Abstract
Although the Th17 subset and its signature cytokine, interleukin (IL)-17A (IL-17), are implicated in certain autoimmune diseases, their role in cancer remains to be further explored. IL-17 has been shown to be elevated in several types of cancer, but how it might contribute to tumor growth is still unclear. We show that growth of B16 melanoma and MB49 bladder carcinoma is reduced in IL-17−/− mice but drastically accelerated in IFN-γ−/− mice, contributed to by elevated intratumoral IL-17, indicating a role of IL-17 in promoting tumor growth. Adoptive transfer studies and analysis of the tumor microenvironment suggest that CD4+ T cells are the predominant source of IL-17. Enhancement of tumor growth by IL-17 involves direct effects on tumor cells and tumor-associated stromal cells, which bear IL-17 receptors. IL-17 induces IL-6 production, which in turn activates oncogenic signal transducer and activator of transcription (Stat) 3, up-regulating prosurvival and proangiogenic genes. The Th17 response can thus promote tumor growth, in part via an IL-6–Stat3 pathway.
A major advance in understanding the role of various T cell subsets in disease has been the recent identification of the Th17 subset, characterized by the production of IL-17A (IL-17), as well as the related IL-17F cytokine (Weaver et al., 2006). Just as with the Th1 and Th2 subsets, whose development and propagation are mediated by specific cytokines, Th17 development is selectively induced by a combination of IL-6 and TGF-β, whereas the IL-12 family member IL-23 supports Th17 propagation (Chen and O’Shea, 2008). The ability of IL-6 to divert TGF-β–stimulated T cells away from regulatory T cell differentiation and toward Th17 cell differentiation, as well as the distinct roles of IL-12 and IL-23 in supporting Th1 and Th17 propagation, respectively, emphasizes the high degree of regulation involved in T cell development and function under various physiological states.
Th17 responses appear to be physiologically important in pulmonary bacterial immunity as well as immunity to certain intestinal pathogens (Khader et al., 2007). The specific role of IL-17 and other cytokines produced by Th17 cells in these responses remains to be completely elucidated, though direct effects on epithelial cells as well as recruitment of neutrophils seem to be important factors, depending on the site and nature of the infection (Khader et al., 2007). Pathologically, Th17 responses are involved in certain inflammatory and autoimmune diseases, including inflammatory bowel disease, rheumatoid arthritis, autoimmune iritis, and central nervous system autoimmune syndromes (Bettelli et al., 2007). CD8 T cells and non–T cells have been reported to produce Th17 cytokines (Weaver et al., 2007), including IL-17, but the role of non–T cell–derived IL-17 remains to be further defined.
The immune system can act as an extrinsic suppressor of tumors, and the importance of Th1, characterized by IFN-γ and type 1 IFNs, in inhibiting tumor incidence and growth has been established (Kaplan et al., 1998; Dunn et al., 2006). Recent studies, however, have also demonstrated a critical role of certain immune cells, via production of specific cytokines or growth factors, in promoting carcinogenesis and tumor growth (Colombo and Mantovani, 2005). This opposing role of the immune system in tumor immune surveillance and cancer promotion is exemplified by a pair of related cytokines: although IL-12 clearly possesses antitumor activity via both NK activation and Th1/IFN-γ induction, IL-23 has been found to promote carcinogenesis (Langowski et al., 2006). Specific STAT pathways regulate the IL-12/IL-23 balance, with Stat3 coordinately activating IL-23/p19 gene transcription while inhibiting expression of the IL-12/p35 gene (Kortylewski et al., 2009). Similar to IL-23, IL-17 expression is also regulated by Stat3 (Chen et al., 2006), which is an oncogene persistently activated in tumor cells and tumor stromal cells, promoting tumor cell survival, proliferation, and tumor angiogenesis (Bromberg et al., 1999; Yu et al., 2007). Persistent activation of Stat3 in tumor cells and in tumor-associated immune cells also promotes accumulation of tumor myeloid-derived suppressor cells and tumor regulatory T cells, leading to tumor immune suppression (Kortylewski et al., 2005). Although IL-17–producing T cells have been found in increased numbers within certain tumors (Miyahara et al., 2008; Sfanos et al., 2008; Zhang et al., 2008), it remains controversial whether IL-17 promotes or inhibits cancer progression (Numasaki et al., 2003; Numasaki et al., 2005; Muranski et al., 2008; Nam et al., 2008; Kryczek et al., 2009; Xiao et al., 2009; Zhang et al., 2009). Importantly, the underlying mechanisms of IL-17 in modulating tumor growth is still poorly understood.
