Abstract
The Wiskott-Aldrich syndrome (WAS) protein (WASp) is a regulator of actin cytoskeleton in hematopoietic cells. Mutations of the WASp gene cause WAS. Although WASp is involved in various immune cell functions, its role in invariant natural killer T (iNKT) cells has never been investigated. Defects of iNKT cells could indeed contribute to several WAS features, such as recurrent infections and high tumor incidence. We found a profound reduction of circulating iNKT cells in WAS patients, directly correlating with the severity of clinical phenotype. To better characterize iNKT cell defect in the absence of WASp, we analyzed was−/− mice. iNKT cell numbers were significantly reduced in the thymus and periphery of was−/− mice as compared with wild-type controls. Moreover analysis of was−/− iNKT cell maturation revealed a complete arrest at the CD44+ NK1.1− intermediate stage. Notably, generation of BM chimeras demonstrated a was−/− iNKT cell-autonomous developmental defect. was−/− iNKT cells were also functionally impaired, as suggested by the reduced secretion of interleukin 4 and interferon γ upon in vivo activation. Altogether, these results demonstrate the relevance of WASp in integrating signals critical for development and functional differentiation of iNKT cells and suggest that defects in these cells may play a role in WAS pathology.
Invariant natural killer T (iNKT) cells constitute a peculiar T lymphocyte subset, which is characterized by the coexpression of NK markers and an invariant TCR-α chain (Vα14Jα18 in mouse or Vα24Jα18 in human), which pairs with a restricted number of TCR-β chains (Vβ8, Vβ7, and Vβ2 in mice and Vβ11 in human). iNKT cells recognize glycolipid antigens, such as α-galactosylceramide (α-GalCer), presented in the context of CD1d molecules (1). iNKT cells develop in the thymus from CD4+ CD8+ double-positive (DP) cells that have randomly rearranged the semiinvariant TCR and are positively selected by recognition of CD1d molecules on DP thymocytes. After positive selection, the most immature iNKT cells (stage 1, CD44− NK1.1−) first differentiate into CD44+ NK1.1− (stage 2) and then are either exported into the periphery or remain in the thymus. In both compartments, iNKT cells complete their maturation, becoming CD44+ NK1.1+ mature cells (stage 3) (2). This differentiation program requires signaling molecules, adapters, and transcription factors that selectively control the development of iNKT and not of mainstream T cells (2).
Mature iNKT cells are strong immunoregulatory elements because they promptly produce a wide range of cytokines upon TCR triggering (1). iNKT cells are indeed involved in the control of pathogen infection and cancer immunosurveillance (3, 4) and play a protective role in many autoimmune diseases, although in some autoimmune mouse models they can exert a detrimental activity (5).
Interestingly, a complete lack of iNKT cells was found in the X-linked lymphoproliferative disease (XLP) (6), a primary immunodeficiency which is caused by mutations in SAP and XIAP genes and characterized by inappropriate response to EBV infection, usually leading to B cell lymphoma. The absence of iNKT cells reveals a role for SAP and XIAP in the regulation of iNKT cell development and implies the contribution of this cell subset to the control of infections and cancer progression. In keeping with the iNKT cell immunoregulatory role, their absence has been recently described in the Omenn syndrome, a primary immunodeficiency characterized by severe autoimmune manifestations (7). All these evidences have prompted us to investigate iNKT cells in Wiskott-Aldrich syndrome (WAS), a primary immunodeficiency associated with thrombocytopenia, recurrent infections, increased risk of developing cancer (mainly B cell lymphoma EBV associated), and autoimmunity (8, 9). WAS is caused by mutations in the gene encoding for the WAS protein (WASp), a key regulator of actin-dependent processes in hematopoietic cells (9). In humans, complete lack of WASp gives rise to the severe WAS phenotype, whereas hypomorphic mutations allowing residual WASp expression usually lead to X-linked thrombocytopenia (XLT), a milder disease characterized by marginal immune defects (8).
Thus far, many cellular defects resulting from the absence of WASp have been described, revealing the involvement of this protein in regulation of migration, cell trafficking and immunological synapse (IS) formation in distinct immune cell types (9). Aside from its role in actin cytoskeleton remodeling, WASp is required in signaling pathways downstream from NK and T cell activation (10–12). Although impaired innate and adaptive immune cell function can account for infections and partially explain the increased susceptibility to developing cancer and autoimmunity, a full comprehension of the cellular mechanisms underlying the pathogenesis of this syndrome still needs to be achieved (9, 13).
In the present work, we provide evidence that iNKT cells are absent in full-blown WAS patients. Moreover, analysis of iNKT cells in was−/− mice revealed defects in iNKT cell maturation and function. Our data point to a new role for WASp as an important regulator of iNKT cell development and function and lead to the hypothesis that these defects could contribute to the immune dysregulation in WAS.
