Abstract
Background
We have investigated predictors of 90-day-mortality in a large cohort of non-specific cancer of unknown primary patients.
Methods
Predictors have been identified by univariate and then logistic regression analysis in a single-center cohort comprising 429 patients (development cohort). We identified four predictors that produced a predictive score that has been applied to an independent multi-institutional cohort of 409 patients (validation cohort). The score was the sum of predictors for each patient (0 to 4).
Results
The 90-day-mortality-rate was 33 and 26% in both cohorts. Multivariate analysis has identified 4 predictors for 90-day-mortality: performance status>1 (OR = 3.03, p = 0.001), at least one co-morbidity requiring treatment (OR = 2.68, p = 0.004), LDH>1.5×the upper limit of normal (OR = 2.88, p = 0.007) and low albumin or protein levels (OR = 3.05, p = 0.007). In the development cohort, 90-day-mortality-rates were 12.5%, 32% and 64% when the score was [0–1], 2 and [3]–[4], respectively. In the validation cohort, risks were 13%, 25% and 62% according to the same score values.
Conclusions
We have validated a score that is easily calculated at the beside that estimates the 90-days mortality rate in non-specific CUP patients. This could be helpful to identify patients who would be better served with palliative care rather than aggressive chemotherapy.
Introduction
Cancer of unknown primary (CUP) site represents about 2% of all invasive cancers diagnosed in adults (in 2006, 27,860 of 1,399,790 new cancer cases in the US) [1]. CUP is defined as a metastatic cancer with no identifiable origin at the time of diagnosis [2]. CUP is an aggressive cancer with generally poor outcomes; overall survival ranges from 4 to 12 months in large series [2]–[8]. Nevertheless, the recognition of particular clinico-pathologic entities and the specific treatments delivered to these patients significantly improved CUP management [8]. More recently, progress in immunochemistry [2]–[9] as well as gene profiling [10]–[11] made a step forward to better CUP diagnosis. However, these promising tools lack evidence in making impact on patient outcome and are of little use in daily practice.
But, 80% of CUP does not fall into favorable subsets [2]–[4]. Non-specific CUP treatment remains debatable, because its prognosis remains very difficult to estimate. Several previous studies have analyzed prognostic factors in such a population [4]–[7]. Nevertheless, these prognostic factors are not used in routine practice, because they are not convenient to use at the bedside [8]. From a physician's point of view it is of major importance to discriminate patients who would benefit from combination chemotherapy from those who would not and would be better served by palliative care.
Due to lack of reliable tools to estimate life-expectancy, we have conducted a new prognostic analysis in order to delineate and validate an easily derived bedside score that predicts risk of early death in CUP patients.
Methods
Development cohort
We retrospectively reviewed medical records of 429 consecutive patients primarily admitted to the Oscar Lambret Cancer Centre from November 1993 to February 2007. The study population consisted of patients who were diagnosed as having non-specific CUP. Inclusion criteria were: histological proof of malignancy, metastatic epithelial cancer, absence of identified primary site at the time of initial diagnostic and pre-treatment work-up. In addition, the following entities were excluded from analysis: adenocarcinoma in an axillary lymph node in women, primary papillary serous peritoneal carcinoma, undifferentiated carcinoma of the mediastinum and retroperitoneum in young men (middle line syndrome), cervical lymph nodes containing squamous cell carcinoma. All patients underwent a basic evaluation consisting on medical history, complete physical examination, biopsy and histopathological examination of the most easily accessible lesion, mammography for women, PSA levels for men, thoracic, abdominal and pelvic computed tomography (CT)-Scan, and, in the context of undifferentiated carcinoma the α-feto-protein and β-human chorionic gonadotrophin levels for both sexes [2].
Validation cohort
This cohort included non-specific CUP referred to the Cross Cancer Institute, Edmonton, Canada from January 1998 to December 2004 (308 cases), to Centre Léon Bérard and Hospices Civils of Lyon, France from January 2000 to December 2004 (79 cases) and to Hospital of Lille University from January 2004 to November 2007 (22 cases) Lille, France.
