Abstract
A three-stimulus auditory oddball series was presented to experienced Vipassana meditators during meditation and a control thought period to elicit event-related brain potentials (ERPs) in the two different mental states. The stimuli consisted of a frequent standard tone (500 Hz), an infrequent oddball tone (1000 Hz), and an infrequent distracter (white noise), with all stimuli passively presented through headphones and no task imposed. The strongest meditation compared to control state effects occurred for the distracter stimuli: N1 amplitude from the distracter was reduced frontally during meditation; P2 amplitude from both the distracter and oddball stimuli were somewhat reduced during meditation; P3a amplitude from the distracter was reduced during meditation. The meditation-induced reduction in P3a amplitude was strongest in participants reporting more hours of daily meditation practice and was not evident in participants reporting drowsiness during their experimental meditative session. The findings suggest that meditation state can decrease the amplitude of neurophysiologic processes that subserve attentional engagement elicited by unexpected and distracting stimuli. Consistent with the aim of Vipassana meditation to reduce cognitive and emotional reactivity, the state effect of reduced P3a amplitude to distracting stimuli reflects decreased automated reactivity and evaluative processing of task irrelevant attention-demanding stimuli.
Keywords: Meditation, event-related potentials (ERPs), P3a, mental state, altered state of consciousness (ASC), Vipassana
1.0 Introduction
The neuroelectric (EEG) effects of meditation on brain activity are as yet not well characterized. There is no consensus as to whether evoked sensory and elicited cognitive event-related potentials (ERPs) are altered systematically from long hours dedicated meditators devote to their practice (Cahn and Polich, 2006). Some meditation effects of increased attention-related activations have been reported for changes in P300 amplitude (Banquet and Lesévre, 1980; Murthy et al., 1997; Sarang and Telles, 2006), contingent negative variation (CNV) amplitude (Travis et al., 2000; Travis et al., 2002), and frontal midline theta power (Aftanas and Golocheikine, 2001; Hebert and Lehmann, 1977). Meditation is most readily conceived as a set of diverse and specific methods of distinct attentional engagement and recent reports have begun to focus specifically on measures of attentional engagement during (state) and from (trait) meditation (Brefczynski-Lewis et al., 2007; Holzel et al., 2007; Jha et al., 2007; Pagnoni and Cekic, 2007; Raz and Buhle, 2006; Slagter et al., 2007; Srinivasan and Baijal, 2007).
The goal of present study was to assess the state effects of meditation in experienced Vipassana meditators (average 13 years of daily meditation practice) using stimulus conditions indexing neurophysiologic processing underlying perception and attentional engagement. A passive three-stimulus auditory oddball task was employed as it did not require participants to disengage from meditation practice to produce a behavioral response, simultaneously allows for characterization of the sensory aspects of audition via the N1/P2 components, and assays attentional engagement via the P3a potential elicited by the o distracter stimulus (Combs and Polich, 2006; Polich, 2007). As the primary goal was to characterize neurocognitive meditation effects, a within-subject meditation vs. control cognitive task paradigm was employed.
1.1. Vipassana meditation
Vipassana meditation is a traditional Buddhist practice that involves focusing on present-moment sensory awareness within an equanimous and non-reactive mental set. This tradition has served as the foundation for the development of contemporary “mindfulness” meditation techniques that are being used clinically (Davidson, 2003; Kabat-Zinn, 1982, 2003). Development of greater awareness of and non-reactivity to intero- and exteroceptive sensory stimuli during formal Vipassana/mindfulness meditation is hypothesized to enhance self-awareness such that selective adaptive responding is facilitated at the expense of automated nonadaptive reactions, thereby promoting more successful management of stressful life situations (Hart, 1987; Lutz et al., 2007; Segal et al., 2002). Vipassana practitioners of the Theravadin Vipassana tradition as taught by S. N. Goenka (Hart, 1987) were assayed. This practice emphasizes deep attentional absorption in subtle somatosensory awareness and associated self-monitoring without mental reactivity to such sensory experience. It was expected that meditation state effects would reflect a decrease in automated cognitive reactivity to the infrequent distracter stimuli of the auditory three-stimulus paradigm.
The neural loci of Vipassana/mindfulness meditation effects are of key empirical and theoretical import. A study using fMRI demonstrated that experienced Vipassana meditators during meditation evinced higher levels of hemodynamic activity in rostral anterior cingulate cortex and medial prefrontal cortex relative to novice meditators (Holzel et al., 2007). Moreover, experienced meditators in the mindfulness-based traditions have consistently demonstrated higher levels of attention-related activity in prefrontal areas. This outcome is consistent with findings that selective attentional control is increased in meditative practice partly through the recruitment of prefrontal cortical activity (Baerentsen, 2001; Cahn and Polich, 2006; Lazar et al., 2003; Ritskes et al., 2003). Following a three-month intensive Vipassana meditation retreat, practitioners but not control participants demonstrated P300 amplitude decreases to the initial stimulus during the attentional blink paradigm suggesting enhanced attentional engagement to the full stimulus train (Slagter, et al., 2007). Furthermore, several investigations of mindfulness meditation practice have reported increased functioning of attentional measures such as executive attention (Chan and Woollacott, 2007; Tang et al., 2007; Wenk-Sormaz, 2005), visual sensitivity (Brown et al., 1984, 1984; Brown, 2007), as well as endogenous orienting and exogenous alerting-related functions (Jha, et al., 2007).
These attention-related meditation effects may stem from physical changes induced in Vipassana meditators, who have increased cortical thickness in regions related to auditory, visual, somatosensory, and interoceptive processing (Lazar et al., 2005). The strongest of these effects have been observed in the right anterior insula, an area related to bodily attention and increased visceral awareness (Craig, 2002, 2003; Critchley et al., 2004). That meditative practice was the cause for these changes in cortical thickness is not definitive, as cross-sectional rather than longitudinal samples were assessed. However, the cortical thickness of the meditation vs. control young participant groups was similar and implies that meditation practice may have slowed the age-related thinning of the insular and prefrontal cortical areas. Assessment of Zen meditators compared to controls yielded similar findings (Pagnoni and Cekic, 2007). The absence of age-related gray matter loss was especially prominent in the putamen and was accompanied by improved sustained attentional functioning in the meditator group as assessed by a rapid visual processing sustained attention. Thus, Vipassana meditation appears to be associated with differences in attentional deployment, brain function, and cortical structures that may underlie meditation’s long-term effects of decreased emotional reactivity, increased well-being and compassion, and reported changes in self-experience (Goleman, 1996; Wallace, 1999) and scientific (Astin, 1997; Farb et al., 2007; Travis et al., 2004; Wallace and Shapiro, 2006).
1.2. Meditation and ERPs
The earliest studies on the effects of meditation on neuroelectric activity associated with stimulus processing were concerned with alpha blocking. Assessment of concentrative Yogic practices indicated that during meditation some highly experienced experts did not demonstrate the characteristic alpha blocking to auditory clicks or aversive stimuli such as placing the hands in cold water (Anand, 1961; Wenger and Bagchi, 1961). The results suggested that practitioners during meditation may be able to tune the relevant neural attentional networks such that brain activity is not activated to the same extent by stimulation. Studies of Japanese Zen monks, schooled in a tradition with similarity to the mindfulness-focus of Vipassana, indicated that with regularly repetitive auditory stimulation the normal habituation of alpha blocking was not observed in meditation masters compared to novices (Hirai, 1974; Kasamatsu and Hirai, 1966). This lack of habituation was thought to indicate that long-term meditation was associated with a “de-automization” of sensory and cognitive processing such that successive auditory stimuli were perceived as fresh. Later studies further indicated that alpha power was less disrupted in meditation than control states during presentation of loud aversive stimuli (Lehrer et al., 1980) and “name calling” (Kinoshita, 1975). Thus, meditation may lead to neurophysiologic states that are less reactive to stimulus-driven automated processing.
