Abstract
Thymus settling by precursor cells is essential for the production of T cells, yet the immigration requirements are poorly defined. P-selectin and CC chemokine receptor-9 (CCR9) are involved, and settling is favored when existing residents have moved on. A new study strengthens the correlation between niche emptying and the induction of thymic P-selectin and CCR9 ligand, and provides evidence for feedback from the periphery to thymic P-selectin expression via sphingosine-1-phosphate.
To support sustained T cell production, the thymus depends on a supply of thymic progenitor cells (TPCs) arriving via the blood. TPCs settle the thymus in waves separated by multiple weeks (1). This has led to the fascinating concept that precursor immigration to the thymus is “gated.” One clue to how thymic gating occurs came from the finding that mice lacking IL-7R, which have dramatically reduced cell numbers in the thymus, had a marked increase in receptivity for settling by transferred precursors (2). Thus, gate opening is promoted when TPC numbers are low. But how gate opening is regulated remains a mystery. On page 761 of this issue, Gossens et al. (3) report on important new findings that identify additional factors that influence thymic gating and precursor immigration.
Immigration requirements: P-selectin ligand and CCR9
It is estimated that as few as 10 TPCs settle the mouse thymus per day (4). These cells give rise to early thymic progenitors (ETPs; characterized as Lin−CD44+CD25−CD117+ cells) that progress though the CD4 and CD8 double-negative (DN) stages (DN2, DN3, and DN4) of development over a period of ∼2 wk before becoming double-positive (DP) cells and, eventually, CD4 or CD8 single-positive (SP) T cells (4). Recent studies have shown that both P-selectin and CCR9 are required for TPCs to enter the thymus (4). Mice lacking these molecules have fewer immature cells in the thymus, yet generate normal numbers of DP and SP thymocytes, suggesting that additional undefined molecules also mediate TPC homing. P-selectin is expressed by thymic vessels (5), most likely in venules (6), and P-selectin glycoprotein ligand-1 (PSGL-1) is its only defined ligand. The P-selectin binding site on PSGL-1 is made up in part by core-2-O–linked sugars (7), and cells deficient in core 2 b1,6-glucosaminyltransferase-I (C2) suffer a partial impairment in P-selectin–dependent rolling (7). The CCR9 ligand CCL25 (TECK) is expressed by thymic endothelial cells and medullary and cortical epithelial cells (3, 8). Although the phenotype of TPCs remains a matter of debate, the emerging consensus is that they express both PSGL-1 and CCR9 (4, 9).
Periodicity in P-selectin and CCL25 expression
The study by Gossens et al. (3) begins with the authors’ previous finding that IL-7R–deficient mice have elevated P-selectin expression on thymic vessels (5). To further explore the correlation between niche occupancy and expression of molecules needed for TPC homing, the authors examined mice deficient in P-selectin, PSGL-1, and C2. Using intrathymic injection of bone marrow cells to measure niche occupancy, they find that all three strains have increased availability of thymic niche space (3). Findings in IL-7R−/− mice suggested that DN3 numbers controlled niche availability (2), but no alterations in DN3 numbers were seen in the three trafficking mutants in this study. Instead, there was a correlation between receptivity and endogenous ETP numbers in the thymus. Thus, the number of ETPs (or a subset within this compartment) may control intrathymic niche availability for incoming thymic precursors.
Supporting a positive relationship between niche occupancy and P-selectin expression, PSGL-1−/− mice had increased levels of P-selectin transcripts and protein, approaching the levels seen in IL-7R−/− mice. Unexpectedly, however, C2-deficient mice showed minimal increases in P-selectin (3). A second thymic homing factor, CCL25, was also increased in IL-7R– and PSGL-1–deficient (but not C2-deficient) thymi. In short-term homing assays with wild-type cells, homing to the thymus was increased in PSGL-1−/− recipients, but was not appreciably increased in C2−/− recipients, which is congruent with the P-selectin and CCL25 expression data. Thus, the relationship between thymic progenitor niche occupancy and endothelial homing molecule expression seemed to hold up for PSGL-1–deficient mice, but not for C2-deficient mice.
