Abstract
Hyper-IgE syndrome (HIES) is a primary immunodeficiency characterized by atopic manifestations and susceptibility to infections with extracellular pathogens, typically Staphylococcus aureus, which preferentially affect the skin and lung. Previous studies reported the defective differentiation of T helper 17 (Th17) cells in HIES patients caused by hypomorphic STAT3 mutations. However, the apparent contradiction between the systemic Th17 deficiency and the skin/lung-restricted susceptibility to staphylococcal infections remains puzzling. We present a possible molecular explanation for this enigmatic contradiction. HIES T cells showed impaired production of Th17 cytokines but normal production of classical proinflammatory cytokines including interleukin 1β. Normal human keratinocytes and bronchial epithelial cells were deeply dependent on the synergistic action of Th17 cytokines and classical proinflammatory cytokines for their production of antistaphylococcal factors, including neutrophil-recruiting chemokines and antimicrobial peptides. In contrast, other cell types were efficiently stimulated with the classical proinflammatory cytokines alone to produce such factors. Accordingly, keratinocytes and bronchial epithelial cells, unlike other cell types, failed to produce antistaphylococcal factors in response to HIES T cell–derived cytokines. These results appear to explain, at least in part, why HIES patients suffer from recurrent staphylococcal infections confined to the skin and lung in contrast to more systemic infections in neutrophil-deficient patients.
The identification of Th17 cells as a third subset of helper T cells has illuminated the fact that distinct subsets of helper T cells have been evolved to protect our body from infections by various types of microorganisms and are involved differently in the induction and exacerbation of various immunological disorders. Th17 cells are characterized and distinguished from IFN-γ–producing Th1 cells and IL-4–producing Th2 cells by their production of so-called Th17 cytokines including IL-17 (IL-17A), IL-17F, and IL-22 (1–4). For their differentiation from naive CD4 T cells, Th17 cells require different cytokines and transcription factors than do Th1, Th2, or regulatory T cells. The roles of Th17 cells in immune responses are also different from those of other helper T cells. In particular, their pathological roles in autoimmune and inflammatory diseases, including multiple sclerosis, rheumatoid arthritis, psoriasis, and inflammatory bowel diseases, have been studied extensively (5–10).
Although the functions of Th17 cells under physiological conditions have not been completely elucidated, accumulating data suggest that Th17 cells play crucial roles in the host defense against extracellular pathogens that are not efficiently cleared by Th1- and Th2-type immune responses. Th17-type cytokines IL-17A and IL-17F are important for the recruitment of neutrophils (11), whereas IL-22 induces the production of antimicrobial peptides β-defensin (BD) 2 and BD3 by keratinocytes, through the activation of STAT3 (12–14). Mice with a homozygous deletion of the gene encoding the IL-17RA (IL-17 receptor A) and mice that do not produce IL-22 are susceptible to lung infection by the Gram-negative bacteria Klebsiella pneumoniae and Mycoplasm pulmonis (15–17). Mice that produce neither IL-17A nor IL-17F are susceptible to skin infection by the Gram-positive bacteria Staphylococcus aureus (18). Administration of anti–IL-17A neutralizing antibodies impairs both the intraabdominal abscess formation in response to Bacteroides fragilis and Escherichia coli (19–21) and the host defense against systemic infection by the fungus Candida albicans (22). These data indicate that Th17 cells play a key role in immune responses to extracellular bacteria and fungi in mice. In contrast, the anti-pathogenic roles of Th17 cells in humans are relatively uncertain.
Recent studies demonstrated that the differentiation of human Th17 cells was defective in patients with hyper-IgE syndrome (HIES) (23–26). HIES is a primary immunodeficiency disease caused by dominant-negative mutations in the DNA-binding domain, SH2 domain, or transactivating domain of STAT3 (26–28). As expected from the important roles of STAT3 in transducing signals for a variety of cytokines, growth factors, and hormones, patients with HIES display complex clinical manifestations in multiple organs, including atopic dermatitis with high serum IgE levels and abnormalities of the bones and teeth (29–32). Most patients suffer from recurrent infections by fungi and bacteria, predominantly the Gram-positive bacteria S. aureus. The presence of these infections suggests that Th17 cells play a crucial role in protection from extracellular pathogens, not only in mice but also in humans. However, curiously, the staphylococcal infections in HIES patients are often confined to the skin and lung and manifest clinically as skin abscesses and cyst-forming pneumonia. These skin- and lung-restricted infections are in sharp contrast to the pattern of infection observed in patients with a neutrophil deficiency. For example, in patients with chronic granulomatous disease (CGD), staphylococcal infections occur in a wide variety of organs including the lung, lymph nodes, skin, liver, bone, gastrointestinal tract, kidney, and brain (33). Thus, it remains elusive why HIES patients suffer from skin- and lung-restricted staphylococcal infections in spite of their systemic Th17 deficiency.
In the present study, we explored possible molecular mechanisms underlying the recurrent staphylococcal infections confined to the skin and lung in HIES patients. We found that primary human keratinocytes and bronchial epithelial cells displayed a much stronger dependence than other cell types on Th17 cytokines in their production of antistaphylococcal factors including the neutrophil-recruiting chemokines and antibacterial peptides. T cells from HIES patients, in spite of their defect in production of Th17 cytokines, showed normal production of other proinflammatory cytokines, including IL-1β, which was insufficient for triggering keratinocytes and bronchial epithelial cells but sufficient for other cell types to produce antistaphylococcal factors. Th17 cytokines and classical proinflammatory cytokines synergistically stimulated keratinocytes and bronchial epithelial cells, but the synergy was not seen in other types of cells. These findings provide a possible molecular explanation for the apparent contradiction between the systemic Th17 deficiency and the skin and lung-restricted staphylococcal infections in HIES patients.
