Abstract
Central tolerance is shaped by the array of self-antigens expressed and presented by various types of thymic antigen-presenting cells (APCs). Depending on the overall signal quality and/or quantity delivered in these interactions, self-reactive thymocytes either apoptose or commit to the T regulatory cell lineage. The cellular and molecular complexity underlying these events has only recently been appreciated. We analyzed the ex vivo presentation of ubiquitous or tissue-restricted self-antigens by medullary thymic epithelial cells (mTECs) and thymic dendritic cells (DCs), the two major APC types present in the medulla. We found that the ubiquitously expressed nuclear neo–self-antigen ovalbumin (OVA) was efficiently presented via major histocompatibility complex class II by mTECs and thymic DCs. However, presentation by DCs was highly dependent on antigen expression by TECs, and hemopoietic cells did not substitute for this antigen source. Accordingly, efficient deletion of OVA-specific T cells correlated with OVA expression by TECs. Notably, OVA was only presented by thymic but not peripheral DCs. We further demonstrate that thymic DCs are constitutively provided in situ with cytosolic as well as membrane-bound mTEC-derived proteins. The subset of DCs displaying transferred proteins was enriched in activated DCs, with these cells being most efficient in presenting TEC-derived antigens. These data provide evidence for a unique, constitutive, and unidirectional transfer of self-antigens within the thymic microenvironment, thus broadening the cellular base for tolerance induction toward promiscuously expressed tissue antigens.
The scope of central tolerance is determined by the recognition of self-antigens presented by various APCs in the thymus. The thymic medulla is the major but not exclusive site of central tolerance induction (McCaughtry et al., 2008). The complexity of this process with regard to the composition and role of distinct APC types, and the sets of self-antigens displayed has only recently become apparent. Self-antigens can be supplied by the proteome of residential epithelial cells (i.e., cortical and medullary thymic epithelial cells [mTECs]) and BM-derived APCs (i.e., DCs, macrophages [MΦ], and B cells). In addition, self-antigens from the periphery can reach the thymus medulla via the blood stream or be imported via immigrating antigen-laden DCs (Klein et al., 2001; Bonasio et al., 2006; Li et al., 2009). Among these different self-antigen pools, the array of promiscuously expressed tissue-restricted self-antigens by mTECs is of particular interest, because it preempts the peripheral self. Qualitative or quantitative alterations of this antigen pool predispose to organ-specific autoimmunity. How central tolerance to these antigens is achieved is closely connected to the questions of which APCs present these antigens and which processing pathways are involved (Klein and Kyewski, 2000).
Previous studies addressing these issues have been limited to broadly expressed self-antigens (i.e., Eα; Humblet et al., 1994), or neo–self-antigens driven by promoters resulting in expression in a major subset of mTECs (i.e., the Aire promoter; Aschenbrenner et al., 2007) or in more restricted expression, e.g., the rat insulin promoter (Zhang et al., 2003; Gallegos and Bevan, 2004; Zehn and Bevan, 2006). In case of the latter transgenes, it remained however unclear whether the frequency of antigen-positive mTECs and the expression levels per cell faithfully reflected the pattern of promiscuously expressed tissue-restricted antigens (TRAs). Both parameters are critical for T cell (TC) selection.
In this report, we analyzed ex vivo presentation of the two native self-antigens, P1A and proteolipid protein (PLP). The tumor rejection antigen P1A is a prototypic cytosolic TRA whose expression is tightly regulated and only detectable in male germ cells and rare mTECs (Derbinski et al., 2001). PLP, an oligodendrocyte-specific myelin protein, is strongly expressed in mTECs and weakly expressed in thymic DCs (Derbinski et al., 2001). Moreover, we wanted to delineate the relative contribution of thymic APC types to tolerance when antigen expression is not confined to a particular cell type. To this end, we chose the neo–self-antigen OVA, expressed ubiquitously and targeted specifically to the nucleus (Kawahata et al., 2002).
We found both thymic DCs and mTECs to present these self-antigens ex vivo and in vivo. Efficient presentation by DCs, however, was contingent on antigen provision by TECs irrespective of their subcellular localization or expression pattern. This efficient self-antigen transfer, which apparently is unique to the thymus, broadens the cellular base of self-tolerance and thus enhances its efficacy.
RESULTS AND DISCUSSION
Ex vivo presentation of an ubiquitous, nuclear self-antigen by thymic APCs
We chose to analyze presentation of the neo–self-antigen OVA driven by the ubiquitous MHC class I (Ld) promotor. In contrast to previous studies that mainly focused on soluble (Klein et al., 2001) or membrane-bound neo–self-antigens (Zhang et al., 2003; Gallegos and Bevan, 2004; Zehn and Bevan, 2006; Aschenbrenner et al., 2007), in this case a nuclear localization sequence targets OVA protein to the nucleus, thus representing self-antigens of the nuclear compartment (Kawahata et al., 2002).