RESULTS AND DISCUSSION
Th17 cells can promote tumor growth
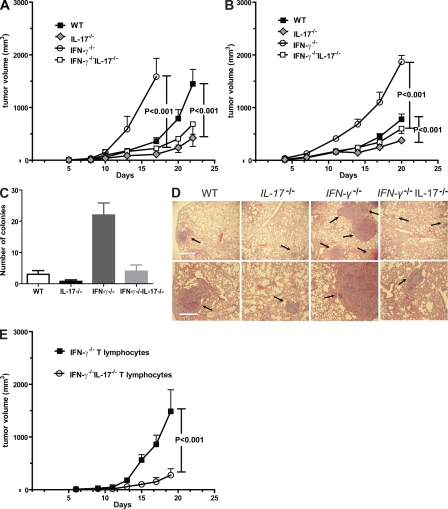
We assessed the role of IL-17 in modulating tumor growth in mice with genetically ablated IL-17 alleles (IL-17 KO). Because IFN-γ–producing Th1 T cells generally provide antitumor immunity (Kaplan et al., 1998; Dunn et al., 2006) and are known to restrain Th17 cell development (Harrington et al., 2005), we also evaluated tumor growth in IFN-γ KO mice and in IL-17IFN-γ double KO mice. Sex- and age-matched WT, IL-17−/−, IFN-γ−/−, and IFN-γ−/−IL-17−/− B6 mice were challenged with B16 melanoma. Results from these experiments showed that tumor growth rate was reduced in the B6 IL-17−/− mice when compared with WT B6 controls (Fig. 1 A). As expected from the antitumor role of IFN-γ (Kaplan et al., 1998; Dunn et al., 2006), in B6 mice lacking IFN-γ alleles, B16 tumors appeared earlier and the tumor growth rate was drastically increased relative to the WT (Fig. 1 A). Importantly, in IFN-γ−/−IL-17−/− mice, tumor growth was significantly reduced compared with IFN-γ−/− mice (Fig. 1 A), supporting a tumor-promoting role of Th17 cells in the B16 subcutaneous tumor model.
Figure 1.
IL-17 promotes tumor growth, which is mainly mediated by T cells. (A) 105 B16 tumor cells were injected subcutaneously into WT, IL-17−/−, IFN-γ−/−, and IFN-γ−/−IL-17−/− B6 mice. Data represent means ± SEM (n = 12 mice per group from three independent experiments). (B) MB49 tumor growth in WT, IL-17−/−, IFN-γ−/−, and IFN-γ−/−IL-17−/− B6 mice (n = 8 mice from two experiments). (C and D) Intratumoral IL-17 promotes cancer metastasis. (C) MB49 tumor cells were injected subcutaneously into the four groups of mice as indicated, and lung colonies were enumerated 3 wk later (n = 4; P = 0.01). (D) Representative photos of the lung tissues from each group with arrows indicating lung metastasis are shown. Bars: (top) 1,000 µm; (bottom) 500 µm. (E) IL-17–mediated tumor-promoting effects are mainly mediated by T cells. Rapid B16 tumor growth in Rag-2−/− mice receiving adoptive transfer of IFN-γ−/− T cells was observed compared with significantly reduced growth rates in mice receiving adoptive-transferred IFN-γ−/−IL-17−/− T cells. Data represent means ± SEM (n = 11 mice from two experiments).
We also evaluated growth of the MB49 bladder carcinoma in WT, IL-17−/−, IFN-γ−/−, and IFN-γ−/−IL-17−/− mice. Similar to B16, we observed growth inhibition of MB49 tumors in IL-17−/− mice relative to WT controls (Fig. 1 B). Furthermore, MB49 tumor growth was significantly accelerated in IFN-γ−/− relative to WT mice. Ablating IL-17 diminished accelerated tumor growth caused by a lack of IFN-γ (Fig. 1 B). However, the tumor growth rate in the IFN-γ−/−IL-17−/− B6 mice was higher than in IL-17−/− mice. Thus, the general pattern of tumor growth in IL-17−/−, IFN-γ−/−, and IFN-γ−/−IL-17−/− mice was similar for B16 and MB49 tumors despite their different tissue origins. Subcutaneous MB49 tumors also naturally metastasize to lungs; we therefore assessed whether levels of lung metastasis correlate with tumor size in the four groups of mice. Ablating IL-17 in both WT and IFN-γ−/− mice was associated with a decreased number of lung metastases (Fig. 1, C and D).
To assess whether T cells were a primary source of IL-17 for promoting tumor growth, we reconstituted Rag2−/− mice by adoptive T cell transfer with purified T cells from IFN-γ−/− mice that display high levels of IL-17 production (Fig. 2), or from IFN-γ−/−IL-17−/− mice. Tumor growth in mice receiving IFN-γ−/−IL-17−/− T cells was significantly reduced compared with mice given IFN-γ−/− T cells (Fig. 1 E), suggesting that the increased tumor growth, caused by elevated IL-17 production, was mainly contributed by T cells.
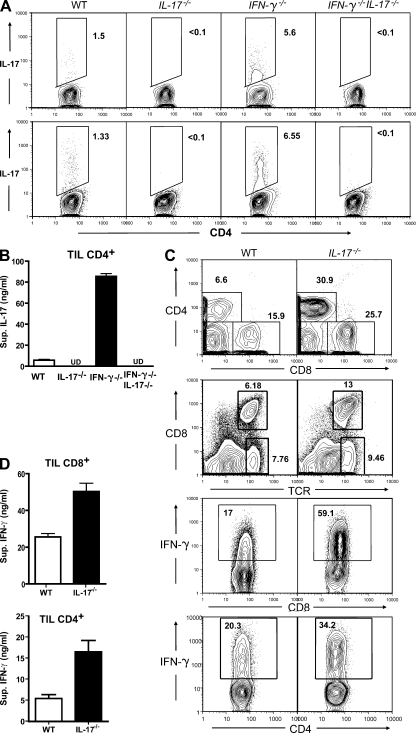
Figure 2.
