Abstract
The activating natural killer (NK) cell receptor Ly49H recognizes the mouse cytomegalovirus (MCMV) m157 glycoprotein expressed on the surface of infected cells and is required for protection against MCMV. Although Ly49H has previously been shown to signal via DAP12, we now show that Ly49H must also associate with and signal via DAP10 for optimal function. In the absence of DAP12, DAP10 enables Ly49H-mediated killing of m157-bearing target cells, proliferation in response to MCMV infection, and partial protection against MCMV. DAP10-deficient Ly49H+ NK cells, expressing only Ly49H–DAP12 receptor complexes, are partially impaired in their ability to proliferate during MCMV infection, display diminished ERK1/2 activation, produce less IFN-γ upon Ly49H engagement, and demonstrate reduced control of MCMV infection. Deletion of both DAP10 and DAP12 completely abrogates Ly49H surface expression and control of MCMV infection. Thus, optimal NK cell–mediated immunity to MCMV depends on Ly49H signaling through both DAP10 and DAP12.
NK cells mediate immunity against tumors and pathogens by producing effector cytokines, such as IFN-γ, and by secreting lytic granules that kill target cells. NK cell activation and function are determined by a balance of signals transmitted by inhibitory and activating NK cell receptors (NKRs) (1). Many of the inhibitory NKRs recognize self-ligands such as MHC class Ia, which are recognized by inhibitory Ly49 receptors in mice and inhibitory killer immunoglobulin-like receptors (KIRs) in humans, and MHC class Ib molecules, which are recognized by CD94/NKG2A in both species. Activating NKRs can recognize host-encoded molecules that are induced by transformation or infection of the host cells (e.g., NKG2D ligands), and some activating NKRs recognize non–self-ligands, for example, the mouse CMV (MCMV) m157 glycoprotein, which is recognized by Ly49H (2–6). Activating NKRs typically have short intracellular domains that lack known signaling motifs and instead associate with signaling subunits, including the immunoreceptor tyrosine-based activation motif (ITAM)–bearing CD3-ζ, FcϵRIγ, and DAP12 (also referred to as KARAP) proteins and the YINM motif-containing DAP10 adaptor (7, 8). In the absence of an appropriate signaling subunit, many of the activating NKRs (e.g., NKG2D, CD16, KIR3DS1, and the CD94/NKG2C heterodimer) are not stably expressed on the cell surface (8–11). In humans, some of the activating NKRs, such as CD16, can associate interchangeably with both the ITAM-bearing FcϵRIγ and CD3-ζ subunits for stable expression and signaling (12).
DAP12 associates with multiple activating NKRs, including the human and mouse CD94/NKG2C heterodimers, the mouse Ly49H and Ly49D receptors, human NKp44, and the activating human KIR (1). Conversely, NKG2D is the only activating NKR known to associate with DAP10 and initiate NK cell immune responses in vivo (13). Based on the structural similarity of DAP10 and DAP12, particularly within their transmembrane domains, which mediate receptor–adaptor association, it is reasonable to expect that receptors known to associate with DAP12 might also complex with DAP10. However, the alternatively spliced isoform of NKG2D, which is designated NKG2D-S, expressed by activated mouse but not human NK cells, is the only receptor known to pair with both DAP10 and DAP12 in vivo (14–16). Signaling downstream of these adaptors differs as ITAM-containing adaptors recruit Syk or ZAP-70, whereas DAP10 recruits the p85 subunit of PI3 kinase and Grb2 (8, 17–21). NK cells activated via ITAM-containing subunits proliferate, produce cytokines, and are cytotoxic, whereas cells activated through DAP10 are triggered to kill but do not efficiently induce the production of cytokines (14, 15, 19, 22, 23). Importantly, in human NK cells, NKG2D signaling via DAP10 augments IFN-γ and GM-CSF production induced by an activating DAP12-associated KIR; thus, signaling through both adaptors induces a more robust immune response (22).
Lack of NK cells renders both humans and mice susceptible to certain infections, particularly the herpesviruses, including human CMVs and MCMVs (24–26). Therefore, experimental infection of mice with MCMV provides an instructive model for studying NK cell responses to viral infection. The Ly49H receptor, which is expressed on a subset of NK cells in C57BL/6 (B6) mice, binds to the m157 glycoprotein encoded by MCMV and is the dominant receptor responsible for NK cell–mediated resistance to MCMV in B6 mice (2, 6, 27–29). Ly49H+ NK cells control MCMV replication by both direct cytotoxic mechanisms and by secretion of IFN-γ (30, 31). Genetic ablation of Ly49H, treatment with Ly49H blocking antibody, or infection with a mutant MCMV lacking m157 (Δm157) renders normally resistant B6 mice susceptible to MCMV (32–34). Dokun et al. (35) have reported that early after infection with MCMV, both Ly49H+ and Ly49H−, NK cells become activated and proliferate, presumably as a result of the proinflammatory cytokine environment. However, by 3 d after infection Ly49H+ NK cells preferentially proliferate, and by 6 d the percentage of Ly49H+ NK cells increases from ∼50 to 80–90% of the total NK cell population (35). In B6 mice in which the ITAM of DAP12 had been altered to prevent association with Syk or ZAP-70 (designated as DAP12ki or KARAPki mice), this preferential expansion of Ly49H+ NK cells was lost (36, 37). Furthermore, these mice exhibited increased viral burden and histopathology (38).
Recently, Coudert et al. (39) suggested an association between Ly49H and DAP10 based on transient transfection experiments and coimmunoprecipitation from IL-2–cultured NK cells, and prior studies have suggested an association of mouse SIRP-β1 with DAP10 or DAP12 in a rat mast cell leukemia transfectant model (40). However, it remains unknown whether Ly49H–DAP10 complexes are functionally active and whether they contribute to host protection in vivo. In this paper, we demonstrate that Ly49H associates with DAP10 in addition to DAP12 and that Ly49H–DAP10 complexes are functional and necessary for optimal control of MCMV infection. Deletion of either signaling subunit reduces surface expression of Ly49H, and loss of both DAP10 and DAP12 completely ablates Ly49H surface expression. In the absence of DAP12, Ly49H can signal via DAP10 to induce NK cell responses that partially control MCMV infection. In contrast, the deletion of both DAP10 and DAP12 ablates control of MCMV to the level observed in NK cell–depleted mice. Although either signaling subunit is sufficient for NK cell–mediated killing of m157-bearing targets, we find that DAP10 is necessary for optimal Ly49H-mediated activation of ERK1/2, NK cell proliferation, IFN-γ production, and control of MCMV infection. Thus, Ly49H must associate with both DAP10 and DAP12 to induce optimal NK cell–mediated immunity to MCMV.
