Abstract
Objective
The aim of this study was to investigate the prevalence of clinical and laboratory metabolic abnormalities during long-term risperidone treatment in children and adolescents.
Methods
Medically healthy 7- to 17-year-old children chronically treated, in a naturalistic setting, with risperidone were recruited through child psychiatry clinics. Anthropometric measurements and laboratory testing were conducted. Developmental and medication histories were obtained from medical records.
Results
In 99 patients treated with risperidone for an average of 2.9 years, a significant increase in age- and gender-adjusted weight and body mass index (BMI) (i.e., z-scores) was observed. Concomitant treatment with psychostimulants did not attenuate this weight gain. Risperidone-associated weight gain was negatively correlated with the BMI z-score obtained at the onset of risperidone treatment. Compared to lean children, overweight and obese children had higher odds of metabolic abnormalities, including increased waist circumference, hypertriglyceridemia, and low high-density lipoprotein cholesterol (HDL-C). They also tended to have a higher insulin level and homeostasis model assessment insulin resistance (HOMA-IR) index. As a result, upon recruitment in the study, children with excessive weight were 12 times more likely to have at least one laboratory metabolic abnormality and seven times more likely to have at least one criterion of the metabolic syndrome compared to lean subjects. In contrast to excessive weight status, gaining ≥0.5 BMI z-score point during risperidone treatment was not associated with a significantly higher occurrence of metabolic disturbances.
Conclusions
The long-term use of risperidone, especially when weight is above normal, is associated with a number of metabolic abnormalities but a low prevalence of the metabolic syndrome phenotype. Future studies should evaluate the stability of these abnormalities over time.
Introduction
With the dramatic increase in the use of atypical antipsychotics (AAPs) in youths over the last decade (Olfson et al. 2006), concerns have been raised regarding their safety (American Diabetes Association 2004; Correll and Carlson 2006; Newcomer and Haupt 2006). Most worrisome among the long-term adverse events associated with AAPs is their potential to cause significant weight gain, dyslipidemia, insulin resistance, and hyperglycemia (American Diabetes Association 2004; Correll and Carlson 2006; Newcomer and Haupt 2006). The impact of these metabolic abnormalities on morbidity and mortality is well established (Daniels et al. 2005).
Weight gain was common in short-term and extension pediatric clinical trials of various AAPs (Aman et al. 2002; Sikich et al. 2004; Croonenberghs et al. 2005). For example, over 6 weeks of treatment, the weight of children receiving risperidone increased by around 7% compared with baseline (Aman et al. 2002; Sikich et al. 2004; Croonenberghs et al. 2005). Although weight gain eventually plateaus, during extended treatment, patients continue to maintain an elevated weight compared to their same-age peers (Croonenberghs et al. 2005; Reyes et al. 2006).
AAPs have been reported to increase triglycerides by up to 45% in adults (Meyer and Koro 2004; Newcomer and Haupt 2006). Likewise, total cholesterol, low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) are negatively affected by antipsychotic treatment, albeit to a lesser extent (Meyer and Koro 2004). However, although much research has been conducted in adults, to our knowledge little is known about the nature and prevalence of the lipid abnormalities that follow prolonged antipsychotic treatment in youths (Martin and L'Ecuyer 2002; McGlashan et al. 2006; Laita et al. 2007). Similarly, data regarding the development of insulin resistance, glucose intolerance, and diabetes in children treated with AAPs remain scant (Croonenberghs et al. 2005; McGlashan et al. 2006; Laita et al. 2007).
In a longitudinal study of youths prescribed risperidone, we set out to evaluate the prevalence of metabolic disturbances during long-term treatment. We hypothesized that risperidone will be associated with significant weight gain and that excessive weight will increase the risk of metabolic abnormalities.
Methods
Subjects
Children and adolescents 7–17 years old and receiving risperidone for a minimum of 6 months were enrolled, irrespective of their primary psychiatric diagnosis or indication for risperidone treatment. Subjects either responded to advertisements in outpatient psychiatric clinics or to letters inviting them to participate. Patients with mental retardation, traumatic brain injury, or other neurological disorders were excluded. Participants could have been receiving, concomitantly, other psychotropics but not other antipsychotics. Pregnant females and those receiving hormonal contraception were not eligible. Finally, medical conditions that could confound the metabolic assessments, such as hypertension, diabetes mellitus, hypercholesterolemia, corticosteroid-dependent diseases (e.g., severe asthma and autoimmune disorders), and significant hormonal abnormalities (e.g., growth hormone deficiency) also led to exclusion. Subjects with minor, untreated thyroid-stimulating hormone (TSH) elevation were enrolled due to the unlikely clinical significance of their abnormality (Surks et al. 2004) (TSH <7.0 μIU/mL; normal range, 0.27–4.20).
Procedures
This study was approved by the local Institutional Review Board. Assent was obtained from children ≤11 years old and consent was obtained from adolescents and parents or guardians. Subjects were compensated for their participation.
The clinical diagnoses were based on chart review. We extracted anthropometric measurements from the medical records and recorded the start and stop dates of each medication as well as changes in the dosage and formulation. This documentation, confirmed by a physician, also reflected any potential deviation from the prescribed treatments. All dosages of psychostimulants were expressed in methylphenidate (MPH) equivalents for amphetamines (× 2) (Swanson et al. 2007). On the basis of the comprehensive psychiatric treatment history of each patient, we summed the duration of exposure to various psychotropics (excluding risperidone and psychostimulants), divided into those with a definite potential to induce weight gain or weight loss (following Vanina et al. 2002 and the extant literature). For example, atomoxetine was considered a weight-reducing agent and olanzapine a weight-inducing one. Benzodiazepines, α-2 agonists, selective serotonin reuptake inhibitors, trazodone, venlafaxine, nortriptyline, carbamazepine, oxcarbazepine, gabapentin, lamotrigine, tiagabine, and anticholinergics were considered weight neutral.
Upon enrollment, vital signs were obtained in the sitting position, after 15 minutes of rest. Height was measured to the nearest 0.1 cm using a stadiometer (Holtain Ltd., UK) while subjects were standing erect, and weight was recorded to the nearest 0.1 kg using a digital scale (Scaletronix, Wheaton, IL) while subjects were wearing indoor clothes without shoes. Triceps and subscapular skinfold thickness was measured with a Lange skinfold caliper to the nearest 0.1 mm (Centers for Disease Control 2000). Waist circumference was measured, to the nearest 0.1 cm, with the measuring tape placed at the uppermost lateral border of the right iliac crest (Centers for Disease Control 2000). The intraclass correlation coefficient between the two dietitians who collected the waist circumference and triceps and subscapular skinfold thickness was >0.95 for each of the three variables (n = 16). The average of two measurements was used.
