Abstract
Objectives
to investigate the relation of plasma lipids to all-cause mortality in a multi-ethnic cohort of non-demented elderly.
Setting
community-based sample of Medicare recipients, 65 years and older, residing in Northern Manhattan.
Participants
about two thousand five hundred and fifty-six non-demented elderly, 65–103 years. Among participants, 66.1% were women, 27.6% were White/non-Hispanic, 31.2% were African-American and 41.2% were Hispanic.
Methods
a standardised assessment, including functional ability, medical history, physical and neurological examination and a neuropsychological battery was conducted. Vital status was ascertained through the National Death Index (NDI). We used survival analyses stratified by race and ethnicity to examine the relation of plasma lipids to subsequent all-cause mortality.
Results
hispanics had the best overall survival, followed by African-Americans and Whites. Whites and African-Americans in the lowest quartiles of total cholesterol, non-HDL cholesterol and low-density lipoprotein cholesterol (LDL cholesterol) were approximately twice as likely to die as those in the highest quartile (White HR: 2.2, for lowest total cholesterol quartile; HR: 2.3, for lowest non-HDL cholesterol quartile; and HR: 1.8, for lowest LDL cholesterol quartile. African-American HR: 1.9, for lowest total cholesterol, HR: 2.0, for lowest non-HDL cholesterol and HR: 1.9, for lowest LDL cholesterol). In contrast, plasma lipid levels were not related to mortality risk among Hispanics.
Conclusions
hispanic ethnicity modifies the associations between lipid levels and all-cause mortality in the elderly.
Keywords: plasma lipids, all-cause mortality, race/ethnicity, ageing, elderly
Introduction
High levels of total cholesterol [1] and low-density lipoprotein cholesterol (LDL cholesterol) are associated with greater cardiovascular and all-cause mortality in middle-aged populations, but the association between lipid levels and mortality among individuals aged 65 and older is inconsistent. Some studies have found no association between plasma lipids and mortality in the elderly [2, 3], while others report a positive [4, 5] or an inverse association [6-8].
The associations between lipid levels and mortality may vary by race and ethnicity as well as by age [9-11]. In the Evans County Heart Study (ECHS) a positive association between total cholesterol and coronary heart disease (CHD) mortality was observed among Caucasian and African-American men, total cholesterol was negatively associated with all-cause mortality among African-American women and no relation was found between total cholesterol and all-cause mortality among Caucasian women [10]. Adult and elderly Hispanics have high levels of diabetes and obesity, but rates of CHD mortality and all-cause mortality similar to or lower than that of non-Hispanic Whites in some [12-14] but not all, studies [15, 16]. Previously we found that low levels of total cholesterol, non-HDL cholesterol and LDL cholesterol were associated with increased all-cause mortality risk in the non-demented elderly [8]. In this study, the relation of plasma lipids to subsequent all-cause mortality in the elderly was examined among non-Hispanic Whites, African-American and Hispanic participants in the Washington Heights Inwood Columbia Aging Project (WHICAP) cohorts, taking demographic factors, cardiovascular risk factors, heart disease, smoking status and treatment with lipid-lowering drugs into account. The aim of the study was to examine whether the relation of lipid levels to all-cause mortality varies by ethnicity.
Methods
Participants and setting
Data were included from subjects participating in WHICAP, a prospective study of ageing and dementia among Medicare recipients 65 years and older residing in northern Manhattan. The population from which participants were drawn consisted of individuals from several different countries of origin representing three broadly defined ethnic categories (i.e. Caribbean Hispanic, African-American, and non-Hispanic White of European ancestry). Subjects were recruited in two waves, one ending in 1992 and the other in 1999. Briefly, a stratified random sample of 50% of individuals aged 65 and older residing in Northern Manhattan was obtained from the Health Care Finance Administration (HCFA). The sampling strategies and recruitment outcomes of these two cohorts have been described in detail elsewhere [17]. Ethnic group was classified by the participant’s self-report using the format of the 1990 US Census [18]. Participants were asked if they considered themselves white, black or other, and then asked if they were Hispanic. About 85% of Hispanic participants are of Caribbean descent, primarily from the Dominican Republic. Participants have been followed at approximately 18-month intervals with similar assessments at each interval. Recruitment, informed consent and study procedures were approved by the Institutional Review Boards of Columbia Presbyterian Medical Center and Columbia University Health Sciences and the New York State Psychiatric Institute.
Of the 4,309 individuals who completed a clinical assessment at their initial visit, we excluded those without a blood sample or measurement of height and weight for calculation of body mass index (BMI) and those with prevalent dementia. Plasma lipids at baseline were unavailable in 1,303 cases. BMI was unavailable in 108 individuals for whom lipids were available. Prevalent dementia was found in 295 cases, while dementia status was unknown in an additional 13 people. In addition, subjects with self-identified ‘Other’ race were excluded from the analysis (n = 34). Because increased mortality in patients with Alzheimer’s disease has been well documented [2], we excluded those with prevalent or unknown dementia status, leaving 2,556 participants for this analysis. We compared demographic and health characteristics of participants with and without plasma lipid levels. Participants with and without lipid levels did not differ in age or level of education, but participants with lipid levels were less likely to be female (66.1 versus 70.5%, respectively, P = 0.018). Participants with lipid levels were also more likely to be Hispanic than those without (42.6 versus 35.5%, P = 0.001). There was no difference between participants with and without lipid levels in the frequency of hypertension, heart disease, diabetes or stroke. Compared to those without lipid levels, participants with lipid levels were less likely to have prevalent dementia (11.5 versus 22.7%, P = 0.001) and more likely to have a history of cancer (14.6 versus 10.6%, P = 0.001).