With two thymic homing molecules apparently responding to niche occupancy signals, Gossens et al. asked whether the periodicity of thymic gating could be associated with a periodicity in homing molecule expression. Remarkably, P-selectin transcript abundance oscillated in the adult thymus with a periodicity of ∼2 wk. CCL25 oscillated to a lesser extent, perhaps reflecting its more widespread expression in the thymus, but a correlation was observed between periods of high P-selectin and high CCL25 expression. These oscillations were less evident in PSGL-1– and C2-deficient mice, suggesting a negative link between the extent of niche occupancy and the propensity for oscillations to occur. Thus, the periodic filling and emptying of thymic niches may induce a corresponding change in P-selectin and CCL25 expression, which rise in occurence when the niches are empty and new precursors are needed, and then fall off once the niches are filled (Fig. 1). Future studies will need to quantitate ETP numbers in parallel with assessment of P-selectin and CCL25 protein abundance to more definitively establish this relationship.
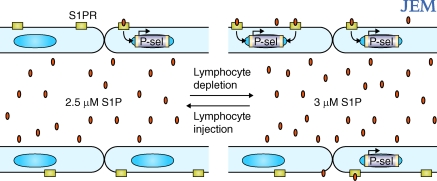
Figure 1.
Model to explain how niche occupancy by ETPs affects endothelial P-selectin and CCL25 expression. When the thymic niche is occupied (left), a signal generated by ETPs or niche cells inhibits P-selectin and CCL25 expression by the endothelium. Over a period of ∼2 wk, some ETP niches become vacant, relieving the inhibitory signal and leading to increased P-selectin and CCL25 expression (right). TPCs are then able to home into the thymus and give rise to ETPs, restarting the “clock.” Alternatively, when a niche is empty, niche cells may release a factor that promotes endothelial P-selectin and CCL25 expression and is consumed by ETPs.
Periphery-thymus cross talk via S1P
The authors next tried to find an explanation for the fact that ETP numbers were similarly reduced in PSGL-1−/− and C2−/− mice, despite the larger elevation in homing molecule expression in the PSGL-1−/− mice. A close look at the thymic subsets in PSGL-1−/− mice revealed an increase in the frequency of semimature (also called immature) SP cells and mature SP cells. This finding, along with the twofold decrease in circulating T cells in the periphery, suggested a possible thymic egress defect. SP thymocytes did not accumulate in the C2-deficient mice. The accumulation of thymocytes at the semimature SP stage is unusual for a thymic egress defect, as other strains of mice with egress defects selectively accumulate mature SP cells (10, 11). However, two additional approaches (intrathymic FITC injection and T cell receptor excision circle analysis) supported the authors’ claim that egress is affected.
The authors suspected that the decreased number of circulating blood cells seen in the PSGL-1–deficient mice somehow facilitated the induction of P-selectin. Indeed, when 20 million lymphocytes were intravenously injected in to PSGL-1−/− mice, thymic P-selectin mRNA levels dropped twofold in 2 d and receptivity for thymic settling was correspondingly diminished. Reciprocally, when peripheral CD4 and CD8 T cells were depleted for 24 h in wild-type mice, P-selectin expression and precursor receptivity increased. These results raise the exciting possibility that thymic receptivity for precursors is responsive to the peripheral demand for T cells. But how do alterations in peripheral T cell numbers alter thymic P-selectin expression? Here, Gossens et al. (3) find evidence for an unexpected link between sphingosine-1-phosphate (S1P) and thymic precursor immigration.