RESULTS
DAP12 is not required for Ly49H-dependent NK cell–mediated cytotoxicity and proliferation
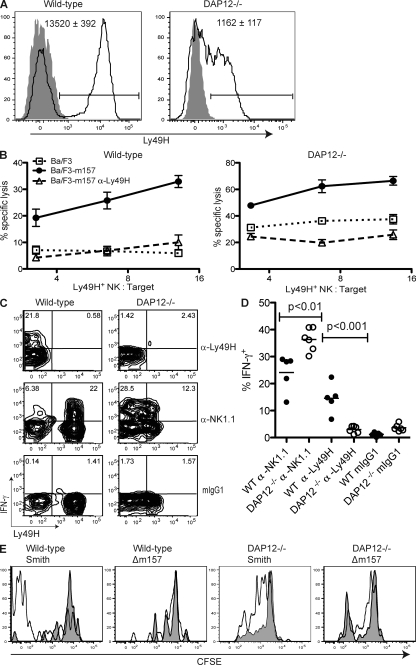
In analyzing NK cells from DAP12-deficient mice, we found that Ly49H was detected on a similar frequency of NK cells in both WT and DAP12-deficient B6 mice, although receptor abundance on DAP12-deficient NK cells was ∼10-fold lower than on WT NK cells, as determined by measuring the mean fluorescence intensity (MFI) of Ly49H staining by flow cytometry (P < 0.001; Fig. 1 A). To determine whether Ly49H is functional in the absence of DAP12 during MCMV infection, we assayed killing of m157-transfected Ba/F3 target cells ex vivo by NK cells directly isolated from MCMV-infected mice. As shown in Fig. 1 B, similar to WT NK cells, NK cells from DAP12-deficient mice preferentially killed m157-bearing Ba/F3 target cells compared with the untransfected Ba/F3 target cells. Although the absolute level of cytotoxicity mediated by WT or mutant NK cells that were assayed immediately ex vivo from MCMV-infected mice varied somewhat between experiments, in all cases we observed significant killing of m157-bearing targets by DAP12-deficient NK cells. Lysis was abrogated in the presence of a blocking anti-Ly49H mAb in both cases, confirming that killing of Ba/F3-m157 by both WT and DAP12-deficient cells was mediated by Ly49H (Fig. 1 B). These results demonstrate that Ly49H is functional and can mediate cytotoxicity in the absence of DAP12. The higher background killing of untransfected targets by DAP12-deficient NK cells has been noted previously (41). In prior studies using NK cells cultured for 1 wk in IL-2, we did not observe Ly49H-dependent cytotoxicity using NK cells from DAP12-deficient mice (2); however, in our present studies we have analyzed NK cells directly ex vivo from MCMV-infected mice, which is more physiologically relevant.
Figure 1.
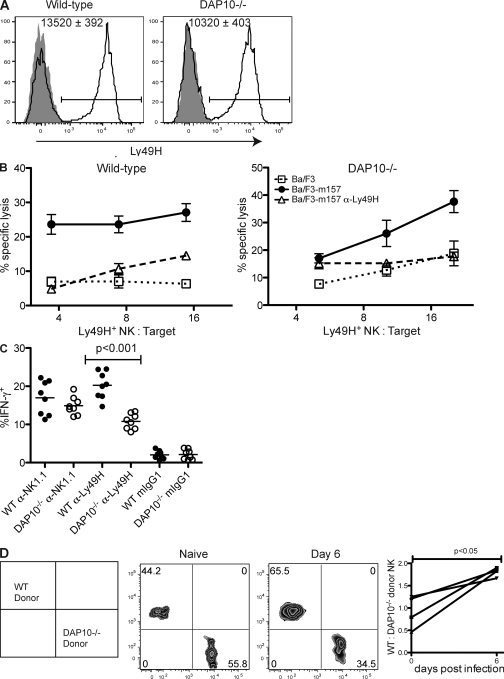
DAP12-independent Ly49H expression and function. (A) Peripheral blood NK cells (NK1.1+ TCR-β−) from uninfected B6 WT and DAP12-deficient mice were stained for Ly49H (solid lines) or with an isotype-matched control mAb (gray fill). The bars indicate the Ly49H+ gate. The MFI ± SEM of Ly49H for the Ly49H+ NK cells are indicated within the graphs (n = 5 mice). Data are representative of three experiments with similar results. (B) Splenocytes from B6 WT or DAP12-deficient mice infected 6 d prior with MCMV-Smith were enriched for NK cells and incubated for 6 h with 51Cr-labeled parental Ba/F3 cells (dotted lines) or Ba/F3 cells expressing MCMV m157 in the presence (dashed lines) or absence (solid lines) of Ly49H blocking antibody. Data are representative of four independent experiments with similar results. Error bars indicate SEM. (C and D) NK cells enriched as in B were incubated in the presence of brefeldin A on plates coated with anti-Ly49H, anti-NK1.1, or an isotype-matched control mAb. After 4 h, cells were stained for intracellular IFN-γ production. (C) Representative graphs are gated on NK cells (DX5+ TCR-β−). In the top panels, stimulation with plate-bound anti-Ly49H prevented subsequent staining with fluorochrome-conjugated anti-Ly49H mAb. (D) Frequency of IFN-γ production by mAb-stimulated NK cells from five WT or six DAP12-deficient mice. Data are pooled from two experiments. The means of the groups are indicated by the horizontal lines. (E) B6 WT or DAP12-deficient splenocytes were labeled with 0.5 µM CFSE and adoptively transferred into CD45.1+CD45.2+ WT recipients. 7 d after infection with MCMV-Smith or -Δm157, mice were analyzed for CFSE dilution in donor Ly49H+ (black lines) or Ly49H− (gray fill) splenic NK (NK1.1+TCR-β−) cells. Data are representative of three experiments.
In addition to lysis of target cells, NK cells can produce effector cytokines, such as IFN-γ, upon activating NKR engagement (42). Unlike WT NK cells, DAP12-deficient NK cells failed to produce IFN-γ upon antibody-mediated cross-linking of the Ly49H receptor ex vivo (Fig. 1, C and D). However, DAP12-deficient NK cells are not universally impaired in IFN-γ production because cross-linking of the FcϵRIγ-associated receptor NK1.1 induced more IFN-γ production by DAP12-deficient NK cells than by WT NK cells, which is consistent with a prior study (43).
To determine whether DAP12-deficient Ly49H+ NK cells proliferate after MCMV infection, we labeled DAP12-deficient or WT splenocytes with CFSE and transferred these cells into separate CD45-congenic recipients. 7 d after infection with MCMV, the transferred Ly49H+ WT NK cells had completely diluted their CFSE, indicating they had undergone at least seven rounds of division. Conversely, WT Ly49H− NK cells retained high amounts of CFSE, indicating that they had not undergone extensive proliferation (Fig. 1 E). Similarly, DAP12-deficient Ly49H+ NK cells diluted CFSE further than DAP12-deficient Ly49H− NK cells from the same host, indicating that DAP12-deficient Ly49H+ NK cells had preferentially proliferated, although not to the same extent as WT cells. Selective proliferation by both WT and DAP12-deficient Ly49H+ NK cells depended on the presence of the cognate m157 ligand because Ly49H+ NK cells of either genotype failed to proliferate when recipient mice were infected with an MCMV virus lacking m157 (MCMV-Δm157; Fig. 1 E).
To confirm that triggering Ly49H is able to drive NK cell proliferation during MCMV infection in a DAP12-independent manner, we assessed BrdU incorporation by NK cells in WT and DAP12-deficient mice during MCMV infection. Similar to WT NK cells, DAP12-deficient Ly49H+ NK cells exhibited a strong preferential incorporation of BrdU compared with Ly49H− cells. This MCMV-driven proliferation depended on triggering Ly49H, as BrdU incorporation was severely reduced in both WT and DAP12-deficient mice infected with MCMV-Δm157 (Fig. S1). These data indicate that cognate Ly49H–m157 interactions can drive NK cell proliferation in a DAP12-independent manner.
