Abstract
The NKG2D receptor stimulates natural killer cell and T cell responses upon engagement of ligands associated with malignancies and certain autoimmune diseases. However, conditions of persistent NKG2D ligand expression can lead to immunosuppression. In cancer patients, tumor expression and shedding of the MHC class I–related chain A (MICA) ligand of NKG2D drives proliferative expansions of NKG2D+CD4+ T cells that produce interleukin-10 (IL-10) and transforming growth factor-β, as well as Fas ligand, which inhibits bystander T cell proliferation in vitro. Here, we show that increased frequencies of functionally equivalent NKG2D+CD4+ T cells are inversely correlated with disease activity in juvenile-onset systemic lupus erythematosus (SLE), suggesting that these T cells may have regulatory effects. The NKG2D+CD4+ T cells correspond to a normally occurring small CD4 T cell subset that is autoreactive, primed to produce IL-10, and clearly distinct from proinflammatory and cytolytic CD4 T cells with cytokine-induced NKG2D expression that occur in rheumatoid arthritis and Crohn's disease. As classical regulatory T cell functions are typically impaired in SLE, it may be clinically significant that the immunosuppressive NKG2D+CD4+ T cells appear functionally uncompromised in this disease.
Receptors that inhibit or activate NK cells can also be expressed on T cells, where they modulate TCR–CD3 complex–dependent responses (1–4). Among these receptors is NKG2D, which interacts with ligands that are absent from most normal cells but can be transcriptionally induced by generic mechanisms of cellular stress and chromatin remodeling (5–9). In humans, NKG2D ligands include the MHC class I–related chain A (MICA), which is frequently associated with epithelial tumors, is induced by microbial infections, and is expressed in certain autoimmune disease target tissues (8, 10–14). Upon ligand engagement, NKG2D conveys directly activating or costimulatory signals via the paired DAP10 adaptor protein (4, 13, 15, 16). NKG2D may thus promote cancer and infectious disease immunity, but worsen autoimmune disease progression. Consistent with its role in effector responses, NKG2D is present on virtually all NK cells and CD8 T cells (6).
However, NKG2D also costimulates proliferative expansions of normally rare NKG2D+CD4+ T cells that have negative regulatory functions and may serve to dampen chronic immune activation resulting from persistent MICA expression (17). Small populations of CD4 T cells with activation-independent, constitutive, NKG2D expression occur in normal peripheral blood (6, 17–19). They appear biased toward an IL-10– and TGF-β–dominated cytokine profile (17). Upon NKG2D costimulation, the NKG2D+CD4+ T cells also produce Fas ligand (FasL), causing growth arrest of bystander T cells in vitro, but are themselves resistant to Fas-mediated homeostatic regulation (17). Hence, in tissue environments providing stimulation of TCR and NKG2D, the NKG2D+CD4+ T cells may expand in numbers relative to other T cells, causing imbalances in the lymphocyte pool and imposing an immunosuppressive cytokine milieu. This model is supported by proliferative expansions, driven by tumor-associated and shed soluble MICA (sMICA), of IL-10, TGF-β, and FasL-producing NKG2D+CD4+ T cells in late-stage cancer patients (17). Because these T cells are stimulated by cancer cells of diverse tissue origins, it seems probable that they recognize normal self-antigens as opposed to malignancy-associated antigens.
By inference, persistent expression of MICA in tissues affected by autoimmune pathology might also result in expansions of the suppressive NKG2D+CD4+ T cells. In this scenario, the NKG2D+CD4+ T cells would be expected to ameliorate disease severity, thus resembling regulatory T (T reg) cells (20, 21). This conceptual view of regulatory NKG2D+CD4+ T cells is complicated by occurrences of effector NKG2D+CD4+ T cells from which they must be distinguished. IFN-γ and TNF-α producing cytotoxic NKG2D+CD4+ T cells occur in rheumatoid arthritis (RA) patients, and frequencies of perforin-positive NKG2D+CD4+ T cells correlate with the severity of Crohn's disease (11, 18). Additional complexity is suggested by observations that cytotoxic NKG2D+CD4+ T cell expansions may be driven by CMV (22). These discrepancies could be explained by the existence of developmentally distinct NKG2D+CD4+ T cell populations, including the normally occurring subset mainly characterized by production of IL-10 and TGF-β, and an effector subset with NKG2D expression that is cytokine induced (11, 18). Alternatively, normally occurring NKG2D+CD4+ T cells could be functionally heterogeneous.
In this study, we show that normally occurring NKG2D+CD4+ T cells in healthy individuals are autoreactive and primed to produce IL-10, but lack proinflammatory cytokine and cytolytic signatures and play no discernible role in antimicrobial recall responses. Large expansions of these T cells in patients with juvenile-onset systemic lupus erythematosus (SLE) are inversely correlated with activity of disease, thus suggesting that they participate in regulation of effector responses that provoke autoimmunity.
RESULTS
Normal peripheral blood NKG2D+CD4+ T cells are autoreactive
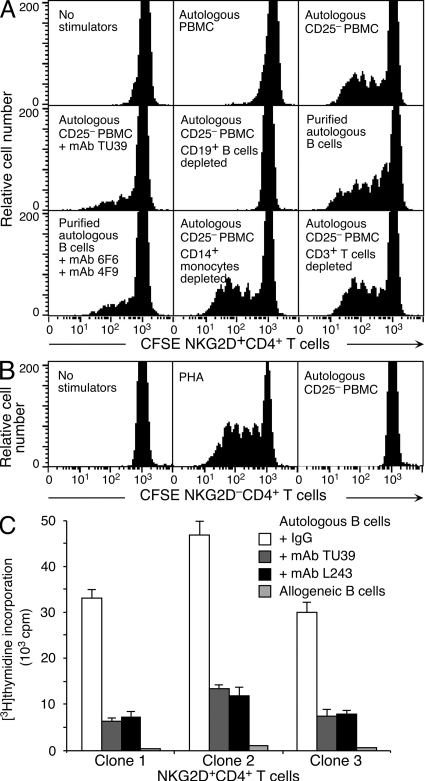
Functional attributes of normal peripheral blood NKG2D+CD4+ T cells, which occur at frequencies of ∼0.5–3%, are insufficiently defined (17). We therefore examined their autoreactivity, cytokine signature, and cytolytic capacity. We have previously shown that NKG2D+CD4+ T cell clones derived from an ovarian cancer patient produce IL-10 and TGF-β upon recognition of MHC class II complexes on autologous tumor and B lymphoblastoid cells (unpublished data) (17). Autoreactivity of normal peripheral blood NKG2D+CD4+ T cells was established in CFSE dilution assays, demonstrating proliferation of these T cells (but not of NKG2D−CD4+ T cells) in the presence of autologous PBMCs depleted of CD25+ T reg cells to unmask T cell reactivity (Fig. 1, A and B) (23). This response was solely dependent on B cells and MHC class II, as separately shown by CD19+ lymphocyte depletion and B cell reconstitution, and by anti–MHC class II antibody blocking. T cell and monocyte depletions had no effect (Fig. 1 A). The costimulatory capacity of the ex vivo–purified B cells was caused by the expression of the UL16-binding protein (ULBP) 2 and 3 ligands of NKG2D (Fig. 1 A) (24). The observed autoreactivity of the NKG2D+CD4+ T cells was confirmed with 32 T cell clones established from three unrelated donors, which incorporated [3H]thymidine in the presence of autologous, but not allogeneic, B cells and were restricted by HLA-DR (Fig. 1 C).
Figure 1.
Autoreactivity of normal peripheral blood NKG2D+CD4+ T cells. (A) NKG2D+CD4+ T cells proliferate in the presence of CD25+ cell–depleted autologous PBMCs. CD3+CD4+CD8−NKG2D+ T cells FACSAria-sorted from magnetic bead–enriched CD4+ T cells to ≥99% purity were labeled with CFSE and cultured with irradiated autologous total or CD25− stimulator PBMCs, or subpopulations thereof, as indicated. CD25+ lymphocytes were depleted to enhance T cell proliferation (23). Autoreactive NKG2D+CD4+ T cell stimulation is solely dependent on B cells as shown separately by B cell depletion and reconstitution. T cell and monocyte depletions have no effect. Blocking antibodies are specific for MHC class II (mAb TU39), and ULBP2 or ULBP3 (mAbs 6F6 and 4F9). The CFSE profiles shown represent gated NKG2D+CD4+ T cells. (B) Purified NKG2D−CD4+ T cells fail to proliferate in the presence of CD25+ cell–depleted autologous PBMCs. Treatment of NKG2D−CD4+ T cells with PHA is shown as positive control. CFSE profiles represent gated NKG2D−CD4+ T cells. Data shown in A and B are representative of results obtained in duplicate experiments with PBMCs from five independent donors. (C) Thymidine incorporation by NKG2D+CD4+ T cell clones in the presence of autologous, but not allogeneic, B cells is inhibited by anti-MHC class II (mAb TU39) and -HLA-DR (mAb L243). Clones 1–3 were derived from 3 donors and are representative of a total of 32 clones. Error bars indicate deviations among triplicate wells. Data shown are representative of three experiments per clone.