Vital status
The National Death Index (NDI) was queried by participant social security number for all subjects reported to be dead or who had been lost to follow-up as of 31 December 2002.
Clinical evaluation
Each participant underwent an in-person interview of general health and functional ability followed by a standardised assessment, including medical history, physical and neurological examination and a neuropsychological battery that included measures of memory, orientation, language, abstract reasoning and visuospatial ability [19]. The neuropsychological test battery and its validity in the diagnosis of dementia has been described in detail in a previous publication [19]. All participants received structured neurologic, medical and functional assessments by physicians. The diagnosis of dementia was based on standard research criteria [20] and was established using all available information gathered from the initial and follow-up assessments and medical records by consensus at a conference of physicians, neurologists, neuropsychologists and psychiatrists. Stroke was defined according to the WHO criteria [21], based on self-report, supplemented by a neurological examination and/or review of medical records.
Diabetes mellitus, heart disease and hypertension
Diabetes, hypertension and heart disease were ascertained by self-report at each visit. Diabetes and hypertension were defined as a history of either disorder at any time during life. Heart disease was defined as a history of myocardial infarction, congestive heart failure or angina pectoris at any time in life. These self-reported diagnoses demonstrated a sensitivity and specificity of over 90% using medical records as a gold standard [22].
Plasma lipids
All lipid assays were conducted by the General Clinical Research Center of Columbia University. Fasting plasma, total cholesterol and triglyceride levels were determined at baseline using standard enzymatic techniques. High-density lipoprotein (HDL) cholesterol levels were determined after precipitation of apolipoprotein B containing lipoproteins with phosphotungstic acid [23]. Low-density lipoprotein (LDL) cholesterol was recalculated using the formula from Friedewald and colleagues [24]. Non-HDL cholesterol was calculated as the difference between total cholesterol and HDL cholesterol. Participants were also asked if they had ever been treated with lipid-lowering drugs.
Apolipoprotein E genotype
Apolipoprotein E (APOE) genotypes were determined using a modification of the methods of Hixson and Vernier [25]. Study participants were classified as either carrying or not carrying at least one copy of the APOE ε4 allele.
Additional covariates
BMI was computed by dividing weight (kg) by height squared (m). Smoking status was defined as currently smoking or not smoking at the initial evaluation. Age was defined as age at the initial visit and was treated as a continuous variable. Participants were asked about the highest level of school completed and education was coded using quartiles of years of education.
Statistical analysis
The baseline characteristics of study participants in each of the three self-reported race/ethnicity groups were compared using chi-square tests for categorical measures and analysis of variance for continuous measures. Quartiles for each of the lipid measures were created within strata defined by self-reported race/ethnicity group based upon the distribution within the data: total cholesterol, non-HDL cholesterol, HDL cholesterol, triglycerides and LDL cholesterol (Table 1). The highest quartile was the reference group. Kaplan—Meier survival curves and Cox proportional hazard models for all-cause mortality were used to estimate survival functions and to compute the hazard ratio (HR) and 95% confidence interval (CI) for mortality by quartile of lipid level within strata defined by self-reported race/ethnicity group. The time to event variable was time since enrollment or time to death for the deceased. The HR for all-cause mortality associated with quartiles of plasma lipids was estimated adjusting for age, sex, education and study cohort (1992 or 99 cohort), BMI, APOE genotype, history of heart disease, hypertension, diabetes, stroke, incident dementia and current smoking status. Since low lipid levels may be associated with malnutrition, acute illness or imminent death, the association of lipid levels and all-cause mortality was examined first in the total sample of 2,556 and then among 2,497 individuals with more than 1 year of follow-up. Analyses were performed using SAS version 9 (SAS Institute Inc., Cary, NC) or SPSS version 11.5 (SPSS, Chicago, IL).
Table 1.