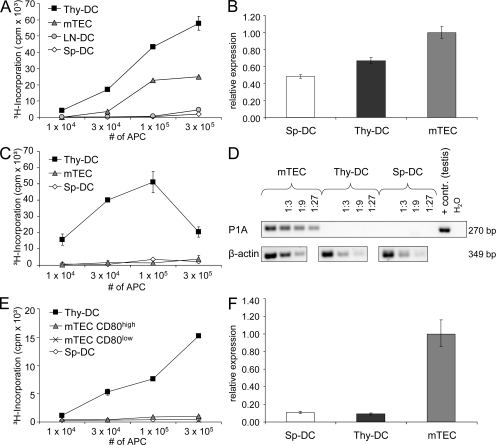
Ex vivo OVA presentation was assayed by co-culture of purified APCs from the thymus, spleen, and mesenteric lymph nodes of Ld-nOVA mice together with naive DO11.10 CD4+ TCs expressing a TCR specific for the OVA peptide 323–339 in conjunction with I-Ad (Murphy et al., 1990; Kawahata et al., 2002). Thymic DCs displayed the most efficient presentation followed by mTECs, whereas splenic and lymph node–derived DCs induced a 10- to 40-fold lower proliferation (Fig. 1 A). At the same time, all four APC populations showed comparable antigen presentation on a per cell basis when loaded with exogenous peptide, excluding overall presentation competence to account for the observed differences (Fig. S1 A). Moreover, thymic and splenic DCs displayed similar OVA expression at the RNA level (Fig. 1 B). One reason for the remarkable difference in efficacy of OVA presentation between thymic and peripheral DCs could reside in their respective composition. 60–90% of thymic DCs belong to the CD8ααpos subset, which was shown to be most efficient in cross-presentation, whereas among splenic DCs, only 10–20% express CD8αα (Vremec et al., 1992; den Haan et al., 2000). Hence, we separately assessed CD8ααpos and CD8ααneg DCs from the thymus and spleen. Enrichment of neither subset rescued splenic OVA presentation, arguing against variable DC subset composition to account for the observed interorgan differences (Fig. S2).
Figure 1.
Efficient presentation of TEC-derived self-antigens by thymic DCs. mTECs and DCs from the thymus (Thy-DC), spleen (Sp-DC), and lymph nodes (LN-DC) of (A) Ld-nOVA, (C) BALB/c, or (E) C57BL/6 mice, respectively, were co-cultured for 3 d with antigen-specific TCs. Presentation of OVA (A), P1A (C), and PLP (E) was assessed by antigen-specific proliferation of naive DO11.10 CD4+ TCs, P1A-TCR transgenic CD8+ TCs, or the PLP-specific TC line TPLP-15-5-2. Shown are the triplicate mean values ± SD of one representative of at least two experiments performed. Transcription of (B) OVA, (D) P1A, and (F) DM20 by the indicated cell populations was evaluated by quantitative (B and F) or semiquantitative (D) PCR analysis. Expression levels were normalized to HPRT/Ubiquitin (B) or β-actin (D and F). Error bars in B and F indicate the SD of triplicates of the same cDNA preparation.
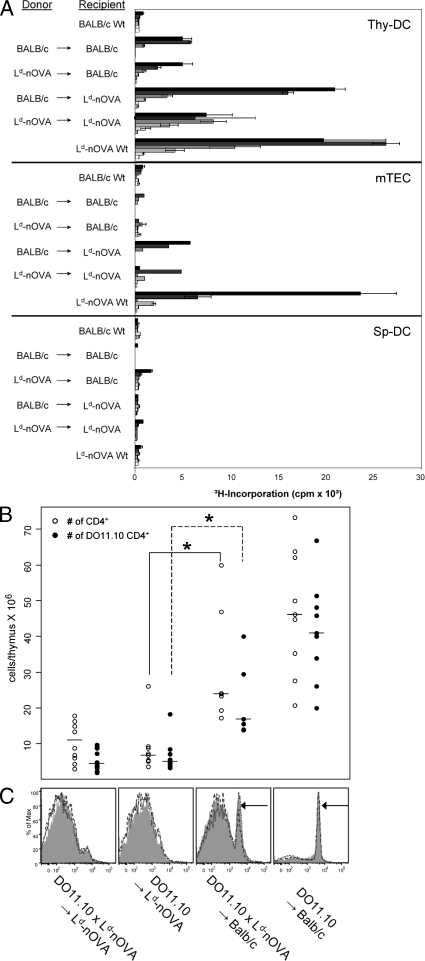
These results suggest that the efficient presentation of the ubiquitously expressed self-antigen OVA by thymic DCs either reflects a property of a specialized thymic DC subset (if presented autonomously), or that antigen is provided specifically within the medullary microenvironment, i.e., by transfer from hemopoietic cells or TECs. To distinguish between these alternatives, we generated BM chimeras in which either BM-derived APCs or TECs expressed OVA exclusively. Remarkably, efficient OVA presentation by thymic DCs was observed only when OVA was expressed by radio-resistant host cells. In contrast, OVA presentation by thymic DCs was inefficient when DCs themselves (and other hemopoietic cells), but not radioresistant stromal cells, were transgenic for Ld-nOVA (Fig. 2 A). At the same time, OVA presentation by mTECs was strictly dependent on endogenous expression (Fig. 2 A). However, antigen presentation was lower in mTECs isolated from chimeras as compared with WT, possibly as a consequence of radiation damage. Conversely, DCs isolated from BALB/c → BALB/c chimeras induced higher TC activation than those from nonmanipulated mice. A possible explanation, not further explored in this report, is the generation of cross-reactive epitopes by radiation, which is not without precedent (Reits et al., 2006). Presentation by splenic DCs remained low, irrespective of the type of chimera (Fig. 2 A). These data show that nuclear OVA is autonomously presented to CD4+ TCs by mTECs, whereas its presentation by thymic DCs mostly relies on antigen transfer from radio-resistant TECs, but not from hemopoietic cells. A similar efficient transfer mechanism obviously does not exist in the microenvironment of the spleen and lymph nodes, at least at the level of resolution attained here. The differences in ex vivo presentation of OVA were also reflected at the level of TC selection. Deletion of OVA-specific DO11.10 TCs was most efficient when OVA was expressed by radio-resistant TECs irrespective of additional OVA expression in BM-derived cells (Fig. 2, B and C). The lower but perceptible degree of deletion by OVA-expressing BM cells indicates that OVA does access MHC class II on DCs, possibly via the secretory pathway followed by endocytosis, a route that has not been strictly excluded by the study of Kawahata et al. (2002).
Figure 2.
