Abstract
Plasmacytoid dendritic cells (pDCs) produce copious type I interferon (IFN) upon sensing nucleic acids through Toll-like receptor (TLR) 7 and TLR9. Uncontrolled pDC activation and IFN production are implicated in lymphopenia and autoimmune diseases; therefore, a mechanism controlling pDC IFN production is essential. Human pDCs specifically express an orphan receptor, immunoglobulin-like transcript 7 (ILT7). Here, we discovered an ILT7 ligand expressed by human cell lines and identified it as bone marrow stromal cell antigen 2 (BST2; CD317). BST2 directly binds to purified ILT7 protein, initiates signaling via the ILT7–FcϵRIγ complex, and strongly inhibits production of IFN and proinflammatory cytokines by pDCs. Readily induced by IFN and other proinflammatory cytokines, BST2 may modulate the human pDC’s IFN responses through ILT7 in a negative feedback fashion.
In humans and other mammals, plasmacytoid DCs (pDCs) are specialized immune cells that selectively express Toll-like receptor (TLR) 7 and TLR9, which are key endosomal sensors of microbial and self-RNA or DNA, respectively (Jarrossay et al., 2001; Kadowaki et al., 2001; Liu, 2005; Gilliet et al., 2008). Activation of TLR7 or TLR9 by nucleic acids in pDCs triggers signal transduction, leading to rapid and robust secretion of type I IFN, inflammatory cytokines, and chemokines (Colonna et al., 2004; Honda and Taniguchi, 2006; Kawai and Akira, 2006; Piqueras et al., 2006; Gilliet et al., 2008). The TLR-induced IFN response is regulated by several immunoreceptor tyrosine-based activation motif (ITAM)–bearing signaling receptors on pDCs (Novak et al., 2004; Fuchs et al., 2005; Blasius et al., 2006a; Cao et al., 2006, 2007; Röck et al., 2007; Cho et al., 2008; Gilliet et al., 2008). One such receptor is ILT7, a member of the Ig-like transcript (ILT) family (also known as leukocyte Ig-like receptors) found in humans and primates (Brown et al., 2004).
ILTs, which are expressed by a variety of immune cell types, are comprised of a group of inhibitory receptors bearing immunoreceptor tyrosine-based inhibitory motifs and a group of stimulatory receptors that signal through their association with adaptor molecules containing ITAM (Brown et al., 2004). ILT7, also known as LILRA4 and CD85g, contains four extracellular Ig-like domains and a positively charged residue within the transmembrane region, allowing ILT7 to form a receptor complex with a signaling adaptor FcϵRIγ. Uniquely expressed by human pDCs, ILT7 suppresses TLR7/9-induced type I IFN secretion by pDCs when cross-linked by anti-ILT7 mAbs that trigger signaling activation (Cao et al., 2006; Cho et al., 2008). Although several members of the inhibitory ILT family regulate innate and adaptive immune responses through interaction with classical and nonclassical MHC class I ligands or virus-encoded MHC class I–like proteins (Chapman et al., 1999; Brown et al., 2004), the natural ligands for the stimulatory ILTs, including ILT7, are unknown.
Here, we report the identification of a surface molecule named bone marrow stromal cell antigen 2 (BST2) as a biological ligand for ILT7 and provide evidence that BST2–ILT7 engagement may serve as a force to negatively regulate human pDCs’ innate immune functions, likely during inflammation, in a negative feedback manner.
RESULTS
Human cancer cells express novel ILT7 ligand
Previously, we constructed an NFAT-GFP reporter cell line expressing ILT7 and FcϵRIγ that expresses GFP in response to ILT7 surface ligation (Cao et al., 2006). To detect the presence of ILT7 ligands (ILT7-L), we used these cells to screen virus-infected cells and a large panel of human tumor cell lines. Co-culture of the ILT7 reporter cells with human breast carcinoma MDA-MB-468, MCF7, and T47D cells, but not with MDA-MB-231 or ZR-75-1 cells, induced GFP expression by the reporter cells (Fig. 1 A). We further tested several human ovarian, colon, melanoma, glioma, and lung cancer cell lines and found that two melanoma lines, WM35 and Mel938, were able to similarly activate the ILT7 reporter cells (Fig. 1 B). Other transformed cell lines, such as HEK293, Vero, CHO, Cos7, and Jurkat, were unable to stimulate the ILT7 reporter cells (unpublished data).
Figure 1.
Human tumor cell lines express a potential ligand for ILT7. (A) Five human breast cancer cell lines were co-cultured with either ILT7+ NFAT-GFP reporter cells or parental NFAT-GFP reporter cells. The percentages of GFP-positive reporter cells were analyzed. Data are representative of four independent experiments. (B) Human carcinoma cells were co-cultured with ILT7+ NFAT-GFP reporter cells. The percentages of GFP-positive reporter cells were analyzed. The categories of the cancer lines are as follows: OVCAR-3, SKOV-3, and DOV-13 (ovarian); HT-29 and HCT-116 (colon); WM35, WM239, MEL526, MEL624, MEL888, and MEL938 (melanoma); U87, LN229, and SNB19 (glioblastoma); A549 (lung cancer). Data are representative of two independent experiments. (C) T47D cells were co-cultured with ILT7+ NFAT-GFP reporter cells in the presence of 1 µg/ml of control IgG1 or anti-ILT7 mAb. The percentages of GFP-positive reporter cells were plotted. Data are representative of four independent experiments. (D) Breast cancer MDA-MB-468 cells were first cultured for 5 d in the presence of medium, 5 ng/ml of TNF-α or 500 U/ml of IFN-α, and then co-cultured with NFAT-GFP reporter cells. The percentages of GFP-positive reporter cells were analyzed. Data are representative of four independent experiments. Error bars represent the mean ± the SEM.
As the breast cancer line T47D is most potent in triggering ILT7, we further characterized these cells. The ability of T47D cells to activate the reporter cells is ILT7 dependent because NFAT-GFP reporter cells expressing only the signaling adaptor FcϵRIγ, and not ILT7, were not activated (Fig. 1 A). In addition, the induction of GFP was completely abolished by a neutralizing anti-ILT7 mAb (Fig. 1 C). Pretreatment with IFN-α and TNF-α enhanced the ability of the breast cancer MDA-MB-468 cells to activate the ILT7 reporters (Fig. 1 D), suggesting that ILT7-L expression is regulated by immune responses.
ILT7 activation by cancer cells requires direct cell–cell contact as cells cultured in separate chambers of a transwell dish fail to induce GFP (unpublished data). Although the two other ILT family members, ILT2 and ILT4, bind to the classical and nonclassical MHC class I (MHC-I) ligands (Navarro et al., 1999; Brown et al., 2004), the putative ILT7-L appears to be unrelated to MHC class I or class II because antibodies against human MHC class I or class II did not block the ability of T47D cells to activate the ILT7 reporter cells (unpublished data), and both the MHC class I–expressing cell lines (e.g., Jurkat and MDA-MB-231) and the MHC class II–expressing cell lines (e.g., EBV-transformed B cells) were unable to activate ILT7 reporter cells (unpublished data).