Pubertal stage was evaluated by a physician. Independently, the subjects, with parental help when necessary, completed a self-assessment form that included age-appropriate instructions and pictures depicting Tanner stages I through V (Marshall and Tanner 1969; Marshall and Tanner 1970). Interrater agreement between the physician and self-rating was high (weighted kappa = 0.81, 95% confidence interval [CI] = 0.74–0.88, n = 74). Self-rating was used for patients (n = 18) who declined to undergo the physical exam.
During the visit, the parent was asked to compare the child's usual level of physical activity to peers using a 5-point Likert scale, and the child and the parent were asked to estimate screen time (i.e., the daily time spent watching television or playing video games).
A morning blood sample was obtained after at least a 9-hour overnight fast and before risperidone was administered, to measure TSH, glucose, total insulin, total cholesterol, HDL-C, triglycerides, risperidone, and 9-hydroxyrisperidone concentrations. In 11% of the sample, the participants were not fasting and their laboratory data were excluded from the analyses. Except in 1 patient, where it was measured directly, LDL-C was calculated following the Friedewald estimation formula (Friedewald et al. 1972).
Data analysis
Weight, height, and body mass index (BMI; kg/m2) measurements were converted into z-scores using the 2000 Center for Disease Control growth norms (Ogden et al. 2002). Blood pressure and waist circumference measurements were converted into percentile ranks using published normative data (National Blood Pressure Working Group of High Blood Pressure in Children and Adolescents 2004; McDowell et al. 2005).
To test whether risperidone contributed to weight gain, we used a linear mixed model with BMI z-score as the dependent variable and baseline BMI z-score (obtained within 1 month before the initiation of risperidone), time, risperidone treatment status (i.e., whether the measurement was collected before or after the onset of risperidone treatment), MPH treatment, and the interactions between them as the independent variables. This model includes two random effects: A random intercept term and a random time by treatment status interaction to account for possible baseline differences across subjects and the fact that measurements were not conducted at preset intervals, this being a naturalistic study. Between 2.0 and 5.5 years of age, BMI declines before it “rebounds” (Ogden et al. 2002). Thus, to minimize the confounding effect of this developmental phenomenon, BMI measurements recorded before age 5.5 were excluded. Because we anticipated a quadratic effect of the duration of risperidone treatment on weight gain and to accurately model the growth curve of each participant, we required that a minimum of three BMI measurements before or three after the onset of risperidone treatment be available for a participant to contribute to this analysis. Also, participants previously treated with other antipsychotics were excluded. Finally, to estimate the effect of co-treatment with stimulants, we computed for each child the average daily dose of MPH equivalent per kilogram of body weight over the course of his/her treatment. Patients were then divided into three groups, which were entered as an independent variable in the mixed model: (1) Those who received, on average, less than 0.2 mg/kg per day of MPH equivalent (No-MPH group, n = 12, with 7 having never received psychostimulants); (2) those who received between 0.2 and 1.2 mg/kg per day (Low-MPH group, n = 26); and (3) those who received ≥1.2 mg/kg per day (High-MPH group, n = 23).
Body fat was estimated using skinfold thickness measurements following Slaughter et al. (Slaughter et al. 1988). The homeostasis model assessment insulin resistance index (HOMA-IR) was estimated by the formula (insulin μUI/ml ×glucose [mg/dL])/405 (Matthews et al. 1985).
To evaluate the rate of metabolic abnormalities in relation to weight, we divided the sample based on whether the BMI percentile was elevated (i.e., corresponding to overweight [85 ≤ BMI percentile <95] and obese [BMI percentile ≥95]) or not. Differences across the two groups were compared using a Student t-test for continuous variables and Fisher exact test for categorical ones. We used the Wilcoxon rank sum test whenever the t-test assumption of normality was violated. The Satterthwaite approximation was used to calculate the t statistic when the two groups had different variances. Finally, we computed the odds ratios (OR) and the 95% CI to evaluate the association between the presence of one or more laboratory metabolic abnormalities or metabolic syndrome criteria and the two patient groups (Stokes et al. 2000; Agresti 2002).
All of the statistical tests performed were two tailed. Given the number of comparisons, we divided the outcomes of interest into four categories of eight related outcomes each (i.e., clinical diagnoses, pharmacotherapy, metabolic measures, and metabolic abnormalities) (Pocock et al. 1987). We then used a Bonferroni adjustment and set the statistical significance at α = 0.05/8 = 0.006. We performed all of the analyses using SAS version 9.1.3 for Windows (SAS Institute Inc., Cary, NC).
Results
Sample characteristics
Of 101 recruited participants (88% males), 2 were excluded due to undetectable risperidone blood levels, reflecting medication nonadherence. One other child, who refused the blood draw, was omitted from those analyses involving the laboratory measures. Nineteen percent of the sample was overweight and 15% was obese (referred to as overweight/obese, henceforth).
Table 1 summarizes the demographic and clinical data of the participants divided in two groups based on the presence of excessive weight (i.e., overweight/obese versus lean group). No demographic differences were found across the two groups. Risperidone was used to target irritability and aggression in 68% of the overweight/obese group and in 80% of the lean group, a nonsignificant difference. In addition to risperidone, psychostimulants, α2-agonists, and selective serotonin reuptake inhibitors were the most commonly prescribed medications. Compared to the lean group, overweight/obese subjects took a 36% lower dose per kilogram of body weight of psychostimulants. Other psychotropics included: nortriptyline, venlafaxine, buspirone, topiramate, lamotrigine, carbamazepine, and oxcarbazepine used in 1 patient each. Divalproex, lithium, and bupropion were prescribed to 2 participants each and trazodone to 5 participants. There were no significant differences across the two groups in the use of these medications. In addition, when all the potentially weight-altering agents (Vanina et al. 2002) were combined (excluding risperidone and psychostimulants), the median exposure was ≤2.3% of the total period of observation and not different between the two groups.
Table 1.
