Abstract
Objective:
Cerebrospinal fluid parameters are of great importance in diagnosing meningitis, but normal values for preterm neonates are based on small, single-center studies. We sought to determine current values for preterm neonate cerebrospinal fluid parameters and assess the association of cerebrospinal fluid parameters with culture proven meningitis.
Study Design:
Cohort study of the first lumbar puncture from 4,632 neonates <34 weeks gestation performed in the years 1997-2004 at 150 neonatal intensive care units managed by the Pediatrix Medical Group.
Results:
We identified 95 cases of meningitis from the 4,632 lumbar punctures. The area under the receiver operating characteristic curves for white blood cell count, glucose, and protein were 0.80, 0.63, and 0.72 respectively for prediction of culture proven meningitis.
Conclusion:
Cerebrospinal fluid parameters used to diagnose meningitis in the absence of dependable cerebrospinal fluid cultures are unreliable. Caution should be employed when interpreting cerebrospinal fluid parameters in the premature neonate.
Keywords: nosocomial infections, central nervous system, diagnosis, preterm
INTRODUCTION
The cumulative incidence of meningitis is highest in the first month of life and is higher in preterm neonates than term neonates.1 For premature infants who develop meningitis, the neurodevelopmental consequences are often profound.2 Despite the relatively high incidence and substantial morbidity, there are only seven published studies describing expected cerebrospinal fluid (CSF) from 220 preterm neonates (Table 1),3-8 and the most recent of these reports 3 appeared prior to the widespread use of surfactant.
Table 1.
Mean values and ranges of CSF parameters previously reported normal CSF parameters for preterm neonates.
| N | Birth weight (g) | WBC/mm3 | Protein (mg/dL) | Glucose (mg/dL) | |
|---|---|---|---|---|---|
| Samson K, 1931 | Unk | 4 (<10) | |||
| Otila E, 1948 | 66 | 10 | |||
| Wolf H, 1961 | 22 | 1880 | 2 (0-13) | 105 (50-180) | |
| Gyllesward A, 1962 | 36 | 2012 | 7 (1-75) | 132 (55-237 | |
| Sarff LD, 1976 | 30 | 9 (0-29) | 115 (65-150) | 50 (24-63) | |
| Pappu LD, 1982 | 22 | 1437 | 7 (0-28) | ||
| Rodriguez AF, 1990 | 43 | 1002 | 5 (0-44) | 142 (45-370) | 60 (29-217) |
CSF parameters (white blood cell (WBC) count, glucose, and protein) are often relied upon to diagnose meningitis in premature infants because the physical exam is unreliable due to the subtle, nonspecific symptoms at presentation.1, 9-11 Reliance upon CSF parameters is especially common in the premature infant because the lumbar puncture (LP) is often delayed until after administration of empirical antibiotics and notification of a positive blood culture.11, 12 CSF cultures are similarly compromised in newborns of mothers given intrapartum antibiotics for chorioamnionitis or group B streptococcal colonization.13, 14
When faced with the need to make therapeutic decisions on the interpretation of CSF parameters, pediatricians, family practice physicians, and neonatologists often use The Harriet Lane Handbook as their guide. The normal “cutoff” values for CSF parameters in preterm neonates found in this guide are ≤25 WBC/mm3, ≥24 mg glucose/dL, and ≤170 mg protein/dL.15 Unfortunately, the diagnostic utility of CSF parameters has not been evaluated for the identification of culture proven meningitis in preterm neonates. Our objective was to provide current values for CSF parameters from a large multi-center cohort of preterm neonates and assess the reliability of CSF parameters to diagnose culture proven meningitis.