RESULTS
HIES T cells produce little or no Th17 cytokines and fail to stimulate keratinocytes to secrete neutrophil-recruiting chemokines and BDs
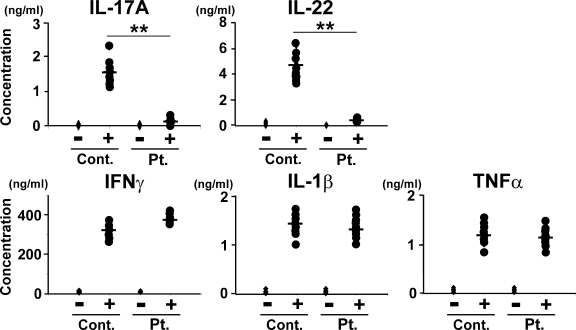
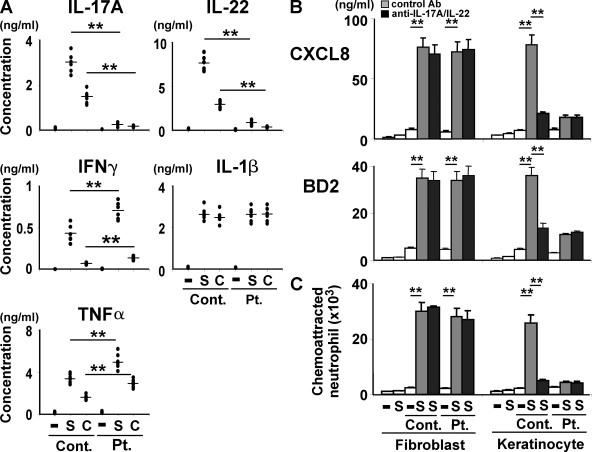
We first examined the profile of cytokines produced by T cells from our cohort of HIES patients whose STAT3 genes carried mutations. The amounts of IL-17A and IL-22 secreted from the patients’ T cells upon stimulation with anti-CD3 and anti-CD28 mAbs were invariably only ∼5–10% of those from healthy control subjects, which is in accordance with previous results (23–26), whereas the production levels of IFN-γ, IL-1β, and TNF-α were comparable in the two groups (Fig. 1). Real-time quantitative RT-PCR demonstrated that the up-regulation of IL-17F expression by the patients’ T cells was also impaired (unpublished data). Thus, the patients’ T cells showed a selective defect in the production of Th17 cytokines.
Figure 1.
HIES T cells produce greatly reduced amounts of Th17 cytokines and normal amounts of classical proinflammatory cytokines upon activation. PBMCs from HIES patients (Pt.) and control subjects (Cont.; n = 8 each, indicated by dots) were stimulated (+) or not (−) with anti-CD3 and anti-CD28 for 72 h, and the concentration of the indicated cytokines in their culture supernatants was determined by ELISA. The results shown are representative of three independent experiments. **, P < 0.01.
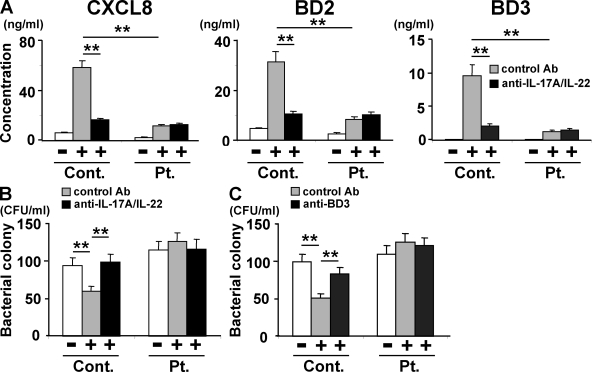
We next investigated the functional consequences of the Th17 deficiency in the context of staphylococcal infections of the skin. Normal human primary epidermal keratinocytes were cultured in vitro with culture supernatants from HIES patients’ or control subjects’ T cells that had been unstimulated or stimulated with anti-CD3 plus anti-CD28. The expression and production of two chemokines, CXCL8 (IL-8) and CCL2, was up-regulated in the keratinocytes cultured with the conditioned medium from activated control T cells (Fig. 2 A and Fig. S1). In contrast, although CCL2 was also up-regulated by the conditioned medium from activated HIES T cells, CXCL8 was not (Fig. 2 A and Fig. S1). Among the three antimicrobial peptides (BDs) examined, at the mRNA and protein level the expression of BD1 but not BD2 or BD3 was up-regulated in keratinocytes when they were stimulated with conditioned medium from the T cells of HIES patients, but all three were up-regulated by the conditioned medium from the control subjects’ T cells (Fig. 2 A and Fig. S1). Thus, the HIES patients’ T cells could not stimulate keratinocytes to produce a significant amount of the neutrophil-recruiting chemokine CXCL8 or the antimicrobial peptides BD2 and BD3, but they could stimulate the up-regulation of CCL2 and BD1.
Figure 2.
Supernatants of activated HIES T cells fail to stimulate keratinocytes to secrete significant amounts of antibacterial factors. In the presence or absence of anti–IL-17A plus anti–IL-22 (A and B), anti-BD3 (C), or isotype-matched control antibodies, primary human keratinocytes were incubated for 48 h with the supernatants of HIES (Pt.) or control (Cont.) T cells that had been stimulated (+) or not (−) with anti-CD3 and anti-CD28 for 72 h as in Fig. 1. (A) The concentration of CXCL8, BD2, and BD3 in keratinocytes supernatants was determined by ELISA. Representative data from one patient and one control are shown (mean ± SD; n = 3), and similar results were obtained from the other patients and controls. (B and C) The culture supernatants of keratinocytes were analyzed for their antistaphylococcal activity by the colony assay (mean ± SD; n = 3). The results shown in are representative of at least three independent experiments. **, P < 0.01.
T cell–derived Th17 cytokines are responsible for the production of CXCL8 and BDs from keratinocytes
When the supernatants from activated control T cells were treated with the combination of anti–IL-17A and anti–IL-22 blocking mAbs before incubation with keratinocytes, their capability of stimulating keratinocytes to up-regulate CXCL8, BD2, and BD3 was diminished to the level displayed by the HIES patients’ T cells (Fig. 2 A and Fig. S1). Either anti–IL-17A or anti–IL-22 alone was less effective than their combination (Fig. S2). Thus, the defective production of chemokines and BDs by keratinocytes in response to the conditioned medium from the patients’ T cells was attributable to the T cells’ defective production of Th17 cytokines.
We next examined the direct effect of the keratinocyte-derived factors on the growth of Staphylococcus aureus using a colony-forming assay (Fig. 2, B and C). When bacteria were cultured with the culture supernatant from keratinocytes stimulated by control T cells, the number of bacterial colonies was reduced to 60% as compared with that when cultured with control medium. However, this was not the case when HIES T cells were used to stimulate keratinocytes. The antibacterial activity was completely abrogated when the culture supernatant from the control T cells was pretreated with the blocking mAbs for IL-17A and IL-22 before it was added to the keratinocytes (Fig. 2 B), and it was attenuated when the keratinocyte supernatants were pretreated with an anti-BD3 mAb before their application to the bacterial culture (Fig. 2 C). These results indicated that control but not HIES T cells produced Th17 cytokines that, in turn, acted on keratinocytes to elicit their secretion of antimicrobial factors including BD3.