Ex vivo presentation of the nuclear neo–self-antigen OVA by thymic DCs and efficient deletion of DO11.10 TCs in vivo depend on antigen transfer from radio-resistant stromal cells. (A) Lethally irradiated BALB/c WT or Ld-nOVA transgenic mice were reconstituted with TC-depleted BM cells of the indicated donor strains. After 5–8 wk, CD11c+ DCs of the thymus (Thy-DC) and spleen (Sp-DC) and mTECs, respectively, were isolated and co-cultured with DO11.10 CD4+ TCs. OVA presentation was assessed by antigen-specific proliferation of TCs. Bars represent threefold titration steps of the indicated APC population ranging from 3 × 105–103 cells/well. Shown are the triplicate mean values ± SD of one representative of two or more experiments performed. (B) 5–6 wk after reconstitution, the number of total CD4 single-positive and DO11.10 clonotype-specific CD4 single-positive thymocytes was assessed. Symbols represent values of 7–10 individual thymi in each group. *, P < 0.005. (C) FACS analysis of clonotypic DO11.10 CD4 single-positive spleen TCs of each chimera type. Shown are overlay histograms of three individuals. Arrows indicate DO11.10 clonotype-specific CD4+ TCs.
Ex vivo presentation of ectopically expressed endogenous TRAs by thymic APCs
To verify whether presentation of promiscuously expressed endogenous antigens follows the same rules as deduced from transgenic models (Zhang et al., 2003; Gallegos and Bevan, 2004; Zehn and Bevan, 2006), we assessed the ex vivo presentation of the TRAs P1A and PLP via MHC class I and II, respectively.
The tumor antigen P1A is only expressed in mTECs but not in thymic or splenic DCs (Fig. 1 D), whereby 1–3% of mTECs express P1A at the protein level (Derbinski et al., 2001). Ex vivo presentation of P1A was assayed by proliferation of freshly isolated naive CD8+ TCs expressing a transgenic TCR specific for the P1A epitope 35–43. Again, thymic DCs proved to be most efficient (Fig. 1 C), pointing toward cross-presentation of mTEC-derived P1A, whereas direct presentation by mTECs was clearly weaker and only revealed by isolating the mature mTEC subset (Fig. S3). No significant proliferation was induced by splenic DCs. Again, this pattern could not be explained by differences in the presentation competence of the various APC populations (Fig. S1 B).
A previous report on in vivo deletion of autoreactive OVA-specific CD8+ and CD4+ TCs showed differences in the requirement for cross-presentation (Gallegos and Bevan, 2004). Although both mTECs and DCs were able to delete MHC class I–restricted OT-1 cells, deletion of MHC class II–restricted OT-2 cells required OVA cross-presentation by thymic DCs in the RIP-mOVA mouse model. In contrast, TECs were sufficient to delete CD4+ TCs specific for soluble human C-reactive protein (Klein et al., 1998, 2001), and mTECs were capable of deleting CD4+ TCs specific for membrane-bound hemagglutinin (Aschenbrenner et al., 2007).
We chose PLP, a myelin sheath component, to assess MHC class II–restricted ex vivo presentation. PLP is promiscuously expressed by both cortical thymic epithelial cells and mTECs and at marginal levels in DCs (Fig. 1 F), with 1–3% of all mTECs expressing the PLP protein (Derbinski et al., 2001). Presentation of PLP was assayed using a CD4+ TC line specific for the epitope 239–250 in the context of I-Ab. Importantly, this epitope is present in the DM20 isoform of PLP, which is predominantly expressed in the thymus (Klein et al., 2000; Derbinski et al., 2001). In line with previous results, thymic DCs efficiently presented PLP (Fig. 1 E). In contrast, neither mTECs, including the mature subset, nor splenic DCs induced proliferation above background levels. The lack of presentation by mature mTECs was unexpected given the strong mRNA signal and detectable protein expression (Derbinski et al., 2001). Possible explanations are a lower PLP protein expression and stability and/or inefficient processing by mTECs when compared with P1A. Irrespective of these considerations, PLP expression and presentation by mTECs and DCs are inversely correlated, which is indicative of antigen transfer from TECs to DCs rather than autonomous presentation by DCs. This interpretation is indirectly supported by the lack of PLP presentation by splenic DCs.
Thus, presentation of these two TRAs, P1A and PLP, follows similar rules as shown for Ld-nOVA. Which features of the medullary microenvironment might account for the efficient transfer of self-antigens?
Transfer routes from mTECs to DCs
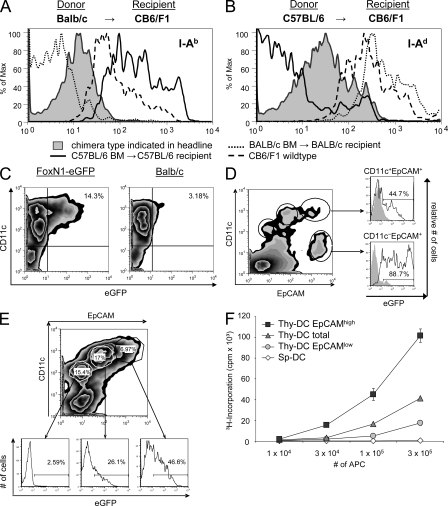
At least four mechanisms might contribute to the intercellular transfer of proteins of different subcellular compartments: exosome transfer, uptake of apoptotic bodies, membrane exchange (“nibbling”), and gap junctions (Albert et al., 1998; Harshyne et al., 2001; Thery et al., 2002; Neijssen et al., 2005). The most direct way to confer MHC-restricted antigen presentation onto another cell is the exchange of surface membrane–bound peptide–MHC complexes. We addressed this option by analyzing the transfer of MHC class II molecules in P → F1 type chimeras, i.e., BALB/c → (BALB/c × C57BL/6)F1 and C57BL/6 → (BALB/c × C57BL/6)F1 mice. 6–8 wk after reconstitution, thymic DCs were isolated and analyzed for expression of host-specific MHC haplotypes. Clearly, DCs stained for MHC class II molecules transcribed by radio-resistant host cells, with the density of expression of these “acquired” MHC molecules being lower than on DCs of heterozygous animals (Fig. 3, A and B). These results concur with a recent report describing a unidirectional transport of MHC class II molecules between thymic APCs in vitro and in vivo (Millet et al., 2008).