MATERIALS AND METHODS
Study Population
We evaluated the first lumbar puncture from neonates <34 weeks gestation discharged from 150 neonatal intensive care units (NICUs) managed by the Pediatrix Medical Group from 1997-2004. The data were obtained from an administrative database as described previously.16 CSF data were triple-checked for accuracy. We excluded 119 neonates with CSF reservoirs and ventriculoperitoneal shunts, 85 neonates with likely contaminated CSF specimens (54 coagulase-negative Staphylococcus (CoNS), 8 mixed species, 6 Gram positive rods, 4 Streptococcus viridans, 13 other), and 12 neonates with viral meningitis diagnosed by viral culture. Contaminants were defined as CSF cultures positive for organisms generally considered contaminants (coagulase-negative staphylococci, other skin flora [viridans streptococci and diptheroids], or mixed organisms)
Statistical Analysis
The primary outcome variable, meningitis, was defined as a positive CSF culture, a positive CSF Gram stain, or a positive CSF antigen test concordant with the blood culture result. Medians and interquartile ranges (IQR) for CSF parameters were determined for neonates with and without meningitis. Independent variables examined for diagnostic performance in predicting meningitis included: CSF WBC count, CSF glucose, and CSF protein. We evaluated the value of CSF parameters in predicting meningitis by using nonparametric methods to produce receiver operator characteristic (ROC) curves. Unlike the other CSF parameters, lower CSF glucose values are more suggestive of meningitis. In order to produce an ROC curve for CSF glucose, the CSF glucose value was multiplied by (−1). The sensitivity, specificity, positive predictive value (PPV), negative predictive value(NPV), positive likelihood ratio, and negative likelihood ratio were calculated for CSF parameters based on commonly accepted cutoff values for CSF parameters in preterm neonates.15, 17-19
We considered blood and CSF cultures concordant if both cultures were positive with the same organism. We considered CSF and blood cultures discordant if the organism isolated in the CSF culture was different than the organism isolated in the blood culture or if the CSF culture was positive and the blood culture was negative. Mortality was defined as death of the neonate prior to discharge or transfer to another institution.
We conducted the analysis with STATA 8.2 (College Station, Texas) and used nonparametric (Kruskal-Wallis) tests to compare the median CSF parameter values between groups. Reported p-values are two-tailed. In order to examine the median, mean, and range of a sample of our cohort without meningitis equivalent in size to previous reports, we used a random number generator to indicate a patient in the dataset and collected the CSF WBC for that patient and consecutive patients in the dataset without meningitis. The Duke University Institutional Review Board provided permission to conduct this analysis.
RESULTS
There were 4,632 neonates <34 weeks gestation who underwent lumbar puncture during the study period (Table 2). The majority of lumbar punctures were performed in the first month of life, 79.0% (3659/4632), and the median postnatal day of lumbar puncture was 15. The overall mortality of the cohort was 4.8% (192/4033), and mortality was higher in the neonates with meningitis, 16.1% (13/81), vs. the neonates without meningitis, 4.5% (179/3952), p<0.001. The mortality rate in neonates with Candida meningitis was 41.2% (7/17), 14.3% (4/28) in neonates with Gram negative meningitis, and 5.6% (2/36) in neonates with Gram-positive meningitis.
Table 2.
Demographics of the cohort
| N (%) | |
|---|---|
| Cohort | 4632 |
| Gestational Age (weeks) | |
| 22-25 | 845 (18) |
| 26-29 | 1965 (42 |
| 30-33 | 1822 (39) |
| Birth weight (grams) | |
| 366-500 | 64 (1) |
| 501-750 | 772 (17) |
| 751-1000 | 1025 (22) |
| 1001-1250 | 844 (18) |
| 1251-1500 | 663 (14) |
| 1501-2000 | 851 (18) |
| >2000 | 413 (9) |
| Method of delivery | |
| Vaginal | 1963 (43) |
| Caesarian section | 2649 (57) |
| Race | |
| White | 2394 (53) |
| Black | 1102 (24) |
| Hispanic | 817 (18) |
| Asian | 76 (2) |
| Native American | 36 (1) |
| Other | 93 (2) |
| Sex | |
| Male | 2577 (56) |
| Female | 2055 (44) |
| Day of lumbar puncture | |
| 0-3 | 875 (19) |
| 4-7 | 434 (9) |
| 8-14 | 933 (20) |
| 15-21 | 764 (16) |
| 22-28 | 542 (12) |
| 29-60 | 824 (18) |
| 60-257 | 260 (6) |
| Death | |
| Yes | 192 (5) |
| No | 3841 (95) |
Ninety-five (2.1%) of the CSF specimens were positive. Staphylococcus aureus (N=19) and Candida (N=19) were the most commonly isolated pathogens in the CSF (Table 3). E. coli (N=16) was the most commonly isolated Gram negative species. The blood culture was positive in 794 cases, and 428 (54%) of those were considered likely pathogens (Table 3). Staphylococcus aureus (N=100) was the most commonly isolated pathogen in blood. Blood culture results were missing in 281 (6.1%) patients.
