Abstract
Differentiation and recruitment of alternatively activated macrophages (AAMacs) are hallmarks of several inflammatory conditions associated with infection, allergy, diabetes, and cancer. AAMacs are defined by the expression of Arginase 1, chitinase-like molecules, and resistin-like molecule (RELM) α/FIZZ1; however, the influence of these molecules on the development, progression, or resolution of inflammatory diseases is unknown. We describe the generation of RELM-α–deficient (Retnla−/−) mice and use a model of T helper type 2 (Th2) cytokine-dependent lung inflammation to identify an immunoregulatory role for RELM-α. After challenge with Schistosoma mansoni (Sm) eggs, Retnla−/− mice developed exacerbated lung inflammation compared with their wild-type counterparts, characterized by excessive pulmonary vascularization, increased size of egg-induced granulomas, and elevated fibrosis. Associated with increased disease severity, Sm egg–challenged Retnla−/− mice exhibited elevated expression of pathogen-specific CD4+ T cell–derived Th2 cytokines. Consistent with immunoregulatory properties, recombinant RELM-α could bind to macrophages and effector CD4+ Th2 cells and inhibited Th2 cytokine production in a Bruton's tyrosine kinase–dependent manner. Additionally, Retnla−/− AAMacs promoted exaggerated antigen-specific Th2 cell differentiation. Collectively, these data identify a previously unrecognized role for AAMac-derived RELM-α in limiting the pathogenesis of Th2 cytokine-mediated pulmonary inflammation, in part through the regulation of CD4+ T cell responses.
The phagocytosis and elimination of microbial pathogens by macrophages is an evolutionarily conserved component of the innate immune system in all metazoa from humans to organisms as primitive as starfish (1, 2). In mammals, it is now recognized that macrophages, beyond their antimicrobial properties, can regulate both innate and adaptive immune responses in multiple inflammatory diseases, including mouse models of airway inflammation, psoriasis, inflammatory bowel disease, and atherosclerosis (3–7).
Macrophage differentiation and function are governed by numerous cell-extrinsic factors that include chemokines, cytokines, and toll-like receptor ligands (8). For example, classically activated macrophages differentiate in response to proinflammatory cytokines such as IFN-γ and microbial stimuli including LPS and CpG. Their production of proinflammatory mediators and microbicidal compounds, such as nitric oxide and reactive oxygen species, is essential for the elimination of intracellular pathogens including Leishmania and Salmonella (9). In contrast, exposure to the Th2 cytokines IL-4 and IL-13 promotes the differentiation of alternatively activated macrophages (AAMacs) that are defined by the expression of a panel of signature genes including Arginase 1, chitinase-like molecules (Ym1/2 and AMCase), and resistin-like molecule (RELM) α (10–13). Although the recruitment of AAMacs is a characteristic feature of a wide range of inflammatory conditions associated with parasite infection, allergy, diabetes, and cancer (7, 14–17), their potential roles in influencing the development, severity, or resolution of inflammatory responses have remained controversial. For example, several beneficial functions for AAMacs have been proposed, which include enhancing host defense against parasite infection (14, 18), the amelioration of diabetes through the regulation of nutrient homeostasis (16), and promotion of tissue repair after injury (10, 19, 20). In contrast, tumor-associated AAMacs and those that are recruited in Th2 cytokine-mediated allergic responses have been implicated in the exacerbation of disease (7, 17, 21–23). The putative pleiotropic functions of AAMacs may relate to heterogeneity in expression of signature molecules such as Arginase 1, chitinase-like molecules, and RELM-α; however, to date there has been no systematic analysis of the roles of these molecules in the regulation of inflammatory responses.
In this study, we examined the functions of RELM-α in Th2 cytokine-mediated lung inflammation. RELM-α belongs to a family of small cysteine-rich secreted proteins that are conserved in mammals (24–26) and it exhibits a broad pattern of expression in hematopoietic and nonhematopoietic cells (11, 24–26). Elevated expression of RELM-α in mouse models of pulmonary inflammation (24, 27–29) and increased expression of the related human protein resistin in inflammatory diseases in patients (30) implicate a putative role in influencing innate and adaptive immune responses. However, previous studies have identified contrasting effects of RELM-α in regulating inflammation. Consistent with a role in promoting pulmonary inflammation, in vitro studies showed that recombinant RELM-α (rRELM-α) could drive proliferation and growth factor expression in lung fibroblast cell lines (31, 32). In contrast, rRELM-α was reported to antagonize the effects of nerve growth factor, a protein associated with the exacerbation of allergic pulmonary responses (33), suggesting that RELM-α may negatively regulate Th2 cytokine-mediated inflammation in the lung.
To investigate these paradoxical findings, we used mice deficient in RELM-α (Retnla−/−) in an in vivo model of Th2 cytokine-dependent pulmonary inflammation and fibrosis (19, 27). In response to challenge with eggs from the helminth parasite Schistosoma mansoni (Sm), Retnla−/− mice exhibited more severe pulmonary inflammation and exacerbated egg-induced granuloma formation associated with significantly elevated expression of Th2 cytokines and Th2 cytokine-responsive genes compared with WT mice. In Th2 cell differentiation assays, rRELM-α could bind to macrophages and effector CD4+ Th2 cells and specifically inhibited expression of Th2 cytokines. This suppressive effect was dependent on Bruton's tyrosine kinase (BTK) signaling. Conversely, Retnla−/− AAMacs promoted exaggerated antigen-specific Th2 cytokine production compared with WT AAMacs, identifying a role for endogenous RELM-α in regulating the expression of Th2 cytokines. Collectively, these studies reveal a previously unrecognized role for AAMac-derived RELM-α in limiting Th2 cytokine-dependent inflammatory responses in the lung through immunoregulatory effects on CD4+ T cell responses.
RESULTS
Generation and characterization of Retnla−/− mice
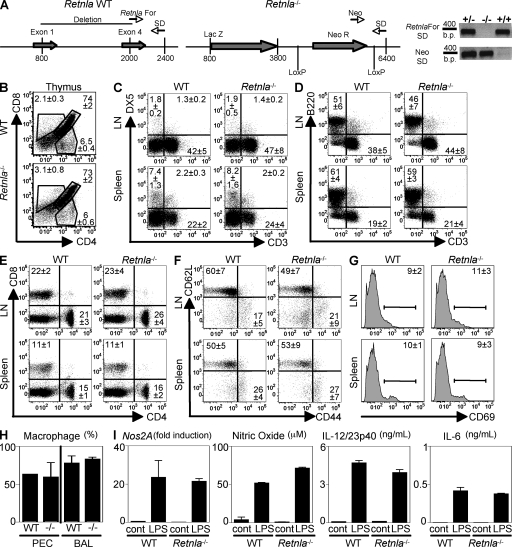
Retnla−/− mice were generated by homologous recombination where all four exons of RELM-α were replaced by a lacZ reporter gene and a neomycin selection cassette (Fig. 1 A). Confirmation of the deletion was determined by genotyping PCR analysis (Fig. 1 A). Naive Retnla−/− mice were healthy and fertile and did not exhibit detectable impairment in immune cell development or composition of lymphoid compartments. This included equivalent frequencies of CD4+, CD8+, and CD4+CD8+ cells in the thymi of WT and Retnla−/− mice (Fig. 1 B). Natural killer cells (Fig. 1 C, DX5+), B cells (Fig. 1 D, B220+), T cells (Fig. 1 D, CD3+), CD4+ (Fig. 1 E), and CD8+ (Fig. 1 E) cells were also present in comparable frequencies in WT and Retnla−/− mice in the peripheral LNs and spleen. CD4+ T cells from naive WT and Retnla−/− mice also exhibited equivalent basal expression of CD62L, CD44, and CD69 (Fig. 1, F and G), indicating no aberrant CD4+ T cell activation. Combined analysis of four WT and four Retnla−/− mice revealed that there were no significant differences in all the compartments (Fig. S1). Finally, RELM-α deficiency did not affect resident macrophage populations, as naive Retnla−/− mice exhibited equivalent proportions of macrophages recovered from the peritoneal cavity (PEC) and bronchoalveolar lavage (BAL) fluid compared with WT counterparts (Fig. 1 H). Macrophages from WT and Retnla−/− mice also exhibited equivalent LPS-induced expression of inducible nitric oxide synthase (Nos2A) messenger RNA (mRNA) and production of nitric oxide, IL-12/23p40, and IL-6 (Fig. 1 I), demonstrating that there was no inherent defect in the innate activation of Retnla−/− macrophages.
Figure 1.
Generation and characterization of Retnla−/− mice. (A) Schematic diagram of the Retnla locus in WT and Retnla−/− mice and primers used to confirm the genotypes by PCR. (B–G) Frequency of CD4, CD8, B lymphocytes, and NK cells (DX5+) in the thymi, spleen, and LN of naive WT and Retnla−/− mice, and CD4+ T cell surface expression of CD62L, CD44, and CD69. (H) Frequency of resident macrophages from the PEC or the BAL of naive WT and Retnla−/− mice. Results (± SEM of four animals) are representative of two independent experiments of a total number of 9–10 individual animals per group. (I) Real-time PCR analysis of Nos2A expression, and secretion of nitric oxide, IL-12/23p40, and IL-6 by control or LPS-stimulated WT and Retnla−/− macrophages. Results (mean ± SEM) are representative of two independent experiments.
