Abstract
Mouse mammary tumor virus has served as a major model for the study of breast cancer since its discovery 1920’s as a milk-transmitted agent. Much is known about in vivo infection by this virus, which initially occurs in lymphocytes that then carry virus to mammary tissue. In addition to the virion proteins, MMTV encodes a number of accessory proteins that facilitate high level in vivo infection. High level infection of lymphoid and mammary epithelial cells ensures efficient passage of virus to the next generation. Since MMTV causes mammary tumors by insertional activation of oncogenes, which is thought to be a stochastic process, mammary epithelial cell transformation is a by-product of the infectious cycle. The envelope protein may also participate in transformation. Although there have been several reports of a similar virus in human breast cancer, the existence of a human MTV has not been definitely established.
Keywords: retrovirus, mammary tumor, mouse model
Introduction
The study of virus-induced cancers in animals has played a critical role in the understanding of oncogenesis in humans. This is especially the case for retroviruses, which were first recognized as transmissible agents that cause cancer shortly after the turn of the previous century. In the 1920’s, J. J. Bittner showed that there was a milk-transmitted agent in mice that was responsible for breast cancer induction (1). Since that time, this transmitted agent, now known to be the betaretrovirus, mouse mammary tumor virus (MMTV), has been used as an in vivo model for the study of mammary carcinogenesis (2,3). Here, I review the biology of MMTV, its in vivo transmission pathway and how it interacts with its host’s biology. I also review the current literature regarding a putative related human mammary tumor virus (HMTV).
MMTV genome structure and proteins
Retroviruses can be classified as simple or complex. The genomes of simple retroviruses, such as murine leukemia virus (MLV), encode only the virion proteins and enzymes required for viral replication. In contrast, complex retroviruses, human immunodeficiency virus (HIV)-1 or human T cell leukemia virus (HTLV) 1, encode in addition a variety of non-structural proteins that facilitate various steps of the replication pathway or counteract cellular and immunological anti-viral host responses. While MMTV was initially classified as a simple retrovirus, it is now clear that it probably lies somewhere in between viruses like MLV and HIV-1 in complexity.
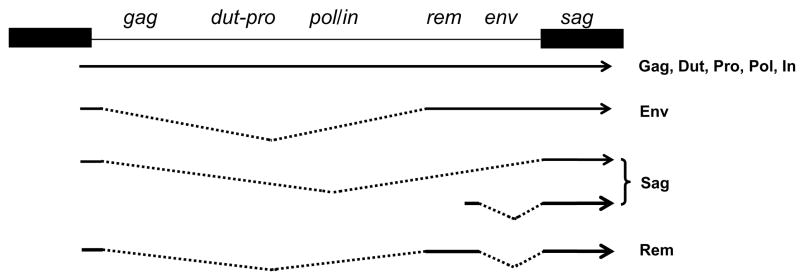
The MMTV genome is approximately 9 kb in size. At least five transcripts are generated from the viral genome, four of which initiate in the 5′ long terminal repeat (LTR) and terminate in the 3′ LTR; the different transcripts are generated by alternative splicing (Fig. 1). The LTR also contains binding sites for transcription factors that determine hormone-responsive and tissue-specific transcription, both of which are necessary for in vivo infection and optimal virus production. Specifically, the LTRs encode sites that regulate both mammary epithelial and lymphoid cell-specific expression, as well as glucorticoid/progesterone response elements that cause increased virus transcription during pregnancy and lactation, when virions are shed into milk (4–8). Because the MMTV LTR encodes transcriptional regulatory elements that direct high level expression in mammary epithelial cells, it has been widely used to drive transgene expression in mouse mammary tissue (reviewed in XXX, this volume).
Fig. 1.
MMTV proviral genome and gene products.
Like all retroviruses, the full-length, unspliced MMTV RNA serves two functions. First, two copies are packaged into virions and thus provide the viral genome. Second, the full-length transcript serves as the mRNA for the gene products encoded by the gag, dut-pro and pol genes (9). The gag translation product is a polyprotein precursor that is processed by the viral protease, PR or Pro, into the capsid (CA) and nucleocapsid (NC) proteins, as well as several other peptides of unknown function. Both the Dut-Pro and Pol polyproteins are translated from the same mRNA as Gag, but in different reading frames, by a process termed ribosomal frameshifting. The pro gene encodes the viral protease and dut, a dUTPase, whose role in virus infection is not known. However, for other retroviruses that encode a dUTPase, such as equine infectious anemia virus (EIAV), it is believed that this protein contributes to pathogenesis by maintaining adequate nucleotide pools and thereby facilitating productive viral replication in non-dividing cells (10). Since MMTV infects dendritic cells (DCs), which are non-dividing in vivo, the dUTPase could play a similar role. The pol gene codes for reverse transcriptase (RT), needed to generate the double-stranded DNA, and the integrase (IN), which is required for integration of this DNA into the host chromosome.
A singly spliced mRNA is translated from the envelope (env) gene, which is cleaved by host furin enzymes to yield two polypeptides, the surface (SU) and transmembrane (TM) domains of the Env protein, required for binding of the virions to cell surface receptor(s) that mediate cell entry (9). The SU domain carries the receptor binding site (RBS), while the TM domain mediates virion-cell membrane fusion required for entry. The MMTV entry receptor is transferrin receptor 1 (TfR1) (11). TfR1 belongs to a class of cell surface receptors that traffic to the acidic endosome upon ligand binding. Unlike MLV or HIV-1, which enter cells via the surface or a neutral compartment, MMTV entry occurs in a late endosomal compartment and probably requires co-trafficking of virions with receptor. The identification of TfR1 as the MMTV entry receptor explains in part the in vivo tissue-specific tropism of this virus, since activated cells of the immune system and dividing mammary epithelial cells express some of the highest levels of this protein in vivo (12–14). However, cell-type restriction in vivo is also probably due to post-entry events. For example, the enhancer elements in the LTR function predominantly in mammary epithelia and lymphoid cells and thus, MMTV is not transcribed in many tissues (15).