DAP12-deficient Ly49H+ NK cells contribute to MCMV clearance
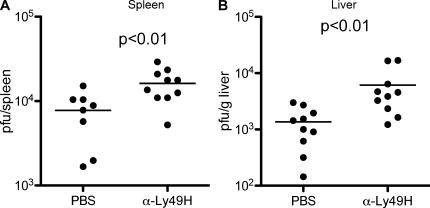
Brown et al. (34) have previously shown that blocking the Ly49H receptor abrogated NK cell control of MCMV infection, elevating viral titers to the level observed when mice had been depleted of NK cells. Although DAP12-deficient mice exhibited high MCMV titers, treatment with an anti-Ly49H blocking mAb significantly increased the viral titers in both the liver and spleen of DAP12-deficient mice compared with PBS-treated DAP12-deficient mice (P < 0.01; Fig. 2). Thus, Ly49H+ NK cells exert a demonstrable level of control of MCMV infection in both organs in a DAP12-independent manner.
Figure 2.
Ly49H-dependent but DAP12-independent control of MCMV. DAP12-deficient mice were treated with PBS or a nondepleting blocking Ly49H mAb 1 d before infection with MCMV. 3 d after infection, MCMV titers in the spleens (A) and liver (B) were determined by plaque assay. The means of the groups are indicated by the horizontal lines. Data are representative of two experiments with similar results.
DAP12-independent Ly49H expression and function is mediated by DAP10
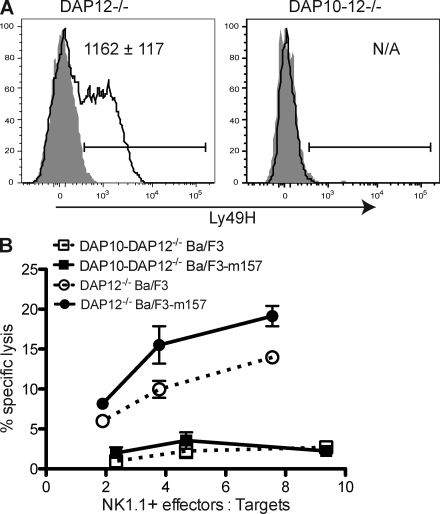
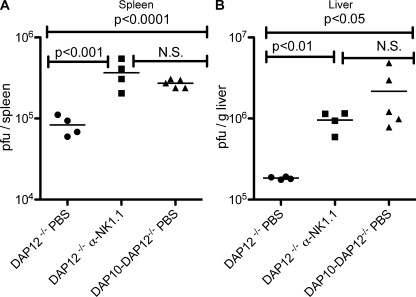
Recently, we have generated mice in which the genes encoding both DAP10 and DAP12 have been deleted (designated DAP10–DAP12-deficient) (44). NK cell numbers, development, and expression of inhibitory receptors in these mice were similar to that in NK cells from WT mice, although minor alterations in expression of certain inhibitory Ly49 receptors were observed (Fig. S2). In contrast to the DAP12-deficient mice, surface expression of Ly49H was totally ablated in mice lacking both DAP10 and DAP12 (Fig. 3 A), indicating that in the absence of DAP12, DAP10 allows for the expression of Ly49H. Unlike DAP12-deficient NK cells, NK cells from DAP10–DAP12-deficient mice failed to kill m157-expressing target cells, confirming that in the absence of DAP12, DAP10 mediates the Ly49H-dependent functions (Fig. 3 B). 4 d after infection, MCMV titers in the spleens and livers of DAP10–DAP12-deficient mice were significantly higher than in DAP12 singly deficient mice (P < 0.0001 and P < 0.05, respectively) and similar to those found in DAP12-deficient mice depleted of NK cells (Fig. 4), confirming that the DAP12-independent protection mediated by Ly49H is DAP10 dependent and that all DAP12-independent NK cell–dependent control requires DAP10. This DAP10 dependency is not caused by NKG2D because all animals were treated with saturating amounts of anti-NKG2D blocking mAb and prior studies have established that NKG2D does not contribute to the control of infection with WT MCMV because the virus ablates expression of NKG2D ligands on MCMV-infected cells (45, 46).
Figure 3.
Ly49H expression and function requires DAP10 or DAP12. (A) Peripheral blood NK cells (NK1.1+ TCR-β−) from uninfected DAP12-deficient and DAP10–DAP12-deficient mice were stained for Ly49H (solid lines) or with an isotype-matched control mAb (gray fill). The bars indicate the Ly49H+ gate. The MFI ± SEM of Ly49H for the Ly49H+ NK cells are indicated within the graphs (n = 5 mice). Data are representative of two experiments with similar results. (B) Splenocytes from DAP12-deficient (circles) and DAP10–DAP12-deficient (squares) mice infected 6 d prior with MCMV-Smith were enriched for NK cells and incubated for 6 h with 51Cr-labeled parental Ba/F3 cells (dashed lines) or Ba/F3 cells expressing MCMV m157 (solid lines). Error bars indicate SEM. Data are representative of two experiments with similar results.
Figure 4.
DAP10 contributes to DAP12-independent control of MCMV. DAP12-deficient mice treated with anti-NKG2D mAb and either undepleted or depleted of NK cells and DAP10–DAP12-deficient mice treated with anti-NKG2D mAb were infected with MCMV. Viral titers in the spleens (A) and livers (B) at 4 d after infection were determined by plaque assay. The means of the groups are indicated by the horizontal lines. Data are representative of two experiments with similar results.
DAP10 is required for optimal Ly49H expression and function
To determine whether DAP10 is involved in Ly49H expression and functions when DAP12 is present, we compared Ly49H expression and functions in WT and DAP10-deficient mice, both of which express DAP12. Although the frequency of Ly49H+ NK cells was similar in WT and DAP10-deficient mice, the intensity of Ly49H staining on NK cells from DAP10-deficient mice was ∼25% lower than on NK cells from WT mice (P < 0.001), indicating that DAP10 enhances Ly49H expression in the presence of DAP12 (Fig. 5 A). As with DAP12-deficient NK cells, DAP10-deficient NK cells killed m157-bearing cells with a similar efficiency to that of WT NK cells (Figs. 1 B and 5 B). Lysis of m157 targets by both WT and DAP10-deficient cells was dependent on Ly49H because killing was abrogated by blocking Ly49H.
Figure 5.
DAP10-dependent functions of Ly49H. (A) Peripheral blood NK cells (NK1.1+ TCR-β−) from uninfected B6 WT and DAP10-deficient mice were stained for Ly49H (solid lines) or with an isotype-matched control mAb (gray fill). The bars indicate the Ly49H+ gate. The mean MFI ± SEM of Ly49H for the Ly49H+ NK cells are indicated within the graphs (n = 5 mice). Data are representative of three experiments with similar results. (B) Splenocytes from B6 WT or DAP10-deficient mice infected 6 d prior with MCMV-Smith were enriched for NK cells and incubated for 6 h with 51Cr-labeled parental Ba/F3 cells (dotted lines) or Ba/F3 cells expressing MCMV m157 in the presence (dashed lines) or absence (solid lines) of Ly49H blocking antibody. Data are representative of three independent experiments with similar results. Error bars indicate SEM. (C) Enriched NK cells from MCMV-infected WT and DAP10-deficient mice were stained for IFN-γ production after incubation for 4 h in the presence of brefeldin A on plates coated with anti-Ly49H, anti-NK1.1, or an isotype-matched control mAb. The means of the groups are indicated by the horizontal lines. Data are pooled from two experiments. (D) WT and DAP10-deficient splenocytes were mixed 1:1 and adoptively transferred into naive hosts. Before infection and 6 d after infection with MCMV-Smith or -Δm157, recipient mice were bled and analyzed for the frequency of WT (CD45.1) and DAP10-deficient (CD45.2) donor NK (NK1.1+ TCR-β−) cells. The plot indicates the ratio of WT to DAP10-deficient donor NK cells in four animals before infection (day 0) and 6 d after infection. Data are representative of five independent experiments with similar results.