Keratinocytes and bronchial epithelial cells display a greater dependence on Th17 cytokines for their production of chemokines and BDs than other cell types
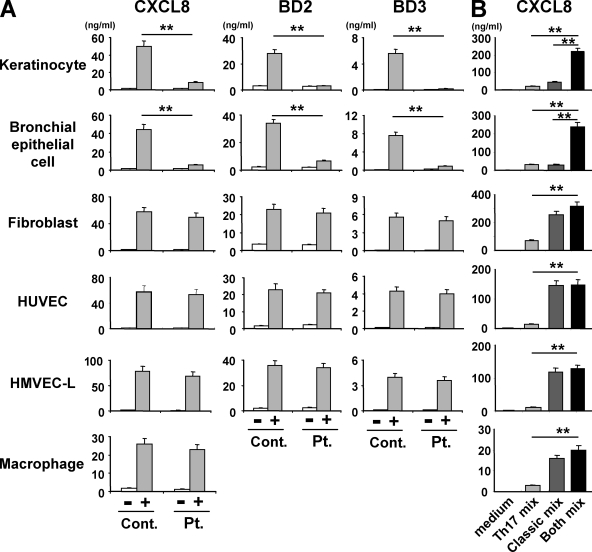
To learn why the staphylococcal infections are confined to the skin and lung in HIES patients, we analyzed different lineages of human primary cells for their ability to secrete chemokines and BDs in response to T cell–derived factors including Th17 cytokines. We first compared their responsiveness to culture supernatants from either control or patient T cells that were activated with anti-CD3 and anti-CD28 mAbs. Primary bronchial epithelial cells responded to the T cell conditioned medium just as the primary keratinocytes did. That is, both cell types up-regulated the expression and production of chemokines (CXCL8 and CXCL1) and BDs (BD2 and BD3) when incubated with the supernatants from control T cells but not from HIES T cells (Fig. 3 A and Fig. S3). Interestingly, primary dermal fibroblasts, human umbilical vein endothelial cells (HUVEC), and human lung microvascular endothelial cells (HMVEC-L) responded equally well, in terms of their secretion of the chemokines and BDs, to the supernatants from control or patient T cells (Fig. 3 A and Fig. S3). This was also true for the expression and production of CXCL8 and CXCL1 by human macrophages (Fig. 3 A and Fig. S3). Human macrophages did not produce detectable amounts of BDs. Thus, keratinocytes and bronchial epithelial cells responded differently to T cell–derived factors than the other cell types tested and appeared to be much more dependent on Th17 cytokines for their induction to secrete chemokines and BDs.
Figure 3.
Keratinocytes and bronchial epithelial cells show greater dependence on Th17 cytokines for the production of chemokines and BDs than other cell types. Primary human keratinocytes, bronchial epithelial cells, dermal fibroblasts, endothelial cells (HUVEC and HMVEC-L), and macrophages were incubated for 48 h with T cell supernatants that were prepared as described in Fig. 1 A or with the Th17 cytokine cocktail (Th17 mix: IL-17A + IL-17F + IL-22), the classical proinflammatory cytokine cocktail (classical mix: TNF-α + IL-1β + IFN-γ), or the combination of both (both mix; B). The concentration of CXCL8, BD2, and BD3 in their culture supernatants was determined by ELISA. Representative data from one patient and one control are shown in A (mean ± SD; n = 3), and similar results were obtained from the other patients and controls. The results shown are representative of at least three independent experiments. **, P < 0.01.
These findings prompted us to examine the responses of different cell types to individual cytokines and their combinations, including the Th17 cytokines (IL-17A, IL-17F, and IL-22), classical proinflammatory cytokines (IL-1β, TNF-α, and IFN-γ), or both. Keratinocytes secreted CXCL8 in response to IL-17A, IL-22, IL-1β, or TNF-α in a dose-dependent manner, but they responded poorly to IL-17F and IFN-γ (Fig. S4 A). Using the Th17 cytokine cocktail or the classical proinflammatory cytokine cocktail resulted in some additive effect on the keratinocytes’ secretion of CXCL8 (Fig. S4, B and C). In contrast, the combination of both types of cytokines dramatically enhanced the CXCL8 production by the keratinocytes (Fig. 3 B and Fig. S5). This was also the case for bronchial epithelial cells (Fig. 3 B). In contrast, fibroblasts, HUVEC, and HMVEC-L secreted a large quantity of CXCL8 in response to the classical proinflammatory cytokine cocktail, but the further addition of Th17 cytokine cocktail caused no significant enhancement of CXCL8 production (Fig. 3 B). Furthermore, the Th17 cytokine cocktail was much less effective in stimulating fibroblasts, HUVEC, and HMVEC-L than the classical cytokine cocktail, and the amount of CXCL8 produced by the Th17 cytokine cocktail-treated fibroblasts, HUVEC, and HMVEC-L was ∼10–30% of that produced by stimulation with the classical proinflammatory cocktail. Macrophages responded to the cytokines in a pattern similar to fibroblasts, HUVEC, and HMVEC-L, although the macrophages produced 10× less CXCL8 than the others.
In keratinocytes and bronchial epithelial cells, the marked synergy caused by combining the Th17 and classical proinflammatory cytokines affected not only the expression of CXCL8 but also that of other chemokines (CXCL1 and CXCL2) and BDs (BD2 and BD3; Fig. S6 A). In accordance with this finding, the supernatants of keratinocytes stimulated with the Th17–classical cytokine combination caused robust neutrophil chemotaxis compared with the supernatants from keratinocytes stimulated with only one of the cocktails (Fig. S6 B).
Previous studies demonstrated that the stimulation of keratinocytes with toll-like receptor (TLR) 2 ligands induces the production of the chemokines and antimicrobial peptides (34, 35). In agreement with this, the keratinocytes showed up-regulated CXCL8 secretion and BD expression in response to lipoteichoic acid, peptidoglycan, or fixed S. aureus, but the extent of up-regulation was <10% of that observed after stimulation with the combination of Th17 and proinflammatory cytokines (Fig. S6 C).