Retroviral Env proteins can have other activities in addition to mediating cellular entry and recent work has indicated that the MMTV Env protein may play additional roles in in vivo infection and MMTV-mediated tumorigenesis. In addition to interacting with TfR1 to mediate viral entry, the Env protein has been shown to activate antigen presenting cells, like DCs and B cells, via Toll-like receptor 4 (TLR4) (16,17). TLR4 is a member of a family of receptors that contribute to innate immune responses to pathogens (18). Env interaction with TLR4 may facilitate initial infection of cells of the immune system (see next section). The Env protein may also participate in the transformation of mammary epithelial cells (see below).
At least two other proteins required for efficient MMTV infection are encoded by additional alternatively spliced mRNAs (Fig. 1). These include a viral protein with HIV-1 rev-like activity required for efficient transport of unspliced MMTV mRNA from the nucleus, termed regulator of export of MMTV (Rem) (19,20) and the viral superantigen (Sag), encoded in the 3′ LTR, which is dispensable for in vitro infection but plays a critical role in virus dissemination in vivo (see next section). Several other alternatively spliced mRNAs originating from the MMTV genome have also been described, but their gene products and role in infection have not been well-studied (21,22).
In summary, since MMTV encodes a number of accessory proteins, as well as an Env that plays multiple roles, it is clearly not a simple retrovirus. Unlike HIV-1 and HTLV-1, viral gene products equivalent to tat and tax, respectively, which function as transcriptional activators of virus and host transcription have not been found in the MMTV genome. However, MMTV does encode proteins that allow it to alter the host immune system, as well as a protein that facilitates transport of unspliced viral RNA out of the nucleus. MMTV may encode these accessory proteins, particularly Sag, because its in vivo infection pathway requires trafficking through diverse cell types, as described in the next section.
MMTV in vivo infection pathway initiates in lymphoid cells
MMTV has two routes of acquisition in vivo. Susceptible strains acquire exogenous virus through milk and can be freed of MMTV by foster-nursing on uninfected mothers, while other strains inherit endogenous copies of the provirus (2). Virtually all laboratory strains have from 2 to 8 endogenous proviruses (termed Mtv loci and numbered in order of their discovery). In general, the endogenous proviruses do not encode functional viruses, although a few inbred strains, such as the GR strain which was selected for high mammary tumor incidence, have retained active endogenous proviruses (23). These active endogenous proviruses probably represent recent germ line integrations, since it has been estimated that endogenous MMTVs have been present in the mouse genome for 20 million years (24,25). Interestingly, while most of the endogenous MMTVs sustain mutations in the coding regions for the virion proteins, almost all retain intact Sag coding regions. This suggests that there is selection for the retention of endogenous sags.
Although mammary epithelial cells are the ultimate targets for MMTV infection, cells of the immune system play multiple critical roles in in vivo infection (3). During milk-borne infection, MMTV first infects DCs in the small intestine and Peyer’s patches (17) (Fig. 2). Retroviral infection is a multi-step process, initiating with the binding of the viral Env protein to one or more cell surface receptors and terminating with the migration of reverse-transcribed viral DNA into the nucleus and integration into the chromosomes. For most retroviruses, including MMTV, this latter step is dependent on the nuclear membrane breakdown that occurs during cell division, because the reverse-transcribed replication complex cannot cross the nuclear membrane. MMTV both infects and activates DCs (26,27). MMTV accomplishes the initial activation of DCs in part because the virion Env interacts with TLR4 (16,17,28). DC activation by MMTV also induces increased expression of the entry receptor TfR1, thereby potentially facilitating their infection (17). Virus interaction with TLR4 also induces their migration to the lymph node, by causing increased expression of CCR7, the receptor for the chemokine macrophage inflammatory protein 3β (26,28). Upon binding to ligand, members of the TLR family also activate signal transduction pathways that result in the production of anti-inflammatory cytokines and interferons, which in turn can influence the adaptive immune response (29). Indeed, there is evidence suggesting the MMTV’s interaction with TLR4 shifts the adaptive immune response from a protective TH1 (cytolytic T cell) to a nonprotective TH2 (antibody) response (30).
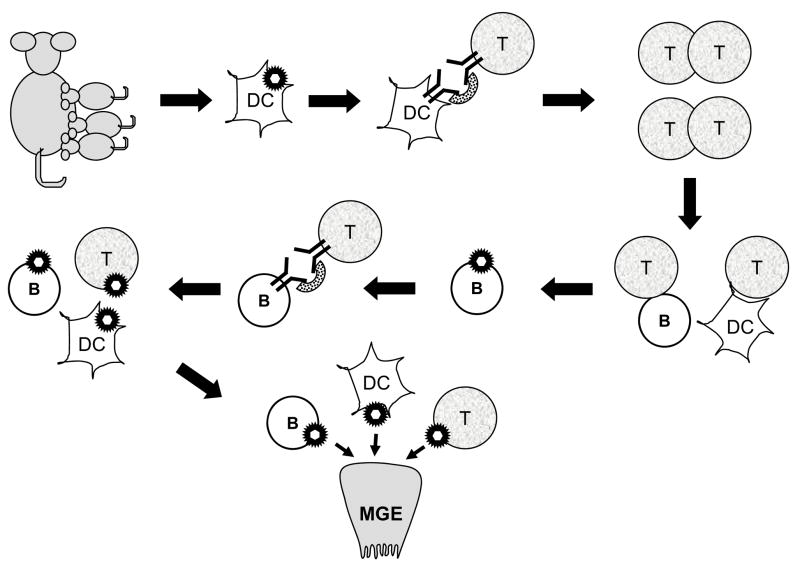
Fig. 2.
In vivo infection by MMTV. DCs in the gut get infected by MMTV, then traffic to the lymph node where they present Sag to cognate T cells. The T cells get activated, secrete cytokines and provide B and DC cell help, thereby creating a reservoir of infection-competent lymphocytes. Infected B cells then further amplify infection by presenting Sag to T cells. The infected lymphoid cells traffic to the mammary gland, where they transmit virus to mammary epithelial cells (MGE).