RESULTS AND DISCUSSION
WAS patients, but not XLT patients, lack circulating iNKT cells
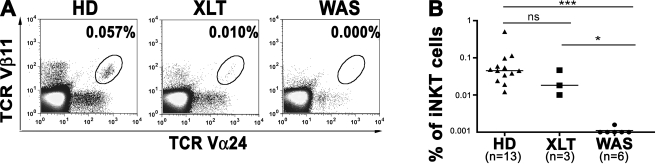
To investigate whether WASp deficiency has an impact on the amount of circulating human iNKT cells, we examined peripheral blood from three patients with a mild clinical phenotype (score of 1–2, XLT), six patients with severe WAS (score 3–5), and 13 age-matched healthy donors (HDs; Table S1). By flow cytometry, iNKT cells were nearly undetectable in WAS patients with a severe score (Fig. 1 A). Interestingly, three patients with XLT presented a detectable number of iNKT cells, even though their median value was within the lower range of HDs (Fig. 1 B). Therefore, impairment in iNKT cell number correlates with the severity of disease. In severe WAS patients lacking iNKT cells, the frequency of T cells expressing either TCR Vα24 or Vβ11, although variable, was in the range of HDs, suggesting that these TCR V regions can be used by WASp-deficient T cells (Fig. S1). Moreover, the analysis of the TCR Vβ repertoire in three WAS patients (WAS1, WAS28, and WAS33) and one case of XLT (WAS30) revealed the presence of all TCR Vβ families, with some alterations probably caused by infections, which are frequent in these patients (Table S2). These findings indicate a profound defect in iNKT cell population, despite minor alterations in the TCR repertoire of mainstream T cells.
Figure 1.
Lack of iNKT cells in WAS patients. (A) Representative flow cytometric analysis of peripheral blood iNKT cells from an age-matched HD control (HD), an XLT patient (XLT), and a WAS patient (WAS). Cells in the density plots are gated on CD3+ cells. Percentages of iNKT cells (TCR-Vβ11+ TCR-Vα24+) are indicated. (B) Frequency of iNKT cells from 13 controls, 3 XLT, and 6 WAS patients. Bars represent the median value of each group. ns, P > 0.05; *, P < 0.05; ***, P < 0.001.
iNKT cell absence could contribute to the increased susceptibility of patients to pathogen infections. Indeed, lack of iNKT cells can impair the immune protection against viral, bacterial, and fungal infections (3). Furthermore, iNKT cells play a key role in tumor immunosurveillance, as suggested by the increased susceptibility of NKT-deficient mice to developing cancer (4) and a decreased iNKT cell number in patients with advanced cancer (14, 15). Therefore, we hypothesize that the absence of iNKT cells might contribute to the high susceptibility to develop EBV+ B cell lymphoma observed in WAS patients, as has also been suggested for XLP (6).
Impaired iNKT cell development in was−/− mice
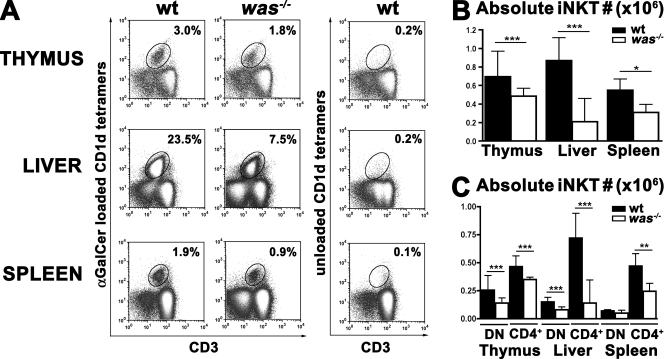
Given the paucity of material derived from WAS patients, we further investigated the role of WASp in the development and function of iNKT cells in was−/− mice (16). This mouse model recapitulates important immune system alterations of the human WAS (16). We first investigated the number and distribution of iNKT cells in the thymus, liver, and spleen by flow cytometric analysis. The percentage of iNKT cells stained specifically with α-GalCer–loaded CD1d tetramers was not significantly decreased in was−/− thymi, whereas it was two- and threefold reduced in spleen and liver, respectively, of was−/− animals compared with WT controls (Fig. 2 A and not depicted). However, the absolute number of iNKT cells was significantly reduced in all three compartments of was−/− mice in comparison with WT mice (Fig. 2 B).
Figure 2.
Reduced iNKT cell number in was−/− mice. (A) iNKT cells were analyzed by flow cytometry in thymus, liver, and spleen of C57BL/6 WT and was−/− mice (was−/−). Thymocytes were stained with anti-CD8, anti-CD3 mAbs, and CD1d tetramers (α-GalCer loaded or unloaded), whereas hepatic leukocytes and splenocytes were stained with anti-B220 and anti-CD3 mAbs and CD1d tetramers (α-GalCer loaded or unloaded). After gating on CD8− or B220− cells, iNKT cells were identified as CD3+ CD1d tetramers+ cells. (B) Absolute numbers of iNKT cells were determined by multiplying their percentage by the absolute cell count within each sample. In A and B, data are representative of at least 10 mice per group analyzed in three independent experiments. (C) iNKT cells were stained with anti-CD4 antibodies. Comparison of the absolute number of CD4+ or CD4− (DN) iNKT cells in thymus, liver, and spleen in WT versus was−/− mice is shown. In B and C, error bars represent the median and interquartile ranges of eight mice per group. *, P < 0.05; **, P < 0.005; ***, P < 0.001.