Reciprocal relationship between IFN-γ and IL-17 production by tumor-infiltrating T lymphocytes. Single-cell suspensions were prepared from B16 or MB49 tumors harvested 20 d after tumor implantation. (A) IL-17 expression in CD4+ T cells from B16 (top) or MB49 (bottom) tumors; flow cytometric patterns shown (one tumor per group) are representative of three independent experiments. Percentages are shown. (B) IL-17 secretion by intratumoral CD4+ T cells sorted from B16 tumors grown in WT, IL-17−/−, IFN-γ−/−, or IFN-γ−/−IL-17−/− mice was assessed by ELISA after a 24-h culture, combining results from three independent experiments of three to four pooled tumor samples (P = 0.001). Data represent means ± SEM. (C) Percentages of CD4+ and CD8+ T cells within the total intratumoral leukocyte population harvested on days 20 (top row) and 14 (second from top row). (second from bottom row) IFN-γ expression in CD8+ T cells derived from tumors of WT or IL-17−/− mice. (bottom row) IFN-γ expression by tumor-infiltrating CD4+ T cells (representative of three independent experiments). Percentages are shown. (D) Increased secretion of IFN-γ by sorted tumor-infiltrating CD8+ (top) and CD4+ (bottom) T cells, combining results from three independent experiments of pooled tumor samples (P = 0.002). Data represent means ± SEM. TIL, tumor-infiltrating T lymphocytes.
Reciprocal relationship between IL-17 and IFN-γ production in tumors
We performed intracellular cytokine staining and in vitro cytokine production assays to compare the cytokine profile of CD4+ and CD8+ T cells in tumors harvested from WT, IL-17−/−, IFN-γ−/−, and IFN-γ−/−IL-17−/− mice. IL-17 production in B16 tumors was markedly augmented in the absence of IFN-γ, with an increase in the number of tumor-infiltrating Th17 cells (Fig. 2 A, top). IL-17 expression was also elevated in MB49 tumors grown in IFN-γ−/− mice (Fig. 2 A, bottom). Further, ELISA analyses indicated a >10-fold increase in secreted IL-17 by B16 tumor–infiltrating CD4+ T cells in IFN-γ−/− mice, which was again abrogated in IFN-γ−/−IL-17−/− double KO mice (Fig. 2 B). Notably, we observed only minimal levels of IL-17 production by CD8+ T cells in both B16 and MB49 tumors (Fig. S1).
We observed an increased infiltration of CD4+ and CD8+ T cells in tumors grown in IL-17−/− mice (Fig. 2 C, top two rows). Furthermore, tumor-infiltrating CD8+ and CD4+ T cells from IL-17−/− mice produced more IFN-γ relative to WT control mice, as assessed by intracellular cytokine staining (Fig. 2 C). The increase in IFN-γ production by both tumor-infiltrating CD4+ and CD8+ T cells from IL-17−/− mice was confirmed by ELISA (Fig. 2 D). IL-17 ablation only modestly increased the percentage of tumor-infiltrating regulatory T cells (Fig. S2 A). It has been reported that IL-17 ablation does not affect the function of regulatory T cells (Yi et al., 2008). No correlation was observed with Th2-type immune responses as measured by CD4+ T cell–mediated IL-4 production caused by IL-17 and IFN-γ ablation (Fig. S2 B).
IL-17 activates Stat3 in both tumor and stromal cells in the tumor microenvironment
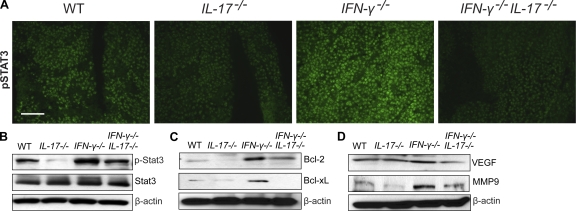
The underlying mechanisms by which Th17 promoted tumor growth was still unknown. Because Stat3 activation in tumor cells and tumor-associated inflammatory cells plays a critical role in tumor progression by augmenting tumor survival and tumor angiogenesis, and suppressing antitumor immunity (Yu et al., 2007), we explored the possibility that IL-17 mediates tumor growth via activation of Stat3. Stat3 activity in B16 tumors from WT, IL-17−/−, IFN-γ−/−, and IFN-γ−/−IL-17−/− mice was examined by immunofluorescence staining of phosphorylated Stat3 (phospho-Stat3) in situ. We observed reduced phospho-Stat3 levels in B16 tumors grown in IL-17−/− mice compared with WT controls. In addition, Stat3 activity was drastically increased in tumors grown in IFN-γ−/− mice, suggesting an inhibitory role of IFN-γ on tumor Stat3 activation. Stat3 phosphorylation was diminished in tumors harvested from IFN-γ−/−IL-17−/− compared with IFN-γ−/− mice (Fig. 3 A). Immunofluorescence staining results were confirmed by Western blotting using an antibody for phospho-Stat3 (Fig. 3 B). Collectively, these results indicated that the levels of IL-17 positively correlated with Stat3 activity in growing tumors.
Figure 3.
