Abstract
Epidermal T cells have been shown to play unique roles in tissue homeostasis and repair in mice through local secretion of distinct growth factors in the skin. Human epidermis contains both αβ+ and γδ+ T cells whose functional capabilities are not understood. We demonstrate that human epidermal T cells are able to produce insulin-like growth factor 1 (IGF-1) upon activation and promote wound healing in a skin organ culture model. Moreover, an analysis of the functional capabilities of T cells isolated from acute versus chronic wounds revealed a striking difference. Both αβ+ and Vδ1+ T cells isolated from acute wounds actively produced IGF-1, demonstrating that they are activated during tissue damage to participate in wound repair. In contrast, IGF-1 production could not be detected in T cells isolated from chronic wounds. In fact, skin T cells isolated from chronic wounds were refractory to further stimulation, suggesting an unresponsive state. Collectively, these results define a novel role for human epidermis–resident T cells in wound healing and provide new insight into our understanding of chronic wound persistence.
The epidermis is a barrier tissue that is exposed to the environment and susceptible to injury. Cooperation between epithelial cells, growth factors, chemokines, and inflammatory cells leads to rapid repair of most injuries. However, increasing numbers of patients suffer from chronic, nonhealing wounds (1). Although much is known about processes leading to effective tissue repair, the role of human epithelial–resident T cells in wound healing has not been examined. γδ+ T cells are found in both the epidermis and dermis of human skin (2–4). In contrast to rodents, there is also a major resident population of epidermal αβ+ T cells (5). Other than analysis of their presence, little is known about these human skin–resident T cell populations. The T cell compartment in mouse epidermis is exclusively composed of γδ+ T cells, with invariant TCRs designated as dendritic epidermal T cells (DETCs) (6). These cells are critical for tumor immunosurveillance (7), skin homeostasis (8), and wound repair (9). Identification of a human skin T cell equivalent with specialized wound healing properties would provide crucial insight into the mechanism of effective repair of acute wounds and elucidate new targets for therapeutic intervention in the treatment of chronic wounds. In this report, we show that human epidermal αβ+ and γδ+ T cells contribute to the effective healing of acute wounds and are functionally defective in patients with chronic wounds, demonstrating a previously unrecognized component of human epidermal wound healing.
RESULTS AND DISCUSSION
Vδ1 and αβ TCR–bearing cells reside in normal human epidermis
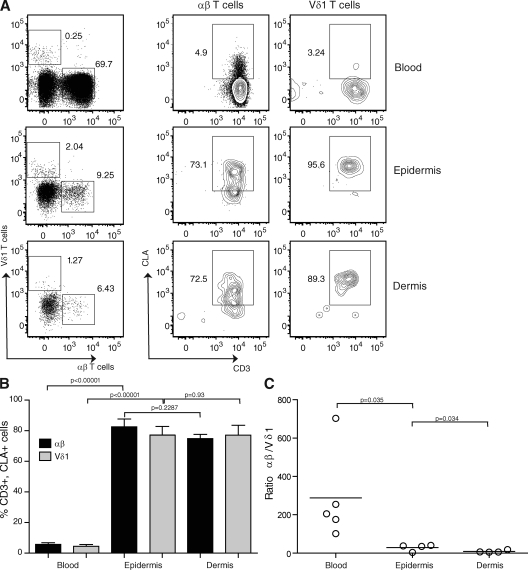
Before examining a role for αβ+ and γδ+ T cells in wound repair and homeostasis, we first investigated the presence of T cell populations in healthy human epidermis. We used a classical method to isolate T cells from the skin (3) that offered the advantage of separating epidermis from dermis, and the ability to study freshly isolated cells. This was especially useful for investigating growth factor production by T cells in wounded skin, a characteristic that might be affected by culturing the T cells or using skin explants (4). Epidermal and dermal T cells were studied and compared with peripheral T cells from healthy donors. It has been previously reported that γδ+ T cells in epithelial tissues primarily express the Vδ1 chain, whereas γδ+ T cells in peripheral blood express the Vδ2 chain (10). Indeed, Vδ1+ T cells, but not Vδ2+ cells, were found in the dermis and epidermis of patient skin samples (unpublished data). We therefore used an anti-Vδ1 antibody for the detection of γδ+ T cells resident in the skin. We detected a substantial number of Vδ1+ T cells compared with αβ+ T cells in the epidermis and dermis compared with the blood, in which the γδ T cell population is mainly composed of Vδ2+ T cells (Fig. 1, A and C) (11). Cutaneous leukocyte antigen (CLA), the ligand for E-selectin that is expressed by endothelial cells, is also present on dermal-resident T cells (4). We found that epidermal αβ+ and Vδ1+ T cells express high levels of CLA compared with T cells isolated from the blood (Fig. 1, A and B). There was no significant difference between the epidermis and dermis in terms of CLA expression in the αβ+ and Vδ1+ subsets (Fig. 1 B), whereas CD28 expression was limited to CLA+, CD3+, αβ+ T cells (not depicted). Overall, our results indicate that human skin contains epidermal-resident αβ+ and γδ+ T cells that have the potential to function in immune surveillance in the skin.