RELM-α is expressed by mannose receptor+ AAMacs in Sm egg-induced pulmonary granulomas
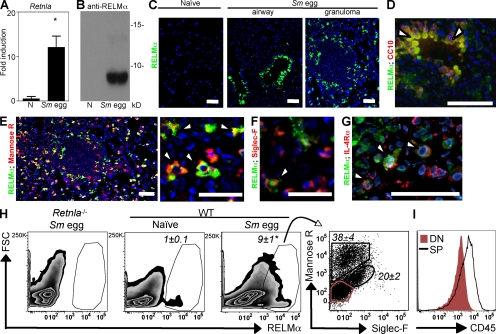
Given previous reports of elevated expression of AAMac-derived RELM-α in Th2 cytokine-associated pulmonary inflammation (12, 27, 28, 33), we used a model of Th2 cytokine-dependent inflammation in the lung to investigate the potential functions of RELM-α in the pathogenesis of pulmonary inflammation. After i.p. sensitization and i.v. challenge, Sm eggs are transported into the lung tissue via the pulmonary arteries where they become trapped within the lung parenchyma by the formation of Th2 cytokine-dependent granulomas composed of AAMacs, eosinophils, and lymphocytes (19, 27). Quantitative real-time PCR of whole lung tissue isolated from C57BL/6 mice at day 8 after Sm egg challenge revealed a 10-fold induction of RELM-α (Retnla) mRNA expression levels in comparison with lung tissue from naive mice (Fig. 2 A). Sm egg challenge also induced robust RELM-α protein secretion, which was detected by Western blot analysis of the BAL fluid (Fig. 2 B).
Figure 2.
RELM-α is expressed after Sm egg challenge. (A) Lung Retnla expression in naive and Sm egg-challenged C57BL/6 mice. *, P <0.05. (B) Western blot analysis of the BAL fluid from naive or day 8 Sm egg-challenged mice. (C) IF staining of lung sections for RELM-α (green) and DAPI (blue). (D–G) Costaining with CC10 (D; red), the mannose receptor (E; red), siglec-F (F; red), or IL-4Rα (G; red). Arrowheads indicate costaining of antibodies with RELM-α. (H) Flow cytometry plots (forward scatter [FSC] vs. RELM-α) of lung cells from naive and Sm egg-challenged mice, and mannose receptor and siglec-F expression by RELM-α–positive cells. *, P <0.05. (I) CD45 expression by the mannose receptor−siglec-F− population (DN; red) and mannose receptor+ or siglec-F+ cells (single positive [SP]). Bars, 50 µm. Results (± SEM of three to four animals) are representative of two to three independent experiments (A–H; n = 9–12) and one experiment (I; n = 4).
To investigate the cellular sources of RELM-α, immunofluorescent (IF) staining with an anti–RELM-α antibody was performed on lung sections from naive and Sm egg-challenged WT mice. In response to Sm egg challenge, RELM-α protein was produced by airway epithelial cells (Fig. 2 C, middle) and in cells recruited into the egg-induced granuloma (Fig. 2 C, right). Costaining for CC10, a marker for secretory Clara cells which line the airway epithelium, confirmed RELM-α expression by Clara cells (Fig. 2 D). In the pulmonary granuloma, costaining with the mannose receptor (Fig. 2 E, red) and siglec-F (Fig. 2 F, red) revealed that mannose receptor+ AAMacs and siglec-F+ eosinophils were the cellular sources of RELM-α. Consistent with studies demonstrating that Retnla gene expression is responsive to Th2 cytokines (12, 20, 24, 27), costaining for IL-4Rα revealed RELM-α+IL-4Rα+ cells within the granuloma (Fig. 2 G, red). To examine whether the mannose receptor+ cells observed in the granulomas were macrophages, we dissociated cells from the lungs of Sm egg-challenged mice and stained for the mannose receptor, for F4/80, and for CD11c, a marker which is elevated in lung macrophages (34, 35). Although there was low surface expression of F4/80 in cells from the lung (Fig. S2 A), which is consistent with previous studies (36), the majority of CD11c+ cells also costained with the mannose receptor (Fig. S2 B), suggesting that the mannose receptor+CD11c+ cells are AAMacs.
To quantify the relative frequencies of RELM-α+ AAMacs and eosinophils, flow cytometric analysis of cells dissociated from lung tissue of naive and Sm egg-challenged mice was performed. Intracellular staining for RELM-α in cells from dissociated lungs revealed a ninefold increase in the frequency of RELM-α+ cells after Sm egg challenge (Fig. 2 H). Of the RELM-α+ cells, 38 ± 4% were mannose receptor+ AAMacs, whereas 20 ± 2% were siglec-F+ eosinophils (Fig. 2 H, right). Therefore, the frequency of RELM-α+ AAMacs was twofold higher than that of eosinophils. Quantification of the total number of RELM-α+ cells within the lung confirmed elevated numbers of RELM-α+ cells (Fig. S2 C), and differential staining for AAMacs and eosinophils demonstrated increased RELM-α+ AAMacs compared with RELM-α+ eosinophils (Fig. S2 D). Staining for CD45 revealed that the remaining mannose receptor−siglec-F− cells (double negative [DN]) were not hematopoietically derived, as they did not express CD45 (Fig. 2 I). Together with the IF staining, which revealed RELM-α expression by CC10+ cells, this DN cell population likely represents lung-resident Clara cells. Collectively, these results demonstrate that after Sm egg challenge in the lung, the dominant cellular sources of RELM-α are resident airway epithelial cells and infiltrating AAMacs within the egg-induced granulomas.
Retnla−/− mice develop exacerbated pulmonary inflammation and fibrosis after exposure to Sm eggs
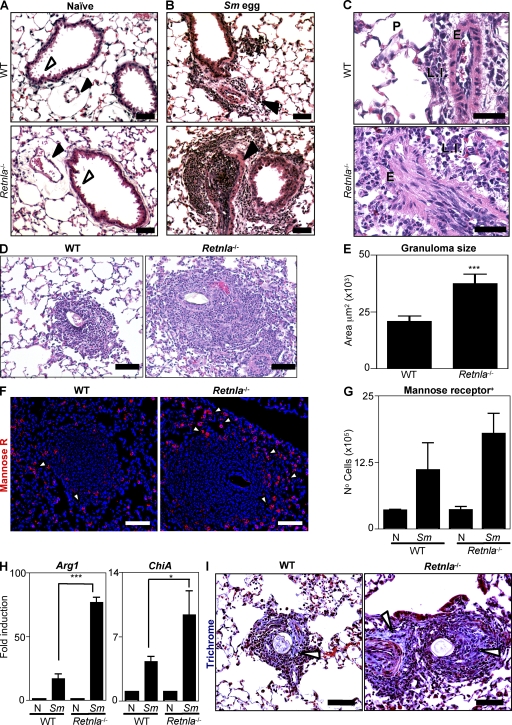
RELM-α+ AAMacs have been implicated in both the promotion and inhibition of inflammatory responses (7, 18). To investigate whether RELM-α could influence Sm egg-induced pulmonary inflammation and granuloma formation, WT and Retnla−/− mice were challenged with Sm eggs. Analysis of hematoxylin and eosin (H&E)–stained lung sections isolated from naive WT and Retnla−/− mice revealed no pathological change in the absence of RELM-α in steady-state conditions (Fig. 3 A). This included comparable architecture of the lung parenchyma in WT and Retnla−/− mice, with equivalent alveolar spaces, endothelial vessel formation (Fig. 3 A, black arrowheads), and bronchiole development (Fig. 3 A, white arrowheads). At day 8 after Sm egg challenge, WT mice exhibited modest airway epithelial cell hyperplasia and increased arterial inflammation characterized by thickening of the endothelium (Fig. 3 B, top, arrowhead). Higher power analysis revealed a perivascular infiltration of lymphocytes, eosinophils, and activated macrophages (Fig. 3 C, top). Strikingly, in comparison with WT mice, Sm egg-challenged Retnla−/− mice exhibited exacerbated pulmonary inflammation (Fig. 3 B, bottom). This included severe arterial inflammation (Fig. 3 B, bottom, arrowhead), characterized by increased cellularity within the arterial lumen, hypertrophy of the endothelium, and the accumulation of lymphocytes, eosinophils, and activated macrophages around the vessel wall (Fig. 3 C, bottom).
Figure 3.
Retnla−/− mice exhibit exacerbated Sm egg-induced pulmonary inflammation. (A–C) H&E-stained lung sections from naive and Sm egg-challenged WT and Retnla−/− mice. Black arrowheads, pulmonary arteries; white arrowheads, bronchiole development. P, lung parenchyma; LI, leukocyte infiltrate; E, endothelium. (D) H&E-stained sections of granulomas from Sm egg-challenged WT and Retnla−/− mice. (E) Size of granulomas surrounding Sm eggs from WT and Retnla−/− mice; n = 30 granulomas from a total of three mice per group. Results are representative of at least two independent experiments with three to four mice per group (n = 6–8 animals per genotype). (F) IF staining of granulomas for the mannose receptor (red) and DAPI (blue). White arrowheads depict mannose receptor+ cells. (G) Flow cytometric quantification of mannose receptor+ cells from dissociated lung tissue. (H) Lung Arg1 and ChiA expression in naive and Sm egg-challenged WT and Retnla−/− mice. ***, P <0.001; *, P <0.05. (I) Masson's trichrome-stained granulomas from WT and Retnla−/− mice. White arrowheads, collagen stain. Bars, 50 µm. Results (mean ± SEM of three to four mice) are representative of two to three independent experiments (n = 6–10 per group).
Histological examination of the egg-induced pulmonary granulomas in WT mice revealed the formation of compact granulomas around the egg, composed of red blood cells, activated macrophages, lymphoid cells, granulocytes, and multinucleated giant cells (Fig. 3 D, left). In contrast, Sm egg-challenged Retnla−/− mice exhibited more severe inflammation surrounding the egg (Fig. 3 D, right), which included a significant increase in the mean area of inflammation surrounding the granuloma (Fig. 3 E). Collectively these data indicate that RELM-α deficiency results in exacerbated Sm egg-induced pulmonary inflammation.