Identification of ILT7-L
To facilitate the identification of the putative ILT7-L, we immunized mice with T47D (ILT7-Lpos) and MDA-MB-231 (ILT7-Lneg) cells and obtained multiple hybridoma clones that bound specifically to T47D cells, but not to MDA-MB-231 cells. To identify mAbs that specifically recognize ILT7-L, we further screened the hybridoma clones for the ability to block T47D-induced ILT7 reporter cell activation. Two such clones (26F8 and 28G4) were identified (Fig. 2 A).
Figure 2.
Characterization of mAbs against a putative ILT7 ligand. (A) Culture supernatants from different hybridoma clones were included in the co-cultures of ILT7 reporter cells and T47D cells. The percentages of GFP-positive reporter cells were determined. Two clones able to inhibit GFP induction are indicated (arrows). Anti-ILT7 mAb (clone 17.2) was included as a positive control. (B) Five human breast cancer cells were stained with mAb 26F8 (red line) or an isotype-matched control mAb (shaded area) and analyzed by flow cytometry. A similar result obtained with mAb 28G4 is not shown. Data are representative of three independent experiments. (C) T47D cells were co-cultured with ILT7 reporter cells in the presence of control IgG1, 26F8, or 28G4 mAbs at different concentrations. The percentages of GFP-positive reporter cells were plotted. Data are representative of four independent experiments. (D) Surface biotinylated MDA-MB-231 and T47D were immunoprecipitated with control IgG1, 26F8, or 28G4. The precipitated proteins were analyzed by Western blotting with NeutrAvidin-HRP. Arrows indicate the specific protein bands obtained from T47D cells. Data are representative of two independent experiments.
Using flow cytometry, we found that these two mAbs stained the breast tumor cell lines MDA-MB-468, MCF7, and T47D, which activated ILT7 reporter cells, but minimally reacted with breast tumor cell lines MDA-MB-231 and ZR-75-1, which failed to activate ILT7 reporters (Fig. 2 B). Other cell lines (e.g., HEK293, Vero, CHO, and Cos 7) that failed to activate the ILT7 reporter cells were not stained by either 26F8 or 28G4 (unpublished data). In addition, these two mAbs, but not the isotype-matched control mAb, strongly inhibited the ability of the tumor cell line T47D to activate the ILT7 reporter cells in a dose-dependent fashion (Fig. 2 C). The two mAbs recognize nonoverlapping epitopes, because one did not block the binding of the other to T47D cells, as determined by flow cytometry (unpublished data). Nevertheless, 26F8 and 28G4 mAbs immunoprecipitated three similar protein bands in T47D cells, but not in MDA-MB-231 cells (Fig. 2 D), suggesting the presence of cellular proteins as the potential ligand for ILT7.
To identify the ligand for ILT7, 26F8 and 28G4 mAbs were used to screen a human cDNA library. Both antibodies specifically recognized the gene product of human BST2 (CD317). BST2 is a 180-aa glycoprotein that was initially identified as a membrane protein expressed by bone marrow stromal cells and later shown to be expressed by plasma cells and multiple types of cancer cells (Ohtomo et al., 1999; Kupzig, 2003; Walter-Yohrling et al., 2003). BST2 is reportedly expressed on many different types of cells after exposure to IFN-α (Blasius et al., 2006b; Neil et al., 2008; Van Damme et al., 2008).
BST2 is an ILT7 ligand
The 26F8 and 28G4 mAbs stained the human BST2 cDNA-transfected HEK293 cells, but not mock-transfected cells, by flow cytometry (Fig. 3 A). In addition, recombinant ILT7 protein directly bound to a recombinant BST2-GST fusion protein, but not GST protein, in a dose-dependent manner (Fig. 3 B). Specific interaction between recombinant BST2-Fc and ILT7-Fc with an estimated affinity of ∼10−6 M was detected by surface plasma resonance with Biacore, an interaction that was completely neutralized by 26F8 mAb (Fig. 3 C).
Figure 3.
BST2 directly binds to ILT7. (A) HEK293 cells transiently transfected with mock control or BST2 cDNA were analyzed by Western blotting for BST2 protein expression. Transfected cells were stained with 26F8 mAb and analyzed by flow cytometry. The staining profile with IgG isotype-matched control mAb is shown in the shaded area. Staining with 28G4 mAb produced identical results (not depicted). Data are representative of five independent experiments. (B) Plate-coated GST or BST2-GST were incubated with different concentrations of recombinant ILT7-Fc and HRP-conjugated anti–human Fc. Shown is absorption at OD 450 nm for each sample after addition of Tetramethyl benzidine (TMB) substrate. Data are representative of three independent experiments. (C, top) Recombinant BST2-Fc, precoated on the surface of Biacore sensor chips, was mixed with different concentrations of injected recombinant ILT7-Fc. The kinetic response data after subtracting the value from precoated Fc are shown. Also shown are control responses with Fc alone or buffer. (bottom) Precoated recombinant BST2-Fc was premixed with buffer, control IgG1, or anti-BST2 mAb 26F8 and then exposed to injected recombinant ILT7-Fc. The kinetic response data after subtracting the value from precoated Fc are shown. Data are representative of two independent experiments.
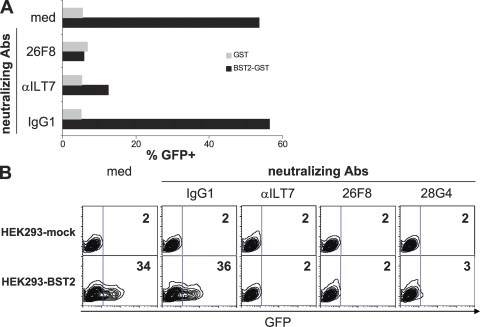
Furthermore, rBST2-GST fusion protein, but not GST alone, strongly activated the ILT7 reporter cells (Fig. 4 A). The specificity of the BST2–ILT7 interaction was demonstrated by the findings that rBST2-GST failed to induce GFP expression in ILT7-negative reporter cells and that BST2-induced GFP expression in the ILT7 reporter cells was abrogated by neutralizing antibodies against either ILT7 or BST2, but not by control antibody. More importantly, BST2 expressed on the surface of HEK293 cells induced GFP expression in ILT7 reporter cells, but not in ILT7-negative reporter cells. Again, activation of the ILT7 reporter was blocked by neutralizing antibodies against either ILT7 or BST2 (Fig. 4 B). These data identify BST2 as a physiological ligand that specifically binds and activates ILT7.
Figure 4.