Figure 1.
T cell distribution in human skin. (A) Epidermal- and dermal-resident αβ+ and Vδ1+ T cells express high levels of CLA. Representative cytometry plots show gated populations from four-color immunofluorescence. Epidermal and dermal cells were stained with anti-CD3, CLA, αβ, and Vδ1 antibodies and were compared with healthy blood. Live lymphocytes were gated according to forward and side scatter. The numbers denote the percentages of gated cells from the live cell gate shown. (B) There is a significant difference in CLA expression between blood and epidermal T cells. There is no difference in terms of CLA expression between epidermal and dermal T cells. Histograms show the percentage of CD3+, CLA+, αβ+, and Vδ1+ cells in the blood, epidermis, and dermis of healthy donors. Results represent means of data ± SEM of four normal skin samples (epidermis and dermis) and five healthy blood samples. (C) The Vδ1/αβ T cell ratio is significantly different between the blood and epidermis as well as between the epidermis and dermis. Plots show the ratio of αβ+ to Vδ1+ T cells in the blood, epidermis, and dermis of individual healthy donors. Ratios were compared using a one-tailed unpaired Student's t test. Horizontal bars represent means of the values from the different patients.
Resident epidermal T cells produce insulin-like growth factor 1 (IGF-1)
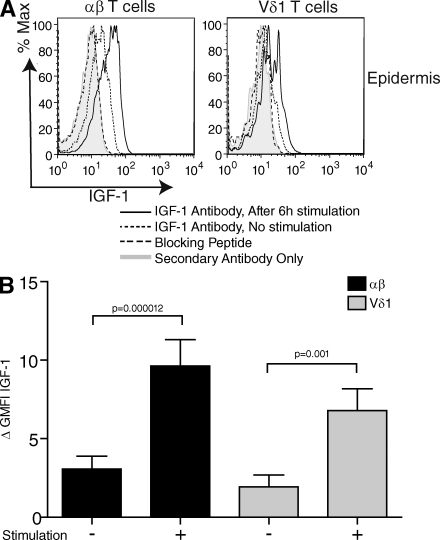
We recently showed that mouse DETCs regulate skin homeostasis through the secretion of IGF-1 (8), a member of the insulin family of growth hormones. IGF-1 is used by keratinocytes in the skin for epidermal development and maintenance (12). In human skin, IGF-1 regulates keratinocyte shape and migration for the mediation of wound epithelialization (13). Fibroblasts have been suggested to be the main IGF-1 source in the skin (14). To investigate whether human epidermal T cells can contribute to this aspect of wound repair, human T cells were isolated from the epidermis of normal skin and assessed for IGF-1 production by flow cytometry. Skin from 17 healthy donors was examined. For each donor, epidermal cells were incubated with Brefeldin A for 6 h in the presence or absence of PMA and ionomycin. We found that epidermal αβ+ and Vδ1+ T cells constitutively produce IGF-1 at low levels (Fig. 2), as previously described for mouse DETCs (8). Moreover, we observed a significant threefold increase of IGF-1 production by skin-resident T cells upon stimulation (Fig. 2 B). No correlation between donor age and IGF-1 production was observed (r < 0.8). Interestingly, previous studies have shown that levels of IGF-1 are increased after epidermal injury, peaking at 3 d after wounding (15), and IGF-1 in human wound fluid reaches maximum levels within 24 h after injury (16). Collectively, our results demonstrate that human skin T cells are a previously unrecognized local source of IGF-1 in the epidermis and have the capacity to up-regulate growth factor expression upon activation, which may affect wound repair.