AAMac responses are enhanced in Sm egg-challenged Retnla−/− mice
Given that RELM-α is a signature gene of AAMacs, we hypothesized that RELM-α deficiency may affect expression of other AAMac-derived genes or the recruitment or function of AAMacs. IF staining of mannose receptor+ cells in the granulomas from Sm egg-challenged WT and Retnla−/− mice indicated that there was no impairment in the recruitment of mannose receptor+ AAMacs in the absence of RELM-α (Fig. 3 F, red), and quantification of the mannose receptor+ cells by flow cytometric analysis of dissociated lung tissue revealed equivalent frequencies of mannose receptor+ AAMacs in the Sm egg-challenged WT and Retnla−/− mice (Fig. 3 G). To examine AAMac responses after Sm egg challenge, lungs from naive and Sm egg-challenged WT and Retnla−/− mice were analyzed for expression of the AAMac genes Arg1 (Arginase 1) and ChiA (acidic mammalian chitinase) by real-time PCR. In WT mice, Sm egg challenge resulted in a 17-fold induction of Arg1 over naive controls (Fig. 3 H). In contrast, there was a >70-fold induction of Arg1 in Sm egg-challenged Retnla−/− mice. Furthermore, although Sm egg challenge of WT mice resulted in a fourfold induction of ChiA, we observed a ninefold induction of ChiA in Retnla−/− mice (Fig. 3 H). The increased recruitment of mannose receptor+ cells into the granulomas, coupled with significant increases in levels of Arg1 and ChiA mRNA in Retnla−/− mice, indicates that the AAMac responses are elevated in the absence of RELM-α. Given that one proposed function of AAMacs is to promote fibrosis (19, 37), in part through mediating collagen synthesis, we tested the hypothesis that Retnla−/− mice may exhibit differences in collagen deposition in the Sm egg-induced granulomas. Consistent with elevated AAMac responses, Masson's trichrome staining of the lung sections revealed that Retnla−/− mice exhibited elevated collagen deposition in the egg-induced granuloma in comparison with WT mice (Fig. 3 I, arrowheads). Together, these data demonstrate that Sm egg-induced AAMac responses were increased in the absence of RELM-α.
Retnla−/− mice exhibit elevated Sm egg-induced CD4+ Th2 cell responses
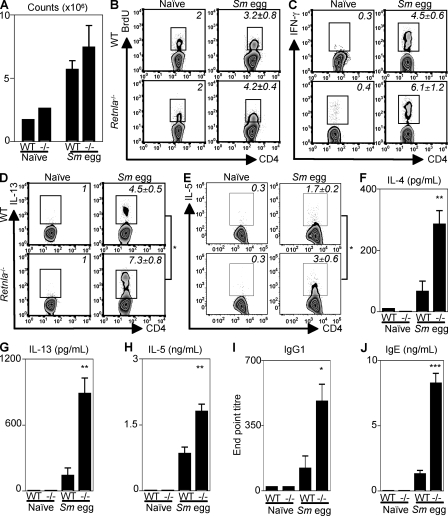
Th2 cytokines play an essential role in Sm egg-induced pulmonary granuloma formation (19). Given the exacerbated Sm egg-induced pulmonary inflammation and granuloma formation in the absence of RELM-α, we sought to determine whether Retnla−/− mice exhibited dysregulated CD4+ T cell responses in comparison with WT mice after Sm egg challenge. In comparison with naive controls, Sm egg-challenged WT and Retnla−/− mice showed equivalent expansion of the draining mediastinal LNs (Fig. 4 A), similar frequencies of lymphocytes in the BAL and lung tissue (Fig. S3, A and B), and an equivalent increase in the frequency of proliferating LN CD4+ T cells (Fig. 4 B) at day 8 after challenge. Furthermore, ex vivo analysis of cytokine production by mediastinal LN cells revealed that Sm egg challenge induced similar up-regulation in the frequency of IFN-γ+CD4+ T cells in WT and Retnla−/− mice (Fig. 4 C). However, although Sm egg-challenged WT mice exhibited a fourfold increase in the frequency of IL-13+ CD4+ T cells in comparison with naive control mice (Fig. 4 D, top), the frequency of IL-13+CD4+ T cells isolated from the draining LN of Sm egg-challenged Retnla−/− mice was increased ninefold over naive mice (Fig. 4 D, bottom). In addition to the elevated frequency of IL-13+CD4+ T cells, Sm egg-challenged Retnla−/− mice exhibited a significantly increased frequency of IL-5+CD4+ T cells in comparison with WT mice (Fig. 4 E), and they exhibited increased levels of Il4 mRNA (Fig. S3 C). Although there were equivalent frequencies of eosinophils in the BAL and lungs of Sm egg-challenged WT and Retnla−/− mice (Fig. S3, A and B), the increased frequency in IL-5+CD4+ T cells in the egg-challenged Retnla−/− mice correlated with elevated Sm egg-induced expression of Ccl11 (eotaxin 1) and Ccl24 (eotaxin 2) mRNA in the absence of RELM-α (Fig. S3 D).
Figure 4.
Exacerbated expression of Th2 cytokines in Sm egg-challenged Retnla−/− mice. (A) Cell counts from the draining LN of naive or Sm egg-challenged WT and Retnla−/− mice. (B) Flow cytometric analysis of CD4+ T cell incorporation of BrdU. (C–E) Ex vivo flow cytometric analysis of CD4+ T cell–derived IFN-γ (C), IL-13 (D), and IL-5 (E). (F–H) Antigen-specific secretion of IL-4 (F), IL-13 (G), and IL-5 (H) by draining LN cells. (I) Antigen-specific IgG1 antibody titers. (J) Serum IgE levels. ***, P < 0.001; **, P < 0.01; *, P < 0.05. Results (mean ± SEM of two to four mice per group) are representative of three independent experiments (naive, n = 6; Sm egg-challenged, n = 11).
To measure parasite-specific Th2 cytokine production, draining LN cells isolated from naive and Sm egg-challenged WT and Retnla−/− mice were restimulated for 48 h with Sm egg antigen. Strikingly, LN cells from Sm egg-challenged Retnla−/− mice secreted significantly higher levels of antigen-specific IL-4 (Fig. 4 F), IL-13 (Fig. 4 G), and IL-5 (Fig. 4 H) than cells isolated from Sm egg-challenged WT mice. This included an over fivefold increase in IL-4 and IL-13 production and a twofold increase in IL-5 production. Consistent with the elevated Th2 cytokine response, Sm egg-challenged Retnla−/− mice also exhibited increased antigen-specific IgG1 antibody titers (Fig. 4 I) and significantly elevated total IgE levels compared with WT mice (Fig. 4 J). Collectively, these results suggest that the production of RELM-α after Sm egg challenge may down-regulate Th2 cytokine responses.
RELM-α inhibits production of Th2 cytokines
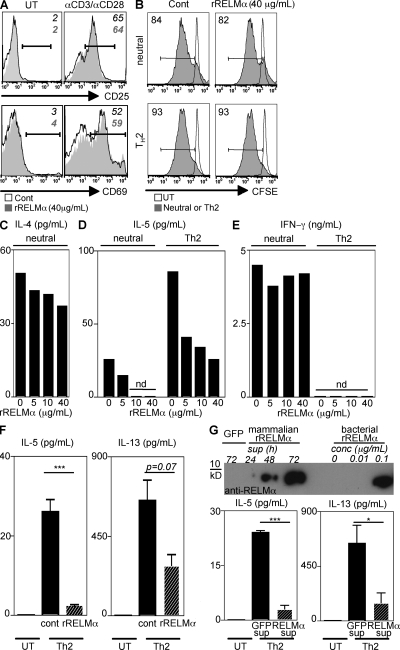
Given that the deletion of RELM-α resulted in enhanced expression of Th2 cytokines and exacerbated lung inflammation and fibrosis after Sm egg challenge, we sought to test the hypothesis that RELM-α may act directly on immune cells to modulate Th2 cell differentiation by using an in vitro CD4+ T cell differentiation assay. CFSE-labeled splenocytes from naive C57BL/6 mice were unstimulated or polyclonally stimulated with α-CD3/α-CD28 under neutral conditions or conditions permissive for Th2 cell differentiation in the absence or presence of recombinant RELM-α (rRELM-α). Treatment with rRELM-α had no effect on T cell activation, as assessed by the surface expression of CD25 or CD69 on CD4+ T cells (Fig. 5 A). Additionally, rRELM-α had no effect on T cell proliferation under neutral or Th2-permissive conditions, as examined by CFSE dilution 3 d after stimulation (Fig. 5 B). However, rRELM-α treatment resulted in a dose-dependent decrease in IL-4 and IL-5 production by splenocytes activated both under neutral and Th2-polarizing conditions (Fig. 5, C and D). Consistent with the in vivo findings with Sm egg challenge of WT and Retnla−/− mice (Fig. 4 C), the inhibitory effect of RELM-α was specific to Th2 cytokines, as there was no difference in IFN-γ production in response to rRELM-α treatment (Fig. 5 E). The effect of RELM-α in modulating expression of Th2 cytokines, but not IFN-γ, was strikingly different from our previous study on the related protein RELM-β, which promoted IFN-γ production (38). These findings indicate pleiotropy in the functions of the RELM protein family. The suppressive effect of rRELM-α was not caused by LPS contamination of the bacterially derived rRELM-α because rRELM-α–induced inhibition of IL-5 and IL-13 was observed in splenocytes from TLR-4 hyporesponsive mice (C3H/HeJ) (Fig. 5 F). In addition, rRELM-α derived from a mammalian expression system also inhibited expression of Th2 cytokines. Transfection of 293T cells with a control (enhanced GFP [eGFP]) or a Retnla-expressing plasmid under the control of the CMV promoter was used for the generation of supernatant enriched for mammalian-derived rRELM-α. No band was detected in the 72-h supernatant from 293T cells transfected with the control eGFP plasmid, indicating specificity in Retnla expression and detection by Western blotting. In contrast, analysis of supernatants from Retnla-transfected cells revealed detectable RELM-α by 48 h and maximal expression at 72 h, with an estimated concentration of 100 ng/ml (Fig. 5 G, top). Addition of the mammalian-derived rRELM-α resulted in the significant reduction in IL-5 and IL-13 production by splenocytes stimulated with α-CD3/α-CD28 under Th2-permissive conditions in comparison with control supernatant (Fig. 5 G, bottom). Collectively with the in vivo results demonstrating elevated Th2 cytokine-induced lung inflammation in the absence of RELM-α, these data indicate that RELM-α can modulate Th2 cytokine production through direct effects on hematopoietic cells.