Cognitive ERPs have been used to assess meditation states and traits, with the P300 elicited to characterize attention and memory processing (Cahn and Polich, 2006). P3a is hypothesized to index frontal neural activity produced by stimulus-driven attention mechanisms, whereas the P3b indexes temporal–parietal activity reflecting resource allocation that contributes subsequent memory processing (Polich, 2007). An early study found shorter response times and increased N1 and P2 as well as P3b amplitudes to visual stimuli after a period of meditation in experienced yoga meditators compared to component amplitude decreases after a period of rest in non-meditators (Banquet and Lesévre, 1980). However, a subsequent investigation obtained no systematic effects of yoga, TM, or Zen meditation on any component from auditory stimuli, although post-hoc analyses of the TM and yoga groups demonstrated increased N1 component amplitudes towards the beginning of the stimulus train (Becker and Shapiro, 1981). A series of reports using Transcendental Meditation participants suggested that increased length of meditation practice was associated with decreased P3b latencies (Cranson et al., 1990; Goddard, 1989, 1992), and that decreased P3b latencies were observed after meditation but not rest periods (Travis and Miskov, 1994). Depressed and dysthymic individuals evinced improved clinical status that occurred with increases in P3b amplitude from an auditory oddball task after a period of concentrative meditation training (Murthy et al., 1997). P3b amplitudes from auditory stimuli were increased after a session of concentrative meditation (Sarang and Telles, 2006). Using an attentional blink paradigm that manipulates fundamental sensory responsivity to visual stimuli demonstrated that after an intensive Vipassana meditation retreat, meditators showed a decrease in visual P3b amplitude to the T1 stimulus and concomitant increase in T2 target detection, reflecting more efficient attentional processing (Slagter, et al., 2007). In sum, the P300 component may be modulated by meditative practice, although whether such findings are consistent across subjects or specific to different sensory domains and particular meditative practices is as yet unclear as is the relative impact on P3a compared to P3b.
1.2. Present study
Although these findings suggest that meditation appears to influence brain function, systematic evaluation of meditation state in comparison to comparable but not meditative thought conditions in long-term practitioners is needed (Lutz et al., 2008). The present study assayed ERP effects between a meditation and control state using an equal-length mental control state through the injunction to let the mind wander freely through non-emotional thoughts and memories. This control task was chosen to induce a state particularly contrasted with the purposeful engagement of attention involved in meditation. Further, this state was designed to mimic a mind-wandering state thought to have good ecologic validity to a common mode of cognitive engagement in normal everyday life (Smallwood and Schooler, 2006).
The auditory three-stimulus paradigm was employed to assess the various components of sensory and cognitive brain functions modulated during meditation. The N1, P2, and P3a components were elicited by a white noise distracter and tone stimuli (cf. Combs and Polich, 2006; Polich, 1989). This paradigm was presented in the absence of a behavioral response task so as to allow the participants to fully engage in the respective meditative and control state. Even with passive presentation instructions, the repetitive presentation of the standard stimuli at fixed interstimulus intervals entrains a state of cognitive/brain expectation that such stimuli will continue (Jeon and Polich, 2001). The inclusion of the oddball tone stimuli engages automated processes of change evaluation and the inclusion of the somewhat aversive white noise distracter tone bursts engages additional frontal cognitive functions (Polich, 2007). Vipassana meditation practice is thought to enhance awareness of internal and external stimuli while reducing reactivity. It was hypothesized that smaller P3a amplitude to distracter stimuli in meditation relative to the control condition would be observed, thereby reflecting decreased evaluative cognitive processing and brain reactivity to attention-demanding stimuli.
2.0 Methods
2.1. Participants
A total of N=16 Vipassana meditators (F=5, M=11) were assessed (age M=45.5, SD=9.8, 24–56 years). These individuals had been meditating for an average of 20 years (M=20.0 SD=12.1, 2.5–40), and had been meditating daily for at least two years (M=13.0, SD=10.7, 1–30), and doing so for at least one-half hour or more each day (M=1.3, SD=0.7, 0.5–3 hours). Participants were recruited from a local Vipassana meditation community through word of mouth and e-mail. Participants were compensated $40 for the three-hour study.
2.2 Recording conditions
EEG data was collected using a 19-channel ECI electrode cap from the following locations: Fp1, Fp2, F3, F4, F7, F8, Fz, C3, C4, T7, T8, Cz, P3, P4, P7, P8, Pz, O1, and O2. These scalp locations were referenced to balanced linked earlobes, with the ground at the forehead. Eye movement (EOG) activity was assessed with electrodes placed at the outer canthi and above/below the left eye in line with the pupil for vertical EOG monitoring using bipolar reference. Impedances were kept below 10 kΩ. The signals were recorded with a bandpass of 0.01–70 Hz (6 dB octave/slope), and digitization rate of 256 Hz.
2.3. Procedure and stimuli
The participants were instructed to meditate in their usual manner as taught within the Theravadin Vipassana meditation tradition as taught by S.N. Goenka (Hart, 1987) or engage in the control neutral thinking state. The Vipassana meditative technique in this tradition involves attentional absorption in the subtle and gross sensations throughout the body in an iterative fashion, scanning body sensations from the top of the head to the toes and back again repeatedly, with the concomitant adoption of an attitude of detached observation and non-reactivity to any sensations and thoughts that may arise. Pilot testing indicated that some experimental participants found it difficult to refrain from engaging in their meditative practice when sitting in the meditative posture with eyes closed. Participants were therefore told to think about emotionally-neutral past events if they noticed themselves slipping into meditative practice state, and to otherwise let their mind wander freely through non-emotional neutral thoughts. This control cognitive engagement was chosen so as to emulate a “mind-wandering” state with high ecologic validity that stands in contrast to the purposeful attentional engagement of the meditation state. Participants were informed that after 25 min of eyes-closed meditation or control thinking they would hear a series of tones and that they were to simply continue their meditation or control cognitive engagement. After the two auditory paradigms, participants again were presented the auditory stimuli but instructed to respond with a button-press to the oddball target stimulus. Following each meditation or control session, participants also completed a CNV task. The data from the active oddball and CNV tasks are reported elsewhere.
The auditory three-stimulus paradigm consisted of pseudorandom presentation of 250 stimuli. Standard tones were 500 Hz and occurred with a probability of 0.80, oddballs were 1000 Hz tones presented with a probability of 0.10, and distracters were white noise bursts that occurred with a probability of 0.10. All stimuli were presented over headphones with an intensity of 80 dB SPL, duration of 60 ms (5 ms r/f), and the interstimulus interval of 1 s. At the conclusion of the first recording period the participants were given the opportunity to stand up and stretch before taking the same posture and seating position for the second recording of equal length. Immediately after each of the two experimental periods they filled out a short form indicating whether they had experienced drowsiness or sleep onset during the session and rating the depth of meditative experience on a 1–10 scale, with 1 the normal waking state and 10 the deepest level of meditative absorption ever experienced. One-half the participants were randomly selected to meditate first, and one-half the participants performed the control task first.
2.4. ERP analysis
The neuroelectric data were first low-pass filtered using zero-phase Butterworth filter (0–20 Hz, 12 dB/octave), and trials were defined from −50 to +950 ms relative to onset of the stimulus. Trials containing voltages of greater magnitude than ±100 μV were excluded from the analyses, and EOG artifacts were corrected according to a correlation procedure (Gratton et al., 1983). The mean voltage from the −50 to 0 ms pre-stimulus interval was subtracted from the waveform for each trial, with the epochs averaged according to stimulus type. Accepted mean numbers of trials for each stimulus type were standards=164.6, oddballs=21.3, and distracters=19.7.
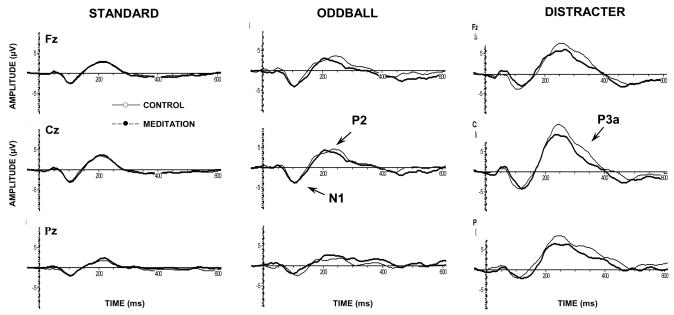
Figure 1 illustrates the grand averages for the meditation and control conditions. As their morphological pattern suggests, the major quantifiable meditation effects occurred for the N1, P2, and P3a potentials. Preliminary analysis yielded no effects of latency of components and numerous subjects evincing highly variable waveforms without clear single maxima within the component latencies of interest. Thus, time periods containing the largest amplitudes for each component at the Fz, Cz, and Pz electrodes were isolated using the global field power measure (Lehmann and Skrandies, 1980, 1984). Mean amplitudes within the following latency windows were computed as the primary dependent measures: N1=85–135 ms for all stimuli; P2=180–240 ms for the standards and 220–280 ms for the oddball and distracter trials; P3a=300–360 ms was clear only for the distracter trials but measured for the standard and oddball stimuli using the same latency window (Katayama and Polich, 1998).