Demographic and Psychiatric Characteristics Of Lean and Overweight/Obese Subjects
| Normal BMI percentile (n = 65) | Overweight/obese (n = 34) | Statistical analysis | p Value | |
|---|---|---|---|---|
| Age, median (quartiles), years | 11.9 | 11.3 | Wilcoxon | p = 0.2 |
| (9.4–14.4) | (9.1–13.4) | S = 1535 | ||
| Males, % (n) | 86 (56) | 91 (31) | Fisher exact | p = 0.5 |
| Pubertal status, % at Tanner stage I, II, III, IV, V | 41/19/5/23/11 | 35/26/12/18/9 | Wilcoxon S = 1695 | p = 0.9 |
| Clinical diagnoses | ||||
| Attention-deficit/hyperactivity disorder % (n) | 91 (59) | 82 (28) | Fisher exact | p = 0.3 |
| Disruptive behavior disorder, % (n) | 65 (42) | 65 (22) | Fisher exact | p = 1.0 |
| Pervasive developmental disorder, % (n) | 12 (8) | 24 (8) | Fisher exact | p = 0.2 |
| Depressive disorder, % (n) | 25 (16) | 9 (3) | Fisher exact | p = 0.07 |
| Bipolar disorder, % (n) | 2 (1) | 6 (2) | Fisher exact | p = 0.3 |
| Anxiety disorder, % (n) | 29 (19) | 53 (18) | Fisher exact | p = 0.03 |
| Psychotic disorder, % (n) | 3 (2) | 0 | Fisher exact | p = 0.5 |
| Tic disorder, % (n) | 18 (12) | 24 (8) | Fisher exact | p = 0.6 |
| Total diagnoses, mean ± SD | 2.6 ± 0.9 | 2.8 ± 1.0 | Fisher exact | p = 0.4 |
| Pharmacotherapy | ||||
| Risperidone dose, median (quartiles), (mg/kg/per day) | 0.03 (0.02–0.04) | 0.02 (0.02–0.04) | Wilcoxon S = 1618 | p = 0.5 |
| Risperidone treatment duration, median (quartiles), years | 2.9 (1.7–3.8) | 2.0 (0.7–3.9) | Wilcoxon S = 1545 | p = 0.3 |
| Psychostimulants, % (n) | 77 (50) 1.4 ± 0.5 | 50 (17) 0.9 ± 0.3 | Fisher exact | p = 0.01 |
| mean ± SD, (mg/kg/per day) | t = 4.8, df = 50.9 | p < 0.0001 | ||
| Psychostimulants treatment duration, median (quartiles), years | 4.0 (2.3–6.1) | 2.2 (1.7–5.7) | Wilcoxon S = 515 | p = 0.4 |
| α-2 Agonists, % (n) | 25 (16) | 47 (16) | Fisher exact | p = 0.02 |
| α-2 Agonists treatment duration, median (quartiles), years | 2.3 (1.4–3.3) | 2.4 (1.7–5.1) | Wilcoxon S = 532 | p = 0.3 |
| Selective serotonin reuptake inhibitors (SSRI), % (n) | 52 (34) | 53 (18) | Fisher exact | p = 1.0 |
| SSRI treatment duration, median (quartiles), years | 2.1 (1.5–3.1) | 2.7 (2.0–3.8) | Wilcoxon S = 240 | p = 0.4 |
| Prior antipsychotic treatment, % (n) | 17 (11) | 21 (7) | Fisher exact | p = 0.8 |
| Antipsychotic treatment duration, median (quartiles), years | 1.2 (0.9–2.7) | 0.5 (0.1–1.4) | Wilcoxon S = 50 | p = 0.1 |
| Activity level, median (quartiles) | 2.0 (1.0–3.0) | 3.0 (2.0–3.0) | Wilcoxon S = 1354 | p = 0.04 |
| Screen time, median (quartiles), hours/day | 2.5 (1.5–4.0) | 2.8 (1.0–4.5) | Wilcoxon S = 1719 | p = 0.9 |
Statistically significant results after Bonferroni correction are in bold (i.e., ≤0.006) and trends are in bolded italics.
Abbreviations: SD = standard deviation; df = degrees of freedom.
Risperidone treatment and weight gain
As expected based on group allocation, the weight and BMI z-scores were higher in the overweight/obese subjects who also had a higher percent body fat (Table 2). Their weight and BMI z-scores at baseline (i.e., obtained in the month prior to risperidone treatment onset) were also higher. However, we found a negative correlation between the baseline BMI z-score and gain in BMI z-score after risperidone was started (Pearson r = −0.54, n = 63, p < 0.0001). In other terms, the lower a child's BMI z-score was at the onset of risperidone treatment, the more weight he/she gained. This last analysis was restricted to patients not treated with other antipsychotics to avoid the confounding effect of prior treatment on weight gain.
Table 2.
Clinical and Laboratory Measurements in Lean and Overweight/Obese Subjects
| Metabolic measures | Normal BMI percentile (n = 65) | Overweight/obese (n = 34) | Statistical analysis | p Value |
|---|---|---|---|---|
| Baseline weight z-score, mean ± SD | −0.3 ± 0.8 [n = 47]a | 0.9 ± 1.0 [n = 24] | t = −5.3, df = 69 | p < 0.0001 |
| Baseline height z-score, mean ± SD | −0.2 ± 0.8 [n = 45] | 0.4 ± 1.0 [n = 23] | t = −2.8, df = 66 | p = 0.006 |
| Baseline BMI z-score, mean ± SD | −0.4 ± 0.9 [n = 45] | 0.9 ± 1.0 [n = 23] | t = −5.2, df = 66 | p < 0.0001 |
| Baseline BMI z-score ≥85 | 7 (3) [n = 45] | 48 (11) [n = 23] | Fisher exact | p = 0.0007 |
| Percentile, % (n) | ||||
| Weight z-score, mean ± SD | 0.0 ± 0.7 | 1.6 ± 0.6 | t = −10.6, df = 97 | p < 0.0001 |
| Height z-score, mean ± SD | 0.0 ± 0.9 | 0.5 ± 0.9 | t = −2.8, df = 97 | p = 0.0005 |
| BMI z-score, mean ± SD | 0.1 ± 0.7 | 1.6 ± 0.4 | t = −13.3, df = 96 | p < 0.0001 |
| Percent body fat, median (quartiles) | 16.5 (12.2–21.3) [n = 62] | 30.3 (25.3–38.2) [n = 32] | Wilcoxon S = 2340 | p < 0.0001 |
| Glucose, median (quartiles), mg/dL | 91.0 (84.0–95.0) [n = 57] | 91.0 (88.0–97.5) [n = 28] | Wilcoxon S = 1310 | p = 0.3 |
| Insulin, median (quartiles), μIU/Ml | 4.9 (3.4–6.6) [n = 57] | 6.6 (4.2–9.9) [n = 27] | Wilcoxon S = 1397 | p < 0.02 |
| HOMA-IR, median (quartiles) | 1.1 (0.7–1.4) [n = 57] | 1.4 (0.9–2.6) [n = 27] | Wilcoxon S = 1390 | p = 0.02 |
| Total cholesterol, mean ± SD, mg/dL | 159.0 ± 23.7 [n = 57] | 160.2 ± 27.9 [n = 28] | t = −0.2, df = 83 | p = 0.9 |
| LDL, mean ± SD, mg/dL | 87.0 ± 21.7 [n = 57] | 88.5 ± 24.5 [n = 28] | t = −0.3, df = 83 | p = 0.8 |
| HDL-C, mean ± SD, mg/dL | 61.3 ± 11.7 [n = 57] | 53.9 ± 14.2 [n = 28] | t = 2.6, df = 83 | p = 0.01 |
| Triglycerides, mean ± SD, mg/mLb | 57.0 ± 23.2 [n = 57] | 76.1 ± 46.0 [n = 27] | t = −2.0, df = 32.4 | p = 0.05 |
| Elevated TSH, % (n) | 16 (10) | 12 (4) | Fisher exact | p = 0.8 |
Only laboratory tests for patients who were fasting for at least 9 hours are reported. Statistically significant results after Bonferroni correction are in bold (i.e., ≤0.006) and trends are in bolded italics.