Lipid quartile range by ethnic group
| Quartiles range (mg/dl) |
Caucasian | African- American |
Hispanic |
|---|---|---|---|
| Quartiles of cholesterol |
|||
| 1 | ≤176.5 | ≤174.0 | ≤171.0 |
| 2 | 176.6–200.0 | 174.1–198.0 | 171.1–196.0 |
| 3 | 200.1–228.5 | 198.1–227.5 | 196.1–223.0 |
| 3 | >228.5 | >227.5 | >223.0 |
| Quartiles of non-HDL cholesterol |
|||
| 1 | ≤127.0 | ≤122.5 | ≤125.0 |
| 2 | 127.1–153.0 | 122.6–146.0 | 125.1–152.0 |
| 3 | 153.1–176.0 | 146.1–171.0 | 152.1–178.0 |
| 4 | >176.0 | >171.0 | >178.0 |
| Quartiles of HDL | |||
| 1 | ≤37.0 | ≤41.0 | ≤35.0 |
| 2 | 37.1–46.0 | 41.1–49.0 | 35.1–43.0 |
| 3 | 46.1–56.0 | 49.1–61.0 | 43.1–52.0 |
| 4 | >56.0 | >61.0 | >52.0 |
| Quartiles of triglycerides |
|||
| 1 | ≤101.0 | ≤83.5 | ≤112.0 |
| 2 | 101.1–137.0 | 83.6–114.0 | 112.1–153.0 |
| 3 | 137.1–188.0 | 114.1–155.0 | 153.1–219.0 |
| 4 | >188.0 | >155.0 | >219.0 |
| Quartiles of LDL | |||
| 1 | ≤100.6 | ≤99.1 | ≤94.0 |
| 2 | 100.7–121.4 | 99.2–122.4 | 94.1–116.8 |
| 3 | 121.5–144.9 | 122.5–145.8 | 116.9–140.8 |
| 4 | >144.9 | >145.8 | >140.8 |
Results
Baseline characteristics
There were 8,846 person years of follow-up and 427 deaths in the cohorts. Table 2 shows the distribution of baseline demographic characteristics within each race/ethnicity group. Mean age of the cohorts at baseline was 77.0 (range 65–103 years), and did not differ by ethnicity. About 66.1% were women, 27.6% White, 31.2% African-American and 41.2% Hispanic. All-cause mortality was highest among the African-Americans (21.1%), while all-cause mortality was similar among Whites and Hispanics (15.7% and 14.0%, respectively). Mean levels of non-HDL cholesterol, HDL cholesterol and triglycerides significantly differed by race and ethnicity (Table 2). The prevalence of comorbidities differed significantly by race and ethnicity for all chronic conditions except stroke (Table 2). The presence of an APOE ε4 allele was highest among African-Americans, as expected, but did not differ among Whites and Hispanics. Current smoking was also highest among African-Americans, but did not differ between Whites and Hispanics (Table 2).
Table 2.
Baseline characteristics
| Characteristics | Caucasian | African-American | Hispanic | Total cohort |
|---|---|---|---|---|
| Sample size, n (%) | 705 (27.6) | 797 (31.2) | 1,054 (41.2) | 2,556 (100) |
| Mortality n (%) | ||||
| Deceased | 111 (15.7) | 168 (21.1) | 148 (14.0) | 427 (16.7) |
| Alive | 594 (84.3) | 629 (78.9) | 906 (86.0) | 2,129 (83.3) |
| Age at baseline, mean ± SD | 77.3 ± 6.9 | 77.2 ± 6.5 | 76.6 ± 6.4 | 77.0 ± 6.6 |
| Sex, n (%)* | ||||
| Male | 271 (38.4) | 250 (31.4) | 346 (32.8) | 867 (33.9) |
| Female | 434 (61.6) | 547 (68.6) | 708 (67.2) | 1,689 (66.1) |
| Education, mean ± SD* | 13.0 ± 3.5 | 10.9 ± 3.8 | 6.7 ± 4.3 | 9.7 ± 4.8 |
| Body mass index, mean ± SD | 26.5 ± 5.1 | 27.8 ± 6.3 | 27.7 ± 5.1 | 27.4 ± 5.5 |
| Cholesterol (mg/dl), mean ± SD | 202.3 ± 38.5 | 200.5 ± 38.0 | 198.4 ± 41.1 | 200.1 ± 39.6 |
| Non-HDL cholesterol (mg/dl), mean ± SD* | 154.6 ± 37.9 | 148.5 ± 36.6 | 154.0 ± 40.5 | 152.4 ± 38.5 |
| HDL (mg/dl), mean ± SD* | 47.8 ± 14.8 | 51.9 ± 16.0 | 44.5 ± 13.7 | 47.7 ± 15.1 |
| Triglycerides (mg/dl), mean ± SD* | 157.3 ± 84.5 | 129.4 ± 67.9 | 176.0 ± 94.7 | 156.3 ± 86.5 |
| LDL (mg/dl), mean ± SD | 123.1 ± 32.6 | 122.7 ± 34.2 | 118.7 ± 36.0 | 121.2 ± 34.6 |
| APOE ε4 allele, n (%)*a | ||||
| 2/4, 3/4, 4/4 | 153 (22.1) | 279 (35.3) | 262 (25.3) | 694 (27.6) |
| 2/2, 2/3, 3/3 | 538 (77.9) | 512 (64.7) | 775 (74.7) | 1,825 (72.4) |
| Heart disease, n (%)*a | ||||
| Yes | 211 (31.2) | 161 (21.0) | 220 (21.8) | 592 (24.2) |
| No heart disease | 465 (68.8) | 605 (79.0) | 788 (78.2) | 1,858 (75.8) |
| Hypertension, n (%)*a | ||||
| Yes | 354 (52.4) | 524 (68.5) | 717 (71.3) | 1,595 (65.2) |
| No hypertension | 321 (47.6) | 241 (31.5) | 289 (28.7) | 851 (34.8) |
| Diabetes, n (%)*a | ||||
| Yes | 80 (11.9) | 168 (21.9) | 260 (25.8) | 508 (20.7) |
| No diabetes | 595 (88.1) | 598 (78.1) | 748 (74.2) | 1,941 (79.3) |
| Stroke, n (%)a | ||||
| Yes | 60 (8.9) | 84 (11.0) | 121 (12.0) | 265 (10.8) |
| No stroke | 618 (91.1) | 682 (89.0) | 888 (88.0) | 2,188 (89.2) |
| Dementia status, n (%)* | ||||
| Incident | 28 (4.0) | 96 (12.1) | 177 (16.8) | 301 (11.8) |
| No | 677 (96.0) | 701 (87.9) | 877 (83.2) | 2,255 (88.2) |
| Smoking status, n (%)* | ||||
| Currently smoking | 59 (8.4) | 137 (17.2) | 84 (8.0) | 280 (10.9) |
| Not currently smoking | 646 (91.6) | 660 (82.8) | 970 (92.0) | 2,276 (89.1) |
P<0.05.