Figure 5.
RELM-α negatively regulates Th2 cytokine production by α-CD3/α-CD28–stimulated splenocytes. (A) Expression of CD25 and CD69 by CD4+ T cells from untreated (UT) or α-CD3/α-CD28–stimulated splenocytes in the presence of rRELM-α (filled histogram). (B) Frequency of CFSE-dim CD4+ T cells from untreated splenocytes or splenocytes stimulated with α-CD3/α-CD28 (filled histogram) under neutral or Th2-permissive conditions in the presence rRELM-α. (C–E) Supernatants were analyzed for IL-4 (C), IL-5 (D), and IFN-γ (E) secretion. (F) C3H/HeJ splenocytes were untreated or stimulated with α-CD3/α-CD28 under Th2-permissive conditions in the presence of 5 µg/ml rRELM-α, followed by measurement of IL-5 and IL-13 secretion. (G) RELM-α Western blotting of supernatants (sup) from 293T cells transfected with a control eGFP plasmid or the Retnla plasmid. WT splenocytes were untreated or stimulated with α-CD3/α-CD28 under Th2-permissive conditions in the presence of 72-h sup at a 1:1 ratio followed by measurement of IL-5 and IL-13 secretion. Results (mean ± SEM; ***, P < 0.001; *, P < 0.05) are representative of two to three independent experiments.
Retnla−/− AAMacs promote Th2 cytokine production by CD4+ T cells
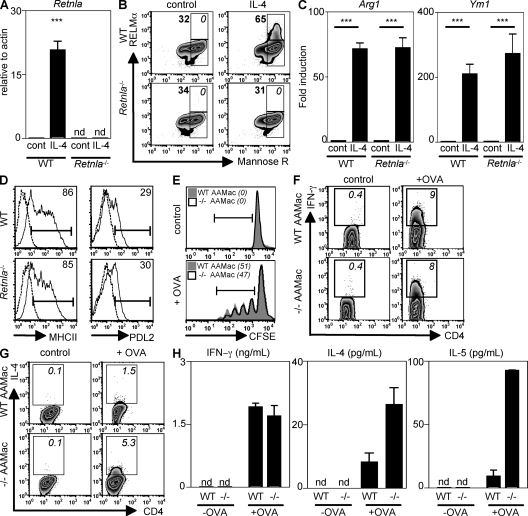
Because AAMacs are a source of RELM-α in the lung and rRELM-α can inhibit Th2 cytokine production, we sought to test whether AAMac-derived RELM-α could influence T cell function in an in vitro T cell proliferation and differentiation assay. WT and Retnla−/− AAMacs were generated by IL-4 treatment of BM-derived macrophages (BMMacs) from WT and Retnla−/− mice. Alternative activation of WT BMMacs resulted in a 20-fold induction of Retnla mRNA levels in contrast with untreated or IL-4–treated Retnla−/− BMMacs, where there was no detectable Retnla mRNA (Fig. 6 A). Costaining for the mannose receptor and RELM-α revealed that WT mannose receptor+ AAMacs exhibited elevated RELM-α protein expression, both in the frequency of RELM-α+ cells and the mean fluorescent intensity, in contrast to Retnla−/− macrophages where there was no detectable RELM-α protein (Fig. 6 B). To investigate if RELM-α deficiency altered the expression of other AAMac signature genes, untreated or IL-4–treated WT and Retnla−/− BMMacs were examined for expression of Arg1 and Ym1 mRNA by real-time PCR. WT and Retnla−/− AAMacs showed comparable up-regulation of Arg1 and Ym1 (Fig. 6 C), which is consistent with no defect in AAMac differentiation in Retnla−/− mice observed in vivo (Fig. 3 F). Further, there was no difference between WT and Retnla−/− AAMacs in the surface expression of MHC class II and PDL2 (Fig. 6 D), both of which are up-regulated in AAMacs in response to IL-4 (39, 40).
Figure 6.
Retnla−/− AAMacs promote exaggerated production of Th2 cytokines by CD4+ T cells. (A and B) Retnla expression by WT or Retnla−/− UT (cont) or AAMacs was measured by real-time PCR (fold induction over actin; A) and intracellular flow cytometry (B). (C) Arg1 and Ym1 expression (fold induction over cont) was measured. ***, P <0.001. (D) Surface expression of MHC class II and PDL2 by WT and Retnla−/− cont (dashed line) or AAMac (solid line). (E–H) Control or OVA-pulsed WT or Retnla−/− AAMacs were cocultured with CFSE-labeled OVA-specific CD4+ T cells. (E) Frequency of CFSE-dim CD4+ T cells (italics). (F and G) Frequency of IFN-γ+ and IL-4+ CFSE-dim CD4+ T cells (italics). (H) Supernatants were recovered for measurement of IFN-γ, IL-4, and IL-5 secretion. Results (mean ± SEM) are representative of two independent experiments.
To address the role of AAMac-derived RELM-α in modulating antigen-specific T cell responses, WT and Retnla−/− AAMacs were left untreated or pulsed overnight with OVA protein and cocultured for 4 d with CFSE-labeled OVA-specific CD4+ T cells. When unpulsed, WT and Retnla−/− AAMacs did not induce T cell proliferation (Fig. 6 E, top). There was equivalent antigen-specific T cell proliferation when OVA-specific CD4+ T cells were cocultured with OVA-pulsed WT and Retnla−/− AAMacs (Fig. 6 E, bottom). WT and Retnla−/− AAMacs also induced equivalent frequencies of antigen-specific CD4+ T cells expressing IFN-γ (Fig. 6 F). Strikingly, OVA-pulsed Retnla−/− AAMacs induced a threefold increase in the frequency of IL-4+CD4+ T cells in comparison with WT AAMacs (Fig. 6 G). The ability of Retnla−/− AAMacs to augment antigen-specific Th2 cell differentiation was confirmed by ELISA. Although equivalent IFN-γ production was observed in cultures with WT and Retnla−/− AAMacs, there was a threefold increase in IL-4 secretion and a 10-fold increase in IL-5 secretion when OTII T cells were cocultured with OVA-pulsed Retnla−/− AAMacs in comparison with WT AAMacs (Fig. 6 H). Collectively, these data demonstrate that the absence of AAMac-derived RELM-α results in exaggerated production of antigen-specific Th2 cytokines by CD4+ T cells.
RELM-α binds CD11c+ DCs, F4/80+ macrophages, and CD4+ Th2 effector cells
Given that rRELM-α treatment of whole splenocyte cultures suppressed Th2 cytokine production and Retnla−/− AAMacs promoted exaggerated antigen-specific CD4+ Th2 cell responses, we sought to determine if the immunomodulatory effects of RELM-α were through direct effects on macrophages and/or CD4+ T cells. rRELM-α treatment of purified CD4+ T cells activated with αCD3/αCD28 under Th2-permissive conditions did not affect cell proliferation or cytokine production (Fig. S4 A). Furthermore, pretreatment of BMMacs with rRELM-α did not alter their ability to stimulate antigen-specific CD4+ T cell proliferation or Th2 cytokine production (Fig. S4 B). These data suggest that the immunomodulatory effects of RELM-α are dependent on its combined action on both macrophages and CD4+ T cells.
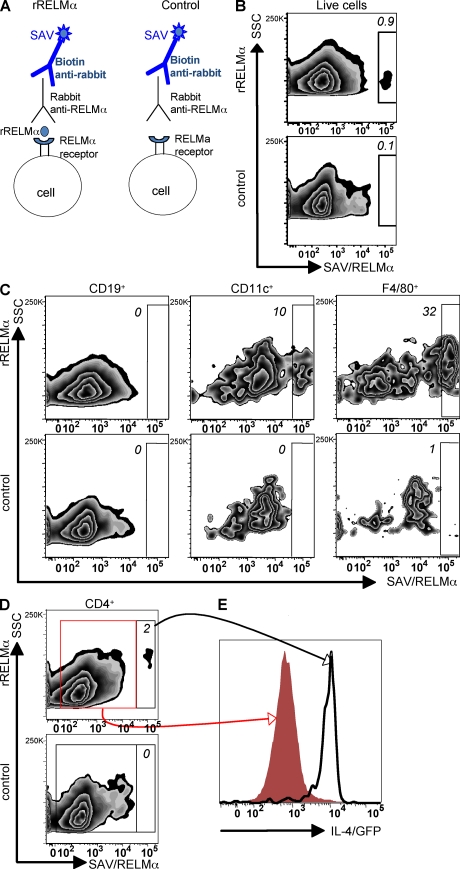
To test which cells bind RELM-α and may be sensitive to its regulation, we designed a RELM-α capture assay. Splenocytes from OVA-specific IL-4 reporter mice (DO11-10/4get) that were stimulated for 3 d with OVA protein were incubated with rRELM-α, followed by detection of the RELM-α–bound cells with an anti–RELM-α antibody (Fig. 7 A). In comparison with the control incubation with no recombinant RELM-α, there was a small but distinct population of cells that exhibited surface-bound RELM-α (Fig. 7 B). Additional controls, which included incubation with the homologous protein rRELM-β or the use of a rabbit isotype control for anti-RELM-α, revealed little or no positive staining, confirming specificity of the RELM-α binding assay (Fig. S5). Surface staining for markers of B cells (CD19+), DCs (CD11c+F4/80−), and macrophages (F4/80+CD11c−) revealed RELM-α binding to DCs and macrophages but not B cells (Fig. 7 C). This observation is indicative of the capacity of RELM-α to influence antigen-presenting cell populations. Given that RELM-α acts to suppress CD4+ T cell–derived Th2 cytokines, we sought to determine if rRELM-α bound directly to CD4+ T cells. The RELM-α capture assay revealed the presence of a distinct population of RELM-α–bound CD4+ T cells that was absent from the control assay (Fig. 7 D). Comparison of the IL-4/GFP expression of RELM-α–unbound CD4+ T cells (Fig. 7 E, red histogram) with RELM-α–bound CD4+ T cells (Fig. 7 E, black histogram) revealed that RELM-α preferentially bound effector CD4+ Th2 cells with 100% of the RELM-α–bound CD4+ cells expressing IL-4/GFP. Collectively, these data implicate that the mechanism by which RELM-α suppresses Th2 cytokines may be dependent on binding and interactions with DCs, macrophages, and effector CD4+ Th2 cells.