BST2 binds and potently activates ILT7. (A) GST or BST2-GST protein was co-cultured with ILT7+ NFAT-GFP reporter cells. The percentages of GFP-positive reporter cells are shown. Neutralizing antibodies αILT7 (5 µg/ml) or 26F8 (25 µg/ml) or an IgG1 control antibody were included in the cultures, as indicated. Data are representative of four independent experiments. (B) HEK293 cells transiently transfected with mock control or BST2 cDNA were co-cultured with ILT7+ NFAT-GFP reporter cells. The percentages of GFP+ reporter cells are shown. Neutralizing antibodies αILT7 (5 µg/ml), 26F8 (25 µg/ml), 28G4 (25 µg/ml), or control IgG1 were included in the cultures, as indicated. Data are representative of three independent experiments.
BST2 negatively regulates the innate immune functions of human pDCs
As previously shown, antibody cross-linking of ILT7 can induce prominent calcium influx in primary pDCs as a result of ITAM-mediated FcϵRIγ signaling (Cao et al., 2006; Cho et al., 2008). Similarly, a rBST2-Fc protein, but not a control Fc protein alone, induced calcium mobilization in human pDCs, which depends on the function of Syk (Fig. 5 A).
Figure 5.
BST2 activates primary pDCs and inhibits IFN and cytokine production by pDCs. (A) pDCs were incubated with anti-ILT7, recombinant Fc, or BST2-Fc proteins and then analyzed for calcium influx. pDCs pretreated with 5 µM of Syk inhibitor were also analyzed. Data are representative of three independent experiments. (B) The amounts of secreted cytokines from pDCs cultured with plate-bound Fc or BST2-Fc are shown. pDCs were activated overnight with either 0.2 µM of CpG 2216 or MOI 6 of Flu. Data are representative of five independent experiments with ten donors. (C) The levels of gene transcripts from pDCs cultured with purified Fc or BST2-Fc are shown. The relative expression of each gene was normalized with S18 and calculated against the value obtained from normal total PBMCs. Data are representative of two independent experiments. (D) BST2 does not affect co-stimulatory molecule expression by pDCs. pDCs cultured with plate-bound Fc or BST2-Fc and then activated with 0.2 µM of CpG 2216 for 48 h. Surface levels of CD80 and CD86 are shown. Data are representative of two independent experiments. (E) pDCs preincubated with medium, 20 µg/ml of IgG1 or 20 µg/ml of neutralizing ILT7 mAb were cultured overnight with plate-bound Fc or BST2-Fc in the presence of CpG 2216. The amounts of secreted IFN-α were measured by ELISA. Percent of BST2-mediated IFN-α suppression was calculated as percent ratio of IFN-α ([Fc minus BST2-Fc]/[Fc]) and plotted. Data are representative of two independent experiments with four donors. (F) The amounts of secreted IFN-α from Flu-challenged pDCs cultured with HEK293 with or without surface HA-tagged BST2 are shown. Data are representative of two independent experiments.
Because mAb cross-linking of ILT7 inhibits the immune response of pDCs, we investigated the effect of BST2 on TLR-induced cytokine responses by pDCs. Freshly isolated pDCs from human peripheral blood were preincubated with plate-bound rBST2-Fc protein or plate-bound Fc protein for 30 min, and were then challenged with influenza virus (Flu) or CpG, which trigger TLR7 and TLR9, respectively. rBST2 protein suppressed the secretion of IFN-α and TNF-α (Fig. 5 B), as well as the transcription of type I IFN subtypes, including IFNα1, IFNα4, IFNα8, and IFN-β, plus IL-6, by pDCs (Fig. 5 C) when activated by TLR agonists. In contrast, BST2 did not alter expression of co-stimulatory molecules, such as CD80 and CD86, by pDCs (Fig. 5, C and D). The effect of BST2-mediated IFN suppression was abolished when ILT7 was neutralized with anti-ILT7 mAb, but not with control IgG1, indicating a direct engagement between ILT7 and rBST2 (Fig. 5 E). Lastly, pDCs co-cultured with HEK293 cells expressing an HA-tagged BST2 secreted reduced levels of IFN-α in response to Flu virus when compared with pDCs in contact with untransfected HEK293 cells (Fig. 5 F), suggesting that the BST2–ILT7 interaction modulates pDCs’ TLR-induced IFN responses.
BST2 expression by human cells and by pDCs
To understand the prevalence of BST2 expression on human cells in response to IFN treatment, we cultured embryonic kidney HEK293 cells, dermal fibroblast line NHDF cells, human umbilical vein endothelial line HUVEC cells, and keratinocyte line HaCat cells in the absence or presence of IFN-α, and then analyzed BST2 surface expression by flow cytometry (Fig. 6 A). Although these cells minimally express BST2 under normal conditions, IFN-α treatment resulted in significant surface BST2 expression in all of them, which is consistent with the reported IFN-mediated BST2 induction (Blasius et al., 2006b; Neil et al., 2008; Van Damme et al., 2008).
Figure 6.
Analysis of BST2 expression and potential BST2–ILT7 cis-interaction. (A) HEK293, NHDF, HUVEC, and HaCat cells, cultured in the absence or presence of 500 units/ml of IFN-α for 48 h, were stained with anti-BST2 mAb 26F8. Staining with isotype-matched control Ig is shown in the gray shaded area. Data are representative of three independent experiments. (B) Fresh isolated pDCs from peripheral blood or cells activated with various stimuli for 48 h were analyzed for surface BST2 expression by flow cytometry. Data are representative of two independent experiments. (C) Human Burkitt’s lymphoma Namalwa cells transduced with different pDC receptor complexes, i.e., BDCA2/FcϵRIγ or ILT7/FcϵRIγ, were analyzed for surface BST2 and ILT7 expression. Data are representative of three independent experiments.
Interestingly, resting mouse pDCs prominently express BST2 (Blasius et al., 2006b), but lack the expression of a direct orthologue of ILT7 (Brown et al., 2004). However, low amounts of surface BST2 were detected on human pDCs that were freshly isolated or cultured with either TLR ligands or cytokines (Fig. 6 B), although BST2 transcripts were found elevated in TLR-activated pDCs (unpublished data). To investigate a potential cis interaction between ILT7 and BST2, we transduced ILT7 and FcϵRIγ into a human Burkitt’s lymphoma cell line expressing endogenous BST2. Interestingly, expression of the ILT7–FcϵRIγ complex, but not BDCA2–FcϵRIγ, another human pDC-specific receptor complex, reduced the levels of surface BST2 (Fig. 6 C). Thus, ILT7–BST2 cis interaction likely results in BST2 internalization and might have functional consequences in human pDCs.