Primary endpoint
The primary endpoint was 90-day mortality. This threshold is believed to be relevant in decision-making for advanced cancer patients in whom the choice of whether to treat with chemotherapy or primary palliative care need to be discussed [12]–[15]
Development of the score predicting the 90-day mortality
This analysis was conducted on the development cohort. We have first identified variables that predicted 90-day mortality using the Student t-test. Continuous variables were analyzed using Student t-test. Variables that predicted 90-day mortality were then dichotomized into binary variables using receiver-operator curves that estimated the cut-off optimizing both sensibility and specificity. Identifying predictors of 90-day-mortality among categorical variables was based on Chi-square tests and calculation of odds ratios and their 95%-confidence intervals (95%-CI).
Variables significantly associated with the 90-day-mortality in univariate analysis were then introduced into a stepwise logistic regression model [16]. Based on these analyses we developed a prognostic score. This score was calculated as the sum of predictors observed for each patient (from 0 to 4). Three categories of patients were defined: patients with high-risk of early death, patients with intermediate risk and patients with low risk according to observed death rates at each value of the score. Its performance was estimated using specificity (Se), specificity (Sp), positive predictive value (PPV), negative predictive value (NPV) and accuracy (rate of well classified) tabulated from a classical 2×2 table.
Validating the model predicting 90-day mortality
This score was then applied to the validation dataset and its performance was estimated using the classical 2×2 table.
Ethical Consideration
This study was conducted according to the principles expressed in the Declaration of Helsinki. The study was approved by the Institutional Review Board of the Oscar Lambret Cancer Center.
Data processing and analyzing
The collected data were entered into computer and analyzed using SPSS version 13.0 statistical software.
The authors had also obtained the approval of Research Ethics Board of Alberta Cancer Board (ETH-21853, February 2006) and the approval of the French “Comission Nationale Informatique et Liberté” (date of approval June 2006)”.
Results
Study population
Development and validation cohorts are described in table 1. Median overall survivals were respectively 189 days (range 1–4,801) and 215 days range 1–3,842). The 90-day-mortality-rates were respectively 142/429 (33%) and 109/409 (26%).
Table 1. Patient's characteristics.
| Categorical data | ||
| Variables | Development dataset 429 cases (%) | Validation dataset 409 Cases (%) |
| Men | 296 (68) | 203 (49) |
| Women | 133 (32) | 206 (51) |
| PS = 0 | 141 (33) | 57 (14) |
| PS = 1 | 138 (32) | 129 (31) |
| PS = 2 | 108 (25) | 103 (25) |
| PS = 3 | 35 (9) | 93 (23) |
| PS = 4 | 4 (1) | 26 (7) |
| Absence of co-morbidity or co- morbidity not requiring treatment | 241 (56) | 264 (66) |
| At least 1 co-morbidity requiring treatment | 172 (44) | 141 (34) |
| Number of met. site = 1 | 168 (39) | 184 (45) |
| Number of met. site = 2 | 107 (25) | 130 (31) |
| Number of met. site = 3 | 85 (20) | 59 (14) |
| Number of met. site = 4 | 43 (10) | 23 (7) |
| Number of met. site≥5 | 26 (6) | 13 (3) |
| Adenocarcinoma | 272 (63) | 210 (51) |
| Undifferentiated carcinoma | 58 (13) | 138 (34) |
| Squamous cell carcinoma | 77 (18) | 24 (6) |
| Others | 22 (6) | 37 (9) |
| Lung met. | 103 (24) | 88 (21) |
| Liver met. | 144 (33) | 174 (42) |
| Bone met. | 156 (36) | 117 (28) |
| Brain met. | 32 (8) | 1 (0) |
| Continuous | ||
| Variables (units) | Development dataset Median (range) | Validation dataset Median (range) |
| Age (years) | 59 (22–91) | 65 (19–92) |
| LDH (IU/l) | 660 (57–10,084) | 428 (86–7,538) |
| Alkaline phosphatase (IU/l) | 280 (31–7,423) | Not done |
| Hemoglobin level (g/dl) | 12.5 (6–17,3) | 12.3 (6–18.2) |
| Platelets (U/mm3) | 320,000 (7,000–830,000) | 374,000 (10,000–736,000) |
| Lymphocytes (U/mm3) | 1,300 (220–6,830) | 1,250 (100–99,2000) |
| Variables | Development dataset 429 cases (%) | Validation dataset Cases (%) |
| Protein levels (g/l) | 68 (49–92) | 69 (42–87) |
| Albumin levels (g/l) | 32 (14–51) | 36 (19–49) |
Abbreviations: PS = performance status, met. = metastasis, LDH = lactate dehydrogenase, ULN = upper limit of normal, IU: international unit, U:unit.