Based on CD4 expression, two iNKT cell subsets can be distinguished: CD4+ and CD4− CD8− double-negative (DN) cells (1). We analyzed whether the lack of WASp could affect the development and/or tissue distribution of one particular iNKT cell subset. Comparison of the absolute number of iNKT cells in was−/− and WT animals revealed a significant reduction of both CD4+ and DN iNKT cells in was−/− mice in all tissues, with the exception of DN cells in the spleen. However no skewing toward one particular subset was observed (Fig. 2 C).
These findings indicate that the lack of WASp causes a significant decrease of iNKT cell number. We thus investigated whether the reduction of iNKT cells in was−/− mice might be the result of an altered thymic development. We first analyzed the expression of CD1d, which is required for iNKT cell positive selection (17), on DP lymphocytes without finding any alteration in was−/− mice in comparison with WT mice (Fig. S2).
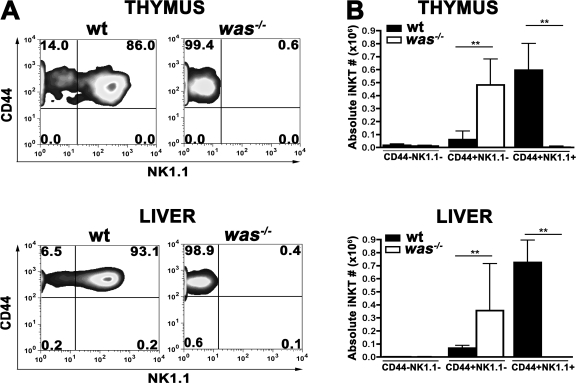
We next investigated the maturation steps of iNKT cells after positive selection, using CD44 and NK1.1 markers. As shown in Fig. 3 A, was−/− iNKT cells were mainly arrested at CD44+ NK1.1− stage, and only a minor fraction became NK1.1+ both in the thymus and in periphery. The analysis of the absolute number of iNKT cells during the different maturation steps confirmed that WASp does not affect the earliest developmental phase, when was−/− iNKT cells are normally present (Fig. 3 B). Conversely was−/− iNKT cells accumulate at stage 2 (CD44+ NK1.1−) without progressing to stage 3 (CD44+NK1.1+), suggesting a potential role of WASp in regulating the late phases of the differentiation process.
Figure 3.
Block iNKT cell maturation in the absence of WASp. (A) Thymocytes and hepatic leukocytes from WT and was−/− mice were stained with anti-CD8, anti-CD3, anti-CD44, and anti-NK1.1 mAbs and α-GalCer–loaded CD1d tetramers. Maturation of iNKT cells (CD1d tetramer+, CD3+, and CD8−) was assessed by CD44 and NK1.1 expression. Data are representative of six mice per group analyzed in two independent experiments. (B) Absolute numbers of iNKT cells (CD1d tetramer+, CD3+, and CD8−) in thymus and liver of WT and was−/− mice. Error bars represent median and interquartile range of six mice per group. **, P < 0.005.
Among the events contributing to the final maturation of iNKT cells in the periphery, a crucial role seems to be played by CD1d recognition because NK1.1− iNKT cells fail to properly complete their maturation in the absence of CD1d (18). To address this point, we examined CD1d expression in the periphery of was−/− mice, finding expression levels comparable to those of the WT (Fig. S2). These data rule out the possibility that an altered CD1d expression in the periphery may have a role in developmental block of was−/− iNKT cells.