DISCUSSION
Here, we identify BST2 as a physiological ligand for a human pDC-specific receptor ILT7. pDCs play a critical role in antiviral innate immune responses by secreting large quantities of IFN-α/β. However, the type 1 IFN responses immediately after viral infection are short lived; if not, massive and prolonged IFN exposure will damage hematopoiesis, leading to lymphopenia (Lin et al., 1998; Kamphuis et al., 2006), and increase the risk of autoimmunity (Gota and Calabrese, 2003). Hence, a mechanism ensuring a specific and transient IFN response to viruses is critical to minimize the possibility of lymphopenia and autoimmune diseases in the host. As BST2 is well known to be robustly induced on the surface of various types of cells after exposure to IFN and other proinflammatory cytokines via STAT activation (Ohtomo et al., 1999; Blasius et al., 2006b; Neil et al., 2008; Van Damme et al., 2008), the BST2–ILT7 interaction, therefore, likely serves as an important negative feedback mechanism for preventing prolonged IFN production after viral infection (Fig. 7).
Figure 7.
A proposed model of BST2–ILT7 mediated regulation of pDC innate immune responses. (top) By sensing viral infection, pDCs can rapidly and rigorously produce large amounts of IFN-I via TLR7 or TLR9 activation. IFN-I then may induce the neighboring cells to express BST2, which in turn engages with ILT7 on pDCs to down-regulate the magnitude of IFN and cytokine responses in a negative feedback manner. (bottom) In a tumor environment where BST2 is endogenously expressed, infiltrating pDCs may be functionally suppressed to elicit normal IFN response to TLR ligands as a result of BST2–ILT7 interaction.
Intracellular TLRs have limited ability to discriminate host versus foreign nucleic acids (Haas et al., 2008). Several host factors, including anti-DNA antibodies, antimicrobial peptide LL37, or the nuclear DNA-binding protein HMGB1, alone or in combination, facilitate entry of self-DNA into the endosomes of pDCs, where they trigger TLR9 to induce type 1 IFN responses (Lande et al., 2007; Marshak-Rothstein and Rifkin, 2007; Tian et al., 2007). Similarly, autoantibody–self small nuclear ribonucleoprotein complexes can activate TLR7 through FcγRII to induce IFN (Vollmer et al., 2005; Savarese et al., 2006). This might lead to the constitutive activation of pDCs, which contributes to the autoimmune pathology of systemic lupus erythematosus and psoriasis. It will be of further interest to study if the BST2–ILT7–mediated controlling mechanism is breached in patients with systemic lupus erythematosus and psoriasis (Blanco et al., 2001; Lande et al., 2007; Marshak-Rothstein and Rifkin, 2007), which may provide an opportunity to develop therapeutics to down-modulate pDC activation during autoimmune disease.
Interestingly, BST2 represents the first non–MHC class I–type ligand for a member of the ILT receptor family (Brown et al., 2004). By sequence analysis, human receptor ILTs are divided into two separate groups—five inhibitory ILTs containing intracellular immunoreceptor tyrosine-based inhibitory motifs and six stimulatory ILTs that would couple with ITAM-bearing adaptors. The inhibitory ILTs, e.g., ILT2 and ILT4, recognize proteins encoded by many MHC class I alleles and UL18, which is a MHC-like molecule associated with human cytomegalovirus (Chapman et al., 1999). However, the ligands for the stimulatory group of ILTs are largely unknown (Cosman et al., 1997; Brown et al., 2004). Structural analysis revealed significant differences in MHC class I–binding sites between the inhibitory and stimulatory ILTs (Shiroishi et al., 2006), suggesting distinct ligand recognition by the two groups of ILTs. Here, we used a NFAT-GFP reporter cell system that reliably senses ILT7 surface ligation in the presence of an ILT7 ligand, which led to the positive identification of BST2, a glycoprotein unrelated to the MHC molecules. BST2 protein has a unique topology, containing an N-terminal transmembrane domain and a C-terminal GPI anchor, and locates within the lipid raft microdomains on plasma membrane (Kupzig, 2003). Further structural analysis of the binding interface between BST2 and ILT7 may shed light on the structural basis of ligand recognition by ILT7 and stimulatory ILTs. The receptor-NFAT reporter system should be also useful for the identification of the relevant ligands for other ILT orphan receptors and a better understanding of ILT’s biological function.
As the innate immune system has widely adopted “paired receptors” to exert tight regulatory control, many of the inhibitory receptors participate in self tolerance by recognizing ubiquitously expressed endogenous molecules, whereas stimulatory receptors may recognize “alert” molecules that are up-regulated after viral infection or immune activation (Lanier, 2008; Yamada and McVicar, 2008). For example, Ly49A, a prototype inhibitory Ly49 receptor expressed on mouse NK cells, recognizes endogenous MHC-I molecule H-2Dd (Karlhofer et al., 1992), whereas stimulatory NK receptor Ly49H binds to glycoprotein M157 encoded by mouse cytomegalovirus (Arase et al., 2002; Smith et al., 2002), Ly49P recognizes the H-2Dk–restricted mouse cytomegalovirus–infected cells (Desrosiers et al., 2005), and NKG2D interacts with ligands with structural homology with MHC class I, including RAE-1α-ϵ, H60, and MULT1 (Cerwenka et al., 2000; Diefenbach et al., 2001, 2003; Carayannopoulos et al., 2002), expression of which is under control of TLR activation, viral infections, and stress (Lodoen et al., 2003; Hamerman et al., 2004; Nice et al., 2009). In human, NKG2D engages with MHC class I–like molecules MICA and MICB (Bauer et al., 1999), inducible by cell stress, and ULBP1-4 (Cosman et al., 2001; Chalupny et al., 2003), expression of which is influenced by TLR activation or viral infections (Ebihara et al., 2007). The fact that BST2 is a gene product whose expression is under control of inflammatory signals is consistent with the notion that stimulatory receptors may regulate immune functions by interacting with inducible or pathogen-associated ligands.
Like NK cells, pDCs express both inhibitory receptors and stimulatory receptors. Human pDC receptors ILT7, BDCA2, high-affinity Fc receptor for IgE, and NKp44, which all signal through an ITAM-mediated pathway, exert negative effects on the pDC’s IFN response to TLR activation (Dzionek et al., 2001; Novak et al., 2004; Fuchs et al., 2005; Cao et al., 2006, 2007). Similarly, activation of Siglec-H, in association with the ITAM-bearing DAP12 subunit, reduces type I IFN production by mouse pDCs in vitro and in vivo (Blasius et al., 2006a). On the other hand, Ly49Q, an inhibitory Ly49 receptor expressed on mouse pDCs, recognizes MHC class I molecules and enhances pDC’s innate immune responses (Tai et al., 2008). Thus, it appears that the so-called stimulatory receptors may exercise potent suppressive regulatory functions on the innate immunity of pDCs.
