Abstract
The empirical and theoretical development of the P300 event-related brain potential (ERP) is reviewed by considering factors that contribute to its amplitude, latency, and general characteristics. The neuropsychological origins of the P3a and P3b subcomponents are detailed, and how target/standard discrimination difficulty modulates scalp topography is discussed. The neural loci of P3a and P3b generation are outlined, and a cognitive model is proffered: P3a originates from stimulus-driven frontal attention mechanisms during task processing, whereas P3b originates from temporal-parietal activity associated with attention and appears related to subsequent memory processing. Neurotransmitter actions associating P3a to frontal/dopaminergic and P3b to parietal/norepinephrine pathways are highlighted. Neuroinhibition is suggested as an overarching theoretical mechanism for P300, which is elicited when stimulus detection engages memory operations.
Keywords: P300, ERP, attention, memory, neurotransmitters, inhibition, theory
1. Introduction
Discovery of the P300 spurred the use of event-related potential (ERP) methods to assess the neural underpinnings of cognition. This quest is pursued today with a convergence of methods that are beginning to hone the fundamental circuitry and identify the neurotransmitter systems activated when the P300 is observed. Although early understanding of the P300 was derived primarily from functional analysis, this once unitary phenomenon is now thought to be composed of several parts that reflect an information-processing cascade when attentional and memory mechanisms are engaged. The present review develops an integrated interpretation of P300 suggested by the current neuroelectric and neuroimaging data. The groundwork is conveyed through citations and summary statements to promote an assessable still-life picture of this evolving research area.
The paper is organized into sections: First, fundamental P300 issues and a theoretical overview are presented. Second, the neuropsychological background of the P3a and P3b distinction is outlined. Third, fMRI data on P300 origins are highlighted to provide the neurophysiological foundations of component neural circuitry. Fourth, the neuropharmacological processes related to P3a and P3b are sketched to suggest how neuroelectric and neurotransmitter systems may interact. Fifth, a model system is proffered in which the P3a and P3b are proposed to result from the operation of inhibitory mechanisms engaged by incoming stimulus events to facilitate memory processing.
To maintain nomenclature consistency, the term “P300” is used to refer to the canonical ERP component, which also is called the “P3” or the more nebulous “late positive component” (LPC). The terms “P3a” and “P3b” denote the distinction between the two subcomponents as defined below. The primary empirical and theoretical approaches to P300 are covered, with early findings reviewed previously (Donchin and Coles, 1988; Hillyard and Kutas, 1983; Hillyard and Picton, 1987; Johnson, 1986; Molnar, 1994; Picton, 1992; Price and Smith, 1974; Verleger, 1988). Not directly addressed are aging, clinical, and developmental P300 studies. These literatures have grown in specialized and cross-disciplinary ways, with summaries available elsewhere (e.g., Cycowicz, 2000;DeBoer et al., 2005; Jeon and Polich, 2003; Oken, 1997; Pan et al., 1999; Polich, 2004; Reinvang, 1999; Verleger, 2003).
2. P300 Characteristics and Theory
2.1. A brief history
The P300 was first reported over 40 years ago (Sutton et al., 1965). Its discovery stemmed from the confluence of increased technological capability for signal averaging applied to human neuroelectric measures and the impact of information theory on psychological research (Sutton, 1979). The original studies manipulated stimulus information to assess how electric brain patterns varied among conditions (see Bashore and van der Molen, 1991). Subsequent results elucidated the roles of stimulus probability and task relevance, which provided the basis for its functional analysis often from data obtained with the “oddball” paradigm (Donchin et al., 1978; Pritchard, 1981).
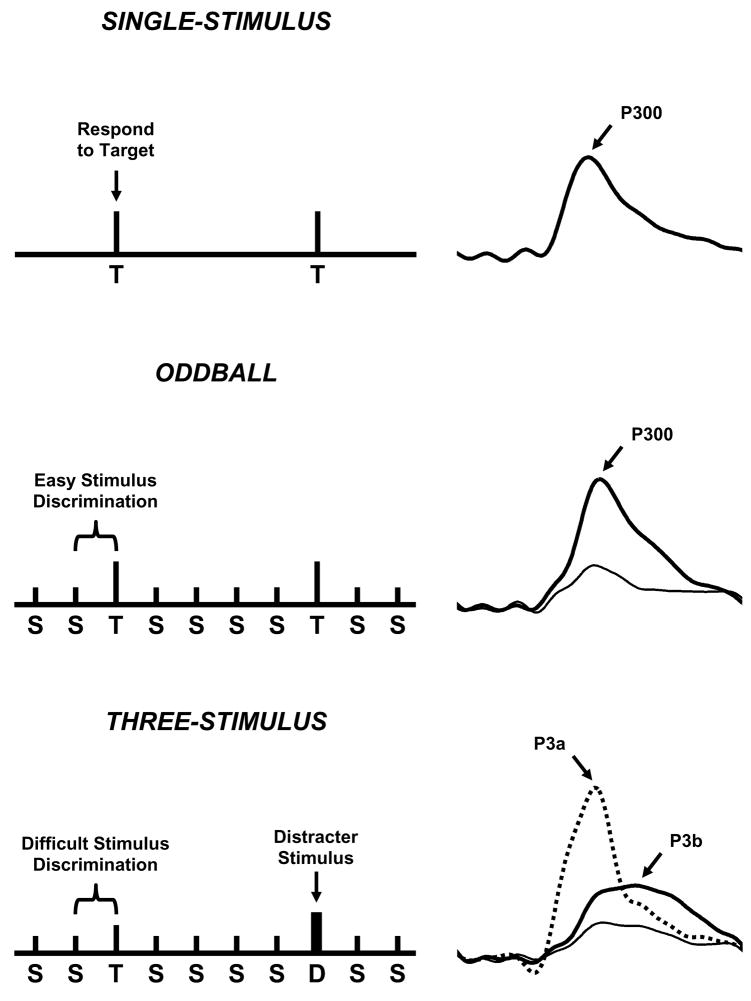
Figure 1 illustrates variants of the oddball task. The single-stimulus procedure infrequently presents the target with no other stimuli occurring (top). The traditional two-stimulus oddball presents an infrequent target in a background of frequent standard stimuli (middle). The three-stimulus oddball presents an infrequent target in a background of frequently occurring standard stimuli and infrequently occurring distracter stimuli (bottom). In each case, the subject is instructed to respond mentally or physically to the target stimulus and not respond otherwise. The P300 component is measured by assessing its amplitude and latency. Amplitude (μV) is defined as the difference between the mean prestimulus baseline voltage and the largest positive-going peak of the ERP waveform within a time window (e.g., 250–500 ms, although the range can vary depending on stimulus modality, task conditions, subject age, etc.). Latency (ms) is defined as the time from stimulus onset to the point of maximum positive amplitude within a time window. P300 scalp distribution is defined as the amplitude change over the midline electrodes (Fz, Cz, Pz), which typically increases in magnitude from the frontal to parietal electrode sites (Johnson, 1993).
Figure 1.
Schematic illustration of the single-stimulus (top), oddball (middle), and three-stimulus (bottom) paradigms, with the elicited ERPs from the stimuli of each task at the right (Polich and Criado, 2006). The single-stimulus task presents an infrequent target (T) in the absence of any other stimuli. The oddball task presents two different stimuli in a random sequence, with one occurring less frequently than the other does (target=T, standard=S). The three-stimulus task is similar to the oddball with a compelling distracter (D) stimulus that occurs infrequently. In each task, the subject is instructed to respond only to the target and otherwise to refrain from responding. The distracter elicits a P3a, and target elicits a P3b (P300). Reprinted with permission of the authors and from Elsevier (Copyright 2006)
2.2. Context updating theory
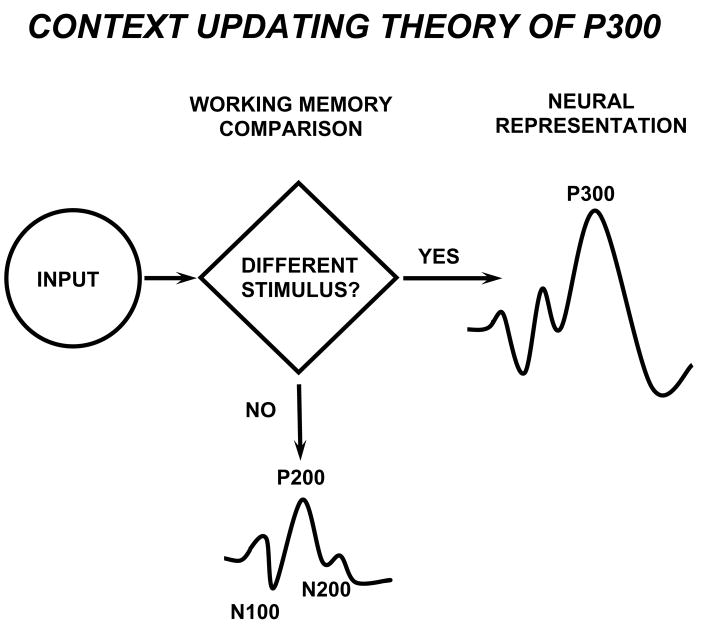
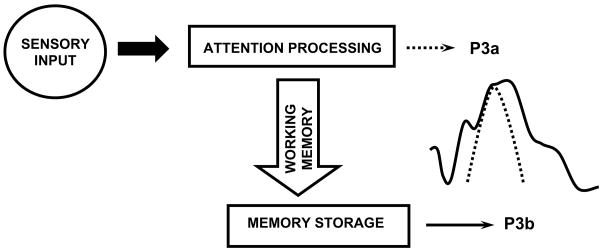
Figure 2 schematically illustrates a theoretical account of oddball processing. In this framework, the P300 component indexes brain activities underlying revision of the mental representation induced by incoming stimuli (Donchin, 1981). After initial sensory processing, an attention-driven comparison process evaluates the representation of the previous event in working memory—a process distinct from although related to sensory stimulus feature mismatch detection (Heslenfeld, 2003; Kujala and Näätänen, 2003). If no stimulus attribute change is detected, the current mental model or “schema” of the stimulus context is maintained, and only sensory evoked potentials are recorded (N100, P200, N200). If a new stimulus is detected, attentional processes govern a change or “updating” of the stimulus representation that is concomitant with P300. These events are similar to the orienting response, and P300 habituation/dishabituation observed (cf. Polich, 1989; Rushby et al., 2005; Yamaguchi et al., 2004).
Figure 2.
Schematic illustration of the P300 context-updating model (Polich, 2003). Stimuli enter the processing system and a memory comparison process is engaged that ascertains whether the current stimulus is either the same as the previous stimulus or not (e.g. in the oddball task, whether a standard or a target stimulus was presented). If the incoming stimulus is the same, the neural model of the stimulus environment is unchanged, and sensory evoked potentials (N100, P200, N200) are obtained after signal averaging. If the incoming stimulus is not the same and the subject allocates attentional resources to the target, the neural representation of the stimulus environment is changed or updated, such that a P300 (P3b) potential is generated in addition to the sensory evoked potentials. Reprinted with permission from Kluwer/Spring Publishing (Copyright 2003).
Despite the simplicity of the task situation and the reliability of observing ERPs in the oddball paradigm, a clear understanding of how and why the brain produces the P300 remains elusive. Indeed, stimulus representations (words, objects) maintained in memory from previous exposures such as in a working memory or recognition task can produce P300 components to the reoccurrence of that stimulus that are larger than those from stimulus items not previously encountered (e.g., Doyle and Rugg, 1992; Guo et al., 2007; McEvoy et al., 2001). Although task demands can readily alter such outcomes, the context is refurbished by updating operations that are apparently sensitive to previous stimulus presentations because the intervening nontarget events engage attention to modify the current neural representation (Donchin et al., 1986).
This versatility of the context-updating hypothesis is a major theoretical strength as 25 years after its proposal and many confirmations, the general nature of the argument has resisted refutation although different theoretical emphases have emerged (e.g., Mecklinger and Ullsperger, 1993, 1995; Nieuwenhuis et al., 2005; Yordanova et al., 2001; Verleger et al., 2005). The context updating approach may reflect relatively strong initial target stimulus processing more related to P3a and diminishes as the repeated target stimuli occur to produce the P3b (Kok, 2001). That the P300 is responsive to habituation and dishabituation procedures suggests this type of interactive association, with each potential indexing attentional allocation at different levels (Kok, 1997; Polich, 1989; Rusby et al., 2005).
2.3. Resource allocation and P300
The context-updating hypothesis of P300 was derived in large measure from manipulating target stimulus probability in two-stimulus oddball tasks. Discriminating the target from the standard stimulus produces a robust P300 that increases in amplitude as the target’s global and local sequence probability decreases (Duncan-Johnson and Donchin, 1977, 1982; Johnson and Donchin, 1982; Squires et al., 1976). Target stimulus probability effects served as the basis for the suggestion that P300 originates from task conditions involving working memory (Donchin et al., 1986), and that conscious awareness may be related to stimulus sequence effects (Leuthold and Sommer, 1993; Sommer et al., 1990). In addition, P300 amplitude is sensitive to the amount of attentional resources engaged during dual-task performance. A primary task that varies cognitive demands is performed while the subject also is engaged in a secondary task of mentally counting target oddball stimuli. As primary task difficulty is increased, P300 amplitude from the oddball task decreases regardless of modality or the motor requirements of the primary task (Isreal et al., 1980; Kramer et al., 1985; Wickens et al., 1983).
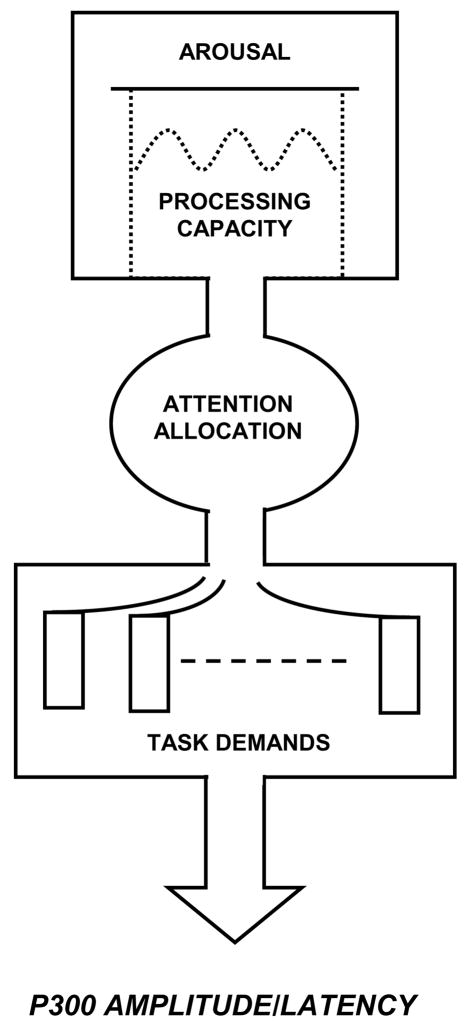
Figure 3 illustrates the conceptual relationship between attentional resource allocation and P300 outcomes. The processing system is modulated by overall arousal level, which governs the amount of attention available for task performance (Kahneman, 1973). When task conditions are undemanding, P300 amplitude is hypothesized to index attentional resources such that amplitude is relatively large and peak latency is relatively short. Hence, for tasks that require greater amounts of attentional resources, P300 amplitude is smaller and peak latency is longer as processing resources are used for task performance (Kok, 2001; Polich, 1987). Passive stimulus processing generally produces smaller P300 amplitudes than active tasks, because stimulus and non-task events engage attentional resources to reduce amplitude. Further, trait and state arousal levels affect the availability of attention processes to modulate P300 (Kok, 1990; Pribram and McGuinness, 1975). Tonic arousal changes are relatively slow manifestations of energetic state fluctuations, whereas phasic arousal changes indicate the organism’s energetic reaction to specific events to affect the availability of attentional resources and P300 measures observed at the scalp (Kok, 2001; Polich and Kok, 1995).