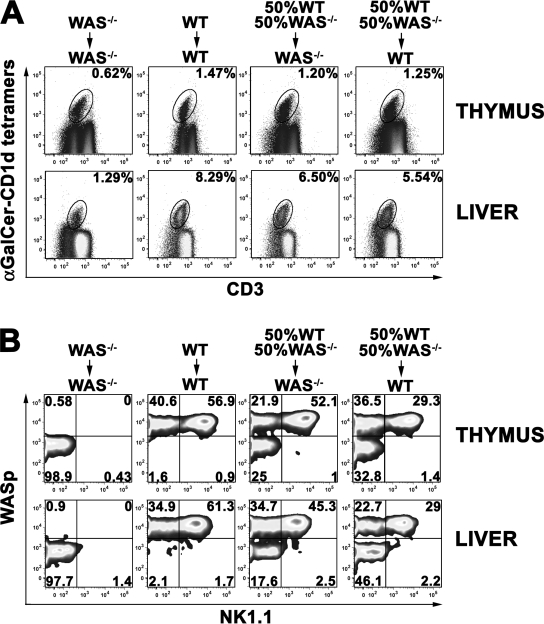
Next, to assess whether the developmental defect of iNKT cells in was−/− mice is cell autonomous, we generated mixed BM chimeras by reconstituting irradiated CD45.1 mice with a mixture of equal numbers of CD45.2 lineage-negative (lin−) cells from the BM of was−/− and WT mice. This way, CD45.2 was−/− iNKT cell precursors would develop in the presence of donor-derived CD45.2 DP thymocytes and hematopoietic cells from either was−/− or WT mice. In case of an iNKT cell-intrinsic defect, the presence of WT cells would not be sufficient to rescue the development of was−/− iNKT cells. BM chimeras were analyzed 7–9 wk after transplantation and evaluated for the expansion and maturation of iNKT cells derived from either was−/− or WT hematopoietic precursors. As shown in Fig. 4 A, the percentage of iNKT cells developing in was−/− mice reconstituted with was−/− lin− cells (WAS−/− →WAS−/− control) was reduced both in the thymus and, more markedly, in the periphery when compared with that observed in WT recipients transplanted with WT precursors (WT→WT control). Both mixed BM chimeras generated in was−/− and WT recipients displayed a level of iNKT cells similar to the WT→WT control, suggesting the same capacity of WT and was−/− recipients to support iNKT cell generation. The analysis of maturation of donor iNKT cells based on NK1.1 expression confirmed the complete developmental block of was−/− iNKT cells in WAS−/− →WAS−/− controls as opposed to successful maturation of WASp+ iNKT cells in the WT→WT control (Fig. 4 B). In the BM chimeras, although WASp+ iNKT cells could acquire the mature phenotype in both recipients, WASp− iNKT cells were unable to up-regulate NK1.1, even in the presence of hematopoietic cells derived from WT progenitors. Altogether, these findings demonstrate that the lack of WASp determines an iNKT cell–autonomous defect that impairs the maturation, survival, and/or expansion of these cells.
Figure 4.
Cell-autonomous developmental defect of was−/− iNKT cells. (A) iNKT cells were analyzed by flow cytometry in thymus and liver of WT or was−/− recipient mice (CD45.1) transplanted with was−/− lin− cells, WT lin− cells, or a mixture of 50% WT and 50% was−/− lin− obtained from CD45.2 mice. Thymocytes and hepatic leukocytes were surface stained with α-GalCer–loaded CD1d tetramers, with anti-CD3 and anti-CD8 (thymocytes) or anti-B220 (hepatic leukocytes) mAbs. The percentage of iNKT cells (CD8− or B220− CD3+CD1d tetramer+ cells) is indicated in each plot. (B) Maturation of iNKT cells in thymus and liver of BM chimera mice. After gating on donor CD45.2+, iNKT cells were further analyzed for NK1.1 and WASp expression. The percentage of mature (NK1.1+) and immature (NK1.1−) iNKT cells from WT donors (WASp+) or from was−/− donors (WASp−) is indicated in each plot. Data are representative of at least three mice per group from two independent experiments.
Many players regulating iNKT cell terminal maturation are molecules involved in the TCR signaling. For instance, a lower iNKT generation and maturation was observed in the absence of PKC-θ, a signal transduction molecule which is known to play an important role in the TCR–NF-κB pathway (19). It is of note that PKC-θ–mediated phosphorylation can activate WASp at the IS (20). Thus, in iNKT cell development, WASp might be a relevant target of the activated PKC-θ in the TCR–NF-κB pathway. In addition, iNKT cell development requires TCR-induced transcriptional factors, such as NF-κB, AP-1, and T-bet (2), whose expression or function are altered in the absence of WASp (10–12). Indeed, a reduced iNKT cell generation and maturation was associated with the absence of the transcriptional factor NF-κB (21), and WASp was demonstrated, at least in NK cells, to be involved in the regulation of NF-κB nuclear translocation (10). Moreover, transgenic mice overexpressing BATF, a negative regulator of AP-1 activity, showed defective maturation of iNKT cells (22, 23). Interestingly, WASp absence was associated with a lower AP-1 binding activity in mouse T cells (12). A severe block in NK1.1 expression was also reported in mice lacking T-bet, a transcriptional factor associated to Th1 immunity (24). A possible role for WASp in the regulation of T-bet is supported by a recent study from our group showing a reduced T-bet expression in TCR-stimulated CD4+ T cell lines from WAS patients (11). Furthermore, very recent evidences have highlighted the critical role of costimulatory signals arising from B7–CD28 interaction in promoting the expansion of mature NK1.1+ iNKT cells (25, 26). Interestingly, WASp was demonstrated to be required for a normal ligation-induced CD28 endocytosis, a process which is relevant to CD28 costimulatory functions (27). These evidences support the hypothesis that WASp acts as an important component in the downstream events of TCR pathway during iNKT cell terminal maturation.