To determine whether Ly49H–DAP10 is able to enhance IFN-γ mediated by Ly49H–DAP12, we compared IFN-γ production induced by antibody-mediated cross-linking of Ly49H on WT and DAP10-deficient NK cells. In the absence of DAP10, cross-linking Ly49H led to diminished IFN-γ production compared with WT NK cells. Fewer cells made IFN-γ, and less cytokine was made by the IFN-γ+ DAP10-deficient NK cells as indicated by the lower MFI of IFN-γ in these cells (Fig. 5 C and Fig. S3). Similar frequencies of DAP10-deficient and WT NK cells produced IFN-γ after stimulation via the NK1.1 receptor, and the amount of cytokine produced was similar between DAP10-deficient and WT NK cells, indicating that the DAP10 requirement for IFN-γ production was specific for the Ly49H receptor (Fig. 5 C and Fig. S3 A). Thus, although Ly49H–DAP10 alone was not sufficient to induce IFN-γ production after receptor cross-linking (Fig. 1 D), the Ly49H–DAP10 receptor complexes synergize with Ly49H–DAP12 receptor complexes to produce optimal IFN-γ in Ly49H-stimulated WT NK cells.
To determine whether DAP10 is required for Ly49H-dependent NK cell proliferation during MCMV infection, we transferred a 1:1 mixture of congenically marked WT and DAP10-deficient splenocytes into naive recipients and then infected with MCMV. 6 d after infection, the WT donor NK cells expanded more than the donor DAP10-deficient NK cells, as indicated by the increased frequency of WT cells compared with DAP10-deficient cells among the donor cell population. This was confirmed by examining CFSE dilution in WT and DAP10-deficient NK cells after MCMV infection. Although both populations exhibited considerable CFSE dilution, the WT NK cells underwent more rounds of division than the DAP10-deficient NK cells, confirming that DAP10 is required for optimal NK cell proliferation during MCMV infection (Fig. S3).
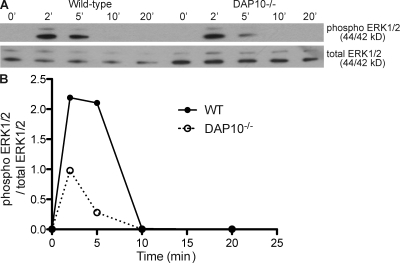
To determine how DAP10 augments DAP12 signaling downstream of Ly49H, we compared signaling events in primary NK cells from MCMV-infected WT and DAP10-deficient mice upon restimulation with an anti-Ly49H mAb. Prior studies have established that ERK1/2 is phosphorylated after stimulation via DAP10 and DAP12 and is critical to ITAM-induced NK cell effector functions (21, 47). Therefore, we analyzed the activation of ERK1/2 in response to Ly49H cross-linking in primary NK cells isolated directly ex vivo from MCMV-infected mice. Compared with WT NK cells, DAP10-deficient NK cells demonstrated reduced phosphorylation of the ERK1/2 kinases early after Ly49H triggering (Fig. 6, A and B). Thus, DAP10 is required for optimal signaling downstream of Ly49H.
Figure 6.
Ly49H signaling is impaired in the absence of DAP10. (A) Splenic NK cells (DX5+ TCR-β−) from B6 WT and DAP10-deficient mice infected 6 d prior with MCMV-Smith were sorted to >93% purity and incubated on Ly49H mAb-coated plates for 0–20 min. Cells were lysed, run on a 10% SDS-PAGE gel, and blotted for total and phosphorylated ERK1/2. ‘, min. (B) Integrated band densities were calculated using ImageJ software and plotted as a ratio of phosphorylated ERK1/2 to total ERK1/2. Data are representative of three experiments with similar results.
DAP10 is required for late control of MCMV infection
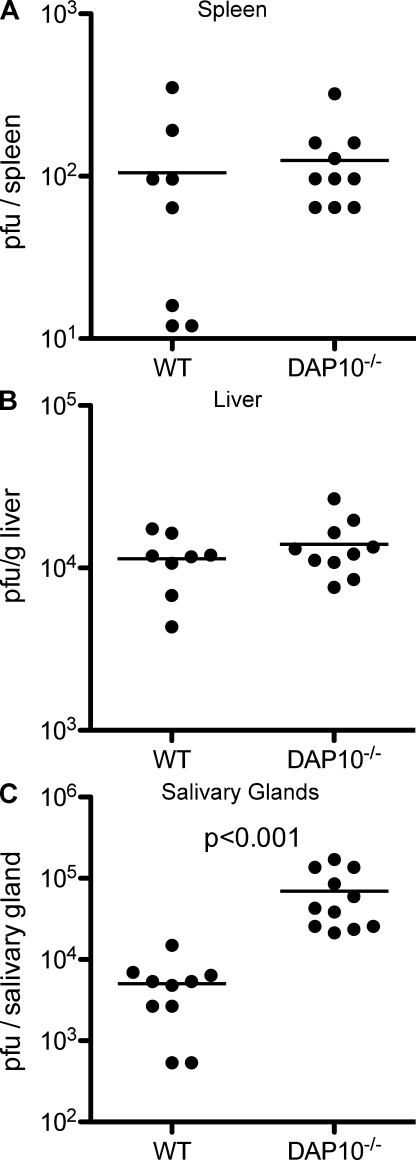
To determine whether DAP10-augmented Ly49H functions contribute to control of MCMV infection, we compared viral titers in the spleens and livers of WT and DAP10-deficient mice 4 d after MCMV infection. Although DAP10-deficient mice demonstrated elevated viral titers in both organs compared with viral titers in WT mice, the differences did not reach the level of statistical significance (Fig. 7, A and B). This is likely a result of the ability of DAP10-deficient NK cells expressing Ly49H–DAP12 complexes to efficiently lyse m157-bearing targets and to produce intermediate amounts of IFN-γ (Fig. 5, B and C).
Figure 7.
DAP10 is required for optimal control of late MCMV infection. B6 WT and DAP10-deficient mice were treated with blocking anti-NKG2D mAb and, 1 d later, infected with MCMV. Viral titers in the spleens (A) and liver (B) at 4 d after infection and in the salivary glands (C) at 7 d after infection were determined by plaque assay. No virus was recovered from the spleens or livers at day 7. The means of the groups are indicated by the horizontal lines. Data are pooled from two experiments with similar results.
To determine whether DAP10-enhanced proliferation of Ly49H+ NK cells contributed to control of MCMV at later time points, we assessed viral titers 1 wk after infection in WT and DAP10-deficient mice. Although both WT and DAP10-deficient mice cleared MCMV from the spleen and liver at this time point, the DAP10-deficient mice had ∼20× higher viral titers in the salivary glands (P < 0.001; Fig. 7 C), which is the site where MCMV persists in the chronically infected host. Thus, DAP10 is required for optimal control of MCMV at later time points in the salivary glands, possibly because of DAP10-dependent enhanced NK cell proliferation and/or IFN-γ production.