Figure 2.
Resident epidermal T cells produce IGF-1 upon stimulation in vitro. (A) Flow cytometry of freshly isolated epidermal cells stimulated with PMA and ionomycin, and stained with anti-IGF-1. (B) Statistical analysis of IGF-1 expression in normal epidermal T cells (n = 17). The population was distributed as follows: sex, 12 females and 5 males; age, 44.8 ± 22.2 yr. There is a significant difference in IGF-1 expression before and after stimulation in the αβ+ and the Vδ1+ subsets according to a one-tailed Student's t test. In all flow cytometry analyses, protein production was determined by subtracting the geometric mean fluorescence intensity (GMFI) of the control secondary antibody from the GMFI of cells treated with specific antibodies. Results represent means of data ± SEM.
Specific T cell stimulation promotes wound healing
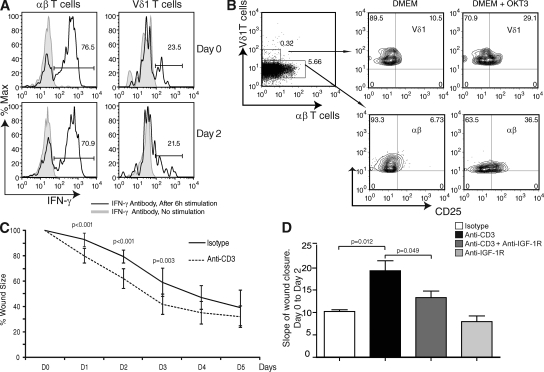
To examine the participation of skin-resident T cells in the reepithelialization phase of wound repair, we used a well-defined model of in vitro skin organ culture (16). Because human lymphocytes have not been studied in this model, we first showed that epidermal-resident T cells do not migrate out of the skin during the first 2 d of culture, and demonstrated that αβ+ and Vδ1+ cells remain functional upon in vitro stimulation by examining IFN-γ production (Fig. 3 A). Next, we directly stimulated resident T cells in situ with an anti-CD3 antibody in skin organ cultures, as previously described for cells in the small intestine (17). Human skin was cultured with or without an antibody specific for CD3ϵ (5 µg/ml OKT3). After 2 d, T cell activation was measured by an increase in cell-surface expression of the IL-2 receptor α chain, CD25, on αβ+ and Vδ1+ T cells by flow cytometry. We observed an up-regulation of cell-surface CD25 expression by both αβ+ and Vδ1+ T cells (Fig. 3 B), demonstrating activation of both T cells subsets. Significantly, addition of anti-CD3 accelerated the wound closure rate at an early stage (days 0–2) compared with untreated skin (Fig. 3 C). Collectively, these data indicate that TCR-specific activation of resident T cells promotes wound healing at an early stage.
Figure 3.
Rate of wound closure in organ culture is increased after activation of skin-resident T cells. (A) T cells remain responsive after 2 d of organ culture. Freshly isolated epidermal cells stimulated with PMA and ionomycin, and stained with IFN-γ for analysis by flow cytometry. IFN-γ expression was assessed on normal skin freshly harvested (day 0) and after being cultured for 2 d (day 2) on gelfoam in DMEM/10% FCS. Skin from the same donor was used for both day 0 and 2 analyses. Data are representative of three independent experiments. Percentages are shown. (B) Anti-CD3 stimulation activates skin-resident T cells in organ culture. The experiment was performed on skin cultured for 2 d on a gelfoam in DMEM/10% FCS in the presence or absence of anti-CD3 (5 µg/ml OKT3) antibody. Flow cytometry of freshly isolated αβ+ and Vδ1+ T cells stained with CD25 is shown. Data are representative of three independent experiments. Percentages are shown. (C) The addition of stimulating antibodies to CD3 increased the rate of wound closure in skin organ culture. Analysis of skin wound closure kinetics in the presence (dashed line) or absence (continuous line) of an mAb to CD3. Data are presented as the means ± SD of three independent experiments with an average of three foreskins per experiment, and are representative of seven total experiments (with a sum of 20 foreskins). (D) Blocking of T cell–mediated wound closure with IGF-1R antibody in skin organ culture. Data are the means ± SD of the slope of wound closure between days 0 and 2 of three independent experiments performed in triplicate. Data were compared using a one-tailed paired Student's t test.