Tumorigenic effects of IL-17 are associated with Stat3 activation and up-regulation of oncogenic genes. B16 tumors were harvested from WT, IL-17−/−, IFN-γ−/−, and IFN-γ−/−IL-17−/− mice 20 d after tumor challenge. (A and B) Stat3 activation in B16 tumors in vivo is notably diminished by IL-17 ablation. (A) Frozen tumor sections were stained with antibodies specific to phospho-Stat3 (pStat3; green). Results are representative of three independent experiments. Bar, 100 µm. (B) Western blot analysis of phospho-Stat3, total Stat3, and β-actin in protein extracts prepared from freshly harvested B16 tumors (representative of three experiments). (C and D) IL-17 deletion inhibits expression of Stat3-downstream oncogenic genes. Western blot analysis of tumor protein extracts to detect Bcl-xL, Bcl-2, vascular endothelial growth factor, MMP9, and β-actin protein levels (representative of two independent experiments).
We next tested whether increased IL-17 expression affected downstream Stat3 target genes. We found that Bcl family members, including Bcl-2 and Bcl-xL, were up-regulated in tumors from IFN-γ−/− mice (Fig. 3 C). In addition, vascular endothelial growth factor and MMP9 were up-regulated in tumors from IFN-γ−/− mice, and their expression was reduced in tumors from IFN-γ−/−IL-17−/− mice (Fig. 3 D). These results suggested that IL-17 promoted Stat3 activity in tumors, leading to up-regulation of antiapoptotic and angiogenic genes.
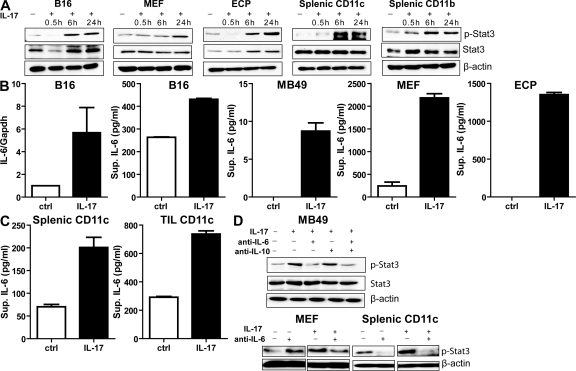
To investigate whether IL-17 signals directly through Stat3, we cultured B16 tumor cells, normal fibroblasts, endothelial cells, splenic DCs, and non-DC myeloid cells in the presence of recombinant mouse IL-17 or control. Results from these experiments showed that IL-17 indeed induced Stat3 activity (Fig. 4 A). Although IL-17 induced Stat3 activity in all tested target cells, it did so with significantly different kinetics (Fig. 4 A). Normal fibroblasts and endothelial cells were included to test IL-17 response, as they are major components of the tumor microenvironment. Similarly, we found that human recombinant IL-17 significantly stimulated Stat3 phosphorylation in several human cancer cell lines (Fig. S3 A). We further confirmed the expression of IL-17 receptor in tumor cells and fibroblasts (Fig. S4). These results suggested that induction of Stat3 activation in tumor cells and elements of the tumor microenvironment by IL-17 occurred through induction of an intermediary factor rather than directly.
Figure 4.
IL-17 activates Stat3 in tumor and tumor stromal cells through an IL-6–dependent mechanism. (A) The kinetics of Stat3 activation in B16 melanoma cells, MEFs, endothelial cells (ECP), splenic CD11c+ DCs, or splenic CD11b+ myeloid cells after stimulation with recombinant IL-17. Western blots of phospho-Stat3, total Stat3, and β-actin are shown and are representative of two independent experiments. (B and C) IL-17 induces IL-6 expression in tumor and various tumor stromal cells. (B) IL-6 levels in B16 cells treated for 24 h with recombinant IL-17 were assessed by quantitative real-time PCR (left) and ELISA (second from left). IL-6 induction in MB49 tumor cells (middle), MEFs (second from right), and endothelial cells (ECP; right) by recombinant IL-17 treatment is shown (from three independent experiments; P < 0.0001). Data represent means ± SEM. (C) Production of IL-6 by CD11c+ DCs upon IL-17 stimulation, as determined by ELISA (from three independent experiments; P = 0.0002). Data represent means ± SEM. (D) IL-17–induced Stat3 activation is IL-6 dependent. (top) MB49 tumor cells were incubated with IL-17 with or without anti–IL-6 or –IL-10 antibodies, and Western blots were performed for phospho-Stat3, total Stat3, and β-actin protein levels. (bottom) MEFs and splenic CD11c+ DCs were stimulated with IL-17 in the presence of IL-6–neutralizing antibodies or control IgG (representative of three independent experiments).
IL-17 activates Stat3 via IL-6 induction
IL-6 is a Stat3 activator and is elevated in diverse cancers (Hirano et al., 2000). IL-17 has recently been reported to stimulate production of IL-6 and Stat3 activation through a positive feedback loop, in inflammatory cells as well as fibroblasts, in an autoimmune disease setting (Ogura et al., 2008). We therefore determined whether IL-6 mediated IL-17–driven Stat3 activation in a tumor setting. We found that IL-17 stimulated IL-6 production by B16 and MB49 tumor cells in vitro, in addition to other cell types that constitute the tumor stroma such as fibroblasts, endothelial cells, and DCs (Fig. 4, B and C). The levels of IL-6 per cell produced by the tested tumor cells were lower than those produced by fibroblasts and endothelial cells. Although treating the tumor cells with recombinant IL-17 only modestly increased their proliferation (Fig. S5 A), which is consistent with another study (Numasaki et al., 2005), similar treatment of endothelial cells led to increased expression of several Stat3 downstream genes that are involved in cell migration (Fig. S5 B). In addition, IL-17–mediated increased secretion of angiogenic factors by endothelial cells induced endothelial cell migration in a Stat3-dependent manner (Fig. S5 C). Importantly, neutralizing IL-6 but not IL-10 (another Stat3 activator) with a blocking mAb reduced Stat3 activation in (mouse embryonic fibroblasts) MEFs, DCs, and MB49 tumor cells after IL-17 stimulation (Fig. 4 D). Similarly, anti–IL-6 antibodies abrogated IL-17–induced Stat3 activation in human cancer cells (Fig. S3 B). These results indicated that IL-17 activated Stat3 through IL-6 in diverse types of cancer and nontransformed cells constituting the tumor stroma.