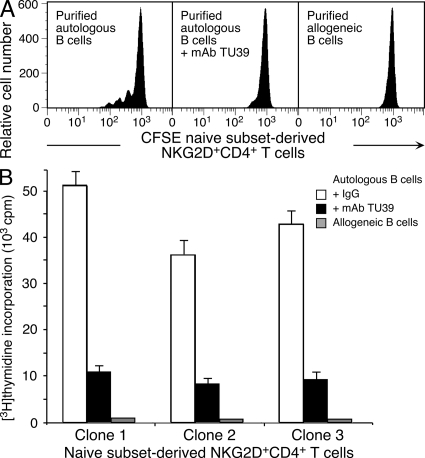
Most normal peripheral blood NKG2D+CD4+ T cells lack conventional markers of antigen exposure, an unexpected phenotype of an autoreactive T cell population that may frequently encounter self (17). However, CFSE dilution experiments with ex vivo–sorted, phenotypically naive CD45RO−CD62L+CD28+CD27+NKG2D+CD4+ bulk responder T cells, and analysis of T cell clones established from single-cell sorted naive NKG2D+CD4+ T cells, confirmed the occurrence of autoreactive and MHC class II–restricted T cell specificities within the naive NKG2D+CD4+ T cell pool (Fig. 2, A and B).
Figure 2.
Autoreactivity of phenotypically naive NKG2D+CD4+ T cells. (A) CD3+CD4+CD8−CD45RA+CD45RO−CD62L+CCR7+CD28+CD27+NKG2D+ T cells FACSAria sorted from magnetic bead–enriched CD4+ T cells to >99% purity dilute CFSE in the presence of autologous, but not allogeneic, B cells. Proliferation is inhibited by anti-MHC class II mAb TU39. CFSE profiles shown represent gated NKG2D+CD4+ T cells and are representative of data obtained in duplicate experiments with PBMCs from five independent donors. (B) NKG2D+CD4+ T cell clones derived from single-cell sorted naive NKG2D+CD4+ T cells incorporate [3H]thymidine in the presence of autologous, but not allogeneic B cells. Proliferative responses are inhibited by mAb TU39. Clones 1–3 are representative of a total of 60 clones established from 3 donors. Error bars indicate deviations among triplicate wells. Data shown are representative of three experiments per clone.
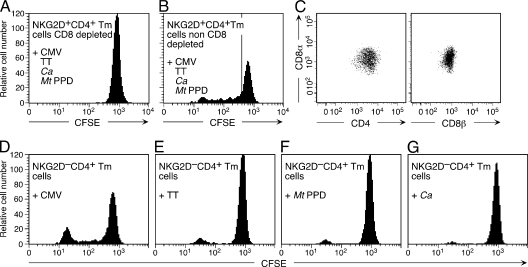
These results were complemented by evidence arguing against a primary role of the NKG2D+CD4+ T cells in host defense, as no memory responses by these T cells, isolated from CMV-seropositive and tuberculin skin test–positive individuals, were discernible against a panel of “recall” antigens, including CMV pp65, tetanus toxoid, Mycobacterium tuberculosis–purified protein derivative, and Candida albicans (Fig. 3 A). In contrast, recall responses against all of these antigens were readily detected with memory NKG2D−CD4+ T cells (Fig. 3, D–G), as well as with NKG2D+CD4+ T cells coexpressing CD8αβ, which comprise significant proportions (∼20–80%; not depicted) of NKG2D+CD4+ T cells if these are purified without taking CD8 into account (Fig. 3, B and C). These results are consistent with the fact that double-positive CD8αβ+CD4+NKG2D+ T cells are fully differentiated CD28− effector memory T cells enriched for antimicrobial specificities, and suggest that the previously recorded CMV-driven expansions of NKG2D+CD4+ T cells may have been limited to proliferation of nonexcluded double-positive T cells (22, 25).
Figure 3.
Absence of recall responses by NKG2D+CD4+ T cells. (A) By CFSE dilution, purified memory NKG2D+CD8−CD4+ T cells are unresponsive to stimulation by autologous monocytes pulsed with CMV pp65, tetanus toxoid (TT), M. tuberculosis–purified protein derivative (Mt PPD), and C. albicans (Ca) antigen cocktail. (B) Proliferation of NKG2D+CD4+ T cells nondepleted for CD8 coexpressing T cells. (C) CFSE diluting T cells seen in B (profile left of vertical line) are double-positive CD8αβ+CD4+ NKG2D+ T cells. (D−G) Proliferation of donor-matched purified memory NKG2D−CD4+ T cells upon stimulation by monocytes pulsed with the individual recall antigens. Data shown are representative of results obtained in duplicate experiments with PBMC from five donors.
Normal peripheral blood NKG2D+CD4+ T cells are primed to produce IL-10
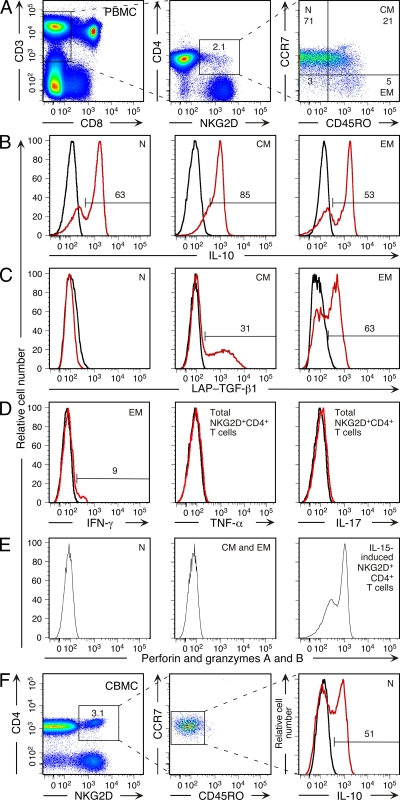
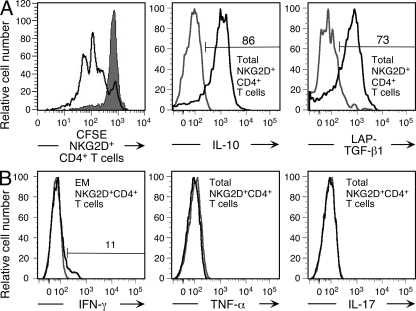
Based on ELISA, bulk cultures of normally occurring NKG2D+CD4+ T cells are biased toward an IL-10– and TGF-β–dominated cytokine profile (17). However, this trait has not been evaluated at the single-cell level or associated with maturational T cell phenotypes. Moreover, the proinflammatory and cytolytic potential of these T cells has not been fully assessed. Using polychromatic flow cytometry, we simultaneously determined cytokine production and CD45RO/CCR7-defined naive and memory phenotypes of peripheral blood NKG2D+CD4+ T cells after short-term polyclonal stimulation. Consistent with the previous data, the T cells produced IL-10, TGF-β, and only a small amount of IFN-γ, and were negative for IL-2 and IL-4. Proinflammatory TNF-α and IL-17 cytokines were not produced (Fig. 4, A–D, and not depicted). Among the positive cytokines, IL-10 was most frequently detected and associated with all T cell differentiation stages representing 48.9 ± 13.8%, 65.7 ± 12.6%, and 51.5 ± 10.2% (mean percentage ± SD for 10 donors) of naive, central memory, and effector memory NKG2D+CD4+ T cells, respectively (Fig. 4 B). TGF-β induction was recorded with central memory (39.8 ± 12.2%) and effector memory (43.7 ± 9%), but not naive NKG2D+CD4+, T cells by surface staining for the latency-associated peptide (LAP), which noncovalently associates with mature TGF-β homodimers (Fig. 4 C) (26). These results were independently confirmed by staining for intracellular LAP-TGF-β (unpublished data). Because of different T cell stimulation time periods required for optimal IL-10 and TGF-β induction, these cytokines could not be simultaneously tested. Minimal IFN-γ responses were recorded with small populations (13.8 ± 8.6%) of IL-10 producing effector memory NKG2D+CD4+ T cells (Fig. 4 D). By flow cytometry testing for perforin, granzymes A and B, and activation-induced surface mobilization of the CD107a degranulation marker, the peripheral blood NKG2D+CD4+ T cells displayed no sign of cytolytic capacity (Fig. 4 E and not depicted). Among these results, the isolated (IL-10 only) and rapid (within 6 h of polyclonal stimulation) cytokine response by the tentatively naive CD45RO−CCR7+NKG2D+CD4+ T cells was highly unusual. However, we made the same observation with CD45RO−CCR7+NKG2D+CD4+ T cells from cord blood and with bona fide naive (CD45RA+CD62L+CD28+CD27+CD11a−) NKG2D+CD4+ T cells from peripheral blood and cord blood (Fig. 4 F and not depicted).
Figure 4.