Table 3.
CSF and blood culture results
| CSF result (%) | Blood result (%) | |
|---|---|---|
| Total | 4632 | 4351 |
| Positive | 95 (2) | 428 (10) |
| Negative | 4537 (98) | 3557 (81) |
| Contaminants | 0 (0)* | 366 (8) |
| Gram positive | 43 | 207 |
| Group B streptococcus | 9 | 30 |
| Enterococcus spp. | 2 | 39 |
| Listeria monocytogenes | 0 | 1 |
| Staphylococcus aureus | 19 | 100 |
| Gram positive unspeciated | 13 | 37 |
| Gram negative | 33 | 156 |
| Citrobacter spp. | 0 | 2 |
| Escherichia coli | 16 | 58 |
| Enterobacter spp. | 3 | 23 |
| Haemophilus influenza | 0 | 3 |
| Klebsiella spp. | 5 | 36 |
| Morganella spp. | 0 | 2 |
| Pseudomonas aeroginosa | 5 | 7 |
| Salmonella spp. | 0 | 1 |
| Serratia spp. | 3 | 10 |
| Fungi | 19 | 65 |
| Candida spp. | 19 | 64 |
| Malasezzia | 0 | 1 |
CSF with contaminants (e.g. CoNS) were excluded from the analysis
Of the neonates with meningitis with available blood culture results, 28/92 (30%) were blood culture negative. Five (5.4%) of the remaining CSF and blood culture pairs were discordant. These included: 2 neonates with Candida in the CSF and CoNS in the blood, 1 neonate with Gram positive cocci in the CSF and Klebsiella in the blood, 1 neonate with Staphylococcus aureus in the CSF and Candida in the blood, and 1 neonate with Staphylococcus aureus in the CSF and Enterococcus in the blood. Fifty-nine neonates (64%) had concordant CSF and blood cultures.
CSF parameters
The median CSF WBC count in neonates with culture proven meningitis was 110 cells/mm3 (IQR, [15, 980], N=55) and 6 cells/mm3 ([2, 16], N=2396) in neonates without meningitis, p=0.0001. The median CSF protein was higher in the neonates with meningitis, 217 mg/dL ([128, 499], N=49), vs. 130 mg/dL ([100, 172], N=2312) in the neonates without meningitis, p=0.0001. The median CSF glucose was lower in neonates with culture proven meningitis, 43 mg/dL ([17, 52], N=50), compared to the neonates without meningitis, 49 mg/dL ([40, 60], N=2329), p=0.0005.
We produced ROC curves using each CSF parameter separately to predict culture proven meningitis (Figures 1-3). The area under the ROC curve was greatest for CSF WBC count (0.80). Using the commonly accepted normal ranges for CSF parameters,15, 18, 19 the sensitivity of a CSF WBC count >25 cells/mm3 in predicting meningitis was 71% (Table 4). The sensitivity decreased to 61% and 32% for CSF protein >170 mg/dL and CSF glucose <24 mg/dL respectively.
Figure 1.
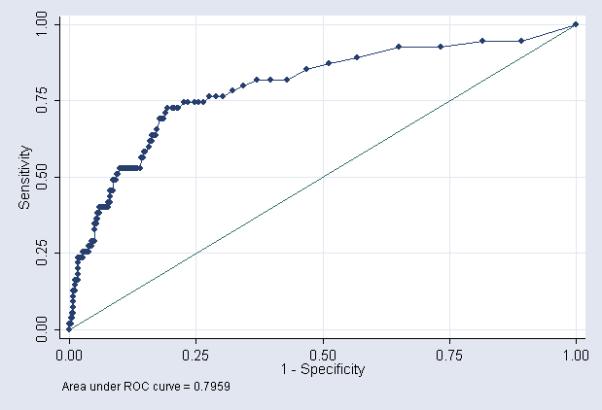
CSF WBC count - ROC curve showing performance of CSF parameters as a diagnostic tool for predicting meningitis
Figure 3.