Impaired function of was−/− iNKT cells
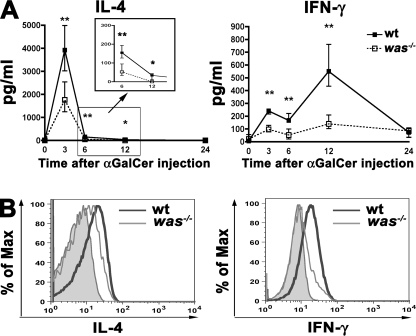
To investigate whether iNKT cells also exhibit functional defects in the absence of WASp, we evaluated the in vivo response to α-GalCer, a synthetic glycosphingolipid that elicits a prompt and selective activation of iNKT cells through the CD1d molecule. Upon α-GalCer stimulation, iNKT cells quickly produce huge amounts of Th1 and Th2 cytokines. We injected 1 µg α-GalCer i.v. into was−/− mice and WT controls and evaluated IL-4 and IFN-γ production in the sera of treated animals 3, 6, 12, and 24 h after stimulation. Although in was−/− mice the kinetics of cytokine production was similar to that in WT mice, the amount of IL-4 and IFN-γ in was−/− sera was significantly reduced at every time point that was tested (Fig. 5 A). The lower cytokine levels could be a result of the reduced iNKT cell number in was−/− mice but also of the impaired ability of was−/− iNKT cells to produce cytokines. To address this issue, we administered α-GalCer to was−/− or WT mice and analyzed ex vivo the intracellular cytokine production by iNKT cells. 45 min after α-GalCer injection, a significant fraction of WT iNKT cells produced a considerable amount of intracellular IL-4 and IFN-γ, whereas was−/− iNKT cells showed an impaired production of both cytokines (Fig. 5 B). In parallel, we evaluated the in vivo iNKT cell expansion induced by α-GalCer injection. The analysis of iNKT cell number at different time points (3, 7, and 11 d) revealed, as expected, a peak of expansion of WT iNKT cell at day 3 followed by a contraction at day 7. Conversely, was−/− iNKT cells showed a delayed kinetics with maximum expansion at day 7 followed by a contraction at day 11 (Fig. S3 A). The increase of was−/− iNKT cell absolute number induced by α-GalCer was significant compared with the untreated mice. However, the capacity of was−/− iNKT cells to expand was substantially lower than WT iNKT cells, as shown by the differences in fold increase values (Fig. S3 B).
Figure 5.
Impaired cytokine production by was−/− iNKT cells. (A) In vivo IL-4 and IFN-γ production upon α-GalCer administration in WT and was−/− mice. Sera were analyzed at 3, 6, 12, and 24 h upon injection. The graphs show the amount of cytokines produced by six WT and six was−/− mice. Black and white squares represent the median values of WT and was−/− mice groups, respectively. The vertical bars represent the interquartile range of each group. *, P < 0.05; **, P < 0.005. (B) IL-4 and IFN-γ production in WT and was−/− mice at the single iNKT cell level. WT and was−/− mice were injected with α-GalCer and, after 45 min, hepatic leukocytes were isolated and stained with α-GalCer–loaded CD1d tetramers and anti-CD3, anti–IL-4, and anti–IFN-γ mAbs. Representative analysis of IL-4 and IFN-γ intracellular production by iNKT cells (CD3+ and CD1d tetramer+) from WT (thick line) and was−/− (thin line) mice is shown. Filled histograms represent IL-4 or IFN-γ production by untreated WT mice. Data are from one representative experiment of three.
The functional defect of was−/− iNKT cells was further confirmed by their inability to help antigen-specific B cell responses in vivo compared with WT-activated iNKT cells (28). The functional impairment of was−/− iNKT cells upon in vivo activation may not be caused by their arrest at the immature stage 2 of differentiation because peripheral WT iNKT with immature phenotype are able to produce both Th1 and Th2 cytokines (29). On the contrary, it may result from various factors such as alterations in antigen presentation, an improper interaction between iNKT cells and APC, or a cell-autonomous defect.
Indeed, the activation of iNKT cells requires TCR recognition of a glycolipid antigens–CD1d complex on the surface of APCs (1). It is possible that a reduced antigen presentation ability of was−/− APC may contribute to the in vivo impaired functionality of was−/− iNKT cells because was−/− DC have a reduced ability to migrate, assemble podosomes, and process particulate antigens (9). Moreover, it is tempting to speculate that iNKT cells, like conventional T lymphocytes, require IS formation to achieve a proper activation. In the absence of WASp, this process has been demonstrated to be defective (30). Furthermore, altered activation of transcriptional factors NF-AT and AP-1, occurring in the absence of WASp (11, 12), may affect not only the development, as discussed in previous paragraphs, but also iNKT cell function. In particular, reduction in NF-AT activation and alteration in nuclear translocation correlates with the impaired IL-2 production observed in was−/− T lymphocytes (11, 12). In agreement with this hypothesis, mouse models carrying defects in molecules involved in the activation of AP-1 present alteration in iNKT cells including a perturbed cytokine profile (22).