Between brackets is the number of subjects for whom data were available, when different from the total number in the subgroup.
One patient with triglycerides levels >600mg/dL was excluded from this analysis.
Abbreviations: SD = standard deviation; df = degrees of freedom; BMI = body mass index; HOMA-IR = homeostasis model assessment of insulin resistance; LDL-C = low-density lipoprotein cholesterol; HDL-C = high-density lipoprotein; TSH = Thyroid-stimulating hormone.
Psychostimulants and risperidone-associated weight gain
We then investigated the effect of concomitant psychostimulant treatment on weight gain associated with risperidone. Following the restrictions described in the data analysis section, 61 participants were included in the analysis comparing the change in BMI z-score over time, as a function of risperidone treatment, across the three psychostimulant groups. After controlling for the effect of baseline BMI z-score (F[1, 46.1] = 269.7, p < 0.0001), we found no main effect for the psychostimulant dose groups but only a significant interaction with quadratic time (F[2, 622] = 5.5, p = 0.004). As depicted in Fig. 1, this latter interaction reflects the fact that, over the observation period, the BMI z-score changed at a different rate for the high-MPH group than for the other two groups. However, at end point, the BMI z-score was similar regardless of concomitant psychostimulant treatment status (F[2, 59] = 0.92, p = 0.4).
FIG. 1.
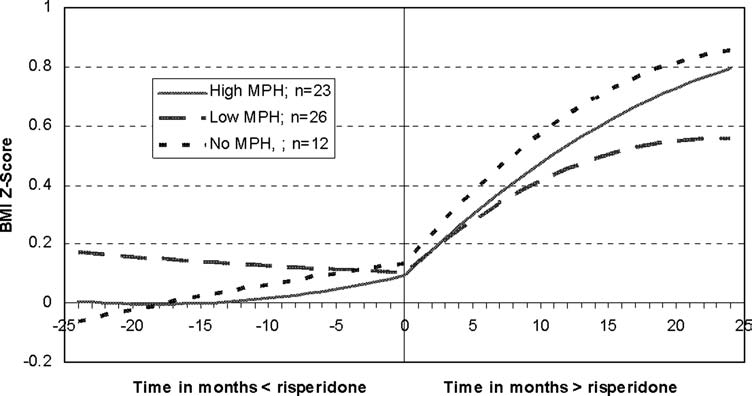
Estimated BMI z-scores over time for three levels of MPH doses. Subjects who had a baseline BMI within 1 month before the onset of risperidone treatment, at least 3 BMI observations before or 3 after risperidone initiation, and who had never been treated with antipsychotic medications, other than risperidone, were included in this analysis (n = 61). They were divided into three subgroups based on their average daily dose per kilogram of body weight of psychostimulants. While there was no change to a minor change in BMI z-score prior to risperidone initiation, all three groups equally gained weight after treatment outset. BMI, Body mass index; MPH, methylphenidate equivalent.
After including patients who had had prior treatment with antipsychotics other than risperidone and controlling for that in the model, this analysis was repeated with similar findings. In addition, as expected, prior antipsychotic treatment significantly altered the BMI z-score curves (F[1, 944] = 11.6, p = 0.0007).
Metabolic abnormalities
Compared to the lean group, overweight/obese participants had a 14% lower mean HDL concentration (Table 2). In addition, there was a trend for their median insulin level and HOMA-IR index to be higher by 35% and 27%, respectively.
We then investigated the prevalence of the metabolic syndrome criteria, as defined by Cook et al. (Cook et al. 2003), in fasting participants across the two groups. Following the new guidelines of the American Diabetes Association (2004), we used a cutoff of 100 mg/dL for fasting blood glucose. As shown in Table 3, compared to lean subjects, the overweight/obese participants were more likely to have a waist circumference ≥90th percentile, a triglyceride concentration ≥110 mg/dL, and an HDL-C concentration ≤40 mg/dL. There was also a trend for overweight/obese participants to have impaired fasting blood glucose, elevated insulin concentration, and insulin resistance more frequently (Lee et al. 2006). There was no difference across the groups in the rate of hypertension. As a result, the odds of having one or more laboratory metabolic abnormalities was nearly 12 times for the overweight/obese group that of the lean one (OR = 11.5, 95% CI = 3.3–40.5, p < 0.0001) and the odds of having at least one metabolic syndrome criteria was seven times (OR = 6.8; 95% CI, 2.5–18.4, p = 0.0002). The prevalence of the metabolic syndrome (i.e., having ≥3 abnormalities), on the other hand, was low in both groups, although the only case identified was in the overweight/obese group. When the analysis was repeated after excluding patients who had previously received other antipsychotics, prior to the initiation of risperidone, we found a similar overall pattern of results.
Table 3.