To identify whether T cell–produced IGF-1 is responsible for the improved wound closure, we attempted to block T cell–mediated IGF-1 responses using IGF-1 receptor (IGF-1R) antibody after anti-CD3 stimulation in skin organ culture. Wound closure was measured between days 0 and 2, because the effect of T cell activation was maximal at this early stage. As shown in Fig. 3 D, anti-CD3 stimulation increases the rate of wound closure. Addition of an anti–IGF-1R antibody significantly blocks this effect, demonstrating that T cell–mediated wound closure is dependent on IGF-1.
T cells are activated after acute injury but are functionally impaired in chronic wounds
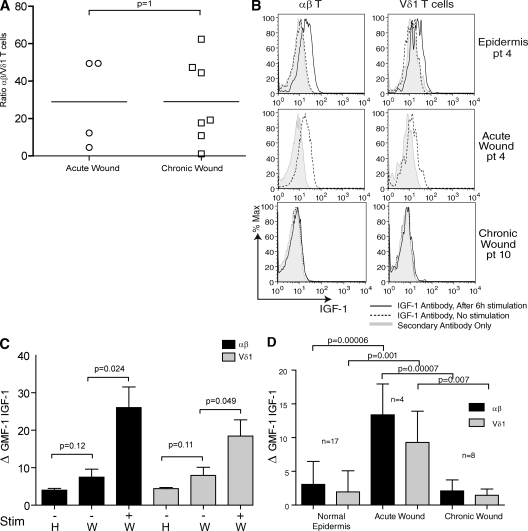
Several pathogenic abnormalities contribute to failure to heal, and the progression of the healing process is impaired in chronic wounds. We therefore hypothesized that T cells might be reduced in number or functionally defective in patients affected by chronic wounds. Skin samples (n = 8) from patients exhibiting wounds that had failed to heal within 8 wk were used to study the presence and function of T cells in chronic wounds, as compared with patients with acute wounds healing normally. No significant differences were found in the ratio of αβ+ to Vδ1+ T cells in chronic as compared with acute wounds (Fig. 4 A). Analysis of absolute cell numbers found an average of 2,890 αβ and 283 Vδ1 T cells/cm2 in normal epidermis, and an average of 10,860 αβ and 300 Vδ1 T cells/cm2 in acute and chronic wounds. These data suggest that there is a recruitment of primarily αβ T cells to wound sites, whereas the Vδ1 T cells found in wounds are a skin-resident population.
Figure 4.
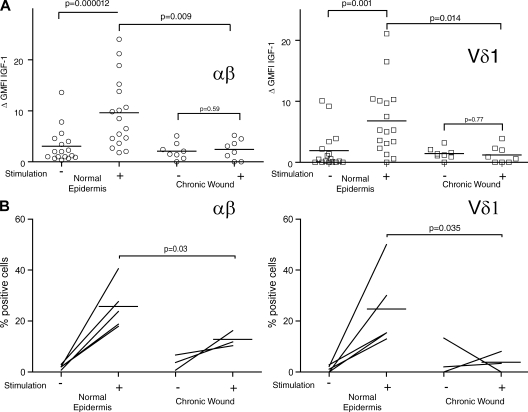
IGF-1 production by T cells in acute versus chronic wounds. (A) Ratio of αβ and Vδ1 T cells in acute (n = 4) versus chronic (n = 8) wounds. Data were compared using a two-tailed unpaired Student's t test. Horizontal bars represent means of the values from the different patients. (B) Cells were isolated from normal epidermis (top), acute wounds (middle), and chronic wounds (bottom) and examined for IGF-1 expression by flow cytometry. (top and bottom) Cells were also stimulated with PMA and ionomycin, and stained with anti–IGF-1. Cells isolated from acute wounds were not stimulated. The acute wound and the healthy epidermis shown were isolated from the same patient and were processed simultaneously. Data are representative of acute wounds obtained from four patients and chronic wounds obtained from eight patients. pt, patient. (C) IGF-1 production in circulating αβ+ and Vδ1+ T cells isolated from the blood of healthy (H; n = 5) and acutely wounded (W; n = 4) patients before and after stimulation with PMA and ionomycin. (D) IGF-1 production is greatly enhanced in acute wounds as compared with chronic wounds and healthy epidermis. There is a significant difference in IGF-1 expression in the αβ+ and the Vδ1+ subsets in acute wounds compared with normal epidermis and chronic wounds. Normal epidermis (n = 17), acute wounds (n = 4), and chronic wounds (n = 8) were examined in the absence of stimulation. Histograms show means ± SD. The GMFI of IGF-1 production by αβ+ and Vδ1+ cells is shown. In all graphs showing flow cytometry analysis, protein expression was determined by subtracting the GMFI of the control secondary antibody from the GMFI of cells treated with specific antibodies. Data are means ± SD.