Immature pDCs have been found in the local tumor environment of patients with ovarian cancer (Zou et al., 2001; Wei et al., 2005), breast cancer (Treilleux et al., 2004), melanoma (Salio et al., 2003; Benitez-Ribas et al., 2006), leukemia (Mohty et al., 2001, 2004), Kaposi’s sarcoma (Della Bella et al., 2006), and head and neck squamous cell carcinoma (Hartmann et al., 2003). Although pDCs only infiltrate 13% of primary breast tumors, their presence strongly correlates with an adverse outcome of the disease (Treilleux et al., 2004). The breast cancer microenvironment profoundly inhibits pDC’s innate immune functions (Sisirak et al., 2008). Because IFN-α is well known to be required for deterring the growth of primary tumors (Wenzel et al., 2005; Dunn et al., 2006), BST2–ILT7 interactions that suppress pDCs’ IFN responses may contribute to the tumor tolerance (Fig. 7). On the other hand, BST2 found in multiple cancer types was identified as a potent inducer of NF-κB (Matsuda et al., 2003) and one of the candidate genes expressed in malignant cells that promote tumor invasion (Ohtomo et al., 1999; Kupzig, 2003; Walter-Yohrling et al., 2003). Thus, BST2–ILT7 interaction might be bidirectional in a cancer environment, and the consequence of ILT7 engagement on BST2-expressing tumor cells should be investigated in the future.
MATERIALS AND METHODS
Reagents and cells.
HEK293 cells were grown in high-glucose DME supplemented with 10% FBS, 50 U/ml penicillin, and 50 µg/ml streptomycin. Breast cancer cells and mouse 2B4 NFAT-GFP reporter cells (Cao et al., 2006) were grown in RPMI 1640 medium supplemented with 10% FBS and antibiotics. NHDF and HUVEC cells (Lonza) were cultured in Clonetics Fibroblast Cell Medium FGM-2 and Endothelial Cell Basal medium, respectively, supplemented with growth factors following the manufacturer’s recommendations. HaCaT cells were provided by S. Ullrich (M.D. Anderson Cancer Center, Houston, TX) and cultured in RPMI 1640 medium supplemented with 10% FBS and antibiotics. Namalwa cells were cultured as previously described (Cao et al., 2007). Human TNF-α was purchased from Peprotech. IFN-α was purchased from Sigma-Aldrich.
ILT7 reporter cell assay.
ELISA plates were coated with 10 µg/ml of protein in PBS or at the concentration specified. 105 cancer cells or transfected HEK293 cells were seeded in 24-well plates the day before the experiment. After 105 NFAT-GFP reporter cells were added to the ELISA plate or the cell monolayer, the plates were spun at 100 g for 2 min. After overnight culture, cells were subjected to flow cytometric analysis to measure GFP expression.
BST2 activation of human primary pDCs.
The institutional review board for human research at the M.D. Anderson Cancer Center approved the use of human blood samples for this study. Primary human pDCs were isolated from blood using a negative selection kit (Miltenyi Biotech) and sorted by flow cytometry as CD3−CD11c−CD14−CD15−CD16−CD19−CD56−CD4+CD123+ cells. pDCs were preincubated with plate-bound control Fc protein or BST2-Fc protein captured with 10 µg/ml of F(ab)2 goat anti–human IgG Fc (Jackson ImmunoResearch Laboratories) on an ELISA plate for 30 min before stimulation with 0.2 µM of CpG 2216 (Sigma-Genosys) or heat-inactivated influenza virus PR8 at a multiplicity of infection (MOI) of 6. 18 h later, the supernatants were harvested and analyzed for cytokines, and the cells were lysed and RNA subjected to RT-PCR analysis, as previously described (Cao et al., 2006). To neutralize ILT7 in this assay, pDCs were preincubated with 20 µg/ml of anti-ILT7 (clone 17.2; Cao et al., 2006), washed, and then incubated with plate-bound Fc or BST2-Fc and stimulated with CpG. In parallel, control pDCs were preincubated with medium or 20 µg/ml of IgG1, washed, and stimulated similarly on Fc- or BST2-Fc–coated plates. To perform pDC and BST2-transfectant cell co-culture, pDCs were preincubated with inactivated influenza virus (MOI = 8) for 30 min, and cells were pelleted by centrifugation for 5 min at 500 g and added to parent HEK293 or BST2-HA–transfected HEK293 monolayers. Cells were packed briefly by centrifuging at 100 g for 2 min. 18 h later, the supernatants were harvested and analyzed for cytokine secretion. To study calcium influx, purified control Fc protein or BST2-Fc protein was incubated with pDCs in the presence of goat anti–human Fc F(ab)2 (Jackson ImmunoResearch Laboratories). Dye loading and flow cytometric analysis were performed as previously described (Cao et al., 2006).
Anti–ILT7-L mAb generation.
6–8-wk-old BALB/c mice were immunized with T47D and MDA-MB-231 cells by the alternate footpad method (Cao et al., 2006). Hybridoma clones secreting mAbs that specifically stained T47D cells, but not MDA-MB-231 cells, were expanded. They were further screened for their ability to block T47D-induced GFP expression from ILT7+ reporter cells. mAbs 26F8 (IgG1) and 28G4 (IgG2a) were affinity purified and fluorochrome conjugated using mAb conjugation kits (Invitrogen).
cDNA library screening.
A library of human full-length cDNA clones was purchased from OriGene Technologies, Inc. Plasmid DNA was prepared using the Wizard plus miniprep kit (Promega). For transfection, HEK293 cells were seeded at 104 cells/well in 96-well plates. 0.6 µl of Fugene 6 (Roche) was added to 15 µl of Opti-MEM (Invitrogen) and mixed with 10 µl of DNA from individual cDNA clone. After 15 min at room temperature, the mixture was added to cells. 48 h later, cells were centrifuged at 200 g for 3 min before the media was removed. 5 µg/ml of mAbs 26F8 or 28G4 was added and mixed with Cy5-labeled goat anti-mIgG (Jackson ImmunoResearch Laboratories). Cells were incubated at room temperature for 2 h in the dark and analyzed by using a fluorometric microvolume assay technology 8100 HTS system (Applied Biosystems).
BST2 transfection and expression analysis.
HEK293 cells were transfected with an expression plasmid containing the full-length human BST2 cDNA (Open Biosystems) with lipofectamine (Invitrogen). 48 h later, cells were either lysed for Western blot analysis using a BST2-specific rabbit polyclonal antibody (FabGennix, Inc.) or subjected to staining with fluorochrome-conjugated anti–ILT7-L mAbs. HEK293 cells stably expressing BST2-HA were transfected with pcDNA3-zeo-BST2-HA, selected with Zeocin, and sorted for high HA expression by flow cytometry.
Generation of recombinant ILT7 and BST2 fusion protein.