IL-10 and TGF-β cytokine signature of peripheral blood and cord blood NKG2D+CD4+ T cells after short-term stimulation with PMA and ionomycin. (A) Polychromatic flow cytometry gating tree for naive (N; CD45RO−CCR7+), central memory (CM; CD45RO+CCR7+), and effector memory (EM; CD45RO−CCR7−) NKG2D+CD8−CD4+CD3+ T cells. Numbers in quadrants and square gate specify T cell frequencies (in percent). All histograms shown in B–D were generated based on this gating tree. (B) Staining for intracellular IL-10 of naive (N) and central (CM) and effector (EM) memory NKG2D+CD4+ T cells. (C) Staining of surface LAP-TGF-β1 on memory (CM and EM), but not naive (N), NKG2D+CD4+ T cells. (D) Minimal detection of intracellular IFN-γ and absence of TNF-α and IL-17 in effector memory (EM) and total NKG2D+CD4+ T cell populations, respectively. (E) Lack of intracellular staining of naive and memory (CM and EM) NKG2D+CD4+ T cells for perforin and granzymes A and B using an antibody cocktail. Gating was as shown in A. Histogram at right shows positive control staining of CD4 T cells with IL-15–induced NKG2D expression (11). (F) Gating tree and intracellular IL-10 staining of naive (CD45RO−CCR7+) NKG2D+CD4+ T cells among CBMCs. Numbers specify T cell frequencies (in percent). Numbers above horizontal bars in histograms B−D and F indicate the percentage of positive cells. Histograms shown in red and black are from stimulated and unstimulated T cells, respectively. Data shown are representative of results obtained in duplicate experiments with samples from 10 donors.
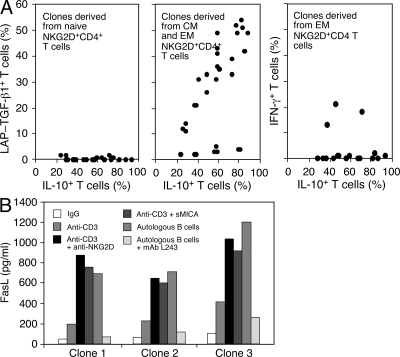
The cytokine profiles of NKG2D+CD4+ T cells were confirmed by clonal analysis of naive and memory subset T cells after FACS sorting into single wells and expansion under nonpolarizing conditions. All clones that originated from phenotypically naive NKG2D+CD4+ T cells produced IL-10 only, whereas most memory subset-derived clones were also positive for intracellular and surface LAP-TGF-β (Fig. 5 A, left and middle, and not depicted). Consistent with the minor IFN-γ responses recorded with some peripheral blood effector memory NKG2D+CD4+ T cells (Fig. 4 D, left), 3 out of 20 IL-10+ effector memory subset-derived clones included small populations of IFN-γ–producing cells (Fig. 5 A, right). All clones were negative for TNF-α and IL-17, lacked cytotoxic proteins, and redirected lysis activity against P815 cells (unpublished data). These and the autoreactive T cell clones tested above (see first paragraph of Results) were further used for confirmation of FasL production, which is the principal mediator of NKG2D+CD4+ T cell regulatory functions, at least in vitro (Fig. 5 B and not depicted) (17). Altogether, these results establish normally occurring NKG2D+CD4+ T cells as a distinct T cell subset that is autoreactive, has no proinflammatory and cytolytic features, and is primed to produce IL-10. These T cells are unusual because they are endowed with an isolated and immediate IL-10 response at a phenotypically naive stage. This quasi innate status is unaligned with the peripheral polarization of conventional T cells and may involve genetic imprinting of the IL-10 locus independent of antigenic stimulation (27, 28).
Figure 5.
IL-10, TGF-β, IFN-γ, and FasL production by normal peripheral blood-derived NKG2D+CD4+ T cell clones. (A) Detection of intracellular IL-10 and surface LAP-TGF-β1, and of IL-10 and IFN-γ among clones established under nonpolarizing conditions from sorted naive (24 T cell clones) and central and effector (CM and EM) memory (30 T cell clones) and effector (CM) memory (20 T cell clones) NKG2D+CD4+ T cells, respectively. Dots in scatter plots represent the percentage of positive cells among a given T cell clone. Clones were derived from three donors. (B) ELISA for FasL in supernatants of NKG2D+CD4+ T cell clones (identical to those used in Fig. 1) generated by autologous B cell stimulation. Conditions of experimental T cell stimulations are indicated in the bar graph. Data in A and B are representative of three separate experiments.
Frequencies of NKG2D+CD4+ T cells are negatively correlated with disease activity in juvenile-onset SLE
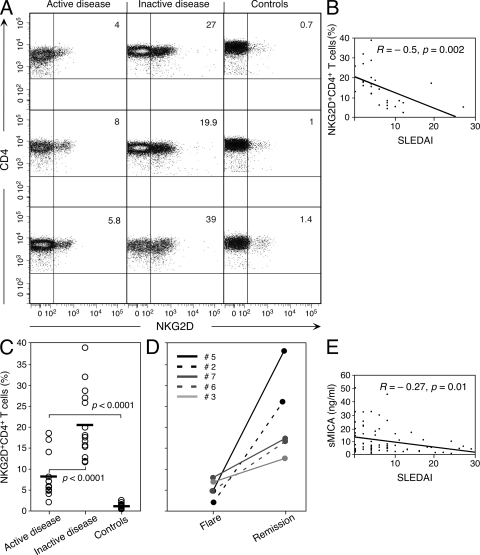
Our observations in advanced tumor settings led us to test whether the NKG2D+CD4+ T cells resemble conventional T reg cells, with similarly opposed negative and protective effects in tumor immunity and autoimmune diseases, respectively. We examined NKG2D+CD4+ T cell frequencies in proportion to all CD4 T cells in juvenile-onset SLE. In the course of this disease, patients undergo spontaneous flares and remissions, thus enabling comparisons of fluctuating disease activity data points, which were graded according to the SLE disease activity index (SLEDAI) (29). We scored proportional increases of NKG2D+CD4+ T cells in 13 active (SLEDAI ≥ 5) and 14 inactive (SLEDAI < 5) SLE patient peripheral blood samples in comparison to 14 age-matched controls (3 representative examples for each category are shown in Fig. 6 A). The overall data point distribution indicated a significant (P = 0.002) inverse correlation (R = –0.5) between NKG2D+CD4+ T cell frequencies and SLEDAI scores (Fig. 6 B). Comparisons of mean T cell frequencies in active and inactive SLE samples and controls revealed highly significant associations (P < 0.0001) with the disease in general, and confirmed the inverse correlation with disease severity in particular (Fig. 6 C). This relationship was further supported by data point pairs from five patients with serial samples obtained during periods of flare and subsequent remission of disease, which illustrated the link between changes in disease activity and NKG2D+CD4+ T cell frequencies (Fig. 6 D). Notably, these fluctuating proportions corresponded to changes in absolute NKG2D+CD4+ T cell numbers, with means of 38.7 ± 21.5 and 287.1 ± 130.6 cells per mm3 in the five serial flare and remission juvenile-onset SLE patient peripheral blood sample pairs, respectively. There was no statistically significant (P = 0.12) association between NKG2D+CD4+ T cell frequencies and the presence or absence of immunosuppressive therapy (Table I). Altogether, these results suggested that the NKG2D+CD4+ T cells may be associated with regulatory effects.
Figure 6.
Increased proportions of NKG2D+CD4+ T cells and inverse correlation with disease activity in juvenile-onset SLE. (A) Frequencies of NKG2D+CD4+ T cells (percentage of CD8−CD4+ T cells) in six disease and three age-matched control samples. (B) Overall data point distribution and statistical evaluation (R = −0.5; P = 0.02) of NKG2D+CD4+ T cell frequencies in relationship to SLEDAI scores in the 27 patient samples studied. (C) Comparison of mean frequencies (positions indicated by short horizontal bars) of NKG2D+CD4+ T cells in 13 active and 14 inactive juvenile-onset SLE and 14 age-matched control samples. Individual comparisons are bracketed, and P values are indicated. (D) NKG2D+CD4+ T cell frequencies in five patients at times of flare or remission of disease. Numbers refer to SLE subjects listed in Table I. (E) Plotting of sMICA concentrations in 72 juvenile-onset SLE plasma samples against SLEDAI scores (R = −0.27; P = 0.01). All data shown in A–E were derived from single analyses of patient and control samples.
Table I.