Altogether, these results show that the lack of WASp leads to profound alterations in iNKT cells, which are absent in severe WAS patients and reduced in the was−/− mouse model. In was−/− mice, accumulation of immature iNKT cells (CD44+ NK1.1−), together with the lack of mature subset, suggests a key role for WASp in iNKT cell maturation process. Moreover, analysis of in vivo stimulation and cytokine production reveals that peripheral iNKT cells are functionally impaired in was−/− mice. WASp is known as a central player in T cell activation by controlling actin polymerization, which in turn favors the generation of a long-lived IS between T cells and APCs (30, 31). We hypothesize that the postselection expansion and differentiation of iNKT cells requires the formation of a proper IS between developing iNKT cells and CD1d-expressing cells. The stable IS may allow developing iNKT cells to receive and integrate various agonist signals, originating from invariant TCR engagement and costimulatory molecules, to achieve full differentiation. Indeed, the defective was−/− iNKT cells resemble the ones generated in the absence of signal transduction molecules or transcription factors, such as PKC-θ, NF-κB, or T-bet. These molecules belong to the complex cascade of events generated upon TCR triggering and IS formation in conventional T cells and regulate iNKT cell expansion or survival, maturation, and cytokine production (19, 21, 24). Furthermore, the genetic deletion of costimulatory molecules, such as CD28, affects expansion and phenotypic and functional differentiation of iNKT cells (25, 26).
In conclusion, these findings provide the first evidence that WASp acts as an important player for the generation of mature and functional iNKT cells. Moreover, our data add a new perspective in the comprehension of the complex immune dysregulation and tumor susceptibility characterizing WAS.
MATERIALS AND METHODS
Patients.
Blood samples from patients and age-matched HDs were obtained according to standard ethical procedures and with the approval of the San Raffaele Scientific Institute Internal Review Board (TIGET02).
Mice.
C57BL/6 (B6) was−/− mice were provided by K.A. Siminovitch (Samuel Lunenfeld Research Institute, Mount Sinai Hospital, Toronto, Canada) (16). CD45.1 was−/− mice were generated in our facility. B6 WT mice were purchased from Charles River Laboratories. All mice were 8–12 wk old. Experiments were performed according to protocols approved by the Animal Care and Use Committee of the San Raffaele Scientific Institute (IACUC318).
Cell preparation and flow cytometry.
Human PBMCs were purified on Ficoll gradient (PoC; Axis-Shield) and stained with anti-CD4 (RPAT4; BD), anti-CD3 (UCHT1; BD), anti–TCR-Vα24 (C15; Beckman Coulter), and anti–TCR-Vβ11 (C21; Beckman Coulter) antibodies. For mouse studies, single cell suspensions were obtained from liver, spleen, and thymus. Hepatic leukocytes were purified using a Percoll (Sigma-Aldrich) gradient. The following mAbs were used for surface staining: anti-B220 (RA3-6B2), anti-CD8a (53–6.7), anti-CD4 (RM4-5), anti-CD44 (IM7), and anti-CD45.2 (104; all purchased from BD); anti-CD3 (17A2) and anti-NK1.1 (PK136; both purchased from BioLegend); and CD1d tetramers (ProImmune). For lipid loading, CD1d tetramers were incubated overnight with a 12 molar excess of α-GalCer (Axxora). Intracytoplasmic staining was performed using the Cytofix/Cytoperm kit (BD) and the following mAbs: anti–IFN-γ (XMG1.2) and anti–IL-4 (11B11; BD). Anti-WASp antibody was provided by H. Ochs (Research Center for Immunity and Immunotherapies, Seattle Children's Research Institute, University of Washington, Seattle, WA). Cells were acquired on a FACS CANTO (BD) and analyzed with FlowJo Software (Tree Star, Inc.).
Generation of BM chimeras.
BM lin− cells from CD45.2 B6 WT or was−/− mice were purified with the mouse hematopoietic progenitor enrichment kit (StemCell Technologies Inc.). Was−/− recipient mice (CD45.1) were irradiated (900 rad) before receiving i.v. 2.5 × 105 lin− cells. Reconstitution was monitored by flow cytometry on blood cells. Mice were sacrificed 7–9 wk after transplantation for the analysis of iNKT cells in thymus and liver. Thymocytes were depleted of CD8+ cells by magnetic beads (Miltenyi Biotec) to enrich iNKT cell fraction.
In vivo activation and cytokine production.
WT and was−/− mice were i.v injected with 1 µg α-GalCer in PBS or not injected as controls. To measure the in vivo IL-4 and IFN-γ production, blood samples were collected 3, 6, 12, and 24 h after injection. Serum cytokine levels were measured by Bio-Plex Technology (Bio-Rad Laboratories). To test the ex vivo IL-4 and IFN-γ intracellular production by liver iNKT cells, mice were sacrificed 45 min after α-GalCer injection.
Statistical analysis.
All data were analyzed with a two-tailed Mann-Whitney U test.
Online supplemental material.