Figure 7.
RELM-α binds to effector CD4+ Th2 cells, DCs, and F4/80+ macrophages. DO11-10/4get splenocytes were incubated with OVA protein, followed by recovery of cells at day 3 to determine which cell types bind RELM-α. (A) Schematic diagram of the RELM-α capture assay. (B) Flow cytometry plot (side scatter [SSC] vs. RELM-α/streptavidin [SAV]) showing frequency of live cells that bind rRELM-α. (C) Frequency of F4/80+ macrophages (right), CD11c+ DCs (middle), and CD19+ B cells (left) that bind RELM-α. (D) Frequency of CD4+ T cells that bind RELM-α (top). (E) IL-4/GFP expression of RELM-α–bound CD4+ T cells (black line) and RELM-α–unbound CD4+ T cells (red histogram). Results are representative of three independent experiments.
RELM-α–induced suppression of Th2 cytokine production is dependent on BTK signaling
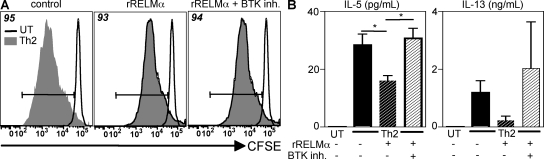
Given that previous studies have demonstrated that RELM-α can signal through the BTK (41), we sought to determine if the inhibition of Th2 cytokine production by RELM-α was dependent on BTK signaling by using a BTK inhibitor (LFM-A13) (41). Treatment of CFSE-labeled splenocytes that were activated with α-CD3/α-CD28 under Th2-permissive conditions with a BTK inhibitor did not affect CD4+ T cell proliferation or Th2 cytokine production (Fig. S6). Furthermore, BTK inhibition of rRELM-α–treated splenocyte cultures did not alter CD4+ T cell proliferation (Fig. 8 A). However, although rRELM-α inhibited the production of IL-5 and IL-13, as previously demonstrated (Fig. 5), this suppressive effect was abrogated in the presence of the BTK inhibitor (Fig. 8 B). Because LFM-A13 can also inhibit Jak2 kinase activity (42), it is possible that the effect of RELM-α may be partly dependent on Jak2 kinase signaling. Nevertheless, given the previous evidence showing that BTK is a binding partner for RELM-α (41), these results implicate BTK signaling as one mechanism by which RELM-α down-regulates Th2 cytokine production. Collectively with the exacerbated Th2 cytokine responses in Sm egg-challenged Retnla−/− mice in vivo, these in vitro studies indicate that one immunoregulatory mechanism of action of AAMac-derived RELM-α involves the direct action of RELM-α on macrophages and CD4+ Th2 effector cells, resulting in the BTK-dependent down-regulation of Th2 cytokine production.
Figure 8.
Suppression of Th2 cytokine production by RELM-α is dependent on BTK signaling. CFSE-labeled splenocytes were left untreated (UT) or stimulated with α-CD3/α-CD28 under Th2-permissive conditions, with or without (+/−) 5 µg/ml rRELM-α, or with or without an inhibitor for BTK (BTK inh.). (A) Frequency of CD4+ T cell proliferation at day 4. (B) IL-5 and IL-13 secretion by cells from A. Results (mean ± SEM; *, P < 0.05) are representative of two independent experiments.
DISCUSSION
The alternative activation of macrophages has been observed in a wide range of disease settings including chronic pulmonary inflammation; however, the functions of AAMacs and AAMac-derived proteins in influencing disease development, progression, or resolution have remained controversial. In this study, we describe the generation of Retnla−/− mice and use a model of Th2 cytokine-mediated pulmonary disease to demonstrate a previously unrecognized immunoregulatory role for RELM-α in limiting Th2 cytokine-mediated inflammation in the lung. After Sm egg challenge, Retnla−/− mice exhibited elevated expression of Th2 cytokines and severe pulmonary inflammation compared with WT counterparts. Furthermore, in in vitro T cell differentiation assays, RELM-α could bind DCs, macrophages, and CD4+ T cells, and it inhibited the production of CD4+ T cell–derived Th2 cytokines in a BTK-dependent manner, suggesting that one mechanism through which AAMac-derived RELM-α inhibits excessive Th2 cytokine-mediated inflammation in vivo may be through the regulation of CD4+ T cell responses.
In addition to an innate role in pathogen killing, there is increasing evidence that macrophages are recruited to a variety of inflammatory settings where they might participate in the down-regulation of inflammation and the subsequent tissue repair process (3, 19, 43). In the lung, alveolar macrophages are proposed to be critical suppressors of excessive inflammatory responses, in part through the action of TGF-β (44, 45). Additionally, macrophages activated by toll-like receptor ligation and immune complexes can protect against lethal endotoxemia (46). In models of colitis, clodronate-mediated depletion of macrophages resulted in disease exacerbation (4), and adoptive transfer of macrophages from Sm-infected mice could limit intestinal inflammation (47).
Consistent with an antiinflammatory role for AAMacs, macrophage-specific deletion of the IL-4Rα in mice, which would prevent AAMac differentiation, resulted in lethal liver and intestinal inflammation after Sm infection (18). However, the molecular mechanisms through which AAMacs can regulate inflammatory responses in these diverse disease settings have remained poorly defined. In this paper, we propose a role for AAMacs in limiting excessive Th2 cytokine-induced pulmonary inflammation, in part through the production of RELM-α.
One mechanism by which AAMac-derived RELM-α limits the magnitude of the Sm egg-induced lung inflammatory response is through action on CD4+ T cells to specifically inhibit the production of Th2 cytokines. Given previous studies demonstrating that AAMacs are present in the LNs draining the site of inflammation (24, 27, 48), our in vitro studies showing that RELM-α+ AAMacs modulate CD4+ T cell function suggest that AAMacs present in the draining LNs may regulate CD4+ Th2 cell function in vivo. In addition to effects of AAMac-derived RELM-α in the LNs, Sm egg challenge induced RELM-α expression in the lung parenchyma by AAMacs, airway epithelial cells, and eosinophils. Although the critical role for epithelial cells at mucosal surfaces as physical barriers to the external environment is well recognized, recent reports demonstrated that epithelial cells are also important modulators of innate and adaptive immune responses at mucosal sites (49–51). In vitro studies demonstrated that human bronchial epithelial cells could inhibit T cell activation and proliferation (49), and eosinophils have been implicated in influencing effector Th2 cell recruitment during allergic pulmonary inflammation (52). Therefore, in addition to AAMac-mediated regulation, Th2 effector cells in the inflamed lung may be influenced by RELM-α derived from airway epithelial cells and infiltrating eosinophils. The generation of cell lineage-specific Retnla−/− mice will be the basis of future studies to elucidate the influence of RELM-α derived from distinct cell types on the development or progression of lung inflammation.
Munitz et al. (53) recently reported that RELM-α was expressed by eosinophils and intestinal epithelial cells, but not macrophages, in the dextran sodium sulfate–induced colitis and that RELM-α exacerbated intestinal inflammation, revealing complexity in RELM-α function at different sites of inflammation. We and others have recently demonstrated a function for the related protein RELM-β in promoting inflammation (38, 54, 55), indicating a dichotomy in the function of this protein family at different mucosal sites. Although i.v. challenge with Sm eggs resulted in the antigen-specific activation of CD4+ Th2 cells and the recruitment and differentiation of RELM-α+ AAMacs, the intestinal inflammation resulting from dextran sodium sulfate administration is caused by activation of innate immune cells in response to the breakdown of the intestinal barrier. Thus, whether RELM-α plays a beneficial or detrimental role in limiting inflammation is likely to be influenced by the immune stimulus and the tissue site.
In addition to exaggerated expression of Th2 cytokines, Sm egg challenge also induced severe pulmonary endothelial inflammation in the absence of RELM-α. Consistent with potential effects of RELM-α in influencing endothelial inflammation, Daley et al. (28) recently demonstrated that pulmonary arterial remodeling occurs as a direct consequence of CD4+ T cell–derived Th2 cytokines and is associated with the recruitment of RELM-α+ macrophages in a model of antigen-specific airway inflammation. Additionally, previous studies showed that RELM-α expression in the lung occurs in response to pulmonary stress, including hypoxia and injury (31, 32, 56), and rRELM-α induced the expression of angiogenic factors such as vascular endothelial growth factor and vascular endothelial cell adhesion molecule-1 (57, 58), leading to the hypothesis that RELM-α may mediate lung vascularization associated with pulmonary inflammation. Although vascularization is essential for leukocyte recruitment to the site of inflammation, it also participates in the subsequent healing process, allowing the recruitment and activation of fibroblasts that will mediate tissue repair and wound contraction. Our findings that Retnla−/− mice exhibit exacerbated Sm egg-induced arterial inflammation suggest that rather than promoting disease, the angiogenic properties of RELM-α are critical to mediate tissue repair and lung regeneration in response to Sm egg-induced lung injury. In addition to activation during an adaptive Th2 cytokine response, the recruitment of AAMacs also occurs as an immediate innate response to injury (20, 59). Thus, through the production of RELM-α, AAMacs may play a pivotal role in mediating tissue repair after injury.