SLE patient characteristics
| Subject | Gender | Age at onset | Age at draw | SLEDAI | Prednisonea | Other immunomodulatory medicationsa | NKG2D+CD4+ T cellsb |
| yr | yr | mg/kg/day | % | ||||
| 1 | F | 13.1 | 13.8 | 4 | 0.30 | HCQ, MTX | 11.6 |
| 15.4 | 4 | 0.13 | AZT | 15.3 | |||
| 2 | F | 11.8 | 16.1 | 2 | 0.14 | HCQ | 27.0 |
| 17.3 | 12 | 0.00 | 2.0 | ||||
| 3 | F | 11.9 | 12.5 | 12 | 0.35 | HCQ | 7.0 |
| 14.5 | 0 | 0.50 | HCQ, dapsone | 12.7 | |||
| 4 | F | 14.9 | 16.5 | 0 | 0.08 | HCQc | 25.8 |
| 17.3 | 2 | 0.28 | HCQc | 15.6 | |||
| 5 | F | 10.0 | 10.2 | 10 | 0.00 | HCQ | 5.0 |
| 11.6 | 4 | 0.00 | HCQ | 39.0 | |||
| 12.1 | 5 | 0.00 | HCQ | 18.5 | |||
| 6 | M | 16.6 | 17.3 | 27 | 0.50 | 4.78 | |
| 18.3 | 4 | 0.04 | HCQ, Cycc | 16.6 | |||
| 7 | M | 15.0 | 15.3 | 11 | 0.00 | Dicloxacillin | 8.08 |
| 16.1 | 2 | 0.36 | HCQ, MMF | 17.53 | |||
| 8 | F | 7.0 | 12.2 | 4 | 0.00 | HCQ | 11.8 |
| 9 | M | 13.9 | 14.2 | 2 | 1.25 | HCQ | 18.0 |
| 10d | M | 7.1 | 11.0 | 2 | 0.14 | HCQ, dapsone | 32.0 |
| 11 | F | 8.9 | 18.2 | 4 | 0.00 | HCQ | 28.7 |
| 12 | F | 9.2 | 17.8 | 8 | 0.00 | HCQ, sulfasalazine | 8.07 |
| 13 | F | 12.2 | 15.7 | 8 | 0.00 | HCQ, MTX | 4.0 |
| 14 | F | 7.1 | 8.1 | 19 | 0.37 | HCQ, MMF | 17.0 |
| 15 | F | 8.1 | 9.2 | 4 | 0.00 | 19.92 | |
| 16 | F | 18.0 | 18.9 | 8 | 1.27 | 5.6 | |
| 17 | F | 11.6 | 11.9 | 6 | 0.00 | HCQ | 14.0 |
| 18 | F | 9.4 | 9.9 | 6 | 0.00 | 5.8 | |
| 19 | F | 14.0 | 15.5 | 8 | 0.13 | HCQ, MMF | 7.02 |
Therapeutic regimens including prednisone ≥0.2 mg/kg/day and/or azathioprine (AZT), dapsone, mycophenolate mofetil (MMF), methotrexate (MTX), or sulfasalazine were considered immunosuppressive.
Percentage of CD8−CD4+ T cells.
Patients enrolled in a placebo-controlled atorvastatin trial.
Patient with C1q deficiency.
Cyc, cyclophosphamide; HCQ, hydroxychloroquine.
Functional correspondence between juvenile-onset SLE patient and normally occurring NKG2D+CD4+ T cells
Immune regulation by NKG2D+CD4+ T cells is likely multifactorial, involving IL-10 and TGF-β cytokine and FasL production in response to self-antigen recognition and NKG2D costimulation, and may be perpetuated by their resistance to Fas-mediated homeostatic regulation. We scrutinized the functional attributes of NKG2D+CD4+ T cells in juvenile-onset SLE, taking into account that they may represent mixed populations that also include the proinflammatory and cytolytic CD4 T cells with cytokine-induced NKG2D expression described in RA and Crohn's disease (11, 18). Autoreactivity was confirmed in CFSE dilution experiments using autologous stimulator B cells (Fig. 7 A, left histogram). As determined by polychromatic flow cytometry, ex vivo SLE NKG2D+CD4+ T cells from all 13 active and 14 inactive disease samples displayed the IL-10 and TGF-β cytokine signature, produced minimal IFN-γ, and were negative for TNF-α, IL-17, and cytolytic markers (Fig. 7, A and B, and not depicted). Consistent with antigen-driven activation, most SLE NKG2D+CD4+ T cells expressed memory phenotypes, whereas, as noted for normal adults, age-matched control T cells were largely naive (Table II). The memory and naive NKG2D+CD4+ T cell subset segregation was similar among samples from active and inactive disease patients. The proportions of IL-10–producing cells among the patient-derived NKG2D+CD4+ T cells (72.6 ± 9.2%) were larger than those among age-matched controls (56 ± 11.7%), but were comparable between the active (72.2 ± 9.1%) and inactive (73 ± 9.2%) disease sample cohorts. Similar T cell proportions and numerical relationships were scored by analysis of all patient and control samples for intracellular and surface LAP–TGF-β (unpublished data). IFN-γ responses were limited to 9.9 ± 9% and 11.4 ± 7.8% effector memory NKG2D+CD4+ T cells from patients with active and inactive disease, respectively (Fig. 7 B).
Figure 7.
Autoreactivity and cytokine signature of NKG2D+CD4+ T cells in juvenile-onset SLE. (A) Left histogram shows CFSE dilution of sorted patient-derived NKG2D+CD4+ T cells in response to stimulation by autologous (open profile), but not allogeneic B cells (shaded profile). Data shown are representative of results obtained from single analyses of two samples each from patients with active or inactive disease. Middle and right histograms show stainings for intracellular IL-10 and surface LAP-TGF-b1 of patient NKG2D+CD4+ T cells stimulated with PMA and ionomycin. Data shown are representative of results obtained with 13 active and 14 inactive disease samples analyzed once. (B) Minimal staining for intracellular IFN-γ, and absence of staining for TNF-α and IL-17 among effector memory (EM) and total patient NKG2D+CD4+ T cell populations, respectively. Data shown are representative of results obtained with 13 active and 14 inactive disease samples analyzed once. All histograms displaying intracellular cytokine stainings were generated based on the gating tree shown in Fig. 4 A. Numbers above horizontal bars in histograms indicate the percentage of positive cells. Histograms shown in black and gray are from stimulated and unstimulated T cells, respectively.
Table II.
NKG2D+CD4+ T cell subset segregation in juvenile-onset SLE and controls
| NKG2D+CD4+ T cells | Active disease | Inactive disease | Controls |
| n = 13 | n = 14 | n = 14 | |
| Naïve (CD45RO−CCR7+) | 5.3% (±2.8)a | 6.1% (±4) | 73.5% (±9) |
| Central memory (CD45RO−CCR7+) | 45.8% (±13.4) | 47.1% (±14.3) | 17.9% (±8.1) |
| Effector memory (CD45RO+CCR7−) | 46.9% (±14.2) | 48.9% (±13.7) | 8.7% (±5.6) |
Mean (in percent) ± SD of NKG2D+CD4+ T cells positive for the indicated phenotype by flow cytometry.
Functional competence of NKG2D+CD4+ T cells in juvenile-onset SLE
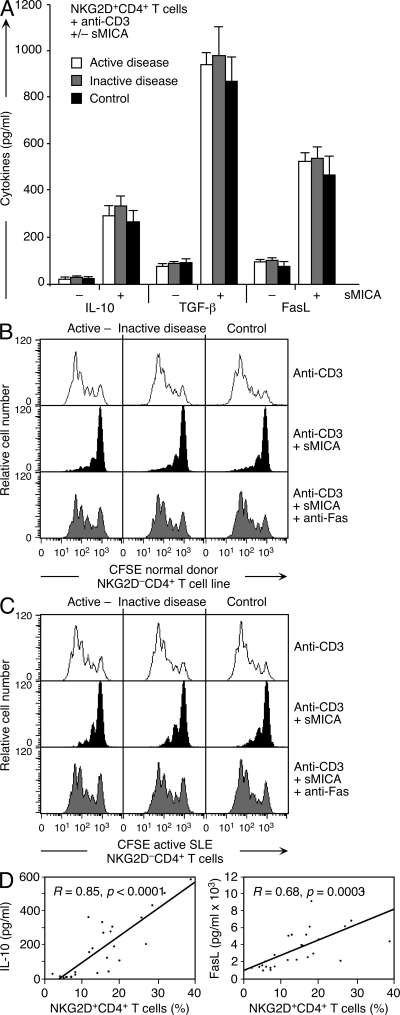
It is well known that T cell signaling and functions are aberrant in SLE, and some studies suggest that CD25+ T reg cells are functionally compromised (30, 31). It was therefore necessary to scrutinize the inverse correlation between disease activity and NKG2D+CD4+ T cell numbers by examining T cell functional competence in the context of population size. As determined by ELISA, purified memory NKG2D+CD4+ T cells from active and inactive disease patients, as well as age-matched controls, secreted similar amounts of IL-10, TGF-β, and FasL after stimulation with solid-phase anti-CD3 and sMICA (Fig. 8 A). Corresponding results were obtained by analysis of T cell clones (unpublished data). As previously shown with NKG2D+CD4+ T cells from tumor patients and normal controls (17), FasL produced by those T cells from juvenile-onset SLE patients representing both ends of the disease spectrum effectively induced Fas-mediated growth arrest of NKG2D−CD4+ T cells from normal controls (Fig. 8 B). NKG2D+CD4+ T cells from active and inactive SLE patients were also equally potent in inducing Fas-mediated inhibition of proliferation of patient-derived NKG2D−CD4+ T cells (Fig. 8 C).
Figure 8.