Figure 3.
Cotransfer of membrane-bound and cytoplasmic proteins from epithelial cells marks DCs with efficient cross-presentation. (A and B) Lethally irradiated CB6/F1 mice were reconstituted with either BALB/c (A) or C57BL/6 (B) TC-depleted BM. After 6 wk, low density TSCs were enriched by Nycodenz density gradient centrifugation. Cells were stained with mAb against CD11c, EpCAM, and the parental MHC class II haplotypes I-Ab and I-Ad. Thymic DCs were defined as CD11chigh EpCAMintermediate cells. Expression of the respective host-specific MHC haplotype by thymic DCs is displayed as shaded histograms, whereas lines represent expression of the indicated haplotypes by C57BL/6 (solid), CB6/F1 (dashed), or BALB/c (dotted) thymic DCs. (C) EGFP-containing cells are detectable within the CD11c+ thymic DC population of FoxN1-eGFP mice. Thymic CD11c+ cells were preenriched using MACS technology and analyzed by flow cytometry, excluding autofluorescent cells. (D) EGFP-expressing TECs are distinguishable from eGFP-containing thymic DCs on the basis of differential CD11c expression, as shown by flow-cytometric analysis of single-cell suspensions. Cells were isolated and pooled from five FoxN1-eGFP thymi. (E) EpCAM expression of FoxN1-eGFP CD11c+ thymic DCs correlates with eGFP fluorescence intensity, as shown for CD45−, nonautofluorescent cells. (F) EpCAM-expressing CD11c+ thymic DCs (Thy-DC) present TEC-derived OVA most efficiently. CD11c+ thymic DCs of Ld-nOVA mice were enriched according to differential CD11c and EpCAM coexpression, resulting in the indicated subpopulations of ≥99% purity. CD11c+ splenic DCs (Sp-DC) were enriched by magnetic separation (purity ≥ 90%). OVA presentation was assessed by proliferation of OVA-specific DO11.10 CD4+ TCs after a 3-d co-culture period. Shown are the means of triplicates ± SD. Each figure (C–F) is representative of two to six independent experiments.
We next explored a second route, namely the transfer of cytoplasmic proteins, that either could occur via uptake of apoptotic bodies or directed exosome traffic. To this end, we analyzed thymi of FoxN1-eGFP reporter mice. These mice express eGFP intrathymically exclusively in TECs under the control of the epithelium-specific FoxN1 promoter, but not in hemopoietic cells (Terszowski et al., 2006). Thymic DCs from this strain displayed a green fluorescence signal clearly above background level but weaker than that of TECs (Fig. 3, C and D). Interestingly, the degree of eGFP staining of thymic DCs correlated with surface staining for epithelial cell adhesion molecule (EpCAM), which presumably, like MHC class II molecules, was acquired from TECs (Fig. 3, D and E). Expression of EpCAM on hemopoietic cells had previously been observed but not further explored (Borkowski et al., 1996). The costaining of eGFP and EpCAM by a subset of DCs implies that the same cells acquire TEC-derived membrane receptors and cytoplasmic proteins.
Phenotype and function of “TEC-tainted” DCs
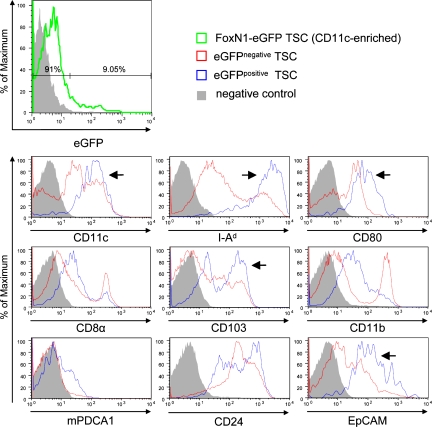
Given the correlation between EpCAM and eGFP expression, we used these surrogate markers to further characterize this DC subset with respect to phenotype and function. Assessing a panel of markers, eGFP/EpCAM-positive DCs were enriched in cells expressing CD80, CD86, CD103, and MHC class II, indicative of a mature phenotype (Fig. 4 and Fig. S4). At the same time, this subset was devoid of CD11b- and F4/80-positive cells, i.e., monocytes/MΦ. Most markers showed a monophasic distribution with the exception of CD103, CD24, and mPDCA1, indicating further heterogeneity among this DC subset.
Figure 4.
EGFP-containing thymic DCs of FoxN1-eGFP mice display a mature phenotype. TSCs of FoxN1-eGFP mice were preenriched for CD11c+ cells using MACS technology (purity ≥ 60%). Subsequently, positively selected cells were stained for CD11c and EpCAM (Fig. 3 B) together with one additional marker and analyzed by flow cytometry. Shaded histograms indicate negative controls representing isotype control or secondary antibody stainings. Expression of indicated marker molecules by eGFP− TSCs is displayed in red and expression by eGFP+ cells is displayed in blue. Arrows indicate marker profiles correlating with eGFP content. For details see Materials and methods. Each histogram is representative of one to three independent experiments.
Next we asked whether TEC-tainted DCs were also enriched in DCs cross-presenting TEC-derived self-antigens, e.g., Ld-nOVA. When separated on the basis of quantitative expression of EpCAM, those DCs displaying the highest level showed the most efficient ex vivo presentation of OVA, whereas EpCAMlow expressors were relatively depleted for cross-presentation (Fig. 3 F). These data reveal a novel phenotype/function correlation: the subset of thymic DCs that most efficiently cross-presents TEC-derived TRA displays a mature phenotype and tracers indicative of recent direct interactions with TECs.