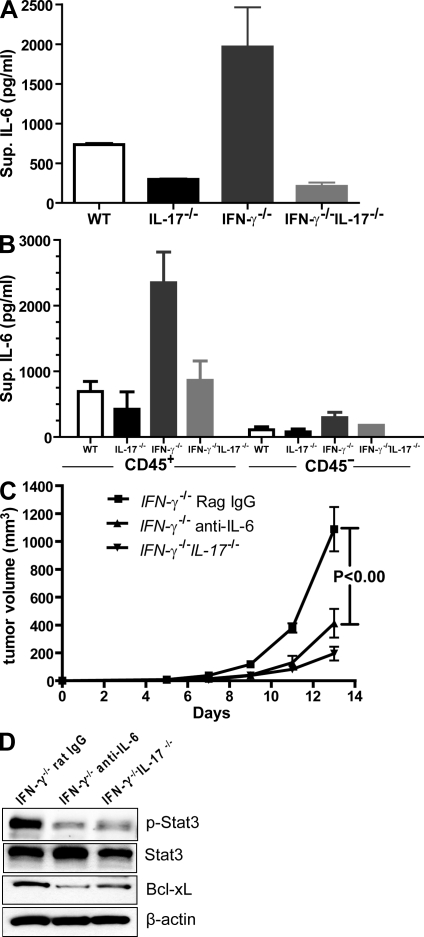
In vivo IL-6 blockade partially reverses tumor progression in the setting of high intratumoral IL-17
We first assessed whether IL-17 affected IL-6 production by tumors in vivo. Freshly harvested B16 tumor cells from WT mice produced relatively high levels of IL-6, which were reduced after IL-17 ablation (Fig. 5 A). In addition, tumors grown in IFN-γ−/− mice produced a markedly increased level of IL-6 when compared with the IFN-γ−/−IL-17−/− mice (Fig. 5 A). More detailed analysis of growing tumors indicated that tumor-associated immune cells (CD45+) were the main source of IL-6 when compared with the tumor cells (Fig. 5 B). To directly test whether IL-17 could promote tumor growth through IL-6–mediated Stat3 activation, IFN-γ−/− mice were challenged with B16 melanoma, followed by administration of IL-6–neutralizing mAbs or control rat IgG. IFN-γ−/−IL-17−/− mice challenged with B16 melanoma but without anti–IL-6 antibody treatment were included as an additional control. Because IL-6 was shown to promote Th17 cell differentiation, we did not start anti–IL-6 treatment until 6 d after tumor inoculation, a time at which tumor-infiltrating Th17 cells were detectable (unpublished data). We found that administration of monoclonal anti–IL-6 but not rat IgG antibodies significantly inhibited tumor growth in IFN-γ−/− mice (Fig. 5 C), suggesting that IL-17–induced tumor progression is at least in part mediated by IL-6. Consistent with a role for IL-6 in mediating tumor Stat3 activation and tumor growth promotion, neutralizing IL-6 in vivo reduced Stat3 activity and the expression of its downstream antiapoptotic gene, Bcl-xL, in tumors (Fig. 5 D).
Figure 5.
Tumor-derived IL-6 mediates tumorigenic effects of IL-17. (A and B) IL-6 secretion in the tumor microenvironment correlates with IL-17 levels. (A) Single-cell suspensions prepared from B16 tumors harvested from the indicated mice were cultured in vitro overnight; supernatants were then collected and assayed for IL-6 levels, combining four mice from two independent experiments (WT vs. IL-17−/−, P = 0.0026; IFN-γ−/− vs. IFN-γ−/−IL-17−/−, P = 0.005). Data are means ± SEM. (B) Single-cell suspensions prepared from MB49 tumors were enriched for CD45. Both CD45+ (immune cells) and CD45− (tumor cells) were cultured overnight before supernatants were collected for ELISA (n = 4 mice representing three independent experiments; IFN-γ−/− vs. IFN-γ−/−IL-17−/−, P = 0.03). Data are means ± SEM. (C) 105 B16 tumor cells were injected subcutaneously into IFN-γ−/− and IFN-γ−/−IL-17−/− mice. 6 d after tumor implantation, tumor-bearing IFN-γ−/− mice were treated with IL-6–neutralizing antibodies or control rat IgG every other day. Data are means ± SEM (n = 7 mice from two independent experiments). (D) Western blot analyses of phospho-Stat3, total Stat3, Bcl-xL, and β-actin levels from protein extracts prepared from B16 tumors harvested from the indicated mice (representative of two independent experiments).