MMTV amplification in the lymphoid compartment also depends on T cells. After milk-borne acquisition of virus, infected DCs present the MMTV Sag via major histocompatibility (MHC) class II proteins expressed on the surface of infected antigen presenting cells, to T cells bearing specific T cell receptor Vβ chains. Different MMTV strains interact with particular Vβ-bearing T cells because they encode Sag proteins with different C-terminal amino acid sequences (termed the hypervariable region); this region of the Sag protein contacts the TCR Vβ molecule. Sag-mediated lymphocyte activation is a requisite step in the infection pathway (3). The Sag-cognate T cells proliferate, provide B cell help and produce cytokines that stimulate and recruit additional DCs, B and T cells, resulting in the establishment of a reservoir of infection-competent and infected cells (31). Sag presentation causes the proliferation of specific Vβ-bearing T cells when it is recognized as foreign but deletion of such T cells when it is recognized as self. Thus, mice infected as neonates show initial activation of Sag-cognate T cells, followed by their gradual deletion (32). Since except for milk there is no cell-free MMTV in vivo, infected lymphocytes are also critical for virus spread to the mammary gland (2,33,34) (Fig. 2).
MMTV-induced tumorigenesis
Infected lymphocytes carry virus to the mammary gland (33,34). Mammary epithelial cells become infected with MMTV at a time when they are driven to divide, that is, during the hormonal stimulation that accompanies puberty and pregnancy. Once MMTV infects mammary cells, virus amplification within this tissue is required both to maximize virion production and to induce mammary tumors. MMTV is a non-acute transforming retrovirus and mammary tumorigenesis takes place after proviral DNA integration near cellular proto-oncogenes that activates their transcription (Fig. 3); indeed, the mammalian Wnt gene family and several other oncogenes were discovered because of their association with MMTV (35) (see the article by Callahan and ? in this volume). Because MMTV integration does not appear to be site-specific (36), the more virus produced, the more likely it is that proviral DNA will integrate near a proto-oncogene. Thus, latency and incidence of tumor formation are proportional to virus load (37). Moreover, since the mammary glands of virgin mice go through fewer cycles of cell division than those of multiparous mice, virgin mice have fewer MMTV-infected epithelial cells and thus a lower incidence and longer latency of tumor induction; estrogen-treated males also develop MMTV-induced mammary tumors (2).
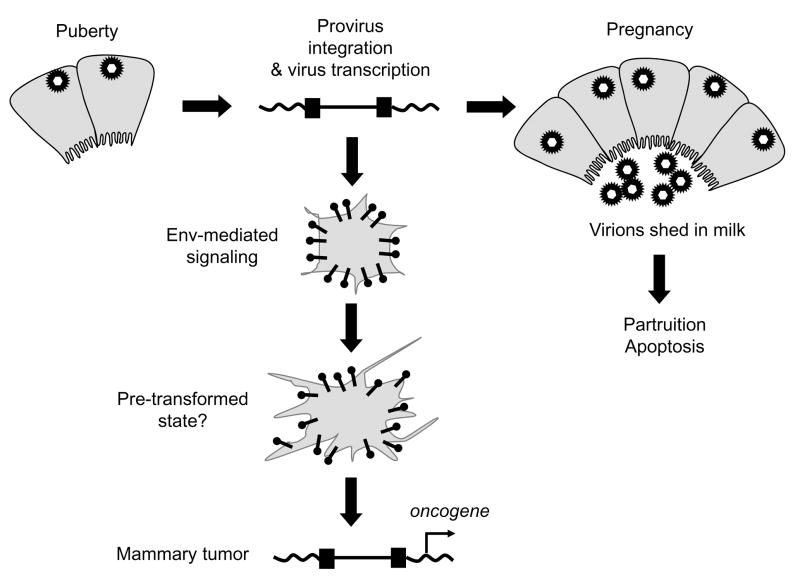
Fig. 3.
MMTV-induced mammary tumors. Virus infects dividing mammary epithelial cells during puberty and viral DNA integrates in the genome. Further proviral integration occurs in mammary epithelial cells stimulated to divide during pregnancy. During lactation, virus is shed into milk and transmitted to the next generation; the fully differentiated mammary epithelial cells undergo apoptosis during partruition. Infected mammary epithelial stem cells express the Env protein, which through ITAM signaling, may predispose mammary cells to more rapid transformation in conjunction with proviral integration near a cellular oncogene.
The Env protein may also play a role in mammary tumorigenesis. Ectopic expression of the MMTV Env in normal mammary epithelial cells results in their phenotypic transformation and an activation motif termed the Immunoreceptor tyrosine-based activation motif (ITAM) in this protein is critical to this activity (38). Expression of the MMTV Env alone in transgenic mice causes increased lobuloalveoar budding in their mammary glands, but not mammary tumors, indicating that ITAM signaling is not sufficient for cellular transformation in vivo (39). However, Env signaling clearly participates in MMTV-mediated transformation, since mutation of the ITAM within the context of an infectious MMTV reduces virus-induced mammary tumorigenesis without affecting infection levels (39). ITAMs are commonly found in receptors expressed in hematopoietic cells and are negatively regulated by cell-type specific modulators. Uncontrolled signaling by the envelope protein in epithelial cells, which lack such negative modulators, may be an early step in the MMTV transformation process (Fig. 3). Interestingly, other oncogenic viruses, such as Epstein Barr Virus (EBV) and Kaposi’s Sarcoma Herpes Virus (KSHV), encode viral proteins with ITAMs that play a role in the transformation of non-hematopoietic cells (40–42) and the Env protein of the Jaagsiekte sheep retrovirus (JSRV), which signals through the Akt pathway, is known to be required for transformation of lung epithelial cells (43–45).
While EBV and KSHV cause both lymphoid and non-lymphoid cancers, MMTV is primarily associated with mammary tumors. However, variant MMTVs have been isolated from T cell lymphomas with altered LTRs (46). These MMTV variants most likely cause lymphomas because the LTR alterations create novel enhancers that allow high level of virus expression in T cells (47,48). Interestingly, the lymphoma-causing MMTVs integrate near c-myc, Notch family and other oncogenes rather than Wnt1 and fibroblast growth factor (fgf) family members (49,50). Although, like EBV, MMTV also infects B cells, it has not been associated with any B cell malignancies.