Molecular mechanisms underlying the unique responsiveness of keratinocytes and bronchial epithelial cells to Th17 cytokines in synergy with other proinflammatory cytokines
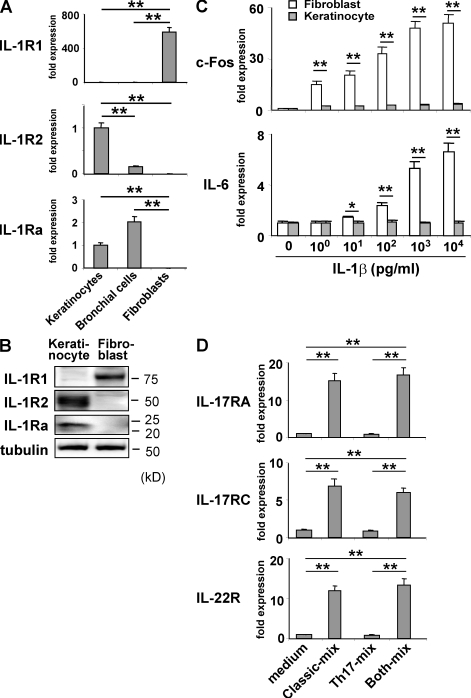
To explore the possible molecular basis of the poorer response of keratinocytes and bronchial epithelial cells to the classical proinflammatory cytokines, as compared with the other types of cells, we analyzed the expression of the classical cytokine IL-1R1 (IL-1 receptor) and its antagonists IL-1R2 and IL-1Ra (Fig. 4, A and B). Compared with fibroblasts, keratinocytes expressed 1/600th of IL-1R1 transcripts, 170-fold more IL-1Ra transcripts, and 260-fold more IL-1R2 (Fig. 4 A). The great difference in their expression between keratinocytes and fibroblasts was also confirmed at the protein level (Fig. 4 B). Bronchial epithelial cells showed the keratinocyte-type expression, whereas HUVEC and HMVEC-L displayed the fibroblast-type expression (Fig. 4 A and not depicted). Consistent with this result, keratinocytes showed a much poorer up-regulation of c-Fos and IL-6 than fibroblasts in response to IL-1β (Fig. 4 C). This appeared to partly explain why keratinocytes and bronchial epithelial cells were less sensitive to the classical proinflammatory cytokines than fibroblasts but did not account for the strong synergy between the Th17 and the classical proinflammatory cytokines in the keratinocytes. Therefore, we next examined the possible cross-talk between the two types of cytokines in terms of the regulation of cytokine receptor expression.
Figure 4.
Distinct expression and regulation of the cytokine receptors in keratinocytes and fibroblasts compared with those in other cell types. (A) The expression of IL-1R1, IL-1Ra, and IL-1R2 in the indicated cells was determined by quantitative RT-PCR. Data shown were normalized to HPRT levels, and the level of expression in keratinocytes was defined as 1.0. (B) IL-1R1, IL-1R2, and IL-1Ra proteins in keratinocytes and fibroblasts were detected by immunoblotting. (C) Keratinocytes and fibroblasts were cultured for 15 min with the indicated concentration of IL-1β and analyzed by quantitative RT-PCR for the expression of c-Fos and IL-6. Data shown were normalized to HPRT levels, and the level of expression in cells cultured without IL-1β was defined as 1.0 for each cell type. (D) Keratinocytes cultured as in Fig. 3 B were analyzed by quantitative RT-PCR for the expression of IL-17RA, IL-17RC, and IL-22R. The data shown were normalized to the HPRT levels, and the level of expression in cells cultured without any added cytokine was defined as 1.0. The results shown are representative of three independent experiments. Error bars show mean ± SD (n = 3). *, P < 0.05; **, P < 0.01.
We found that the expression of the Th17 cytokine receptors IL-17RA, IL-17RC, and IL-22R was up-regulate, in keratinocytes incubated with the classical proinflammatory cytokines but not the Th17 cytokines (Fig. 4 D and Fig. S7). Conversely, in keratinocytes incubated with the Th17 cytokines, the expression of the receptors for the classical proinflammatory cytokines was up-regulated, albeit less markedly (unpublished data). This reciprocal up-regulation of cytokine receptor expression was also observed in bronchial epithelial cells (unpublished data). These findings could account, at least in part, for the synergistic effect of the Th17 and the classical proinflammatory cytokines on the production of antibacterial factors by keratinocytes and bronchial epithelial cells.
HIES T cells show poor ability of stimulating keratinocytes in response to staphylococcal superantigens and candida antigens
We next investigated the responses of HIES T cells under more clinically relevant conditions to obtain a better insight into the susceptibility to staphylococcal infections observed in HIES patients. When stimulated with the S. aureus–derived superantigens for T cells, staphylococcal enterotoxin B (SEB), HIES patients’ T cells produced drastically reduced amounts of IL-17A and IL-22, <10% of those produced by control T cells (Fig. 5 A). In contrast, the IL-1β production was normal, and the IFN-γ and TNF-α production was even enhanced in SEB-stimulated HIES T cells (Fig. 5 A). It is of note that the supernatants of SEB-stimulated HIES T cells showed much poorer ability to induce the production of CXCL8 and BD2 in keratinocytes compared with those from control T cells (Fig. 5 B). In contrast, both supernatants from HIES and control T cells almost equally well stimulated fibroblasts to produce CXCL8 and BD2. The combination of anti–IL-17A and –IL-22 efficiently inhibited the CXCL8/BD2-inducing activity of control T cells’ supernatants in keratinocytes but showed no significant inhibition in the CXCL8/BD2 production from fibroblasts that were stimulated with the supernatants from either control or HIES T cells (Fig. 5 B). These results strongly suggested that Th17 cytokines secreted by SEB-stimulated T cells played a critical role in the induction of CXCL8 and BD2 production in keratinocytes but not in fibroblasts. In accord with these results, supernatants of keratinocytes stimulated with HIES T cells showed little or no ability of neutrophil chemoattraction, whereas those of keratinocytes stimulated with control T cells and those of fibroblasts stimulated with either control or HIES T cells induced robust neutrophil chemotaxis (Fig. 5 C). These in vitro findings may account in part, if not entirely, for the skin/lung-confined susceptibility to staphylococcal infections observed in HIES patients.
Figure 5.