Although the receptor for RELM-α is unknown at present, we have demonstrated that hematopoietic cells are responsive to RELM-α and that RELM-α can bind to DCs, macrophages, and CD4+ effector Th2 cells, suggesting that the immunomodulatory effects of RELM-α observed after Sm egg challenge may be through direct action on DCs, AAMacs, and CD4+ T cells. Furthermore, we show that the suppression of Th2 cytokine production mediated by RELM-α is dependent on BTK signaling, which is consistent with previous studies demonstrating that RELM-α can bind BTK (58). BTK, a non–receptor-associated tyrosine kinase of the Tec family, is a downstream target of the phosphatidylinositol 3-kinase (PI3K) pathway (60). Interestingly, mice deficient in the Src homology 2–containing inositol-5′phosphatase (SHIP), a negative regulator of the PI3K pathway, exhibited a similar phenotype to Sm egg-challenged Retnla−/− mice, including increased Th2 cytokine-associated lung fibrosis (21, 61), suggesting that through its modulation of BTK signaling, RELM-α may act in a similar manner to SHIP.
Comparative phylogenomic analysis of the RELM family has revealed the existence of two closely related human RELM proteins: resistin and RELM-β (24, 25, 33). Although mouse resistin expression is restricted to adipocytes (62), human resistin shows a similar expression pattern to that of mouse RELM-α and is expressed by leukocytes and myeloid cells recruited in inflammatory diseases including rheumatoid arthritis and diabetes (30, 63). Thus, the investigation of whether human resistin shares similar properties to RELM-α and can negatively regulate CD4+ Th2 cell responses warrants further investigation. In summary, the data presented in this paper identify a previously unrecognized role for AAMac-derived RELM-α in regulating CD4+ Th2 cell–mediated lung inflammation. Because activation and recruitment of AAMacs is a dominant feature in inflammatory responses associated with diseases as diverse as cancer, diabetes, and asthma, the manipulation of RELM-α expression may offer novel therapeutic strategies for the treatment of multiple inflammatory conditions.
MATERIALS AND METHODS
Mice.
WT C57BL/6 and C3H/HeJ were purchased from The Jackson Laboratory. OTII transgenic mice and DO11-10/4get transgenic mice were bred at the University of Pennsylvania. VelociGene technology was used to generate the Retnla−/− mice (64) (Fig. 1 A). For genotyping, a PCR-based method was used with primers 5′-TCATTCTCAGTATTGTTTTGCC-3′ and 5′-TTCTCCCTATGTTTCCTAACC-3′ (384 bp; −/− allele) or primers 5′-TTGCCTGTGGATCTTGGGAG-3′ and 5′-TTCTCCCTATGTTTCCTAACC-3′ (382 bp; WT allele). Heterozygous female offspring were backcrossed to the C57BL/6 background (n > 5 generations). Mice were maintained in a specific pathogen-free facility. Animal protocols were approved by the University of Pennsylvania Institutional Animal Care and Use Committee (IACUC), and all experiments were performed according to the guidelines of the University of Pennsylvania IACUC.
Analysis of immune cell compartments in Retnla−/− mice.
Spleens, thymi, and LN were isolated from 12–14-wk-old mice and single cell suspensions were prepared. Cells were analyzed by flow cytometry with antibodies to CD4, CD8, CD3, DX5, B220, CD62L, CD44, and CD69 (eBioscience) using the Canto Flow cytometer (BD), followed by analysis using FlowJo software (Tree Star, Inc.). Cytometry plots depict log10 fluorescence. Cytocentrifuge preparations of cells from the BAL and PEC were prepared and stained with H&E (Thermo Fisher Scientific).
Sm egg granuloma model.
WT C57BL/6 or Retnla−/− mice were immunized i.p. with 5,000 Sm eggs followed by i.v. challenge with 5,000 eggs 14 d later. Naive WT or Retnla−/− mice were used as controls. For measurement of BrdU incorporation, mice were injected with 0.8 mg BrdU (Sigma-Aldrich) in PBS at days −3 and −1 before sacrifice. At day 8 after challenge, animals were euthanized, followed by cardiac bleeding for serum recovery. BAL cells were recovered for flow cytometric analysis or cytocentrifuge preparations. Lung tissue was recovered for RNA extraction, or lung dissociation was performed to obtain single cell suspensions. For histology, lungs were inflated with 4% paraformaldehyde, embedded in paraffin, and 5-µm sections were used for staining with H&E, Masson's trichrome, and IF. Measurement of the egg-induced granulomas was performed as previously described (65). For IF, sections were stained with rabbit polyclonal anti–RELM-α (1:1,000; PeproTech), biotinylated anti-mannose receptor (1:100; AbD Serotec), anti-CC10 (1:400; Santa Cruz Biotechnology, Inc.), anti–siglec-F (1:100; BD), and anti–IL-4Rα (1:200; eBioscience), followed by incubation with the appropriate fluorochrome-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories) and counterstaining with DAPI (Invitrogen). Single cell suspensions from the lung were prepared by digestion for 30 min at 37°C with 1 mg/ml collagenase d (Roche) and 20 µg/ml DNase Type IV (Sigma-Aldrich) in complete media, followed by a Percoll gradient (Thermo Fisher Scientific). For intracellular RELM-α staining, cells were incubated with 10 µg/ml brefeldin A for 6 h at 37°C, fixed, permeabilized (Cytofix/Cytoperm kit; BD), and then stained with anti–RELM-α (1:200), followed by incubation with Alexa 488–conjugated anti–rabbit antibody (1:400; Invitrogen). Western blot analysis for RELM-α was performed by incubation with anti–RELM-α (1:1,000), and secondary staining was performed with peroxidase-conjugated anti–rabbit antibody (1:2,000; Jackson ImmunoResearch Laboratories).
Analysis of lymphocyte responses.
Draining mediastinal LN cells were stimulated ex vivo by incubation for 4 h with 50 ng/ml PMA, 750 ng/ml ionomycin, and 10 µg/ml Brefeldin A (all obtained from Sigma-Aldrich), followed by surface staining for CD4 and intracellular staining for IFN-γ, IL-5, and IL-13 (eBioscience). LN cells were plated at 1.5 million cells/well in complete medium alone or with 20 µg/ml of soluble Sm egg antigen. Supernatants were assayed for IL-4, IL-5, and IL-13 using standard sandwich ELISA protocols. Serum dilutions were assayed by ELISA for total IgE using OptEIA IgE kit (BD) or for antigen-specific IgG1 using Sm egg antigen–coated plates (5 µg/ml). For in vitro assays, single cell suspensions from peripheral LN and spleen were CFSE labeled (5 µg/ml; Invitrogen) and cultured with 1 µg/ml each of α-CD3/α-CD28 (eBioscience) alone or in the presence of 1–40 µg/ml rRELM-α (PeproTech). Th2-permissive conditions were the following: 40 ng/ml rIL-4 (R&D Systems), 50 µg/ml of anti–IL-12 (C17.8), and 50 µg/ml of anti–IFN-γ (XMG 1.2). Cells were recovered at day 1 for surface expression analysis of CD25 and CD69 on CD4+ T cells. Supernatants were recovered at day 4 for cytokine measurement by ELISA. 25 µM of the inhibitor LFM-A13 (EMD) was used to inhibit BTK signaling. Where indicated, CD4+ T cells were purified as described previously (65) and stimulated with plate-bound α-CD3/α-CD28 under Th2-permissive conditions. For macrophage T cell coculture assays, BMMacs were generated as described previously (50, 65) and cultured overnight with medium alone or 500 µg/ml OVA protein (Worthington Biochemical Corporation) in the presence or absence of 40 ng/ml IL-4 or 5 µg/ml rRELM-α, followed by two washes and 4 d of coculture with CFSE-labeled OTII CD4+ T cells, purified as previously described (65), at a 4:1 T cell to macrophage ratio.
Generation of mammalian-derived RELM-α.
293T cells (American Type Culture Collection) were transfected with pCMVSPORT6-Retnla (Open Biosystems) or a control eGFP plasmid using the Lipofectamine reagent (Invitrogen) according to manufacturer's instructions. Transfection efficiency was assessed at >80% according to GFP expression, and supernatants were recovered at the time points indicated for Western blot analysis for RELM-α secretion. Where indicated, splenocytes were treated with supernatants from cells transfected with eGFP or Retnla plasmids for 72 h (maximal RELM-α expression) at a 1:1 ratio (final concentration estimated at 50 ng/ml).
RELM-α capture assay.
Splenocytes from OVA-specific DO11-10/4get reporter mice were cultured with 200 µg/ml OVA protein. At day 3, splenocytes were washed in FACS buffer (PBS supplemented with 2 mM EDTA, 0.5% BSA, and 0.1% azide), followed by a 60-min incubation on ice alone or with 0.5 µg rRELM-α. Cells were washed in FACS buffer and incubated with 10 µg of Fc blocking antibody (α-CD16/32) for 15 min, followed by the addition of 1 µg of anti–RELM-α for 30 min. Cells were then washed and stained for 30 min with biotinylated anti–rabbit antibody (Vector Laboratories), followed by detection with APC-conjugated streptavidin (BD) and surface staining for 15 min with antibodies of interest. Additional controls included incubation with the homologous protein rRELM-β (Peprotech), followed by capture with anti–RELM-α or capture with rabbit IgG isotype instead of rabbit anti–RELM-α.
Analysis of macrophage responses.
106 BMMacs were innately activated by overnight treatment with 100 ng/ml LPS (Sigma-Aldrich), followed by recovery of cells for RNA analysis. Cell-free supernatants were analyzed by ELISA and by the Greiss assay for nitric oxide production. For alternative activation, BMMacs were stimulated overnight with 40 ng/ml IL-4 and recovered for RNA analysis and flow cytometry. Cells were surface stained with antibodies to F4/80, mannose receptor (both obtained from AbD Serotec), MHC class II, and PDL2 (both obtained from BD) and stained intracellularly for RELM-α.
RNA isolation and real-time PCR.
RNA was isolated from cells using an RNeasy kit (QIAGEN) and from lung tissue using TRIzol (Invitrogen), according to manufacturer's instructions. Complementary DNA was generated as per standard protocol with Superscript reverse transcription (Invitrogen). Quantitative PCR was performed on complementary DNA using SYBR green chemistry (Applied Biosystems) and customized primer sets (QIAGEN). Reactions were run on a real-time PCR system (ABI 7500; Applied Biosystems). Samples were normalized to β-actin and displayed as fold induction over naive or untreated controls unless otherwise stated.