In summary, we have shown that IL-17 can promote tumor growth. Our results suggested that the IL-17–mediated tumor-promoting role involves a direct effect on tumor through IL-6 induction, which in turn activates Stat3 in both tumor as well as nontransformed cells constituting the tumor microenvironment. IL-6 activation of Stat3 in tumor cells results in increases in antiapoptotic, proproliferation, and proangiogenic genes, as well as suppression of certain proinflammatory genes (Yu et al., 2007). IL-6–induced Stat3 signaling in T cells is also critical in promoting Th17 cell differentiation and expression (Chen et al., 2006; Harris et al., 2007). Given that TGF-β, the other cytokine involved in Th17 cell differentiation, is also expressed at high levels in tumors, IL-17–induced IL-6 production can generate an autoamplification loop for Th17 cells in the tumor microenvironment. Consistent with our findings, several publications suggested a role of IL-17 in promoting tumor growth (Numasaki et al., 2003; Numasaki et al., 2005; Nam et al., 2008; Xiao et al., 2009), although the mechanism was not well addressed in these studies. However, a recent paper showed that, in a different tumor system (MC38 sarcoma), tumors growth was increased in IL-17−/− mice (Kryczek et al., 2009). These findings suggest that the role of IL-17 in cancer is context and system dependent, like many other cytokines such as TNF-α. Additional studies are required to reveal why IL-17 can have opposite roles in modulating growth in different tumor systems.
The opposing functions of IFN-γ and IL-17 as anticarcinogenic and protumor growth effector cytokines extend an emerging concept that the qualitative nature of the immune response determines whether it will predominantly inhibit tumor growth or promote cancer development. Recently, analogous opposing effects of the two IL-12 family members, IL-12 and IL-23, in tumor inhibition versus promotion have been ascertained based on enhanced tumor formation in IL-12p35 KO mice versus diminished tumor formation in IL-23p19 KO mice (Langowski et al., 2006). We subsequently reported that Stat3 signaling in tumors and their microenvironment determined the balance between these cytokines by transcriptionally activating IL-23p19 and inhibiting IL-12p35 (Kortylewski et al., 2009). The current work is among the first examples by which a qualitatively distinct (Th17) endogenous T cell immune response can be cancer promoting. Further, it adds a new element to the link between immune responses that either depend on or induce Stat3 signaling and Stat3-mediated protumor growth.
MATERIALS AND METHODS
Mice.
WT and IFN-γ−/− C57BL/6 mice were purchased from the National Cancer Institute and the Jackson Laboratory, respectively. The generation of C57BL/6 IL-17−/− mice has been previously reported (Nakae et al., 2002), and the mice were provided by Y. Iwakura (University of Tokyo, Tokyo, Japan). Mouse care and experimental procedures were performed under pathogen-free conditions in accordance with established institutional guidance and approved protocols from the Research Animal Care Committee of the City of Hope Medical Center. For tumor challenge, 105 B16 tumor cells were injected subcutaneously into 8–12-wk-old WT or transgenic mice, and tumor growth was monitored every other day. For IL-6 neutralization experiments in vivo, mice were treated with 125 µg anti–IL-6 antibodies (eBioscience) or control rat IgG (Jackson ImmunoResearch Laboratories) injected i.v. on days 6, 8, 10, 12, and 14 after tumor inoculation. Mice were sacrificed after 2–3 wk from tumor inoculation or when tumor volume exceeded 1.5 cm in diameter.
Cell lines.
B16 and MB49 cell lines were originally obtained from American Type Culture Collection and were maintained in our laboratory. Endothelial cell lines derived from mouse prostate were provided by J. Fidler (M.D. Anderson Cancer Center, Houston, TX).
T cell adoptive transfer.
T cells were enriched from IFN-γ−/− or IFN-γ−/−IL-17−/− mice. Specifically, non–T cells were depleted with biotin-labeled mAbs, including anti-B220, Gr-1, Mac-1, CD11c, DX5, and Ter119, using a magnetic purification system from Miltenyi Biotec. TCRβ+ T cell purity was >90%, as determined by flow cytometry.
Preparation of tumor-infiltrating immune cells.
The procedure for isolating immune cells from tumors has been described previously (Kortylewski et al., 2005). In brief, freshly excised tumor tissues were gently minced and digested for 30 min at 37°C by collagenase D/DNase I (Roche). Cell suspensions were filtered through a 70-µm cell strainer, and dead cells were removed by centrifugation at 600 g over a Histopaque gradient (Sigma-Aldrich).
Flow cytometry.
The FITC-, PE-, allophycocyanin (APC)-, or Cy7-APC–conjugated antibodies specific to CD4, CD8, IL-17, and IFN-γ were purchased from BD; PE-labeled antibody against mouse Foxp3 was purchased from eBioscience, and PE-labeled antibody for IL-17R was purchased from R&D Systems. Intracellular staining for IL-17 and IFN-γ was performed according to the manufacturer’s instructions (BD; Yi et al., 2008). In brief, cells were stimulated for 5 h with PMA and ionomycin (Sigma-Aldrich) in the presence of Golgi-Stop reagent (BD). Cells were harvested, washed, and stained with anti-CD4 or -CD8 in the presence of FcR-Block (BD). After the wash, cells were fixed, permeabilized, and stained with cytokine-specific or control isotype antibodies for 30 min on ice. Multiple-color FACS analysis was performed using a three-laser CyAn Immunocytometry System (Dako). Dead cells were excluded by the Fixable Aqua Dead Cell Stain Kit (Invitrogen).