The current model for thymic egress posits that after SP thymocytes up-regulate S1P receptor-1 (S1P1), they encounter S1P associated with the blood vasculature and are triggered to emigrate (10). S1P is abundant in the blood and is kept low within the thymus by the action of S1P lyase (12). Using an internally standardized mass spectrometry assay, Gossens et al. measured the abundance of S1P in the blood and found a small but apparently significant reduction of S1P (∼20%) in PSGL-1−/− mice compared with wild-type mice—a reduction that is unlikely to cause an egress defect. P-selectin–deficient mice, on the other hand, had increased plasma S1P (3). Red blood cells (RBCs) are the source of ∼90% of blood S1P, and yet mice lacking this S1P source have normal thymic egress (13). S1P produced by a radiation-resistant source is more potent than RBC-derived S1P in promoting egress (13), and it will be interesting to explore whether the former source is affected by PSGL-1 deficiency.
Although the significance of the reduced plasma S1P in PSGL-1–deficient mice is not yet clear, it prompted the authors to make a potentially important connection. Endothelial cells express multiple S1P receptors, and S1P has been shown to augment P-selectin expression in vitro (3, 14). The authors pursued this relationship in vivo and discovered a correlation between S1P plasma abundance and thymic P-selectin mRNA levels in wild-type mice. Moreover, 3 d of treatment with the S1P agonist FTY720, which blocks thymic egress, lead to a reduction in P-selectin mRNA levels. P-selectin mRNA levels also decreased when S1P lyase was inhibited, increasing thymic S1P levels to amounts previously shown to decrease thymocyte S1P1 expression (12). Both of these treatments may cause desensitization (or degradation) of S1P receptors—perhaps those basolaterally exposed on endothelial cells—and thus reduce S1P-mediated P-selectin induction (Fig. 2). Future studies will need to explore the specific S1P receptors involved and whether the action is directly on the endothelial cell or at another site. Because P-selectin is expressed by venules in most tissues, it will be important to determine if its expression is also modulated in other vascular beds.
Figure 2.
Model for how changes in plasma S1P affect the expression of P-selectin by thymic endothelium. The micromolar concentrations of S1P in plasma may continuously saturate luminal S1P receptors (left), but small increases in plasma S1P (or local changes in S1P production by endothelial cells) may increase the engagement of receptors on the ablumenal side of the enodothelium, where S1P concentrations are low, leading to changes in expression of genes such as P-selectin (right).
Exploring whether alterations in circulating lymphocyte number might influence thymic P-selectin expression by altering S1P abundance, the authors found that S1P was elevated in plasma of T cell–depleted mice (contrary to what happens in the lymphopenic PSGL-1−/− mice). Reciprocally, when T or B cells were infused into PSGL-1−/− mice, the S1P concentration was further reduced (3). In these cases, the small increases or decreases in plasma S1P are concordant with the corresponding changes in thymic P-selectin expression (Fig. 2).
Ongoing interrogations at the border
In summary, the study by Gossens et al. (3) suggests that oscillating thymic expression of P-selectin and CCL25 may contribute to thymic gating. The new data reveal a possible role for PSGL-1 in thymic egress and provide evidence for an in vivo relationship between plasma S1P abundance and thymic P-selectin expression. This work not only provides insights into factors regulating thymic precursor immigration, it also has implications for understanding the interplay between S1P and the vascular system. However, the work also leaves us with some unresolved issues and opens up many new questions. For example, how is the periodic P-selectin expression achieved? This study and earlier work suggests that maturation of the limited pool of ETPs is involved, but how niche emptying triggers elevations in endothelial P-selectin is not yet defined. One study showed that Egr1 is needed in the ETP to efficiently deliver this signal (15). The role of PSGL-1 in thymocyte maturation and how it affects thymic egress is also unclear. PSGL-1 promotes T cell migration toward the CCR7 ligand CCL21 by enhancing chemokine binding (16), raising the question of whether it modulates responsiveness to S1P in a similar way. And how does PSGL-1 deficiency cause a reduction in plasma S1P abundance? This is especially perplexing in the context of the finding that peripheral lymphocyte depletion was otherwise associated with an increase in plasma S1P. The S1P elevation seen in P-selectin–deficient mice may well fit with data showing that P-selectin deficiency causes an increase in circulating HDL, given that HDL carry plasma S1P (17, 18). However, a relationship between PSGL-1 and HDL has not been reported.