DISCUSSION
Recent biochemical studies have suggested that DAP10 may associate with NKRs other than NKG2D, including Ly49D and Ly49H (39). We now demonstrate that not only do these Ly49H–DAP10 complexes form but that they are able to induce NK cell functions, including proliferation, cytotoxicity, and cytokine production, and, most importantly, that Ly49H–DAP10 complexes are required for optimal control of MCMV infection. By comparing WT, DAP10-deficient, DAP12-deficient, and DAP10–DAP12-deficient mice, we found that DAP10 alone is sufficient to mediate some Ly49H-dependent NK cell functions and that DAP10 and DAP12 together are necessary for optimal Ly49H-mediated NK activity and control of MCMV infection. In the absence of DAP12, DAP10 was sufficient to support the surface expression of Ly49H, Ly49H-dependent NK cell–mediated cytotoxicity, NK cell proliferation, and control of MCMV. However, DAP10 alone was not sufficient for Ly49H-induced IFN-γ production. Even in the presence of DAP12, DAP10 was required for optimal Ly49H expression, IFN-γ production, proliferation, and control of MCMV infection. Thus, we hypothesize that Ly49H signaling via DAP10 augments the DAP12-mediated proliferation and cytokine production of Ly49H+ NK cells, resulting in optimal NK cell control of MCMV infection.
Surface expression of Ly49H on NK cells requires either DAP10 or DAP12, as demonstrated by the total absence of Ly49H on NK cells in DAP10–DAP12 doubly deficient mice. In singly deficient NK cells, the reduction in Ly49H surface level is more pronounced in DAP12-deficient cells than in DAP10-deficient cells. At least two possibilities might explain this difference. First, Ly49H–DAP10 receptor complexes might be less stable than Ly49H–DAP12 receptor complexes. Second, the amount of DAP10 protein in NK cells might be limiting or being preferentially used by other receptors, such as NKG2D. Given the sequence similarity of the transmembrane domains of DAP10 and DAP12, which determine receptor–adaptor pairing, it is anticipated that other DAP12-associated receptors will be found to associate with DAP10. Indeed, we have also observed an association of Ly49D and mouse CD94/NKG2C with DAP10 (unpublished data), which is in agreement with recent findings (39). It is important to note that our results indicate that DAP10 deficiency not only abrogates the functions of the NKG2D-L receptor but also affects the functions of other activating NKRs that were previously only known to associate with DAP12 in vivo.
During MCMV infection, the Ly49H+ subset of NK cells proliferates extensively. Using DAP12ki mice in which the ITAM of DAP12 was mutated to be nonfunctional, French et al. (36) showed that the selective BrdU incorporation by Ly49H+ NK cells during MCMV infection required DAP12 signaling. Conversely, we found that DAP12-deficient Ly49H+ NK cells preferentially incorporated BrdU during MCMV infection. It is possible that the DAP12ki homodimer might be acting as a dominant negative, preventing DAP10 from associating with Ly49H and thus accounting for the differences between the DAP12ki and the DAP12-deficient NK cells. By adoptively transferring CFSE-labeled cells, we found that either DAP10 or DAP12 was sufficient to mediate proliferation of Ly49H+ NK cells during MCMV infection. However, maximal proliferation required both signaling subunits because WT NK cells outcompeted DAP10-deficient NK cells when cells of both genotypes were transferred into the same host.
Engaging receptors associated with ITAM adaptors (DAP12, CD3-ζ, or FcϵRIγ), such as the KIR2DS1–DAP12 receptor complex, results in both cytotoxicity and cytokine secretion. In contrast, triggering the DAP10-associated NKG2D receptor leads to cytotoxicity but not efficient cytokine secretion (19, 23). We previously reported that when we simultaneously cross-linked both KIR2DS2–DAP12 and NKG2D–DAP10 on human NK cells, DAP10 activation augmented DAP12-induced IFN-γ production (22). We now show that by triggering a single receptor, i.e., Ly49H, both the DAP10 and DAP12 signaling pathways are engaged and lead to optimal IFN-γ production in WT NK cells, whereas either signaling subunit is sufficient to trigger NK cell–mediated cytotoxicity. Signaling downstream of DAP10 and DAP12 engages some of the same pathways, including the ERK1/2 pathway (21, 47, 48). Compared with WT NK cells, cross-linking Ly49H on DAP10-deficient NK cells lead to only a modest induction of ERK1/2, suggesting that the DAP10 and DAP12 signaling cascades intersect at this molecule in an additive or synergistic manner. ERK1/2 activation is known to be upstream of IFN-γ transcription; thus, the partial defect in IFN-γ production by DAP10-deficient NK cells downstream of Ly49H might be caused by inefficient activation of ERK1/2 (48, 49). Similarly, CD45-deficient NK cells exhibit minimal phosphorylation of ERK1/2 and IFN-γ production upon triggering of multiple ITAM-associated receptors; however, cytolytic activity induced by these same receptors was maintained in the Cd45−/− NK cells, suggesting that weak activation of ERK1/2 may be sufficient for NK cell–mediated killing but not IFN-γ production (50).
Ly49H is the dominant receptor in NK cell–mediated control of MCMV in B6 mice (34). In addition to the previously reported DAP12-dependent mechanism of MCMV control (38), we find a DAP12-independent mechanism of control of MCMV that is dependent on both Ly49H and DAP10. Compared with DAP12-deficient mice, mice lacking both DAP10 and DAP12 demonstrated elevated MCMV titers in both the spleen and the liver similar to that seen in DAP12-deficient mice treated with blocking Ly49H antibody or depleted of NK cells. The most striking defect in MCMV control in DAP10-deficient mice is evident in the salivary glands 1 wk after infection. As Ly49H+ NK cells in DAP10-deficient mice are partially impaired in their proliferative response to MCMV, it is possible that efficient control of MCMV in the salivary glands is dependent on expansion of Ly49H+ NK cells. Furthermore, a DAP10-dependent contribution to IFN-γ production might also be involved in control of viral replication in the salivary gland. The DAP10-dependent control of MCMV in the absence of DAP12 is unlikely to be caused by NKG2D because MCMV encodes at least four proteins that prevent expression of NKG2D ligands on the surface of infected cells (45, 46, 51–53), and NKG2D-deficient mice control MCMV infection as efficiently as WT mice (Dan Serna and David Raulet, personal communication). Thus, although DAP12-deficient mice are impaired in their Ly49H-dependent response against MCMV infection (54), we now show that DAP10 enables some Ly49H function in the absence of DAP12 and is necessary for optimal activity in the presence of DAP12.
Although DAP10 and DAP12 make unequal contributions to Ly49H-mediated function, it is clear that both are required for optimal NK cell responses to MCMV. The signaling events known to be induced by DAP10 receptor triggering are also induced by DAP12 (48). Thus, DAP12 activation could theoretically supersede DAP10 activation. However, it is possible that engaging both pathways induces a quantitatively more robust signal, as demonstrated by the stronger ERK1/2 activation seen in WT NK cells compared with DAP10-deficient NK cells triggered via Ly49H. Alternatively, DAP10 triggering might engage an unknown signaling pathway that is not shared with DAP12. Regardless, we demonstrate that DAP12-induced DAP10-augmented NK cell responses initiated by Ly49H-mediated recognition of m157 provide for the optimal NK cell response during MCMV infection. Thus, our findings provide a new paradigm for adaptor usage, signaling, and function of activating NKRs, specifically the Ly49H receptor which is necessary for NK cell–mediated resistance to MCMV and potentially other DAP12-associated receptors.