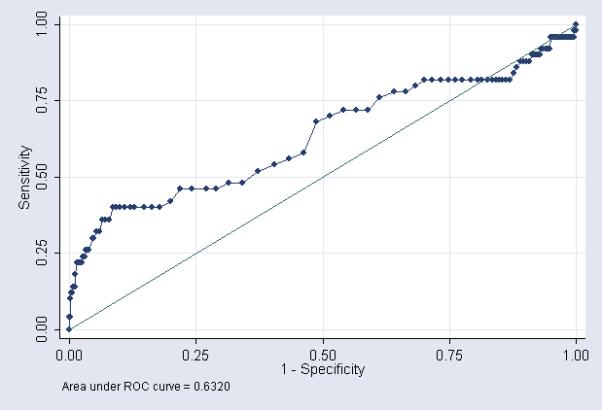
CSF glucose - ROC curve showing performance of CSF parameters as a diagnostic tool for predicting meningitis
Table 4.
CSF parameter comparison – (WBC cells/mm3, glucose mg/dL, protein mg/dL)
| Sensitivity | Specificity | PPV | NPV | + LR | − LR | |
|---|---|---|---|---|---|---|
| WBC>0 | 95 | 12 | 2 | 99 | 1.1 | 0.4 |
| WBC>10 | 80 | 67 | 5 | 99 | 2.4 | 0.3 |
| WBC>20 | 73 | 79 | 7 | 99 | 3.5 | 0.3 |
| *WBC>25 | 69 | 82 | 8 | 99 | 3.9 | 0.4 |
| WBC>100 | 51 | 91 | 11 | 99 | 5.7 | 0.5 |
| WBC>1000 | 24 | 97 | 17 | 98 | 8 | 0.8 |
| glucose<10 | 18 | 99 | 33 | 98 | 23.0 | 0.8 |
| *glucose<24 | 32 | 96 | 14 | 98 | 8.0 | 0.7 |
| protein>90 | 96 | 18 | 2 | 99 | 1.2 | 0.2 |
| *protein >170 | 61 | 75 | 5 | 99 | 2.4 | 0.5 |
| All Harriet Lane values abnormal | 26 | 97 | 13 | 98 | 8.7 | 0.8 |
| Any Harriet Lane value abnormal | 78 | 65 | 5 | 99 | 2.2 | 0.3 |
| WBC>25, glucose<10, protein>250 | 18 | 99.5 | 41 | 98 | 164.0 | 0.8 |
Harriet Lane value
CSF contaminants
There were 54 neonates excluded from the study with CoNS meningitis. Although often a pathogen in preterm neonates, the organism is a common contaminant and was excluded in our primary analysis in order to concentrate on those neonates with known true pathogens present in their CSF. Including these 54 neonates decreased the median WBC count from 110 cells /mm3 to 37 cells/mm3, decreased the median CSF protein from 217 mg/dL to 194 mg/dL, and increased the median CSF glucose from 43 mg/dL to 45 mg/dL. Similarly the area under the ROC curves decreased from 0.80 to 0.71 for CSF WBC count, 0.72 to 0.67 for CSF protein, and 0.63 to 0.59 for CSF glucose.
Using the commonly accepted normal ranges for CSF parameters,15, 18, 19 the sensitivity of a CSF WBC count >25 cells/mm3 for predicting CoNS meningitis was only 37% (Table 5). Similarly, cutoff values for glucose and protein demonstrated lower sensitivity and specificity for predicting CoNS meningitis when compared to their predictive values for other pathogens.
Table 5.