Prevalence of Metabolic Abnormalities Across Lean and Overweight/Obese Subjects
| Metabolic abnormality | Normal BMI percentile (n = 65) | Overweight/obese (n = 34) | Statistical analysis | p Value |
|---|---|---|---|---|
| Waist circumference ≥90th percentile, % (n) | 0 [n = 63]a | 25 (8) [n = 32] | Fisher exact | p < 0.0001 |
| SBP ≥90th percentile, % (n) | 16 (10) [n = 64] | 12 (4) | Fisher exact | p = 0.8 |
| DBP ≥90th percentile, % (n) | 3 (2) [n = 64] | 3 (1) | Fisher exact | p = 1.0 |
| Glucose ≥100 mg/dL, % (n)b | 7 (4) [n = 57] | 21 (6) [n = 28] | Fisher exact | p = 0.07 |
| Insulin >20 μIU/mL, % (n) | 0 [n = 57] | 11 (3) [n = 27] | Fisher exact | p = 0.03 |
| HOMA-IR >4.39, % (n)c | 0 [n = 27] | 18 (2) [n = 11] | Fisher exact | p = 0.08 |
| Triglycerides ≥110 mg/dL, % (n) | 0 [n = 57] | 18 (5) [n = 28] | Fisher exact | p = 0.003 |
| HDL-C ≤40 mg/dL, % (n) | 0 [n = 57] | 21 (6) [n = 28] | Fisher exact | p = 0.0008 |
| ≥1 Laboratory metabolic abnormalities, % (n)d | 7 (4) [n = 57] | 46 (13) [n = 28] | Fisher exact | p < 0.0001 |
| ≥1 Metabolic syndrome criteria, % (n)e | 21 (12) [n = 57] | 64 (18) [n = 28] | Fisher exact | p = 0.0002 |
| Metabolic syndrome, % (n) | 0 [n = 57] | 4 (1) [n = 28] | Fisher exact | p = 0.3 |
Statistically significant results after Bonferroni correction are in bold (i.e., ≤0.006) and trends are in bolded italics.
Between brackets is the number of subjects for whom data were available, when different from the total number in the subgroup. Only laboratory data for patients who were fasting for at least 9 hours are reported.
In no instances was fasting blood glucose >126 mg/dL.
HOMA-IR was defined categorically (i.e., >4.39) in adolescent subjects (age ≥12) only (Lee et al. 2006).
This variable includes abnormal glucose, total insulin, triglycerides, or HDL-C as defined in Table 1. HOMA-IR was not included because the 2 adolescents with elevated values also had total insulin concentration >20 μIU/mL.
This variable includes abnormal waist circumference, SBP and DBP, glucose, triglycerides, or HDL-C as defined in Table 1.
Abbreviations: SBP = Systolic blood pressure; DBP = diastolic blood pressure; HOMA-IR = homeostasis model assessment of insulin resistance; HDL-C = high-density lipoprotein cholesterol.
Correll and Carlson have proposed that an increase in BMI z-score of ≥0.5 point in children treated with AAPs should be considered clinically significant (Correll and Carlson 2006). Thus, we evaluated the prevalence of the metabolic syndrome criteria in those patients who gained more than 0.5 BMI z-score point (n = 31) and those who did not (n = 26). Here, we restricted the analysis to those subjects with a BMI measurement obtained within 30 days before starting risperidone and who had not received other antipsychotics because this could mask the effect of risperidone on weight gain. The findings across those who gained ≥0.5 BMI z-score and those who did not, none of which reached statistical significance, were as follows: 10% (n = 3) versus 8% (n = 2) for waist circumference ≥90th percentile, 9% (n = 3) versus 22% (n = 6) for systolic blood pressure ≥90th percentile, 3% (n = 1) versus 0% for systolic blood pressure ≥90th percentile, 14% (n = 4) versus 5% (n = 1) for impaired fasting glucose, 10% (n = 3) versus 0% for triglycerides ≥110 mg/dL, and 14% (n = 4) versus 5% (n = 1) for HDL-C ≤40 mg/dL.
Discussion
To our knowledge, this is the largest study to investigate the prevalence of metabolic abnormalities in children and adolescents in extended risperidone treatment. Over an average 2.9 years of treatment, we found that the participants gained a mean 0.6 BMI z-score point and 35% had at least one criterion of the metabolic syndrome phenotype (Cook et al. 2003).
Before discussing the results, we will review the study limitations. First, this study is observational; thus, unlike controlled studies, it cannot establish causality. In fact, because adverse events often result in attrition (Aman et al. 2005; Croonenberghs et al. 2005), our cross-sectional design likely underestimates the actual rate and severity of weight gain and metabolic abnormalities during risperidone treatment. Conversely, it is also possible that our recruitment method led to self-selection bias, exaggerating the metabolic risks. In addition, although risperidone was the first AAP to have pediatric Food and Drug Adminstration (FDA) indications, other antipsychotics are widely prescribed in youths (Olfson et al. 2006). Because these drugs vary with regard to their receptor-binding profiles (Richelson and Souder 2000) and their potential to cause metabolic abnormalities in adults (American Diabetes Association 2004; Newcomer and Haupt 2006), our findings might not be generalizable to other AAPs. Third, most of the anthropometric data were collected from chart review and were not measured in a standardized manner. Nevertheless, measurement errors are equally likely to occur in either group, which reduces their potential impact on the findings. Fourth, we included patients with minor TSH elevation because that is unlikely to have clinical significance (Surks et al. 2004); in fact, excluding them did not alter the prevalence of the metabolic abnormalities across the two groups. Also, in our analysis, we controlled for the effect of risperidone and psychostimulants, but the patients had received a variety of other drugs, some of which could potentially affect weight. This effect might be considered negligible, however, because the overall exposure to weight-altering psychotropics, other than risperidone and psychostimulants, was short relative to the total duration of treatment (median ≤ 2.3%). Sixth, our sample was not evaluated using a standardized clinical assessment. Although weight gain associated with AAPs has been described in various clinical samples (Aman et al. 2002; Sikich et al. 2004), it is possible that psychopathology exerts a moderating effect on this adverse drug event, as we discuss below. Last, our findings could have been strengthened by the use of invasive metabolic studies directly evaluating insulin resistance or lipolysis. However, the clinical utility of such studies would have been limited because the current definitions of the metabolic syndrome in children do not involve such measurements (Zimmet et al. 2007; Ford and Li 2008).
Extended risperidone pediatric clinical trials have described a significant increase in weight beyond that expected for normal development (Aman et al. 2005; Croonenberghs et al. 2005; Reyes et al. 2006). In agreement with these reports, 54% of our participants gained at least 0.5 BMI z-score point, with 34% being either overweight or obese. These findings are particularly striking when one considers that, after adjusting for the effects of age and gender, the rate of excessive weight was only 22% prior to the initiation of risperidone and that, over the course of the treatment, the prevalence of obesity nearly doubled with that of overweight increasing by 35%.