The extracellular domain of ILT7 or extracellular domain of BST2 (excluding GPI anchor) were cloned into an expression vector containing a mutated human Fc fragment (Arase et al., 2002). HEK293 cells were transiently transfected with the expression plasmids or the empty vector to produce recombinant protein, which was purified by using a Protein A column (GE Healthcare). The extracellular domain of BST2 (excluding GPI anchor) was constructed as a GST-fusion protein, which was stably expressed in CHOK1SV cells and purified by glutathione–Sepharose affinity chromatography (GE Healthcare).
Surface plasma resonance analysis.
Surface plasma resonance was performed using a Biacore 3000 (GE Healthcare). BST2-Fc and Fc were covalently immobilized onto flow cells of a CM5 sensor chip by amine coupling according to the manufacturer’s instruction. ILT7-Fc, Fc, and 26F8 in 10 mM Hepes (pH 7.4), 150 mM NaCl, and 0.005% P20 were passed over the chip at 20 µl/min. To block the interactions between BST2 and ILT7, 26F8 (120 µg/ml), IgG1 (120 µg/ml), or buffer was injected over BST2-Fc–coated sensor chip before injection of ILT7-Fc (120 µg/ml).
Acknowledgments
We thank David Son and Ran Zhang for excellent technical assistance and Dr. Margaret Kripke for critiques of the manuscript.
This work is supported by grants from the National Institutes of Health (AI068129 to L.L. Lanier, AI074809 to W. Cao, and AI 059718 to Y.-J. Liu). L.L. Lanier is an American Cancer Society Research Professor.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used: BST2, bone marrow stromal cell antigen 2; ILT, Ig-like transcript; ITAM, immunoreceptor tyrosine-based activation motif; MOI, multiplicity of infection; pDC, plasmacytoid DC; TLR, Toll-like receptor.
References
- Arase H., Mocarski E.S., Campbell A.E., Hill A.B., Lanier L.L. 2002. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors.Science. 296:1323–1326 [DOI] [PubMed] [Google Scholar]
- Bauer S., Groh V., Wu J., Steinle A., Phillips J.H., Lanier L.L., Spies T. 1999. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA.Science. 285:727–729 [DOI] [PubMed] [Google Scholar]
- Benitez-Ribas D., Adema G.J., Winkels G., Klasen I.S., Punt C.J.A., Figdor C.G., de Vries I.J.M. 2006. Plasmacytoid dendritic cells of melanoma patients present exogenous proteins to CD4+ T cells after FcγRII-mediated uptake.J. Exp. Med. 203:1629–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco P., Palucka A.K., Gill M., Pascual V., Banchereau J. 2001. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus.Science. 294:1540–1543 [DOI] [PubMed] [Google Scholar]
- Blasius A.L., Cella M., Maldonado J., Takai T., Colonna M. 2006a. Siglec-H is an IPC-specific receptor that modulates type I IFN secretion through DAP12.Blood. 107:2474–2476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasius A.L., Giurisato E., Cella M., Schreiber R.D., Shaw A.S., Colonna M. 2006b. Bone marrow stromal cell antigen 2 is a specific marker of type I IFN-producing cells in the naive mouse, but a promiscuous cell surface antigen following IFN stimulation.J. Immunol. 177:3260–3265 [DOI] [PubMed] [Google Scholar]
- Brown D., Trowsdale J., Allen R. 2004. The LILR family: modulators of innate and adaptive immune pathways in health and disease.Tissue Antigens. 64:215–225 [DOI] [PubMed] [Google Scholar]
- Cao W., Rosen D.B., Ito T., Bover L., Bao M., Watanabe G., Yao Z., Zhang L., Lanier L.L., Liu Y.-J. 2006. Plasmacytoid dendritic cell–specific receptor ILT7-FcϵRIγ inhibits Toll-like receptor–induced interferon production.J. Exp. Med. 203:1399–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W., Zhang L., Rosen D.B., Bover L., Watanabe G., Bao M., Lanier L.L., Liu Y.-J. 2007. BDCA2/FcϵRIγ complex signals through a novel BCR-like pathway in human plasmacytoid dendritic cells.PLoS Biol. 5:e248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carayannopoulos L.N., Naidenko O.V., Fremont D.H., Yokoyama W.M. 2002. Cutting edge: murine UL16-binding protein-like transcript 1: a newly described transcript encoding a high-affinity ligand for murine NKG2D.J. Immunol. 169:4079–4083 [DOI] [PubMed] [Google Scholar]
- Cerwenka A., Bakker A.B.H., McClanahan T., Wagner J., Wu J., Phillips J.H., Lanier L.L. 2000. Retinoic acid early inducible genes define a ligand family for the activating NKG2D receptor in mice.Immunity. 12:721–727 [DOI] [PubMed] [Google Scholar]
- Chapman T.L., Heikema A.P., Bjorkman P.J. 1999. The inhibitory receptor LIR-1 uses a common binding interaction to recognize class I MHC molecules and the viral homolog UL18.Immunity. 11:603–613 [DOI] [PubMed] [Google Scholar]
- Chalupny N.J., Sutherland C.L., Lawrence W.A., Rein-Weston A., Cosman D. 2003. ULBP4 is a novel ligand for human NKG2D.Biochem. Biophys. Res. Commun. 305:129–135 [DOI] [PubMed] [Google Scholar]
- Cho M., Ishida K., Chen J., Ohkawa J., Chen W., Namiki S., Kotaki A., Arai N., Arai K.-i., Kamogawa-Schifter Y. 2008. SAGE library screening reveals ILT7 as a specific plasmacytoid dendritic cell marker that regulates type I IFN production.Int. Immunol. 20:155–164 [DOI] [PubMed] [Google Scholar]
- Colonna M., Trinchieri G., Liu Y.J. 2004. Plasmacytoid dendritic cells in immunity.Nat. Immunol. 5:1219–1226 [DOI] [PubMed] [Google Scholar]
- Cosman D., Fanger N., Borges L., Kubin M., Chin W., Peterson L., Hsu M.-L. 1997. A novel immunoglobulin superfamily receptor for cellular and viral MHC class I molecules.Immunity. 7:273–282 [DOI] [PubMed] [Google Scholar]
- Cosman D., Müllberg J., Sutherland C.L., Chin W., Armitage R., Fanslow W., Kubin M., Chalupny N.J. 2001. ULBPs, novel MHC class I related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor.Immunity. 14:123–133 [DOI] [PubMed] [Google Scholar]
- Della Bella S., Nicola S., Brambilla L., Riva A., Ferrucci S., Presicce P., Boneschi V., Berti E., Villa M.L. 2006. Quantitative and functional defects of dendritic cells in classic Kaposi’s sarcoma.Clin. Immunol. 119:317–329 [DOI] [PubMed] [Google Scholar]