Table S1 describes the gene mutations and clinical status of patients. Table S2 shows the TCR-Vβ repertoire of HD and WAS patients. Fig. S1 displays the analysis of TCR-Vα24 and TCR-Vβ11 single-positive cells in HD, XLT, and WAS patients. Fig. S2 depicts CD1d expression in DP thymocytes and in splenic B cells and DCs. Fig. S3 shows iNKT cell in vivo expansion upon α-GalCer injection. Fig. S4 describes iNKT cell help to antigen-specific antibody responses. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20081773/DC1.
Acknowledgments
We thank K.A. Siminovitch for providing was−/− mouse strain.
This work was supported by OTKA 17049 to L. Marodi; Ministero Saulte RF2007:Giovani Ricercatori grant to M. Bosticardo; Italian Telethon Foundation to M.G. Roncarolo and A. Villa; N.O.B.E.L. (Network Operativo per la Biomedicina di Eccellenza in Lombardia) Program from Fondazione Cariplo to M.G. Roncarolo; and FIRB (Fondo per gli Investimenti della Ricerca di Base) to M.G. Roncarolo.
The authors have no conflicting financial interests.
References
- Bendelac A., Savage P.B., Teyton L. 2007. The biology of NKT cells.Annu. Rev. Immunol. 25:297–336 [DOI] [PubMed] [Google Scholar]
- Godfrey D.I., Berzins S.P. 2007. Control points in NKT-cell development.Nat. Rev. Immunol. 7:505–518 [DOI] [PubMed] [Google Scholar]
- Tupin E., Kinjo Y., Kronenberg M. 2007. The unique role of natural killer T cells in the response to microorganisms.Nat. Rev. Microbiol. 5:405–417 [DOI] [PubMed] [Google Scholar]
- Crowe N.Y., Smyth M.J., Godfrey D.I. 2002. A critical role for natural killer T cells in immunosurveillance of methylcholanthrene-induced sarcomas.J. Exp. Med. 196:119–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kaer L. 2007. NKT cells: T lymphocytes with innate effector functions.Curr. Opin. Immunol. 19:354–364 [DOI] [PubMed] [Google Scholar]
- Latour S. 2007. Natural killer T cells and X-linked lymphoproliferative syndrome.Curr. Opin. Allergy Clin. Immunol. 7:510–514 [DOI] [PubMed] [Google Scholar]
- Matangkasombut P., Pichavant M., Saez D.E., Giliani S., Mazzolari E., Finocchi A., Villa A., Sobacchi C., Cortes P., Umetsu D.T., Notarangelo L.D. 2008. Lack of iNKT cells in patients with combined immune deficiency due to hypomorphic RAG mutations.Blood. 111:271–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai K., Morio T., Zhu Y., Jin Y., Itoh S., Kajiwara M., Yata J., Mizutani S., Ochs H.D., Nonoyama S. 2004. Clinical course of patients with WASP gene mutations.Blood. 103:456–464 [DOI] [PubMed] [Google Scholar]
- Ochs H.D., Thrasher A.J. 2006. The Wiskott-Aldrich syndrome.J. Allergy Clin. Immunol. 117:725–738 [DOI] [PubMed] [Google Scholar]
- Huang W., Ochs H.D., Dupont B., Vyas Y.M. 2005. The Wiskott-Aldrich syndrome protein regulates nuclear translocation of NFAT2 and NF-kappa B (RelA) independently of its role in filamentous actin polymerization and actin cytoskeletal rearrangement.J. Immunol. 174:2602–2611 [DOI] [PubMed] [Google Scholar]
- Trifari S., Sitia G., Aiuti A., Scaramuzza S., Marangoni F., Guidotti L.G., Martino S., Saracco P., Notarangelo L.D., Roncarolo M.G., Dupre L. 2006. Defective Th1 cytokine gene transcription in CD4+ and CD8+ T cells from Wiskott-Aldrich syndrome patients.J. Immunol. 177:7451–7461 [DOI] [PubMed] [Google Scholar]
- Cianferoni A., Massaad M., Feske S., de la Fuente M.A., Gallego L., Ramesh N., Geha R.S. 2005. Defective nuclear translocation of nuclear factor of activated T cells and extracellular signal-regulated kinase underlies deficient IL-2 gene expression in Wiskott-Aldrich syndrome.J. Allergy Clin. Immunol. 116:1364–1371 [DOI] [PubMed] [Google Scholar]
- Torgerson T.R., Ochs H.D. 2007. Regulatory T cells in primary immunodeficiency diseases.Curr. Opin. Allergy Clin. Immunol. 7:515–521 [DOI] [PubMed] [Google Scholar]
- Tahir S.M., Cheng O., Shaulov A., Koezuka Y., Bubley G.J., Wilson S.B., Balk S.P., Exley M.A. 2001. Loss of IFN-gamma production by invariant NK T cells in advanced cancer.J. Immunol. 167:4046–4050 [DOI] [PubMed] [Google Scholar]
- Fujii S., Shimizu K., Klimek V., Geller M.D., Nimer S.D., Dhodapkar M.V. 2003. Severe and selective deficiency of interferon-gamma-producing invariant natural killer T cells in patients with myelodysplastic syndromes.Br. J. Haematol. 122:617–622 [DOI] [PubMed] [Google Scholar]