MATERIALS AND METHODS
Mice, viruses, and infections.
C57BL/6 mice were purchased from the National Cancer Institute. DAP10-deficient mice were generated on a B6 background as previously described (55). DAP12-deficient mice were backcrossed 12 generations to the C57BL/6 background (41). DAP10–DAP12 double knockout mice were generated by standard techniques and backcrossed 13 generations onto the C57BL/6 background (44). All mice were maintained in the University of California, San Francisco specific pathogen-free animal facility. Animal protocols were approved by the University of California, San Francisco Institutional Animal Care and Use Committee. Mice were infected i.p. with 5 × 104 pfu MCMV-Smith or MCMV-Δm157 (33). No lethality was observed with this inoculum of MCMV in any of the animals studied.
Cytotoxicity and cytokine production.
6 d after MCMV infection, splenic NK cells from WT, DAP10-deficient, DAP12-deficient, or DAP10–DAP12-deficient mice were enriched by labeling splenocytes with rat anti–mouse IgG mAbs against CD4, CD8, TER119, and Gr-1 and magnetically depleting labeled cells with anti–rat IgG and anti–mouse IgG (to deplete B cells) beads. For cytotoxicity assays, enriched NK cells were incubated in triplicate with 51Cr-labeled Ba/F3 cells or Ba/F3 cells stably expressing MCMV m157. The blocking anti-Ly49H mAb (clone 3D10) or an isotype-matched control mAb was added to the indicated cultures at 5 µg/ml. 6 h later, supernatants were harvested and assayed for 51Cr release. Spontaneous lysis was determined by incubating target cells without effectors. Maximum lysis was determined by incubating target cells in 1% Triton X-100 in water. The percentage of specific lysis = (lysis − spontaneous lysis)/(maximum lysis − spontaneous lysis) × 100. The anti-Ly49H mAb was a gift from W. Yokoyama (Washington University, St. Louis, MO). IFN-γ production was determined by incubating 2 × 105 enriched splenic NK cells on plates coated with 10 µg/ml of anti-Ly49H, anti-NK1.1, or an isotype-matched control mAb for 4 h in the presence of brefeldin A. Cells were surface stained for Ly49H, TCR-β, DX5 (CD49b), and intracellular IFN-γ using the Intracellular Staining kit (BD). Antibodies were purchased or were gifts from eBioscience, BD, and BioLegend.
CFSE labeling.
CD45.1+ or CD45.2+ naive donor B6 WT, DAP10-deficient, or DAP12-deficient splenocytes were treated with ACK lysis buffer to remove red blood cells, labeled for 10 min with 0.5 µM CFSE in PBS, and washed twice in PBS. 4 × 107 labeled splenocytes were transferred i.v. into naive recipient mice either separately or mixed 1:1 before transfer. Recipient mice were infected with Smith or Δm157 strain MCMV and sacrificed 7 d later. Adoptively transferred NK cells were analyzed for Ly49H expression and CFSE dilution by flow cytometry. The Δm157 strain MCMV was a gift from U. Koszinowski (Ludwig Maximilians University Munich, Munich, Germany).
MCMV titers.
Mice were injected i.p. with PBS, 200 µg of anti-Ly49H (clone 3D10; eBioscience), anti-NK1.1 (clone PK136), and/or 500 µg of anti-NKG2D (clone CX5) in PBS 1 d before infection with 5 × 104 pfu MCMV-Smith. These anti-Ly49H and anti-NKG2D mAbs block receptor function but are nondepleting. In prior studies, we have observed no difference in viral titers in control mice injected with PBS or an irrelevant IgG. 3–7 d later, salivary glands, whole spleens, and one lobe of each liver were harvested and snap frozen on dry ice in 1:1 DME/skim milk media. Samples were stored at −80°C, thawed, weighed, sonicated, plated on Ma/My MEF cells in fivefold serial dilutions in DME without FCS, and incubated for 2 h at 37°C. DME with 10% FCS and 0.75% carboxymethyl cellulose was added and samples were incubated for 7 d. Plaques were visualized by staining with crystal violet dye. Splenic and salivary gland titers were calculated for the whole organ and liver titers were adjusted for weight of the tissue.
Western blot analysis.
Splenic NK cells from WT and DAP10-deficient mice infected with MCMV 6 d prior were sorted as DX5+ TCR-β− to >93% purity based on NK1.1 expression. Sorted cells were incubated with 10 µg/ml of purified anti-CD16+CD32 mAb 2.4G2 to block Fc receptors and then rested for 1 h without serum at 37°C. Cells were added to plates coated with Ly49H mAb, incubated at 37°C, and lysed with Laemmli Sample buffer (Bio-Rad Laboratories). Lysates were boiled, separated on a 10% SDS-PAGE gel, and transferred to an Immobilon P membrane. Blots were blocked with 5% BSA in TBS-Tween and then probed with anti–phospho-ERK1/2 or anti–total ERK1/2 (Cell Signaling Technology). Primary antibodies were detected with HRP-conjugated anti–rabbit IgG antibody (Cell Signaling Technology) and visualized with ECL Plus (GE Healthcare). Quantitation of the integrated density of bands minus background was performed using ImageJ software (National Institutes of Health).
Statistical analysis.
Statistical differences in MFIs and percentages of IFN-γ+ NK cells were determined by the unpaired two-tailed Student's t test. Statistical differences in viral titers were determined by the unpaired two-tailed Mann-Whitney U test. Statistical differences in the ratio of WT and DAP10-deficient NK cells before and after infection were determined by the paired two-tailed Student's t test. Statistics were determined with Prism software (GraphPad Software, Inc.).
Online supplemental material.
Fig. S1 shows in vivo BrdU incorporation by WT and DAP12-deficient Ly49H+ NK cells during MCMV infection. Fig. S2 shows expression of NKRs, maturation, and activation status of NK cells from naive DAP10–DAP12-deficient mice. Fig. S3 shows IFN-γ production and CFSE dilution by WT and DAP10-deficient Ly49H+ NK cells during MCMV infection. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20090168/DC1.
Acknowledgments
We thank Dr. Wayne Yokoyama and Dr. Ulrich Koszinowski for generously providing reagents.
M.T. Orr and J.C. Sun are Irvington Postdoctoral Fellows of the Cancer Research Institute and D.G.T. Hesslein is a Special Fellow of the Leukemia and Lymphoma Society. This study was supported by National Institutes of Health grant AI066897, and L.L. Lanier is an American Cancer Society Professor. Schering-Plough Corp. supports J.H. Phillips.
J.H. Phillips is an employee of Schering-Plough Biopharma. The authors have no other competing financial interests.
Footnotes
Abbreviations used: ITAM, immunoreceptor tyrosine-based activation motif; KIR, killer immunoglobulin-like receptors; MCMV, mouse CMV; MFI, mean fluorescence intensity; NKR, NK cell receptor.