One of the hallmarks of the early stage of wound repair is increased IGF-1 at the wound site, and levels of IGF-1 have been shown to be decreased in nonhealing wounds from diabetic patients (18). We therefore hypothesized that skin-resident T cells are activated upon injury in vivo to produce IGF-1. We investigated IGF-1 expression in αβ+ and Vδ1+ T cells from acute wounds. Skin specimens from patients suffering from recent injury were used to study IGF-1 production in the context of acute wounds. Cells were isolated from acute wounds and examined for IGF-1 production by flow cytometry. Levels of IGF-1 produced by both αβ+ and Vδ1+ T cells from wounded skin were similar to IGF-1 levels in T cells stimulated with PMA and ionomycin (Fig. 4 B). In addition to PMA and ionomycin, anti-CD3–stimulating antibody induced IGF-1 production in T cells, whereas IL-2 treatment did not (unpublished data). This data implies that T cells up-regulate IGF-1 in response to tissue injury, which likely promotes wound repair.
To determine if this is a unique response of skin-resident T cells during injury, αβ+ and Vδ1+ T cells were isolated from the blood of both normal healthy donors and from acutely wounded patients 2–7 d after surgery. Basal IGF-1 levels were similar between T cells isolated from the acutely wounded patients as compared with the normal healthy donors. We observed a small but not statistically significant increase in the wounded patients that may represent αβ+ or Vδ1+ T cells that have exited the wound site. In all cases, IGF-1 levels were increased upon ex vivo stimulation with PMA and ionomycin. Our data suggests that up-regulated T cell production of IGF-1 during wound repair is limited to the local environment (Fig. 4 C).
However, an analysis of the functional capabilities of T cells isolated from chronic versus acute wounds revealed a striking difference. Both αβ+ and Vδ1+ T cells isolated from nonhealing chronic skin wounds did not produce IGF-1 compared with T cells isolated from acute wounds (Fig. 4, B and D). No significant differences were observed in CD69 expression on αβ and Vδ1 T cells from patients with chronic wounds (n = 4) compared with normal epidermis (n = 4; not depicted). This indicates that, although present, T cells in chronic wounds are functionally impaired and are unable to produce IGF-1 during the tissue repair process.
Epidermal T cells from chronic wounds are less responsive to stimulation
To better characterize whether skin-resident T cells in chronic wounds retain the ability to produce IGF-1 and cytokines upon stimulation, we isolated αβ+ and Vδ1+ T cells from the epidermis of normal and chronic wounds, and stimulated them in vitro for 6 h with PMA and ionomycin. As shown in Fig. 5 A, neither αβ+ or Vδ1+ T cells isolated from chronic wounds could be restored by stimulation with PMA and ionomycin (Fig. 5 A). Additionally, αβ+ and Vδ1+ T cells isolated from chronic wounds produce significantly less IL-2 after stimulation compared with cells isolated from normal epidermis (Fig. 5 B). This suggests that the T cells in a chronic wound environment become less responsive to activation.
Figure 5.
IGF-1 and IL-2 production by T cells in normal epidermis and chronic nonhealing wounds. (A) IGF-1 production by αβ+ and Vδ1+ T cells is significantly higher after stimulation in normal epidermis (n = 17) compared with chronic wounds (n = 8). Plots represent IGF-1 production by individual donors with or without stimulation with PMA and ionomycin. (B) The percentage of αβ+ (left) and Vδ1+ (right) T cells producing IL-2 before and after stimulation with PMA and ionomycin in normal epidermis (n = 5) and nonhealing chronic wounds (n = 3). Although no difference in IL-2 production by αβ+ and Vδ1+ T cells in normal epidermis compared with chronic wounds was seen before stimulation, the percentage of αβ+ and Vδ1+ cells producing IL-2 after stimulation is significantly lower in chronic wounds. Data were compared using a one-tailed unpaired Student's t test. Horizontal bars represent means of the values from the different patients.