Our data document constitutive cross-presentation of (m)TEC-derived nuclear, cytosolic, and membrane-bound self-antigens by thymic DCs along with autonomous presentation by mTECs. Of particular interest are three observations. First, only TEC- but not hemopoietic cell–derived (nuclear) antigens are efficiently cross- presented by thymic DCs. Second, this antigen transfer is unidirectional (Millet et al., 2008). Third, the neo–self-antigen OVA, which is expressed in the nucleus under an ubiquitous promoter, is autonomously presented via MHC class II only by mTECs but not hemopoietic APCs of the thymus or the periphery, a finding consistent with a previous study (Oukka et al., 1996). We consider this autonomous presentation of OVA by mTECs to be most likely caused by constitutive macroautophagy in TECs (Nedjic et al., 2008), which, in addition to cytosolic proteins, also includes nuclear self-antigens (Riedel et al., 2008). Indeed, peptides derived from nuclear proteins can be eluted from MHC class II of mTECs (unpublished data). Several explanations could account for the fact that OVA-expressing hemopoietic cells (including TCs, DCs, MΦ, and B cells) are poor in providing antigenic material for cross-presentation, depending on the mechanism of self-antigen transfer. If processed antigens were transferred, as recently shown in vitro (Millet et al., 2008), hemopoietic cells might lack the ability to generate the specific peptide–MHC class II complex to be transferred onto DCs (i.e., they lack constitutive autophagy). If antigen were transferred in its native form and processing occurred in the target cell, generation of transfer vehicles by hemopoietic cells might be less efficient than by TECs. Irrespective of these considerations, the observed antigen transfer could occur via membrane transfer of peptide–MHC complexes, exosomes, or apoptotic bodies. At present we cannot distinguish between these different modes. The fact that DCs expressing the highest amount of EpCAM also show the strongest GFP staining speaks in favor of cotransfer of cytosolic and membrane constituents i.e., via directed exosome trafficking or apoptotic bodies. However, we consider uptake of apoptotic bodies as the main mechanism less likely, because negative selection of autoreactive TCs directly interacting with mTECs (Irla et al., 2008) should provide plenty of antigenic material, yet such a transfer is not observed. Moreover, the extensive transfer of membrane complexes between mTECs and DCs would comply with the similar in vivo half-life of peptide–MHC complexes on both cell types, which is in the range of 25 h (unpublished data).
Our results imply that the self-antigen repertoire specifically generated by mTECs is also available to DCs for presentation. This concurrent presentation ensures that any particular tolerance mode linked to a special APC type will cover the array of promiscuously expressed genes. Thus, mTECs have been shown to be more efficient in selecting T reg cells, whereas DCs are the better deleters (Apostolou et al., 2002; Aschenbrenner et al., 2007). This preference, however, is relative rather than absolute, because bone marrow-derived APCs can also generate T reg cells under contrived experimental conditions (Watanabe et al., 2005; Spence and Green, 2008; Wirnsberger et al. 2009). In this context it is worth noting that DCs interacting with mTECs display a phenotype similar to intestinal DCs, which induce T reg cells (Coombes et al., 2007). Furthermore, TEC-interacting DCs are still phenotypically heterogeneous and include plasmacytoid- and myeloid-type DCs, both of which have recently been proposed to induce different subsets of T reg cells (Ito et al., 2008).
Our data characterize a constitutive and unidirectional transfer route for self-antigens between mTECs and DCs, which is possibly unique to the microenvironment of the thymic medulla. By this way, the set of tissue-restricted self-antigens exclusively expressed by mTECs also becomes available to the second major APC type involved in TC repertoire selection, thus ensuring that this critical set of self-antigens is “double checked” for tolerance induction.
MATERIALS AND METHODS
Animals
C57BL/6, BALB/c, and CB6/F1 mice were obtained from Charles River Laboratories. Ld-nOVA mice (Kawahata et al., 2002) on the BALB/c background were provided by K. Yamamoto (University of Tokyo, Tokyo, Japan) and were further interbred. FoxN1-eGFP mice (Terszowski et al., 2006) were provided by T. Boehm (Max-Planck-Institute for Immunobiology, Freiburg, Germany) and backcrossed for at least five generations on the BALB/c background. DO11.10 TCR transgenic mice have been described previously (Murphy et al., 1990) and were kept as an inbred strain. TCRP1A transgenic mice were provided by A.-M. Schmitt-Verhulst (University of Marseille, Marseille, France), and were generated on a RagO/O B10.D2 background, as described previously (Shanker et al., 2004), and kept as an inbred strain.
All animals were kept under specific pathogen-free conditions in the animal facilities of the German Cancer Research Center. For preparation of APCs, mice of both genders were used at the age of 4–12 wk. TCR transgenic TCs were isolated from 12–16-wk-old mice. Animal experiments were approved by local authorities (Regierungspräsidium Karlsruhe, permit nos. DKFZ 155, DKFZ 169, DKFZ 196, and G-66/02).
Cell preparation and analysis
Isolation of mouse thymic stromal cells (TSCs).
TSCs were purified as described previously (Derbinski et al., 2001) or with the following modifications: after three rounds of collagenase digestion and one round of collagenase/dispase digestion, thymic rosettes were pooled, washed in PBS containing 0.5% BSA and 5 µM EDTA, and stained with anti-CD11c microbeads (Miltenyi Biotec). Subsequently, CD11c+ thymic DCs were isolated using MACS technology according to the manufacturer's protocol (Miltenyi Biotec), resulting in a suspension containing 90–95% CD11c+ cells. For subdivision of thymic DCs, CD11c-enriched TSCs were stained with various combinations of biotinylated anti-CD11c (HL3) and streptavidin-PE or streptavidin-allophycocyanin-Cy7 (BD), anti–EpCAM–Alexa Fluor 647 (G8.8), anti-CD45–PE or CD45-PerCP, respectively (both 30-F11; BD), and anti-CD8α–PE (53-6.7; BD). The TEC-containing fraction (collagenase/dispase digestion rounds two to five) was further enriched by MACS depletion of CD45+ hemopoietic cells using anti-CD45 microbeads (Miltenyi Biotec), followed by staining with either anti-CDR1–Alexa Fluor 488 or anti-Ly51–FITC (6C3; BD), anti–EpCAM–Alexa Fluor 647 (G8.8), and anti-CD45–PE (30-F11). After staining, cells were resuspended in FACS buffer containing 1 µg/ml propidium iodide to exclude dead cells. For subdivision of mTECs, the following combinations were used: anti-CD80–PE (16-10A1) or anti-KLH–PE (Ha 4/8; isotype control), anti-CD45–PerCP (30-F11), anti-Ly51–FITC (6C3; all were obtained from BD), and anti–EpCAM–Alexa Fluor 647 (G8.8). FcR blocking with the anti-FcR mAb 2.4G2 preceded all stainings. Cell sorting was performed with a cell sorter (FACSDiVa; BD). Autofluorescent cells were excluded from the sorted populations.