CSF parameter comparison for prediction of CoNS meningitis – (WBC cells/mm3, glucose mg/dL, protein mg/dL)
| Sensitivity | Specificity | PPV | NPV | + LR | − LR | |
|---|---|---|---|---|---|---|
| *WBC>25 | 37 | 78 | 2 | 99 | 1.7 | 0.8 |
| *glucose<24 | 11 | 94 | 2 | 99 | 2.0 | 0.9 |
| *protein >170 | 38 | 73 | 2 | 99 | 1.4 | 0.8 |
Harriet Lane value
DISCUSSION
This paper reports CSF data from over 4600 neonates born during the years 1997-2004, and thus represents a major advance in the state of our knowledge. The most recent report of normal CSF parameters in preterm neonates involved 71 CSF specimens from 43 very low birth weight (birth weight <1500 grams) neonates, and the highest CSF WBC count observed was 44 cells/mm3.3 In the group of neonates in our study with negative CSF cultures, 333/2396 (14%) of the neonates had CSF WBC counts >44 cells/mm3 and 437/2396 (18%) of the neonates had CSF WBC counts >25 cells/mm3, the commonly accepted upper limit of normal.15, 18, 19 Excluding the neonates with >100 RBC in the CSF (possible traumatic specimens), 37/1209 (3.1%) of the neonates with negative CSF cultures had CSF WBC counts >44 cells/mm3, and 62/1209 (5.1%) had CSF WBC counts >25 cells/mm3. However, the median values for CSF parameters in our study were similar to previously reported mean values (Table 1).
In contrast to prior studies, we were able to examine the diagnostic value of CSF parameters in predicting culture proven meningitis by generating ROC curves for each individual CSF parameter. The area under the ROC curve was greatest for CSF WBC count (0.80, [0.73, 0.86]) followed by CSF protein (0.72 [0.64, 0.80]), and CSF glucose (0.63 [0.54, 0.73]). Diagnostic tests with an area under the ROC curve >0.9 are generally considered excellent, and those with an area under the ROC curve between 0.8 and 0.9 are considered good. Although ROC curves are often used to determine cutoff points for diagnostic tests, the process of developing a cutoff point must take into account the costs of treating the disease (financial costs, patient discomfort, drug side effects) in patients without the disease (false positives) with the resulting morbidity and mortality of not treating the disease in patients who truly have the disease (false negatives). Neonatal meningitis is a disease with substantial mortality and morbidity even among treated neonates.1, 20 Costs associated with treating false positive patients include prolonged hospital stay, exposure of the neonate to broad spectrum antibiotics, and insertion of central venous catheters for prolonged antibiotic administration.
Using the normal ranges provided in several reference texts,15, 17-19 22% of preterm neonates with culture proven meningitis had normal CSF WBC count, glucose, and protein levels. The PPV of having an abnormality in the CSF was low for each of the CSF parameters (4-10%). The NPV was high for each CSF parameter (98-99%) due to the low incidence of meningitis in the cohort. The likelihood ratios observed for each of the parameters are modest at best (Table 4). For example, a preterm neonate with a 10% pretest probability of having meningitis based on one's clinical experience that has a CSF WBC count >25 cells/mm3 [positive likelihood ratio of 3.7] would have a post-test probability of culture positive meningitis of 29%.
Many clinicians often use the CSF parameters to “rule out” meningitis in the neonate. According to table 4, the CSF parameter most useful for this purpose is CSF protein. Ninety-six percent of neonates with meningitis have a CSF protein level >90 mg/dL. The negative likelihood ratio for culture proven meningitis of a neonate having a protein level ≤90 mg/dL is 0.2. CSF glucose appears to be more useful to “rule in” meningitis. The positive likelihood ratio of a glucose level <24 mg/dL is 8.0 and increases to 23.0 for a level <10 mg/dL (Table 4). However, the sensitivity of a glucose level of <24 mg/dL is only 32% and falls to 18% for a level <10 mg/dL. A combination of all 3 parameters provides for a more robust way of “ruling in” meningitis. A neonate undergoing a lumbar puncture with >25 WBC cells/mm3, a glucose of <10 mg/dL, and >250 mg/dL of CSF protein has a 164 fold increase in odds of having a positive CSF culture. However, only 18% of neonates with positive CSF cultures were identified using these cutoff values.