Collectively, these data indicate that T cells are functionally impaired in chronic wounds, and bypassing TCR stimulation is unable to rescue function. These data are in line with the hypothesis proposed by Falanga et al. (19), which states that debridement, often an effective treatment for resolving chronic wounds, could aid in the release of trapped growth factors unavailable for wound healing. Moreover, the hypothesis that debridement resets the stage for wounds proceeding toward the normal sequence of healing (20) is supported by patient 4 (Fig. 4 B) from our acute wound group. This patient received a first debridement surgery 3 d before our detection of high T cell specific IGF-1 production.
Cutaneous wound healing is a complex process requiring constant communication between cells in the form of cytokine release, cell-to-cell contacts, and cell-to-matrix interactions. Chronic wounds are a serious clinical problem affecting numerous elderly and diabetic patients. Many cell types are involved in wound healing, but the role of resident T cells has been neglected. This report demonstrates for the first time that skin-resident T cell populations (αβ+ and Vδ1+) are able to produce IGF-1, which can contribute to skin homeostasis and promote wound healing. It will now be important to define the trigger of αβ+ and Vδ1+ T cell activation in acute wounds and address the mechanism of dysregulation of T cell–mediated wound repair in chronic wounds. Some of the healthy patients examined in this study did exhibit a low level of IGF-1 production by resident T cells after stimulation. It is possible that this phenotype, in addition to chronic disease such as diabetes, increases susceptibility to chronic wounds. On the other hand, perhaps activating antigens are masked or overstimulate the T cells in a chronic wound, leading to their unresponsiveness to activation signaling. T cell dysfunction might also be caused by increased expression of inhibitory receptors such as programmed death 1, which have been shown to become elevated in response to severe injury (21) and HIV infection (22). From a clinical aspect, T cell impairment at an early stage of the wound healing process could be a predictive factor for chronic wound formation. In addition, these results may explain why wound debridement is often effective for the treatment of chronic wounds. This new evidence supports the idea that skin-resident T cells play a critical role in skin homeostasis and the wound repair mechanism in humans, suggesting possible therapeutic targets that could be considered in the future to enhance chronic wound management.
MATERIALS AND METHODS
Subjects.
Acquisition of skin samples and all scientific studies were approved by the Institutional Review Boards of Scripps Health and the University of California, San Diego. Informed consent was obtained from all subjects. Samples of normal human skin and wounded skin were obtained from discarded human tissue from cutaneous surgery. Skin specimens from patients suffering from recent injury were used to study acute wounds. None of the patients had systemic disease or were currently receiving immunosuppressive treatment. Discarded tissue from patients affected by wounds that failed to heal within 2 mo was used to study chronic wounds. More details on patients enrolled in the study are given in Table S1. Foreskin was obtained from the Cooperative Human Tissue Network for use in skin organ culture. Normal human blood was obtained from the Scripps Research Institute Normal Blood Donor Services. Blood samples from patients after breast reduction surgery (2–7 d) were used to investigate IGF-1 production by T cells from acutely wounded patients.
Cell isolation.
Separation of the epidermis and dermis was accomplished by floating small skin strips on 1.25 U/ml Dispase II solution (Roche) overnight at 4°C. Epidermal sheets were separated from the dermis by mechanical separation and vigorously agitated in fresh Dispase II for 30 min at 37°C. The dermis was further treated with 1 mg/ml collagenase D (Roche) and Dispase II for 30 min at 37°C, isolating T cells from the dermis. Wounded skin was processed according to the same enzymatic digestion: Dispase II overnight at 4°C followed by Dispase II and collagenase D for 30 min at 37°C. Digestion was halted by the addition of 10 mM EDTA. Cell suspensions were filtered, enriched for T cells using a Ficoll-Paque gradient, and rested in complete medium (RPMI 1640, 10% FCS, 25 mM Hepes, 100 U penicillin, 100 µg streptomycin, 2 mM glutamine, 100 µM of nonessential amino acids, 1 mM sodium pyruvate) for 6 h. To assess IGF-1 production, cells were cultured in the presence or absence of 50 ng/ml PMA and 500 ng/ml ionomycin, and 5 µg/ml Brefeldin A (Sigma-Aldrich) for 6 h. Cells were fixed and permeabilized with Fix & Perm reagent (Invitrogen) according to manufacturer's instructions.
Flow cytometry.
