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet. 2007;369:1603–1613. doi: 10.1016/S0140-6736(07)60747-8. [DOI] [PubMed] [Google Scholar]
- 17.Cuzick J, Otto F, Baron JA. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol. 2009;10:501–507. doi: 10.1016/S1470-2045(09)70035-X. [DOI] [PubMed] [Google Scholar]
- 18.Berger JS, Roncaglioni MC, Avanzini F, Pangrazzi I, Tognoni G, Brown DL. Aspirin for the primary prevention of cardiovascular events in women and men. JAMA. 2006;295:306–313. doi: 10.1001/jama.295.3.306. [DOI] [PubMed] [Google Scholar]
- 19.Cholesterol Treatment Trialists' (CTT) Collaboration Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90 056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 20.Ridker PM, Danielson E, Fonseca FAH, for the JUPITER Study Group Rosuvastatin to prevent vascular events in men and women with raised C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 21.Buse JB, Ginsberg HN, Bakris GL. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care. 2007;30:162–172. doi: 10.2337/dc07-9917. [DOI] [PubMed] [Google Scholar]
- 22.ETDRS Investigators Aspirin effects on mortality and morbidity in patients with diabetes mellitus. Early treatment diabetic retinopathy study report 14. JAMA. 1992;268:1292–1300. doi: 10.1001/jama.1992.03490100090033. [DOI] [PubMed] [Google Scholar]
- 23.Belch J, MacCuish A, Campbell I, Prevention of Progression of Arterial Disease and Diabetes Study Group. Diabetes Registry Group. Royal College of Physicians Edinburgh The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ. 2008;337:a1840. doi: 10.1136/bmj.a1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogawa H, Nakayama M, Morimoto T. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial. JAMA. 2008;300:2134–2141. doi: 10.1001/jama.2008.623. [DOI] [PubMed] [Google Scholar]
- 25.A Study of Cardiovascular Events in Diabetes (ASCEND) Study. 2008. http://www.ctsu.ox.ac.uk/ascend/ (accessed April 23, 2009).
- 26.De Berardis G, Sacco M, Evangelista V. Aspirin and Simvastatin Combination for Cardiovascular Events Prevention Trials in Diabetes (ACCEPT-D): design of a randomized study of the efficacy of low-dose aspirin in the prevention of cardiovascular events in subjects with diabetes mellitus treated with statins. Trials. 2007;8:21. doi: 10.1186/1745-6215-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aspirin to Reduce Risk of Initial Vascular Events (ARRIVE) Study. 2008. http://www.arrive-study.com/EN/study.cfm (accessed April 23, 2009).
- 28.Nelson MR, Reid CM, Beilin LJ. Rationale for a trial of low-dose aspirin for the primary prevention of major adverse cardiovascular events and vascular dementia in the elderly. Aspirin in Reducing Events in the Elderly (ASPREE) Drugs Aging. 2003;20:897–903. doi: 10.2165/00002512-200320120-00004. [DOI] [PubMed] [Google Scholar]
- 29.Greving JP, Buskens E, Koffijberg H, Algra A. Cost-effectiveness of aspirin in the primary prevention of cardiovascular disease in subgroups based on age, gender, and varying cardiovascular risk. Circulation. 2008;117:2875–2883. doi: 10.1161/CIRCULATIONAHA.107.735340. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.