Figure 3.
Schematic illustration of how attentional resources affect P300 (after Kahneman, 1973). This model reflects a general framework for viewing how attentional resources can affect P300 measures. Overall arousal level determines the amount of processing capacity available for attention allocation to on-going tasks. More difficult or multiple task demands reduce P300 amplitude and lengthen peak latency. Reprinted with permission from Academic Press (Copyright 1973).
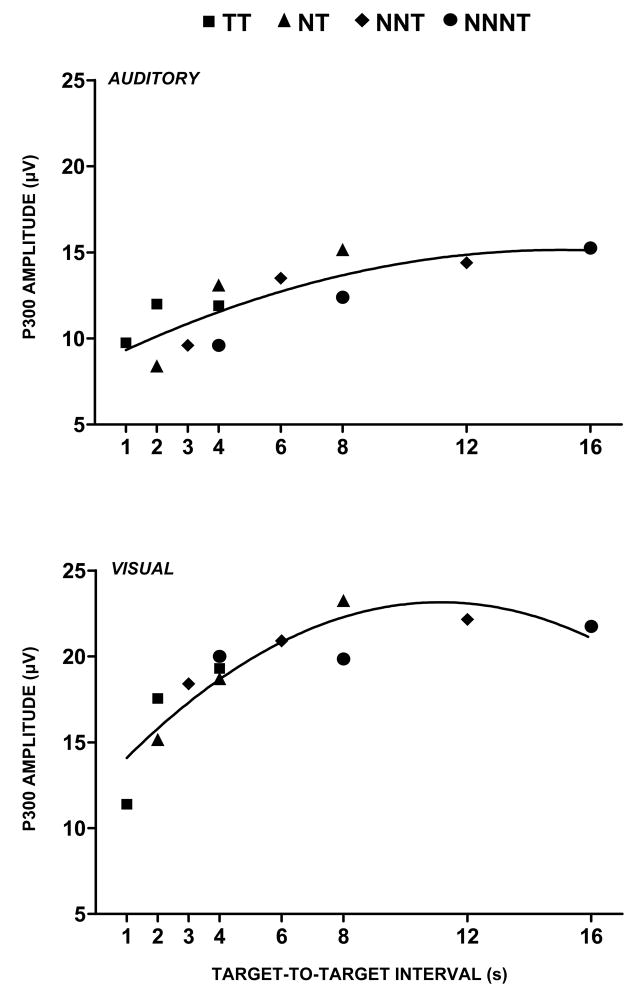
2.4. Target-to-target interval
Figure 4 illustrates the influence of time-induced limitations on P300 amplitude as a function of target-to-target interval (TTI) for stimulus sequences defined by the number of nontarget (standard) stimuli that occur before the detected target (Gonsalvez and Polich, 2002; Gonsalvez et al., 2007). These findings reflect a major empirical qualification of probability and stimulus sequence on P300 outcomes (Squires et al., 1977; Woods and Courchesne, 1986), as TTI determines how quickly resources can be redirected to process target stimuli (Pashler, 1994). Short intervals produce smaller P300 components than longer intervals (Fitzgerald and Picton, 1984), with TTIs of 6–8 seconds or greater eliminating probability effects (Polich, 1990; Polich and Bondurant, 1997). Temporal limitations therefore may originate from memory trace development governing the event representational quality that underlies P300 generation (cf. Gonsalvez et al., 2007; Kok, 2001).
Figure 4.
P300 amplitude plotted as a function of target-to-target interval (TTI) for the target (T) stimulus in an oddball task across sequences of preceding nontarget (N) standard stimuli. The legend defines the symbols used to depict various nontarget and target sequences. The subject is instructed to respond only to the target stimulus. P300 amplitude increases independently of local sequence and global target probability. The regression lines reflect curvilinear best fit for a second order polynomial. Similar results have been found for the single-stimulus paradigm when only target stimuli are presented (Gonsalvez et al., 2007). Adapted from Gonsalvez and Polich (2002) with permission of the authors and Blackwell Publishing (Copyright 2002)
This theoretical interpretation is supported by P300 findings from “single-stimulus” paradigms in which only the target stimulus occurs randomly and variably in time (Mertens and Polich, 1997a; Polich and Heine, 1996; Polich et al., 1994). This task produces P300 components comparable to the oddball paradigm (Katayama and Polich, 1996; Polich and Margala, 1997; Strüber and Polich, 2002). Topographic localization methods have demonstrated similar waveforms, amplitude distributions, and dipole coordinates across tasks (Croft et al., 2003; Tarkka and Stokic, 1998). Thus, even when the target stimulus probability is unitary, the time between events is the primary determinant of P300 amplitude.
2.5. Memory and P300
The orienting response origins and the attention allocation effects for P300 led to ERP assessment of recall memory to assess whether P300 was associated with recall performance (Karis et al., 1984). Words presented sequentially and ERPs were recorded. In some lists, one of the words was presented either in smaller or larger font size than the other words, so that stimulus distinctiveness would affect encoding and facilitate recall memory. Distinct word stimuli that were subsequently recalled elicited larger P300 components during encoding than those that were not recalled. However, P300 was affected by the rehearsal strategy such that component amplitude was larger for subsequent recall when participants used rote rehearsal (Fabiani et al., 1986, 1990). When participants employed an elaborative strategy, P300 amplitude was unassociated with subsequent recall performance.
These findings suggested tasks that alter stimulus attention and require fundamental memory processing affect P300 amplitude (Donchin, 1980). In addition, subsequent attentional resource engagement contributes to P300 amplitude as increases in memory load reduce component size because task-processing demands increase (Kok, 2001; Wijers et al., 1989). Although the scalp topographic changes with attention and memory requirements vary with task requirements, memory for items that elicit a late positive potential is larger than for items that do not elicit such a potential (Paller et al., 1988; Rushby et al., 2002). Studied word stimuli that receive full attention also are recognized with more confidence and associated with greater P300 amplitude (Curran and Cleary, 2003; Curran, 2004). Furthermore, the close relationship of encoding mechanisms with P300 amplitude when retrieval is delayed in a serial position paradigm is consistent with the view that this potential is associated with memory engagement (Azizian and Polich, 2007). Thus, stimulus encoding that promotes successful memory storage to facilitate retrieval and recognition produces increased P300-like amplitude.
2.6. Summary: P300 amplitude
Early accounts of P300 emphasized stimulus information and probability sequence. Subsequent findings described the role of attentional resource allocation, thereby implying that cognitive demands during task processing influence P300. TTI results demonstrated that component size is small for relatively rapid stimulus presentations, whereas target stimulus items occurring at longer intervals yield maximum component amplitudes. This empirical framework is consonant with the link between P300 and attentional processing of target stimulus events—phenomena that appear related to memory processing.
2.7. P300 latency
P300 latency is thought to index classification speed, which is proportional to the time required to detect and evaluate a target stimulus (Kutas et al., 1977; Magliero et al., 1984). Like P300 amplitude, component latency changes across the scalp and is shorter over frontal areas but longer over parietal areas (Mertens and Polich, 1997a; Polich et al., 1997). Stimulus and task requirements contribute to the association between P300 latency and response time, but the strength or sensitivity of the relationship between latency and response time varies across stimulus-response compatibility and Stroop choice-response tasks (Duncan-Johnson and Kopell, 1981; McCarthy and Donchin., 1981). Semantic-based compatibility tasks produce a larger P300 latency/response time difference compared to spatial compatibility tasks. Furthermore, P300 latency has been used as a metric for timing mental events producing other ERP components (Renault et al., 1982; Ritter et al., 1983). Inferences based on component timing and behavioral output therefore must take into account the type of stimulus and decision process underlying response activation (Pfefferbaum et al., 1986; Ragot and Renault, 1981, 1985). An insightful review found that P300 timing is sensitive to both stimulus- and response-related processing when responding is fast (Verleger, 1997). This conclusion has evolved into the intriguing possibility that P300 may originate from the neural linkage between stimulus perception and the response to that event (Verleger et al., 2005).
Individual differences for P300 latency are correlated with mental function speed, such that shorter latencies are related to superior cognitive performance (e.g., Emmerson et al., 1989; Johnson et al., 1985; Pelosi et al., 1992a; Polich et al., 1983). The neuropsychological tests that produce the strongest correlation between P300 latency and cognitive capability assess how rapidly subjects can allocate attentional resources (Houlihan et al., 1998; Pelosi et al., 1992b; Reinvang, 1999). P300 latency decreases as children develop (Howard and Polich, 1985; Polich et al., 1990a) and increases with normal aging (Fjell and Walhovd, 2001; Polich, 1996). Component latency also becomes longer as dementia level increases (O’Donnell et al., 1992; Polich and Corey-Bloom, 2005; Polich et al., 1986, 1990b; Potter and Barrett, 1999), although few reports suggest how brain insult or disease prolong ERP timing (Bashore and Ridderinkhof, 2002; Polich, 2004).
2.8. Summary: P300 latency
P300 peak latency is proportional to stimulus evaluation timing, is sensitive to task processing demands, and varies with individual differences in cognitive capability. However, most studies report only a single peak and response time for specific paradigms rather than fostering a wider theoretical framework. Evaluation of normal aging and cognitive impairment have used P300 latency, but fundamental measurement issues on defining individual peaks is complicated by topographic timing variation, single-trial variability, multiple intra-component peaks, and an absence of clinical guidelines.
2.9. Applied P300 characteristics
Test-retest correlation coefficients for oddball task P300 amplitude range from 0.50 to 0.80 and for peak latency from 0.40 to 0.70 (Fabiani et al., 1987; Polich, 1986; Segalowitz and Barnes, 1993; Walhovd and Fjell, 2002). Latency jitter can contribute to amplitude effects, but it is relatively minimal for oddball tasks (cf. Cohen and Polich, 1997; Karniski and Blair, 1989; Michalewski et al., 1986). Correlation strength is influenced by ultradian rhythms that contribute to ERP individual differences (Lin and Polich, 1999; Ravden and Polich, 1999). Regardless, P300 measures are as reliable as clinical assays, can assess cognitive capability, and are relatively inexpensive to obtain (Polich and Herbst, 2000).
Neuroelectric signals are genetically transmitted (van Beijsterveldt and van Baal, 2002). EEG spectral characteristics are highly similar for identical twins (Lykken, 1974; Stassen et al., 1987), and strong spectral power similarities are observed for biological family members (Eischen et al., 1995; Vogel et al., 1979). P300 is virtually identical for pairs of monozygotic twins, less so for dyzygotic twins, and different for unrelated controls (Katsanis et al., 1997; O’Connor et al., 1994; Polich and Burns, 1987). P300 heritability also is evidenced by biologically related family members who demonstrate significant inter-family member correlations for ERP measures (Eischen and Polich, 1994; Polich and Bloom, 1999). Specific loci on the human genome have been identified that may determine ERP characteristics (Begleiter et al., 1998). These observations suggest that P300 may be a marker of disease phenotype (e.g., Carlson et al., 1999; Jeon and Polich, 2003; Porjesz et al., 2005).
The genetic underpinnings for P300 are consonant with findings for ERPs and personality attributes such as introversion/extraversion, sensation seeking, and impulsivity (Gurrera et al., 2001; Stelmack and Houlihan, 1994). Although the relationship among ERP measures and personality is murky, a correlation between individual differences for personality-related arousal levels and P300 is generally observed: Low arousal individuals have smaller P300 amplitudes compared to high-arousal individuals who have larger P300 components (cf. Brocke, 2004; Brocke et al., 1997; De Pascalis, 2004; Stenberg, 1992). This relationship is modulated by biological factors (Polich and Kok, 1995), differences among paradigms (DiTraglia and Polich, 1991; Stenberg, 1994), and psychopathology (Iacono et al., 2002, 2003; Justus et al., 2001). These effects could be related to individual differences for attentional resource capabilities that may stem from variability for neurotransmitter function (Hill et al., 1998, 1999; Polich and Criado, 2006).
2.10. Summary: Applied P300 characteristics
P300 amplitude/latency measurement reliability, genetic origins, and personality variables are important for interpreting individual ERP differences. Genetic factors strongly affect P300, and biological determinants contribute to component variability. Although the story is complex, this natural variation occurs across studies and implies that the neuroelectric circuit underlying P300 is a fundamental physiological attribute of individual CNS reactivity.
3. Neuropsychology of P3a and P3b
3.1. Background
An infrequent distinct tone presented in a series of frequent tones without a task can produce a positive-going waveform having a central/parietal maximum amplitude distribution and relatively short peak latency. This component was dubbed the “P3a” to distinguish it from the task-relevant “P3b” potential elicited during target stimulus processing (Snyder and Hillyard, 1976; Squires et al., 1975). P3a from an auditory oddball task can be readily observed in about 10–15% of normal young adults (Polich, 1988), which indicates that observation of subcomponent generation varies across individuals. Appropriate visual stimuli without a task also can produce a P3a-like potential (Jeon and Polich, 2001).
Several ERPs have been reported that appear related to the P3a. These components are elicited by an infrequent distracter stimulus inserted randomly into the target/standard sequence (see Figure 1c). When perceptually novel distracters (dog barks, color forms, etc.) occur in a series of more typical (tones, letters of the alphabet, etc.) stimuli, a frontal/central P300 can be elicited with a relatively short peak latency that habituates rapidly (Courchesne et al., 1975; Knight, 1984). This potential has been called the “novelty P300” and is interpreted as reflecting frontal lobe activity related to the hippocampus (Grunwald et al., 1998; Knight, 1996). It is observed across modalities (Fabiani et al., 1998; Yamaguchi and Knight, 1991) and populations (Friedman et al., 1998; Knight, 1987; Yamaguchi et al., 2000). With repeated stimulus presentation, novelty P300 decreases in amplitude so that it may be more directly related to the orienting response than the P3b (Knight, 1984; Kok, 2001; Riggins and Polich, 2003; Rushby et al., 2006).