Statistical analysis.
Results represent the mean ± SEM of individual animals or individual triplicate conditions. Statistical significance was determined by the two-tailed Student's t test. Results were considered significant when P < 0.05.
Online supplemental material.
Fig. S1 examines the immune cell compartments of WT and Retnla−/− mice. Fig. S2 shows that CD11c+MR+F4/80− macrophages are a dominant cellular source of RELM-α in the lung. Fig. S3 shows cell recruitment in naive and Sm egg-challenged WT and Retnla−/− mice and lung mRNA levels of Il4, Ccl11, and Ccl24. Fig. S4 shows that rRELM-α has no effect on purified CD4+ T cells and BM Macs. Fig. S5 shows specificity of RELM-α binding. Fig. S6 shows that BTK inhibitor alone does not affect CD4+ T cell responses. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20082048/DC1.
Acknowledgments
We would like to acknowledge Thomas Kirn, Paul Giacomin, Betsy Taylor, Chris Carbone, and Michael Abt for helpful discussions and critical reading of the manuscript.
This work was supported by the National Institutes of Health (AI61570 and AI74878 to D. Artis; AI32573 and AI53825 to E.J. Pearce; T32AI007532-08 to J.G. Perrigoue), the Burroughs Wellcome Fund (Investigator in Pathogenesis of Infectious Disease Award to D. Artis), the Crohn's and Colitis Foundation of America (William and Shelby Model Family Foundation Research Award to M. Nair and D. Artis), the Morphology Core and Pilot Feasibility Program of the National Institute of Diabetes and Digestive and Kidney Diseases Center (DK50306 to D. Artis and G.P. Swain), and pilot grants from the University of Pennsylvania (Center for Infectious Diseases and University Research Fund to D. Artis). C. Zaph is funded by the Irvington Institute Fellowship Program of the Cancer Research Institute. M. Karow is employed by Amgen; G.D. Yancopoulos, D.M. Valenzuela, A. Murphy, and S. Stevens are employed by Regeneron Pharmaceuticals.
The authors have no further conflicting financial interests.
Footnotes
Abbreviations used: AAMac, alternatively activated macrophage; BAL, bronchoalveolar lavage; BMMac, BM-derived macrophage; BTK, Bruton's tyrosine kinase; DN, double negative; eGFP, enhanced GFP; IF, immunofluorescent; mRNA, messenger RNA; PEC, peritoneal cavity; RELM, resistin-like molecule; Sm, Schistosoma mansoni.
References
- Janeway C.A., Jr., Medzhitov R. 2002. Innate immune recognition.Annu. Rev. Immunol. 20:197–216 [DOI] [PubMed] [Google Scholar]
- Stuart L.M., Ezekowitz R.A. 2008. Phagocytosis and comparative innate immunity: learning on the fly.Nat. Rev. Immunol. 8:131–141 [DOI] [PubMed] [Google Scholar]
- Lawrence T., Bebien M., Liu G.Y., Nizet V., Karin M. 2005. IKKalpha limits macrophage NF-kappaB activation and contributes to the resolution of inflammation.Nature. 434:1138–1143 [DOI] [PubMed] [Google Scholar]
- Qualls J.E., Kaplan A.M., van Rooijen N., Cohen D.A. 2006. Suppression of experimental colitis by intestinal mononuclear phagocytes.J. Leukoc. Biol. 80:802–815 [DOI] [PubMed] [Google Scholar]
- Swirski F.K., Libby P., Aikawa E., Alcaide P., Luscinskas F.W., Weissleder R., Pittet M.J. 2007. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata.J. Clin. Invest. 117:195–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Peters T., Kess D., Sindrilaru A., Oreshkova T., Van Rooijen N., Stratis A., Renkl A.C., Sunderkotter C., Wlaschek M., et al. 2006. Activated macrophages are essential in a murine model for T cell-mediated chronic psoriasiform skin inflammation.J. Clin. Invest. 116:2105–2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z., Zheng T., Homer R.J., Kim Y.K., Chen N.Y., Cohn L., Hamid Q., Elias J.A. 2004. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation.Science. 304:1678–1682 [DOI] [PubMed] [Google Scholar]
- Taylor P.R., Martinez-Pomares L., Stacey M., Lin H.H., Brown G.D., Gordon S. 2005. Macrophage receptors and immune recognition.Annu. Rev. Immunol. 23:901–944 [DOI] [PubMed] [Google Scholar]
- MacMicking J., Xie Q.W., Nathan C. 1997. Nitric oxide and macrophage function.Annu. Rev. Immunol. 15:323–350 [DOI] [PubMed] [Google Scholar]
- Gordon S. 2003. Alternative activation of macrophages.Nat. Rev. Immunol. 3:23–35 [DOI] [PubMed] [Google Scholar]
- Nair M.G., Guild K.J., Artis D. 2006. Novel effector molecules in type 2 inflammation: lessons drawn from helminth infection and allergy.J. Immunol. 177:1393–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loke P., Nair M.G., Parkinson J., Guiliano D., Blaxter M., Allen J.E. 2002. IL-4 dependent alternatively-activated macrophages have a distinctive in vivo gene expression phenotype.BMC Immunol. 3:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raes G., De Baetselier P., Noel W., Beschin A., Brombacher F., Hassanzadeh Gh G. 2002. Differential expression of FIZZ1 and Ym1 in alternatively versus classically activated macrophages.J. Leukoc. Biol. 71:597–602 [PubMed] [Google Scholar]
- Anthony R.M., Urban J.F., Jr., Alem F., Hamed H.A., Rozo C.T., Boucher J.L., Van Rooijen N., Gause W.C. 2006. Memory T(H)2 cells induce alternatively activated macrophages to mediate protection against nematode parasites.Nat. Med. 12:955–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condeelis J., Pollard J.W. 2006. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis.Cell. 124:263–266 [DOI] [PubMed] [Google Scholar]
- Odegaard J.I., Ricardo-Gonzalez R.R., Goforth M.H., Morel C.R., Subramanian V., Mukundan L., Eagle A.R., Vats D., Brombacher F., Ferrante A.W., Chawla A. 2007. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance.Nature. 447:1116–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E.Y., Battaile J.T., Patel A.C., You Y., Agapov E., Grayson M.H., Benoit L.A., Byers D.E., Alevy Y., Tucker J., et al. 2008. Persistent activation of an innate immune response translates respiratory viral infection into chronic lung disease.Nat. Med. 14:633–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert D.R., Holscher C., Mohrs M., Arendse B., Schwegmann A., Radwanska M., Leeto M., Kirsch R., Hall P., Mossmann H., et al. 2004. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology.Immunity. 20:623–635 [DOI] [PubMed] [Google Scholar]
- Wynn T.A. 2004. Fibrotic disease and the T(H)1/T(H)2 paradigm.Nat. Rev. Immunol. 4:583–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loke P., Gallagher I., Nair M.G., Zang X., Brombacher F., Mohrs M., Allison J.P., Allen J.E. 2007. Alternative activation is an innate response to injury that requires CD4+ T cells to be sustained during chronic infection.J. Immunol. 179:3926–3936 [DOI] [PubMed] [Google Scholar]
- Rauh M.J., Ho V., Pereira C., Sham A., Sly L.M., Lam V., Huxham L., Minchinton A.I., Mui A., Krystal G. 2005. SHIP represses the generation of alternatively activated macrophages.Immunity. 23:361–374 [DOI] [PubMed] [Google Scholar]
- Zimmermann N., King N.E., Laporte J., Yang M., Mishra A., Pope S.M., Muntel E.E., Witte D.P., Pegg A.A., Foster P.S., et al. 2003. Dissection of experimental asthma with DNA microarray analysis identifies arginase in asthma pathogenesis.J. Clin. Invest. 111:1863–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallina G., Dolcetti L., Serafini P., De Santo C., Marigo I., Colombo M.P., Basso G., Brombacher F., Borrello I., Zanovello P., et al. 2006. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells.J. Clin. Invest. 116:2777–2790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair M.G., Gallagher I.J., Taylor M.D., Loke P., Coulson P.S., Wilson R.A., Maizels R.M., Allen J.E. 2005. Chitinase and Fizz family members are a generalized feature of nematode infection with selective upregulation of Ym1 and Fizz1 by antigen-presenting cells.Infect. Immun. 73:385–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steppan C.M., Brown E.J., Wright C.M., Bhat S., Banerjee R.R., Dai C.Y., Enders G.H., Silberg D.G., Wen X., Wu G.D., Lazar M.A. 2001. A family of tissue-specific resistin-like molecules.Proc. Natl. Acad. Sci. USA. 98:502–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S.D., Rajala M.W., Rossetti L., Scherer P.E., Shapiro L. 2004. Disulfide-dependent multimeric assembly of resistin family hormones.Science. 304:1154–1158 [DOI] [PubMed] [Google Scholar]
- Sandler N.G., Mentink-Kane M.M., Cheever A.W., Wynn T.A. 2003. Global gene expression profiles during acute pathogen-induced pulmonary inflammation reveal divergent roles for Th1 and th2 responses in tissue repair.J. Immunol. 171:3655–3667 [DOI] [PubMed] [Google Scholar]
- Daley E., Emson C., Guignabert C., de Waal Malefyt R., Louten J., Kurup V.P., Hogaboam C., Taraseviciene-Stewart L., Voelkel N.F., Rabinovitch M., et al. 2008. Pulmonary arterial remodeling induced by a Th2 immune response.J. Exp. Med. 205:361–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., Jin H., Ullenbruch M., Hu B., Hashimoto N., Moore B., McKenzie A., Lukacs N.W., Phan S.H. 2004. Regulation of found in inflammatory zone 1 expression in bleomycin-induced lung fibrosis: role of IL-4/IL-13 and mediation via STAT-6.J. Immunol. 173:3425–3431 [DOI] [PubMed] [Google Scholar]
