Abstract
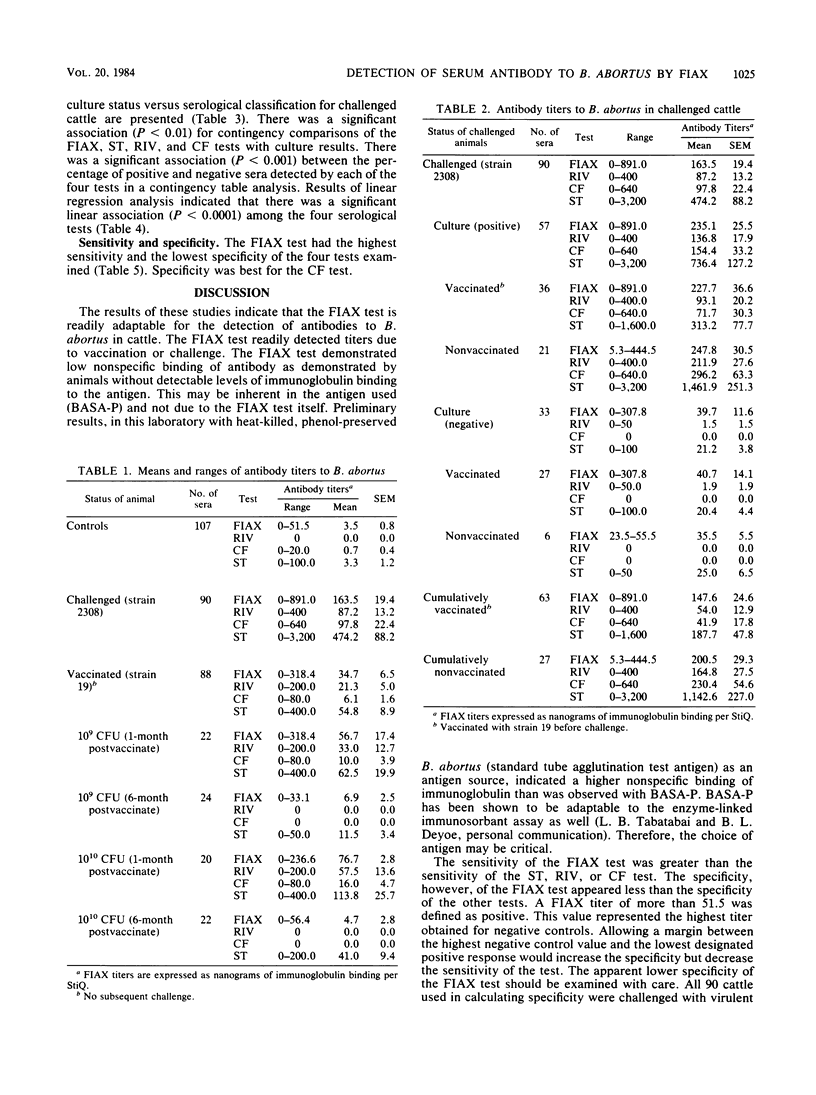
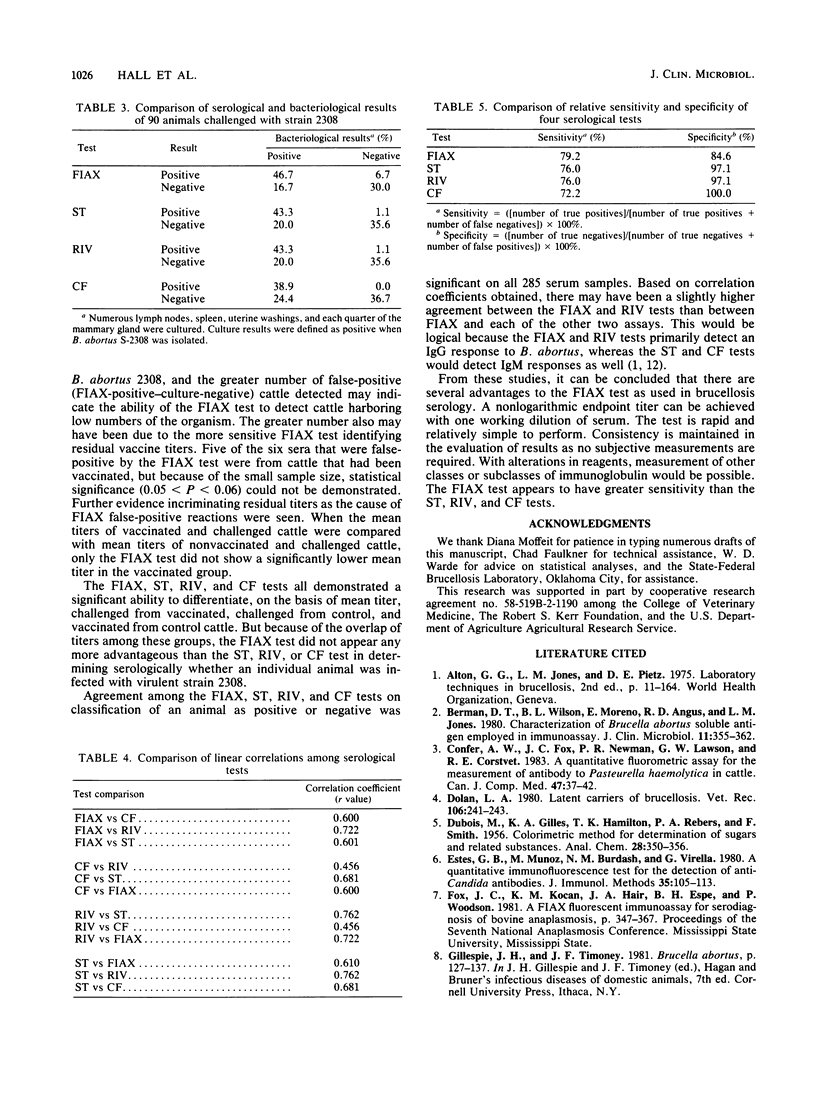
A quantitative fluorometric immunoassay (FIAX) was adapted for the detection of serum antibodies to Brucella abortus in cattle. Results are expressed in nanograms of immunoglobulin binding the antigen carrier. The FIAX was compared with the standard tube agglutination, Rivanol precipitation, and complement fixation tests, using 285 serum samples from vaccinated, challenged, or control cattle. Linear regression analysis indicated a significant correlation among all four serological tests; the FIAX test correlated best with the Rivanol test. Ninety sera were from vaccinated and nonvaccinated cattle that were challenged with virulent B. abortus 2308. The sensitivity and specificity of each serological test were determined based on culture results from these cattle. The FIAX was the most sensitive of the four serological tests, detecting 79.2% of the culture-positive animals. The FIAX was the least specific, with 15.4% of the culture-negative animals being classified as positive. Eighty-eight sera were from cattle vaccinated with strain 19 but not challenged. All four serological tests had a statistically significant ability to distinguish sera from control and vaccinates on the basis of mean titers. The mean titer of vaccinates was also significantly different from that of challenged animals. Advantages and disadvantages of the FIAX test for bovine brucellosis are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berman D. T., Wilson B. L., Moreno E., Angus R. D., Jones L. M. Characterization of Brucella abortus soluble antigen employed in immunoassay. J Clin Microbiol. 