If non-novel repeated stimuli (tones, letters, etc.) are used as distracters in a three-stimulus oddball, a “no-go” P300 is elicited. Subjects do not respond to the infrequent distracter and only respond to the targets (Kok, 1986; Pfefferbaum et al., 1985). The P300 from this type of distracter has maximum amplitude over the central/parietal areas (Falkenstein et al., 1999; Katayama and Polich, 1998). The topographic distribution for this component is somewhat more central than the parietal P300 from the target stimulus. The no-go P300 has been linked to response inhibition mechanisms, although this hypothesis is debated (Azizian et al., 2006; Eimer, 1993; Falkenstein et al., 2000; Salisbury et al., 2004).
3.2. P3a and stimulus context
The P3a, novelty, and no-go P300 findings suggest that the type of nontarget distracter and task demands determine component amplitude portraiture (cf. Donchin et al., 1997; Friedman et al., 2001; Gaeta et al., 2003). Historically, the experimental paradigms used to elicit these potentials fostered the idea that they were distinct entities, but this view was modified when the critical factor of task difficulty was assessed systematically in the three-stimulus oddball paradigm. Katayama and Polich (1998) varied perceptual discrimination difficulty between the auditory target and standard stimulus to manipulate the amount of focal attention engaged, with a repeated tone used as the distracter stimulus. For the easy task, P300 amplitude from the distracter and target was largest over the parietal electrodes; for the difficult task, P300 from the distracter was larger than that from the target over the frontal/central electrodes. Target and distracter amplitude was largest over the parietal electrodes for all conditions. The same outcomes were observed for auditory and visual non-novel distracter stimuli evaluated separately. Easy discrimination tasks produced scalp topography similar to no-go P300 potentials, whereas difficult discrimination tasks produced P3a potentials that were similar to novelty P300 (Comerchero and Polich, 1998, 1999; Hagen et al., 2005).
Subsequent studies supported the interpretation that the P3a, novelty P300, and no-go P300 are variations of the same potential. Spencer et al. (1999) compared oddball target P300 with those from novel distracters in an auditory three-stimulus task by employing a large electrode array and Principal Components Analysis (PCA). The findings indicated that the novelty P3 was a different component from the classic P300. Simons et al. (2001) carefully replicated the original non-novel auditory P3a and novelty P300 tasks, found no differences between the two components using PCA, and concluded that previous distinctions between the P3a and novelty P300 were not supported. Polich and Comerchero (2003) compared novel non-repeating abstract color stimuli and non-novel repetitive blue-square distracters. The easy task yielded a central maximum distribution for the novel stimuli and a central/parietal maximum P300 potential for the non-novel stimuli—i.e., the same topography as the no-go P300. The hard task produced virtually identical central maximum topographies for both the novel and non-novel distracters. Combs and Polich (2006) obtained similar results for the auditory modality when distracters were white noise bursts, different novel sounds, and a high frequency tone: White noise and novel stimuli produced P3a components that were larger over the central electrodes compared to the tone distracters. Taken together, the findings suggest that the P3a, novelty, and no-go P300 are most likely variants of the same ERP that varies in scalp topography as a function of attentional and task demands.
3.3. Theoretical perspective
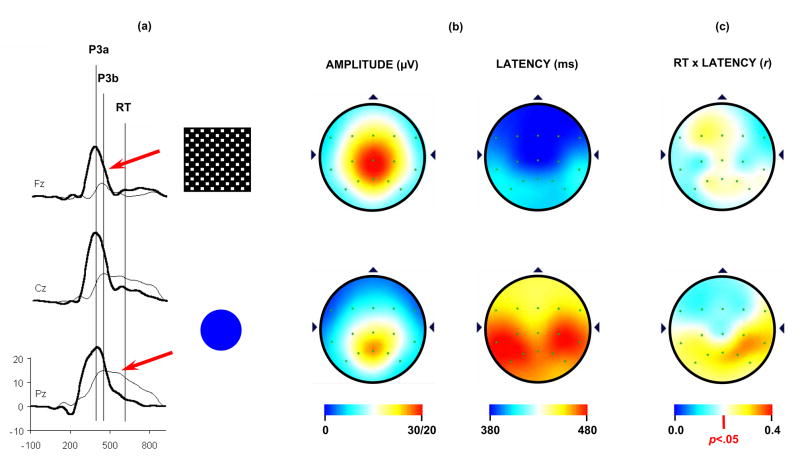
Figure 5 illustrates P3a and P3b data using a difficult target/standard discrimination task in a three-stimulus paradigm from 120 healthy young adults. The distracter was a large black/white checkerboard square, and the target was a blue circle that was slightly larger than the standard (not shown) blue circle (Conroy and Polich, 2007). Figure 5a illustrates the grand averages for the P3a from the checkerboard distracter (thick line) and P3b from the target circle (thin line); the vertical lines indicate the peak latencies and mean response time. Figure 5b illustrates the topographic distributions of the mean amplitude and latency for the distracter (top) and target (bottom) stimuli. P3a has a central maximum, whereas P3b has a parietal maximum. Peak latency for both potentials was shorter over the frontal and longer over the parietal electrode sites. Figure 5c illustrates the topographic distributions of the correlation coefficients between P3a (top) and P3b (bottom) peak latencies and response time calculated across subjects for each electrode position. P3a latency and response time are weakly and widely associated, whereas P3b latency and response time are positively and significantly correlated over the parietal areas.
Figure 5.
(a) Grand averages of the P3a, P3b, and response time (RT) from a three-stimulus oddball task (N=120). Subjects were instructed to press a button whenever an infrequent target (5.0 cm diameter) circle was detected in a series of standard (4.5 cm diameter) stimuli (not shown). Infrequently presented distracter checkerboard patterns (18 cm2) were employed to elicit the P3a. (b) Topography distributions for the mean P3a (upper) and P3b (lower) amplitude and latency. Note the distinct patterns for amplitude and latency from each component. Amplitude scales on upper end (30/20) refers to GV for P3a and P3b, respectively. (c) Topographic distributions of the correlation between P3a (top) and P3b (lower) latency and response time. The subject responded only to the target stimuli. P3a and response time were moderately correlated, whereas P3b and response time were strongly correlated over parietal areas. Adapted from Conroy and Polich (2007) with permission of the authors and Hogrefe & Huber Publishers (Copyright 2007)
These results demonstrate that P3a and P3b have distinct topographic amplitude distributions. Their scalp topography latency distributions are similar but covary differently with response time. The topographic variation may be induced by different stimulus/task contexts to produce overlapping neural activation patterns that are functionally distinct (Gaeta et al., 2003; He et al., 2001; Spencer et al., 2001; Yago et al., 2003). This perspective is consonant with the view that novelty processing is modulated by contextual and familiarity effects: Non-repeating stimulus events define novel items, but repeating stimulus events engage top-down processing (Ranganath and Rainer, 2003). Hence, novelty P300 and P3a may differ with respect to how attentional processes are engaged for distracter stimuli (Demiralp et al., 2001; Hagen et al., 2005; Jeon and Polich, 2001). As distracter stimuli do not receive an overt response, topographic differences among potentials necessarily reflect stimulus-driven attributes that can be manipulated by discrimination task demands. Whether the resulting components from a wider range of stimulus configurations, probabilities, or multiple target responses would yield similar outcomes is unknown. However, given the relatively simple paradigms assessed to date, it is reasonable to infer that stimulus evaluation engages focal attention (P3a) to facilitate context maintenance (P3b), which is associated with memory operations (Hartikainen and Knight, 2003; Kok, 2001; Polich, 2003).
3.4. Neural origins of P3a and P3b
The neural generators of P300 are imprecisely delineated, although appreciable progress has been made in the last 25 years (Eichele et al., 2005; Linden, 2005; Soltani and Knight, 2000). Patients with frontal lobe lesions demonstrated diminution of P3a amplitude, whereas the same patients demonstrated a parietal maximum for the P3b. Frontal lobe integrity is therefore necessary for P3a generation (Knight, 1984; Knight et al., 1995). Moreover, patients with focal hippocampal lesions evinced reduced P3a amplitude from novel distracters but normal P3b components from targets (Knight, 1996). Initial studies of the hippocampal formation using depth electrodes in humans suggested that at least some portion of the P300 (P3b) is generated in the medial temporal lobe (Halgren et al., 1980; McCarthy et al., 1989). However, subsequent reports of scalp recordings from individuals after temporal lobectomy (Johnson, 1988b; Smith and Halgren, 1989), experimental excisions in monkeys (Paller et al., 1988, 1992), and patients with severe medial temporal lobe damage (Onofrj et al., 1992; Rugg et al., 1991) found that the hippocampal formation does not contribute directly to P300 generation (Molnar, 1994). Indeed, assessment of patients with bilateral hippocampal lesions without surgical intervention demonstrated no reliable P300 differences relative to controls (Polich and Squire, 1993). P300 amplitude is affected by temporal-parietal junction integrity as its absence greatly reduces component size over the parietal area (Knight et al., 1989; Verleger et al., 1994; Yamaguchi and Knight, 1992). This connection implies that the P3a and P3b indicate a circuit pathway between frontal and temporal/parietal brain areas (Soltani and Knight, 2000; Polich, 2003).
Figure 6 presents a neuropsychological model for P3a and P3b based on these results (Polich, 2003). Discrimination between target and standard stimuli in an oddball paradigm is hypothesized to initiate frontal lobe activity that is sensitive to the attentional demands induced by task performance (Pardo et al., 1991; Posner, 1992; Posner and Petersen, 1990). fMRI and ERP findings have demonstrated frontal lobe activity for the detection of rare or physically alerting stimuli (McCarthy et al., 1997; Potts et al., 1996; Verbaten et al., 1997). P3a may be generated when such stimuli are processed if sufficient attentional focus is engaged. P3b appears to occur when subsequent attentional resources activations promote memory operations in temporal-parietal areas (Brázdil et al., 2001, 2003; Knight, 1996; Squire and Kandel, 1999). Indeed, elegant cellular recording studies in primates indicate that information induced by changes in frontal activation during a matching-to-sample task is shunted to infero-temporal structures that index task context updating for future stimulus presentations (Desimone et al., 1995). Thus, it is reasonable to suppose that P3a and P3b generation stems from frontal and temporal/parietal activations (Ebmeier et al., 1995; Kirino et al., 2000).
Figure 6.
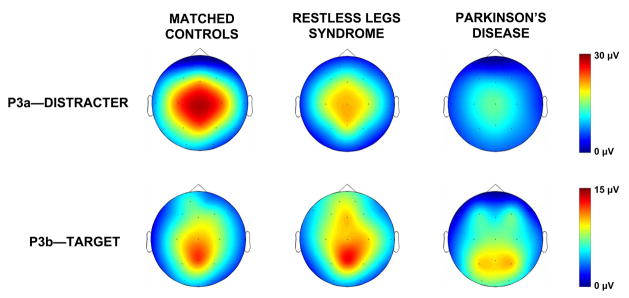
Topographic amplitude mappings from the distracter and target stimuli (after Poceta et al., 2006). Each subject in the three groups of unaffected matched control, restless leg syndrome, and Parkinson’s disease patients performed the same task (see Figure 6). The patients were matched on age, gender, and education with n=7 individuals per group. The P3a amplitudes illustrate increasing dopaminergic deficits from left to right; the P3b amplitudes demonstrated little difference between controls and the restless leg syndrome patients, with Parkinson’s disease patients demonstrating appreciably smaller amplitudes.
This view is congenial with the neurocognitive assumptions that incoming stimuli invoke top-down attention switching, and that bottom-up memory-driven operations guide response organization and production (cf. Debener et al., 2005; Escera et al., 1998; Goldstein et al., 2002). ERP and fMRI studies suggest that a frontal attention mechanism governs neural responsivity to novelty (Daffner et al., 2000a, 2000b, 2000c; Suwazono et al., 2000), thereby implying top-down control (Bledowski et al., 2004a; Dien et al., 2004; Kiehl et al., 2005; Opitz et al., 1999; Opitz, 2003). Attentional resources used to maintain memory items in parietal regions may result from response organization produced by bottom-up processing (Conroy and Polich, 2007; Nieuwenhuis et al., 2005; Verleger et al., 2005). In sum, stimulus characteristics and task demands are determinants of distracter evaluation and contribute to the different topographic and timing outcomes observed at the scalp (Berti et al., 2004; Debener et al., 2002; Gaeta et al., 2003; Polich and Comerchero, 2003).
3.5 Summary: Neuropsychology of P3a and P3b
The functional and neuropsychological origins of different P300 potentials suggest that the P3a, novelty P300, and no-go potentials elicited by distracter stimuli are variants of the same generation system. The differences among these distracter components appear to arise from variation in top-down monitoring by frontal attention mechanisms engaged to evaluate incoming stimuli. Processing of such stimulus events produces P3b activity related to context updating operations and subsequent memory storage. The proposed model is speculative but the fundamental neural pathways are consistent with empirical findings, data from lesion studies, and theoretical constraints. A major issue is how the memory storage operations are related to P300. The large number of resulting ERP memory studies has produced a complex set of outcomes that are only sketched here. The general assertion that P300 is generated in the service of memory storage remains supported by these findings, even though a comprehensive view has not emerged.
4. Neuropharmacology of P300
4.1. Dual-transmitter hypothesis
The neurotransmitter systems underlying P300 generation are yet unclear, with various mechanisms implicated (Frodl-Bauch et al., 1999; Hansenne, 2000). However, available data suggest that P3a is related to frontal focal attention and working memory mediated by dopaminergic activity, and that P3b is related to temporal-parietal activity where dense norepinephrine inputs are found (cf. Braver and Cohen, 2002; Nieuwenhuis et al., 2005; Pineda, 1995; Pineda et al., 1989; Polich and Criado, 2006).
Figure 7 illustrates examples of “natural” neuropharmacological interventions (Poceta et al., 2006). The P3a and P3b amplitude data were obtained using a three-stimulus paradigm to compare unaffected controls, patients with restless leg syndrome, and patients with Parkinson’s disease. Restless leg syndrome is thought to originate from dopaminergic deficits, with greater such deficits found for Parkinson’s disease patients (Trenkwalder and Winkelmann, 2003). As indicated by the topographic mappings, P3a amplitude from the distracter stimulus is robust for the controls, decreased for the restless leg syndrome patients, and virtually eliminated for the Parkinson’s patients. P3b from the target stimulus for the controls and restless leg patients is comparable, but greatly reduced for the Parkinson’s patients. These data suggest that at least the P3a and some portion of the P3b are affected by dopaminergic activity (Polich and Criado, 2006).
Figure 7.