Genetic resistance MMTV-induced mammary tumors
Interest in genetic predisposition to human breast cancer led to breeding experiments between mouse strains with high mammary tumor incidence and those with low tumor incidence, as a means of identifying breast cancer susceptibility genes (51,52). A number of mouse strains were identified that were resistant to MMTV-induced mammary tumors, but thus far, most have been shown to control susceptibility to virus infection rather than tumorigenesis itself. In some strains, resistance to virus infection is due to the inability of certain major MHC class II genes to present Sag, thereby aborting the in vivo infection process at an early step (53). For example, C57BL/6 mice (MHC haplotype H-2b) that lack the I-E chain required for efficient presentation of most MMTV Sags are not readily infected by virus. This resistance phenotype can overcome by the introduction of the I-E molecule as a transgene, although only infection and not mammary tumorigenesis was examined in this study (54). Similarly, the retention of endogenous sag genes with the same Vβ-specificity as those encoded by infectious virus precludes infection because mice delete Sag-responsive T cells during the shaping of the immune repertoire and thus, lack a reservoir of infection-competent cells (3). Not surprisingly, both B cells and DCs are also required for efficient MMTV infection, most likely because they both present Sag and serve as virus reservoirs (28,55). Interestingly, while there is an absolute requirement for DCs, B and T cells, the requirement for lymphocyte activation, either via TLR4 or Sag, is not absolute. Mice with mutant Tlr4 genes still get infected with virus, although the kinetics is somewhat delayed (30)(Rassa and Ross, unpublished observations). Similarly, Sag activity seems to be required primarily for lymphocyte amplification but not initial infection in the gut (56). This may be because MMTV can enter lymphocytes or DCs that are activated by commensal organisms in the gut.
Other mechanisms of resistance to MMTV infection also occur. The I/LnJ strain shows wild-type infection of lymphocytes, yet little or no transfer of virus to mammary tissue because these mice develop high titer anti-MMTV antibodies as they age that block efficient mammary gland infection (57). B10.BR mice are resistant to MMTV infection because their T cells have an attenuated, MHC-independent signaling response to the viral Sag and thus, there is little amplification of lymphocyte infection (58). Similarly, MMTV can infect and by transmitted by YBR/Ei mice, but virus production is severely attenuated through an as-of-yet uncharacterized T cell-mediated restriction (59). It has also recently been shown that BALB/c congenic mice lacking endogenous Mtv loci are resistant to infection; the mechanism of this resistance is not yet known (60).
There are a few indications that there may also be genetic differences in tumorigenesis. Impaired recognition of tumor cells by the cellular immune system might be responsible for the increased susceptibility of BALB/c mice to MMTV-induced mammary tumors (61). Finally, there is evidence that MMTV integration into the commonly targeted integration sites (CIS) are different in tumors induced in various inbred mouse strains (35,39). This suggests that the biology of the mammary epithelial cells of different inbred strains dictates which particular MMTV insertionally-altered oncogenes will result in mammary tumors.
Is there a HMTV?
Shortly after MMTV was shown to be a retrovirus, the search for an equivalent human virus began. Early studies reported finding MMTV-like proteins in human breast cancer biopsies and antibodies against the mouse virus proteins in human breast cancer patients (62,63). However, a more recent study that tested sera from almost 100 patients with breast cancer, using Western blots as the readout for the presence of antibodies against MMTV proteins rather than indirect methods, indicated no immunological reactivity against MMTV proteins (64).
Several groups have reported MMTV-like sequences in up to 30% of human breast cancers (65–68). Unlike previous reports that showed a high degree of homology between the pol genes of human endogenous retroviruses such as HERV-K and MMTV, these investigators found MMTV-like env sequences by PCR amplification of DNA from human breast cancer tissue. Although MMTV-induced mammary tumors in mice are associated with high level infection of normal as well as transformed mammary tissue, which is necessary to achieve insertional activation of cellular oncogenes, the HMTV sequences have only been found in tumor and not normal tissue from the same patients (66). While HMTV sequences have been reported by several investigators, others have been unable to replicate these studies (69–71).
There have also been a number of reports that continuous passage of certain strains of MMTV on human breast cancer cell lines results in adapted viruses that infect human cells (72,73). The mechanism by which MMTV infection of human cells occurs is unclear. The human TfR1 does not function as an MMTV entry receptor (11,74). The HMTV sequences are highly related to MMTV, particularly the endogenous Mtv loci, and there are no consistent changes in the Env protein of the HMTVs that would predict a changed tropism for the human TfR1. Moreover, some of the human cell-adapted MMTV envelopes still show tropism for mouse and not human TfR1 and these viruses appear not to have spread in the human cultures (75). More recently, one group has shown that MMTV can infect cultured human mammary breast cancer cells by an undefined mechanism (76,77). Taken together, these data suggest that neither the HMTVs nor MMTV use TfR1 for entry into human cells.
The mode of transmission of a potential HMTV has also not been described. If HMTV was transmitted through milk, then breast feeding should be associated with increase cancer risk in daughters. However, a large epidemiological study showed that there is no increase in breast cancer incidence in the breast-fed daughters of mothers who developed breast cancer compared those who were not breast-fed (78). Moreover, as described above, pregnancy in MMTV-infected mice is associated with greatly increased tumor incidence, yet in humans, pregnancy appears to have a protective effect (79) and breast cancer rates have gone up at the same time that breast feeding rates have decreased (80). Based on an epidemiological analysis showing that high breast cancer incidence in humans geographically co-localizes with the prevalence of mus domesticus in the environment, it has been suggested that MMTV may spread to humans from feral mice (81). However, the only clearly established mode of transmission of infectious MMTV in mice is through nursing, most likely because with the exception of milk, all of the virus in vivo appears to be cell-associated; there is little evidence for virions in blood, saliva or seminal fluid (2,33). Additionally, mice infected as adults have life-long, high-titer antibodies against MMTV (82). Thus, if HMTV was a zoonotic transmission from mice, humans with breast cancer should have anti-MMTV antibodies, which at least in a recent study, have not been detected (64).