References
- Lanier L.L. 2005. NK cell recognition.Annu. Rev. Immunol. 23:225–274 [DOI] [PubMed] [Google Scholar]
- Arase H., Mocarski E.S., Campbell A.E., Hill A.B., Lanier L.L. 2002. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors.Science. 296:1323–1326 [DOI] [PubMed] [Google Scholar]
- Gasser S., Orsulic S., Brown E.J., Raulet D.H. 2005. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor.Nature. 436:1186–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raulet D.H. 2003. Roles of the NKG2D immunoreceptor and its ligands.Nat. Rev. Immunol. 3:781–790 [DOI] [PubMed] [Google Scholar]
- Bauer S., Groh V., Wu J., Steinle A., Phillips J.H., Lanier L.L., Spies T. 1999. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA.Science. 285:727–729 [DOI] [PubMed] [Google Scholar]
- Smith H.R., Heusel J.W., Mehta I.K., Kim S., Dorner B.G., Naidenko O.V., Iizuka K., Furukawa H., Beckman D.L., Pingel J.T., et al. 2002. Recognition of a virus-encoded ligand by a natural killer cell activation receptor.Proc. Natl. Acad. Sci. USA. 99:8826–8831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier L.L., Corliss B.C., Wu J., Leong C., Phillips J.H. 1998. Immunoreceptor DAP12 bearing a tyrosine-based activation motif is involved in activating NK cells.Nature. 391:703–707 [DOI] [PubMed] [Google Scholar]
- Wu J., Song Y., Bakker A.B., Bauer S., Spies T., Lanier L.L., Phillips J.H. 1999. An activating immunoreceptor complex formed by NKG2D and DAP10.Science. 285:730–732 [DOI] [PubMed] [Google Scholar]
- Carr W.H., Rosen D.B., Arase H., Nixon D.F., Michaelsson J., Lanier L.L. 2007. Cutting Edge: KIR3DS1, a gene implicated in resistance to progression to AIDS, encodes a DAP12-associated receptor expressed on NK cells that triggers NK cell activation.J. Immunol. 178:647–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier L.L., Corliss B., Wu J., Phillips J.H. 1998. Association of DAP12 with activating CD94/NKG2C NK cell receptors.Immunity. 8:693–701 [DOI] [PubMed] [Google Scholar]
- Lanier L.L., Yu G., Phillips J.H. 1989. Co-association of CD3 zeta with a receptor (CD16) for IgG Fc on human natural killer cells.Nature. 342:803–805 [DOI] [PubMed] [Google Scholar]
- Lanier L.L., Yu G., Phillips J.H. 1991. Analysis of Fc gamma RIII (CD16) membrane expression and association with CD3 zeta and Fc epsilon RI-gamma by site-directed mutation.J. Immunol. 146:1571–1576 [PubMed] [Google Scholar]
- Lanier L.L. 2009. DAP10- and DAP12-associated receptors in innate immunity.Immunol. Rev. 227:150–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diefenbach A., Tomasello E., Lucas M., Jamieson A.M., Hsia J.K., Vivier E., Raulet D.H. 2002. Selective associations with signaling proteins determine stimulatory versus costimulatory activity of NKG2D.Nat. Immunol. 3:1142–1149 [DOI] [PubMed] [Google Scholar]
- Gilfillan S., Ho E.L., Cella M., Yokoyama W.M., Colonna M. 2002. NKG2D recruits two distinct adapters to trigger NK cell activation and costimulation.Nat. Immunol. 3:1150–1155 [DOI] [PubMed] [Google Scholar]
- Rosen D.B., Araki M., Hamerman J.A., Chen T., Yamamura T., Lanier L.L. 2004. A structural basis for the association of DAP12 with mouse, but not human, NKG2D.J. Immunol. 173:2470–2478 [DOI] [PubMed] [Google Scholar]
- Isakov N. 1998. Role of immunoreceptor tyrosine-based activation motif in signal transduction from antigen and Fc receptors.Adv. Immunol. 69:183–247 [PubMed] [Google Scholar]
- MacFarlane A.W., 4th, Campbell K.S. 2006. Signal transduction in natural killer cells.Curr. Top. Microbiol. Immunol. 298:23–57 [DOI] [PubMed] [Google Scholar]
- Billadeau D.D., Upshaw J.L., Schoon R.A., Dick C.J., Leibson P.J. 2003. NKG2D-DAP10 triggers human NK cell-mediated killing via a Syk-independent regulatory pathway.Nat. Immunol. 4:557–564 [DOI] [PubMed] [Google Scholar]
- Upshaw J.L., Arneson L.N., Schoon R.A., Dick C.J., Billadeau D.D., Leibson P.J. 2006. NKG2D-mediated signaling requires a DAP10-bound Grb2-Vav1 intermediate and phosphatidylinositol-3-kinase in human natural killer cells.Nat. Immunol. 7:524–532 [DOI] [PubMed] [Google Scholar]
- McVicar D.W., Taylor L.S., Gosselin P., Willette-Brown J., Mikhael A.I., Geahlen R.L., Nakamura M.C., Linnemeyer P., Seaman W.E., Anderson S.K., et al. 1998. DAP12-mediated signal transduction in natural killer cells. A dominant role for the Syk protein-tyrosine kinase.J. Biol. Chem. 273:32934–32942 [DOI] [PubMed] [Google Scholar]
- Wu J., Cherwinski H., Spies T., Phillips J.H., Lanier L.L. 2000. DAP10 and DAP12 form distinct, but functionally cooperative, receptor complexes in natural killer cells.J. Exp. Med. 192:1059–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zompi S., Hamerman J.A., Ogasawara K., Schweighoffer E., Tybulewicz V.L., Di Santo J.P., Lanier L.L., Colucci F. 2003. NKG2D triggers cytotoxicity in mouse NK cells lacking DAP12 or Syk family kinases.Nat. Immunol. 4:565–572 [DOI] [PubMed] [Google Scholar]
- Biron C.A., Byron K.S., Sullivan J.L. 1989. Severe herpesvirus infections in an adolescent without natural killer cells.N. Engl. J. Med. 320:1731–1735 [DOI] [PubMed] [Google Scholar]
- Lanier L.L. 2008. Evolutionary struggles between NK cells and viruses.Nat. Rev. Immunol. 8:259–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukowski J.F., Woda B.A., Habu S., Okumura K., Welsh R.M. 1983. Natural killer cell depletion enhances virus synthesis and virus-induced hepatitis in vivo.J. Immunol. 131:1531–1538 [PubMed] [Google Scholar]
- Gosselin P., Mason L.H., Willette-Brown J., Ortaldo J.R., McVicar D.W., Anderson S.K. 1999. Induction of DAP12 phosphorylation, calcium mobilization, and cytokine secretion by Ly49H.J. Leukoc. Biol. 66:165–171 [DOI] [PubMed] [Google Scholar]
- Smith K.M., Wu J., Bakker A.B., Phillips J.H., Lanier L.L. 1998. Ly-49D and Ly-49H associate with mouse DAP12 and form activating receptors.J. Immunol. 161:7–10 [PubMed] [Google Scholar]
- Daniels K.A., Devora G., Lai W.C., O'Donnell C.L., Bennett M., Welsh R.M. 2001. Murine cytomegalovirus is regulated by a discrete subset of natural killer cells reactive with monoclonal antibody to Ly49H.J. Exp. Med. 194:29–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh J., Chu D.T., O'Guin A.K., Yokoyama W.M., Virgin H.W., 4th 2005. Natural killer cells utilize both perforin and gamma interferon to regulate murine cytomegalovirus infection in the spleen and liver.J. Virol. 79:661–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay C.H., Welsh R.M. 1997. Distinct organ-dependent mechanisms for the control of murine cytomegalovirus infection by natural killer cells.J. Virol. 71:267–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.H., Girard S., Macina D., Busa M., Zafer A., Belouchi A., Gros P., Vidal S.M. 2001. Susceptibility to mouse cytomegalovirus is associated with deletion of an activating natural killer cell receptor of the C-type lectin superfamily.Nat. Genet. 28:42–45 [DOI] [PubMed] [Google Scholar]