- Bokarewa M., Nagaev I., Dahlberg L., Smith U., Tarkowski A. 2005. Resistin, an adipokine with potent proinflammatory properties.J. Immunol. 174:5789–5795 [DOI] [PubMed] [Google Scholar]
- Li D., Fernandez L.G., Dodd-o J., Langer J., Wang D., Laubach V.E. 2005. Upregulation of hypoxia-induced mitogenic factor in compensatory lung growth after pneumonectomy.Am. J. Respir. Cell Mol. Biol. 32:185–191 [DOI] [PubMed] [Google Scholar]
- Teng X., Li D., Champion H.C., Johns R.A. 2003. FIZZ1/RELMalpha, a novel hypoxia-induced mitogenic factor in lung with vasoconstrictive and angiogenic properties.Circ. Res. 92:1065–1067 [DOI] [PubMed] [Google Scholar]
- Holcomb I.N., Kabakoff R.C., Chan B., Baker T.W., Gurney A., Henzel W., Nelson C., Lowman H.B., Wright B.D., Skelton N.J., et al. 2000. FIZZ1, a novel cysteine-rich secreted protein associated with pulmonary inflammation, defines a new gene family.EMBO J. 19:4046–4055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siracusa M.C., Reece J.J., Urban J.F., Jr., Scott A.L. 2008. Dynamics of lung macrophage activation in response to helminth infection.J. Leukoc. Biol. 84:1422–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijt L.S., Kuipers H., Vos N., Hijdra D., Hoogsteden H.C., Lambrecht B.N. 2004. A rapid flow cytometric method for determining the cellular composition of bronchoalveolar lavage fluid cells in mouse models of asthma.J. Immunol. Methods. 288:111–121 [DOI] [PubMed] [Google Scholar]
- Hume D.A., Robinson A.P., MacPherson G.G., Gordon S. 1983. The mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80. Relationship between macrophages, Langerhans cells, reticular cells, and dendritic cells in lymphoid and hematopoietic organs.J. Exp. Med. 158:1522–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse M., Modolell M., La Flamme A.C., Schito M., Fuentes J.M., Cheever A.W., Pearce E.J., Wynn T.A. 2001. Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: granulomatous pathology is shaped by the pattern of L-arginine metabolism.J. Immunol. 167:6533–6544 [DOI] [PubMed] [Google Scholar]
- Nair M.G., Guild K.J., Du Y., Zaph C., Yancopoulos G.D., Valenzuela D.M., Murphy A., Stevens S., Karow M., Artis D. 2008. Goblet cell-derived resistin-like molecule beta augments CD4+ T cell production of IFN-gamma and infection-induced intestinal inflammation.J. Immunol. 181:4709–4715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loke P., Allison J.P. 2003. PD-L1 and PD-L2 are differentially regulated by Th1 and Th2 cells.Proc. Natl. Acad. Sci. USA. 100:5336–5341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein M., Keshav S., Harris N., Gordon S. 1992. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation.J. Exp. Med. 176:287–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Q., Zhou Y., Johns R.A. 2007. Bruton's tyrosine kinase (BTK) is a binding partner for hypoxia induced mitogenic factor (HIMF/FIZZ1) and mediates myeloid cell chemotaxis.FASEB J. 21:1376–1382 [DOI] [PubMed] [Google Scholar]
- van den Akker E., van Dijk T.B., Schmidt U., Felida L., Beug H., Lowenberg B., von Lindern M. 2004. The BTK inhibitor LFM-A13 is a potent inhibitor of Jak2 kinase activity.Biol. Chem. 385:409–413 [DOI] [PubMed] [Google Scholar]
- Fadok V.A., Bratton D.L., Konowal A., Freed P.W., Westcott J.Y., Henson P.M. 1998. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF.J. Clin. Invest. 101:890–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris D.G., Huang X., Kaminski N., Wang Y., Shapiro S.D., Dolganov G., Glick A., Sheppard D. 2003. Loss of integrin alpha(v)beta6-mediated TGF-beta activation causes Mmp12-dependent emphysema.Nature. 422:169–173 [DOI] [PubMed] [Google Scholar]
- Thepen T., Van Rooijen N., Kraal G. 1989. Alveolar macrophage elimination in vivo is associated with an increase in pulmonary immune response in mice.J. Exp. Med. 170:499–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber J.S., Mosser D.M. 2001. Reversing lipopolysaccharide toxicity by ligating the macrophage Fc gamma receptors.J. Immunol. 166:6861–6868 [DOI] [PubMed] [Google Scholar]
- Smith P., Mangan N.E., Walsh C.M., Fallon R.E., McKenzie A.N., van Rooijen N., Fallon P.G. 2007. Infection with a helminth parasite prevents experimental colitis via a macrophage-mediated mechanism.J. Immunol. 178:4557–4566 [DOI] [PubMed] [Google Scholar]
- Taylor M.D., Harris A., Nair M.G., Maizels R.M., Allen J.E. 2006. F4/80+ alternatively activated macrophages control CD4+ T cell hyporesponsiveness at sites peripheral to filarial infection.J. Immunol. 176:6918–6927 [DOI] [PubMed] [Google Scholar]
- Mayer A.K., Bartz H., Fey F., Schmidt L.M., Dalpke A.H. 2008. Airway epithelial cells modify immune responses by inducing an anti-inflammatory microenvironment.Eur. J. Immunol. 38:1689–1699 [DOI] [PubMed] [Google Scholar]
- Zaph C., Troy A.E., Taylor B.C., Berman-Booty L.D., Guild K.J., Du Y., Yost E.A., Gruber A.D., May M.J., Greten F.R., et al. 2007. Epithelial-cell-intrinsic IKK-beta expression regulates intestinal immune homeostasis.Nature. 446:552–556 [DOI] [PubMed] [Google Scholar]
- Nenci A., Becker C., Wullaert A., Gareus R., van Loo G., Danese S., Huth M., Nikolaev A., Neufert C., Madison B., et al. 2007. Epithelial NEMO links innate immunity to chronic intestinal inflammation.Nature. 446:557–561 [DOI] [PubMed] [Google Scholar]
- Jacobsen E.A., Ochkur S.I., Pero R.S., Taranova A.G., Protheroe C.A., Colbert D.C., Lee N.A., Lee J.J. 2008. Allergic pulmonary inflammation in mice is dependent on eosinophil-induced recruitment of effector T cells.J. Exp. Med. 205:699–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munitz A., Waddell A., Seidu L., Cole E.T., Ahrens R., Hogan S.P., Rothenberg M.E. 2008. Resistin-like molecule alpha enhances myeloid cell activation and promotes colitis.J. Allergy Clin. Immunol. 122:1200–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan S.P., Seidu L., Blanchard C., Groschwitz K., Mishra A., Karow M.L., Ahrens R., Artis D., Murphy A.J., Valenzuela D.M., et al. 2006. Resistin-like molecule beta regulates innate colonic function: barrier integrity and inflammation susceptibility.J. Allergy Clin. Immunol. 118:257–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVay L.D., Keilbaugh S.A., Wong T.M., Kierstein S., Shin M.E., Lehrke M., Lefterova M.I., Shifflett D.E., Barnes S.L., Cominelli F., et al. 2006. Absence of bacterially induced RELMbeta reduces injury in the dextran sodium sulfate model of colitis.J. Clin. Invest. 116:2914–2923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Q., Zheng L., Kang Q., Dodd O.J., Langer J., Li B., Wang D., Li D. 2006. Upregulation of hypoxia-induced mitogenic factor in bacterial lipopolysaccharide-induced acute lung injury.FEBS Lett. 580:2207–2215 [DOI] [PubMed] [Google Scholar]
- Tong Q., Zheng L., Lin L., Li B., Wang D., Li D. 2006. Hypoxia-induced mitogenic factor promotes vascular adhesion molecule-1 expression via the PI-3K/Akt-NF-kappaB signaling pathway.Am. J. Respir. Cell Mol. Biol. 35:444–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Q., Zheng L., Lin L., Li B., Wang D., Huang C., Li D. 2006. VEGF is upregulated by hypoxia-induced mitogenic factor via the PI-3K/Akt-NF-kappaB signaling pathway.Respir. Res. 7:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reece J.J., Siracusa M.C., Scott A.L. 2006. Innate immune responses to lung-stage helminth infection induce alternatively activated alveolar macrophages.Infect. Immun. 74:4970–4981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane J.A., Fruman D.A. 2004. Phosphoinositide 3-kinase: diverse roles in immune cell activation.Annu. Rev. Immunol. 22:563–598 [DOI] [PubMed] [Google Scholar]
- Xiao W., Hong H., Kawakami Y., Lowell C.A., Kawakami T. 2008. Regulation of myeloproliferation and M2 macrophage programming in mice by Lyn/Hck, SHIP, and Stat5.J. Clin. Invest. 118:924–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R.Z., Huang Q., Xu A., McLenithan J.C., Eisen J.A., Shuldiner A.R., Alkan S., Gong D.W. 2003. Comparative studies of resistin expression and phylogenomics in human and mouse.Biochem. Biophys. Res. Commun. 310:927–935 [DOI] [PubMed] [Google Scholar]
- Nagaev I., Bokarewa M., Tarkowski A., Smith U. 2006. Human resistin is a systemic immune-derived proinflammatory cytokine targeting both leukocytes and adipocytes.PLoS One. 1:e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela D.M., Murphy A.J., Frendewey D., Gale N.W., Economides A.N., Auerbach W., Poueymirou W.T., Adams N.C., Rojas J., Yasenchak J., et al. 2003. High-throughput engineering of the mouse genome coupled with high-resolution expression analysis.Nat. Biotechnol. 21:652–659 [DOI] [PubMed] [Google Scholar]
- Perrigoue J.G., Li J., Zaph C., Goldschmidt M., Scott P., de Sauvage F.J., Pearce E.J., Ghilardi N., Artis D. 2007. IL-31–IL-31R interactions negatively regulate type 2 inflammation in the lung.J. Exp. Med. 204:481–487 [DOI] [PMC free article] [PubMed] [Google Scholar]