Concluding Remarks
Much has been learned about the biology of MMTV since its discovery early in the last century, largely through the use of classical genetics and the more modern use of genetically altered mice. Indeed, the MMTV in vivo infection pathway is probably one of the best-characterized both with regard to the virus and the host. While MMTV has served as a valuable model, particularly through the use of its LTR to direct transgene expression to mammary tissue, it is currently unclear whether a similar transmissible agent exists in humans. Confirmation of such a virus awaits the cloning of HMTV insertion sites in human mammary tumors and the identification of the means by which this virus infects, both at the level of the cell and organism.
Abbreviations
- MMTV
mouse mammary tumor virus
- HMTV
human mammary tumor virus
- Sag
superantigen
- Env
envelope
- MLV
murine leukemia virus
- HIV-1
human immunodeficiency virus-1
- HTLVI
human T cell leukemia virus I
- CA
capsid
- NC
nucleocapsid
- LTR
long terminal repeat
- EIAV
equine infectious anemia virus
- Env
envelope
- SU
surface
- TM
transmembrane
- TfR1
transferrin receptor 1
- Rem
regulator of export of MMTV
- TLR4
Toll-like receptor 4
- DCs
dendritic cells
- ITAM
immunoreceptor tyrosine-based activation motif
- EBV
Epstein Barr Virus
- KSHV
Kaposi’s Sarcoma Herpes Virus
- CIS
common integration site
- fgf
fibroblast growth factor
References
- 1.Bittner JJ. Some possible effects of nursing on the mammary gland tumor incidence in mice. Science. 1936;84:162. doi: 10.1126/science.84.2172.162. [DOI] [PubMed] [Google Scholar]
- 2.Nandi S, McGrath CM. Mammary neoplasia in mice. Adv Canc Res. 1973;17:353–414. [Google Scholar]
- 3.Ross SR. Using genetics to probe host-virus interactions: the mouse mammary tumor virus model. Microbes and Inf. 2000;2:1215–23. doi: 10.1016/s1286-4579(00)01275-2. [DOI] [PubMed] [Google Scholar]
- 4.Mink S, Hartig E, Jennewein P, Doppler W, Cato ACB. A mammary cell-specific enhancer in mouse mammary tumor virus DNA is composed of multiple regulatory elements including binding sites for CTF/NF-1 and novel transcription factor, mammary cell-activating factor. Mol Cell Biol. 1992;11:4906–18. doi: 10.1128/mcb.12.11.4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mok E, Golovkina TV, Ross SR. A mouse mammary tumor virus (MMTV) mammary gland enhancer confers tissue-specific, but not lactation-dependent expression in transgenic mice. J Virol. 1992;66:7529–32. doi: 10.1128/jvi.66.12.7529-7532.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reuss FU, Coffin JM. Stimulation of mouse mammary tumor virus superantigen expression by an intragenic enhancer. Proc Natl Acad Sci USA. 1995;92:9293–7. doi: 10.1073/pnas.92.20.9293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu Q, Maitra U, Johnston D, Lozano M, Dudley JP. The homeodomain protein CDP regulates mammary-specific gene transcription and tumorigenesis. Mol Cell Biol. 2004;24:4810–23. doi: 10.1128/MCB.24.11.4810-4823.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vicent GP, Ballare C, Zaurin R, Saragueta P, Beato M. Chromatin remodeling and control of cell proliferation by progestins via cross talk of progesterone receptor with the estrogen receptors and kinase signaling pathways. Ann NY Acad Sci. 2006;1089:59–72. doi: 10.1196/annals.1386.025. [DOI] [PubMed] [Google Scholar]
- 9.Coffin JM, Hughes SH, Varmus HE, editors. Retroviruses. Cold Spring Harbor (NY): CSHL Press; 1997. [PubMed] [Google Scholar]
- 10.Payne SL, Elder JH. The role of retroviral dUTPases in replication and virulence. Curr Protein Pept Sci. 2001;2:381–8. doi: 10.2174/1389203013381008. [DOI] [PubMed] [Google Scholar]
- 11.Ross SR, Schofield JJ, Farr CJ, Bucan M. Mouse transferrin receptor 1 is the cell entry receptor for mouse mammary tumor virus. Proc Natl Acad Sci USA. 2002;99:12386–90. doi: 10.1073/pnas.192360099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schulman HMPPWA, Gauthier Y, Shyamala G. Transferrin receptor and ferritin levels during murine mammary gland development. Biochim Biophys Acta. 1989;1010:1–6. doi: 10.1016/0167-4889(89)90176-6. [DOI] [PubMed] [Google Scholar]
- 13.Futran J, Kemp JD, Field EH, Vora A, Ashman RF. Transferrin receptor synthesis is an early event in B cell activation. J Immunol. 1989;143:787–92. [PubMed] [Google Scholar]
- 14.Brekelmans P, van Soest P, Voerman J, Platenburg PP, Leenen PJ, van Ewijk W. Transferrin receptor expression as a marker of immature cycling thymocytes in the mouse. Cell Immunol. 1994;159:331–9. doi: 10.1006/cimm.1994.1319. [DOI] [PubMed] [Google Scholar]
- 15.Ross SR. MMTV and the immune system. Adv Pharm. 1997;39:21–46. doi: 10.1016/s1054-3589(08)60068-x. [DOI] [PubMed] [Google Scholar]