Numbers are less than 2,556 due to missing data.
Overall survival
Figure 1 shows the Kaplan—Meier survival estimates by ethnic group. Hispanics had the best overall survival, followed by African-Americans and Whites. Among Hispanics, median survival time was 10.7 years, while among African-Americans median survival was 9.4 years and 8.4 years among Whites (P<0.0001).
Figure 1.
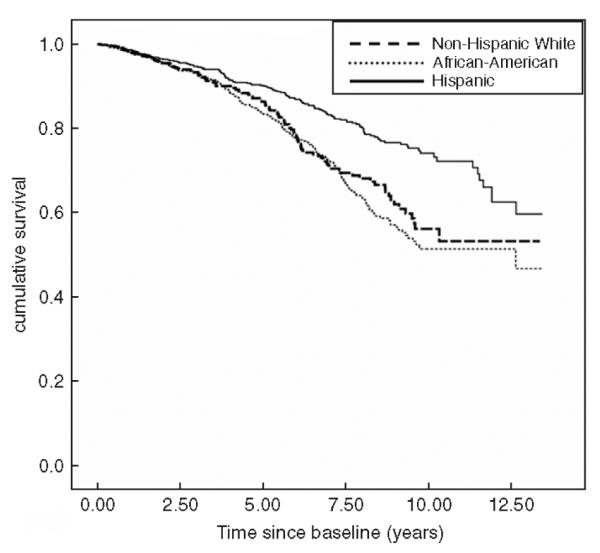
All-cause mortality by race/ethnicity.
Plasma lipid levels and all-cause mortality
Table 3 shows the relation of plasma lipids and all-cause mortality stratified by self-reported race/ethnicity. Whites in the lowest quartile of total cholesterol, the three lowest quartiles of non-HDL cholesterol and the lowest quartile of LDL cholesterol were approximately twice as likely to die as those in the highest quartile after adjusting for covariates (Among Whites, HR: 2.2, 95% CI: 1.2–4.1 for lowest total cholesterol quartile versus highest quartile; HR: 2.3, 95% CI: 1.2–4.3, for lowest non-HDL cholesterol quartile; HR: 1.8, 95% CI: 1.0–3.4, for lowest LDL cholesterol quartile (Table 3). Similarly, African-Americans in the lowest quartiles of total cholesterol, non-HDL cholesterol and LDL cholesterol were almost twice as likely to die as individuals with total cholesterol, non-HDL cholesterol and LDL cholesterol levels in the highest quartile (HR: 1.9, 95% CI: 1.2–3.0, for total cholesterol: HR: 2.0, 95% CI: 1.3–3.2, for non-HDL cholesterol and HR: 1.9, 95% CI: 1.2–3.0 for LDL cholesterol) (Table 3). In contrast, plasma lipids were not significant predictors of all-cause mortality among Hispanics (Table 3).
Table 3.