Our longitudinal analysis also revealed that once risperidone is initiated, concomitant psychostimulant treatment may not protect against weight gain. Although this finding might be due to a type II error, similar results have been reported by Aman et al., who pooled data from two identically designed pediatric trials and found that, on average, the children gained 2.2 kg over the 6-week duration of the study, regardless of whether they were also taking psychostimulants (Aman et al. 2004). The appetite-suppressing effect of psychostimulants is dose-related and might contribute to the growth delay observed during extended treatment (Swanson et al. 2007). On the other hand, emerging evidence suggests a stimulating effect of AAPs on appetite (Gothelf et al. 2002). This likely occurs through the modulation of histaminergic H1 receptors, activating the phosphokinase messenger in the hypothalamus (Kim et al. 2007). Thus, not only does it appear that psychostimulants and antipsychotics modulate different pathways of appetite regulation (Woods et al. 1998), the data presented here and elsewhere (Aman et al. 2004) suggest that the weight-inducing effect of antipsychotics also overrides the weight-suppressing effect of stimulants. That physiological mechanisms promoting energy storage, through weight gain, are more potent than those preventing it should not be surprising because evolutionary pressures are geared toward avoiding starvation (Woods et al. 1998).
Although our findings suggest that psychostimulants do not attenuate the amount of weight gain associated with risperidone, it is nevertheless significant that, compared to the overweight/obese group, lean children received a higher dose of psychostimulants per kilogram of body weight, even though they were as likely to have attention-deficit/hyperactivity disorder (ADHD). Correcting psychostimulant dose for weight might not be appropriate for obese children (Spencer et al. 2003). In fact, this could underlie the differences in dosage across the two subgroups. Alternatively, it is possible that the leaner group had more severe ADHD symptoms, requiring higher psychostimulant doses. We did not administer any psychiatric rating scales; thus, it is not possible to determine to what extent the participants' symptoms were well controlled. We speculate that poorly controlled hyperactivity may have attenuated risperidone-induced weight gain due to the resultant increase in nonexercise activity thermogenesis (Levine et al. 1999). This refers to thermogenesis associated with physical activity other than volitional exercise, such as fidgeting (Levine et al. 1999). In fact, compared to overweight/obese participants, leaner children were rated as more active by their parents (Table 1). On the other hand, the two groups were not different with regard to the number of hours they watch television or play video games.
The excessive weight we found during risperidone treatment appears associated with metabolic disturbances. As a group, children and adolescents who were overweight/obese had higher percentage of body fat and lower HDL-C levels than the lean group. In addition, their insulin level and HOMA-IR index reflected a higher level of insulin resistance. When the metabolic disturbances were defined categorically, allowing a more direct clinical application, these participants were nearly seven times more likely to have at least one metabolic syndrome criterion. It has been suggested that when obesity develops in childhood, compensatory mechanisms might operate, resulting in “metabolically healthy” obesity (Karelis et al. 2004). Metabolically healthy obese individuals account for up to 20% of the obese population and appear more resistant to the development of the metabolic disturbances associated with excessive weight (Karelis et al. 2004). In our sample, most overweight/obese children and adolescents exhibited metabolic abnormalities, placing them at higher risk for future cardiovascular morbidity (Daniels et al. 2005). Longitudinal monitoring is required to determine whether adaptation to this risperidone-associated excessive weight develops over time, and our group is currently carrying out such monitoring. This issue is particularly significant because the precise definition of the metabolic syndrome in children and its long-term sequelae continue to be debated (Zimmet et al. 2007; Ford and Li 2008).
Despite finding a significant increase in weight, two relatively long-term studies identified no lipid abnormalities in youths and young adults treated with risperidone (Martin and L'Ecuyer 2002) or olanzapine (McGlashan et al. 2006). However, these reports involved small samples. In addition, one of them suffered from significant attrition during the year-long pharmacological intervention, whereas the second group of recruited youths was treated with risperidone for an average duration of only 4.9 months (Martin and L'Ecuyer 2002; McGlashan et al. 2006). On the other hand, a recent study comparing patients treated with various antipsychotic medications for less than 1 month with those treated for more than a year revealed significantly elevated weight and BMI in the chronically medicated group. These patients also had higher LDL and total cholesterol levels (Laita et al. 2007). Similarly, in hospitalized youths treated with a variety of antipsychotics for at least 1 month, Patel et al. found that 51% had one or more lipid abnormalities (Patel et al. 2007).
Insulin resistance could ensue secondary to weight gain, change in body fat composition, or could be due to a direct effect of the antipsychotics on insulin sensitivity (American Diabetes Association 2004; Amamoto et al. 2006). Data are again scant in children and adolescents. In the olanzapine trial cited earlier, no abnormalities in glucose metabolism were found (McGlashan et al. 2006), which is similar to the finding from the Spanish study comparing short- and long-term exposure to antipsychotics (Laita et al. 2007). The divergence between these studies and ours is likely due to differences in design, sample size, duration of risperidone treatment, prior exposure to other antipsychotics, and age of the patients. In addition, these studies did not measure insulin concentration, which increases years before hyperglycemia develops (Leahy 1990).
Clinical trials are establishing the efficacy of AAPs in an increasing number of pediatric psychiatric disorders. Thus, the use of these psychotropics will likely continue to rise. Although only some have been found to be associated with excessive weight gain in adults, preliminary evidence suggests that all psychotropics could cause this side effect in AAP-naïve children (Correll et al. 2005). In addition, as our data show, youths maintained in extended risperidone treatment frequently present with metabolic abnormalities, especially if they are obese (Correll et al. 2005). Thus, clinicians have a pivotal role to play in carefully evaluating the extant literature regarding efficacy and safety of AAPs when prescribing them to children and adolescents and in closely monitoring the treatment to minimize the morbidity and mortality associated with their adverse events, including weight gain, diabetes, and dyslipidemia (American Diabetes Association 2004; Correll and Carlson 2006).
Due to ethical and practical considerations, it will likely never be possible to conduct long-term, placebo-controlled trials to investigate the safety of AAPs in a pediatric population. Nevertheless, future research could compare different antipsychotics with regard to their potential to cause weight gain and metabolic disturbances.
Footnotes
The statistical consultant for this study was Laura Acion, M.S.
This study was funded by a 2005 Young Investigator Award to Chadi Calarge and by the National Institute of Health Through the Clinical Research Centers Mechanism (RR00059).
Disclosures
Drs. Calarge, Kuperman, Tansey, and Schlechte and Ms. Acion have no financial ties or conflicts of interest to report.
Acknowledgments
The authors thank the families and the staff in the University of Iowa Child and Adolescent Psychiatry clinic and Clinical Research Center. We also acknowledge the contributions of Dr. Jen McWilliams and the research team to data collection.