Why does lymphocyte transfer reduce plasma S1P and lymphocyte depletion increase it? A straightforward argument is that lymphocytes are a major compartment for plasma S1P degradation, but this explanation does not readily fit with the finding that Rag1−/− and IL-7R−/− mice have normal amounts of plasma S1P (3, 13). Perhaps lymphocyte transfers and depletion treatments cause fluctuations in a cell type or cytokine that in turn modulates S1P production or degradation. Another conundrum is that blood S1P is present at micromolar concentrations, yet S1P receptors have nanomolar affinities (19). How could small (20–30%) changes in plasma S1P lead to alterations in endothelial behavior? Most S1P in the blood is bound to albumen or HDL and may not be bioavailable. Is it possible that the bioavailable amounts of plasma S1P fluctuate within a meaningful range? The finding that S1P1 surface expression on blood T cells is fully down-modulated even in mice with 5–10% of normal plasma S1P makes this unlikely (13). Perhaps the S1P elevations saturate degradation systems that normally keep tissue concentrations low, and this allows increased access of S1P to ablumenally distributed S1P receptors (Fig. 2). Or maybe the changes in plasma S1P are indirect readouts of more relevant local changes in S1P production by radiation-resistant cell types (perhaps endothelial cells). Clearly, much more work is needed, but the study by Gossens et al. (3) highlights the need to consider the physiological correlates of even small changes in plasma S1P.
Finally, we come back to the initial puzzle of why PSGL-1 deficiency, but not C2 deficiency, causes marked elevations in thymic P-selectin expression. Despite the many studies dedicated to this issue, the explanation for this discrepancy remains unclear. The deficiency in circulating T cells in PSGL-1−/− mice seems to play a role, as transferring in large numbers of lymphocytes decreases P-selectin expression. Perhaps the presence of transferred PSGL-1+ cells in the mice compensates for a missing signal. Certainly, a variety of experiments can now be planned to better define where PSGL-1 acts to control thymic P-selectin expression.
The authors leave us with the important concept that reductions in peripheral T cell numbers can lead to elevations in the expression of thymic homing molecules. Future analyses will help to determine whether the lymphopenia that can occur after viral infection or in other diseases is associated with elevations in thymic P-selectin and CCL25 expression and receptivity for TPCs.
References
- Foss D.L., Donskoy E., Goldschneider I. 2001. The importation of hematogenous precursors by the thymus is a gated phenomenon in normal adult mice.J. Exp. Med. 193:365–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop S.E., Petrie H.T. 2004. Regulation of thymus size by competition for stromal niches among early T cell progenitors.J. Immunol. 173:1604–1611 [DOI] [PubMed] [Google Scholar]
- Gossens K., Naus S., Corbel S.Y., Lin S., Rossi F.M., Kast J., Ziltener H.J. 2009. Thymic progenitor homing and lymphocyte homeostasis are linked via S1P controlled expression of thymic P-selectin/CCL25.J. Exp. Med. 206:761–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotoff D.A., Schwarz B.A., Bhandoola A. 2008. The long road to the thymus: the generation, mobilization, and circulation of T-cell progenitors in mouse and man.Sem. Immunopathol. 30:371–382 [DOI] [PubMed] [Google Scholar]