LPs are often delayed because of the clinical instability of the neonate or the low perceived risk of meningitis, and thus, clinicians often defer the LP until after blood culture results are known to be positive and the neonate has been exposed to empirical antibiotics. Although only 2.1% (95/4632) of the neonates in our cohort had meningitis, 30% (28/92) of these neonates had negative blood cultures. These findings are consistent with previous multicenter reports in term and preterm neonates.11, 13 In contrast to previous studies describing the causative organisms of sepsis in preterm neonates, we found Staphylococcus aureus and Candida to be the most commonly isolated species in the CSF.21, 22
Although the administrative database used for this study was collected prospectively, the analysis was retrospective. CSF parameters were missing in the dataset in 45% of neonates. Our analysis of the diagnostic utility of CSF glucose was limited as we did not have temporally related serum glucose levels. We acknowledge that the major weakness of this study was the lack of information regarding type and timing of empirical antibiotics to which the neonates were exposed. It is acknowledged that a number of the neonates with negative CSF cultures likely had a central nervous system infection; however, this acknowledgement does not conflict with our primary hypothesis. Chiefly, that these data suggest that the clinician should employ caution when interpreting “negative” CSF parameter results.
This is the largest collection of CSF parameters in preterm neonates presented to date. These data suggest that CSF parameters relied upon to diagnose meningitis in preterm neonates in the absence of dependable cultures are of marginal diagnostic use, and CSF parameters, either alone or in combination, cannot be used to exclude meningitis. In order to avoid diagnosing this potentially devastating infection on marginally useful CSF parameters, our findings support the argument that the LP should be performed during the initial evaluation of the clinically stable neonate with a suspected systemic infection. A prospective study using mortality and neurodevelopmental follow up will better define the utility of CSF parameters in the premature neonate. Until such study is completed, caution should be employed when interpreting CSF parameters.
Figure 2.
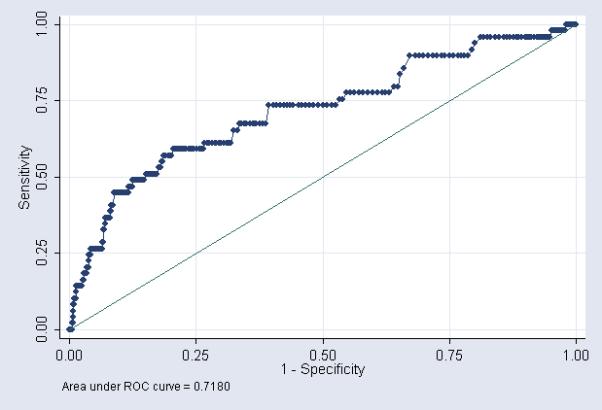
CSF protein - ROC curve showing performance of CSF parameters as a diagnostic tool for predicting meningitis
Acknowledgements
Dr. Smith received support from NIH T32 (HD-043728-01A2)
Dr. Benjamin received support from HD044799-01 and the Thrasher Research Fund
Abbreviations
- CSF
cerebrospinal fluid
- WBC
white blood cell
- LP
lumbar puncture
- NICU
neonatal intensive care unit
- CoNS
coagulase negative Staphylococcus
- IQR
interquartile range
- ROC
receiver operating characteristic
- PPV
positive predictive value
- NPV
negative predictive value
References
- 1.Overall JC., Jr. Neonatal bacterial meningitis. Analysis of predisposing factors and outcome compared with matched control subjects. J Pediatr. 1970;76:499–511. doi: 10.1016/s0022-3476(70)80399-7. [DOI] [PubMed] [Google Scholar]
- 2.Stoll BJ, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292:2357–65. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez AF, Kaplan SL, Mason EO., Jr. Cerebrospinal fluid values in the very low birth weight infant. J Pediatr. 1990;116:971–4. doi: 10.1016/s0022-3476(05)80663-8. [DOI] [PubMed] [Google Scholar]