ELISA assays.
105 sorted tumor-infiltrating CD4+, CD8+ T cells were cultured for 24 h in a U-bottom 96-well plate with plate-bound CD3 and 2 µg/ml of soluble CD28. 106 enriched splenic CD11c+ or cultured cells/ml were stimulated for 24 h with 10 ng/ml of recombinant IL-17. IFN-γ, IL-17, IL-6, and IL-4 concentrations in culture supernatants were measured using ELISA kits from R&D Systems.
Immunofluorescence staining.
5-µm sections of flash-frozen tumor specimens were fixed in acetone, permeabilized with methanol, stained with antibodies specific to tyrosine–phospho-Stat3 (Santa Cruz Biotechnology, Inc.) and detected with secondary antibodies conjugated Alexa Fluor 488 (Invitrogen), as previously described (Kortylewski et al., 2005). After staining with Hoechst 33342 (Invitrogen) to visualize cell nuclei, slides were mounted and analyzed by fluorescence microscopy.
Statistical analysis.
The unpaired t test was used to calculate the two-tailed p-value. The two-way analysis of variance test was used to calculate the p-value for tumor growth. Data were analyzed using Prism software (GraphPad Software, Inc.).
Online supplemental material.
Fig. S1 shows the production of IL-17 in tumor-infiltrating CD8+ T cells, determined by intracellular staining and ELISA. Fig. S2 indicates that ablating IFN-γ or IL-17 did not significantly affect the T reg cell percentage and IL-4 production. Fig. S3 shows that IL-17 activates Stat3 in human tumor cell lines in an IL-6–dependent manner. Fig. S4 confirms IL-17 receptor expression on B16 tumor cells and MEFs. Fig. S5 depicts the effect of IL-17 on tumor cell proliferation and endothelial cell migration. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20090207/DC1.
Acknowledgments
We thank Dr. Y. Iwakura for generously providing us with IL-17−/− mice; Dr. Y. Liu for superb assistance; the members of the Flow Cytometry Core, the Pathology Core, and the Animal Facility at the City of Hope Medical Center for their contributions; and Dr. S. da Costa for editing the manuscript.
This study was supported by the National Institutes of Health (grants R01CA122976, R01CA115815, and R01 AI066008).
The authors have no conflicting financial interests.
Footnotes
Abbreviations used: MEF, mouse embryonic fibroblast; phospho-Stat3, phosphorylated Stat3.
References
- Bettelli E., Oukka M., Kuchroo V.K. 2007. T(H)-17 cells in the circle of immunity and autoimmunity.Nat. Immunol. 8:345–350 [DOI] [PubMed] [Google Scholar]
- Bromberg J.F., Wrzeszczynska M.H., Devgan G., Zhao Y., Pestell R.G., Albanese C., Darnell J.E., Jr 1999. Stat3 as an oncogene.Cell. 98:295–303 [DOI] [PubMed] [Google Scholar]
- Chen Z., O’Shea J.J. 2008. Th17 cells: a new fate for differentiating helper T cells.Immunol. Res. 41:87–102 [DOI] [PubMed] [Google Scholar]
- Chen Z., Laurence A., Kanno Y., Pacher-Zavisin M., Zhu B.M., Tato C., Yoshimura A., Hennighausen L., O’Shea J.J. 2006. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells.Proc. Natl. Acad. Sci. USA. 103:8137–8142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo M.P., Mantovani A. 2005. Targeting myelomonocytic cells to revert inflammation-dependent cancer promotion.Cancer Res. 65:9113–9116 [DOI] [PubMed] [Google Scholar]
- Dunn G.P., Koebel C.M., Schreiber R.D. 2006. Interferons, immunity and cancer immunoediting.Nat. Rev. Immunol. 6:836–848 [DOI] [PubMed] [Google Scholar]
- Harrington L.E., Hatton R.D., Mangan P.R., Turner H., Murphy T.L., Murphy K.M., Weaver C.T. 2005. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages.Nat. Immunol. 6:1123–1132 [DOI] [PubMed] [Google Scholar]
- Harris T.J., Grosso J.F., Yen H.R., Xin H., Kortylewski M., Albesiano E., Hipkiss E.L., Getnet D., Goldberg M.V., Maris C.H., et al. 2007. Cutting edge: An in vivo requirement for STAT3 signaling in TH17 development and TH17-dependent autoimmunity.J. Immunol. 179:4313–4317 [DOI] [PubMed] [Google Scholar]
- Hirano T., Ishihara K., Hibi M. 2000. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors.Oncogene. 19:2548–2556 [DOI] [PubMed] [Google Scholar]
- Kaplan D.H., Shankaran V., Dighe A.S., Stockert E., Aguet M., Old L.J., Schreiber R.D. 1998. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice.Proc. Natl. Acad. Sci. USA. 95:7556–7561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khader S.A., Bell G.K., Pearl J.E., Fountain J.J., Rangel-Moreno J., Cilley G.E., Shen F., Eaton S.M., Gaffen S.L., Swain S.L., et al. 2007. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge.Nat. Immunol. 8:369–377 [DOI] [PubMed] [Google Scholar]