- 16.Rassa JC, Meyers JL, Zhang Y, Kudaravalli R, Ross SR. Murine retroviruses activate B cells via interaction with Toll-like receptor 4. Proc Natl Acad Sci USA. 2002;99:2281–6. doi: 10.1073/pnas.042355399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burzyn D, Rassa JC, Kim D, Nepomnaschy I, Ross SR, Piazzon I. Toll-like receptor 4-dependent activation of dendritic cells by a retrovirus. J Virol. 2004;78:576–584. doi: 10.1128/JVI.78.2.576-584.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 19.Mertz JA, Simper MS, Lozano MM, Payne SM, Dudley JP. Mouse mammary tumor virus encodes a self-regulatory RNA export protein and is a complex retrovirus. J Virol. 2005;79:14737–47. doi: 10.1128/JVI.79.23.14737-14747.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Indik S, Gunzburg WH, Salmons B, Rouault F. A novel, mouse mammary tumor virus encoded protein with Rev-like properties. Virol. 2005;337:1–6. doi: 10.1016/j.virol.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 21.Salmons B, Erfle V, Brem G, Günzburg WH. Naf, a trans-regulating negative-acting factor within the mouse mammary tumor virus open reading frame region. J Virol. 1990;64:6355–59. doi: 10.1128/jvi.64.12.6355-6359.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rouault F, Nejad Asl SB, Rungaldier S, Fuchs E, Salmons B, Gunzburg WH. Promoter complex in the central part of the mouse mammary tumor virus long terminal repeat. J Virol. 2007;81:12572–81. doi: 10.1128/JVI.00351-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michalides R, van Nie R, Nusse R, Hynes NE, Groner B. Mammary tumor virus induction loci in GR and DBAf mice contain one provirus of the mouse mammary tumor virus. Cell. 1981;23:165–73. doi: 10.1016/0092-8674(81)90281-6. [DOI] [PubMed] [Google Scholar]
- 24.Morris VL, Medeiros E, Ringold GM, Bishop JM, Varmus HE. Comparison of mouse mammary tumor virus-specific DNA in inbred, wild and Asian mice, and in tumors and normal organs from inbred mice. J Mol Biol. 1977;114:73–91. doi: 10.1016/0022-2836(77)90284-4. [DOI] [PubMed] [Google Scholar]
- 25.Baillie GJ, van de Lagemaat LN, Baust C, Mager DL. Multiple groups of endogenous betaretroviruses in mice, rats and other mammals. J Virol. 2004;78:5784–98. doi: 10.1128/JVI.78.11.5784-5798.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin P, Ruiz SR, Martinez del Hoyo G, Anjuere F, Vargas HH, Lopez-Bravo M, Ardavin C. Dramatic increase in lymph node dendritic cell numbers during infection by the mouse mammary tumor virus occurs by a CD62L-dependent blood-borne DC recruitment. Blood. 2002;99:1282–8. doi: 10.1182/blood.v99.4.1282. [DOI] [PubMed] [Google Scholar]
- 27.Vacheron S, Luther SJ, Acha-Orbea H. Preferential infection of immature dendritic cells and B cells by mouse mammary tumor virus. J Immunol. 2002;168:3470–6. doi: 10.4049/jimmunol.168.7.3470. [DOI] [PubMed] [Google Scholar]
- 28.Courreges MC, Burzyn D, Nepomnaschy I, Piazzon I, Ross SR. Critical role of dendritic cells in mouse mammary tumor virus in vivo infection. J Virol. 2007;81:3769–77. doi: 10.1128/JVI.02728-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Neill LAJ. The interleukin-1 receptor/toll-like receptor superfamily: signal transduction during inflammation and host defense. Scie Stke. 2000:1–11. doi: 10.1126/stke.442000re1. [DOI] [PubMed] [Google Scholar]
- 30.Jude BA, Pobezinskaya Y, Bishop J, Parke S, Medzhitov RM, Chervonsky AV, Golovkina TV. Subversion of the innate immune system by a retrovirus. Nat Immunol. 2003;4:573–8. doi: 10.1038/ni926. [DOI] [PubMed] [Google Scholar]
- 31.Held W, Waanders G, Shakhov AN, Scarpellino L, Acha-Orbea H, MacDonald HR. Superantigen-induced immune stimulation amplifies mouse mammary tumor virus infection and allows virus transmission. Cell. 1993;74:529–40. doi: 10.1016/0092-8674(93)80054-i. [DOI] [PubMed] [Google Scholar]
- 32.Ignatowicz L, Kappler J, Marrack P. The effects of chronic infection with a superantigen-producing virus. J Exp Med. 1992;175:917–23. doi: 10.1084/jem.175.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Golovkina TV, Dudley JP, Ross SR. Superantigen activity is need for mouse mammary tumor virus spread within the mammary gland. J Immunol. 1998;161:2375–82. [PubMed] [Google Scholar]
- 34.Finke D, Acha-Orbea H. Differential migration of in vivo primed B and T lymphocytes to lymphoid and non-lymphoid organs. Eur J Immunol. 2001;31:2603–11. doi: 10.1002/1521-4141(200109)31:9<2603::aid-immu2603>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 35.Callahan R, Smith GH. MMTV-induced mammary tumorigenesis: gene discovery, progression to malignancy and cellular pathways. Oncogene. 2000;19:992–1001. doi: 10.1038/sj.onc.1203276. [DOI] [PubMed] [Google Scholar]
- 36.Faschinger A, Rouault F, Sollner J, Lukas A, Salmons B, Gunzburg WH, Indik S. Mouse mammary tumor virus integration site selection in human and mouse genomes. J Virol. 2008;82:13601–07. doi: 10.1128/JVI.02098-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Golovkina TV, Prescott JA, Ross SR. Mouse mammary tumor virus-induced tumorigenesis in sag transgenic mice: a laboratory model of natural selection. J Virol. 1993;67:7690–94. doi: 10.1128/jvi.67.12.7690-7694.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Katz E, Lareef MH, Rassa JC, Grande SM, King LB, Russo J, Ross SR, Monroe JG. MMTV Env encodes an ITAM responsible for transformation of mammary epithelial cells in three-dimensional culture. J Exp Med. 2005;201:431–9. doi: 10.1084/jem.20041471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ross SR, Schmidt JW, Katz E, Cappelli L, Hultine S, Gimmotty P, Monroe JG. An immunoreceptor tyrosine activation motif in the Mouse Mammary Tumor Virus envelope protein plays a role in virus-induced mammary tumors. J Virol. 2006;80:9000–8. doi: 10.1128/JVI.00788-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu J, Lin W-H, Chen S-Y, Longnecker R, Tsai S-C, Chen C-L, Tsai C-H. Syk tyrosine kinase mediates Epstein-Barr Virus latent membrane protein 2A-induced cell migration in epithelial cells. J Biol Chem. 2006;281:8806–14. doi: 10.1074/jbc.M507305200. [DOI] [PubMed] [Google Scholar]