HIES T cells show poor ability of stimulating keratinocytes in response to staphylococcal superantigens and candida antigens. (A) PBMCs from HIES patients (Pt.) and control subjects (Cont.; n = 6 each, indicated by dots) were stimulated or not (−) with SEB (S, 100 ng/ml) or CAA (C, 1/20,000 vol/vol) for 5 d, and the concentration of the indicated cytokines in their culture supernatant was determined by ELISA. (B and C) Fibroblasts and keratinocytes were cultured for 48 h in the absence (−) or presence (S) of SEB or with the supernatants of patients (Pt.) or control (Cont.) PBMCs that had been unstimulated (−) or stimulated with SEB (S) as in A, in the presence or absence of anti–IL-17A + anti–IL-22 or isotype-matched control antibodies. Their culture supernatants were analyzed by ELISA for the secretion of CXCL8 and BD2 (B) and evaluated for their neutrophil chemotactic activity (C). The results shown are representative of two independent experiments. Error bars show mean ± SD (n = 3). **, P < 0.01.
We further examined the responsiveness of HIES T cells to Candida albicans antigen (CAA). HIES T cells showed impaired cytokine production in response to CAA in the essentially same pattern as observed in response to SEB (Fig. 5 A). This may also explain in part the incidence of mucocutaneous infections with C. albicans that is often observed in HIES patients.
DISCUSSION
In the present study, we demonstrated that skin and lung epithelial cells displayed an unusual pattern of responsiveness to Th17 and other proinflammatory cytokines that was distinct from that of the other cell types tested. This previously unrecognized modes of cytokine responses could fill in the apparent gap between the systemic Th17 deficiency and the tissue-dependent susceptibility to staphylococcal infections in the HIES patients. Both Th17 cytokines and other classical proinflammatory cytokines stimulate a variety of cells to produce neutrophil-recruiting chemokines and antimicrobial peptides, which are important for providing protection against bacterial infections (7). We found that skin and lung epithelial cells efficiently secreted antibacterial factors only when stimulated with a combination of Th17 cytokines and classical proinflammatory cytokines. These observations were made using primary cells that were grown on plastic. In contrast, fibroblasts, endothelial cells, and macrophages efficiently secreted antibacterial factors when stimulated with the classical proinflammatory cytokines alone. Thus, skin and lung epithelial cells showed a much higher dependence on Th17 cytokines, in synergy with the classical proinflammatory cytokines, than the other cell types. The classical proinflammatory cytokines up-regulated the expression of Th17 cytokine receptors and, conversely, the Th17 cytokines up-regulated the expression of receptors for the classical proinflammatory cytokines, albeit less strongly. This reciprocal up-regulation of cytokine receptor expression could be one of the molecular mechanisms underlying the strong synergy between the Th17 and classical proinflammatory cytokines in skin and lung epithelial cells.
This synergistic action of the cytokines appears to account in part, if not entirely, for the skin- and lung-restricted staphylococcal infections of HIES patients. S. aureus produces enterotoxins, including SEB, that function as superantigens to stimulate the bulk of T cells. The HIES patients’ T cells showed impaired production of Th17 cytokines in response to SEB but normal production of the classical proinflammatory cytokines. Therefore, the skin and lung epithelial cells of HIES patients, unlike other cell types, probably do not secrete sufficient amounts of neutrophil-recruiting chemokines and the antimicrobial peptide BDs to fend off staphylococcal infection. With regard to the sites of bacterial infections, it is important to consider the pathogen’s characteristics. The tendency of S. aureus to colonize the skin and upper respiratory tract may explain the skin/lung-restricted staphylococcal infections observed in HIES patients. It is of note, however, that CGD patients suffer from staphylococcal infections that occur in a wide variety of organs, including the lung, lymph nodes, skin, liver, bone, gastrointestinal tract, kidney, and brain (33). The difference in the spectrum of affected tissues between HIES and CGD patients strongly suggests that the host factors, in addition to the pathogen’s intrinsic factors, would determine the preferential sites of infections. In HIES patients, unlike in CGD patients, the neutrophils themselves are normal in their number and function; however, they probably cannot be recruited to the skin and lung because HIES T cells cannot induce the skin and lung epithelial cells to produce neutrophil-recruiting chemokines like CXCL8, even though we cannot formally exclude the possibility that the STAT3 mutation in the epithelial cells of HIES patients also contributes to impaired production of antistaphylococcal factors including CXCL8. Mice deficient for IL-17RA or IL-22 and mice treated with an anti–IL-17A blocking antibody are susceptible to infections with Gram-negative bacteria, such as K. pneumoniae, M. pulmonis, B. fragilis, E. coli, and Citrobacter rodentium, which are rarely observed in HIES patients (16, 17, 20, 21, 36, 37). The reason for this difference between human and mouse in bacterial susceptibility remains to be determined.
TLR-mediated signals are known to be important for immune protection from staphylococcal infections. The outer cell wall of Staphylococcus aureus is composed of exposed peptidoglycan and lipoteichoic acid, which are recognized by TLR2 (38–40). Mice deficient in TLR2 and patients deficient in IRAK4, a transducer of TLR signaling, show increased susceptibility to staphylococcal infections (41–43). Importantly, TLR2 signaling is intact in HIES patients (44, 45). We demonstrated in the present study that keratinocytes indeed produced antibacterial factors in response to TLR2 ligands, but the amounts were <10% of those induced when the cells were stimulated with the combination of Th17 and classical proinflammatory cytokines. Thus, TLR2-mediated signaling alone appears to be insufficient for the full protection against staphylococcal infection of the human skin and lung.
Skin and lung epithelial cells are located, respectively, at the major outer and inner surface barriers of the body, and are constantly exposed to agents from the environment. Therefore, these cells probably need to discriminate between infectious and noninfectious agents to avoid unnecessary inflammation. The present study demonstrated that they secrete antibacterial factors only when they receive stimuli from both classical proinflammatory cytokines delivered by innate immunity–type cells and Th17 cytokines delivered by T cells. Thus, an attractive hypothesis is that epithelial cells have been equipped by evolution to respond poorly to the first alert signal, i.e., the classical proinflammatory cytokines produced by innate immunity cell types, which might be evoked even by noninfectious agents. Infectious agents, such as S. aureus, could evoke the production of the second alert signal, i.e., Th17 cytokines produced by T cells, in addition to the first alert signal. This would allow the epithelial cells to respond selectively to pathogens. In HIES patients, the second alert signal is not delivered because of the Th17 deficiency, which probably results in skin- and lung-restricted staphylococcal infections.