Functional competence of NKG2D+CD4+ T cells in juvenile-onset SLE. (A) IL-10, TGF-β, and FasL in supernatants of NKG2D+CD4+ T cells from active and inactive disease patients and age-matched controls. Sorted T cells were stimulated as indicated, and mediators were determined by ELISA. Data shown represent means of values obtained with each of five active and inactive SLE patient and control samples that were analyzed once. Error bars indicate SDs. (B and C) CFSE dilution histograms of gated NKG2D−CD4+ T cells (B, normal donor T cell line; C, ex vivo–sorted patient T cells) co-cultured with purified patient or control NKG2D+CD4+ T cells activated with solid-phase anti-CD3 alone and exposed to sMICA alone or together with the antagonist anti-Fas. Data shown are representative of each of five active and inactive SLE patient and control samples analyzed once. (D) Plotting of IL-10 and FasL plasma concentrations against NKG2D+CD4+ T cell frequencies (R = 0.85, P < 0.0001; and R = 0.68, P = 0.0003) from 27 juvenile-onset SLE patient samples analyzed once.
Together, these results indicate that most, if not all, NKG2D+CD4+ T cells in juvenile-onset SLE are progeny of the normally occurring NKG2D+CD4+ T cell subset. The functional disposition of these T cells appears relatively invariant and independent of disease activity, with the caveat that our in vitro analysis cannot account for factors that may modulate T cell functions in physiological environments. It remains unclear which of the NKG2D+CD4+ T cell functions, individually or in combination, may be related to ameliorating disease. However, IL-10 and FasL, determined in SLE patient plasma samples by ELISA, were positively correlated with NKG2D+CD4+ T cell frequencies (R = 0.85 and P < 0.0001 for IL-10; R = 0.68 and P = 0.0003 for FasL), and thus inversely correlated with SLEDAI scores in all of the 27 matched sample pairs tested (Fig. 8 D). These results suggest that the NKG2D+CD4+ T cells are a prominent source of immunosuppressive activity in the patient cohort in this study. This evidence contrasts results from earlier studies that identified monocytes, macrophages, and B cells as the main producers of IL-10, and noted positive correlations between disease activity and serum concentrations of IL-10 (32, 33). Possible reasons underlying these discrepancies are unknown.
Expression of NKG2D ligands in juvenile-onset SLE
In cancer patients, population expansions of the NKG2D+CD4+ T cells are dependent on the presence of tumor-associated MICA and correlate with serum concentrations of sMICA (17). Because of lack of access to tissue biopsies, we were unable to explore disease-associated NKG2D ligand expression in juvenile-onset SLE. However, we detected sMICA in all of the 72 patient plasma samples tested, but in none of 20 age-matched controls. Although an inverse trend relationship between sMICA and SLEDAI scores was apparent, plotting sMICA concentrations against NKG2D+CD4+ T cell frequencies revealed no correlation among the available 27 matched sample pairs (Fig. 6 E and not depicted). This disparity could be caused by the presence of anti-MICA autoantibodies, which have been found in cancer patients with anti-CTLA-4 therapy-induced autoimmunity and may obscure ELISA detection of sMICA (34). In fact, using a microbead-based screening assay, we detected anti-MICA antibodies in all of the 27 SLE patient plasma samples whereas all of 20 cancer patient samples containing sMICA were negative (unpublished data). In addition or alternatively to MICA, other NKG2D ligands such as its close relative MICB, or ULBP2 and ULBP3, which are normally expressed on B cells, could provide for NKG2D+CD4+ T cell costimulation in juvenile-onset SLE (Fig. 1 A).
DISCUSSION
The normal peripheral CD4 T lymphocyte compartment harbors small numbers of T cells with activation-independent, constitutive NKG2D expression. Our results establish that these T cells constitute a discrete T cell subset characterized by autoreactivity, an immunosuppressive IL-10 and TGF-β cytokine signature, and regulatory effects. We originally detected expansions of these T cells in cancer patients and inferred a regulatory role from their ability to cause FasL/Fas-mediated inhibition of bystander T cell proliferation as a result of NKG2D costimulation in vitro (17). This idea is supported by the inverse correlation between NKG2D+CD4+ T cell frequencies and disease activity in juvenile-onset SLE demonstrated in this study.
Previous data obtained with T cell clones from an ovarian cancer patient suggested that NKG2D+CD4+ T cells recognize normal self-antigens (17). On the other hand, CMV-driven expansions of NKG2D+CD4+ T cells in CMV-seropositive individuals pointed toward a role of these T cells in host defense (22). Our study shows that normally occurring NKG2D+CD4+ T cells are autoreactive and unresponsive toward classical recall antigens.
Although NKG2D+CD4+ T cells with specificities for microbial determinants other than those tested may exist, their proportionally large responses to autologous B cells imply that autoreactivity is a predominant if not all-inclusive characteristic. Moreover, NKG2D+CD4+ T cells that recognize recall antigens are set apart from the autoreactive subset because they coexpress CD8αβ, and thus correspond to double-positive CD8+CD4+ effector T cells that are known to be enriched for microbial specificities (25). The identity and expression of the self-antigens recognized by the autoreactive NKG2D+CD4+ T cells remain unknown, although their expression in peripheral blood B cells and diverse cancer cells may suggest a ubiquitous tissue distribution and ample opportunity for T cell priming. Under this assumption, the predominantly naive phenotype of the NKG2D+CD4+ T cells in normal individuals seems unusual, but is not without precedent as, for example, normal donor-derived CD8 T cells specific for the broadly expressed melanocyte Melan-A/MART-1 antigen are mostly naive as well (35). On the other hand, it is possible that NKG2D+CD4+ T cell priming is rare because of limited antigen expression and/or specific conditions in tissue microenvironments or that these T cells are not truly naive despite a phenotype conventionally associated with that stage. It may be somewhat puzzling that only B cells among PBMCs stimulated NKG2D+CD4+ T cell in vitro proliferation, an observation that cannot be analytically addressed without knowledge of T cell antigens, their expression, and processing.
Previously, immunosuppressive and proinflammatory and cytolytic functions have been associated with CD4 T cells that express NKG2D (11, 17, 18). We addressed this heterogeneity by examining the functional signature of NKG2D+CD4 T cells from normal peripheral blood. The results indicate that this T cell population is homogeneously immunosuppressive and lacks a proinflammatory profile and cytolytic capacity. Although some of the NKG2D+CD4 T cells produce IFN-γ, these responses are minimal. Normal peripheral blood NKG2D+CD4+ T cells are thus clearly distinct from the proinflammatory and cytolytic CD28−CD4+ T cells with cytokine-induced NKG2D expression in peripheral blood and synovia of RA patients, and from lamina propria NKG2D+CD4+ T cells in Crohn's disease, which are positive for perforin (11, 18).
The association of the immediate, quasi–effector-like IL-10 response with a naive NKG2D+CD4+ T cell phenotype is highly unusual. At least we are not aware of precedent for a similar uncoupling of functional differentiation from T cell maturation. Our results thus invoke an unconventional, possibly antigen-independent, mechanism for IL-10 imprinting of these T cells, which may involve epigenetic derepression of IL-10 loci early in T cell ontogeny, perhaps together with constitutive expression of transcription factors (36, 37). Alternatively, as discussed earlier, these T cells may not be truly naive. It is unclear whether NKG2D+CD4+ T cells are irreversibly IL-10 committed. However, these T cells retain some cytokine flexibility as they acquire the ability to produce TGF-β and IFN-γ with functional maturation. In regard to the IL-10/TGF-β profile, the NKG2D+CD4+ T cells superficially resemble naturally occurring and inducible T reg cells. However, as we have previously reported, they are clearly distinct (17).
Based on their in vitro functional attributes and inverse correlation with disease severity in juvenile-onset SLE, the NKG2D+CD4+ T cells appear to be associated with regulatory effects. Direct evidence for a role in attenuating disease activity cannot be readily obtained because equivalent T cells have not been observed in mice. It also remains unknown which NKG2D+CD4+ T cell functions, alone or combined, may affect disease activity. However, FasL-dependent effects are most likely relevant because Fas expression is unusually abundant among lupus patient T and B cells (38, 39). At least in vitro, lupus T cells are susceptible to NKG2D+CD4+ T cell–derived FasL-dependent growth arrest, and preliminary data suggest similar effects on patient B cells as well (unpublished data). An immunosuppressive role for NKG2D+CD4+ T cell–derived IL-10 in lupus patients may be controversial because IL-10 is thought to promote disease (32, 33, 40). TGF-β, on the other hand, is generally considered antiinflammatory, although its pleiotropic effects include a role in the differentiation of inflammatory Th17 cells and tissue damage (41).