Relation of plasma lipids and mortality by race/ethnicitya
| Caucasian |
African-American |
Hispanic |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Quartiles range (mg/dl) |
At-risk | No. of deaths (%) |
Hazard ratioa (95% CI) |
At-risk | No. of deaths (%) |
Hazard ratioa (95% CI) |
At-risk | No. of deaths (%) |
Hazard ratioa (95% CI) |
| Quartiles of cholesterol |
|||||||||
| 1 | 176 | 36 (20.5) | 2.2 (1.2–4.1)* | 201 | 52 (25.9) | 1.9 (1.2–3.0)a | 268 | 40 (14.9) | 0.8 (0.5–1.4) |
| 2 | 177 | 27 (15.3) | 1.7 (0.9–3.3) | 200 | 49 (24.5) | 1.2 (0.8–1.9) | 267 | 36 (13.5) | 0.8 (0.5–1.3) |
| 3 | 176 | 27 (15.3) | 1.4 (0.7–2.8) | 197 | 30 (15.2) | 1.0 (0.6–1.6) | 258 | 32 (12.4) | 0.7 (0.4–1.2) |
| 4 | 176 | 21 (11.9) | 1.0 | 199 | 37 (21.1) | 1.0 | 261 | 40 (15.3) | 1.0 |
| Quartiles of non-HDL cholesterol |
|||||||||
| 1 | 172 | 32 (18.6) | 2.3 (1.2–4.3)* | 199 | 55 (27.6) | 2.0 (1.3–3.2)a | 257 | 45 (17.5) | 1.3 (0.8–2.1) |
| 2 | 178 | 30 (16.9) | 2.3 (1.2–4.4)* | 192 | 41 (21.4) | 1.2 (0.7–1.9) | 267 | 32 (12.0) | 0.9 (0.5–1.5) |
| 3 | 176 | 28 (15.9) | 2.3 (1.2–4.4)* | 202 | 36 (17.8) | 1.0 (0.6–1.6) | 258 | 34 (13.2) | 0.9 (0.5–1.4) |
| 4 | 179 | 21 (11.7) | 1.0 | 204 | 36 (17.6) | 1.0 | 272 | 37 (13.6) | 1.0 |
| Quartiles of HDL | |||||||||
| 1 | 158 | 33 (17.9) | 0.8 (0.4–1.6) | 209 | 47 (22.5) | 1.3 (0.8–2.0) | 248 | 40 (16.1) | 0.9 (0.5–1.4) |
| 2 | 179 | 30 (16.8) | 0.8 (0.4–1.4) | 193 | 44 (22.3) | 1.2 (0.7–2.0) | 260 | 28 (10.8) | 0.6 (0.3–1.0) |
| 3 | 163 | 20 (12.3) | 0.8 (0.4–1.4) | 209 | 46 (22.0) | 1.0 (0.6–1.7) | 282 | 40 (14.2) | 0.7 (0.4–1.2) |
| 4 | 179 | 28 (15.6) | 1.0 | 186 | 32 (17.2) | 1.0 | 264 | 40 (15.2) | 1.0 |
| Quartiles of triglycerides |
|||||||||
| 1 | 156 | 25 (14.5) | 1.2 (0.6–2.2) | 199 | 30 (15.1) | 0.6 (0.4–1.0) | 262 | 40 (15.3) | 1.2 (0.7–2.0) |
| 2 | 191 | 31 (17.3) | 1.3 (0.7–2.4) | 199 | 43 (21.6) | 0.8 (0.5–1.3) | 262 | 33 (12.6) | 0.9 (0.6–1.5) |
| 3 | 185 | 32 (18.4) | 1.2 (0.7–2.3) | 199 | 50 (25.1) | 1.0 (0.6–1.5) | 264 | 29 (11.0) | 0.6 (0.4–1.03) |
| 4 | 173 | 23 (12.8) | 1.0 | 200 | 45 (22.5) | 1.0 | 265 | 45 (17.0) | 1.0 |
| Quartiles of LDL | |||||||||
| 1 | 177 | 33 (18.6) | 1.8 (1.0–3.4)* | 199 | 52 (26.1) | 1.9 (1.2–3.0)a | 265 | 46 (17.4) | 1.4 (0.8–2.3) |
| 2 | 178 | 30 (16.9) | 1.6 (0.8–2.9) | 200 | 50 (25.0) | 1.5 (1.0–2.4) | 263 | 36 (13.7) | 1.1 (0.6–1.8) |
| 3 | 174 | 25 (14.4) | 1.3 (0.7–2.5) | 202 | 32 (15.8) | 1.1 (0.6–1.7) | 260 | 33 (12.7) | 1.1 (0.7–1.9) |
| 4 | 176 | 23 (13.1) | 1.0 | 196 | 34 (17.3) | 1.0 | 265 | 32 (12.1) | 1.0 |
Cox proportional hazards model with time to death as the time variable: 95% confidence interval.
P-value <0.05. The baseline quartile, quartile 4 includes individuals with 75–100th percentile.
Adjusted for age, sex, education, study cohort, body mass index, APOE genotype, heart disease, hypertension, diabetes, stroke, dementia and smoking status.
Among 2,497 participants with more than 1 year of follow-up, risk of dying was the same as for the total cohort. Among Whites and African-Americans, individuals with total cholesterol levels in the lowest quartile were twice as likely to die compared with individuals with cholesterol levels in the highest quartile (HR = 2.3, 95% CI: 1.2–4.3 for Whites; HR = 2.1, 95% CI: 1.3–3.2 for African-Americans). Among Whites, individuals with non-HDL cholesterol levels in the three lowest quartiles were twice as likely to die as those in the highest quartile (HR = 2.4, 95% CI: 1.2–4.5 for the lowest quartile; HR = 2.4, 95% CI: 1.3–4.6 for the second lowest quartile and HR = 2.4, 95% CI: 1.2–4.6 for the next lowest quartile). Among African-Americans, those in the lowest quartile of non-HDL cholesterol were twice as likely to die as those in the highest quartile (HR = 2.1, 95% CI: 1.3–3.2). Among both Whites and African-Americans, individuals with LDL cholesterol levels in the lowest quartile were almost two times more likely to die compared to those with levels in the highest quartile [HR 1.9, 95% CI: 1.0–3.4, for Whites and HR = 1.9, 95% CI: 1.2–3.1 for African-Americans (data not shown)]. As in the full dataset, there was no association between plasma lipid levels and all-cause mortality among Hispanics.