Accumulating evidence indicates that Th17 cells and their products are very important in the induction and propagation of autoimmunity (5–10). Therefore, the neutralization of Th17 cytokines appears to be a promising therapeutic strategy for the control of inflammation in autoimmune disorders. Moreover, antagonists of STAT3 are considered good candidates for the treatment of tumors because a variety of tumor cells show up-regulated STAT3 expression (46). However, our data indicate that such treatments would render patients susceptible to staphylococcal infection, particularly of the skin and lung, as observed in HIES. Fortunately, our study also suggests that this undesirable side effect might be prevented or treated by the local application of neutrophil-recruiting chemokines and BDs or their derivatives.
In summary, we demonstrated in the present study that T cells from HIES patients, in spite of their defect in production of Th17 cytokines, showed normal production of other proinflammatory cytokines including IL-1β in response to staphylococcal antigens, which was insufficient for triggering keratinocytes and bronchial epithelial cells but sufficient for other cell types to produce antistaphylococcal factors. This provides a possible molecular explanation for the apparent contradiction between the systemic Th17 deficiency and the skin- and lung-restricted staphylococcal infections in HIES patients.
MATERIALS AND METHODS
Patients.
All eight patients enrolled in this study had typical findings associated with HIES and a National Institutes of Health score >40 points (27). The diagnosis was confirmed by the identification of the mutations in the STAT3 gene. The study was approved by the Tokyo Medical and Dental University Ethics Committee, and written informed consent was obtained from the patients. All of the patients were in a healthy state when their blood samples were collected. Blood samples from patients and age-matched healthy subjects were obtained and PBMCs were prepared by density-gradient centrifugation.
Cell culture.
PBMCs were cultured in 96-well plates in RPMI medium 1640 supplemented with 1% penicillin/streptomycin, 1% glutamine, and 10% heat-inactivated FCS. Cultures were stimulated with a 1:100 (vol/vol) dilution of anti-CD3 and anti-CD28 beads (Invitrogen). For some experiments, the following mAbs, cytokines, and TLR ligands were added: 20 ng/ml IL-17A, 200 ng/ml IL-17F, and 200 ng/ml IL-22 (R&D Systems); 10 ng/ml TNF-α, 10 ng/ml IL-1β, 10 ng/ml IFN-γ (PeproTech); neutralizing antibodies against IL-17, IL-22, and BD3 (R&D Systems); TLR ligands (InvivoGen); fixed S. aureus (EMD); SEB (Toxin Technology); and C. albicans skin test antigen (Torii Pharmaceutical Co., Ltd).
Culture of human keratinocytes, bronchial epithelial cells, fibroblasts, endothelial cells, and macrophages.
Human epidermal keratinocytes and bronchial epithelial cells (Lonza) were propagated as adherent cells to plastic in RPMI 1640 medium containing bovine pituitary extract, human epidermal growth factor, insulin, hydrocortisone, gentamicin, and amphotericin at 37°C in a 5% CO2 incubator. Human primary dermal fibroblasts, HUVEC, HMVEC-L were obtained from Lonza. Macrophages were derived from adherent cells in PBMCs cultured in the presence of 30 ng/ml GM-CSF for 7 d.
RNA isolation and real-time quantitative RT-PCR.
Cells were harvested for total RNA isolation using the RNeasy Miniprep kit (QIAGEN), according to the manufacturer’s instructions. Total RNA was reverse transcribed using the PrimeScript transcription kit (Takara Bio Inc.). An aliquot of the RT reaction was used as a template for real-time PCR in triplicate using a SYBR Green MasterMix (Takara Bio Inc.) on an Mx3005P thermocycler (Agilent Technologies) with SYBR green I dye as the amplicon detector and ROX as the passive reference. The gene for HPRT was amplified as an endogenous reference. Quantification was determined using both a standard curve and comparative ΔΔCT methods.
ELISA.
Conditioned medium from cultured cells was collected after the cells were stimulated and stored at −80°C until use. IL-17A (eBioscience), IL-22 (R&D Systems), IFN-γ, TNF-α, IL-1β, CXCL8 (BD), BD2 (KOMABIOTECH), and BD3 (Alpha Diagnostics) were measured in triplicate by ELISA according to the manufacturers’ instructions.
Bactericidal activity against S. aureus.
S. aureus (strain Rosenbach 1884) was obtained from the National Biological Resource Center. Bactericidal activity was evaluated by plating serial dilutions of S. aureus mixed with the supernatant from keratinocytes or bronchial epithelial cells, and the CFUs were determined in triplicate on the next day. In some experiments, a neutralizing antibody to BD3 was added to the supernatant.
Chemotaxis.
Chemotaxis of neutrophils was determined in triplicate by the Boyden chamber technique. The migration chamber was divided into upper and lower compartments by a membrane with a pore size of 3 µm. The neutrophils were placed into the upper compartment at a concentration of 106/ml, and the lower compartment contained the supernatant from the keratinocytes or fibroblasts grown under the conditions indicated. The chambers were incubated at 37° C for 1 h, and the number of neutrophils that migrated to the lower chamber was counted.
Immunoblotting.
Cells were lysed on ice for 30 min in lysis buffer containing 1% Triton X-100, 50 mM Tris, pH 8.0, 150 mM NaCl, 2 mM EDTA, 2 µg/ml aprotinin, and 100 µg/ml PMSF. The cell lysates were subjected to SDS-PAGE, followed by electrotransfer to PVDF membranes and immunoblotting with antibodies for IL-1R1, IL-1R2, and IL-1Ra (R&D Systems) and for tubulin (Sigma-Aldrich).
Statistical analysis.
Data were compared by a two-tailed Mann-Whitney U test or unpaired Student’s t test. P-values < 0.05 were considered significant.
Online supplemental material.
Fig. S1 shows the quantitative RT-PCR analysis for chemokine and BD expression in activated keratinocytes. The importance of IL-17A and IL-22 in stimulating keratinocytes to produce antistaphylococcal factors is demonstrated in Fig. S2. Fig. S3 shows the quantitative RT-PCR analysis for chemokine and BD expression in various types of cells. Production of CXCL8 by keratinocytes and fibroblasts in response to various cytokines is displayed in Figs. S4 and S5. Fig. S6 shows the expression and production of antistaphylococcal factors by keratinocytes in response to various stimuli. Up-regulation of the Th17 cytokine receptors in keratinocytes in response to classical inflammatory cytokines is displayed in Fig. S7. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20082767/DC1.