- 4.Sarff LD, Platt LH, McCracken GH., Jr. Cerebrospinal fluid evaluation in neonates: comparison of high-risk infants with and without meningitis. J Pediatr. 1976;88:473–7. doi: 10.1016/s0022-3476(76)80271-5. [DOI] [PubMed] [Google Scholar]
- 5.Otila E. Studies on the cerebrospinal fluid in premature infants. Acta Paediatrica. 1948;1948:7–99. [Google Scholar]
- 6.Gyllensward A, Malmstrom S. The cerebrospinal fluid in immature infants. Acta Paediatr. 1962:54–62. [PubMed] [Google Scholar]
- 7.Wolf H, Hoepffner L. The cerebrospinal fluid in the newborn and premature infant. World Neurol. 1961;2:871–8. [PubMed] [Google Scholar]
- 8.Samson K. Die liquordiagnostik im kindesalter. Ergeb Inn Med Kinderheilkd. 1931;1931:551–778. [Google Scholar]
- 9.Feigin RD, McCracken GH, Jr., Klein JO. Diagnosis and management of meningitis. Pediatr Infect Dis J. 1992;11:785–814. doi: 10.1097/00006454-199209000-00039. [DOI] [PubMed] [Google Scholar]
- 10.Schwersenski J, McIntyre L, Bauer CR. Lumbar puncture frequency and cerebrospinal fluid analysis in the neonate. Am J Dis Child. 1991;145:54–8. doi: 10.1001/archpedi.1991.02160010058016. [DOI] [PubMed] [Google Scholar]
- 11.Stoll BJ, et al. To tap or not to tap: high likelihood of meningitis without sepsis among very low birth weight infants. Pediatrics. 2004;113:1181–6. doi: 10.1542/peds.113.5.1181. [DOI] [PubMed] [Google Scholar]
- 12.Fielkow S, Reuter S, Gotoff SP. Cerebrospinal fluid examination in symptom-free infants with risk factors for infection. J Pediatr. 1991;119:971–3. doi: 10.1016/s0022-3476(05)83058-6. [DOI] [PubMed] [Google Scholar]
- 13.Wiswell TE, et al. No lumbar puncture in the evaluation for early neonatal sepsis: will meningitis be missed? Pediatrics. 1995;95:803–6. [PubMed] [Google Scholar]
- 14.Weiss MG, Ionides SP, Anderson CL. Meningitis in premature infants with respiratory distress: role of admission lumbar puncture. J Pediatr. 1991;119:973–5. doi: 10.1016/s0022-3476(05)83059-8. [DOI] [PubMed] [Google Scholar]
- 15.Robertson J, Shilkofski N. The Harriet Lane Handbook. 17th ed. Mosby; Philadelphia: 2005. p. 557. [Google Scholar]
- 16.Garges HP, et al. Neonatal meningitis: what is the correlation among cerebrospinal fluid cultures, blood cultures, and cerebrospinal fluid parameters? Pediatrics. 2006;117:1094–100. doi: 10.1542/peds.2005-1132. [DOI] [PubMed] [Google Scholar]
- 17.Katz SL, Gershon AA, Hotez PJ. Krugman's Infectious Diseases of Children. 10th ed. Mosby; St. Louis: 1998. pp. 769–770. [Google Scholar]
- 18.McMillan JA, et al. Oski's Pediatrics. 3rd ed. Lippincott Williams and Wilkens; Philadelphia: 1999. pp. 414–420. [Google Scholar]
- 19.Fanaroff AA, Martin RJ. Neonatal-Perinatal Medicine Diseases of the Fetus and Newborn. 7th ed. Vol. 2. Mosby; St. Louis: 2002. pp. 719–720. [Google Scholar]
- 20.Harvey D, Holt DE, Bedford H. Bacterial meningitis in the newborn: a prospective study of mortality and morbidity. Semin Perinatol. 1999;23:218–25. doi: 10.1016/s0146-0005(99)80066-4. [DOI] [PubMed] [Google Scholar]
- 21.Stoll BJ, et al. Changes in pathogens causing early-onset sepsis in very-low-birth-weight infants. N Engl J Med. 2002;347:240–7. doi: 10.1056/NEJMoa012657. [DOI] [PubMed] [Google Scholar]
- 22.Stoll BJ, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110:285–91. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]