For phenotype analysis of DC subpopulations, in addition to the aforementioned reagents the following antibodies were used: anti-CD86–PE (GL1; BD), anti–MHC class II–PE I-Ad (AMS-32.1; BD), anti-CD24–PE I-Ad (M1/69; BD), anti-F4/80–FITC (CI:A3-1; AbD Serotec), and anti-mPDCA1–PE (JF05-1C2.4.1; Miltenyi Biotec).
The frequency of clonotype-specific TCs was assessed by costaining with anti-CD4–FITC (H129.19; BD), anti-CD8α–PE (53-6.7; BD), and biotinylated anti-DO11.10 clonotype (KJ1-26; eBioscience) antibodies, followed by streptavidin-allophycocyanin-Cy7 (BD).
Flow cytometric measurements were performed using a FACSCalibur or FACSCanto II (BD). Data were collected from viable lymphocytes by appropriate forward and side scatter gating. Data analysis was performed with FlowJo software (version 6.4.1; Tree Star, Inc.).
Isolation of lymphoid cells from the spleen.
Myeloid and lymphoid DCs from the spleen were isolated as previously described (den Haan et al., 2000), with minor modifications. In brief, a collagenase-digested suspension of four to eight spleens was subjected to erythrocyte lysis and filtered through a 70-µm cell strainer. Cells were collected in PBS containing 0.5% BSA and 5 µM EDTA and stained with anti-CD11c microbeads (Miltenyi Biotec). Subsequently, CD11c+ DCs were isolated using MACS technology according to the manufacturer's protocol (Miltenyi Biotec), resulting in a suspension containing 90–95% CD11c+ cells.
For isolation of TCs, spleens of one to two mice were minced into small pieces and passed through a metal sieve. After erythrocyte lysis, the cell suspension was washed in PBS containing 0.5% BSA and 5 µM EDTA and filtered through a 70-µm cell strainer. For enrichment of CD4+ TCs, cells were incubated with anti-CD4 (L3T4) microbeads, followed by magnetic separation of CD4+ cells according to manufacturer's protocol (Miltenyi Biotec). CD8+ TCs were isolated by magnetic depletion of CD8− cell populations using the CD8α+ T cell isolation kit (Miltenyi Biotec). For both TC-populations, purity was ≥95%, as confirmed by flow cytrometrical analysis.
RNA preparation and cDNA synthesis
Whole-tissue RNA was isolated using DNaseI digestion on column with an Ultra-Turrax T8 (IKA) and the RNeasy Mini Kit (QIAGEN), or from a single-cell suspension with the High Pure RNA Isolation Kit (Roche). Total RNA (3 µg of tissue-extracted RNA or an equivalent of 105–106 single cells) was reverse transcribed into cDNA with Oligo(dT)20 primer and Superscript II RT (Invitrogen), followed by RNase H digestion (Fermentas).
PCR analysis
PCRs were performed as previously described (Derbinski et al., 2001). Primer pairs were synthesized by the oligonucleotide synthesis facility of the German Cancer Research Center and, when possible, were designed to span at least one intron.
Amplification was performed in a DNA Engine Dyad ThermoCycler (Biozym) under standard thermal cycler conditions. Reaction products were separated on 1 × TAE (40 mM Tris-acetate, 1 mM EDTA) 1.5% agarose gels that contained 30 ng/ml ethidium bromide (Roth). PCR products were revealed with the Lumi-Imager F1 Workstation (Roche) or a video documentation system (BioVision; Peqlab), and bands were quantified with LumiAnalyst software (version 3.0; Roche) or Vision-Capture software (version 12.9; Peqlab). For semiquantitative PCR, cDNA preparations were normalized to β-actin expression before testing expression of the gene of interest.
Quantitative PCR
Real-time PCR reactions were performed in a final volume of 25 µl with optimal concentrations of forward and reverse primers (50–900 nM) using the qPCR core kit for SybrGreen I (Eurogentec) containing Hot GoldStar polymerase and Uracil-N-Glycosylase. Probes were used at a concentration of 200 nM in combination with the qPCR core kit. Reactions were run on a sequence detection system (GeneAmp 7300; Applied Biosystems) in triplicates, and expression values were normalized to β-actin or HPRT/ubiquitin expression using the comparative Ct method. Primers were purchased from Eurofins MWG Operon and, whenever possible, were designed to span at least one intron. Probes were purchased from Eurogentec.
Generation of radiation BM chimeras
4–5-wk-old recipients were lethally irradiated (8.5–9.5 Gy) and reconstituted within 24 h with TC-depleted BM (5 × 106 cells/animal). Functional analysis of antigen presentation and phenotype analysis of grafted animals was performed after another 5–8 wk.