- Rossi F.M., Corbel S.Y., Merzaban J.S., Carlow D.A., Gossens K., Duenas J., So L., Yi L., Ziltener H.J. 2005. Recruitment of adult thymic progenitors is regulated by P-selectin and its ligand PSGL-1.Nat. Immunol. 6:626–634 [DOI] [PubMed] [Google Scholar]
- Thurston G., Baluk P., McDonald D.M. 2000. Determinants of endothelial cell phenotype in venules.Microcirculation. 7:67–80 [PubMed] [Google Scholar]
- Ellies L.G., Tsuboi S., Petryniak B., Lowe J.B., Fukuda M., Marth J.D. 1998. Core 2 oligosaccharide biosynthesis distinguishes between selectin ligands essential for leukocyte homing and inflammation.Immunity. 9:881–890 [DOI] [PubMed] [Google Scholar]
- Wurbel M.A., Philippe J.M., Nguyen C., Victorero G., Freeman T., Wooding P., Miazek A., Mattei M.G., Malissen M., Jordan B.R., et al. 2000. The chemokine TECK is expressed by thymic and intestinal epithelial cells and attracts double- and single-positive thymocytes expressing the TECK receptor CCR9.Eur. J. Immunol. 30:262–271 [DOI] [PubMed] [Google Scholar]
- Benz C., Martins V.C., Radtke F., Bleul C.C. 2008. The stream of precursors that colonizes the thymus proceeds selectively through the early T lineage precursor stage of T cell development.J. Exp. Med. 205:1187–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab S.R., Cyster J.G. 2007. Finding a way out: lymphocyte egress from lymphoid organs.Nat. Immunol. 8:1295–1301 [DOI] [PubMed] [Google Scholar]
- Shiow L.R., Roadcap D.W., Paris K., Watson S.R., Grigorova I.L., Lebet T., An J., Xu Y., Jenne C.N., Foger N., et al. 2008. The actin regulator coronin 1A is mutant in a thymic egress-deficient mouse strain and in a patient with severe combined immunodeficiency.Nat. Immunol. 9:1307–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab S.R., Pereira J.P., Matloubian M., Xu Y., Huang Y., Cyster J.G. 2005. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients.Science. 309:1735–1739 [DOI] [PubMed] [Google Scholar]
- Pappu R., Schwab S.R., Cornelissen I., Pereira J.P., Regard J.B., Xu Y., Camerer E., Zheng Y.W., Huang Y., Cyster J.G., Coughlin S.R. 2007. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate.Science. 316:295–298 [DOI] [PubMed] [Google Scholar]
- Matsushita K., Morrell C.N., Lowenstein C.J. 2004. Sphingosine 1-phosphate activates Weibel-Palade body exocytosis.Proc. Natl. Acad. Sci. USA. 101:11483–11487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell F.J., Zoller A.L., Patel S.R., Williams I.R., Kersh G.J. 2006. Early growth response gene 1 provides negative feedback to inhibit entry of progenitor cells into the thymus.J. Immunol. 176:4740–4747 [DOI] [PubMed] [Google Scholar]
- Veerman K.M., Williams M.J., Uchimura K., Singer M.S., Merzaban J.S., Naus S., Carlow D.A., Owen P., Rivera-Nieves J., Rosen S.D., Ziltener H.J. 2007. Interaction of the selectin ligand PSGL-1 with chemokines CCL21 and CCL19 facilitates efficient homing of T cells to secondary lymphoid organs.Nat. Immunol. 8:532–539 [DOI] [PubMed] [Google Scholar]
- Nageh M.F., Sandberg E.T., Marotti K.R., Lin A.H., Melchior E.P., Bullard D.C., Beaudet A.L. 1997. Deficiency of inflammatory cell adhesion molecules protects against atherosclerosis in mice.Arterioscler. Thromb. Vasc. Biol. 17:1517–1520 [DOI] [PubMed] [Google Scholar]
- Aoki S., Yatomi Y., Ohta M., Osada M., Kazama F., Satoh K., Nakahara K., Ozaki Y. 2005. Sphingosine 1-phosphate-related metabolism in the blood vessel.J. Biochem. (Tokyo). 138:47–55 [DOI] [PubMed] [Google Scholar]
- Hla T. 2003. Signaling and biological actions of sphingosine 1-phosphate.Pharmacol. Res. 47:401–407 [DOI] [PubMed] [Google Scholar]