Figure 1.
Grand averaged event-related potentials for each experimental condition, stimulus type, and midline electrode (N=16).
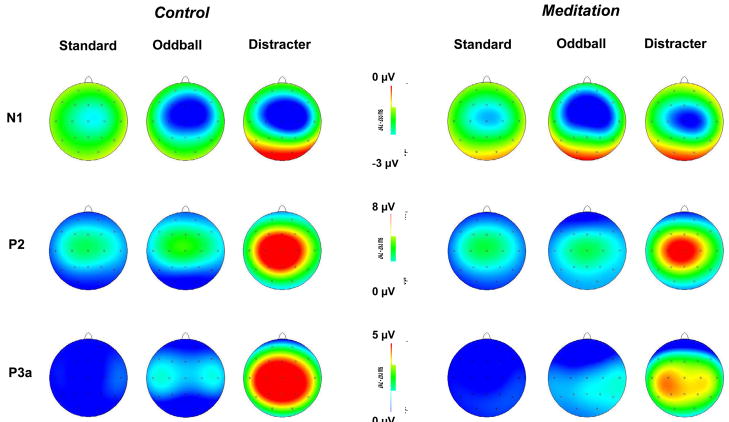
Figure 2 illustrates topographic plots for each component, stimulus type, and electrode for the meditation and control conditions. Preliminary analyses indicated no reliable meditation effects from the lateral electrodes, so that only midline electrodes were employed for the major statistical evaluation. Mean amplitude values were assessed with a two-factor repeated measures analysis of variance, with 2 conditions (meditation vs. control) × 3 electrodes (Fz, Cz, Pz). Greenhouse-Geisser corrections were applied to the df as needed and Tukey post-hoc means comparisons used to assess reliable interactions.
Figure 2.
Topographic voltage maps for each experimental condition, stimulus type, and major component.
3.0 Results
3.1. Self-report scales
The three-stimulus task was presented passively, so that no behavioral data were obtained. “Depth of meditative state” from the self-report 0–10 scale completed after each of the two experimental conditions indicated that the mean meditative depth experienced during the rest state was 1.7±1.4 and meditative depth experience during the meditative state was 4.5±1.4. The difference between the reported depth of meditative experience comparing meditation to control period was significant at the p<0.00001 level.
Drowsiness was reported by 7 of the 16 participants during the meditation and 10 of the 16 during the control thought condition. There was a correlation obtained between the order of experimental session and the self-reported experience of drowsiness during the control state wherein those individuals meditating first were less likely to experience drowsiness during the control state (r=0.52, p<0.05). There was no correlation between the number of years of daily practice and either the self-reported depth of meditative state (r=0.24, p>0.36) or the incidence of reported drowsiness during the meditative state (r=0.00, p>0.98). A negative correlation between the number of years of daily practice and reported drowsiness during the control state (r=−0.60, p<0.05) was found, which seems to reflect individuals with more years of daily meditation practices were less likely to report drowsiness during the control cognitive condition. A negative correlation between current number of hours of daily practice reported and drowsiness during meditative state also was obtained (r=−0.60, p<0.05), although no association was observed for the control state and number of hours of daily practice (r=0.02, p>0.93).
3.2. Event-related potentials
Figures 3, 4, and 5 illustrate the mean amplitude data as a function of midline electrode for the standard, oddball, and distracter stimuli, respectively. The data from each stimulus type were assessed with separate 2 conditions (meditation vs. control) × 3 electrodes (Fz, Cz, Pz) repeated measures analysis of variance.
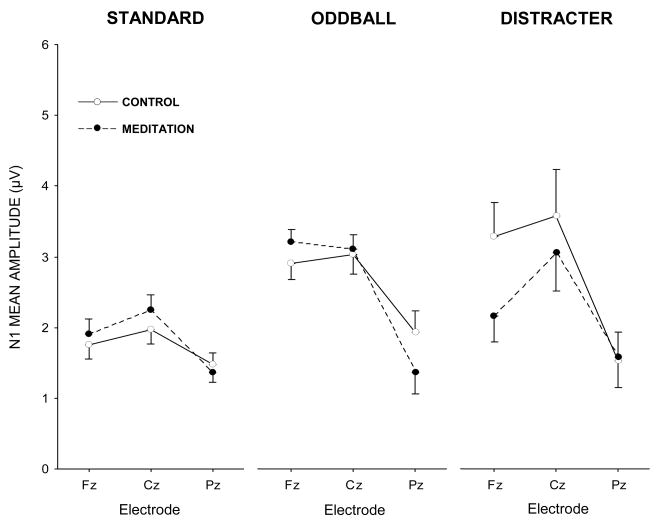
Figure 3.
N1 component mean amplitude (μV) for each experimental condition and stimulus type as a function of midline electrode.
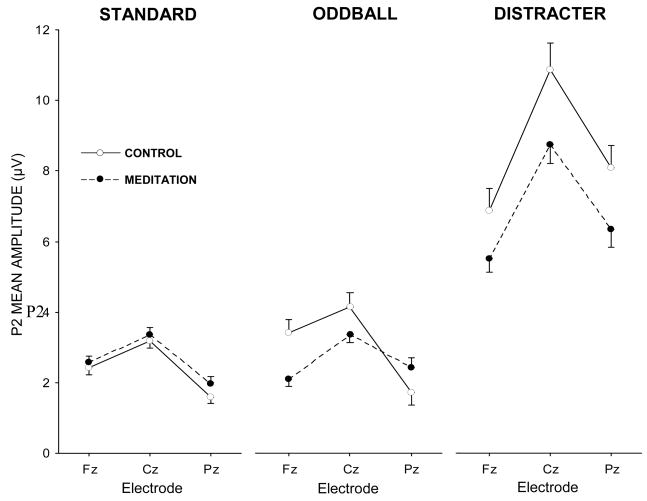
Figure 4.
P2 component mean amplitude (μV) for each experimental condition and stimulus type as a function of midline electrode.
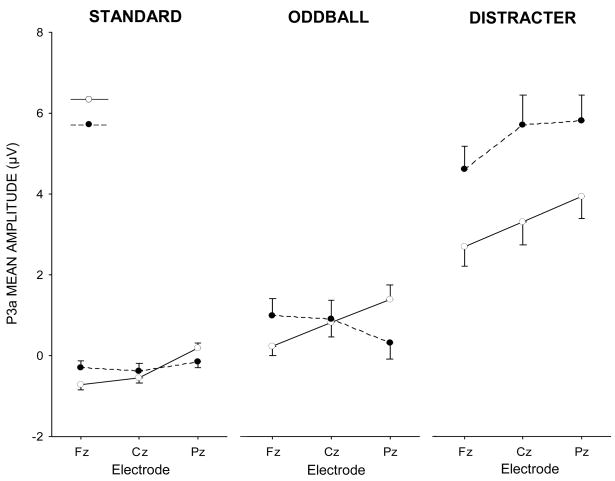
Figure 5.
P3a component mean amplitude (μV) for each experimental condition and stimulus type as a function of midline electrode.
N1 component
The auditory N1 and P2 components are known to reflect stimulus encoding strength generated largely by activity in the auditory (superior temporal) gyrus, with an additional frontal contribution to the N1 shown to be related to change detection. Standard stimuli demonstrated no meditation state effects (p>0.60), although amplitude was largest at the Cz electrode, F(2,30)=4.98, p<0.05, η2=0.25, which was larger than Pz (p<0.01) but not Fz (p>0.40). The state × electrode interaction reflected a slight trend towards a relatively greater frontal distribution for meditation than control state, F(2,30)=2.00, p=0.17, η2=0.12. Oddball stimuli produced no meditation state effect (p>0.90), with larger component amplitudes at the Fz and Cz electrodes, F(2,30)=9.55, p<0.01, η2=039. A marginal meditation state × electrode interaction also was found, F(2,30)=3.04, p=0.10, η2=0.17, indicating an N1 distribution with greater relative frontal vs. parietal scalp map amplitude in meditation compared to control.