Schematic model of cognitive P300 activity (Polich, 2003). Sensory input is processed, with frontal lobe activation from attention-driven working memory changes producing P3a and temporal/parietal lobe activation from memory updating operations producing P3b. Reprinted with permission from Kluwer/Spring Publishing (Copyright 2003).
Several lines of evidence imply catecholaminergic mediation of frontal P300 (P3a) generation: (1) Parkinson disease patients who have decreased levels of dopamine demonstrate deficient P300 measures (Hansch et al., 1982; Stanzione et al., 1991). (2) The dopamine antagonist sulpiride increases P300 in low-amplitude subjects and decreases it in high-amplitude subjects (Takeshita and Ogura, 1994). (3) Pharmacological studies have found dopaminergic mediation of P300 amplitude and latency (Hansenne et al., 1995; Wang et al., 2000). (4) Children at elevated risk for alcoholism demonstrate dopamine-related genetic differences and P300 amplitude deficits (Hill et al., 1998), which may be associated with an “endophenotype of alcoholism” that likely originate from externalizing disorders (cf. Hesselbrock et al., 2001; Hicks et al., 2007).
A comprehensive review of the wide-ranging P300 neuropharmacology literature suggests that the locus-coeruleus-norepinephrine (LC-NE) system underlies parietal P300 (P3b) generation for a target detection task (Nieuwenhuis et al., 2005). Since the neuropharmacological evidence stems primarily from ERPs elicited in rat, cat, and monkey populations, differences in paradigm and task performance need to be considered in evaluating these outcomes. However, the suggestion that LC-NE contributes to P300 generation is consonant with attention resource allocation and arousal-related effects in humans (Intriligator and Polich, 1995; Kok, 1997, 2001). The topographic LC-NE activation of temporal-parietal areas also is in agreement with overall P300 characteristics (Aston-Jones and Cohen, 2005).
4.2. Summary: P300 neurotransmitters
Given that frontal/attention P3a and temporal-parietal/memory P3b origins are associated with dopaminergic and LC-NE activity, respectively, individual ERP differences for acute and chronic drug intake could be associated with specific transmitter responsivity variation. One approach to these issues in humans is to assay ERP effects before and after acute drug intake and compare individuals who vary in chronic drug-use frequency. If P3a and P3b topographic distributions change as a function of acute and/or chronic drug use, development of a metric for assessing drug effects can be pursued. Neuropharmacological ERP studies in humans are difficult to execute, with stimulus/task considerations (cue reactivity, performance variation, response difficulty), neurobiological variables (circadian rhythms, dose level, gender differences), administrative challenges (pre-test deprivation, retest reliability, task fatigue), and necessary control groups (no drug use, only target drug use, frequency/amount use level) all possibly affecting the assessment. Characterizing ERP neurochemical foundations should greatly further the understanding of their neurophysiological mechanisms and yield important clinical applications.
5. What does the P300 do?
The P300 is produced by a distributed network of brain processes associated with attention and memory operations. However, specifying a singular overarching explanation for this neuroelectric phenomenon has proven difficult, primarily because the P300 is observed in any task that requires stimulus discrimination—a fundamental psychological event that determines many aspects of cognition. Differentiation between the P3a and P3b subcomponents has begun to elucidate the interaction between initial and subsequent P300 processes, but the resulting component’s ontology still is unknown.
5.1. P300 as inhibition: A hypothesis
Given the nature of the P300, generation of a neuroelectric event linked to attention and memory-related operations might be caused by brain mechanisms engaged to inhibit extraneous brain activation. The implication of this hypothesis is that the P300 and its underlying subprocesses could reflect rapid neural inhibition of ongoing activity to facilitate transmission of stimulus/task information from frontal (P3a) to temporal-parietal (P3b) locations. P300 signals could occur from the initial need to enhance focal attention during stimulus detection relative to the contents of working memory (Knight, 1997; Soltani and Knight, 2000b). Hence, a minimization of extraneous stimulus processing would facilitate the transference of incoming stimulus information from frontal to temporal-parietal areas to sharpen memory operations. This speculation is supported broadly by neurophysiological results (Friedman et al., 2001; Knight and Nakada, 1998; Nieuwenhuis et al., 2005; Ranganath and Rainer, 2003), experimental findings (Birbaumer and Elbert, 1988; Birbaumer et al., 1990), and personality ERP variation (DiTraglia and Polich, 1991; Orlebeke et al., 1989; Polich and Martin, 1992; Stenberg, 1994). Although these results are diverse, such an inhibition hypothesis cognitively encompasses the aggregate findings using a unitary mechanism.
An inhibition hypothesis is consistent with the functional descriptions of P300 outlined above: (1) Since infrequent, low probability stimuli can be biologically important, it is adaptive to inhibit unrelated activity to promote processing efficiency thereby yielding large P300 amplitudes. (2) Difficult and dual-processing tasks that induce high cognitive demand limit attentional resources to resist inhibitory control and produce smaller P300 components. (3) Arousal would modulate the level of neural inhibition engaged, as it governs the amount of attention resources available for task performance to affect P300 measures. Since endogenous arousal level is a proposed major difference among personality characteristics, ERP differences across individual profiles also could be accounted for by this scheme. (4) The relationship between P300 peak latency and cognitive capability indexes the celerity with which extraneous processes are inhibited—a highly advantageous quality associated with intelligence. (5) Declines in P300 amplitude and lengthening of latency with aging and dementing illness stem from breakdowns in cortical processes underlying inhibitory signals. (6) The postulated neurotransmitter systems for P3a and P3b are congenial with an inhibition hypothesis, as these neurochemical effects influence inhibitory signals to affect P300. Thus, a general neural inhibition approach seems to work as an underlying P300 generation mechanism when stimuli garner attention are detected and engage memory operations.
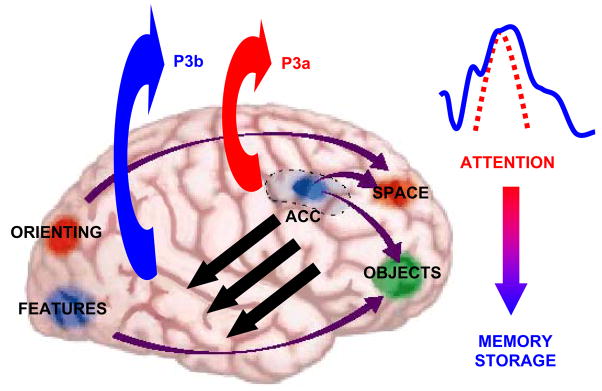
Figure 8 presents a schematic outline of how these processes might occur. Attention-demanding stimuli elicit a P3a when the contents of working memory change. These events are hypothesized to initiate neural activity towards the areas associated with P3b production and subsequent memory storage. If the resultant ERP waveforms originate from inhibitory signals derived from stimulus and task processing, a major question is how P3a information is transmitted to structures used for P3b generation (suggested by the black arrows in the figure). Communication of the output from frontal attention and working memory processes to temporal/parietal activation has been documented in primates and findings from intra-cranial as well as neuroimaging recordings in humans (cf. Brázdil et al., 2001, 2003; Desimone et al., 1995; Halgren et al., 1998; Simons and Spiers, 2003). A neuroelectric connection between frontal and temporal/parietal structures could modulate P3a and P3b activity at the scalp. As suggested above, variegated P300 data sets can be subsumed by inhibitory modulation in this fashion.
Figure 8.
Schematic representation of brain activation patterns underlying P3a and P3b generation (after Gazzaniga et al., 2000). The model suggests that stimulus information is maintained in frontal lobe working memory and monitored by anterior cingulate structures. When focal attention for the standard stimulus is disrupted by the detection of a distracter or a target (stimuli that garner attention automatically or purposefully from task demands), the P3a is perhaps generated by the activation pattern of the anterior cingulate and related structures. The attention-driven neural activity signal may be transmitted to temporal-parietal areas. Memory-related storage operations are engaged and P3b is generated via temporal/parietal cortical structures. As indicated by the ERP waveform and arrow to the right, every “P300” is composed of the P3a and P3b subcomponents, but the resulting ERP scalp topographies vary with the stimulus and task conditions that elicit them. Reprinted with permission from W.W. Norton & Company (Copyright, 2000).
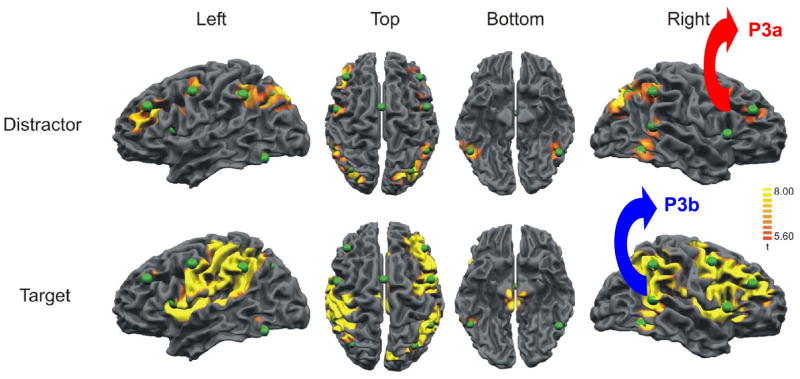
Figure 9 illustrates fMRI activation from a three-stimulus oddball task using non-novel distracter stimuli and a difficult target/standard task (Bledowski et al., 2004b). The pattern of fMRI hemodynamic responses indicates that strong frontal lobe activation occurs for distracter stimulus processing, with minimal temporal/parietal activation observed. The target stimulus produces activation in both frontal and temporal/parietal areas. These patterns occur in cortical areas associated with P3a and P3b generation. Whether the fMRI signals reflect the sequelae of rapid neuroelectric inhibitory mechanisms is unknown, but the similarity of the magnetic and electric signal locales is suggestive. Indeed, structural gray matter volume imaging found P3a amplitude variation is positively correlated with frontal lobe area size, whereas P3b amplitude variation is positively correlated with parietal lobe area size (Ford et al., 1994). Such individual differences in cortical area may contribute to normative P3a and P3b amplitude and latency variability (Conroy and Polich, 2007; Polich, 1988; Squires et al., 1975). Thus, functional and structural imaging data mimic the neuroelectric morphological signatures of P3a and P3b.
Figure 9.
Brain activation patterns from a visual three-stimulus oddball task modeled after that described in Figure 6 used with fMRI and EEG recordings (after Bledowski et al., 2004). Arrows and labels have been added to the images on the right for emphasis. The green spheres reflect dipole generator sources and appeared on the original figure. Reprinted with permission of the Society for Neuroscience (Copyright 2004).
Figure 9 also indicates greater activation for the right compared to left hemisphere. Given a frontal-parietal right-hemisphere attentional network system, wider activation for right-hemisphere processing would be expected (Posner and Dehaene, 1994). These imaging findings support observations of larger P300 amplitude over the right compared to left frontal/central areas for a variety of stimulus types in an oddball task (Alexander et al., 1995; Mertens and Polich, 1997b). Communication between the frontal to temporal/parietal areas appears to be propagated across the corpus callosum to affect ERP morphology (Barcelo et al., 2000; Baudena et al., 1995; Satomi et al., 1995). This pathway contributes to P300 differences between handedness groups: Left-handers have larger callosal pathways than right-handers (Driesen and Raz, 1995; Witelson, 1992), and larger P300 amplitudes with shorter latencies have been found for left- compared to right-handed individuals (Alexander and Polich, 1995, 1997; Polich and Hoffman, 1998). Taken together, these findings suggest that P300 measures are hemispherically similar to these fMRI activation patterns.
5.2. Neuroelectric underpinnings of P3a and P3b
Conventional ERP analyses are performed in the time domain by assessing the amplitudes and latencies of prominent peaks and associating the resulting measures with information processing variables. The distinct time-scales for brain stem, middle latency, and endogenous potentials indicate that frequency is an important parameter for ERP interpretation (Hillyard and Picton, 1987; Makeig, 2002; Polich and Starr, 1983; Regan, 1989). Procedures such as Principal Component Analysis (PCA) and Independent Component Analysis (ICA) are based on mathematical transformations of ERP data and can be employed to separate functionally distinct events that occur simultaneously in time (Kayser and Tenke, 2003, 2005; Makeig et al., 2004). Additional analytical methods are also available (cf. Johnson, 1993; McCarthy and Wood, 1985; Urbach and Kutas, 2002). In contrast, EEG/ERP frequency domain analyses have revealed that EEG rhythms in specific frequency ranges are functionally related to cognitive processing and behavior (Anokhin et al., 1999; Başar et al., 1997; McEvoy et al., 2000; Pfurtscheller and Lopes da Silva, 1999).
These methods were first introduced by Başar in animals (Başar, 1980; Başar et al., 1979), with similar techniques applied to humans (Başar et al., 1980, 1984; Başar and Stampfer, 1985; Stampfer and Başar, 1986). Wavelet transform (WT) is an efficient time-frequency decomposition method (Daubechies, 1990), which has been used on ERP signals (e.g., Ademoglu et al., 1997; Kolev et al., 1997; Yordanova et al., 2000). The major advantage of WT is its multi-resolution property that employs shorter time windows for higher frequencies and longer time windows for lower frequencies—an attribute that closely matches the structural properties of ERP signals (Ademoglu et al., 1998; Samar et al., 1995). The variable time-frequency localization method therefore takes into consideration the overlapping of ERP components and provides for the efficient analysis of the transient non-stationary ERP signals (Demiralp et al., 1999).
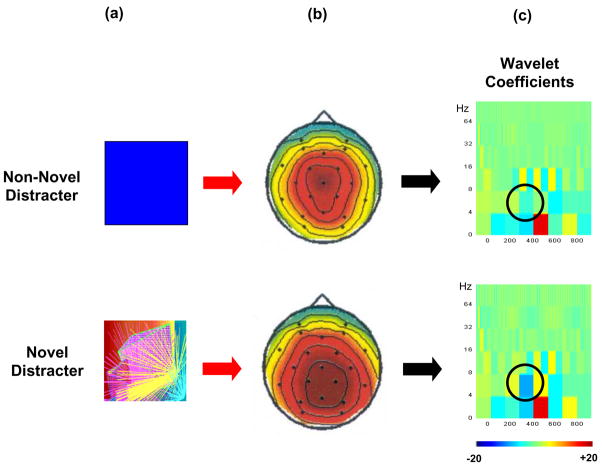
Figure 10 illustrates an application of the WT to assess non-novel and novel distracter stimuli when P3a is generated by a difficult oddball task (Demiralp et al., 2001). The topographic amplitude distributions are similar for the two distracter types, as typically found for perceptually demanding discrimination tasks (cf. Combs and Polich, 2006; Polich and Comerchero, 2003). The spectral density plots of the WT coefficients also are similar for both distracter types (see figure caption for details). However, the novel visual stimuli produced significantly more theta band activity during the P3a time interval than did the non-novel stimuli. These results suggest that theta band frequencies are engaged more for novel compared to non-novel stimuli. In addition, spectral analysis of the P3b from an auditory oddball task has demonstrated that cognitive variables strongly affect theta band activity (Spencer and Polich, 1999). If the P3a stems from the initial inhibitory activation elicited by focal attention to a distracting stimulus and P3b indexes subsequent inhibition related to memory, theta frequency modulations may provide inhibitory control of these processes.