- Kortylewski M., Kujawski M., Wang T., Wei S., Zhang S., Pilon-Thomas S., Niu G., Kay H., Mule J., Kerr W.G., et al. 2005. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity.Nat. Med. 11:1314–1321 [DOI] [PubMed] [Google Scholar]
- Kortylewski M., Xin H., Kujawski M., Lee H., Liu Y., Harris T., Drake C., Pardoll D., Yu H. 2009. Regulation of the IL-23 and IL-12 balance by Stat3 signaling in the tumor microenvironment.Cancer Cell. 15:114–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryczek I., Wei S., Szeliga W., Vatan L., Zou W. 2009. Endogenous IL-17 contributes to reduced tumor growth and metastasis.Blood. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langowski J.L., Zhang X., Wu L., Mattson J.D., Chen T., Smith K., Basham B., McClanahan T., Kastelein R.A., Oft M. 2006. IL-23 promotes tumour incidence and growth.Nature. 442:461–465 [DOI] [PubMed] [Google Scholar]
- Miyahara Y., Odunsi K., Chen W., Peng G., Matsuzaki J., Wang R.F. 2008. Generation and regulation of human CD4+ IL-17-producing T cells in ovarian cancer.Proc. Natl. Acad. Sci. USA. 105:15505–15510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muranski P., Boni A., Antony P.A., Cassard L., Irvine K.R., Kaiser A., Paulos C.M., Palmer D.C., Touloukian C.E., Ptak K., et al. 2008. Tumor-specific Th17-polarized cells eradicate large established melanoma.Blood. 112:362–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae S., Komiyama Y., Nambu A., Sudo K., Iwase M., Homma I., Sekikawa K., Asano M., Iwakura Y. 2002. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses.Immunity. 17:375–387 [DOI] [PubMed] [Google Scholar]
- Nam J.S., Terabe M., Kang M.J., Chae H., Voong N., Yang Y.A., Laurence A., Michalowska A., Mamura M., Lonning S., et al. 2008. Transforming growth factor beta subverts the immune system into directly promoting tumor growth through interleukin-17.Cancer Res. 68:3915–3923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numasaki M., Fukushi J., Ono M., Narula S.K., Zavodny P.J., Kudo T., Robbins P.D., Tahara H., Lotze M.T. 2003. Interleukin-17 promotes angiogenesis and tumor growth.Blood. 101:2620–2627 [DOI] [PubMed] [Google Scholar]
- Numasaki M., Watanabe M., Suzuki T., Takahashi H., Nakamura A., McAllister F., Hishinuma T., Goto J., Lotze M.T., Kolls J.K., Sasaki H. 2005. IL-17 enhances the net angiogenic activity and in vivo growth of human non-small cell lung cancer in SCID mice through promoting CXCR-2-dependent angiogenesis.J. Immunol. 175:6177–6189 [DOI] [PubMed] [Google Scholar]
- Ogura H., Murakami M., Okuyama Y., Tsuruoka M., Kitabayashi C., Kanamoto M., Nishihara M., Iwakura Y., Hirano T. 2008. Interleukin-17 promotes autoimmunity by triggering a positive-feedback loop via interleukin-6 induction.Immunity. 29:628–636 [DOI] [PubMed] [Google Scholar]
- Sfanos K.S., Bruno T.C., Maris C.H., Xu L., Thoburn C.J., DeMarzo A.M., Meeker A.K., Isaacs W.B., Drake C.G. 2008. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing.Clin. Cancer Res. 14:3254–3261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver C.T., Harrington L.E., Mangan P.R., Gavrieli M., Murphy K.M. 2006. Th17: an effector CD4 T cell lineage with regulatory T cell ties.Immunity. 24:677–688 [DOI] [PubMed] [Google Scholar]
- Weaver C.T., Hatton R.D., Mangan P.R., Harrington L.E. 2007. IL-17 family cytokines and the expanding diversity of effector T cell lineages.Annu. Rev. Immunol. 25:821–852 [DOI] [PubMed] [Google Scholar]
- Xiao M., Wang C., Zhang J., Li Z., Zhao X., Qin Z. 2009. IFNgamma promotes papilloma development by up-regulating Th17-associated inflammation.Cancer Res. 69:2010–2017 [DOI] [PubMed] [Google Scholar]
- Yi T., Zhao D., Lin C.L., Zhang C., Chen Y., Todorov I., LeBon T., Kandeel F., Forman S., Zeng D. 2008. Absence of donor Th17 leads to augmented Th1 differentiation and exacerbated acute graft-versus-host disease.Blood. 112:2101–2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Kortylewski M., Pardoll D. 2007. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment.Nat. Rev. Immunol. 7:41–51 [DOI] [PubMed] [Google Scholar]
- Zhang B., Rong G., Wei H., Zhang M., Bi J., Ma L., Xue X., Wei G., Liu X., Fang G. 2008. The prevalence of Th17 cells in patients with gastric cancer.Biochem. Biophys. Res. Commun. 374:533–537 [DOI] [PubMed] [Google Scholar]
- Zhang J.P., Yan J., Xu J., Pang X.H., Chen M.S., Li L., Wu C., Li S.P., Zheng L. 2009. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients.J. Hepatol. 50:980–989 [DOI] [PubMed] [Google Scholar]