Distracter stimuli yielded no overall N1 meditation state effect (p>0.30), but did produce a reliable electrode location difference, F(2,30)=6.65, p<0.01, η2=0.31, with amplitudes greater at Cz compared to Pz (p<0.01) and a trend towards greater magnitudes at Fz compared to Pz (p=0.06). The interaction between meditation state and electrode location was reliable, F(2,30)=4.20, p<0.05, η2=0.22. N1 mean amplitude was smaller for the meditation compared to control condition over Fz (p<0.01, M±95% CI: control, −3.3+1.9 μV, meditation, −2.2+1.4 μV), with no state difference found for Cz (p>0.45) or Pz (p>0.95). Moreover, N1 amplitude during the meditation state was greater at Cz than either Fz (p<0.05) and Pz (p<0.001), whereas Fz and Pz were not significantly different (p>0.35). N1 amplitude during the control state for Fz and Cz yielded larger amplitude values than Pz (p<0.001, in each case), whereas Fz and Cz amplitudes were similar (p>0.90).
P2 component
Standard stimuli yielded no meditation state effect (p>0.30), with electrode location demonstrating maximal amplitude for the frontal and central electrodes, F(2,30)=18.36, p<0.001, η2=0.55. Both Fz (p<0.05) and Cz (p<0.001) evinced greater amplitudes than Pz, and Cz exhibited greater amplitude than Fz (p<0.05). Oddball stimuli produced no meditation state effect (p>0.45), with electrode location demonstrating an overall main effect for fronto-central maximum amplitudes, F(2,30)=13.91, p<0.001, η2=0.48. However, a reliable interaction between meditation state and electrode location was obtained, F(2,30)=5.31, p<0.05, η2=0.26. Post-hoc tests revealed that P2 amplitudes were marginally smaller during meditation than the control condition at Fz (p=0.07, M+95% CI: control, 3.4±1.6 μV; meditation, 2.1±0.9 μV) but not Cz or Pz (p>0.50, both cases). Further, during the control state Fz (p<0.05) and Cz (p<0.001) exhibited greater amplitude values than Pz, but during meditation no electrode effects were obtained.
Distracter stimuli yielded marginally smaller P2 amplitudes during the meditation compared to the control state, F(1,15)=3.97, p=0.065, η2=0.21 (M+95% CI: control, 8.6±4.4 μV; meditation, 6.9±3.1 μV). Electrode location was reliable, F(2,30)=19.89, p<0.0001, with Cz larger than Fz and Pz (p<0.001, both cases) but no difference between Fz and Pz (p>0.20).
P3a component
Standard stimuli demonstrated no meditation state effect (p>0.80). Electrode location yielded a reliable main effect, F(2,30)=9.20, p<0.01, as Fz and Cz amplitudes were smaller than those from Pz (p<0.01, both cases). The interaction between meditation state and electrode was reliable, F(2,30)=5.64, p<0.05, η2=0.27. P3a amplitude to standard stimuli during meditation exhibited decreased amplitudes at Fz (p<0.001) and Cz (p<0.01) relative to Pz whereas in the control condition no electrode effects were obtained. Oddball stimuli demonstrated no meditation state (p>0.90) or electrode location (p>0.60) effects. Meditation state condition and electrode location yielded a reliable interaction, F(2,30)=8.44, p<0.01, η2=0.36. Component amplitude was larger at Pz for the meditation relative to control state (p<0.05), with no electrode effect in the control condition whereas the meditation condition for Pz demonstrated larger amplitude than Fz (p<0.01).
Distracter stimuli yielded smaller P3a amplitudes in the meditation compared to control state, F(1,15)=6.84, p<0.05, η2=0.31 (M+95% CI: control, 5.4±4.2 μV; meditation, 3.3±3.4 μV). Electrode location demonstrated marginally smaller P3a amplitudes for Fz compared to Cz and Pz, F(2,30)=2.83, p>0.10, η2=0.16. No state × electrode interaction was found.
3.3. Additional analyses
Several additional analyses were conducting using various covariates to characterize individual differences. No reliable outcomes were obtained when the self-report score for meditative depth between meditation and control sessions was used as a covariate for any of the ERP measures. However, a positive correlation was obtained between self-reported meditative depth (difference between rating in meditation and rest) and the distracter P3a amplitude (Pz) in both the control (r=0.51, p<0.05) and meditation (r=0.56, p<0.05) states. No consistent effect obtained when order of experimental sessions (control→meditation vs. meditation→control) was used as a covariate. Using self-reported drowsiness as a covariate for ANOVA analysis of P3a amplitude yielded a significant interaction between self-reported drowsiness during meditation and the meditation effect (F(1,14)=4.66, p<0.05). P3a amplitude reduction only was found for those participants not reporting drowsiness during meditation (p<0.05), and was absent for the group who did report drowsiness (p>0.95).
Categorization of participants into those meditating 1+ hours/day (n=6, M=2.1, SD=0.49 hours) and those meditating about 1 hour/day (n=10, M=0.85, SD=0.24 hours) demonstrated a significant interaction between the covariate hrs/day and the meditation state × electrode location interaction (F(2,28)=4.07, p<0.05). The sub-group reporting greater daily meditation practice length exhibited more P3a amplitude reduction at Fz (p<0.01 vs. p>0.95), and Cz (p<0.05 vs. p>0.15), with an opposite trend observed at Pz (p>0.90 vs. p>0.05) compared to the group reporting less daily meditation practice.
4.0 Discussion
Vipassana meditative practice involves the adoption of a mindful and receptive mental awareness, with attentional absorption on present-moment sensations in the body and meta-cognitive reframing of ongoing experience as impersonal phenomena to be observed but not reacted upon (Gunaratana, 2002; Hart, 1987; Lutz et al., 2007). Cognitive ERP measures were obtained from experienced Vipassana meditators with conditions that contrasted the meditative state with a control condition designed to mimic “everyday thinking”. The pattern of meditation-induced alterations of ERP components suggests that sensory and cognitive processing were altered during meditation relative to the control state.
Previous assessments have not obtained neuroelectric measures during meditation vs. cognitive control period of equal length (Cahn and Polich, 2006). Although a number of participants reported difficulty in avoiding engagement in meditative practice with eyes-closed and the posture used during meditation the use of instructions to keep the mind purposefully engaged with neutral mental activity during the control period appears reasonably successful.. A few participants reported approximately the same meditation depth in both periods, which may have resulted from the combined challenge of trying to avoid entering a meditative state during control period and the unfamiliar laboratory set-up. Despite these challenges, both the introspective meditative depth and neurophysiologic measures significantly differentiated between meditation and the control condition.
The experience of drowsiness during the meditation and control periods was affected by meditative expertise. The negative correlation observed between years of daily meditation and drowsiness rating during the control period indicated that meditators with longer periods of daily practice were less susceptible to control-condition drowsiness, an effect that may have general applicability reflecting a trait effect of meditation on increased vigilance during periods of low sensory stimulation. Further, the negative correlation between duration of daily meditation and drowsiness during the meditation period suggested that current intensity of practice was related to the individual’s ability to maintain meditative alertness even during the unfamiliar experience of wearing a tight-fitting cap in a small enclosed room with bulky headphones on the head.