- Zhang J., Shehabeldin A., da Cruz L.A., Butler J., Somani A.K., McGavin M., Kozieradzki I., dos Santos A.O., Nagy A., Grinstein S., et al. 1999. Antigen receptor–induced activation and cytoskeletal rearrangement are impaired in Wiskott-Aldrich syndrome protein–deficient lymphocytes.J. Exp. Med. 190:1329–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendelac A. 1995. Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes.J. Exp. Med. 182:2091–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNab F.W., Berzins S.P., Pellicci D.G., Kyparissoudis K., Field K., Smyth M.J., Godfrey D.I. 2005. The influence of CD1d in postselection NKT cell maturation and homeostasis.J. Immunol. 175:3762–3768 [DOI] [PubMed] [Google Scholar]
- Stanic A.K., Bezbradica J.S., Park J.J., Van Kaer L., Boothby M.R., Joyce S. 2004. Cutting edge: the ontogeny and function of Va14Ja18 natural T lymphocytes require signal processing by protein kinase C theta and NF-kappa B.J. Immunol. 172:4667–4671 [DOI] [PubMed] [Google Scholar]
- Sasahara Y., Rachid R., Byrne M.J., de la Fuente M.A., Abraham R.T., Ramesh N., Geha R.S. 2002. Mechanism of recruitment of WASP to the immunological synapse and of its activation following TCR ligation.Mol. Cell. 10:1269–1281 [DOI] [PubMed] [Google Scholar]
- Stanic A.K., Bezbradica J.S., Park J.J., Matsuki N., Mora A.L., Van Kaer L., Boothby M.R., Joyce S. 2004. NF-kappa B controls cell fate specification, survival, and molecular differentiation of immunoregulatory natural T lymphocytes.J. Immunol. 172:2265–2273 [DOI] [PubMed] [Google Scholar]
- Williams K.L., Zullo A.J., Kaplan M.H., Brutkiewicz R.R., Deppmann C.D., Vinson C., Taparowsky E.J. 2003. BATF transgenic mice reveal a role for activator protein-1 in NKT cell development.J. Immunol. 170:2417–2426 [DOI] [PubMed] [Google Scholar]
- Zullo A.J., Benlagha K., Bendelac A., Taparowsky E.J. 2007. Sensitivity of NK1.1-negative NKT cells to transgenic BATF defines a role for activator protein-1 in the expansion and maturation of immature NKT cells in the thymus.J. Immunol. 178:58–66 [DOI] [PubMed] [Google Scholar]
- Townsend M.J., Weinmann A.S., Matsuda J.L., Salomon R., Farnham P.J., Biron C.A., Gapin L., Glimcher L.H. 2004. T-bet regulates the terminal maturation and homeostasis of NK and Valpha14i NKT cells.Immunity. 20:477–494 [DOI] [PubMed] [Google Scholar]
- Williams J.A., Lumsden J.M., Yu X., Feigenbaum L., Zhang J., Steinberg S.M., Hodes R.J. 2008. Regulation of thymic NKT cell development by the B7-CD28 costimulatory pathway.J. Immunol. 181:907–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X., Zhang H., Yin L., Wang C.R., Liu Y., Zheng P. 2008. Modulation of NKT cell development by B7-CD28 interaction: an expanding horizon for costimulation.PLoS One. 3:e2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badour K., McGavin M.K., Zhang J., Freeman S., Vieira C., Filipp D., Julius M., Mills G.B., Siminovitch K.A. 2007. Interaction of the Wiskott-Aldrich syndrome protein with sorting nexin 9 is required for CD28 endocytosis and cosignaling in T cells.Proc. Natl. Acad. Sci. USA. 104:1593–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli G., Pittoni P., Tonti E., Malzone C., Uematsu Y., Tortoli M., Maione D., Volpini G., Finco O., Nuti S., et al. 2007. Invariant NKT cells sustain specific B cell responses and memory.Proc. Natl. Acad. Sci. USA. 104:3984–3989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNab F.W., Pellicci D.G., Field K., Besra G., Smyth M.J., Godfrey D.I., Berzins S.P. 2007. Peripheral NK1.1 NKT cells are mature and functionally distinct from their thymic counterparts.J. Immunol. 179:6630–6637 [DOI] [PubMed] [Google Scholar]
- Dupre L., Aiuti A., Trifari S., Martino S., Saracco P., Bordignon C., Roncarolo M.G. 2002. Wiskott-Aldrich syndrome protein regulates lipid raft dynamics during immunological synapse formation.Immunity. 17:157–166 [DOI] [PubMed] [Google Scholar]
- Sims T.N., Soos T.J., Xenias H.S., Dubin-Thaler B., Hofman J.M., Waite J.C., Cameron T.O., Thomas V.K., Varma R., Wiggins C.H., et al. 2007. Opposing effects of PKCtheta and WASp on symmetry breaking and relocation of the immunological synapse.Cell. 129:773–785 [DOI] [PubMed] [Google Scholar]