- Desrosiers M.-P., Kielczewska A., Loredo-Osti J.C., Adam S.G., Makrigiannis A.P., Lemieux S., Pham T., Lodoen M.B., Morgan K., Lanier L.L., Vidal S.M. 2005. Epistasis between mouse Klra and major histocompatibility complex class I loci is associated with a new mechanism of natural killer cell-mediated innate resistance to cytomegalovirus infection.Nat. Genet. 37:593–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diefenbach A., Jensen E.R., Jamieson A.M., Raulet D.H. 2001. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity.Nature. 413:165–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diefenbach A., Hsia J.K., Hsiung M.-Y., Raulet D.H. 2003. A novel ligand for the NKG2D receptor activates NK cells and macrophages and induces tumor immunity.Eur. J. Immunol. 33:381–391 [DOI] [PubMed] [Google Scholar]
- Dunn G.P., Koebel C.M., Schreiber R.D. 2006. Interferons, immunity and cancer immunoediting.Nat. Rev. Immunol. 6:836–848 [DOI] [PubMed] [Google Scholar]
- Dzionek A., Sohma Y., Nagafune J., Cella M., Colonna M., Facchetti F., Gunther G., Johnston I., Lanzavecchia A., Nagasaka T., et al. 2001. BDCA-2, a novel plasmacytoid dendritic cell–specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon α/β induction.J. Exp. Med. 194:1823–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara T., Masuda H., Akazawa T., Shingai M., Kikuta H., Ariga T., Matsumoto M., Seya T. 2007. Induction of NKG2D ligands on human dendritic cells by TLR ligand stimulation and RNA virus infection.Int. Immunol. 19:1145–1155 [DOI] [PubMed] [Google Scholar]
- Fuchs A., Cella M., Kondo T., Colonna M. 2005. Paradoxic inhibition of human natural interferon-producing cells by the activating receptor NKp44.Blood. 106:2076–2082 [DOI] [PubMed] [Google Scholar]
- Gilliet M., Cao W., Liu Y.-J. 2008. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases.Nat. Rev. Immunol. 8:594–606 [DOI] [PubMed] [Google Scholar]
- Gota C., Calabrese L. 2003. Induction of clinical autoimmune disease by therapeutic interferon-alpha.Autoimmunity. 36:511–518 [DOI] [PubMed] [Google Scholar]
- Haas T., Metzger J., Schmitz F., Heit A., Müller T., Latz E., Wagner H. 2008. The DNA sugar backbone 2’ deoxyribose determines Toll-like receptor 9 activation.Immunity. 28:315–323 [DOI] [PubMed] [Google Scholar]
- Hamerman J.A., Ogasawara K., Lanier L.L. 2004. Cutting edge: Toll-like receptor signaling in macrophages induces ligands for the NKG2D receptor.J. Immunol. 172:2001–2005 [DOI] [PubMed] [Google Scholar]
- Hartmann E., Wollenberg B., Rothenfusser S., Wagner M., Wellisch D., Mack B., Giese T., Gires O., Endres S., Hartmann G. 2003. Identification and functional analysis of tumor-infiltrating plasmacytoid dendritic cells in head and neck cancer.Cancer Res. 63:6478–6487 [PubMed] [Google Scholar]
- Honda K., Taniguchi T. 2006. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors.Nat. Rev. Immunol. 6:644–658 [DOI] [PubMed] [Google Scholar]
- Jarrossay D., Napolitani G., Colonna M., Sallusto F., Lanzavecchia A. 2001. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells.Eur. J. Immunol. 31:3388–3393 [DOI] [PubMed] [Google Scholar]
- Kadowaki N., Ho S., Antonenko S., de Waal Malefyt R., Kastelein R.A., Bazan F., Liu Y.-J. 2001. Subsets of human dendritic cell precursors express different Toll-like receptors and respond to different microbial antigens.J. Exp. Med. 194:863–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamphuis E., Junt T., Waibler Z., Forster R., Kalinke U. 2006. Type I interferons directly regulate lymphocyte recirculation and cause transient blood lymphopenia.Blood. 108:3253–3261 [DOI] [PubMed] [Google Scholar]
- Karlhofer F.M., Ribaudo R.K., Yokoyama W.M. 1992. MHC class I alloantigen specificity of Ly-49+ IL-2-activated natural killer cells.Nature. 358:66–70 [DOI] [PubMed] [Google Scholar]
- Kawai T., Akira S. 2006. Innate immune recognition of viral infection.Nat. Immunol. 7:131–137 [DOI] [PubMed] [Google Scholar]
- Kupzig S. 2003. Bst-2/HM1.24 is a raft-associated apical membrane protein with an unusual topology.Traffic. 4:694–709 [DOI] [PubMed] [Google Scholar]
- Lande R., Gregorio J., Facchinetti V., Chatterjee B., Wang Y.-H., Homey B., Cao W., Wang Y.-H., Su B., Nestle F.O., et al. 2007. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide.Nature. 449:564–569 [DOI] [PubMed] [Google Scholar]
- Lanier L.L. 2008. Up on the tightrope: natural killer cell activation and inhibition.Nat. Immunol. 9:495–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q., Dong C., Cooper M.D. 1998. Impairment of T and B cell development by treatment with a type I interferon.J. Exp. Med. 187:79–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.-J. 2005. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors.Annu. Rev. Immunol. 23:275–306 [DOI] [PubMed] [Google Scholar]
- Lodoen M., Ogasawara K., Hamerman J.A., Arase H., Houchins J.P., Mocarski E.S., Lanier L.L. 2003. NKG2D-mediated natural killer cell protection against cytomegalovirus is impaired by viral gp40 modulation of retinoic acid early inducible 1 gene molecules.J. Exp. Med. 197:1245–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshak-Rothstein A., Rifkin I.R. 2007. Immunologically active autoantigens: the role of Toll-Like receptors in the development of chronic inflammatory Disease.Ann. Rev. Immunol. 25:419–441 [DOI] [PubMed] [Google Scholar]
- Matsuda A., Suzuki Y., Honda G., Muramatsu S., Matsuzaki O., Nagano Y., Doi T., Shimotohno K., Harada T., Nishida E., et al. 2003. Large-scale identification and characterization of human genes that activate NF-kappaB and MAPK signaling pathways.Oncogene. 22:3307–3318 [DOI] [PubMed] [Google Scholar]
- Mohty M., Jarrossay D., Lafage-Pochitaloff M., Zandotti C., Briere F., de Lamballeri X.-N., Isnardon D., Sainty D., Olive D., Gaugler B. 2001. Circulating blood dendritic cells from myeloid leukemia patients display quantitative and cytogenetic abnormalities as well as functional impairment.Blood. 98:3750–3756 [DOI] [PubMed] [Google Scholar]