4.1. Meditation and neuroelectric measures
N1 amplitude in meditation compared to control condition demonstrated decreased frontal amplitude to the distracter stimuli. In contrast, N1 amplitude did not differ across conditions for the standard or oddball stimuli, although a modest trend towards a greater relative frontal distribution for the standard and oddball stimuli was observed. It is known that the auditory N1 component is composed of at least two major subcomponents—a bilateral temporal component generated on the supratemporal (Heschl’s) gyrus, and a frontal component generated by the supplementary motor area (SMA) and/or cingulate cortex (Atcherson et al., 2006; Giard et al., 1994; Näätänen and Picton, 1987; Picton et al., 1999). The temporal cortex component of N1 has been described as reflecting the sensory processing of the auditory stimuli in primary auditory cortex and is modulated by specific aspects of the physical parameters of auditory stimuli whereas the frontal contribution to the N1 has been described as related to attention-switching mechanisms and automated orienting (Alcaini et al., 1994; Näätänen and Picton, 1987). This specific reduction of frontal N1 amplitude to the distracters suggests that the meditation condition involved decreased engagement of the frontal contribution, possibly reflecting decreases in the orienting response to the distracting stimuli. P2 amplitude did not vary with meditation for the standards but did demonstrate a marked trend towards frontal amplitude decrease to the oddballs and overall decrease to the distracter stimuli. Hence, the N1 and P2 potentials were sensitive to meditation state in conjunction with stimulus type, with a tendency for meditation-induced frontal decrease of component strength in the N1 and decreased P2 amplitude specifically to the distracter. The P2 component to the oddball stimulus also demonstrated moderately decreased frontal component strength, further implying that the frontal attentional system of the brain was relatively disengaged from the detection of change in the sensory field during meditative practice.
P3a amplitude from the distracter stimulus was reduced in meditation relative to the control condition. Given that the P3a reflects frontal cortical activity due to focal attentional system engagement, it seems likely that the present outcomes may reflect a disengagement of the attentional networks to stimulus-driven activation (Polich, 2007). In this view, the meditation state neural activity was less responsive to the ongoing stimulus train compared to the control state perhaps because stimulus context was weakly developed. Given that meditation imposed an attentional tuning on each passing stimulus event without regard to the previous stimulus trace, the distracter stimuli were perceived as less incongruent than the control condition because the attentional system was engaged similarly for all stimuli.
Attentive set and stimulus receptivity may be related to the increased mismatch negativity amplitudes reported for concentrative meditation on the breath and mantra (Srinivasan and Baijal, 2007). The effects of meditation on the ERP measures for different stimulus types suggests that background standard stimuli were not strongly influenced by meditation, with the oddball stimuli somewhat affected. Distracter stimuli demonstrated the strongest amplitude changes between the meditation and control states, with an ERP pattern of an alteration for N1 amplitude topography to a less frontal distribution, a trend for P2 amplitude decrease, and clear P3a amplitude reduction. This pattern also is consistent with a decreased involvement of frontal cortex in response to unexpected and distracting stimuli during meditation.
Target stimulus meditation effects typically have shown that meditative practice increases P3b amplitude as a state and/or trait effect in tasks involving active responding to target stimuli (Banquet and Lesévre, 1980; Murthy et al., 1997; Sarang and Telles, 2006). One exception is reflected by the attentional blink task where intense Vipassana meditation practice was associated with decreased P3b amplitude to the first of two targets in conjunction with improved performance in detecting a second target, suggesting an improved efficiency of neural processing for the task (Slagter et al., 2007). In the present study, experienced Vipassana meditators produced a decrease in overall P3a amplitude to distracting stimuli in meditation relative to the control condition, which may index decreased attentional engagement by the frontal cortex.
One limitation of the present study is the lack of a control group of non-meditators, such that possible ERP trait measures were not assessed and the meditation state effects cannot be unequivocally linked to meditation expertise. It is possible that non-meditators might have shown a similar “meditation” state effect reflected by some aspects of demand characteristics for the two cognitive tasks assayed. However, participants with more hours of daily meditation experience contributed more to the P3a amplitude decrements, supporting the interpretation that the meditation state effect likely would not have been observed in a non-meditator cohort.
Despite explicit instructions to ignore the subsequent stimuli at the beginning of each of the 30 min blocks, the meditators may have covertly and selectively attended to the auditory stimuli during the control but not meditation condition. This possibility is unlikely because participants were given no information as to what the stimuli represented or of the study’s hypotheses regarding the neurophysiologic processing. It is also possible that motivation for staying alert may have varied across the two experimental periods due to some sort of “performance” pressure in the meditation period, thereby leading to higher levels of arousal and possibly confounding the results. This outcome also is unlikely given that, (1) analysis of the spontaneous EEG data prior to presentation of the tone stimuli indicated no changes in power for delta, theta, and alpha frequencies between the two states, and (2) increased arousal is generally associated with higher P3B amplitudes, whereas the meditation effects demonstrated decreased P3a amplitude (Polich, 2007; Polich and Kok, 1995).
Effects of mind-wandering on ERP measures indicate that in moments of mind-wandering, response-related P3b amplitudes were decreased (Smallwood et al., 2008), emphasizing that the selection of a control mind-wandering condition may make the meditation findings of particular significance. However, it is possible that the sort of mind-wandering state experienced by the present meditators was not consistent with the off-task mind-wandering assayed in the literature on mind-wandering to date (Smallwood and Schooler, 2006; Smallwood et al., 2007, 2008).
4.2. Theoretical perspectives
Early studies suggested that meditation may produce a state of brain processing less susceptible to stimulus-driven processing as indexed by alpha blocking (Anand, 1961; Kinoshita, 1975; Lehrer et al., 1980). The P3a amplitude decrease is consistent with the interpretation of a decrease in stimulus-driven cognitive processing during meditation (Lutz et al., 2008). It is possible to interpret this decrease in terms of altered sensory processing of the attention-demanding distracter stimuli. However, P3a amplitude reduction from a meditation-induced processing state reflects a mental condition that fulfills the goal of purposefully engaging attention in the present-moment without regard for past conditioning or future expectations (Gunaratana, 2002; Hart, 1987). The auditory attentional system would reflect sensory stimulation by maintained N1 amplitude to the standard and oddball stimuli and less affected by unexpected distracting events such as a burst of white noise. Frontal N1 and subsequent P2 and P3a amplitudes from the distracter stimuli would be decreased, because evaluative processing of the distracter relative to the standards and oddballs was minimized during meditation. Although the oddball stimuli were not demanding attention enough to elicit strong P3b potentials, that the frontal contribution to P2 also would show some decrease is further evidence for the relative disengagement of frontal processing of unexpected stimuli during meditation.
A number of studies have reported increased frontal cortex activation in meditation (Baerentsen, 2001; Herzog et al., 1990; Jevning et al., 1996; Khushu et al., 2000; Lazar et al., 2000; Newberg et al., 2001). fMRI assessment of experienced Vipassana meditators indicated that the experts engaged medial prefrontal cortex (MPFC) and rostral anterior cingulate (rACC) during meditation to a greater extent than novice meditators (Holzel et al., 2007). Further, concentrative Tibetan Buddhist meditators demonstrated that experts relative to novice meditators during meditation yielded decreased activation in posterior cingulate and amygdala to distracting sounds (Brefczynski-Lewis et al., 2007). A related fMRI report of Transcendental Meditation found that meditation training induced a decreased activation from painful stimuli in the anterior cingulate, prefrontal cortex, thalamus, and whole brain (Orme-Johnson et al., 2006). The meditative state may therefore induce a brain activity with increased baseline activation of frontal attentional circuits wherein these circuits also are less responsive to unexpected attention-demanding stimuli. Thus, as a state effect the frontal attentional network may be directed inward and become less reactive to external stimuli, whereas long-term meditation practice may be related to trait effects reflecting the purposeful engagement of attention that preserves neural sources of attentional and interoceptive processing (Lazar et al., 2005; Pagnoni and Cekic, 2007)
4.3. Additional meditation outcomes
Assessment of the relationship between the meditation-induced neurophysiologic changes and psychometric measures indicated a positive correlation between self-reported experienced meditative depth and parietal distracter P3a amplitude during both meditation and control conditions, possibly related to individual differences in sensation seeking. Perhaps the meditators reporting greater meditative depth are those who find it easier to enter into deep quiescence due to low sensation-seeking trait, which has been shown previously to correlate with passive P3a amplitude (Wang and Wang, 2001). Had the decrease in P3a amplitude been related to changes during the meditative state stemming from a shift towards a reduction in vigilance and attentiveness as is produced by sleep onset, the opposite pattern would have obtained. Additional studies incorporating more detailed reports on the subjective perception of stimuli in and out of meditative state may yield more detailed correlative understanding between internal state and brain measures for these types of paradigms.