References
- Expert Commitee on the Diagnosis and Classification of Diabetes. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004;27(Suppl 1):S5–S10. doi: 10.2337/diacare.27.2007.s5. [DOI] [PubMed] [Google Scholar]
- National High Blood Pressure Working Group of High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- Agresti A. Hoboken (New Jersey): Wiley & Sons, Inc; 2002. Categorical Data Analysis. [Google Scholar]
- Amamoto T. Kumai T. Nakaya S. Matsumoto N. Tsuzuki Y. Kobayashi S. The elucidation of the mechanism of weight gain and glucose tolerance abnormalities induced by chlorpromazine. J Pharmacol Sci. 2006;102:213–219. doi: 10.1254/jphs.fp0060673. [DOI] [PubMed] [Google Scholar]
- Aman MG. De Smedt G. Derivan A. Lyons B. Findling RL. Double-blind, placebo-controlled study of risperidone for the treatment of disruptive behaviors in children with subaverage intelligence. Am J Psychiatry. 2002;159:1337–1346. doi: 10.1176/appi.ajp.159.8.1337. [DOI] [PubMed] [Google Scholar]
- Aman MG. Arnold LE. McDougle CJ. Vitiello B. Scahill L. Davies M. McCracken JT. Tierney E. Nash PL. Posey DJ. Chuang S. Martin A. Shah B. Gonzalez NM. Swiezy NB. Ritz L. Koenig K. McGough J. Ghuman JK. Lindsay RL. Acute and long-term safety and tolerability of risperidone in children with autism. J Child Adolesc Psychopharmacol. 2005;15:869–884. doi: 10.1089/cap.2005.15.869. [DOI] [PubMed] [Google Scholar]
- Aman MG. Binder C. Turgay A. Risperidone effects in the presence/absence of psychostimulant medicine in children with ADHD, other disruptive behavior disorders, and subaverage IQ. J Child Adolesc Psychopharmacol. 2004;14:243–54. doi: 10.1089/1044546041649020. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association (ADA) Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27:596–601. doi: 10.2337/diacare.27.2.596. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control. National Health and Nutrition Examination Survey: Anthropometry Procedures Manual (Revised December 2000) 2000.
- Cook S. Weitzman M. Auinger P. Nguyen M. Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: Findings from the third National Health and Nutrition Examination Survey, 1988–1994. Arch Pediatr Adolesc Med. 2003;157:821–827. doi: 10.1001/archpedi.157.8.821. [DOI] [PubMed] [Google Scholar]
- Correll CU. Carlson HE. Endocrine and metabolic adverse effects of psychotropic medications in children and adolescents. J Am Acad Child Adolesc Psychiatry. 2006;45:771–791. doi: 10.1097/01.chi.0000220851.94392.30. [DOI] [PubMed] [Google Scholar]
- Correll CU. Parikh UH. Mughal T. Olshanskiy V. Moroff M. Pleak RR. Foley C. Shah M. Gutkovich Z. Kane JM. Malhotra AK. Body composition changes associated with second-generation antipsychotic treatment of children, adolescents. Abstract from the Biological Psychiatry Conference; Atlanta, Georgia. May 19–21;2005 . [Google Scholar]
- Croonenberghs J. Fegert JM. Findling RL. De Smedt G. Van Dongen S. Risperidone in children with disruptive behavior disorders and subaverage intelligence: A 1-year, open-label study of 504 patients. J Am Acad Child Adolesc Psychiatry. 2005;44:64–72. doi: 10.1097/01.chi.0000145805.24274.09. [DOI] [PubMed] [Google Scholar]
- Daniels SR. Arnett DK. Eckel RH. Gidding SS. Hayman LL. Kumanyika S. Robinson TN. Scott BJ. St Jeor S. Williams CL. Overweight in children and adolescents: Pathophysiology, consequences, prevention, and treatment. Circulation. 2005;111:1999–2012. doi: 10.1161/01.CIR.0000161369.71722.10. [DOI] [PubMed] [Google Scholar]
- Ford ES. Li C. Defining the metabolic syndrome in children and adolescents: Will the real definition please stand up? J Pediatr. 2008;152:160–164. doi: 10.1016/j.jpeds.2007.07.056. [DOI] [PubMed] [Google Scholar]
- Friedewald WT. Levy RI. Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- Gothelf D. Falk B. Singer P. Kairi M. Phillip M. Zigel L. Poraz I. Frishman S. Constantini N. Zalsman G. Weizman A. Apter A. Weight gain associated with increased food intake and low habitual activity levels in male adolescent schizophrenic inpatients treated with olanzapine. Am J Psychiatry. 2002;159:1055–1057. doi: 10.1176/appi.ajp.159.6.1055. [DOI] [PubMed] [Google Scholar]
- Karelis AD. St-Pierre DH. Conus F. Rabasa-Lhoret R. Poehlman ET. Metabolic and body composition factors in subgroups of obesity: What do we know? J Clin Endocrinol Metab. 2004;89:2569–2575. doi: 10.1210/jc.2004-0165. [DOI] [PubMed] [Google Scholar]
- Kim SF. Huang AS. Snowman AM. Teuscher C. Snyder SH. From the cover: Antipsychotic drug-induced weight gain mediated by histamine H1 receptor-linked activation of hypothalamic AMP-kinase. Proc Natl Acad Sci USA. 2007;104:3456–3459. doi: 10.1073/pnas.0611417104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laita P. Cifuentes A. Doll A. Llorente C. Cortes I. Parellada M. Moreno D. Ruiz-Sancho A. Graell M. Arango C. Antipsychotic-related abnormal involuntary movements and metabolic and endocrine side effects in children and adolescents. J Child Adolesc Psychopharmacol. 2007;17:487–502. doi: 10.1089/cap.2006.0039. [DOI] [PubMed] [Google Scholar]
- Leahy JL. Natural history of beta-cell dysfunction in NIDDM. Diabetes Care. 1990;13:992–1010. doi: 10.2337/diacare.13.9.992. [DOI] [PubMed] [Google Scholar]
- Lee JM. Okumura MJ. Davis MM. Herman WH. Gurney JG. Prevalence, and determinants of insulin resistance among U.S. adolescents: A population-based study. Diabetes Care. 2006;29:2427–2432. doi: 10.2337/dc06-0709. [DOI] [PubMed] [Google Scholar]