- Bubic I., Wagner M., Krmpotic A., Saulig T., Kim S., Yokoyama W.M., Jonjic S., Koszinowski U.H. 2004. Gain of virulence caused by loss of a gene in murine cytomegalovirus.J. Virol. 78:7536–7544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M.G., Dokun A.O., Heusel J.W., Smith H.R., Beckman D.L., Blattenberger E.A., Dubbelde C.E., Stone L.R., Scalzo A.A., Yokoyama W.M. 2001. Vital involvement of a natural killer cell activation receptor in resistance to viral infection.Science. 292:934–937 [DOI] [PubMed] [Google Scholar]
- Dokun A.O., Kim S., Smith H.R., Kang H.S., Chu D.T., Yokoyama W.M. 2001. Specific and nonspecific NK cell activation during virus infection.Nat. Immunol. 2:951–956 [DOI] [PubMed] [Google Scholar]
- French A.R., Sjolin H., Kim S., Koka R., Yang L., Young D.A., Cerboni C., Tomasello E., Ma A., Vivier E., et al. 2006. DAP12 signaling directly augments proproliferative cytokine stimulation of NK cells during viral infections.J. Immunol. 177:4981–4990 [DOI] [PubMed] [Google Scholar]
- Tomasello E., Desmoulins P.O., Chemin K., Guia S., Cremer H., Ortaldo J., Love P., Kaiserlian D., Vivier E. 2000. Combined natural killer cell and dendritic cell functional deficiency in KARAP/DAP12 loss-of-function mutant mice.Immunity. 13:355–364 [DOI] [PubMed] [Google Scholar]
- Sjolin H., Tomasello E., Mousavi-Jazi M., Bartolazzi A., Karre K., Vivier E., Cerboni C. 2002. Pivotal role of KARAP/DAP12 adaptor molecule in the natural killer cell–mediated resistance to murine cytomegalovirus infection.J. Exp. Med. 195:825–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coudert J.D., Scarpellino L., Gros F., Vivier E., Held W. 2008. Sustained NKG2D engagement induces cross-tolerance of multiple distinct NK cell activation pathways.Blood. 111:3571–3578 [DOI] [PubMed] [Google Scholar]
- Anfossi N., Lucas M., Diefenbach A., Buhring H.J., Raulet D., Tomasello E., Vivier E. 2003. Contrasting roles of DAP10 and KARAP/DAP12 signaling adaptors in activation of the RBL-2H3 leukemic mast cell line.Eur. J. Immunol. 33:3514–3522 [DOI] [PubMed] [Google Scholar]
- Bakker A.B., Hoek R.M., Cerwenka A., Blom B., Lucian L., McNeil T., Murray R., Phillips L.H., Sedgwick J.D., Lanier L.L. 2000. DAP12-deficient mice fail to develop autoimmunity due to impaired antigen priming.Immunity. 13:345–353 [DOI] [PubMed] [Google Scholar]
- Kim S., Poursine-Laurent J., Truscott S.M., Lybarger L., Song Y.J., Yang L., French A.R., Sunwoo J.B., Lemieux S., Hansen T.H., Yokoyama W.M. 2005. Licensing of natural killer cells by host major histocompatibility complex class I molecules.Nature. 436:709–713 [DOI] [PubMed] [Google Scholar]
- Takaki R., Watson S.R., Lanier L.L. 2006. DAP12: an adapter protein with dual functionality.Immunol. Rev. 214:118–129 [DOI] [PubMed] [Google Scholar]
- Yamanishi Y., Kitaura J., Izawa K., Matsuoka T., Oki T., Lu Y., Shibata F., Yamazaki S., Kumagai H., Nakajima H., et al. 2008. Analysis of mouse LMIR5/CLM-7 as an activating receptor: differential regulation of LMIR5/CLM-7 in mouse versus human cells.Blood. 111:688–698 [DOI] [PubMed] [Google Scholar]
- Lodoen M., Ogasawara K., Hamerman J.A., Arase H., Houchins J.P., Mocarski E.S., Lanier L.L. 2003. NKG2D-mediated natural killer cell protection against cytomegalovirus is impaired by viral gp40 modulation of retinoic acid early inducible 1 gene molecules.J. Exp. Med. 197:1245–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodoen M.B., Abenes G., Umamoto S., Houchins J.P., Liu F., Lanier L.L. 2004. The cytomegalovirus m155 gene product subverts natural killer cell antiviral protection by disruption of H60–NKG2D interactions.J. Exp. Med. 200:1075–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M., Fujikawa K., Tassi I., Kim S., Latinis K., Nishi S., Yokoyama W., Colonna M., Swat W. 2004. Differential requirements for Vav proteins in DAP10- and ITAM-mediated NK cell cytotoxicity.J. Exp. Med. 200:817–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier L.L. 2008. Up on the tightrope: natural killer cell activation and inhibition.Nat. Immunol. 9:495–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotta R., Puorro K.A., Paroli M., Azzoni L., Abebe B., Eisenlohr L.C., Perussia B. 1998. Dependence of both spontaneous and antibody-dependent, granule exocytosis-mediated NK cell cytotoxicity on extracellular signal-regulated kinases.J. Immunol. 161:6648–6656 [PubMed] [Google Scholar]
- Hesslein D.G., Takaki R., Hermiston M.L., Weiss A., Lanier L.L. 2006. Dysregulation of signaling pathways in CD45-deficient NK cells leads to differentially regulated cytotoxicity and cytokine production.Proc. Natl. Acad. Sci. USA. 103:7012–7017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krmpotic A., Busch D.H., Bubic I., Gebhardt F., Hengel H., Hasan M., Scalzo A.A., Koszinowski U.H., Jonjic S. 2002. MCMV glycoprotein gp40 confers virus resistance to CD8+ T cells and NK cells in vivo.Nat. Immunol. 3:529–535 [DOI] [PubMed] [Google Scholar]
- Krmpotic A., Hasan M., Loewendorf A., Saulig T., Halenius A., Lenac T., Polic B., Bubic I., Kriegeskorte A., Pernjak-Pugel E., et al. 2005. NK cell activation through the NKG2D ligand MULT-1 is selectively prevented by the glycoprotein encoded by mouse cytomegalovirus gene m145.J. Exp. Med. 201:211–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenac T., Budt M., Arapovic J., Hasan M., Zimmermann A., Simic H., Krmpotic A., Messerle M., Ruzsics Z., Koszinowski U.H., et al. 2006. The herpesviral Fc receptor fcr-1 down-regulates the NKG2D ligands MULT-1 and H60.J. Exp. Med. 203:1843–1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J.C., Beilke J.N., Lanier L.L. 2009. Adaptive immune features of natural killer cells.Nature. 457:557–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyka-Nouspikel N., Phillips J.H. 2006. Physiological roles of murine DAP10 adapter protein in tumor immunity and autoimmunity.Immunol. Rev. 214:106–117 [DOI] [PubMed] [Google Scholar]