Figure 10.
(a) Illustrations of non-novel blue-square distracter and novel distracter stimuli used in a three-stimulus oddball task (after Demiralp et al., 2001). Both squares were relatively large (18 cm2) and presented on a monitor. Blue-squares were always the same stimulus, whereas the novel stimuli varied in form and color across trials. (b) Topographic distributions of the grand average P3a components (μV) from the blue-square and novel distracter stimuli. (c) Time-frequency wavelet analysis representation of the blue square and novel distracter stimuli. The amplitudes of the wavelet coefficients are encoded in color, with brighter colors indicating greater spectral power. Circles indicate theta activity. Reprinted with permission from Springer Publishers (Copyright, 2001).
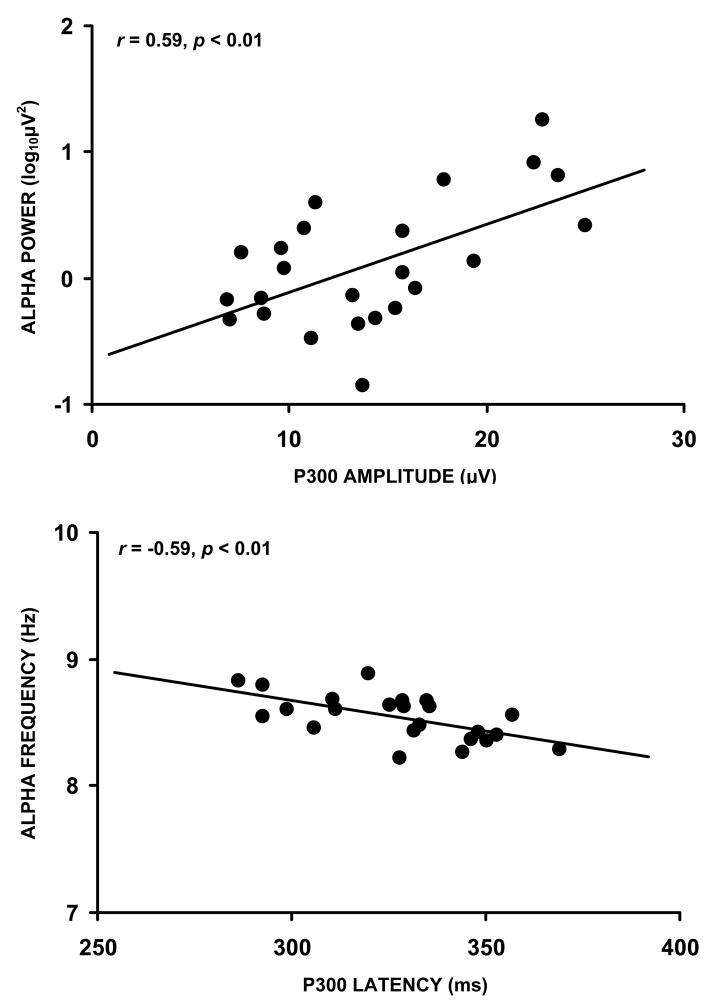
Figure 11 illustrates the relationship between slow alpha (8–10 Hz) spectral power and mean frequency measures with auditory P300 amplitude and latency from normal young adults (Intriligator and Polich, 1994). Other frequencies demonstrated similar associations, but the slow alpha activity yielded the strongest correlation. Furthermore, pre-stimulus EEG and post-stimulus ERP measures are most strongly related to the theta and alpha EEG bands (Brandt et al., 1991; Jansen and Brandt, 1991), and positive correlations have been obtained between pre-stimulus theta/alpha spectral power and P300 amplitude (Başar et al., 1989; Jasiukaitis and Hakerem, 1988; Pritchard et al., 1985). These results may reflect attentional mechanisms that modulate slow alpha (Klimesch, 1997; Klimesch et al., 1992, 1993), and episodic memory operations that alter fast alpha (10–12 Hz) activity (Hanslmayr et al., 2007; Klimesch, 2003). Thus, alpha band spectral power covaries with P300, which suggests this ERP is related to attention and memory neuroelectric effects (Intriligator and Polich, 1995; Polich, 1997).
Figure 11.
Upper panel: Mean alpha power plotted against P300 amplitude (after Intriligator and Polich, 1994). Lower panel: Mean alpha frequency plotted against P300 latency. EEG was collected with eyes open. ERP data were elicited using an auditory oddball task (n=24). Reprinted with permission from Elsevier (Copyright 1994).
5.3. Event-related desynchronization and P300
Alpha suppression (or desynchronization) was first observed by Berger (1929). Phasic alpha event-related desynchronization (ERD) can be induced by sensory stimulation across modalities (Aranibar and Pfurtscheller, 1978; Krause et al., 1994; Mann et al., 1996). Alpha ERD also occurs during cognitive processing invoked by task conditions requiring attention and memory (Boiten et al., 1992; Pfurtscheller and Klimesch, 1991, 1992; Sergeant et al., 1987). ERD originates from a reduction of fast non-phase-locked oscillations, whereas P300 is composed of phase-locked delta and theta-range synchronized oscillations (Başar-Eroglu et al., 1992; Kolev et al., 1997; Lopes da Silva, 1999; Pfurtscheller et al., 1996; Yordanova and Kolev, 1998a). Task-induced alpha frequencies have specific functional meanings: slow alpha ERD (8–10 Hz) is associated with attention and fast alpha ERD (10–12 Hz) is associated with memory (Klimesch, 2003; Schack et al., 2005) operations, although stimulus and task demands affect specific outcomes (cf. Klimesch et al., 2006; Ward, 2003). ERD could therefore index the effects of cognitive operations on alpha activity power and frequency (Klimesch et al., 1998; Spencer and Polich, 1999; Yordanova and Kolev, 1998b; Yordanova et al., 2000).
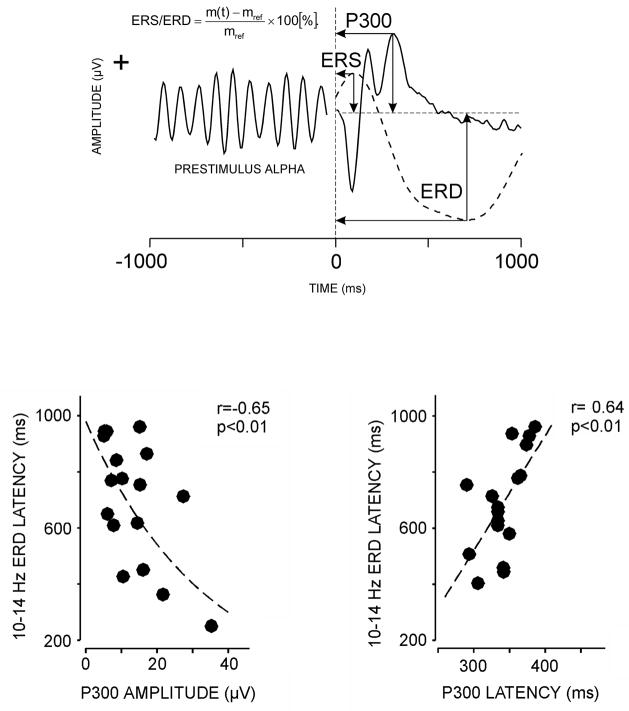
Figure 12 summarizes how ERD is calculated and illustrates the relationship of alpha frequency deactivation to P300 amplitude and latency elicited with an auditory oddball task (Yordanova et al., 2001). ERD measures were obtained from post-stimulus late alpha power. Strong relationships between ERD and P300 (P3b) were found: (1) ERD onset was negatively associated with P300 amplitude—the shorter the onset time, the larger the component amplitude. (2) ERD onset was positively associated with P300 latency—the shorter the onset time, the shorter the component latency. (3) Homogenous variability for each measurement pairing was observed across subjects. Assuming that ERD is related to neural inhibition by virtue of its desynchronization origins, P300 amplitude and latency could therefore stem from alpha-band deactivation. Whether P3a is regulated by this process is unknown, but P3b is clearly related to desynchronization of late alpha frequency EEG.
Figure 12.
Upper panel: Illustration of event-related synchronization (ERS) and event-related desynchronization (ERD) from stimulus-related alpha activity calculations obtained during and ERP auditory oddball task (after Yordanova et al., 2001). Lower panel: ERD latency plotted against P300 amplitude (left) and amplitude (right) from normal young adults. ERP data were elicited using an auditory oddball task. Reprinted with permission from Blackwell Publishing (Copyright 2001).
5.4. Summary: P300 and neuroinhibition
The P300 waveform may result from the operation of neural inhibition generated when cognitive mechanisms are engaged by stimulus and task demands. The neuroelectric architecture of the phenomenon appears related to the activity and desynchronization of the theta and alpha frequency bands. These activation patterns also may reflect the effects of gamma band signals (Canolty et al., 2006). It is notable in this context that theta and alpha frequencies have been strongly linked to meditation effects—a self-induced neuroelectric inhibition (Cahn and Polich, 2006). Thus, the P300 may be the neuronal consequence of stimulus events important enough to inhibit concomitant brain activity and therefore index processes that are generated by brain mechanisms underlying various types of attention and memory operations.
6. Conclusions
The present review has attempted to assess the diverse P300 literature by integrating the background findings of the P3a and P3b subcomponents. The approach traced the historical development of P300 and emphasized the functional, neurophysiological, and neuropsychological mechanisms associated with component generation. Neuroimaging and neuropharmacological investigations were highlighted to substantiate how P3a and P3b might interact. The empirical findings and developed theoretical perspective suggest that the P300 may stem from neural inhibitory activity organized to delimit task-extraneous events to sculpt attentional focus and promote memory operations for target stimuli.
The proposed model posits that “the P300” comprises an early attention process stemming from a frontal working memory representational change to produce the P3a. The attention-driven stimulus signal is then transmitted to temporal and parietal structures related to P3b. These resulting potentials can be dissociated with paradigmatic manipulations and are generated when perceptual stimulus discrimination occurs. That fundamental EEG changes are connected to P300 variability is consistent with a neural inhibition hypothesis of stimulus processing. The fundamental issues of what neuroelectric mechanisms structure and convey these signals are being explored, with the findings indicating that theta and alpha band activity may govern the relationship of the P3a to attention and the P3b to memory processing.
If these processes determine P3a and P3b generation, understanding the origin of neuroelectric information and its transmission are important next steps in discerning the meaning of P300. As the role of neurotransmitter contributions to neuroelectric signals recorded at the scalp becomes better known, how this factor contributes to EEG desynchronization will elucidate the emerging neural organizational portrait more precisely. Articulating the way in which these variables interact, even in broad terms, will help clarify the underlying mechanisms. Achieving this goal will fulfill the cognitive promise that the P300 inspired when it was discovered over 40 years ago.