In cancer patients, expansions of the NKG2D+CD4+ T cells are dependent on tumor expression of MICA, and their frequencies are correlated with serum concentrations of sMICA providing for NKG2D costimulation of T cell proliferation (17). Although sMICA was present in all juvenile-onset SLE patients studied, its tissue source could not be determined. It is thus unclear which tissue environments combine MHC class II self-antigen presentation and NKG2D ligand expression. Other conditions conducive for stimulation of NKG2D+CD4+ T cell expansions and functions likely include the unmasking of their autoreactivity by breakdown of peripheral tolerance. As suggested by our results and other studies of autoreactive T cells (23, 42), loss of CD25+ T reg cell functions may be one requirement—a condition that may be met by T reg cell impairments in SLE (30). Gradually expanding NKG2D+CD4+ T cell populations may thus, in some ways, compensate for T reg cell deficiencies. This is supported by our confirmation of NKG2D+CD4+ T cell functional competence in juvenile-onset SLE. However, this observation raises the question of how these T cells can remain uncompromised by the multitude of lymphocyte functional abnormalities that include TCR “rewiring” and signaling and gene regulation defects in this disease (31).
As with other autoimmune diseases, there is considerable interest in restoring immune tolerance by reconstitution of functionally active T reg cells in SLE (43). However, adoptive transfer approaches are hampered by difficulties, including the absence of surface markers for unequivocal identification and the isolation of conventional T reg cells. In contrast, the NKG2D+CD4+ T cells can be readily identified and in vitro expanded, and may thus offer new treatment modalities.
MATERIALS AND METHODS
Subjects and blood samples.
Normal peripheral blood samples were obtained from healthy adult volunteers who consented in accordance with a protocol approved by the Internal Review Board (IRB) at Fred Hutchinson Cancer Research Center. Blood samples for assessment of recall responses were from CMV-seropositive and tuberculin skin test–positive donors. Umbilical cord blood from normal deliveries was collected according to IRB guidelines. 19 patients diagnosed before (n = 18) or at age 18 (n = 1) who fulfilled the American College of Rheumatology criteria for the classification of SLE and age-matched healthy volunteers were recruited and consented under a research protocol approved by the IRB of the Children's Hospital and Regional Medical Center, Seattle. Peripheral blood and clinical and laboratory data were collected for each individual, and disease activity was determined according to the modified SLEDAI 2000 (29). Demographic characteristics, disease activity status, and immunomodulatory medications of all subjects are listed in Table I. Disease activity scores of five or greater were considered to represent active disease or “flare”; scores of less than five were indicative of inactive disease or “remission” (44, 45). All blood samples were processed within 6 h of collection (46). PBMCs and cord blood mononuclear cells (CBMCs) were isolated by density centrifugation over Ficoll-Hypaque and cryopreserved or directly analyzed. Patient and age-matched control plasma was collected before PBMC isolation and cryopreserved. 27 PBMCs and matched plasma samples were collected from 19 SLE patients. Serial samples obtained at times of high or low disease activity were available from six patients. Additional plasma or serum samples were available from 44 juvenile-onset SLE patients and 20 tumor patients positive for sMICA (17).
Lymphocyte subset purifications, T cell cloning, and CFSE labeling.
All cell purifications involved FACSAria sorting (BD) and/or microbead-facilitated (MACS methodology; Miltenyi Biotech) cell selection, and yielded >99% pure cell populations. Unless otherwise indicated, all mAbs were obtained from BD. Antibody coupling to quantum dots (Quantum Dot Corporation) was according to protocol (http://drmr.com/abcon/index.html).
NKG2D+CD8−CD4+ T cells and control NKG2D−CD8−CD4+ T cells were sorted from negatively bead-enriched (CD4+ T Cell Isolation kit II) CD4 T cells using the following mAb cocktail: anti-CD3–Alexa Fluor 700 (clone UCHT1), anti-CD8α–PerCPCy5.5 (clone SK1), anti-CD4–FITC (clone RPA-T4), and anti-NKG2D–APC (clone 1D11; 6). Naive and memory subsets of NKG2D+CD8−CD4+ T cells were sorted as CD45RO−CD62L+CD28+CD27+ (naive) and CD45RO+CD62L+/− (memory) or CD45RO+CD62L− (effector memory) T cells by adding anti-CD45RO–ECD (PE–TR; clone UCHL1; Beckman Coulter), anti-CD62L–PE (clone SK11), anti-CD28–PECy5 (clone CD28.2), and anti-CD27–QDot 605 (clone M-T271) to the antibody cocktail. For select recall response assays, double-positive CD4+CD8+ NKG2D+ T cells were copurified together with NKG2D+CD8−CD4+ T cells. NKG2D− memory CD4 T cells were sorted from negatively bead-selected memory CD4 T cells (Memory CD4+ T Cell Isolation kit) using anti-NKG2D–PE. Depletion of T cells, CD25+ lymphocytes, B cells, or monocytes from autologous stimulator PBMCs used CD3 MicroBeads, CD25 MicroBeads II, CD19 MicroBeads, and CD14 MicroBeads, respectively. CD19+ B cells (B Cell Isolation kit II) and CD14+ monocytes (Monocyte Isolation kit II) were purified from PBMCs by negative selection. Antigen-specific T cell clones were generated from single-cell sorted CD3+CD4+CD8−NKG2D+ or CD3+CD4+CD8−CD45RO−CD62L+CD28+CD27+NKG2D+ cells exposed to two rounds of weekly stimulation with irradiated (40 Gy) autologous CD19+ B cells in the presence of IL-2 (% IU/ml) and IL-7 (10 ng/ml), as previously described (47). To determine NKG2D+CD4+ T cell cytokine signatures, naive and memory subset-derived clones were established from single cells in the presence of 105 irradiated allogeneic PBMC feeders and 1 µg/ml PHA in nonpolarizing conditions (10 ng/ml TGF-β, 1 µg/ml anti–IL-4, and 2 µg/ml anti–IL-12; all from R&D Systems) (48); juvenile-onset SLE patient– and normal donor–derived NKG2D−CD4+ T cell lines were established as previously described (17).
T cell stimulations, proliferation and cytotoxicity assays, and ELISA of soluble mediators.
All T cell stimulations were in AIM V serum-free medium (Invitrogen). To test for autoreactivity, CFSE-labeled purified NKG2D+CD4+ and control NKG2D−CD4+ responder T cells (5 × 104 cells per U-bottom well) were cultured with PHA (1 µg/ml, positive control) or irradiated autologous PBMC stimulator populations, with or without additional lymphocyte depletions as indicated in Fig. 1, at a responder-to-stimulator cell ratio of 1:1. Purified B cell stimulators were used at a ratio of 1 per 10 T cells. The anti–MHC class II, -ULBP2, and -ULBP3 blocking mAbs, all used at 10 µg/ml, were TU39 (BD) and 6F6 and 4F9 (our reagents), respectively. Cells were harvested after 4–5 d of culture and analyzed for NKG2D and CD4 expression and CFSE dilution by multicolor flow cytometry. T cell clones established in the presence of autologous B cells were tested for [3H]thymidine incorporation after exposure to irradiated autologous or allogeneic B cells in the presence or absence of mAb TU39 (10 µg/ml) or L243 (anti-HLA-DR; 5 µg/ml; American Type Culture Collection). For recall response assays, monocytes (5 × 104 cells/U-bottom well) were cultured with purified CFSE-labeled NKG2D+CD4+ or NKG2D−CD4+ memory T cells at a ratio of 1:1 in the presence of recombinant CMV pp65 (2 µl/ml; Miltenyi Biotech), tetanus toxoid (1 µg/ml; Calbiochem), PPD from M. tuberculosis (5 µg/ml; Statens Serum Institute), and heat-inactivated C. albicans (10 µg/ml; Greer Laboratories), added individually or as a cocktail as indicated in Fig. 3. CFSE dilution of NKG2D+ and NKG2D−CD4+ T cells and coexpression of CD8α and CD8β were evaluated by multicolor flow cytometry using anti-CD3–Alexa Fluor 700, anti-CD8α–PerCPCy5.5, anti-CD8β–PE (clone 2ST8.5H7; BD), anti-CD4–APC–Alexa 750 (clone S3.2; Invitrogen), and anti-NKG2D–APC after 6–10 d of culture. T cell stimulations (106 cells/ml) for cytokine profiling and CD107a mobilization assays were with PMA (50 ng/ml; Sigma-Aldrich) and ionomycin (0.5 µg/ml; Sigma-Aldrich) at 37°C. Stimulation times were optimized for each cytokine and CD107a. Monensin (0.7 µg/ml; BD) and/or brefeldin A (10 µg/ml; Sigma-Aldrich) were present throughout the final 4 h of stimulation. For ELISA for cytokines and FasL, T cells (5 × 104 per flat-bottom well) were plated onto solid-phase anti-CD3 (OKT3; Ortho Biotech) alone or together with anti-NKG2D (mAb 1D11) or endotoxin-free recombinant sMICA (20 ng/ml) (49), or stimulated with irradiated autologous B cells in the presence or absence of anti-HLA-DR (mAb L243) (17). IL-10, TGF-β1, and FasL were quantified in 24-h culture supernatants by commercial ELISA (R&D Systems). Redirected lysis assays using anti-CD3 and mouse mastocytoma P815 cells, and the assay for FasL-mediated inhibition of proliferation of NKG2D−CD4+ T cells by NKG2D+CD4+ T cells have been previously described (6, 17). IL-15–mediated NKG2D induction in NKG2D−CD4+ T cells was as described and was monitored by flow cytometry (11). Co-culture experiments assessing FasL-mediated regulatory functions of NKG2D+CD4+ T cells were as previously described (17). In brief, sorted patient- or control donor–derived NKG2D+CD4+ T cells were mixed with CFSE-labeled NKG2D−CD4+ responder T cells (normal donor-derived T cell lines or ex vivo–sorted active and inactive SLE patient T cells) and activated with solid-phase anti-CD3 alone or together with sMICA in the presence or absence of antagonist anti-Fas (17).