Predictors for 90-day mortality
This analysis was conducted on the development cohort. Three continuous variables were not predictive for 90-day-mortality: age (p = 0.090), lymphocyte count (p = 0.2206) and platelet count (p = 0.7535). Five continuous variables were predictive of 90-day mortality and then were dichotomized into binary variables using the cut-off value that optimized both sensibility and specificity in ROC curves: number of metastatic sites with a cut-off fixed at>2 sites, LDH level with a cut-off fixed at>1.5 times the upper limit of normal (ULN), alkaline phosphatase levels with a cut-off fixed at>ULN, hemoglobin levels with a cut-off fixed at<12 g/dl, hypoproteinemia with a cut-off fixed at<70 g/l and hypoalbuminemia with a cut-off fixed at<35 g/l. In further analysis, patients with low protein or albumin levels have been combined into a single group.
Under univariate analysis, thirteen categorical variables were predictive for 90-day-mortality: Performance status (PS)>1, at least one co-morbidity requiring treatment, presence of lung, liver, bone, adrenal, brain or rare metastases, presence of more than 2 metastatic sites, LDH>1.5×ULN, alkaline phosphatase>ULN, hemoglobin less than 12 g/dl and low albumin or protein levels (Table 2). These variables were then introduced in a logistic regression model that identified 4 independent predictive factors for early death: PS>1, at least one co-morbidity requiring treatment, LDH>1.5×ULN and low protein or albumin levels.
Table 2. Identification of predictive factors for 90-day-mortality.
| Univariate analysis | Logistic regression | model | ||
| Variables not introduced in multivariate analysis | Odds Ratio and [95%-CI] | P value | - | - |
| Men | 1.03 [0.60–1.76] | 0.8204 | - | - |
| Lymph nodes | 0.68 [0.45–1.02] | 0.063 | - | - |
| Pleural met. | 1.58 [0.78–3.18] | 0.2006 | - | - |
| Peritoneal met. | 1.79 [0.89–3.60] | 0.0980 | - | - |
| Cutaneous met. | 1.36 [0.38–4.89] | 0.6398 | - | - |
| Other histology than adenocarcinoma | 1.03 [0.60–1.76] | 0.3280 | - | - |
| Variables introduced in multivariate analysis | Odds Ratio and [95%-CI] | P value | Adjusted Odds Ratio [95%-CI] | p |
| PS>1 | 4.70 [2.91–7.61] | <0.0001 | 3.03 [2.64–6.81] | 0.0010 |
| At least 1 co-morbidity requiring treatment | 2.04 [1.29–3.23] | 0.0015 | 2.68 [1.47–3.47] | 0.0040 |
| Lung met. | 2.94 [1.80–4.83] | <0.0001 | - | 0.1580 |
| Liver met. | 2.59 [1.52–4.42] | 0.0004 | - | 0.5640 |
| Bone met. | 1.47 [0.94–2.30] | 0.0084 | - | 0.7000 |