Among this sample, 517, or 20.2%, of study participants reported using lipid-lowering medication. The inclusion of lipid-lowering medication use in the models stratified by race/ethnicity did not change the association between total cholesterol levels, non-HDL cholesterol, LDL cholesterol and all-cause mortality among Whites. Among African-Americans, adjustment for lipid-lowering medication use slightly attenuated the association between low lipid levels and all-cause mortality (data not shown).
We also examined the relation of total cholesterol level to vascular morbidity. Among Whites there was no relation between total cholesterol and vascular morbidity. Among African-Americans, the prevalence of hypertension was higher among those in the lowest quartile of total cholesterol compared with those in the highest quartile (72.2 versus 62.6%, P = 0.001). Similarly, the prevalence of heart disease was higher in those in the lowest quartile of total cholesterol versus the highest quartile (28.9 versus 14.9%, P = 0.001). There was no relation of quartile of total cholesterol to the frequency of diabetes or stroke. Among Hispanics, those in the lowest quartile of total cholesterol were more likely to have diabetes than those in the highest quartile (33 versus 20.2%, P = 0.003), but there was no difference in the frequency of hypertension, heart disease or stroke by quartile of total cholesterol (data not shown). Thus, in this cohort, low levels of cholesterol were related to an increased risk of vascular morbidity in African-American and in Hispanics and to all-cause mortality among Whites and African-Americans.
Discussion
We found low total cholesterol, non-HDL cholesterol and LDL-cholesterol levels were associated with an increased risk of death among White and African-American elders, while similar lipid levels were not associated with an increased mortality among Hispanic elders. Levels of HDL-cholesterol and triglycerides were not related to risk for subsequent mortality in any ethnic group. The effects of total cholesterol, non-HDL cholesterol and LDL cholesterol were similar among White and African-American elders, despite a higher prevalence of hypertension, diabetes, and current smoking among African-Americans. Hispanic participants, who had the highest prevalence of hypertension, diabetes, stroke and dementia, showed the lowest risk of all-cause mortality and showed no relation of plasma lipid level with all-cause mortality. Restriction of the study group to those with more than 1 year of follow-up did not change the association between lipid levels and mortality. Additional adjustment for treatment with lipid-lowering drugs slightly attenuated the results among African-Americans, but not among Whites. Results were similar in analyses using ethnic-group specific cut points for quartiles of lipid levels or using cut points generated from the overall sample, suggesting that these findings are robust.
Studies of the association between cholesterol and mortality by race and ethnicity have been inconsistent. In the ECHS, a J-shaped relationship of cholesterol with all-cause mortality was found among White men, while an inverse association between total cholesterol and all-cause mortality was observed among African-American women [10]. Serum cholesterol levels were not related to all-cause or CHD mortality among black men or White women [10]. An analysis of the combined Charleston and ECHS studies found that African-American men experienced significantly less coronary disease mortality than white men, despite a similar distribution of CHD risk factors [26]. Among African-Americans in the Cardiovascular Health Study (CHS), total cholesterol was positively associated with incident CHD, while LDL cholesterol and HDL cholesterol were not associated with incident CHD [11]. A recent follow-up of the Charleston Heart Study cohort suggested that differences in all-cause mortality among Whites and African-Americans were largely explained by the low socio-economic status and increased frequency of cardiovascular disease risk factors among African-Americans compared to Whites [27]. In our cohort, the effect of low lipids levels on all-cause mortality was similar among White and African-American participants, despite differences in level of education and cardiovascular disease risk factors.
We found both an overall mortality advantage and no relation of lipid levels to mortality among Caribbean Hispanics compared with a significant relation of lipid levels and mortality in White and African-American elders. An overall mortality advantage has been reported for Hispanic elders, termed the ‘Hispanic Paradox’ and has been most consistently found among foreign-born Mexicans and foreign-born Hispanics other than Cubans or Puerto Ricans [12-14, 16, 28]. Sociocultural ‘buffering’ effects such as the social support provided by living with family members, inaccurate age reporting in immigrant populations, ‘healthy migrant’ effects and reduced ascertainment of death resulting from elderly migrants retiring to their native country have been suggested to explain the paradox [15, 28-30]. Our results confirm the survival advantage experienced by Hispanics.