Ex vivo proliferation assay
Purified TSCs were co-cultured with naive transgenic OVA-specific (DO11.10; Murphy et al., 1990) or P1A-specific (TCR-P1Atg; Shanker et al., 2004) TCs, or PLP-specific TC lines (TPLP15-5-2; Klein et al., 2000), respectively. TCs were cultured for 72 h in triplicates at a density of 5 × 104 cells/well, with titered numbers of purified APCs in flat-bottom 96-well plates in 200 µl of culture medium in the presence of 1 µg/ml of the respective peptide, as indicated in the figures. The following antigen-specific peptides have been used: OVA323-339, P1A35-43, and PLP239-250 (all synthesized in the Peptide-Synthesis Core Facility of the German Cancer Research Center). Antigen-specific proliferation was measured by incorporation of 3H[TdR], which was added for an additional 16–20 h of culture (1 µCi/well).
Statistics
The Wilcoxon exact sum-rank test was used for statistical analysis. Calculations were performed using SAS software (version 9.1; SAS Institute Inc.).
Online supplemental material
Fig. S1 shows a comparison of the capacity of mTECs and DCs from thymus and peripheral lymphoid organs of different mouse strains to present exogenous peptides. Fig. S2 shows the presentation of endogenous and exogenous OVA by CD8α+ and CD8α− DC subpopulations from thymus and spleen. Fig. S3 shows the presentation of endogenous and exogenous P1A by CD80high and CD80low mTEC subsets in comparison to thymic and splenic DCs. Fig. S4 shows phenotype analysis of thymic DCs based on differential CD11c and EpCAM expression. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20082449/DC1.
Acknowledgments
We are most grateful to K. Hexel for excellent cell sorting, S. Rösch and E. Rezavandy for expert technical assistance, and the staff of our animal facilities for competent animal husbandry. We would like to thank Drs. A.-M. Schmitt-Verhulst and B. van den Eynde for critical comments on the manuscript, and A. Kopp-Schneider for help with the statistics. Drs. K. Yamamoto, A.-M. Schmitt-Verhulst, and T. Boehm generously provided us with different mouse lines.
This work was supported by the German Cancer Research Center and the European Union–funded consortia Thymaide (grant 14362), Immunotherapy (grant 14363), and Tolerage (grant 14364).
The authors declare no conflicting financial interests.
Footnotes
Abbreviations used: EpCAM, epithelial cell adhesion molecule; MΦ, macrophages; mTEC, medullary thymic epithelial cell; PLP, proteolipid protein; TC, T cell; TRA, tissue-restricted antigen; TSC, thymic stromal cell.
References
- Albert M.L., Pearce S.F., Francisco L.M., Sauter B., Roy P., Silverstein R.L., Bhardwaj N. 1998. Immature dendritic cells phagocytose apoptotic cells via αvβ5 and CD36, and cross-present antigens to cytotoxic T lymphocytes.J. Exp. Med. 188:1359–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolou I., Sarukhan A., Klein L., von Boehmer H. 2002. Origin of regulatory T cells with known specificity for antigen.Nat. Immunol. 3:756–763 [DOI] [PubMed] [Google Scholar]
- Aschenbrenner K., D'Cruz L.M., Vollmann E.H., Hinterberger M., Emmerich J., Swee L.K., Rolink A., Klein L. 2007. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells.Nat. Immunol. 8:351–358 [DOI] [PubMed] [Google Scholar]
- Bonasio R., Scimone M.L., Schaerli P., Grabie N., Lichtman A.H., von Andrian U.H. 2006. Clonal deletion of thymocytes by circulating dendritic cells homing to the thymus.Nat. Immunol. 7:1092–1100 [DOI] [PubMed] [Google Scholar]
- Borkowski T.A., Nelson A.J., Farr A.G., Udey M.C. 1996. Expression of gp40, the murine homologue of human epithelial cell adhesion molecule (Ep-CAM), by murine dendritic cells.Eur. J. Immunol. 26:110–114 [DOI] [PubMed] [Google Scholar]
- Coombes J.L., Siddiqui K.R., Arancibia-Carcamo C.V., Hall J., Sun C.M., Belkaid Y., Powrie F. 2007. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β– and retinoic acid–dependent mechanism.J. Exp. Med. 204:1757–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Haan J.M., Lehar S.M., Bevan M.J. 2000. CD8+ but not CD8− dendritic cells cross-prime cytotoxic T cells in vivo.J. Exp. Med. 192:1685–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbinski J., Schulte A., Kyewski B., Klein L. 2001. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self.Nat. Immunol. 2:1032–1039 [DOI] [PubMed] [Google Scholar]
- Gallegos A.M., Bevan M.J. 2004. Central tolerance to tissue-specific antigens mediated by direct and indirect antigen presentation.J. Exp. Med. 200:1039–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harshyne L.A., Watkins S.C., Gambotto A., Barratt-Boyes S.M. 2001. Dendritic cells acquire antigens from live cells for cross-presentation to CTL.J. Immunol. 166:3717–3723 [DOI] [PubMed] [Google Scholar]
- Humblet C., Rudensky A., Kyewski B. 1994. Presentation and intercellular transfer of self antigen within the thymic microenvironment: expression of the E alpha peptide-I-Ab complex by isolated thymic stromal cells.Int. Immunol. 6:1949–1958 [DOI] [PubMed] [Google Scholar]
- Irla M., Hugues S., Gill J., Nitta T., Hikosaka Y., Williams I.R., Hubert F.X., Scott H.S., Takahama Y., Hollander G.A., Reith W. 2008. Autoantigen-specific interactions with CD4+ thymocytes control mature medullary thymic epithelial cell cellularity.Immunity. 29:451–463 [DOI] [PubMed] [Google Scholar]