| Brain met. | 2.61 [1.20–5.69] | 0.0038 | - | 0.3300 |
| Adrenal met. | 4.34 [1.07–17.68] | 0.0122 | - | 0.8890 |
| Rare met. | 2.42 [1.48–3.97] | 0.0004 | - | 0.3430 |
| Number of met. Site>2 | 2.94 [1.86–4.65] | 0.0015 | - | 0.4400 |
| LDH>1.5×ULN | 3.18 [1.98–5.24] | <0.0001 | 2.88 [1.65–5.02] | 0.0070 |
| AP>ULN | 2.01 [1.22–3.32] | <0.0001 | - | 0.8055 |
| Hemoglobin<12 g/dl | 2.67 [1.65–4.32] | <0.0001 | - | 0.3060 |
| Low albumin or protein levels | 3.93 [2.36–6.56] | <0.0001 | 3.05 [1.98–5.12] | 0.0070 |
Abbreviations: 95%-CI: 95%-confidence intervals, PS = performance status, met. = metastasis, LDH = lactates dehydrogenase, AP = Alkaline phosphatase, ULN = upper limit of normal.
Score and performance
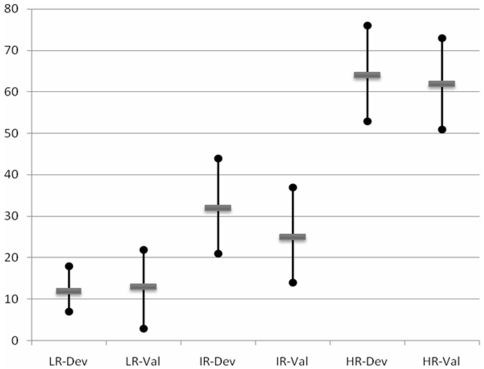
In the development cohort, 274 patients were fully assessable for the four predictive factors and the primary endpoint. In order to develop a simple and bedside model, patients with score [0–1], 2 and [3]–[4] points were respectively considered at low risk, intermediate risk and high risk of 90-day mortality. Rates of 90-day-mortality were 12.5% for “low-risk patients”, 32% for “intermediate-risk patients” and 64% for “high-risk patients”. The 95%-confidence intervals (CI) of these three rates did not overlap (Table 3 and Figure 1). Performance of this score for prediction of 90-day-mortality were calculated in Table 3; accuracy and specificity were superior to 75% with a threshold set at score≥3 (that is to say when considering patients at high risk of 90 days mortality).
Table 3. Predictive score and its performance.
| Development cohort | Validation cohort | |
| Assessable patients | 274 | 174 |
| 90-day-mortality-rate | 31% [25–36] | 37% [30–44] |
| 90-day-mortality-rates | according to the score | |
| Score = [0–1] | ||
| Rate, 95%-CI | 17/136 (12%), [7–18] | 6/47 (13%), [3–22] |
| Score = 2 | ||
| Rate, 95%-CI | 22/67 (32%), [21–44] | 14/55 (25%), [14–37] |
| Score = [3–4] | ||
| Rate, 95%-CI | 46/71 (64%), [53–76] | 45/72 (62%), [51–73] |
| Performance of the score | for prediction of 90-day- mortality | (Score≥3) |
| Sensitivity | 0.54 [0.43–0.65] | 0.69 [0.58–0.80] |
| Specificity | 0.86 [0.82–0.91] | 0.75 [0.67–0.83] |
| Positive predictive value | 0.64 [0.53–0.75] | 0.62 [0.51–0.73] |
| Negative predictive value | 0.80 [0.75–0.86] | 0.80 [0.72–0.88] |
| Accuracy | 0.76 |0.68–0.82] | 0.73 [0.66–0.79] |
Figure 1. 90-day-mortality-rates and 95%-confidence intervals according to the predicitve score.