Antibodies to IGF-1 (G-17) and the accompanying blocking peptides were purchased from Santa Cruz Biotechnology, Inc. Secondary antibody–staining reagents were purchased from Jackson ImmunoResearch Laboratories. mAbs to CD3 (UCHT1), αβ (T10B9.1A-31), Vδ2 (B6), and CLA (HECA-452) were obtained from BD, and mAbs to CD3 (UCHT1), αβ (IP26), and IFN-γ (4S.B3) were purchased from eBioscience. The mAb to Vδ1 (TS8.2) was obtained from Thermo Fisher Scientific, and the mAb to CD25 (0479) was purchased from Beckman Coulter. Analysis of flow cytometry samples was performed on a FACSCalibur or an LSR-II (both from BD). Data were analyzed with FlowJo software (Tree Star, Inc.).
Skin organ culture.
Skin organ culture was performed as previously described (23) using human foreskin samples. This method can be effectively used to examine keratinocyte proliferation and migration during the reepithelialization phase of wound repair. In brief, each sample was trimmed into four pieces with a 6-mm diameter each, and a punch biopsy with a 3-mm diameter including the epidermis and upper dermis was removed from the center. Each piece was placed with the dermis down on gelfoam (Savmart Pharmaceuticals) in a 24-well plate containing DMEM/10% FCS in the presence or absence of an anti-CD3 antibody (5 µg/ml OKT3; eBioscience) or the isotype control. These cultures were incubated at 37°C with 5% CO2 for 5 d, with the media changed every other day. For blocking experiments, 12 µg/ml IGF-1 receptor antibody (R&D Systems) was added to skin organ cultures. Digital images were acquired every day, and the wound surface area was measured and analyzed with ImageJ software (available at http://rsbweb.nih.gov/ij/). For each experiment, two to three foreskin samples were processed. To address T cell activation (CD25 expression and IFN-γ production) after 2 d of culture, healthy skin specimens were used and processed as described.
Statistical analysis.
Data were analyzed using an unpaired Student's t test to determine significant differences between two groups of patients and a paired t test to determine significant differences within the same group. Tests were two-tailed except when noted in the text. All findings were considered significant at P < 0.05. Power analysis based on previous mouse cell work indicated that a minimum sample size of two provided power of >95% at α = 0.05.
Online supplemental material.
Table S1 shows the main features of the subjects with wounds presented in Fig. 4. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20081787/DC1.
Acknowledgments
We thank Drs. A. Theofilopoulos, L. Teyton, and D. Witherden for critical review of the manuscript, and R. Mills, G. Cauvi, and A. Webster for technical assistance.
This work was supported by L'Oreal and the National Institutes of Health (grants AI36964 and GM80301 to W.L. Havran).
The authors have no conflicting financial interests.
References
- Kuehn B.M. 2007. Chronic wound care guidelines issued.JAMA. 297:938–939 [DOI] [PubMed] [Google Scholar]
- Dupuy P., Heslan M., Fraitag S., Hercend T., Dubertret L., Bagot M. 1990. T-cell receptor-γδ bearing lymphocytes in normal and inflammatory human skin.J. Invest. Dermatol. 94:764–768 [DOI] [PubMed] [Google Scholar]
- Ebert L.M., Meuter S., Moser B. 2006. Homing and function of human skin γδ T cells and NK cells: relevance for tumor surveillance.J. Immunol. 176:4331–4336 [DOI] [PubMed] [Google Scholar]
- Clark R.A., Chong B., Mirchandani N., Brinster N.K., Yamanaka K., Dowgiert R.K., Kupper T.S. 2006. The vast majority of CLA+ T cells are resident in normal skin.J. Immunol. 176:4431–4439 [DOI] [PubMed] [Google Scholar]
- Tamaki K., Sugaya M., Tada Y., Yasaka N., Uehira M., Nishimoto H., Nakamura K. 2001. Epidermal and dermal γδ T cells.Chem. Immunol. 79:43–51 [DOI] [PubMed] [Google Scholar]