- Ito T., Hanabuchi S., Wang Y.H., Park W.R., Arima K., Bover L., Qin F.X., Gilliet M., Liu Y.J. 2008. Two functional subsets of FOXP3+ regulatory T cells in human thymus and periphery.Immunity. 28:870–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahata K., Misaki Y., Yamauchi M., Tsunekawa S., Setoguchi K., Miyazaki J., Yamamoto K. 2002. Peripheral tolerance to a nuclear autoantigen: dendritic cells expressing a nuclear autoantigen lead to persistent anergic state of CD4+ autoreactive T cells after proliferation.J. Immunol. 168:1103–1112 [DOI] [PubMed] [Google Scholar]
- Klein L., Kyewski B. 2000. Self-antigen presentation by thymic stromal cells: a subtle division of labor.Curr. Opin. Immunol. 12:179–186 [DOI] [PubMed] [Google Scholar]
- Klein L., Klein T., Ruther U., Kyewski B. 1998. CD4 T cell tolerance to human C-reactive protein, an inducible serum protein, is mediated by medullary thymic epithelium.J. Exp. Med. 188:5–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein L., Klugmann M., Nave K.A., Tuohy V.K., Kyewski B. 2000. Shaping of the autoreactive T-cell repertoire by a splice variant of self protein expressed in thymic epithelial cells.Nat. Med. 6:56–61 [DOI] [PubMed] [Google Scholar]
- Klein L., Roettinger B., Kyewski B. 2001. Sampling of complementing self-antigen pools by thymic stromal cells maximizes the scope of central T cell tolerance.Eur. J. Immunol. 31:2476–2486 [DOI] [PubMed] [Google Scholar]
- Li J., Park J., Foss D., Goldschneider I. 2009. Thymus-homing peripheral dendritic cells constitute two of the three major subsets of dendritic cells in the steady-state thymus.J. Exp. Med. 206:607–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaughtry T.M., Baldwin T.A., Wilken M.S., Hogquist K.A. 2008. Clonal deletion of thymocytes can occur in the cortex with no involvement of the medulla.J. Exp. Med. 205:2575–2584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet V., Naquet P., Guinamard R.R. 2008. Intercellular MHC transfer between thymic epithelial and dendritic cells.Eur. J. Immunol. 38:1257–1263 [DOI] [PubMed] [Google Scholar]
- Murphy K.M., Heimberger A.B., Loh D.Y. 1990. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo.Science. 250:1720–1723 [DOI] [PubMed] [Google Scholar]
- Nedjic J., Aichinger M., Emmerich J., Mizushima N., Klein L. 2008. Autophagy in thymic epithelium shapes the T-cell repertoire and is essential for tolerance.Nature. 455:396–400 [DOI] [PubMed] [Google Scholar]
- Neijssen J., Herberts C., Drijfhout J.W., Reits E., Janssen L., Neefjes J. 2005. Cross-presentation by intercellular peptide transfer through gap junctions.Nature. 434:83–88 [DOI] [PubMed] [Google Scholar]
- Oukka M., Colucci-Guyon E., Tran P.L., Cohen-Tannoudji M., Babinet C., Lotteau V., Kosmatopoulos K. 1996. CD4 T cell tolerance to nuclear proteins induced by medullary thymic epithelium.Immunity. 4:545–553 [DOI] [PubMed] [Google Scholar]
- Reits E.A., Hodge J.W., Herberts C.A., Groothuis T.A., Chakraborty M., Wansley E.K., Camphausen K., Luiten R.M., de Ru A.H., Neijssen J., et al. 2006. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy.J. Exp. Med. 203:1259–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel A., Nimmerjahn F., Burdach S., Behrends U., Bornkamm G.W., Mautner J. 2008. Endogenous presentation of a nuclear antigen on MHC class II by autophagy in the absence of CRM1-mediated nuclear export.Eur. J. Immunol. 38:2090–2095 [DOI] [PubMed] [Google Scholar]
- Shanker A., Auphan-Anezin N., Chomez P., Giraudo L., Van den Eynde B., Schmitt-Verhulst A.M. 2004. Thymocyte-intrinsic genetic factors influence CD8 T cell lineage commitment and affect selection of a tumor-reactive TCR.J. Immunol. 172:5069–5077 [DOI] [PubMed] [Google Scholar]
- Spence P.J., Green E.A. 2008. Foxp3+ regulatory T cells promiscuously accept thymic signals critical for their development.Proc. Natl. Acad. Sci. USA. 105:973–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terszowski G., Muller S.M., Bleul C.C., Blum C., Schirmbeck R., Reimann J., Pasquier L.D., Amagai T., Boehm T., Rodewald H.R. 2006. Evidence for a functional second thymus in mice.Science. 312:284–287 [DOI] [PubMed] [Google Scholar]
- Thery C., Duban L., Segura E., Veron P., Lantz O., Amigorena S. 2002. Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes.Nat. Immunol. 3:1156–1162 [DOI] [PubMed] [Google Scholar]
- Vremec D., Zorbas M., Scollay R., Saunders D.J., Ardavin C.F., Wu L., Shortman K. 1992. The surface phenotype of dendritic cells purified from mouse thymus and spleen: investigation of the CD8 expression by a subpopulation of dendritic cells.J. Exp. Med. 176:47–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N., Wang Y.H., Lee H.K., Ito T., Wang Y.H., Cao W., Liu Y.J. 2005. Hassall's corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus.Nature. 436:1181–1185 [DOI] [PubMed] [Google Scholar]
- Wirnsberger G., Mair F., Klein L. 2009. Regulatory T cell differentiation of thymocytes does not require a dedicated antigen resenting cell but is under T cell intrinsic developmental control.Proc. Natl. Acad. Sci. USA. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehn D., Bevan M.J. 2006. T cells with low avidity for a tissue-restricted antigen routinely evade central and peripheral tolerance and cause autoimmunity.Immunity. 25:261–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Vacchio M.S., Vistica B.P., Lesage S., Egwuagu C.E., Yu C.R., Gelderman M.P., Kennedy M.C., Wawrousek E.F., Gery I. 2003. T cell tolerance to a neo-self antigen expressed by thymic epithelial cells: the soluble form is more effective than the membrane-bound form.J. Immunol. 170:3954–3962 [DOI] [PubMed] [Google Scholar]