LR-Dev: Low-risk patients among the developpement cohort (score = [0–1]). LR-Val: Low-risk patients among the validation cohort (score = [0–1]). IR-Dev: Intermediate-risk patients among the developpement cohort (score = 2). IR-Val: Intermediate-risk patients among the validation cohort (score = 2). HR-Dev: High-risk patients among the developpement cohort (score = [3–4]). HR-Val: High-risk patients among the validation cohort (score = [3–4]).
Validation of the score
This score was then applied to the validation cohort. Only 174 patients were fully assessable for the four predictive factors and the primary endpoint. The separation of patients into the three groups was similar to that of the development cohort (Table 3 and Figure 1). In the validation cohort, 90-day-mortality-rates were 13%, 25% and 62% according to the score (Figure 1).
Discussion
This retrospective analysis was conducted on a large database of patients with non-specific CUP. This study has generated an easily obtained at bedside score that estimate the risk of 90-day-mortality in such a population. Our multivariate analysis has identified four independent predictive factors: PS>1, presence of at least one underlying co-morbidity requiring treatment, elevated LDH and low albumin or protein levels. The 90-day-mortality rate in patients having at least 3 factors was about 62–64% (see Figure 1). This group of poor prognosis patients was well identified; the 95%-CI of the rate did not overlap the 95%-CI of other categories (see Figure 1). This is a reliable guidance to estimate the risk of early death and for rational decision making shared with patient.
Patient's characteristics were consistent with the literature on CUP patients. The 90-day mortality was 26% (120/350) in the Van der Gaast's series [5] and 33% (134/401) in the Culine's series [6]. Culine et al. has shown that LDH levels and PS constitute two major prognostic factors for CUP [6]. Van de Gaast et al. has also identified PS as major prognostic factor for CUP [5]. Seve et al. has previously shown that co-morbidity was also an important prognostic factor [17].
In the present study, LDH appears as one of the independent predictors for 90-day mortality. Although LDH is related to tumor burden, LDH is also high in liver diseases, in hemolysis and in other situations with massive cells destruction. Despite its lack of specificity, LDH remains a well-established prognostic factor for many metastatic diseases [6], [18]–[21].
Low albumin and protein levels are associated with both weight loss and induction of systemic inflammatory responses. These elements are interlinked in the metastatic setting, and hypoalbuminemia is a frequent biological sign of advanced disease. Serum albumin is a well-established marker of nutritional status and general patient status [18], [21], [23]–[24]. The prognostic value of this parameter is also well-established [12], [18], [21].
Despite its subjective nature, estimation of general condition by PS remains one of the most powerful prognostic factors in CUP patients [5]–[6]. Biological markers (LDH, albumin) that constitute more objective variables did not outperform PS in our model and in previously published ones [5]–[6].
As previously reported [17]–[18], co-morbidity requiring treatment constitutes the fourth predictor for 90-day mortality. This relationship we believe relates to our ability to treat the patient. It is noteworthy that in the present study and in previously published ones that age is not a prognostic factor in CUP patients. Nevertheless, severe underlying diseases limit our ability to administer optimally chemotherapy. Evaluation of co-morbidities could be done using the ACE-27 score; ACE-27>2 represented the cut-off used in the present study [17].
This study presents several limitations due to its retrospective nature. First of all, missing data did not allow analysis of the entire cohorts [7]. Extensive immunohistochemical analysis and gene profiling were not available. Several recent studies have shown the importance of molecular and histological expertise in this field, histological review of case must be discussed [25–27]. But despite these modern investigations, the vast majority of CUP remains without identifiable or highly-suspected underlying primary. Lastly, treatments were heterogeneous across study periods and study sites. Nevertheless, there is no consensus on treating non-specific CUP.