- Levine JA. Eberhardt NL. Jensen MD. Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science. 1999;283:212–214. doi: 10.1126/science.283.5399.212. [DOI] [PubMed] [Google Scholar]
- Marshall WA. Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA. Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. L'Ecuyer S. Triglyceride, cholesterol and weight changes among risperidone-treated youths. A retrospective study. Eur Child Adolesc Psychiatry. 2002;11:129–133. doi: 10.1007/s00787-002-0255-5. [DOI] [PubMed] [Google Scholar]
- Matthews DR. Hosker JP. Rudenski AS. Naylor BA. Treacher DF. Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and Insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- McDowell MA. Fryar CD. Hirsch R. Ogden CL. Anthropometric Reference Data for Children and Adults: U.S. Population, 1999–2002. 2005. Retrieved August, 29, 2006. [PubMed]
- McGlashan TH. Zipursky RB. Perkins D. Addington J. Miller T. Woods SW. Hawkins KA. Hoffman RE. Preda A. Epstein I. Addington D. Lindborg S. Trzaskoma Q. Tohen M. Breier A. Randomized, double-blind trial of olanzapine versus placebo in patients prodromally symptomatic for psychosis. Am J Psychiatry. 2006;163:790–799. doi: 10.1176/ajp.2006.163.5.790. [DOI] [PubMed] [Google Scholar]
- Meyer JM. Koro CE. The effects of antipsychotic therapy on serum lipids: A comprehensive review. Schizophr Res. 2004;70:1–17. doi: 10.1016/j.schres.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Newcomer JW. Haupt DW. The metabolic effects of antipsychotic medications. Can J Psychiatry. 2006;51:480–491. doi: 10.1177/070674370605100803. [DOI] [PubMed] [Google Scholar]
- Ogden CL. Kuczmarski RJ. Flegal KM. Mei Z. Guo S. Wei R. Grummer-Strawn LM. Curtin LR. Roche AF. Johnson CL. Centers for Disease Control and Prevention 2000 growth charts for the United States: Improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109:45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- Olfson M. Blanco C. Liu L. Moreno C. Laje G. National trends in the outpatient treatment of children and adolescents with antipsychotic drugs. Arch Gen Psychiatry. 2006;63:679–685. doi: 10.1001/archpsyc.63.6.679. [DOI] [PubMed] [Google Scholar]
- Patel NC. Hariparsad M. Matias-Akthar M. Sorter MT. Barzman DH. Morrison JA. Stanford KE. Strakowski SM. DelBello MP. Body mass indexes and lipid profiles in hospitalized children and adolescents exposed to atypical antipsychotics. J Child Adolesc Psychopharmacol. 2007;17:303–311. doi: 10.1089/cap.2006.0037. [DOI] [PubMed] [Google Scholar]
- Pocock SJ. Geller NL. Tsiatis AA. The analysis of multiple endpoints in clinical trials. Biometrics. 1987;43:487–498. [PubMed] [Google Scholar]
- Reyes M. Croonenberghs J. Augustyns I. Eerdekens M. Long-term use of risperidone in children with disruptive behavior disorders and subaverage intelligence: Efficacy, safety, and tolerability. J Child Adolesc Psychopharmacol. 2006;16:260–272. doi: 10.1089/cap.2006.16.260. [DOI] [PubMed] [Google Scholar]
- Richelson E. Souder T. Binding of antipsychotic drugs to human brain receptors focus on newer generation compounds. Life Sci. 2000;68:29–39. doi: 10.1016/s0024-3205(00)00911-5. [DOI] [PubMed] [Google Scholar]
- Sikich L. Hamer RM. Bashford RA. Sheitman BB. Lieberman JA. A pilot study of risperidone, olanzapine, and haloperidol in psychotic youth: A double-blind, randomized, 8-week trial. Neuropsychopharmacology. 2004;29:133–145. doi: 10.1038/sj.npp.1300327. [DOI] [PubMed] [Google Scholar]
- Slaughter MH. Lohman TG. Boileau RA. Horswill CA. Stillman RJ. Van Loan MD. Bemben DA. Skinfold equations for estimation of body fatness in children and youth. Hum Biol. 1988;60:709–723. [PubMed] [Google Scholar]
- Spencer T. Biederman J. Wilens T. Greene R. Attention-deficit hyperactivity disorder. In: Martin A, editor; Scahill L, editor; Charney DS, editor; Leckman JF, editor. Pediatric Psychopharmacology, Principles and Practice. New York: Oxford University Press, Inc.; 2003. pp. 447–465. [Google Scholar]
- Stokes ME. Davis CS. Koch GG. Categorical Data Analysis Using the SAS System. Cary (North Carolina): SAS Institute Inc.; 2000. [Google Scholar]
- Surks MI. Ortiz E. Daniels GH. Sawin CT. Col NF. Cobin RH. Franklyn JA. Hershman JM. Burman KD. Denke MA. Gorman C. Cooper RS. Weissman NJ. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. JAMA. 2004;291:228–238. doi: 10.1001/jama.291.2.228. [DOI] [PubMed] [Google Scholar]
- Swanson JM. Elliott GR. Greenhill LL. Wigal T. Arnold LE. Vitiello B. Hechtman L. Epstein JN. Pelham WE. Abikoff HB. Newcorn JH. Molina BS. Hinshaw SP. Wells KC. Hoza B. Jensen PS. Gibbons RD. Hur K. Stehli A. Davies M. March JS. Conners CK. Caron M. Volkow ND. Effects of stimulant medication on growth rates across 3 years in the MTA follow-up. J Am Acad Child Adolesc Psychiatry. 2007;46:1015–1027. doi: 10.1097/chi.0b013e3180686d7e. [DOI] [PubMed] [Google Scholar]
- Vanina Y. Podolskaya A. Sedky K. Shahab H. Siddiqui A. Munshi F. Lippmann S. Body weight changes associated with psychopharmacology. Psychiatr Serv. 2002;53:842–847. doi: 10.1176/appi.ps.53.7.842. [DOI] [PubMed] [Google Scholar]
- Woods SC. Seeley RJ. Porte D., Jr. Schwartz MW. Signals that regulate food intake and energy homeostasis. Science. 1998;280:1378–1383. doi: 10.1126/science.280.5368.1378. [DOI] [PubMed] [Google Scholar]
- Zimmet P. Alberti KG. Kaufman F. Tajima N. Silink M. Arslanian S. Wong G. Bennett P. Shaw J. Caprio S. The metabolic syndrome in children and adolescents—An IDF consensus report. Pediatr Diabetes. 2007;8:299–306. doi: 10.1111/j.1399-5448.2007.00271.x. [DOI] [PubMed] [Google Scholar]