Acknowledgments
This work is supported by Grants-in-Aid 16616004, 17047013, and 18659299 from the Japanese Ministry of Education, Culture, Sports, Science and Technology and Research on Intractable Diseases from the Ministry of Health, Labor and Welfare, the Uehara Foundation, Naito Foundation, and the Mother and Child Health Foundation.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used: BD, β-defensin; CAA, Candida albicans antigen; CGD, chronic granulomatous disease; HIES, hyper-IgE syndrome; HMVEC-L, human lung microvascular endothelial cell; HUVEC, human umbilical vein endothelial cell; SEB, staphylococcal enterotoxin B; TLR, Toll-like receptor.
References
- Dong C. 2008. TH17 cells in development: an updated view of their molecular identity and genetic programming.Nat. Rev. Immunol. 8:337–348 [DOI] [PubMed] [Google Scholar]
- Ivanov I.I., Zhou L., Littman D.R. 2007. Transcriptional regulation of Th17 cell differentiation.Semin. Immunol. 19:409–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Laurence A., O’Shea J.J. 2007. Signal transduction pathways and transcriptional regulation in the control of Th17 differentiation.Semin. Immunol. 19:400–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeachy M.J., Bak-Jensen K.S., Chen Y., Tato C.M., Blumenschein W., McClanahan T., Cua D.J. 2007. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology.Nat. Immunol. 8:1390–1397 [DOI] [PubMed] [Google Scholar]
- Cua D.J., Sherlock J., Chen Y., Murphy C.A., Joyce B., Seymour B., Lucian L., To W., Kwan S., Churakova T., et al. 2003. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain.Nature. 421:744–748 [DOI] [PubMed] [Google Scholar]
- Korn T., Oukka M., Bettelli E. 2007. Th17 cells: effector T cells with inflammatory properties.Semin. Immunol. 19:362–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aujla S.J., Dubin P.J., Kolls J.K. 2007. Th17 cells and mucosal host defense.Semin. Immunol. 19:377–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang W., Kolls J.K., Zheng Y. 2008. The biological functions of T helper 17 cell effector cytokines in inflammation.Immunity. 28:454–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diveu C., McGeachy M.J., Cua D.J. 2008. Cytokines that regulate autoimmunity.Curr. Opin. Immunol. 20:663–668 [DOI] [PubMed] [Google Scholar]
- Iwakura Y., Nakae S., Saijo S., Ishigame H. 2008. The roles of IL-17A in inflammatory immune responses and host defense against pathogens.Immunol. Rev. 226:57–79 [DOI] [PubMed] [Google Scholar]
- Kolls J.K., Linden A. 2004. Interleukin-17 family members and inflammation.Immunity. 21:467–476 [DOI] [PubMed] [Google Scholar]
- Kolls J.K., McCray P.B., Jr., Chan Y.R. 2008. Cytokine-mediated regulation of antimicrobial proteins.Nat. Rev. Immunol. 8:829–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreymborg K., Etzensperger R., Dumoutier L., Haak S., Rebollo A., Buch T., Heppner F.L., Renauld J.C., Becher B. 2007. IL-22 is expressed by Th17 cells in an IL-23-dependent fashion, but not required for the development of autoimmune encephalomyelitis.J. Immunol. 179:8098–8104 [DOI] [PubMed] [Google Scholar]
- Wolk K., Kunz S., Witte E., Friedrich M., Asadullah K., Sabat R. 2004. IL-22 increases the innate immunity of tissues.Immunity. 21:241–254 [DOI] [PubMed] [Google Scholar]
- Toy D., Kugler D., Wolfson M., Vanden Bos T., Gurgel J., Derry J., Tocker J., Peschon J. 2006. Cutting edge: interleukin 17 signals through a heteromeric receptor complex.J. Immunol. 177:36–39 [DOI] [PubMed] [Google Scholar]
- Aujla S.J., Chan Y.R., Zheng M., Fei M., Askew D.J., Pociask D.A., Reinhart T.A., McAllister F., Edeal J., Gaus K., et al. 2008. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia.Nat. Med. 14:275–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Valdez P.A., Danilenko D.M., Hu Y., Sa S.M., Gong Q., Abbas A.R., Modrusan Z., Ghilardi N., de Sauvage F.J., Ouyang W. 2008. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens.Nat. Med. 14:282–289 [DOI] [PubMed] [Google Scholar]
- Ishigame H., Kakuta S., Nagai T., Kadoki M., Nambu A., Komiyama Y., Fujikado N., Tanahashi Y., Akitsu A., Kotaki H., et al. 2009. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses.Immunity. 30:108–119 [DOI] [PubMed] [Google Scholar]
- Higgins S.C., Jarnicki A.G., Lavelle E.C., Mills K.H. 2006. TLR4 mediates vaccine-induced protective cellular immunity to Bordetella pertussis: role of IL-17-producing T cells.J. Immunol. 177:7980–7989 [DOI] [PubMed] [Google Scholar]
- Chung D.R., Kasper D.L., Panzo R.J., Chitnis T., Grusby M.J., Sayegh M.H., Tzianabos A.O. 2003. CD4+ T cells mediate abscess formation in intra-abdominal sepsis by an IL-17-dependent mechanism.J. Immunol. 170:1958–1963 [DOI] [PubMed] [Google Scholar]
- Shibata K., Yamada H., Hara H., Kishihara K., Yoshikai Y. 2007. Resident Vdelta1+ gammadelta T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production.J. Immunol. 178:4466–4472 [DOI] [PubMed] [Google Scholar]
- Huang W., Na L., Fidel P.L., Schwarzenberger P. 2004. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice.J. Infect. Dis. 190:624–631 [DOI] [PubMed] [Google Scholar]