To conclude, we have developed and validated a score that is easily obtained at bedside that helps physicians to manage patients with non-specific CUP in a more rationale way. Further studies are required to combine this score with more current biological parameters (such as gene profiling). Use of large multi-institutional database could be useful to further narrow 95%-confidence intervals of each predictor and refine their roles in the final score. In a further analysis we plan to compare this score to the others published predictive tools [4]–[7]. A randomized trial comparing benefits of palliative chemotherapy versus best supportive care in patients having 3 or 4 predictors for early-death should be performed.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The study has been funded by the Oscar Lambret Cancer Center. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. CA Cancer J Clin. Cancer Statistics, 2009. 2009 May 27.
- 2.Bugat R, Bataillard A, Lesimple T, Voigt JJ, Culine S, Lhortolary A, et al. Summary of the unknwn primary site (2002). Br J Cancer. 2003;89:S559–S566. doi: 10.1038/sj.bjc.6601085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abbruzzese JL, Abbruzzese MC, Lenzi R, et al. Analysis of a diagnostic strategy for patients with suspected tumours of unknown origin. J Clin Oncol. 1995;13:2094–2103. doi: 10.1200/JCO.1995.13.8.2094. [DOI] [PubMed] [Google Scholar]
- 4.Abbruzzese JL, Abbruzzese MC, Hess KR, et al. Unknown primary carcinoma: natural history and prognostic factors in 657 consecutive patients. J Clin Oncol. 1994;12:1272–1280. doi: 10.1200/JCO.1994.12.6.1272. [DOI] [PubMed] [Google Scholar]
- 5.Van der Gaast A, Verweij J, et al. Simple prognostic model to predict survival in patients with undifferentiated carcinoma of unknown primary site. J Clin Oncol. 1995;3:1720–1725. doi: 10.1200/JCO.1995.13.7.1720. [DOI] [PubMed] [Google Scholar]
- 6.Culine S, Kramar A, Saghatchian M, et al. Development and validation of a prognostic model to predict the length of survival in patients with carcinomas of an unknown primary site. J Clin Oncol. 200;20:4679–4683. doi: 10.1200/JCO.2002.04.019. [DOI] [PubMed] [Google Scholar]
- 7.Seve P, Ray-Coquard I, Trillet-Lenoir V, et al. Low serum albumin levels and liver metastasis are powerful prognostic markers for survival in patients with unknown primary site. Cancer. 2006;107:2698–2705. doi: 10.1002/cncr.22300. [DOI] [PubMed] [Google Scholar]
- 8.Briasoulis E, Pavlidis N, Felip E On behalf of the ESMO Guidelines Working Group. Cancers of unknown primary site: ESMO Clinical Recommendation for diagnosis, treatment and follow-up. Annals Oncol. 2008;S2:ii106–ii107. doi: 10.1093/annonc/mdn104. [DOI] [PubMed] [Google Scholar]
- 9.Harlings HM, Van Laar RK, Kerst JM, Helgasar HM, Wessling J, Van der Hoeven JJ, et al. Gene expression profiling to identify the histogenetic origin of metastases adenocarcinoma of unknown primary. J Clin Oncol. 2008;26:4435–4441. doi: 10.1200/JCO.2007.14.6969. [DOI] [PubMed] [Google Scholar]
- 10.Varadhachary GR, Raber MN, Matamoros A, Abbruzzese JL. Carcinoma of unknown primary with a colon-specific profile- changing paradigm and emerging definitions. Lancet Oncol. 2008;9:596–599. doi: 10.1016/S1470-2045(08)70151-7. [DOI] [PubMed] [Google Scholar]