Acknowledgments
This work was supported by NIH Grants RO1-DA018262 and 3 P50 AA06420. It is manuscript number 18235 from The Scripps Research Institute. I thank Brendan Allison, Allen Azizian, Maya Cano, and Jonas Olofsson for helpful comments. Special thanks go to Brian Lopez for his astute editorial suggestions and figure making artistry. Sincere gratitude is expressed to the reviewers for their very perspicacious critiques.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ademoglu A, Demiralp T, Yordanova J, Kolev V, Devrim M. Decomposition of event-related brain potentials into multicomponents using wavelet transform. App Signal Process. 1998;5:142–151. [Google Scholar]
- Ademoglu A, Micheli-Tzanakou E, Istefanopulos Y. Analysis of pattern reversal visual evoked potentials (PRVEP’s) by spline wavelets. IEEE Trans Biomed Eng. 1997;44:881–890. doi: 10.1109/10.623057. [DOI] [PubMed] [Google Scholar]
- Alexander J, Bauer L, Kuperman S, Morzorati S, O’Connor S, Rohrbaugh J, Porjesz B, Begleiter H, Polich J. Hemispheric differences for P300 amplitude from an auditory oddball task. Int J Psychophysiol. 1996;21:189–196. doi: 10.1016/0167-8760(95)00047-x. [DOI] [PubMed] [Google Scholar]
- Alexander J, Polich J. P300 differences between sinistrals and dextrals. Cogn Brain Res. 1995;2:277–282. doi: 10.1016/0926-6410(95)90019-5. [DOI] [PubMed] [Google Scholar]
- Alexander J, Polich J. Handedness and P300 from auditory stimuli. Brain Cogn. 1997;35:259–270. doi: 10.1006/brcg.1997.0941. [DOI] [PubMed] [Google Scholar]
- Alexander J, Porjesz B, Bauer L, Kuperman S, Morzorati S, O’Connor S, Rohrbaugh J, Begleiter, Polich J. P300 amplitude hemispheric asymmetries from a visual discrimination task. Psychophysiology. 1995;32:467–475. doi: 10.1111/j.1469-8986.1995.tb02098.x. [DOI] [PubMed] [Google Scholar]
- Anokhin AP, Rohrbaugh JW, Todorov AA, Vedeniapin AB. The P300 event-related brain potential in neuropsychiatric disorders: a moderator of genetic risk? Behav Genet. 1999;29:349–349. [Google Scholar]
- Aranibar A, Pfurtscheller G. On and off effects in background EEG activity during one-second photic stimulation. Electroencephalogr Clin Neurophysiol. 1978;44:307–316. doi: 10.1016/0013-4694(78)90306-1. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Ann Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Azizian A, Freitas AL, Watson TD, Squires NK. Electrophysiological correlates of categorization: P300 amplitude as an index of target similarity. Biol Psychol. 2006;71:278–288. doi: 10.1016/j.biopsycho.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Azizian A, Polich J. Evidence for attentional gradient in the serial position memory curve from ERPs. J Cog Neurosci. doi: 10.1162/jocn.2007.19.12.2071. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcelo F, Suwazono S, Knight R. Prefrontal modulation of visual processing in humans. Nature Neurosci. 2000;3:399–403. doi: 10.1038/73975. [DOI] [PubMed] [Google Scholar]
- Başar E. EEG-brain dynamics: relation between EEG and brain evoked potentials. Amsterdam: Elsevier/North Holland Biomedical Press; 1980. [Google Scholar]
- Başar E, Başar-Eroglu C, Röschke J, Schütt A. The EEG is a quasi-deterministic signal anticipating sensory-cognitive tasks. In: Başar E, Bullock TH, editors. Brain dynamics: progress and perspectives. Berlin: Springer-Verlag; 1989. pp. 43–71. [Google Scholar]
- Başar-Eroglu C, Başar E, Demiralp T, Schürmann M. P300-response: possible psychophysiological correlates in delta and theta frequency channels. a review Int J Psychophysiol. 1992;13:161–179. doi: 10.1016/0167-8760(92)90055-g. [DOI] [PubMed] [Google Scholar]
- Başar E, Başar-Eroglu C, Rosen B, Schütt A. A new approach to endogenous event-related potentials in man: relation between EEG and P300-wave. Int J Neurosci. 1984;24:1–21. doi: 10.3109/00207458409079530. [DOI] [PubMed] [Google Scholar]
- Başar E, Demir N, Gönder A, Ungan P. Combined dynamics of EEG and evoked-potentials. Studies of simultaneously recorded EEG-epograms in the auditory pathway, reticular-formation, and hippocampus of the cat brain during the waking stage. Biol Cybern. 1979;34:1–19. doi: 10.1007/BF00336852. [DOI] [PubMed] [Google Scholar]
- Başar E, Gönder A, Ungan P. Comparative frequency-analysis of single EEG-evoked potential records. J Biomed Eng. 1980;2:9–14. doi: 10.1016/0141-5425(80)90086-2. [DOI] [PubMed] [Google Scholar]
- Başar E, Hari R, Lopes da Silva FH, Schürmann M. Brain alpha activity: new aspects and functional correlates. Int J Psychophysiol. 1997;26:353–368. [Google Scholar]
- Başar E, Stampfer HG. Important associations among EEG-dynamics, event-related potentials, short-term-memory, and learning. Int J Neurosci. 1985;26:161–180. doi: 10.3109/00207458508985615. [DOI] [PubMed] [Google Scholar]
- Bashore TR, Ridderinkhof KR. Older age, traumatic brain injury, and cognitive slowing: some convergent and divergent findings. Psychol Bull. 2002;128:151–198. doi: 10.1037/0033-2909.128.1.151. [DOI] [PubMed] [Google Scholar]
- Bashore T, van der Molen M. Discovery of P300: a tribute. Biol Psychol. 1991;32:155–171. doi: 10.1016/0301-0511(91)90007-4. [DOI] [PubMed] [Google Scholar]
- Baudena P, Halgren E, Heit G, Clarke J. Intracerebral potentials to rare target and distractor auditory and visual stimuli. III. Frontal cortex. Electroencephalogr Clin Neurophysiol. 1995;94:251–264. doi: 10.1016/0013-4694(95)98476-o. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Reich T, Edenberg HJ, Goate A, Blangero J, Almasy L, Foroud T, Van Eerdewegh P, Polich J, Rohrbaugh J, Kuperman S, Bauer LO, O’Connor SJ, Chorlian DB, Li T-K, Conneally PM, Hesselbrock V, Rice JP, Schuckit MA, Cloninger R, Nurnberger J, Crowe R, Bloom FE. Quantitative trait loci analysis of human event-related brain potentials: P3 voltage. Electroencephalogr Clin Neurophysiol. 1998;108:244–250. doi: 10.1016/s0168-5597(98)00002-1. [DOI] [PubMed] [Google Scholar]
- Berger H. Über das Elektrenkephalogramm des Menschen. Arch Psychiatr Nervenkr. 1929;87:527–570. [Google Scholar]
- Berti S, Roeber U, Schröger E. Bottom-up influences on working memory: behavioral and electrophysiological distraction varies with distractor strength. Exp Psychol. 2004;51:249–257. doi: 10.1027/1618-3169.51.4.249. [DOI] [PubMed] [Google Scholar]
- Birbaumer N, Elbert T. P3, by-product of a by-product. Behav Brain Sci. 1988;11:375–377. [Google Scholar]
- Birbaumer N, Elbert T, Canavan AGM, Rockstroh B. Slow potentials of the cerebral-cortex and behavior. Physiol Rev. 1990;70:1–41. doi: 10.1152/physrev.1990.70.1.1. [DOI] [PubMed] [Google Scholar]
- Bledowski C, Prvulovic D, Goebel R, Zanella F, Linden D. Attentional systems in target and distractor processing: a combined ERP and fMRI study. Neuroimage. 2004a;22:530–540. doi: 10.1016/j.neuroimage.2003.12.034. [DOI] [PubMed] [Google Scholar]
- Bledowski C, Prvulovic D, Hoechstetter K, Scherg M, Wibral M, Goebel R, Linden DEJ. Localizing P300 generators in visual target and distracter processing: a combined event-related potential and functional magnetic resonance imaging study. J Neurosci. 2004b;24:9353–9360. doi: 10.1523/JNEUROSCI.1897-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiten F, Sergeant J, Geuze R. Event-related desynchronization: the effects of energetic and computational demands. Electroencephalogr Clin Neurophysiol. 1992;82:302–309. doi: 10.1016/0013-4694(92)90110-4. [DOI] [PubMed] [Google Scholar]
- Brandt ME, Jansen BH, Carbonari JP. Pre-stimulus spectral EEG patterns and the visual evoked-response. Electroencephalogr Clin Neurophysiol. 1991;80:16–20. doi: 10.1016/0168-5597(91)90037-x. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD. On the control of control: the role of dopamine in regulating prefrontal function and working memory. In: Monsell S, Driver J, editors. Control of cognitive processes: Attention and performance XVIII. Cambridge, MA: MIT Press; pp. 713–737. [Google Scholar]
- Brázdil M, Rektor I, Daniel P, Dufek M, Jurak P. Intracerebral event-related potentials to subthreshold target stimuli. Clin Neurophysiol. 2001;112:650–661. doi: 10.1016/s1388-2457(01)00463-1. [DOI] [PubMed] [Google Scholar]
- Brázdil M, Roman R, Daniel P, Rektor I. Intracerebral somatosensory event-related potentials: effect of response type (button pressing versus mental counting) on P3-like potentials within the human brain. Clin Neurophysiol. 2003;114:1489–1496. doi: 10.1016/s1388-2457(03)00135-4. [DOI] [PubMed] [Google Scholar]
- Brocke B. The multilevel approach in sensation seeking: potentials and findings of a four-level research program. In: Stelmack RM, editor. On the psychobiology of personality. Amsterdam: Elsevier; 2004. pp. 267–293. [Google Scholar]
- Brocke B, Tasche KG, Beauducel A. Biopsychological foundations of extraversion: differential effort reactivity and state control. Pers Ind Diff. 1997;22:447–485. [Google Scholar]
- Cahn BR, Polich J. Meditation states and traits: EEG, ERP, and neuroimaging studies. Psychol Bull. 2006;132:180–211. doi: 10.1037/0033-2909.132.2.180. [DOI] [PubMed] [Google Scholar]
- Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, Berger MS, Barbaro NM, Knight RT. High gamma power is phase-locked to theta oscillations in human neocortex. Science. 2006;313:1626–1628. doi: 10.1126/science.1128115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SR, Katsanis J, Iacono WG, Mertz AK. Substance dependence and externalizing psychopathology in adolescent boys with small, average, or large P300 event-related potential amplitude. Psychophysiology. 1999;36:583–590. [PubMed] [Google Scholar]
- Cohen J, Polich J. On the number of trials needed for P300. Int J Psychophysiol. 1997;25:249–255. doi: 10.1016/s0167-8760(96)00743-x. [DOI] [PubMed] [Google Scholar]
- Combs LA, Polich J. P3a from auditory white noise stimuli. Clin Neurophysiol. 2006;117:1106–1112. doi: 10.1016/j.clinph.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Comerchero MD, Polich J. P3a, perceptual distinctiveness, and stimulus modality. Cogn Brain Res. 1998;7:41–48. doi: 10.1016/s0926-6410(98)00009-3. [DOI] [PubMed] [Google Scholar]
- Comerchero MD, Polich J. P3a and P3b from typical auditory and visual stimuli. Clin Neurophysiol. 1999;110:24–30. doi: 10.1016/s0168-5597(98)00033-1. [DOI] [PubMed] [Google Scholar]
- Conroy MA, Polich J. Normative variation of P3a and P3b from a large sample (N=120): Gender, topography, and response time. J Psychophysiology. 2007;21:22–32. [Google Scholar]
- Courchesne E, Hillyard SA, Galambos R. Stimulus novelty, task relevance, and visual evoked-potential in man. Electroencephalogr Clin Neurophysiol. 1975;39:131–143. doi: 10.1016/0013-4694(75)90003-6. [DOI] [PubMed] [Google Scholar]
- Croft RJ, Gonsalvez CJ, Gabriel C, Barry RJ. Target-to-target interval versus probability effects on P300 in one- and two-tone tasks. Psychophysiology. 2003;40:322–328. doi: 10.1111/1469-8986.00036. [DOI] [PubMed] [Google Scholar]
- Curran T. Effects of attention and confidence on the hypothesized ERP correlates of recollection and familiarity. Neuropsychologia. 2004;42:1088–1106. doi: 10.1016/j.neuropsychologia.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Curran T, Cleary AM. Using ERPs to dissociate recollection from familiarity in picture recognition. Brain Research Cognitive Brain Research. 2003;15:191–205. doi: 10.1016/s0926-6410(02)00192-1. [DOI] [PubMed] [Google Scholar]
- Cycowicz YM. Memory development and event-related brain potentials in children. Biol Psychol. 2000;54:145–174. doi: 10.1016/s0301-0511(00)00055-7. [DOI] [PubMed] [Google Scholar]
- Daffner K, Mesulam M, Holcomb P, Calvo V, Acar D, Chabrerie A, Kikinis R, Jolesz F, Rentz D, Seinto L. Disruption of attention to novel events after frontal lobe injury in humans. J Neurol Neurosurg Psychiatry. 2000a;68:18–24. doi: 10.1136/jnnp.68.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daffner K, Mesulam M, Seinto L, Acar D, Calvo V, Faust R, Chabrerie A, Kennedy B, Holcomb P. The central role of the prefrontal cortex in directing attention to novel events. Brain. 2000b;123:927–939. doi: 10.1093/brain/123.5.927. [DOI] [PubMed] [Google Scholar]
- Daffner K, Mesulam M, Seinto L, Calvo V, Faust R, Holcomb P. An electrophysiological index of stimulus unfamiliarity. Psychophysiology. 2000c;37:737–747. [PubMed] [Google Scholar]
- Daubechies I. The wavelet transform, time-frequency localization, and signal analysis. IEEE Trans Info Theory. 1990;36:961–1005. [Google Scholar]
- Debener S, Kranczioch C, Herrman C, Engel A. Auditory novelty oddball allows reliable distinction of top-down and bottom-up processes of attention. Int J Psychophysiol. 2002;46:77–84. doi: 10.1016/s0167-8760(02)00072-7. [DOI] [PubMed] [Google Scholar]
- Debener S, Makeig S, Delorme A, Engel A. What is novel in the novelty oddball paradigm? Functional significance of the novelty P3 event-related potential as revealed by independent component analysis. Cogn Brain Res. 2005;22:309–321. doi: 10.1016/j.cogbrainres.2004.09.006. [DOI] [PubMed] [Google Scholar]
- DeBoer T, Scott LS, Nelsaon CA. ERPs in developmental populations. In: Handy TC, editor. Event-related potentials: a methods handbook. Cambridge, MA: MIT Press; pp. 263–297. [Google Scholar]
- Demiralp T, Ademoglu A, Comerchero M, Polich J. Wavelet analysis of P3a and P3b. Brain Topogr. 2001;13:251–267. doi: 10.1023/a:1011102628306. [DOI] [PubMed] [Google Scholar]
- Demiralp T, Yordanova J, Kolev V, Ademoglu A, Devrim M, Samar VJ. Time-frequency analysis of single-sweep event-related potentials by means of fast wavelet transform. Brain Lang. 1999;66:129–145. doi: 10.1006/brln.1998.2028. [DOI] [PubMed] [Google Scholar]
- De Pascalis V. On the psychophysiology of extraversion. In: Stelmack RM, editor. On the psychobiology of personality. Amsterdam: Elsevier; 2004. pp. 297–329. [Google Scholar]
- Desimone R, Miller EK, Chelazzi L, Lueschow A. Multiple memory systems in the visual cortex. In: Gazzaniga MS, editor. The cognitive neurosciences. Cambridge:MA: MIT Press; 1995. pp. 475–486. [Google Scholar]
- Dien J, Spencer K, Donchin E. Parsing the late positive complex: mental chronometry and the ERP components that inhabit the neighborhood of the P300. Psychophysiology. 2004;41:665–678. doi: 10.1111/j.1469-8986.2004.00193.x. [DOI] [PubMed] [Google Scholar]