- 41.Morrison JA, Raab-Traub N. Roles of the ITAM and PY motifs of Epstein-Barr Virus latent membrane protein 2A in the inhibition of epithelial cell differentiation and activation of β-catenin signaling. J Virol. 2005;79:2375–82. doi: 10.1128/JVI.79.4.2375-2382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee H, Guo J, Li M, Choi JK, DeMaria M, Rosenzweig M, Jung JU. Identification of an immunoreceptor tyrosine-based activation motif of K1 transforming protein of Kaposi’s sarcoma-associated herpesvirus. Mol Cell Biol. 1998;18:5219–28. doi: 10.1128/mcb.18.9.5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maeda N, Palmarini M, Murgia C, Fan H. Direct transformation of rodent fibroblasts by jaagsiekte sheep retrovirus DNA. Proc Natl Acad Sci USA. 2001;98:4449–54. doi: 10.1073/pnas.071547598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rai SK, Duh FM, Vigdorovich V, Danilkovitch-Miagkova A, Lerman MI, Miller AD. Candidate tumor suppressor HYAL2 is a glycosylphosphatidylinositol (GPI)-anchored cell-surface receptor for jaagsiekte sheep retrovirus, the envelope protein of which mediates oncogenic transformation. Proc Natl Acad Sci USA. 2001;98:4443–48. doi: 10.1073/pnas.071572898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wootton SK, Halbert CL, Miller AD. Sheep retrovirus structural protein induces lung tumours. Nature. 2005;434:904–7. doi: 10.1038/nature03492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Michalides R, Wagenaar E, Hilkens J, Hilgers J, Groner B, Hynes NE. Acquisition of proviral DNA of mouse mammary tumor virus in thymic leukemia cells from GR mice. J Virol. 1982;43:819–829. doi: 10.1128/jvi.43.3.819-829.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yanagawa SI, Kakimi K, Tanaka H, Murakami A, Nakagawa Y, Kubo Y, Yamada Y, Hiai H, Kuribayashi K, Amsuda T, Ishimoto A. Mouse mammary tumor virus with rearranged long terminal repeats causes murine lymphomas. J Virol. 1993;67:112–18. doi: 10.1128/jvi.67.1.112-118.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhadra S, Lozano MM, Dudley JP. Conversion of mouse mammary tumor virus to a lymphomagenic virus. J Virol. 2005;79:12592–6. doi: 10.1128/JVI.79.19.12592-12596.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yanagawa S, Lee JS, Kakimi K, Matsuda Y, Honjo T, Ishimoto A. Identification of Notch1 as a frequent target for provirus insertional mutagenesis in T-cell lymphomas induced by leukemogenic mutants of mouse mammary tumor virus. J Virol. 2000;74:9786–91. doi: 10.1128/jvi.74.20.9786-9791.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Broussard DR, Mertz JA, Lozano M, Dudley JP. Selection for c-myc integration sites in polyclonal T-cell lymphomas. J Virol. 2002;76:2087–99. doi: 10.1128/jvi.76.5.2087-2099.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bentvelzen P, Brinkhof J, Haaijman JJ. Genetic control of endogenous murine mammary tumour viruses reinvestigated. Eur J Canc. 1978;14:1137–47. doi: 10.1016/0014-2964(78)90070-1. [DOI] [PubMed] [Google Scholar]
- 52.Outzen HC, Morrow D, Shultz LD. Attenuation of exogenous murine mammary tumor virus virulence in the C3H/HeJ mouse substrain bearing the Lps mutation. J Natl Canc Inst. 1985;75:917–23. doi: 10.1093/jnci/75.5.917. [DOI] [PubMed] [Google Scholar]
- 53.Held W, Waanders GA, MacDonald HR, Acha-Orbea H. MHC class II hierarchy of superantigen presentation predicts efficiency of infection with mouse mammary tumor virus. Int Immunol. 1994;6:1403–1407. doi: 10.1093/intimm/6.9.1403. [DOI] [PubMed] [Google Scholar]
- 54.Pucillo C, Cepeda R, Hodes RJ. Expression of a MHC Class II transgene determines superantigenicity and susceptibility to mouse mammary tumor virus infection. J Exp Med. 1993;178:1441–5. doi: 10.1084/jem.178.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beutner U, Draus E, Kitamura D, Rajewsky K, Huber BT. B cells are essential for murine mammary tumor virus transmission, but not for presentation of endogenous superantigens. J Exp Med. 1994;179:1457–66. doi: 10.1084/jem.179.5.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pobezinskaya Y, Chervonsky AV, Golovkina TV. Initial stages of mammary tumor virus infection are superantigen independent. J Immunol. 2004;172:5582–7. doi: 10.4049/jimmunol.172.9.5582. [DOI] [PubMed] [Google Scholar]
- 57.Purdy A, Case L, Duvall M, Overstrom-Coleman M, Monnier N, Chervonsky A, Golovkina T. Unique resistance of I/LnJ mice to a retrovirus is due to sustained IFN-gamma dependent production of virus-neutralizing antibodies. J Exp Med. 2003;197:233–43. doi: 10.1084/jem.20021499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Okeoma CM, Shen M, Ross SR. A novel block to mouse mammary tumor virus infection of lymphocytes in B10. BR mice. J Virol. 2008;82:1314–22. doi: 10.1128/JVI.01848-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.MacDearmid CC, Case LK, Starling CL, Golovkina TV. Gradual elimination of retroviruses in YBR/Ei mice. J Virol. 2006;80:2206–15. doi: 10.1128/JVI.80.5.2206-2215.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bhadra S, Lozano MM, Payne SM, Dudley JP. Endogenous MMTV proviruses induce susceptibility to both viral and bacterial pathogens. PLoS Pathog. 