- 11.Varadhachary GR, Talantov D, Raber MV, Meng C, Hess KR, Jatkoe T, et al. Molecular profiling of carcinoma of unknown primary and correlation with clinical evaluation. J Clin Oncol. 2008;26:4442–4448. doi: 10.1200/JCO.2007.14.4378. [DOI] [PubMed] [Google Scholar]
- 12.Penel N, Vanseymortier M, Bonneterre ME, et al. Prognostic factors among cancer patients with good performance status screened for phase I trials. Invest New Drugs. 2008;26:53–58. doi: 10.1007/s10637-007-9088-x. [DOI] [PubMed] [Google Scholar]
- 13.Geraci JM, Tsang W, Valdres RV, et al. Progressive disease in patients with cancer presenting to an emergency room with acute symptoms predicts short-term mortality. Supp Care Cancer. 2006;14:1038–45. doi: 10.1007/s00520-006-0053-6. [DOI] [PubMed] [Google Scholar]
- 14.Kelly L, White S, Stone PC. The B12/CRP index as a simple prognostic indicator in patients with advanced cancer: a confirmatory study. Ann Oncol. 2007;18:1395–1399. doi: 10.1093/annonc/mdm138. [DOI] [PubMed] [Google Scholar]
- 15.Sessa C, Roggero E, Pampallona, et al. The 3 last months of life cancer patients: medical aspects and role of home-care services in southern Switzerland. Supp Care Cancer. 1996;4:180–183. doi: 10.1007/BF01682337. [DOI] [PubMed] [Google Scholar]
- 16.Lemeshow S, Hosmer DW. A review of goodness of fit statistics for use in the development of logistic regression models. Am J Epidemiol. 1982;115:92–106. doi: 10.1093/oxfordjournals.aje.a113284. [DOI] [PubMed] [Google Scholar]
- 17.Sève P, Sawyer M, Hanson J, Broussole C, Dumontet C, Mackey JR. The influence of comorbidities, age, and performance status on the prognosis and treatment of patients with metastatic carcinomas of unknown primary site. J Clin Oncol. 2002;20:4679–4683. doi: 10.1002/cncr.21833. [DOI] [PubMed] [Google Scholar]
- 18.Vigano A, Bruera E, Jhangri GS, Newman SC, Fields AL, Suarez-Almazor ME. Clinical survival predictors in patients with advanced cancer. Arch Intern Med. 2000;160:861–868. doi: 10.1001/archinte.160.6.861. [DOI] [PubMed] [Google Scholar]
- 19.Manola J, Atkins M, Ibrahim J, Kirkwood J. Prognostic factors in metastatic melanoma: a pooled analysis of Eastern Cooperative Oncology Group trials. J Clin Oncol. 2000;18:3782–3793. doi: 10.1200/JCO.2000.18.22.3782. [DOI] [PubMed] [Google Scholar]
- 20.Suh SY, Ahn HY. Lactate deshydrogenase as a prognostic factor for survival time of terminally ill cancer patients: a preliminary study. Eur J Cancer. 2007;43:1051–1059. doi: 10.1016/j.ejca.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 21.Barbot AC, Mussault P, Ingrand P, Tourani JM. Assessing 2-months clinical prognosis in hospitalized patients with advanced solid tumors. J Clin Oncol; 2008;26:2538–43. doi: 10.1200/JCO.2007.14.9518. [DOI] [PubMed] [Google Scholar]
- 22.Mc Millian DC, Watson WS, O'Gorman P, Preston T, Scott HR, McArdle CS. Albumin concentrations are primarily determined by the body cell mass and the systemaic inflammatory response in cancer patients with weight loss. Nut Cancer. 2001;39:210–213. doi: 10.1207/S15327914nc392_8. [DOI] [PubMed] [Google Scholar]
- 23.Coates RJ, Clark WS, Eley JW, et al. Race, nutritional status, and survival from breast cancer. J Natl Cancer Inst. 1990;82:1684–1692. doi: 10.1093/jnci/82.21.1684. [DOI] [PubMed] [Google Scholar]
- 24.Liu SA, Tsai WC, Wong YK, Wong YK, Lin JC, Poon CK, et al. Nutritional factors and survival of patients with oral cancer. Head Neck. 2006;2028:998–1007. doi: 10.1002/hed.20461. [DOI] [PubMed] [Google Scholar]