Another covariate found to associate with a P3a decrease in meditation was the daily number of hours spent meditating consistent with the findings with concentrative and mindfulness-based meditative practitioners demonstrating improved measures of executive attention function as assessed by the Stroop task (Chan and Woollacott, 2007). Stroop task performance was correlated with meditation time/day rather than the length of time engaged in practice—similar to the P3a amplitude effects observed in the present study. This association may be of particular relevance given the known importance of anterior cingulate activity in both P3a generation and Stroop task performance.
The decreased P3a amplitude exhibited in meditation relative to control conditions can be related to the finding that after a Vipassana meditation retreat, experienced meditators demonstrated decreased P3b amplitude to the T1 stimulus in an attentional blink paradigm. This outcome was associated with behavioral measures indicating that the meditation intervention decreased the attentional disengagement induced by T1 processing (Slagter et al., 2007). As in the present study, Vipassana meditation appears to mediate a decrease in the automated recruitment of attentional resources when such recruitment is not expedient for the task at hand. Complementarily, the attentional blink findings suggest that engagement in meditative practice induced a brain state wherein decreased attentional resources were devoted to processing of the first stimulus, which contributed to greater preparation to perceive further environmental cues presented in rapid succession. In the present study, the meditative engagement with present-moment awareness of body sensation presumably induced a brain state with decreased reactivity to unrelated distractions.
4.4. Clinical implications
Vipassana/mindfulness meditation practice appears to produce significant effects on the attentional systems of the brain, consistent with significant findings of increased attentional capacities elicited from meditative practice (Chan and Woollacott, 2007; Jha et al., 2007; Slagter et al., 2007; Tang et al., 2007). A study of ADHD in children employed a similar passive auditory oddball design presented while participants were involved in a visual task and found that in the ADHD cohort P3a amplitudes to auditory distracters were increased relative to control participants (van Mourik et al., 2007). Thus, the present findings of decreased P3a amplitude from an auditory distracter in meditation are consonant with a pattern of increased attentional focus with brain effects that may be therapeutic for pathological deficits in attention such as those seen in ADHD (Zylowska et al., 2007).
The observed pattern that Vipassana meditation induces a brain state of decreased automated frontal cognitive processing of distracting stimuli may help to shed light on the mechanistic explanation for the clinical efficacy of mindfulness training, shown to encompass broad-ranging effects on psychological well-being, decreased anxiety, and improved immune functioning (Carlson et al., 2003, 2004; Davidson et al., 2003; Kabat-Zinn et al., 1992; Kabat-Zinn, 2003; Segal et al., 2002). Decreased brain reactivity to aversive distracting external stimuli may be related to meditation helping to alleviate habitual reactive thought patterns and ruminative processes (Evans et al., 2007; Kabat-Zinn et al., 1992; Koszycki et al., 2007; Ma and Teasdale, 2004; Mason and Hargreaves, 2001; Rickels and Rynn, 2001), psychological components seen as central targets in the search for therapeutic intervention in the spectrum of anxiety and depression-related illness.
4.3. Conclusions
Appreciation for the variety of mental practices subsumed by the name “meditation” has recently become a salient research topic, as observation of the various types of attentional engagement across meditative practices may promote different neurophysiologic outcomes (Cahn and Polich, 2006; Depraz et al., 2003; Lutz et al., 2007). Vipassana meditation induces a number of changes in ERP component amplitudes consistent with decreased frontal engagement in the processing of unexpected and aversive stimuli during meditation in experienced meditators. In contrast to the mindfulness-based approach of Vipassana, more concentrative forms of meditative practice could yield stronger alterations of ERPs as these practices promote narrowing of attentional focus in a fashion that may remove the attentional systems even further from the immediate sensory surround (Cahn and Polich, 2006; Lutz et al., 2008). It therefore should be fruitful to apply a similar paradigm to meditators practicing more concentrative forms of meditation such as mantra or visualization-based methods. Assessment of clinical populations with concomitant assessment of sensory and cognitive processing as impacted by meditative training might help to elucidate the important neurophysiologic changes that contribute to positive health outcomes due to meditation and mindfulness intervention.
Acknowledgments
This work was supported by NIH grants DA018262 and P50 AA06420. The Fetzer Institute and NIGMS Medical Scientist Training Grant in part supported BRC, who is also affiliated with the Laboratory for Psychopharmacology and Brain Imaging, University of Zurich Hospital of Psychiatry. The help and guidance of Drs. Mark Geyer and Franz Vollenweider are gratefully acknowledged. We thank Dr. Arnaud Delorme for helpful comments and Brian Lopez for technical assistance. We thank the meditator participants and Mr. John Beary of Vipassana Research International for assistance in recruiting meditation participants. This paper is 19248 from The Scripps Research Institute..
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aftanas LI, Golocheikine SA. Human anterior and frontal midline theta and lower alpha reflect emotionally positive state and internalized attention: high-resolution EEG investigation of meditation. Neurosci Let. 2001;310:57–60. doi: 10.1016/s0304-3940(01)02094-8. [DOI] [PubMed] [Google Scholar]
- Alcaini M, Giard MH, Thevenet M, Pernier J. Two separate frontal components in the N1 wave of the human auditory evoked response. Psychophysiology. 1994;31:611–615. doi: 10.1111/j.1469-8986.1994.tb02354.x. [DOI] [PubMed] [Google Scholar]
- Anand B, Chhina GS, Singh B. Some aspects of electroencephalographic studies in yogis. Electroencephalography and Clin Neurophysiol. 1961;13:452–456. [Google Scholar]
- Astin JA. Stress reduction through mindfulness meditation. Effects on psychological symptomatology, sense of control, and spiritual experiences. Psychother Psychosom. 1997;66:97–106. doi: 10.1159/000289116. [DOI] [PubMed] [Google Scholar]
- Atcherson SR, Gould HJ, Pousson MA, Prout TM. Long-Term Stability of N1 Sources Using Low-Resolution Electromagnetic Tomography. Brain Topogr. 2006;19 doi: 10.1007/s10548-006-0008-8. [DOI] [PubMed] [Google Scholar]
- Baerentsen KB. Onset of meditation explored with fMRI. NeuroImage. 2001;13:S297. [Google Scholar]
- Banquet JP, Lesévre N. Event-related potentials in altered states of consciousness. Prog Brain Res. 1980;54:447–453. doi: 10.1016/S0079-6123(08)61659-3. [DOI] [PubMed] [Google Scholar]
- Brefczynski-Lewis JA, Lutz A, Schaefer HS, Levinson DB, Davidson RJ. Neural correlates of attentional expertise in long-term meditation practitioners. Proc Natl Acad Sci U S A. 2007;104:11483–11488. doi: 10.1073/pnas.0606552104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D, Forte M, Dysart M. Visual sensitivity and mindfulness meditation. Percept Mot Skills. 1984;58:775–784. doi: 10.2466/pms.1984.58.3.775. [DOI] [PubMed] [Google Scholar]
- Brown D, Forte M, Dysart M. Differences in visual sensitivity among mindfulness meditators and non-meditators. Percept Mot Skills. 1984;58:727–733. doi: 10.2466/pms.1984.58.3.727. [DOI] [PubMed] [Google Scholar]