- Milner J.D., Brenchley J.M., Laurence A., Freeman A.F., Hill B.J., Elias K.M., Kanno Y., Spalding C., Elloumi H.Z., Paulson M.L., et al. 2008. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome.Nature. 452:773–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C.S., Chew G.Y., Simpson N., Priyadarshi A., Wong M., Grimbacher B., Fulcher D.A., Tangye S.G., Cook M.C. 2008. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3.J. Exp. Med. 205:1551–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Beaucoudrey L., Puel A., Filipe-Santos O., Cobat A., Ghandil P., Chrabieh M., Feinberg J., von Bernuth H., Samarina A., Janniere L., et al. 2008. Mutations in STAT3 and IL12RB1 impair the development of human IL-17–producing T cells.J. Exp. Med. 205:1543–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner E.D., Rylaarsdam S., Anover-Sombke S., Rack A.L., Reichenbach J., Carey J.C., Zhu Q., Jansson A.F., Barboza J., Schimke L.F., et al. 2008. Novel signal transducer and activator of transcription 3 (STAT3) mutations, reduced T(H)17 cell numbers, and variably defective STAT3 phosphorylation in hyper-IgE syndrome.J. Allergy Clin. Immunol. 122:181–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minegishi Y., Saito M., Tsuchiya S., Tsuge I., Takada H., Hara T., Kawamura N., Ariga T., Pasic S., Stojkovic O., et al. 2007. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome.Nature. 448:1058–1062 [DOI] [PubMed] [Google Scholar]
- Holland S.M., DeLeo F.R., Elloumi H.Z., Hsu A.P., Uzel G., Brodsky N., Freeman A.F., Demidowich A., Davis J., Turner M.L., et al. 2007. STAT3 mutations in the hyper-IgE syndrome.N. Engl. J. Med. 357:1608–1619 [DOI] [PubMed] [Google Scholar]
- Grimbacher B., Holland S.M., Puck J.M. 2005. Hyper-IgE syndromes.Immunol. Rev. 203:244–250 [DOI] [PubMed] [Google Scholar]
- Minegishi Y., Karasuyama H. 2007. Hyperimmunoglobulin E syndrome and tyrosine kinase 2 deficiency.Curr. Opin. Allergy Clin. Immunol. 7:506–509 [DOI] [PubMed] [Google Scholar]
- Minegishi Y., Karasuyama H. 2008. Genetic origins of hyper-IgE syndrome.Curr. Allergy Asthma Rep. 8:386–391 [DOI] [PubMed] [Google Scholar]
- Minegishi Y., Karasuyama H. 2009. Defects in Jak-STAT-mediated cytokine signals cause hyper-IgE syndrome: lessons from a primary immunodeficiency.Int. Immunol. 21:105–112 [DOI] [PubMed] [Google Scholar]
- Winkelstein J.A., Marino M.C., Johnston R.B., Jr., Boyle J., Curnutte J., Gallin J.I., Malech H.L., Holland S.M., Ochs H., Quie P., et al. 2000. Chronic granulomatous disease. Report on a national registry of 368 patients.Medicine (Baltimore). 79:155–169 [DOI] [PubMed] [Google Scholar]
- Sumikawa Y., Asada H., Hoshino K., Azukizawa H., Katayama I., Akira S., Itami S. 2006. Induction of beta-defensin 3 in keratinocytes stimulated by bacterial lipopeptides through toll-like receptor 2.Microbes Infect. 8:1513–1521 [DOI] [PubMed] [Google Scholar]
- Pivarcsi A., Bodai L., Rethi B., Kenderessy-Szabo A., Koreck A., Szell M., Beer Z., Bata-Csorgoo Z., Magocsi M., Rajnavolgyi E., et al. 2003. Expression and function of Toll-like receptors 2 and 4 in human keratinocytes.Int. Immunol. 15:721–730 [DOI] [PubMed] [Google Scholar]
- Happel K.I., Dubin P.J., Zheng M., Ghilardi N., Lockhart C., Quinton L.J., Odden A.R., Shellito J.E., Bagby G.J., Nelson S., Kolls J.K. 2005. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae.J. Exp. Med. 202:761–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W., Huang W., Zhong Q., Schwarzenberger P. 2006. IL-17 receptor knockout mice have enhanced myelotoxicity and impaired hemopoietic recovery following gamma irradiation.J. Immunol. 176:6186–6193 [DOI] [PubMed] [Google Scholar]
- Yoshimura A., Lien E., Ingalls R.R., Tuomanen E., Dziarski R., Golenbock D. 1999. Cutting edge: recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2.J. Immunol. 163:1–5 [PubMed] [Google Scholar]
- Lien E., Sellati T.J., Yoshimura A., Flo T.H., Rawadi G., Finberg R.W., Carroll J.D., Espevik T., Ingalls R.R., Radolf J.D., Golenbock D.T. 1999. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products.J. Biol. Chem. 274:33419–33425 [DOI] [PubMed] [Google Scholar]
- Schwandner R., Dziarski R., Wesche H., Rothe M., Kirschning C.J. 1999. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2.J. Biol. Chem. 274:17406–17409 [DOI] [PubMed] [Google Scholar]
- Takeuchi O., Hoshino K., Kawai T., Sanjo H., Takada H., Ogawa T., Takeda K., Akira S. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components.Immunity. 11:443–451 [DOI] [PubMed] [Google Scholar]
- Takeuchi O., Hoshino K., Akira S. 2000. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection.J. Immunol. 165:5392–5396 [DOI] [PubMed] [Google Scholar]
- Ku C.L., von Bernuth H., Picard C., Zhang S.Y., Chang H.H., Yang K., Chrabieh M., Issekutz A.C., Cunningham C.K., Gallin J., et al. 2007. Selective predisposition to bacterial infections in IRAK-4–deficient children: IRAK-4–dependent TLRs are otherwise redundant in protective immunity.J. Exp. Med. 204:2407–2422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawn T.R., Ozinsky A., Williams L.M., Rodrigues S., Clark A., Pham U., Hill H.R., Ochs H., Aderem A., Liles W.C. 2005. Hyper-IgE syndrome is not associated with defects in several candidate toll-like receptor pathway genes.Hum. Immunol. 66:842–847 [DOI] [PubMed] [Google Scholar]
- Renner E.D., Pawlita I., Hoffmann F., Hornung V., Hartl D., Albert M., Jansson A., Endres S., Hartmann G., Belohradsky B.H., Rothenfusser S. 2005. No indication for a defect in toll-like receptor signaling in patients with hyper-IgE syndrome.J. Clin. Immunol. 25:321–328 [DOI] [PubMed] [Google Scholar]
- Yu H., Kortylewski M., Pardoll D. 2007. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment.Nat. Rev. Immunol. 7:41–51 [DOI] [PubMed] [Google Scholar]