Polychromatic flow cytometry.
Functional profiling in conjunction with maturational phenotypes was done by surface and intracellular staining of unstimulated (to assess presence of perforin and granzymes A and B) or short-term stimulated (to assess surface mobilization of CD107a and cytokine production) PBMCs and CBMCs. Cell viability was assessed using a LIVE/DEAD Fixable Violet Dead Cell Stain kit (Invitrogen). For surface staining, cells were incubated in PBS/10% human serum/0.15 sodium azide for 15 min at room temperature, exposed to pretitrated mAb cocktails for 20 min at 4°C, and washed in PBS/1% BSA/0.15 sodium azide. For intracellular stainings, cells were permeabilized, stained for cytotoxic proteins or intracellular cytokines, and washed using Cytofix/Cytoperm kit reagents (BD). Unless otherwise specified, all mAbs were purchased from BD. QDot and Alexa Fluor 680 (Zenon Alexa 680 Mouse IgG1 Labeling kit; Invitrogen) conjugates were made in-house. The surface marker antibody cocktail included anti-CD3–Alexa Fluor 700, anti-CD4–APC–Alexa 750 (clone S3.2; Invitrogen), anti-CD8α–PerCPCy5.5, anti NKG2D–APC, anti-CD45RO–ECD, and anti-CCR7–PECy7 (clone 3D12). More extensive maturation phenotyping used anti-CD3–QDot 565, anti-CD4–APC–Alexa Fluor 750, anti-CD8α–PerCPCy5.5, anti-NKG2D–APC, anti-CD45RA–FITC (clone HI 100), anti-CD62L–PE, anti-CD28–PECy5, anti-CD27–QDot 605, and anti-CD11a–Alexa Fluor 680 (clone 27). CD107a surface mobilization was determined using anti-CD107a–FITC (clone H4A3) as previously described (50). Cytotoxic proteins were monitored with anti-perforin–FITC (clone deltaG9), anti-granzyme A–FITC (clone CB9), and anti-granzyme B–FITC (clone GB11). Anti-cytokine mAbs were anti-IL-2–FITC (clone MQ1-17H12), anti-IL-4–FITC (clones 8D 4–8 and MP4-252D), anti-IL-10–PE (clone JES3-19F1), anti-IL-17–PE (clone CAP17), anti-IFN-γ–FITC (clone 4S.B3), anti-TGF-β1–PE (clone TB21; IQ Products), anti-LAP TGF-β–PE (clone 27232; R&D Systems), and anti-TNF-α–FITC (clone Mab11; BioLegend). HiCK-1 and -2 control cell (BD) stainings were included in all analyses. Cells were examined with a LSR II (BD) flow cytometer. Between 0.5–1 × 106 events were collected from each sample. Electronic compensation was with single stains. Cut-offs for background fluorescence were based on “fluorescence minus one” gating controls. Data analysis was with FlowJo version 8.5 (Tree Star, Inc.). Gating for each sample was based on forward scatter area versus a forward scatter height plot to eliminate aggregates and to isolate lymphocytes, followed by exclusion of dead cells. Additional gating was on CD3+CD8− cells to exclude double-positive CD4+CD8+ T cells.
ELISA for sMICA and MICA autoantibody detection.
sMICA in juvenile-onset SLE patient plasma was determined by ELISA as previously described (49). Screening for anti-MICA autoantibodies, including MICA alleles *001, *002, *004, *007, *012, *018, *019, and *027, used the LABScreen MICA Single Antigen product (One Lambda) and Luminex technology according to manufacturer's instructions.
Statistics.
The correlation between NKG2D+CD4+ T cell frequencies and each of the factors SLEDAI, IL-10, and FasL was estimated from linear regression. The null hypothesis that the correlation coefficient is equal to zero was tested using generalized estimating equations, as some subjects contributed multiple samples. The generalized estimating equations approach takes the multiple samples per patient (as opposed to treating all samples as independent observations) into account when estimating the variance of the slope coefficient. T cell frequencies among patients with active disease were compared with those among patients with inactive disease and with those among control subjects using the two-sample Student's t test.
Acknowledgments
We thank Dr. Helen Horton, Emilie Jalbert, and Andrew Berger for help with polychromatic flow cytometry.
This work was supported by an Arthritis Foundation Investigator Award (A.M. Stevens), the Lupus Research Institute (V. Groh), and National Institutes of Health grants AI30581 and AI52319 (T. Spies). C.J. Turtle was supported by a Thomsen Family Postdoctoral Fellowship.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used: CBMC, cord blood mononuclear cell; FasL, Fas ligand; IRB, Internal Review Board; LAP, latency-associated peptide; MICA, MHC class I–related chain A; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; SLEDAI, SLE disease activity index; sMICA, soluble MICA; T reg cell, regulatory T cell; ULBP, UL16-binding protein.
References
- Long E.O. 1999. Regulation of immune responses through inhibitory receptors.Annu. Rev. Immunol. 17:875–904 [DOI] [PubMed] [Google Scholar]
- Huard B., Karlsson L. 2000. KIR expression on self-reactive CD8+ T cells is controlled by T-cell receptor engagement.Nature. 403:325–328 [DOI] [PubMed] [Google Scholar]
- Moser J.M., Gibbs J., Jensen P.E., Lukacher A.E. 2002. CD94-NKG2A receptors regulate antiviral CD8(+) T cell responses.Nat. Immunol. 3:189–195 [DOI] [PubMed] [Google Scholar]
- Lanier L.L. 2008. Up on the tightrope: natural killer cell activation and inhibition.Nat. Immunol. 9:495–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh V., Steinle A., Bauer S., Spies T. 1998. Recognition of stress-induced MHC molecules by intestinal epithelial gammadelta T cells.Science. 279:1737–1740 [DOI] [PubMed] [Google Scholar]
- Bauer S., Groh V., Wu J., Steinle A., Phillips J.H., Lanier L.L., Spies T. 1999. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA.Science. 285:727–729 [DOI] [PubMed] [Google Scholar]
- Raulet D.H. 2003. Roles of the NKG2D immunoreceptor and its ligands.Nat. Rev. Immunol. 3:781–790 [DOI] [PubMed] [Google Scholar]
- Gonzalez S., Groh V., Spies T. 2006. Immunobiology of human NKG2D and its ligands.Curr. Top. Microbiol. Immunol. 298:121–138 [DOI] [PubMed] [Google Scholar]
- Venkataraman G.M., Suciu D., Groh V., Boss J.M., Spies T. 2007. Promoter region architecture and transcriptional regulation of the genes for the MHC class I-related chain A and B ligands of NKG2D.J. Immunol. 178:961–969 [DOI] [PubMed] [Google Scholar]
- Groh V., Rhinehart R., Secrist H., Bauer S., Grabstein K.H., Spies T. 1999. Broad tumor-associated expression and recognition by tumor-derived gamma delta T cells of MICA and MICB.Proc. Natl. Acad. Sci. USA. 96:6879–6884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh V., Bruhl A., El-Gabalawy H., Nelson J.L., Spies T. 2003. Stimulation of T cell autoreactivity by anomalous expression of NKG2D and its MIC ligands in rheumatoid arthritis.Proc. Natl. Acad. Sci. USA. 100:9452–9457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hue S., Mention J.J., Monteiro R.C., Zhang S., Cellier C., Schmitz J., Verkarre V., Fodil N., Bahram S., Cerf-Bensussan N., Caillat-Zucman S. 2004. A direct role for NKG2D/MICA interaction in villous atrophy during celiac disease.Immunity. 21:367–377 [DOI] [PubMed] [Google Scholar]
- Meresse B., Chen Z., Ciszewski C., Tretiakova M., Bhagat G., Krausz T.N., Raulet D.H., Lanier L.L., Groh V., Spies T., et al. 2004. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease.Immunity. 21:357–366 [DOI] [PubMed] [Google Scholar]
- Saikali P., Antel J.P., Newcombe J., Chen Z., Freedman M., Blain M., Cayrol R., Prat A., Hall J.A., Arbour N. 2007. NKG2D-mediated cytotoxicity toward oligodendrocytes suggests a mechanism for tissue injury in multiple sclerosis.J. Neurosci. 27:1220–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Song Y., Bakker A.B., Bauer S., Spies T., Lanier L.L., Phillips J.H. 1999. An activating immunoreceptor complex formed by NKG2D and DAP10.Science. 285:730–732 [DOI] [PubMed] [Google Scholar]