- Brown DP. Mastery of the Mind East and West: Excellence in Being and Doing and Everyday Happiness. Ann N Y Acad Sci. 2007 doi: 10.1196/annals.1393.018. [DOI] [PubMed] [Google Scholar]
- Cahn BR, Polich J. Meditation states and traits: EEG, ERP, and neuroimaging studies. Psychol Bull. 2006;132:180–211. doi: 10.1037/0033-2909.132.2.180. [DOI] [PubMed] [Google Scholar]
- Carlson LE, Speca M, Patel KD, Goodey E. Mindfulness-based stress reduction in relation to quality of life, mood, symptoms of stress, and immune parameters in breast and prostate cancer outpatients. Psychosom Med. 2003;65:571–581. doi: 10.1097/01.psy.0000074003.35911.41. [DOI] [PubMed] [Google Scholar]
- Carlson LE, Speca M, Patel KD, Goodey E. Mindfulness-based stress reduction in relation to quality of life, mood, symptoms of stress and levels of cortisol, dehydroepiandrosterone sulfate (DHEAS) and melatonin in breast and prostate cancer outpatients. Psychoneuroendocrinology. 2004;29:448–474. doi: 10.1016/s0306-4530(03)00054-4. [DOI] [PubMed] [Google Scholar]
- Chan D, Woollacott M. Effects of level of meditation experience on attentional focus: is the efficiency of executive or orientation networks improved? J Altern Complement Med. 2007;13:651–657. doi: 10.1089/acm.2007.7022. [DOI] [PubMed] [Google Scholar]
- Combs LA, Polich J. P3a from auditory white noise stimuli. Clin Neurophysiol. 2006;117:1106–1112. doi: 10.1016/j.clinph.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Cranson R, Goddard PH, Orme-Johnson D. P300 under conditions of temporal uncertainty and filter attenuation: Reduced latency in long-term practitioners of TM. Psychophysiology. 1990;27:S23. [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Affective neuroscience and psychophysiology: toward a synthesis. Psychophysiology. 2003;40:655–665. doi: 10.1111/1469-8986.00067. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Kabat-Zinn J, Schumacher J, Rosenkranz M, Muller D, Santorelli SF, Urbanowski F, Harrington A, Bonus K, Sheridan JF. Alterations in brain and immune function produced by mindfulness meditation. Psychosomatic Medicine. 2003;65:564–570. doi: 10.1097/01.psy.0000077505.67574.e3. [DOI] [PubMed] [Google Scholar]
- Depraz N, Varela JF, Vermersch P. On becoming aware: A pragmatics of experiencing. John Benjamins Publishing Company; Amsterdam: 2003. [Google Scholar]
- Evans S, Ferrando S, Findler M, Stowell C, Smart C, Haglin D. Mindfulness-based cognitive therapy for generalized anxiety disorder. J Anxiety Disord. 2007 doi: 10.1016/j.janxdis.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Farb NAS, Segal Z, Mayberg H, Bean J, McKeon D, Fatima Z, Anderson AK. Attending to the present: mindfulness meditation reveals distinct neural modes of self-reference. Soc Cogn Affect Neurosci. 2007;2:313–322. doi: 10.1093/scan/nsm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giard M, Perrin F, Echallier J, Thevenet M, Froment J, Pernier J. Dissociation of temporal and frontal components in the human auditory N1 wave: a scalp current density and dipole model analysis. Electroencephalogr Clin Neurophysiol. 1994;92:238–252. doi: 10.1016/0168-5597(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Goddard PH. Reduced age-related declines of P300 latencies in elderly practicing Transcendental Meditation. Psychophysiology. 1989;26:S29. [Google Scholar]
- Goddard PH. Transcendental Meditation as an intervention in the aging of neurocognitive function: Reduced age-related declines of P300 latencies in elderly practitioners. U.S.A. Dissertation Abstracts International. 1992;53:3189B. [Google Scholar]
- Goleman D. The meditative mind: Varieties of meditative experience. Penguin Putnam; New York: 1996. [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Gunaratana H. Mindfulness in plain English. Wisdom Publications; Boston, MA: 2002. [Google Scholar]
- Hart W. In: The Art of Living: Vipassana Meditation As Taught. Goenka SN, editor. HarperOne; New York, NY: 1987. [Google Scholar]
- Hebert R, Lehmann D. Theta bursts: An EEG pattern in normal subjects practicing the Transcendental Meditation technique. Electroencephalogr Clin Neurophysiol. 1977;42:397–405. doi: 10.1016/0013-4694(77)90176-6. [DOI] [PubMed] [Google Scholar]
- Herzog H, Lele VR, Kuwert T, Langen KJ, Kops ER, Feinendegen LE. Changed pattern of regional glucose metabolism during Yoga meditative relaxation. Neuropsychobiology. 1990;23:182–187. doi: 10.1159/000119450. [DOI] [PubMed] [Google Scholar]
- Hirai T. Psychophysiology of Zen. Igaku Shoin; Tokyo: 1974. [Google Scholar]
- Holzel BK, Ott U, Hempel H, Hackl A, Wolf K, Stark R, Vaitl D. Differential engagement of anterior cingulate and adjacent medial frontal cortex in adept meditators and non-meditators. Neurosci Lett. 2007;421:16–21. doi: 10.1016/j.neulet.2007.04.074. [DOI] [PubMed] [Google Scholar]
- Jeon YW, Polich J. P3a from a passive visual stimulus task. Clin Neurophysiol. 2001;112:2202–2208. doi: 10.1016/s1388-2457(01)00663-0. [DOI] [PubMed] [Google Scholar]
- Jevning R, Anand R, Biedebach M, Fernando G. Effects on regional cerebral blood flow of Transcendental Meditation. Physiol Behav. 1996;59:399–402. doi: 10.1016/0031-9384(95)02006-3. [DOI] [PubMed] [Google Scholar]
- Jha AP, Krompinger J, Baime MJ. Mindfulness training modifies subsystems of attention. Cogn Affect Behav Neurosci. 2007;7:109–119. doi: 10.3758/cabn.7.2.109. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: Theoretical considerations and preliminary results. Gen Hosp Psychiatry. 1982;4:33–47. doi: 10.1016/0163-8343(82)90026-3. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J, Massion AO, Kristeller J, Peterson LG, Fletcher KE, Pbert L, Lenderking WR, Santorelli SF. Effectiveness of a meditation-based stress reduction program in the treatment of anxiety disorders. Am J Psychiatry. 1992;149:936–943. doi: 10.1176/ajp.149.7.936. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. Mindfulness-based interventions in context: Past, present, and future. Clinical Psychology: Science and Practice. 2003;10:144–158. [Google Scholar]
- Kasamatsu A, Hirai T. An electroencephalographic study on the Zen meditation (Zazen) Folia Psychiatr Neurol Jpn. 1966;20:315–336. doi: 10.1111/j.1440-1819.1966.tb02646.x. [DOI] [PubMed] [Google Scholar]
- Katayama J, Polich J. Stimulus context determines P3a and P3b. Psychophysiology. 1998;35:23–33. [PubMed] [Google Scholar]
- Khushu S, Telles S, Kumaran S, Naveen KV, Tripathi RP. Frontal activation during meditation based on functional magnetic resonance imaging (fMRI) Indian Journal of Physiology and Pharmacology. 2000;44:34. [Google Scholar]
- Kinoshita K. A study on response of EEG during Zen meditation--alpha-blocking to name calling (in Japanese) Seishin Shinkeigaku Zasshi. 1975;77:623–658. [PubMed] [Google Scholar]
- Koszycki D, Benger M, Shlik J, Bradwejn J. Randomized trial of a meditation-based stress reduction program and cognitive behavior therapy in generalized social anxiety disorder. Behav Res Ther. 2007;45:2518–2526. doi: 10.1016/j.brat.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Lazar SW, Bush G, Gollub RL, Fricchione GL, Khalsa G, Benson H. Functional brain mapping of the relaxation response and meditation. Neuroreport. 2000;11:1581–1585. [PubMed] [Google Scholar]
- Lazar SW, Rosman IS, Vangel M, Rao V, Dusek H, Benson H, Bush G, Gollub RL. Functional brain imaging of mindfulness and mantra-based meditation. Society for Neuroscience. 2003;86.11 Online. [Google Scholar]
- Lazar SW, Kerr CE, Wasserman RH, Gray JR, Greve DN, Treadway MT, McGarvey M, Quinn BT, Dusek JA, Benson H, Rauch SL, Moore CI, Fischl B. Meditation experience is associated with increased cortical thickness. Neuroreport. 2005;16:1893–1897. doi: 10.1097/01.wnr.0000186598.66243.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann D, Skrandies W. Reference-free identification of components of checkerboard-evoked multichannel potential fields. Electroencephalogr Clin Neurophysiol. 1980;48:609–621. doi: 10.1016/0013-4694(80)90419-8. [DOI] [PubMed] [Google Scholar]