Our general hypothesis that there are ethnic differences in the relation between lipids and mortality was generated from the previous observations summarised in the introduction. We did not have a hypothesis based on specific mechanisms as to how these differences might occur. Given our findings and the reported ‘Hispanic Paradox’, we can now hypothesize that a mechanism for this paradox may be a differential effect of lipids in this ethnic group. Ethnic differences in insulin resistance, which underlies lipid profiles, are well known [31], which lends plausibility to this hypothesis that the effect of lipids in Hispanics could be different compared with other ethnic groups.
Healthy survivor effects may contribute to differences in all-cause mortality in our cohort of elders. The mean age of our sample was 77 years old at baseline. It is possible that subjects with higher lipid levels and with high risk of acute cardiovascular events died prior to recruitment, and our sample represents a group of healthy survivors with traits that make them less susceptible to disease caused by high lipid levels. Further, this survival bias might be more pronounced for African-American and Hispanic participants. In the United States, Whites born in 1900 had an estimated life expectancy of 47.6 years, while non-White individuals born in 1900 had an estimated life expectancy of 33.0 years [32]. Among individuals born in 1921, Whites had an estimated life expectancy of 61.8 years, while non-White individuals had an estimated life expectancy of 51.5 years [32]. Some of this earlier mortality may have been related to lipid-related conditions. However, the effect of cholesterol on mortality was similar among Whites and African-Americans, after adjustment for demographic factors and comorbid conditions, but not among Hispanics, suggesting that healthy survival bias among non-Whites cannot entirely account for the observed results.
Our study has several limitations, notably that we did not identify disease-specific mortality for study participants. We did not have data on the subtype of lipid-lowering agents. However, our experience is that over 95% of persons on lipid-lowering medications use statins [33], and we would not be powered to examine differences among classes of lipid-lowering drugs. It would be of interest to examine whether causes of death differed among ethnic groups. To better understand the observed ethnic differences in the association between lipid levels and mortality, a prospective study is needed to examine the risk factors associated with disease-specific mortality.
Acknowledgments
Funding This research was supported by Federal grants AG18732 and AG07232 (Mayeux, P.I.). Laboratory work was supported by Federal grant RR00645 to the Irving Center for Clinical Research.
References
- 1.Stamler J, Stamler R, Neaton JD, et al. Low risk-factor profile and long-term cardiovascular and noncardiovascular mortality and life expectancy: findings for 5 large cohorts of young adult and middle-aged men and women. JAMA. 1999;282:2012–8. doi: 10.1001/jama.282.21.2012. [DOI] [PubMed] [Google Scholar]
- 2.Fried LP, Kronmal RA, Newman AB, et al. Riskfactors for 5-year mortality in older adults: the Cardiovascular Health Study. JAMA. 1998;279:585–92. doi: 10.1001/jama.279.8.585. [DOI] [PubMed] [Google Scholar]
- 3.Krumholtz HM, Seeman TE, Merrill SS, et al. Lack of association between cholesterol and coronary heart disease mortality and morbidity and all-cause mortality in persons older than 70 years. JAMA. 1994;272:1335–40. [PubMed] [Google Scholar]
- 4.Benfante R, Reed D. Is elevated serum cholesterol level a risk factor for coronary heart disease in the elderly? JAMA. 1990;263:393–6. [PubMed] [Google Scholar]
- 5.Frost PH, Davis BR, Burlando AJ, et al. Serum lipids and incidence of coronary heart disease. Findings from the Systolic Hypertension in the Elderly Program (SHEP) Circulation. 1996;94:2381–8. doi: 10.1161/01.cir.94.10.2381. [DOI] [PubMed] [Google Scholar]
- 6.Manolio TA, Ettinger WH, Tracy RP, et al. The Cardiovascular Health Study. Epidemiology of low cholesterol levels in older adults. Circulation. 1993;87:728–37. doi: 10.1161/01.cir.87.3.728. [DOI] [PubMed] [Google Scholar]
- 7.Onder G, Landi F, Volpato S, et al. Serum cholesterol levels and in-hospital mortality in the elderly. Am J Med. 2003;115:265–71. doi: 10.1016/s0002-9343(03)00354-1. [DOI] [PubMed] [Google Scholar]
- 8.Schupf N, Costa R, Luchsinger J, et al. Relationship between plasma lipids and all-cause mortality in nondemented elderly. J Am Geriatr Soc. 2005;53:219–26. doi: 10.1111/j.1532-5415.2005.53106.x. [DOI] [PubMed] [Google Scholar]
- 9.Keil JE, Sutherland SE, Knapp RG, et al. Does equal socioeconomic status in black and white men mean equal risk of mortality? Am J Public Health. 1992;82:1133–6. doi: 10.2105/ajph.82.8.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White AD, Hames CG, Tyroler HA. Serum cholesterol and 20-year mortality in black and white men and women aged 65 and older in the Evans County Heart Study. Ann Epidemiol. 1992;2:85–91. doi: 10.1016/1047-2797(92)90041-n. [DOI] [PubMed] [Google Scholar]