1980 Apr;11(4):355–362. doi: 10.1128/jcm.11.4.355-362.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Confer A. W., Fox J. C., Newman P. R., Lawson G. W., Corstvet R. E. A quantitative fluorometric assay for the measurement of antibody to Pasteurella haemolytica in cattle. Can J Comp Med. 1983 Jan;47(1):37–42. [PMC free article] [PubMed] [Google Scholar]
- Dolan L. A. Latent carriers of brucellosis. Vet Rec. 1980 Mar 15;106(11):241–243. doi: 10.1136/vr.106.11.241. [DOI] [PubMed] [Google Scholar]
- Estes G. B., Muñoz M., Burdash N. M., Virella G. A quantitative immunofluorescence test for the detection of anti-Candida antibodies. J Immunol Methods. 1980;35(1-2):105–113. doi: 10.1016/0022-1759(80)90155-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Morgan W. J. The serological diagnosis of bovine brucellosis. Vet Rec. 1967 May 27;80(21):612–620. doi: 10.1136/vr.80.21.612. [DOI] [PubMed] [Google Scholar]
- Nicoletti P. Problems in the diagnosis of bovine brucellosis. Dev Biol Stand. 1976;31:131–135. [PubMed] [Google Scholar]
- Weissfeld A. S., Gehle W. D., Sonnenwirth A. C. Comparison of several test systems used for determination of rubella immune status. J Clin Microbiol. 1982 Jul;16(1):82–85. doi: 10.1128/jcm.16.1.82-85.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]