- Lehmann D, Skrandies W. Spatial analysis of evoked potentials in man--a review. Prog Neurobiol. 1984;23:227–250. doi: 10.1016/0301-0082(84)90003-0. [DOI] [PubMed] [Google Scholar]
- Lehrer PM, Schoicket S, Carrington P, Woolfolk RL. Psychophysiological and cognitive responses to stressful stimuli in subjects practicing progressive relaxation and clinically standardized meditation. Behaviour Research & Therapy. 1980;18:293–303. doi: 10.1016/0005-7967(80)90088-1. [DOI] [PubMed] [Google Scholar]
- Lutz A, Dunne JD, Davidson RJ. Meditation and the neuroscience of consciousness. The Cambridge Handbook of Consciousness 2007 [Google Scholar]
- Lutz A, Slagter HA, Dunne JD, Davidson RJ. Attention regulation and monitoring in meditation. Trends Cog Sci. 2008;xxx:xx–xx. doi: 10.1016/j.tics.2008.01.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma SH, Teasdale JD. Mindfulness-based cognitive therapy for depression: Replication and exploration of differential relapse prevention effects. Journal of Consulting & Clinical Psychology. 2004;72:31–40. doi: 10.1037/0022-006X.72.1.31. [DOI] [PubMed] [Google Scholar]
- Mason O, Hargreaves I. A qualitative study of mindfulness-based cognitive therapy for depression. British Journal of Medical Psychology. 2001;74:197–212. [PubMed] [Google Scholar]
- Murthy PJ, Gangadhar BN, Janakiramaiah N, Subbakrishna DK. Normalization of P300 amplitude following treatment in dysthymia. Biol Psychiatry. 1997;42:740–743. doi: 10.1016/s0006-3223(97)00296-5. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Picton T. The N1 wave of the human electric and magnetic response to sound: a review and an analysis of the component structure. Psychophysiology. 1987;24:375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Newberg A, Alavi A, Baime M, Pourdehnad M, Santanna J, d’Aquili E. The measurement of regional cerebral blood flow during the complex cognitive task of meditation: A preliminary SPECT study. Psychiatry Res. 2001;106:113–122. doi: 10.1016/s0925-4927(01)00074-9. [DOI] [PubMed] [Google Scholar]
- Orme-Johnson DW, Schneider RH, Son YD, Nidich S, Cho ZH. Neuroimaging of meditation’s effect on brain reactivity to pain. Neuroreport. 2006;17:1359–1363. doi: 10.1097/01.wnr.0000233094.67289.a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagnoni G, Cekic M. Age effects on gray matter volume and attentional performance in Zen meditation. Neurobiol Aging. 2007;28:1623–1627. doi: 10.1016/j.neurobiolaging.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Picton TW, Alain C, Woods DL, John MS, Scherg M, Valdes-Sosa P, Bosch-Bayard J, Trujillo NJ. Intracerebral sources of human auditory-evoked potentials. Audiol Neurootol. 1999;4:64–79. doi: 10.1159/000013823. [DOI] [PubMed] [Google Scholar]
- Polich J. P300 from a passive auditory paradigm. Electroencephalogr Clin Neurophysiol. 1989;74:312–320. doi: 10.1016/0168-5597(89)90061-0. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz A, Buhle J. Typologies of attentional networks. Nat Rev Neurosci. 2006;7:367–379. doi: 10.1038/nrn1903. [DOI] [PubMed] [Google Scholar]
- Rickels K, Rynn M. Overview and clinical presentation of generalized anxiety disorder. Psychiatr Clin North Am. 2001;24:1–17. doi: 10.1016/s0193-953x(05)70203-3. [DOI] [PubMed] [Google Scholar]
- Ritskes R, Ritskes-Hoitinga M, Stodkilde-Jorgensen H, Baerentsen K, Hartman T. MRI scanning during Zen meditation: The picture of enlightenment? Constructivism in the Human Sciences. 2003;8:85–90. [Google Scholar]
- Sarang SP, Telles S. Changes in P300 following two yoga-based relaxation techniques. Int J Neurosci. 2006;116:1419–1430. doi: 10.1080/00207450500514193. [DOI] [PubMed] [Google Scholar]
- Segal ZV, Williams JMG, Teasdale JD. Mindfulness-based Cognitive Therapy for depression: A new approach to preventing relapse. Guilford Press: New York; 2002. [Google Scholar]
- Slagter HA, Lutz A, Greischar LL, Francis AD, Nieuwenhuis S, Davis JM, Davidson RJ. Mental Training Affects Distribution of Limited Brain Resources. PLoS Biol. 2007;5:1228–1235. doi: 10.1371/journal.pbio.0050138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood J, Schooler JW. The restless mind. Psychol Bull. 2006;132:946–958. doi: 10.1037/0033-2909.132.6.946. [DOI] [PubMed] [Google Scholar]
- Smallwood J, McSpadden M, Schooler JW. The lights are on but no one’s home: meta-awareness and the decoupling of attention when the mind wanders. Psychon Bull Rev. 2007;14:527–533. doi: 10.3758/bf03194102. [DOI] [PubMed] [Google Scholar]
- Smallwood J, Beach E, Schooler JW, Handy TC. Going AWOL in the Brain: Mind Wandering Reduces Cortical Analysis of External Events. J Cogn Neurosci. 2008;20:458–469. doi: 10.1162/jocn.2008.20037. [DOI] [PubMed] [Google Scholar]
- Srinivasan N, Baijal S. Concentrative meditation enhances preattentive processing: a mismatch negativity study. Neuroreport. 2007;18:1709–1712. doi: 10.1097/WNR.0b013e3282f0d2d8. [DOI] [PubMed] [Google Scholar]
- Tang YY, Ma Y, Wang J, Fan Y, Feng S, Lu Q, Yu Q, Sui D, Rothbart MK, Fan M, Posner MI. Short-term meditation training improves attention and self-regulation. Proc Natl Acad Sci U S A. 2007;104:17152–17156. doi: 10.1073/pnas.0707678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis F, Miskov S. P300 latency and amplitude during eyes-closed rest and Transcendental Meditation practice. Psychophysiology. 1994;31:S67. [Google Scholar]
- Travis F, Tecce JJ, Guttman J. Cortical plasticity, contingent negative variation, and transcendent experiences during practice of the Transcendental Meditation technique. Biol Psychol. 2000;55:41–55. doi: 10.1016/s0301-0511(00)00063-6. [DOI] [PubMed] [Google Scholar]
- Travis F, Tecce J, Arenander A, Wallace RK. Patterns of EEG coherence, power, and contingent negative variation characterize the integration of transcendental and waking states. Biol Psychol. 2002;61:293–319. doi: 10.1016/s0301-0511(02)00048-0. [DOI] [PubMed] [Google Scholar]
- Travis F, Arenander A, DuBois D. Psychological and physiological characteristics of a proposed object-referral/self-referral continuum of self-awareness. Conscious Cogn. 2004;13:401–420. doi: 10.1016/j.concog.2004.03.001. [DOI] [PubMed] [Google Scholar]
- van Mourik R, Oosterlaan J, Heslenfeld DJ, Konig CE, Sergeant JA. When distraction is not distracting: a behavioral and ERP study on distraction in ADHD. Clin Neurophysiol. 2007;118:1855–1865. doi: 10.1016/j.clinph.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Wallace BA. The Buddhist tradition of Samatha: Methods for refining and examining consciousness. Journal of Consciousness Studies. 1999;6:175–187. [Google Scholar]
- Wallace BA, Shapiro SL. Mental balance and well-being: building bridges between Buddhism and Western psychology. Am Psychol. 2006;61:690–701. doi: 10.1037/0003-066X.61.7.690. [DOI] [PubMed] [Google Scholar]
- Wang W, Wang YH. Sensation seeking correlates of passive auditory P3 to a single stimulus. Neuropsychologia. 2001;39:1188–1193. doi: 10.1016/s0028-3932(01)00051-3. [DOI] [PubMed] [Google Scholar]
- Wenger MA, Bagchi BK. Studies of autonomic functions in practitioners of Yoga in India. Behav Sci. 1961;6:312–323. doi: 10.1002/bs.3830060407. [DOI] [PubMed] [Google Scholar]
- Wenk-Sormaz H. Meditation can reduce habitual responding. Altern Ther Health Med. 2005;11:42–58. [PubMed] [Google Scholar]
- Zylowska L, Ackerman DL, Yang MH, Futrell JL, Horton NI, Hale S, Pataki C, Smalley SL. Mindfulness Meditation Training in Adults and Adolescents With ADHD: A Feasibility Study. J Atten Disord. 2007 doi: 10.1177/1087054707308502. [DOI] [PubMed] [Google Scholar]