- 11.Jackson SA, Burke GL, Thach C, et al. Incidence and predictors of coronary heart disease among older African Americans—the Cardiovascular Health Study. J Natl Med Assoc. 2001;93:423–9. [PMC free article] [PubMed] [Google Scholar]
- 12.Liao Y, Cooper RS, Cao G, et al. Mortality from coronary heart disease and cardiovascular disease among adult U.S. Hispanics: findings from the National Health Interview Survey (1986 to 1994) J Am Coll Cardiol. 1997;30:1200–5. doi: 10.1016/s0735-1097(97)00278-7. [DOI] [PubMed] [Google Scholar]
- 13.Sorlie PD, Backlund E, Johnson NJ, et al. Mortality by Hispanic status in the United States. JAMA. 1993;270:2464–8. [PubMed] [Google Scholar]
- 14.Swenson CJ, Trepka MJ, Rewers MJ, et al. Cardiovascular disease mortality in Hispanics and non-Hispanic whites. Am J Epidemiol. 2002;156:919–28. doi: 10.1093/aje/kwf140. [DOI] [PubMed] [Google Scholar]
- 15.Hunt KJ, Resendez RG, Williams K, et al. All-cause and cardiovascular mortality among Mexican-American and non-Hispanic White older participants in the San Antonio Heart Study- evidence against the “Hispanic paradox”. Am J Epidemiol. 2003;158:1048–57. doi: 10.1093/aje/kwg249. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell BD, Haffner SM, Hazuda HP, et al. Diabetes and coronary heart disease risk in Mexican Americans. Ann Epidemiol. 1992;2:101–6. doi: 10.1016/1047-2797(92)90043-p. [DOI] [PubMed] [Google Scholar]
- 17.Tang MX, Cross P, Andrews H, et al. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001;56:49–56. doi: 10.1212/wnl.56.1.49. [DOI] [PubMed] [Google Scholar]
- 18.Technical Documentation. Bureau of the Census; Washington, DC: 1991. Census of Population and Housing Summary Tape File1. [Google Scholar]
- 19.Stern Y, Andrews H, Pittman J, et al. Diagnosis of dementia in a heterogeneous population. Development of a neuropsychological paradigm-based diagnosis of dementia and quantified correction for the effects of education. Arch Neurol. 1992;49:453–60. doi: 10.1001/archneur.1992.00530290035009. [DOI] [PubMed] [Google Scholar]
- 20.Association AP . Diagnostic and Statistical Manual of Mental Disorders. 3rd edition. American Psychiatric Association; Washington, DC: 1987. Revised. [Google Scholar]
- 21.Hatano S. Experience from a multicenter stroke register: a preliminary report. Bull World Health Organ. 1976;54:541–53. [PMC free article] [PubMed] [Google Scholar]
- 22.Luchsinger JA, Reitz C, Honig LS, et al. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology. 2005;65:545–51. doi: 10.1212/01.wnl.0000172914.08967.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopez-Virella MF, Stone P, Ellis S, et al. Cholesterol determination in high-density lipoproteins separated by three different methods. Clin Chem. 1977;23:882–4. [PubMed] [Google Scholar]
- 24.Friedewald WT, Levy RI, Fredickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without the use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 25.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31:545–8. [PubMed] [Google Scholar]
- 26.Keil JE, Sutherland SE, Hames CG, et al. Coronary disease mortality and risk factors in black and white men. Results from the combined Charleston, SC, and Evans County, Georgia, heart studies. Arch Intern Med. 1995;155:1521–7. [PubMed] [Google Scholar]
- 27.Nietert PJ, Sutherland SE, Keil JE, et al. Demographic and biologic influences on survival in whites and blacks: 40 years of follow-up in the Charleston heart study. Int J Equity Health. 2006;5:8. doi: 10.1186/1475-9276-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palloni A, Arias E. Paradox lost: explaining the Hispanic adult mortality advantage. Demography. 2004;41:385–415. doi: 10.1353/dem.2004.0024. [DOI] [PubMed] [Google Scholar]
- 29.Boyle CA, Decoufle P. National sources of vital status information: extent of coverage and possible selectivity in reporting. Am J Epidemiol. 1990;131:160–8. doi: 10.1093/oxfordjournals.aje.a115470. [DOI] [PubMed] [Google Scholar]
- 30.Stern MP, Wei M. Do Mexican Americans really have low rates of cardiovascular disease? Prev Med. 1999;29:S90–5. doi: 10.1006/pmed.1998.0464. [DOI] [PubMed] [Google Scholar]
- 31.McKeigue P. Ethnic variation in insulin resistance and risk of type 2 diabetes. In: Reaven G, Laws A, editors. Insulin Resistance: The Metabolic Syndrome X. Humana Press; Totowa, NJ: 1999. pp. 19–34. [Google Scholar]
- 32.National Vital Statistics Report. 2004;53:33–4. http://www.cdc.gov/nchs/data/nvsr/nvsr53/nvsr53_06.pdf.
- 33.Reitz C, Tang MX, Luchsinger J, et al. Relation of plasma lipids to Alzheimer disease and vascular dementia. Arch Neurol. 2004;61:705–14. doi: 10.1001/archneur.61.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]