2006;2:e128–e128. doi: 10.1371/journal.ppat.0020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Czarneski J, Meyers J, Peng T, Abraham V, Ross SR. Interleukin-4 up-regulates mouse mammary tumor virus expresssion but is not required for in vivo virus spread. J Virol. 2001;75:11886–90. doi: 10.1128/JVI.75.23.11886-11890.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Day NK, Witkin SS, Sarkar NH, Kinne D, Jussawalla DJ, Levin A, Hsia CC, Geller N, Good RA. Antibodies reactive with murine mammary tumor virus in sera of patients with breast cancer: geographic and family studies. Proc Natl Acad Sci USA. 1981;78:2483–87. doi: 10.1073/pnas.78.4.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mesa-Tejada R, Oster MW, Fenoglio CM, Magidson J, Spiegelman S. Diagnosis of primary breast carcinoma through immunohistochemical detection of antigen related to mouse mammary tumor virus in metastatic lesions: a report of two cases. Cancer. 1982;49:261–8. doi: 10.1002/1097-0142(19820115)49:2<261::aid-cncr2820490211>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 64.Goedert JJ, Rabkin CS, Ross SR. Prevalence of serologic reactivity against four strains of mouse mammary tumor virus among U.S. women with breast cancer. Br J Canc. 2006;94:548–51. doi: 10.1038/sj.bjc.6602977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pogo BGT, Melana SM, Holland JF, Mandeli JF, Polotti S, Casalini P, Menard S. Sequences homologous to the mouse mammary tumor virus env gene in human breast cancer correlate with overexpression of laminin receptor. Clin Canc Res. 1999;5:2108–11. [PubMed] [Google Scholar]
- 66.Wang Y, Holland JF, Bleiweiss IJ, Melana S, Liu X, Pelisson I, Cantarella A, Stellrecht K, Mani S, Pogo BG. Detection of mammary tumor virus ENV gene-like sequences in human breast cancer. Canc Res. 1995;35:5173–9. [PubMed] [Google Scholar]
- 67.Etkind P, Du J, Khan A, Pillitteri J, Wiernik PH. Mouse mammary tumor virus-like ENV gene sequences in human breast tumors and in a lymphoma of a breast cancer patient. Clin Canc Res. 2000;6:1273–78. [PubMed] [Google Scholar]
- 68.Liu B, Wang Y, Melana SM, Pelisson I, Najfeld V, Holland JF, Pogo BG. Identification of a proviral structure in human breast cancer. Canc Res. 2001;61:1754–59. [PubMed] [Google Scholar]
- 69.Mant C, Hodgson S, Hobday R, D’Arrigo C, Cason J. A viral aetiology for breast cancer: time to re-examine the postulate. Intervirol. 2003;47:2–13. doi: 10.1159/000076636. [DOI] [PubMed] [Google Scholar]
- 70.Witt A, Hartmann B, Marton E, Zeillinger R, Schreiber M, Kubista E. The mouse mammary tumor virus-like env gene sequence is not detectable in breast cancer tissue of Austrian patients. Oncol Rep. 2003;10:1025–9. [PubMed] [Google Scholar]
- 71.Bindra A, Muradrasoli S, Kisekka R, Nordgren H, Warnberg F, Blomberg J. Search for DNA of exogenous mouse mammary tumor virus-related virus in human breast cancer samples. Journal of General Virology. 2007;88:1806–9. doi: 10.1099/vir.0.82767-0. [DOI] [PubMed] [Google Scholar]
- 72.Lasfargues EY, Coutinho WG, Dion AS. A human breast tumor cell line (BT474) that supports mammary tumor virus replication. In Vitro. 1979;15:723–28. doi: 10.1007/BF02618252. [DOI] [PubMed] [Google Scholar]
- 73.Howard DK, Schlom J. Isolation of a series of novel variants of murine mammary tumor viruses with broadened host range. Int J Canc. 1980;25:647–54. doi: 10.1002/ijc.2910250515. [DOI] [PubMed] [Google Scholar]
- 74.Wang E, Albritton L, Ross SR. Identification of the segments of the mouse transferrin receptor 1 required for mouse mammary tumor virus infection. J Biol Chem. 2006;281:10243–9. doi: 10.1074/jbc.M511572200. [DOI] [PubMed] [Google Scholar]
- 75.Zhang Y, Rassa JC, deObaldia EM, Albritton L, Ross SR. Identification of the mouse mammary tumor virus envelope receptor-binding domain. J Virol. 2003;77:10468–78. doi: 10.1128/JVI.77.19.10468-10478.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Indik S, Gunzburg WH, Salmons B, Rouault F. Mouse mammary tumor virus infects human cells. Canc Res. 2005;65:6651–9. doi: 10.1158/0008-5472.CAN-04-2609. [DOI] [PubMed] [Google Scholar]
- 77.Indik S, Gunzburg WH, Kulich P, Salmons B, Rouault F. Rapid spread of mouse mammary tumor virus in cultured human breast cells. Retrovirol. 2007;4:73. doi: 10.1186/1742-4690-4-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Titus-Ernstoff L, Egan KM, Newcomb PA, Baron JA, Stampfer M, Greenberg ER, et al. Exposure to breast milk in infancy and adult breast cancer risk. J Natl Canc Inst. 1998;12:921–4. doi: 10.1093/jnci/90.12.921. [DOI] [PubMed] [Google Scholar]
- 79.Kampert JB, Whittemore AS, Paffenbarger RS., Jr Combined effect of childbearing, menstrual events, and body size on age-specific breast cancer risk. Am J Epidemiol. 1988;128:962–79. doi: 10.1093/oxfordjournals.aje.a115070. [DOI] [PubMed] [Google Scholar]
- 80.MacMahon B, Cole P, Brown J. Etiology of human breast cancer: a review. Natl Canc Inst. 1973;50:21–42. doi: 10.1093/jnci/50.1.21. [DOI] [PubMed] [Google Scholar]
- 81.Stewart THM, Sage RD, Stewart AFR, Cameron DW. Breast cancer incidence highest in the range of one species of house mouse, Mus domesticus. Brit J Canc. 2000;82:446–51. doi: 10.1054/bjoc.1999.0941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Luther SA, Maillard I, Luthi F, Scarpellino L, Diggelmann H, Acha-Orbea H. Early neutralizing antibody response against mouse mammary tumor virus; critical role of viral infection and superantigen-reactive T cells. J Immunol. 1997;159:2807–14. [PubMed] [Google Scholar]