- Mohty M., Olive D., Gaugler B. 2004. Plasmacytoid DCs and cancer: a new role for an enigmatic cell.Trends Immunol. 25:397–398 [DOI] [PubMed] [Google Scholar]
- Navarro F., Llano M., Bellón T., Colonna M., Geraghty D.E., López-Botet M. 1999. The ILT2(LIR1) and CD94/NKG2A NK cell receptors respectively recognize HLA-G1 and HLA-E molecules co-expressed on target cells.Eur. J. Immunol. 29:277–283 [DOI] [PubMed] [Google Scholar]
- Neil S.J.D., Zang T., Bieniasz P.D. 2008. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu.Nature. 451:425–430 [DOI] [PubMed] [Google Scholar]
- Nice T.J., Coscoy L., Raulet D.H. 2009. Posttranslational regulation of the NKG2D ligand Mult1 in response to cell stress.J. Exp. Med. 206:287–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak N., Allam J.-P., Hagemann T., Jenneck C., Laffer S., Valenta R., Kochan J., Bieber T. 2004. Characterization of FcϵRI-bearing CD123+ blood dendritic cell antigen-2+ plasmacytoid dendritic cells in atopic dermatitis.J. Allergy Clin. Immunol. 114:364–370 [DOI] [PubMed] [Google Scholar]
- Ohtomo T., Sugamata Y., Ozaki Y., Ono K., Yoshimura Y., Kawai S., Koishihara Y., Ozaki S., Kosaka M., Hirano T., Tsuchiya M. 1999. Molecular cloning and characterization of a surface antigen preferentially overexpressed on multiple myeloma cells.Biochem. Biophys. Res. Commun. 258:583–591 [DOI] [PubMed] [Google Scholar]
- Piqueras B., Connolly J., Freitas H., Palucka A.K., Banchereau J. 2006. Upon viral exposure, myeloid and plasmacytoid dendritic cells produce 3 waves of distinct chemokines to recruit immune effectors.Blood. 107:2613–2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röck J., Schneider E., Grün J.R., Grützkau A., Küppers R., Schmitz J., Winkels G. 2007. CD303 (BDCA-2) signals in plasmacytoid dendritic cells via a BCR-like signalosome involving Syk, Slp65 and PLCγ2.Eur. J. Immunol. 37:3564–3575 [DOI] [PubMed] [Google Scholar]
- Salio M., Cella M., Vermi W., Facchetti F., Palmowski M.J., Smith C.L., Shepherd D., Colonna M., Cerundolo V. 2003. Plasmacytoid dendritic cells prime IFN-γ-secreting melanoma-specific CD8 lymphocytes and are found in primary melanoma lesions.Eur. J. Immunol. 33:1052–1062 [DOI] [PubMed] [Google Scholar]
- Savarese E., Chae O.-w., Trowitzsch S., Weber G., Kastner B., Akira S., Wagner H., Schmid R.M., Bauer S., Krug A. 2006. U1 small nuclear ribonucleoprotein immune complexes induce type I interferon in plasmacytoid dendritic cells through TLR7.Blood. 107:3229–3234 [DOI] [PubMed] [Google Scholar]
- Shiroishi M., Kajikawa M., Kuroki K., Ose T., Kohda D., Maenaka K. 2006. Crystal Structure of the Human Monocyte-activating Receptor, “Group 2” Leukocyte Ig-like Receptor A5 (LILRA5/LIR9/ILT11).J. Biol. Chem. 281:19536–19544 [DOI] [PubMed] [Google Scholar]
- Sisirak V., Gobert M., Renaudineau S., Menetrier-Caux C., Aspord C., Banchereau J., Palucka A.K., Blay J.-Y., Caux C., Berdriss-Vermare N. 2008. Breast tumor environment inhibits human plasmacytoid dendritic cells functions. In The 10th International Symposium on Dendritic Cells (Kobe, Japan). pp. P-4-03 [Google Scholar]
- Smith H.R.C., Heusel J.W., Mehta I.K., Kim S., Dorner B.G., Naidenko O.V., Iizuka K., Furukawa H., Beckman D.L., Pingel J.T., et al. 2002. Recognition of a virus-encoded ligand by a natural killer cell activation receptor.Proc. Natl. Acad. Sci. USA. 99:8826–8831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai L.-H., Goulet M.-L., Belanger S., Toyama-Sorimachi N., Fodil-Cornu N., Vidal S.M., Troke A.D., McVicar D.W., Makrigiannis A.P. 2008. Positive regulation of plasmacytoid dendritic cell function via Ly49Q recognition of class I MHC.J. Exp. Med. 205:3187–3199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J., Avalos A.M., Mao S.-Y., Chen B., Senthil K., Wu H., Parroche P., Drabic S., Golenbock D., Sirois C., et al. 2007. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE.Nat. Immunol. 8:487–496 [DOI] [PubMed] [Google Scholar]
- Treilleux I., Blay J.-Y., Bendriss-Vermare N., Ray-Coquard I., Bachelot T., Guastalla J.-P., Bremond A., Goddard S., Pin J.-J., Barthelemy-Dubois C., Lebecque S. 2004. Dendritic cell infiltration and prognosis of early stage breast cancer.Clin. Cancer Res. 10:7466–7474 [DOI] [PubMed] [Google Scholar]
- Van Damme N., Goff D., Katsura C., Jorgenson R.L., Mitchell R., Johnson M.C., Stephens E.B., Guatelli J. 2008. The Interferon-Induced Protein BST-2 Restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein.Cell Host Microbe. 3:245–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer J., Tluk S., Schmitz C., Hamm S., Jurk M., Forsbach A., Akira S., Kelly K.M., Reeves W.H., Bauer S., Krieg A.M. 2005. Immune stimulation mediated by autoantigen binding sites within small nuclear RNAs involves Toll-like receptors 7 and 8.J. Exp. Med. 202:1575–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter-Yohrling J., Cao X., Callahan M., Weber W., Morgenbesser S., Madden S.L., Wang C., Teicher B.A. 2003. Identification of genes expressed in malignant cells that promote invasion.Cancer Res. 63:8939–8947 [PubMed] [Google Scholar]
- Wei S., Kryczek I., Zou L., Daniel B., Cheng P., Mottram P., Curiel T., Lange A., Zou W. 2005. Plasmacytoid dendritic cells induce CD8+ regulatory t cells in human ovarian carcinoma.Cancer Res. 65:5020–5026 [DOI] [PubMed] [Google Scholar]
- Wenzel J., Bekisch B., Uerlich M., Haller O., Bieber T., Tuting T. 2005. Type I interferon-associated recruitment of cytotoxic lymphocytes: a common mechanism in regressive melanocytic lesions.Am. J. Clin. Pathol. 124:37–48 [DOI] [PubMed] [Google Scholar]
- Yamada E., McVicar D. 2008. Paired receptor systems of the innate immune system.Curr. Protoc. Immunol. Appendix 1X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W., Machelon V., Coulomb-L’Hermin A., Borvak J., Nome F., Isaeva T., Wei S., Krzysiek R., Durand-Gasselin I., Gordon A., et al. 2001. Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells.Nat. Med. 7:1339–1346 [DOI] [PubMed] [Google Scholar]