- DiTraglia GM, Polich J. P300 and introverted/extraverted personality types. Psychophysiology. 1991;28:177–184. doi: 10.1111/j.1469-8986.1991.tb00410.x. [DOI] [PubMed] [Google Scholar]
- Donchin E. Surprise!…. Surprise? Psychophysiology. 1981;18:493–513. doi: 10.1111/j.1469-8986.1981.tb01815.x. [DOI] [PubMed] [Google Scholar]
- Donchin E, Coles MGH. Is the P300 component a manifestation of context updating? Behav Brain Sci. 1988;11:357–374. [Google Scholar]
- Donchin E, Karis D, Bashore TR, Coles MGH, Gratton G. Cognitive psychophysiology: systems, processes, and applications. In: Coles MGH, Donchin E, Porges S, editors. Psychophysiology: systems, processes, and applications. New York: The Guilford Press; 1986. pp. 244–267. [Google Scholar]
- Donchin E, Ritter W, McCallum C. Cognitive psychophysiology: the endogenous components of the ERP. In: Callaway P, Tueting P, Koslow S, editors. Brain-event related potentials in man. New York: Academic Press; 1978. pp. 349–411. [Google Scholar]
- Donchin E, Spencer KM, Dien J. The varieties of deviant experience: ERP manifestations of deviance processors. In: van Boxtel GJM, Bocker KBE, editors. Brain and behavior: past, present, and future. of deviance detectors in the brain. Tilburg: The Netherlands University Press; 1997. pp. 67–91. [Google Scholar]
- Doyle MC, Rugg MD. Event-related potentials and recognition memory for low- and high-frequency words. J Cog Neurosci. 1992;4:69–79. doi: 10.1162/jocn.1992.4.1.69. [DOI] [PubMed] [Google Scholar]
- Driesen N, Raz N. The influence of sex, age and handedness of corpus callosum morphology: a meta-analysis. Psychobiology. 1995;23:240–247. [Google Scholar]
- Duncan-Johnson CC. P300 latency: a new metric of information processing. Psychophysiology. 1981;18:207–215. doi: 10.1111/j.1469-8986.1981.tb03020.x. [DOI] [PubMed] [Google Scholar]
- Duncan-Johnson CC, Donchin E. Effects of a priori and sequential probability of stimuli on event-related potential. Psychophysiology. 1977;14:95. doi: 10.1111/j.1469-8986.1977.tb01312.x. [DOI] [PubMed] [Google Scholar]
- Duncan-Johnson CC, Donchin E. The P300 component of the event-related brain potential as an index of information processing. Biol Psychol. 1982;14:1–52. doi: 10.1016/0301-0511(82)90016-3. [DOI] [PubMed] [Google Scholar]
- Duncan-Johnson CC, Kopell BS. The Stroop effect: brain potentials localize the source of interference. Science. 1981;20:938–40. doi: 10.1126/science.7302571. [DOI] [PubMed] [Google Scholar]
- Ebmeier KP, Steele JD, MacKenzie DM, O’Carroll RE, Kydd RR, Glabus MF, Backwood DHR, Rugg MD, Goodwin GM. Cognitive brain potentials and regional cerebral blood flow equivalents during two- and three-sound auditory “oddball tasks”. Electroencephalogr Clin Neurophysiol. 1995;95:434–443. doi: 10.1016/0013-4694(95)00173-5. [DOI] [PubMed] [Google Scholar]
- Eichele T, Specht K, Moosmann M, Jongsma MLA, Quiroga RQ, Hordby H, Hugdahl K. Assessing the spatiotemporal evolution of neuronal activation with single-trial event-related potentials and functional MRI. Proc Nat Acad Sci. 2005;102:17798–17803. doi: 10.1073/pnas.0505508102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimer M. Spatial cueing, sensory gating, and selective response preparation: an ERP study on visuospatial orienting. Electroencephalogr Clin Neurophysiol. 1993;88:408–420. doi: 10.1016/0168-5597(93)90017-j. [DOI] [PubMed] [Google Scholar]
- Eischen SE, Luckritz JY, Polich J. Spectral analysis of EEG from families. Biol Psychol. 1995;41:61–68. doi: 10.1016/0301-0511(95)05129-x. [DOI] [PubMed] [Google Scholar]
- Eischen SE, Polich J. P300 from families. Electroencephalogr Clin Neurophysiol. 1994;92:369–372. doi: 10.1016/0168-5597(94)90105-8. [DOI] [PubMed] [Google Scholar]
- Emmerson RY, Dustman RE, Shearer DE, Turner CW. P3 latency and symbol digit performance correlations in aging. Exp Aging Res. 1989;15:151–159. doi: 10.1080/03610738908259769. [DOI] [PubMed] [Google Scholar]
- Escera C, Alho K, Winkler I, Näätänen R. Neural mechanisms of involuntary attention to acoustic novelty and change. J Cogn Neurosci. 1998;10:590–604. doi: 10.1162/089892998562997. [DOI] [PubMed] [Google Scholar]
- Fabiani M, Friedman D, Cheng JC. Individual differences in P3 scalp distribution in older adults, and their relationship to frontal lobe function. Psychophysiology. 1998;35:698–708. [PubMed] [Google Scholar]
- Fabiani M, Gratton G, Karis D, Donchin E. The definition, identification and reliability of measurement of the P300 component of the event-related brain potential. In: Ackles P, Jennings J, Coles MGH, editors. Advances in psychophysiology. Vol. 2. Greenwich, CT: JAI Press; 1987. pp. 1–78. [Google Scholar]
- Fabiani M, Karis D, Donchin E. P300 and recall in an incidental memory paradigm. Psychophysiology. 1986;23:298–308. doi: 10.1111/j.1469-8986.1986.tb00636.x. [DOI] [PubMed] [Google Scholar]
- Fabiani M, Karis D, Donchin E. Effects of mnemonic strategy manipulation in a Von Restorff paradigm. Electroenceph Clin Neurophysiol. 1990;75:2–35. doi: 10.1016/0013-4694(90)90149-e. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Christ S, Hohnsbein J. ERP components on reaction errors and their functional significance: a tutorial. Biol Psychol. 2000;51:87–107. doi: 10.1016/s0301-0511(99)00031-9. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Hohnsbein J. ERP components in go/no-go tasks and their relation to inhibition. Acta Psychol. 1999;101:267–291. doi: 10.1016/s0001-6918(99)00008-6. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PG, Picton TW. The effects of probability and discriminability on the evoked potentials to unpredictable stimuli. Ann N Y Acad Sci. 1984;425:199–203. doi: 10.1111/j.1749-6632.1984.tb23533.x. [DOI] [PubMed] [Google Scholar]
- Fjell A, Walhovd K. P300 and neuropsychological tests as measures of aging: scalp topography and cognitive changes. Brain Topogr. 2001;14:25–40. doi: 10.1023/a:1012563605837. [DOI] [PubMed] [Google Scholar]
- Ford J, Sullivan E, Marsh L, White P, Lim K, Pfefferbaum A. The relationship between P300 amplitude and regional gray matter volumes depends on the attentional system engaged. Electroencephalogr Clin Neurophysiol. 1994;90:214–228. doi: 10.1016/0013-4694(94)90093-0. [DOI] [PubMed] [Google Scholar]
- Friedman D, Cycowicz YM, Gaeta H. The novelty P3: an event-related brain potential (ERP) sign of the brain’s evaluation of novelty. Neurosci Biobehav Rev. 2001;25:355–373. doi: 10.1016/s0149-7634(01)00019-7. [DOI] [PubMed] [Google Scholar]
- Friedman D, Kazmerski VA, Cycowicz YM. Effects of aging on the novelty P3 during attend and ignore oddball tasks. Psychophysiology. 1998;35:508–520. doi: 10.1017/s0048577298970664. [DOI] [PubMed] [Google Scholar]
- Friedman D, Simpson GV. ERP amplitude and scalp distribution to target and novel events: effects of temporal-order in young, middle-aged and older adults. Cogn Brain Res. 1994;2:49–63. doi: 10.1016/0926-6410(94)90020-5. [DOI] [PubMed] [Google Scholar]
- Frodl-Bauch T, Bottlender R, Hegerl U. Neurochemical substrates and neuroanatomical generators of the event-related P300. Neuropsychobiol. 1999;40:86–94. doi: 10.1159/000026603. [DOI] [PubMed] [Google Scholar]
- Gaeta H, Friedman D, Hunt G. Stimulus characteristics and task category dissociate the anterior and posterior aspects of novelty P3. Psychophysiology. 2003;40:198–208. doi: 10.1111/1469-8986.00022. [DOI] [PubMed] [Google Scholar]
- Goldstein A, Spencer K, Donchin E. The influence of stimulus deviance and novelty on the P300 and novelty P3. Psychophysiology. 2002;39:781–790. [PubMed] [Google Scholar]
- Gonsalvez CJ, Barry RJ, Rushby JA, Polich J. Target-to-target interval, intensity, and P300 from an auditory single-stimulus task. Psychophysiology. 2007;44:245–250. doi: 10.1111/j.1469-8986.2007.00495.x. [DOI] [PubMed] [Google Scholar]
- Gonsalvez CJ, Polich J. P300 amplitude is determined by target-to-target interval. Psychophysiology. 2002;39:388–396. doi: 10.1017/s0048577201393137. [DOI] [PubMed] [Google Scholar]
- Grunwald T, Lehnertz K, Heinze HJ, Helmstaedter C, Elger CE. Verbal novelty detection within the hippocampus proper. Proceedings of the National Academy of Science. 1998;95:3193–3197. doi: 10.1073/pnas.95.6.3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Duan L, Li W, Paller KA. Distinguishing source memory and item memory: brain potentials at encoding and retrieval. Brain Research. 2006;1118:142–154. doi: 10.1016/j.brainres.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Gurrera RJ, O’Donnell BF, Nestor PG, Gainski J, McCarley RW. The P3 auditory event-related brain potential indexes major personality traits. Biol Psychiat. 2001;49:922–929. doi: 10.1016/s0006-3223(00)01067-2. [DOI] [PubMed] [Google Scholar]
- Hagen GF, Gatherwright JR, Lopez BA, Polich J. P3a from visual stimuli: primary task difficulty effects. Int J Psychophysiol. 2006;59:8–14. doi: 10.1016/j.ijpsycho.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Halgren E, Marinkovic K, Chauvel P. Generators of the late cognitive potentials in auditory and visual oddball tasks. Electroencephalogr Clin Neurophysiol. 1998;106:156–164. doi: 10.1016/s0013-4694(97)00119-3. [DOI] [PubMed] [Google Scholar]
- Halgren E, Squires N, Wilson C, Rohbraugh J, Bab T, Crandall P. Endogenous potentials in the human hippocampal formation and amygdala by infrequent events. Science. 1980;210:803–805. doi: 10.1126/science.7434000. [DOI] [PubMed] [Google Scholar]
- Hansch E, Syndulko K, Cohen S, Goldberg Z, Potvin A, Tourtellotte W. Cognition in Parkinson disease: an event-related potential perspective. Ann Neurol. 1982;11:599–607. doi: 10.1002/ana.410110608. [DOI] [PubMed] [Google Scholar]
- Hansenne M. The P300 cognitive event-related potential. II. Individual variability and clinical application in psychopathology. Neurophysiol Clin. 2000;30:211–231. doi: 10.1016/s0987-7053(00)00224-0. [DOI] [PubMed] [Google Scholar]
- Hansenne M, Pitchot W, Gonzalez-Moreno A, Papart P, Timsit-Berthier M, Ansseau M. Catecholaminergic function and P300 amplitude in major depressive disorder (P300 and catecholamines) Electroencephalogr Clin Neurophysiol. 1995;96:194–196. doi: 10.1016/0168-5597(94)00317-8. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Klimesch W, Sauseng P, Gruber W, Doppelmayr M, Freunberger R, Pecherstorfer T, Birbaumer N. Alpha phase reset contributes to the generation of ERPs. Cereb Cortex. 2007;17:1–8. doi: 10.1093/cercor/bhj129. [DOI] [PubMed] [Google Scholar]
- Hartikainen K, Knight RT. Lateral and orbital prefrontal cortex contributions to attention. In: Polich J, editor. Detection of change: event-related potential and fMRI findings. Boston, MA: Kluwer; 2003. pp. 99–116. [Google Scholar]
- He B, Lian J, Spencer K, Dien J, Donchin E. A cortical potential imaging analysis of the P300 and novelty P3 components. Hum Brain Map. 2001;12:120–130. doi: 10.1002/1097-0193(200102)12:2<120::AID-HBM1009>3.0.CO;2-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslenfeld D. Visual mismatch negativity. In: Polich J, editor. Detection of change: event-related potential and fMRI findings. Boston, MA: Kluwer; 2003. pp. 41–59. [Google Scholar]
- Hesselbrock V, Begleiter H, Porjesz B, O’Connor S, Bauer L. P300 event-related potential amplitude as an endophenotype of alcoholism-evidence from the Collaborative Study on the Genetics of Alcoholism. J Biomed Sci. 2001;8:77–82. doi: 10.1007/BF02255974. [DOI] [PubMed] [Google Scholar]
- Hicks BM, Bernaat E, Malone SM, Iacono WG, Patrick CJ, Krueger R, McGue M. Genes mediate the association between P3 amplitude and externalizing disorders. Psychophysiology. 2007;44:98–105. doi: 10.1111/j.1469-8986.2006.00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill S, Locke J, Zezza N, Kaplan B, Neiswanger K, Steinhauer S, Wipprecht G, Xu J. Genetic association between reduced P300 amplitude and the DRD2 dopamine receptor A1 allele in children at high risk for alcoholism. Biol Psychiatry. 1998;43:40–51. doi: 10.1016/s0006-3223(97)00203-5. [DOI] [PubMed] [Google Scholar]
- Hill SY, Shen S, Locke J, Steinhauer S, Konicky C, Lowers L, Connolly J. Developmental delay in P300 production in children at high risk for developing alcohol-related disorders. Biol Psychiat. 1999;46:970–981. doi: 10.1016/s0006-3223(99)00032-3. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Kutas M. Electrophysiology of cognitive processing. Ann Rev Psychol. 1983;34:33–61. doi: 10.1146/annurev.ps.34.020183.000341. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Picton TW. Electrophysiology of cognition. In: Plum F, editor. Handbook of physiology Section 1: Neurophysiology. New York: American Physiological Society; 1987. pp. 187–211. [Google Scholar]
- Houlihan M, Stelmack R, Campbell KB. Intelligence and the effects of perceptual processing demands, task difficulty, and processing speed on P300, reaction time, and movement time. Intelligence. 1998;26:9–25. [Google Scholar]
- Howard L, Polich J. P300 latency and memory span development. Dev Psychol. 1985;21:283–289. [Google Scholar]
- Iacono WG, Carlson SR, Malone SM, McGue M. P3 event-related potential amplitude and the risk for disinhibitory disorders in adolescent boys. Arch Gen Psychiatry. 2002;59:750–757. doi: 10.1001/archpsyc.59.8.750. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Malone SM, McGue M. Substance use disorders, externalizing psychopathology, and P300 event-related potential amplitude. Int J Psychophysiol. 2003;48:147–178. doi: 10.1016/s0167-8760(03)00052-7. [DOI] [PubMed] [Google Scholar]
- Intriligator J, Polich J. On the relationship between background EEG and the P300 event-related potential. Biol Psychol. 1994;37:207–218. doi: 10.1016/0301-0511(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Intriligator J, Polich J. On the relationship between EEG and P300 variability. Int J Psychophys. 1995;20:59–74. doi: 10.1016/0167-8760(95)00028-q. [DOI] [PubMed] [Google Scholar]
- Isreal JB, Chesney GL, Wickens CD, Donchin E. P300 and tracking difficulty: evidence for multiple resources in dual-task performance. Psychophysiology. 1980;17:259–273. doi: 10.1111/j.1469-8986.1980.tb00146.x. [DOI] [PubMed] [Google Scholar]