- Upshaw J.L., Leibson P.J. 2006. NKG2D-mediated activation of cytotoxic lymphocytes: unique signaling pathways and distinct functional outcomes.Semin. Immunol. 18:167–175 [DOI] [PubMed] [Google Scholar]
- Groh V., Smythe K., Dai Z., Spies T. 2006. Fas-ligand-mediated paracrine T cell regulation by the receptor NKG2D in tumor immunity.Nat. Immunol. 7:755–762 [DOI] [PubMed] [Google Scholar]
- Allez M., Tieng V., Nakazawa A., Treton X., Pacault V., Dulphy N., Caillat-Zucman S., Paul P., Gornet J.M., Douay C., et al. 2007. CD4+NKG2D+ T cells in Crohn's disease mediate inflammatory and cytotoxic responses through MICA interactions.Gastroenterology. 132:2346–2358 [DOI] [PubMed] [Google Scholar]
- Sundstrom Y., Nilsson C., Lilja G., Karre K., Troye-Blomberg M., Berg L. 2007. The expression of human natural killer cell receptors in early life.Scand. J. Immunol. 66:335–344 [DOI] [PubMed] [Google Scholar]
- Baecher-Allan C., Hafler D.A. 2006. Human regulatory T cells and their role in autoimmune disease.Immunol. Rev. 212:203–216 [DOI] [PubMed] [Google Scholar]
- Shevach E.M. 2006. From vanilla to 28 flavors: multiple varieties of T regulatory cells.Immunity. 25:195–201 [DOI] [PubMed] [Google Scholar]
- Saez-Borderias A., Guma M., Angulo A., Bellosillo B., Pende D., Lopez-Botet M. 2006. Expression and function of NKG2D in CD4+ T cells specific for human cytomegalovirus.Eur. J. Immunol. 36:3198–3206 [DOI] [PubMed] [Google Scholar]
- Danke N.A., Koelle D.M., Yee C., Beheray S., Kwok W.W. 2004. Autoreactive T cells in healthy individuals.J. Immunol. 172:5967–5972 [DOI] [PubMed] [Google Scholar]
- Cosman D., Mullberg J., Sutherland C.L., Chin W., Armitage R., Fanslow W., Kubin M., Chalupny N.J. 2001. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor.Immunity. 14:123–133 [DOI] [PubMed] [Google Scholar]
- Nascimbeni M., Shin E.C., Chiriboga L., Kleiner D.E., Rehermann B. 2004. Peripheral CD4(+)CD8(+) T cells are differentiated effector memory cells with antiviral functions.Blood. 104:478–486 [DOI] [PubMed] [Google Scholar]
- Li M.O., Wan Y.Y., Sanjabi S., Robertson A.K., Flavell R.A. 2006. Transforming growth factor-beta regulation of immune responses.Annu. Rev. Immunol. 24:99–146 [DOI] [PubMed] [Google Scholar]
- Gerosa F., Paganin C., Peritt D., Paiola F., Scupoli M.T., Aste-Amezaga M., Frank I., Trinchieri G. 1996. Interleukin-12 primes human CD4 and CD8 T cell clones for high production of both interferon-γ and interleukin-10.J. Exp. Med. 183:2559–2569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic D., Trinchieri G. 2007. IL-10 or not IL-10: that is the question.Nat. Immunol. 8:1281–1283 [DOI] [PubMed] [Google Scholar]
- Gladman D.D., Ibanez D., Urowitz M.B. 2002. Systemic lupus erythematosus disease activity index 2000.J. Rheumatol. 29:288–291 [PubMed] [Google Scholar]
- Valencia X., Yarboro C., Illei G., Lipsky P.E. 2007. Deficient CD4+CD25high T regulatory cell function in patients with active systemic lupus erythematosus.J. Immunol. 178:2579–2588 [DOI] [PubMed] [Google Scholar]
- Crispin J.C., Kyttaris V.C., Juang Y.T., Tsokos G.C. 2008. How signaling and gene transcription aberrations dictate the systemic lupus erythematosus T cell phenotype.Trends Immunol. 29:110–115 [DOI] [PubMed] [Google Scholar]
- Capper E.R., Maskill J.K., Gordon C., Blakemore A.I. 2004. Interleukin (IL)-10, IL-1ra and IL-12 profiles in active and quiescent systemic lupus erythematosus: could longitudinal studies reveal patient subgroups of differing pathology? Clin. Exp. Immunol. 138:348–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun H.Y., Chung J.W., Kim H.A., Yun J.M., Jeon J.Y., Ye Y.M., Kim S.H., Park H.S., Suh C.H. 2007. Cytokine IL-6 and IL-10 as biomarkers in systemic lupus erythematosus.J. Clin. Immunol. 27:461–466 [DOI] [PubMed] [Google Scholar]
- Jinushi M., Hodi F.S., Dranoff G. 2006. Therapy-induced antibodies to MHC class I chain-related protein A antagonize immune suppression and stimulate antitumor cytotoxicity.Proc. Natl. Acad. Sci. USA. 103:9190–9195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittet M.J., Valmori D., Dunbar P.R., Speiser D.S., Lienard D., Lejeune F., Fleischhauer K., Cerundolo V., Cerottini J.-C., Romero P. 1999. High frequencies of naïve Melan-A/MART-1–specific CD8+ T cells in a large proportion of human histocompatibility leukocyte antigen (HLA)-A2 individuals.J. Exp. Med. 190:705–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K.W., de Waal Malefyt R., Coffman R.L., O'Garra A. 2001. Interleukin-10 and the interleukin-10 receptor.Annu. Rev. Immunol. 19:683–765 [DOI] [PubMed] [Google Scholar]
- de Lalla C., Festuccia N., Albrecht I., Chang H.D., Andolfi G., Benninghoff U., Bombelli F., Borsellino G., Aiuti A., Radbruch A., et al. 2008. Innate-like effector differentiation of human invariant NKT cells driven by IL-7.J. Immunol. 180:4415–4424 [DOI] [PubMed] [Google Scholar]
- Bijl M., Horst G., Limburg P.C., Kallenberg C.G. 2001. Fas expression on peripheral blood lymphocytes in systemic lupus erythematosus (SLE): relation to lymphocyte activation and disease activity.Lupus. 10:866–872 [DOI] [PubMed] [Google Scholar]
- Liphaus B.L., Kiss M.H., Carrasco S., Goldenstein-Schainberg C. 2007. Increased Fas and Bcl-2 expression on peripheral blood mononuclear cells from patients with active juvenile-onset systemic lupus erythematosus.J. Rheumatol. 34:1580–1584 [PubMed] [Google Scholar]
- Llorente L., Richaud-Patin Y. 2003. The role of interleukin-10 in systemic lupus erythematosus.J. Autoimmun. 20:287–289 [DOI] [PubMed] [Google Scholar]
- Veldhoen M., Stockinger B. 2006. TGFβ1, a ‘Jack of all trades': the link with pro-inflammatory IL-17-producing T cells.Trends Immunol. 27:358–361 [DOI] [PubMed] [Google Scholar]
- Wing K., Lindgren S., Kollberg G., Lundgren A., Harris R.A., Rudin A., Lundin S., Suri-Payer E. 2003. CD4 T cell activation by myelin oligodendrocyte glycoprotein is suppressed by adult but not cord blood CD25+ T cells.Eur. J. Immunol. 33:579–587 [DOI] [PubMed] [Google Scholar]
- Roncarolo M.G., Battaglia M. 2007. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans.Nat. Rev. Immunol. 7:585–598 [DOI] [PubMed] [Google Scholar]
- Moroni G., Quaglini S., Maccario M., Banfi G., Ponticelli C. 1996. “Nephritic flares” are predictors of bad long-term renal outcome in lupus nephritis.Kidney Int. 50:2047–2053 [DOI] [PubMed] [Google Scholar]
- Boumpas D.T., Balow J.E. 1998. Outcome criteria for lupus nephritis trials: a critical overview.Lupus. 7:622–629 [DOI] [PubMed] [Google Scholar]
- Horton H., Thomas E.P., Stucky J.A., Frank I., Moodie Z., Huang Y., Chiu Y.L., McElrath M.J., De Rosa S.C. 2007. Optimization and validation of an 8-color intracellular cytokine staining (ICS) assay to quantify antigen-specific T cells induced by vaccination.J. Immunol. Methods. 323:39–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Bleakley M., Yee C. 2005. IL-21 influences the frequency, phenotype, and affinity of the antigen-specific CD8 T cell response.J. Immunol. 175:2261–2269 [DOI] [PubMed] [Google Scholar]
- Messi M., Giacchetto I., Nagata K., Lanzavecchia A., Natoli G., Sallusto F. 2003. Memory and flexibility of cytokine gene expression as separable properties of human T(H)1 and T(H)2 lymphocytes.Nat. Immunol. 4:78–86 [DOI] [PubMed] [Google Scholar]
- Groh V., Wu J., Yee C., Spies T. 2002. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation.Nature. 419:734–738 [DOI] [PubMed] [Google Scholar]
- Alter G., Malenfant J.M., Altfeld M. 2004. CD107a as a functional marker for the identification of natural killer cell activity.J. Immunol. Methods. 294:15–22 [DOI] [PubMed] [Google Scholar]