- Havran W.L., Chien Y.H., Allison J.P. 1991. Recognition of self antigens by skin-derived T cells with invariant γδ antigen receptors.Science. 252:1430–1432 [DOI] [PubMed] [Google Scholar]
- Girardi M., Oppenheim D.E., Steele C.R., Lewis J.M., Glusac E., Filler R., Hobby P., Sutton B., Tigelaar R.E., Hayday A.C. 2001. Regulation of cutaneous malignancy by γδ T cells.Science. 294:605–609 [DOI] [PubMed] [Google Scholar]
- Sharp L.L., Jameson J.M., Cauvi G., Havran W.L. 2005. Dendritic epidermal T cells regulate skin homeostasis through local production of insulin-like growth factor 1.Nat. Immunol. 6:73–79 [DOI] [PubMed] [Google Scholar]
- Jameson J., Ugarte K., Chen N., Yachi P., Fuchs E., Boismenu R., Havran W.L. 2002. A role for skin γδ T cells in wound repair.Science. 296:747–749 [DOI] [PubMed] [Google Scholar]
- Hayday A., Theodoridis E., Ramsburg E., Shires J. 2001. Intraepithelial lymphocytes: exploring the Third Way in immunology.Nat. Immunol. 2:997–1003 [DOI] [PubMed] [Google Scholar]
- Hayday A.C. 2000. γδ cells: a right time and a right place for a conserved third way of protection.Annu. Rev. Immunol. 18:975–1026 [DOI] [PubMed] [Google Scholar]
- Edmondson S.R., Thumiger S.P., Werther G.A., Wraight C.J. 2003. Epidermal homeostasis: the role of the growth hormone and insulin-like growth factor systems.Endocr. Rev. 24:737–764 [DOI] [PubMed] [Google Scholar]
- Haase I., Evans R., Pofahl R., Watt F.M. 2003. Regulation of keratinocyte shape, migration and wound epithelialization by IGF-1- and EGF-dependent signalling pathways.J. Cell Sci. 116:3227–3238 [DOI] [PubMed] [Google Scholar]
- Rudman S.M., Philpott M.P., Thomas G.A., Kealey T. 1997. The role of IGF-I in human skin and its appendages: morphogen as well as mitogen? J. Invest. Dermatol. 109:770–777 [DOI] [PubMed] [Google Scholar]
- Brown D.L., Kane C.D., Chernausek S.D., Greenhalgh D.G. 1997. Differential expression and localization of insulin-like growth factors I and II in cutaneous wounds of diabetic and nondiabetic mice.Am. J. Pathol. 151:715–724 [PMC free article] [PubMed] [Google Scholar]
- Vogt P.M., Lehnhardt M., Wagner D., Jansen V., Krieg M., Steinau H.U. 1998. Determination of endogenous growth factors in human wound fluid: temporal presence and profiles of secretion.Plast. Reconstr. Surg. 102:117–123 [DOI] [PubMed] [Google Scholar]
- MacDonald T.T., Spencer J. 1988. Evidence that activated mucosal T cells play a role in the pathogenesis of enteropathy in human small intestine.J. Exp. Med. 167:1341–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakytny R., Jude E.B., Martin Gibson J., Boulton A.J., Ferguson M.W. 2000. Lack of insulin-like growth factor 1 (IGF1) in the basal keratinocyte layer of diabetic skin and diabetic foot ulcers.J. Pathol. 190:589–594 [DOI] [PubMed] [Google Scholar]
- Falanga V., Eaglstein W.H. 1993. The “trap” hypothesis of venous ulceration.Lancet. 341:1006–1008 [DOI] [PubMed] [Google Scholar]
- Falanga V. 2005. Wound healing and its impairment in the diabetic foot.Lancet. 366:1736–1743 [DOI] [PubMed] [Google Scholar]
- Laudanski K., Miller-Graziano C., Xiao W., Mindrinos M.N., Richards D.R., De A., Moldawer L.L., Maier R.V., Bankey P., Baker H.V., et al. 2006. Cell-specific expression and pathway analyses reveal alterations in trauma-related human T cell and monocyte pathways.Proc. Natl. Acad. Sci. USA. 103:15564–15569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautmann L., Janbazian L., Chomont N., Said E.A., Gimmig S., Bessette B., Boulassel M.R., Delwart E., Sepulveda H., Balderas R.S., et al. 2006. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction.Nat. Med. 12:1198–1202 [DOI] [PubMed] [Google Scholar]
- Moll I., Houdek P., Schmidt H., Moll R. 1998. Characterization of epidermal wound healing in a human skin organ culture model: acceleration by transplanted keratinocytes.J. Invest. Dermatol. 111:251–258 [DOI] [PubMed] [Google Scholar]