- Jansen BH, Brandt ME. The effect of the phase of prestimulus alpha-activity on the averaged visual evoked-response. Electroencephalogr Clin Neurophysiol. 1991;80:241–250. doi: 10.1016/0168-5597(91)90107-9. [DOI] [PubMed] [Google Scholar]
- Jasiukaitis P, Hakerem G. The effect of prestimulus alpha-activity on the P300. Psychophysiology. 1988;25:157–165. doi: 10.1111/j.1469-8986.1988.tb00979.x. [DOI] [PubMed] [Google Scholar]
- Jeon Y-W, Polich J. P3a from a passive visual stimulus task. Clin Neurophysiol. 2001;112:2202–2208. doi: 10.1016/s1388-2457(01)00663-0. [DOI] [PubMed] [Google Scholar]
- Jeon Y-W, Polich J. Meta-analysis of P300 and schizophrenia: patients, paradigms, and practical implications. Psychophysiology. 2003;40:684–701. doi: 10.1111/1469-8986.00070. [DOI] [PubMed] [Google Scholar]
- Johnson R. A triarchic model of P300 amplitude. Psychophysiology. 1986;23:367–384. doi: 10.1111/j.1469-8986.1986.tb00649.x. [DOI] [PubMed] [Google Scholar]
- Johnson R. The amplitude of the P300 component of the event-related potentials: review and synthesis. In: Ackles P, Jennings J, Coles MGH, editors. Advances in psychophysiology. 2. Greenwich, CT: JAI Press, Inc; 1988. pp. 69–137. [Google Scholar]
- Johnson R. Scalp-recorded P300 activity in patients following unilateral temporal lobectomy. Brain. 1988b;111:1517–1529. doi: 10.1093/brain/111.6.1517. [DOI] [PubMed] [Google Scholar]
- Johnson R. On the neural generators of the P300 component of the event-related potential. Psychophysiology. 1993;30:90–97. doi: 10.1111/j.1469-8986.1993.tb03208.x. [DOI] [PubMed] [Google Scholar]
- Johnson R, Donchin E. Sequential expectancies and decision-making in a changing environment: an electrophysiological approach. Psychophysiology. 1982;19:183–200. doi: 10.1111/j.1469-8986.1982.tb02545.x. [DOI] [PubMed] [Google Scholar]
- Johnson R, Pfefferbaum A, Kopell BS. P300 and long-term-memory: latency predicts recognition performance. Psychophysiology. 1985;22:497–507. doi: 10.1111/j.1469-8986.1985.tb01639.x. [DOI] [PubMed] [Google Scholar]
- Justus AN, Fin PR, Steinmetz JE. P300, disinhibited personality, and early-onset alcohol problems. Alc: Clin Exp Res. 2001;25:1457–1466. doi: 10.1097/00000374-200110000-00008. [DOI] [PubMed] [Google Scholar]
- Kahneman D. Attention and effort. Englewood-Cliffs: Prentice Hall; 1973. [Google Scholar]
- Karis D, Fabiani M, Donchin E. “P300” and memory: Individual differences in the von Restorff effect. Cog Psychol. 1984;16:117–216. [Google Scholar]
- Karniski W, Blair RC. Topographical and temporal stability of the P300. Electroencephalogr Clin Neurophysiol. 1989;72:373–383. doi: 10.1016/0013-4694(89)90043-6. [DOI] [PubMed] [Google Scholar]
- Katayama J, Polich J. P300 from one-, two-, and three-stimulus auditory paradigms. Int J Psychophysiol. 1996;23:33–40. doi: 10.1016/0167-8760(96)00030-x. [DOI] [PubMed] [Google Scholar]
- Katayama J, Polich J. Stimulus context determines P3a and P3b. Psychophysiology. 1998;35:23–33. [PubMed] [Google Scholar]
- Katsanis JG, Iacono WG, McGue MK, Carlson SR. P300 event-related potential heritability in monozygotic and dizygotic twins. Psychophysiology. 1997;34:47–58. doi: 10.1111/j.1469-8986.1997.tb02415.x. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE. Optimizing PCA methodology for ERP component identification and measurement: theoretical rationale and empirical evaluation. Clin Neurophysiol. 2003;114:2307–2325. doi: 10.1016/s1388-2457(03)00241-4. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE. Trusting in or breaking with convention: towards a renaissance of principal components analysis in electrophysiology. Clin Neurophysiol. 2005;116:1747–1753. doi: 10.1016/j.clinph.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Stevens MC, Laurens KR, Pearlson G, Calhoun VD, Liddle PF. An adaptive reflexive processing model of neurocognitive function: supporting evidence from a large scale (n=100) fMRI study of an auditory oddball task. Neuroimage. 2005;25:899–915. doi: 10.1016/j.neuroimage.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Kirino E, Belger A, Goldman-Rakic P, McCarthy G. Prefrontal activation evoked by infrequent target and novel stimuli in a visual target detection task: an event-related functional magnetic resonance study. J Neurosci. 2000;20:6612–6618. doi: 10.1523/JNEUROSCI.20-17-06612.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W. EEG-alpha rhythms and memory processes. Int J Psychophysiol. 1997;26:319–340. doi: 10.1016/s0167-8760(97)00773-3. [DOI] [PubMed] [Google Scholar]
- Klimesch W. Interindividual differences in oscillatory EEG activity and cognitive performance. In: Reinvang I, Greenlee MW, Herrmann M, editors. The cognitive neuroscience of individual differences. Oldenburg: BIS; 2003. pp. 87–99. [Google Scholar]
- Klimesch W, Doppelmay M, Russegger H, Pachinger T. Induced alpha band power changes in the human EEG and attention. Neurosci Let. 1998;244:73–76. doi: 10.1016/s0304-3940(98)00122-0. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Hanslmayr S, Sauseng P, Gruber W, Brozinsky CJ, Kroll NEA, Yonelinas AP, Doppelmayr M. Oscillatory EEG correlates of episodic trace decay. Cereb Cortex. 2006;16:280–290. doi: 10.1093/cercor/bhi107. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Pfurtscheller G, Schimke H. Pre- and post-stimulus processes in category judgement tasks as measured by event-related desynchronization (ERD) J Psychophysiol. 1992;6:185–203. [Google Scholar]
- Klimesch W, Schimke H, Pfurtscheller G. Alpha frequency, cognitive load and memory performance. Brain Topogr. 1993;5:241–251. doi: 10.1007/BF01128991. [DOI] [PubMed] [Google Scholar]
- Knight RT. Decreased response to novel stimuli after prefrontal lesions in man. Electroencephalogr Clin Neurophysiol. 1984;59:9–20. doi: 10.1016/0168-5597(84)90016-9. [DOI] [PubMed] [Google Scholar]
- Knight RT. Aging decreases auditory event-related potentials to unexpected stimuli in humans. Neurobiol Aging. 1987;8:109–113. doi: 10.1016/0197-4580(87)90019-4. [DOI] [PubMed] [Google Scholar]
- Knight R. Neural mechanisms of event-related potentials from human lesion studies. In: Rohbraugh J, Parasuraman R, Johnson R, editors. Event-related brain potentials: basic issues and applications. New York: Oxford University Press; 1990. pp. 3–18. [Google Scholar]
- Knight RT. Contribution of human hippocampal region to novelty detection. Nature. 1996;383:256–259. doi: 10.1038/383256a0. [DOI] [PubMed] [Google Scholar]
- Knight RT. Distributed cortical network for visual attention. J Cogn Neurosci. 1997;9:75–91. doi: 10.1162/jocn.1997.9.1.75. [DOI] [PubMed] [Google Scholar]
- Knight RT, Grabowecky M, Scabini D. Role of human prefrontal cortex in attention control. Adv Neurol. 1995;66:21–34. [PubMed] [Google Scholar]
- Knight RT, Nakada T. Cortico-limbic circuits and novelty: a review of EEG and blood flow data. Rev Neurosci. 1998;9:57–70. doi: 10.1515/revneuro.1998.9.1.57. [DOI] [PubMed] [Google Scholar]
- Knight RT, Scabini D, Woods D, Clayworth C. Contributions of temporal parietal junction to the human auditory P3. Brain Res. 1989;502:109–116. doi: 10.1016/0006-8993(89)90466-6. [DOI] [PubMed] [Google Scholar]
- Kok A. Effects of degradation of visual stimulation on components of the event-related potential (ERP) in go/nogo reaction tasks. Biol Psychol. 1986;23:21–38. doi: 10.1016/0301-0511(86)90087-6. [DOI] [PubMed] [Google Scholar]
- Kok A. Internal and external control: a two-factor model of amplitude change of event-related potentials. Biol Psychol. 1990;74:203–236. [PubMed] [Google Scholar]
- Kok A. Event-related potential (ERP) reflections of mental resources: a review and synthesis. Biol Psychol. 1997;45:19–56. doi: 10.1016/s0301-0511(96)05221-0. [DOI] [PubMed] [Google Scholar]
- Kok A. On the utility of P3 amplitude as a measure of processing capacity. Psychophysiology. 2001;38:557–577. doi: 10.1017/s0048577201990559. [DOI] [PubMed] [Google Scholar]
- Kolev V, Demiralp T, Yordanova J, Ademoglu A, Isoglu-Alkac U. Time-frequency analysis reveals multiple functional components during oddball P300. Neuroreport. 1997;8:2061–2065. doi: 10.1097/00001756-199705260-00050. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Wickens CD, Donchin E. Processing of stimulus properties: evidence for dual-task integrality. J Exp Psychol Hum Percept Perform. 1985;11:393–408. doi: 10.1037//0096-1523.11.4.393. [DOI] [PubMed] [Google Scholar]
- Krause CM, Lang HA, Laine M, Helle SI, Kuusisto MJ, Porn B. Event-related desynchronization evoked by auditory stimuli. Brain Topogr. 1994;7:107–112. doi: 10.1007/BF01186768. [DOI] [PubMed] [Google Scholar]
- Kujala A, Näätänen R. Auditory environment and change detection as indexed by the mismarch gegativity (MMN) In: Polich J, editor. Detection of change: event-related potential and fMRI findings. Boston, MA: Kluwer; 2003. pp. 1–22. [Google Scholar]
- Kutas M, McCarthy G, Donchin E. Augmenting mental chronometry: P300 as a measure of stimulus evaluation time. Science. 1977;197:792–795. doi: 10.1126/science.887923. [DOI] [PubMed] [Google Scholar]
- Leuthold H, Sommer W. Stimulus presentation rate dissociates sequential effects in event-related potentials and reaction times. Psychophysiology. 1993;30:510–517. doi: 10.1111/j.1469-8986.1993.tb02074.x. [DOI] [PubMed] [Google Scholar]
- Lin E, Polich J. P300 habituation patterns: individual differences from ultradian rhythms. Percept Mot Skills. 1999;88:1111–1125. doi: 10.2466/pms.1999.88.3c.1111. [DOI] [PubMed] [Google Scholar]
- Linden D. The P300: where in the brain is it produced and what does it tell us? The Neuroscientist. 2005;6:563–576. doi: 10.1177/1073858405280524. [DOI] [PubMed] [Google Scholar]
- Lopes da Silva FH. Dynamics of EEG as signals of neuronal populations: Models and theoretical considerations. In: Niedermeyer E, Lopes da Silva FH, editors. Electroencephalography: basic principles, applications, and related fields. Baltimore-Munich: Urban & Schwarzenberg; 1999. pp. 76–92. [Google Scholar]
- Lykken DT. Genetic determination of EEG frequency spectra. Biol Psychol. 1:245–259. doi: 10.1016/0301-0511(74)90001-5. [DOI] [PubMed] [Google Scholar]
- Magliero A, Bashore TR, Coles MGH, Donchin E. On the dependence of P300 latency on stimulus evaluation processes. Psychophysiology. 1984;21:171–186. doi: 10.1111/j.1469-8986.1984.tb00201.x. [DOI] [PubMed] [Google Scholar]
- Makeig S, Westerfield M, Jung TP, Enghoff J, Townsend J, Courchesne E, Sejnowski TJ. Dynamic brain sources of visual evoked responses. Science. 2002;295:1466–1466. doi: 10.1126/science.1066168. [DOI] [PubMed] [Google Scholar]
- Makeig S, Debener S, Onton J, Delorme A. Mining event-related brain dynamics. Trends Cogn Sci. 2004;8:204–210. doi: 10.1016/j.tics.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Mann CA, Sterman MB, Kaiser DA. Suppression of EEG rhythmic frequencies during somato-motor and visuo-motor behavior. Int J Psychophysiol. 1996;23:1–7. doi: 10.1016/0167-8760(96)00036-0. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Donchin E. A metric for thought: a comparison of P300 latency and reaction time. Science. 1981;211:77–80. doi: 10.1126/science.7444452. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Luby M, Gore J, Goldman-Rakic P. Infrequent events transiently activate human prefrontal and parietal cortex as measured by functional MRI. J Neurophysiol. 1997;77:1630–1634. doi: 10.1152/jn.1997.77.3.1630. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Wood C, Williamson P, Spencer D. Task-dependent field potentials in human hippocampal formation. J Neurosci. 1989;9:4253–4266. doi: 10.1523/JNEUROSCI.09-12-04253.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy G, Wood CC. Scalp distributions of event-related potentials: an ambiguity associated with analysis of variance models. Electroencephalogr Clin Neurophysiol. 1985;61:S226–S227. doi: 10.1016/0168-5597(85)90015-2. [DOI] [PubMed] [Google Scholar]
- McEvoy LK, Smith ME, Gevins A. Test-retest reliability of cognitive EEG. Clin Neurophysiol. 2000;111:457–463. doi: 10.1016/s1388-2457(99)00258-8. [DOI] [PubMed] [Google Scholar]
- McEvoy LK, Smith ME, Gevins A. Neuophysiological signals of working memory in normal aging. Cog Brain Res. 2001;11:363–376. doi: 10.1016/s0926-6410(01)00009-x. [DOI] [PubMed] [Google Scholar]
- Mecklinger A, Ullsperger P. What makes a category a category? ERP correlates of stimulus-to-category assignments. Electroencephalogr Clin Neurophysiol Suppl. 1995;44:255–60. [PubMed] [Google Scholar]
- Mecklinger A, Ullsperger P. P3 varies with stimulus categorization rather than probability. Electroencephalogr Clin Neurophysiol. 1993;86:395–407. doi: 10.1016/0013-4694(93)90135-i. [DOI] [PubMed] [Google Scholar]
- Mertens R, Polich J. P300 from a single-stimulus paradigm: passive versus active tasks and stimulus modality. Electroencephalogr Clin Neurophysiol. 1997a;104:488–497. doi: 10.1016/s0168-5597(97)00041-5. [DOI] [PubMed] [Google Scholar]
- Mertens R, Polich J. P300 hemispheric differences from oddball, verbal, and spatial tasks. Psychophysiology. 1997b;34:S64. [Google Scholar]
- Michalewski HJ, Prasher DK, Starr A. Latency variability and temporal interrelationships of the auditory event-related potentials (N1, P2, N2, and P3) in normal subjects. Electroencephalogr Clin Neurophysiol. 1986;65:59–71. doi: 10.1016/0168-5597(86)90037-7. [DOI] [PubMed] [Google Scholar]
- Molnár M. On the origin of the P3 event-related potential component. Int J Psychophysiol. 1994;17:129–144. doi: 10.1016/0167-8760(94)90028-0. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Aston-Jones G, Cohen J. Decision making, the P3, and the locus coeruleus-norepinephrine system. Psychol Bull. 2005;131:510–532. doi: 10.1037/0033-2909.131.4.510. [DOI] [PubMed] [Google Scholar]
- O’Connor SO, Morzorati S, Li T-K. Heritable features of the auditory oddball event-related potential: peaks, latencies, morphology and topography. Electroencephalogr Clin Neurophysiol. 1994;59:238–248. doi: 10.1016/0168-5597(94)90052-3. [DOI] [PubMed] [Google Scholar]
- O’Donnell B, Friedman S, Swearer J, Drachman D. Active and passive P3 latency and psychometric performance: influence of age and individual differences. Int J Psychophysiol. 1992;12:187–195. doi: 10.1016/0167-8760(92)90010-9. [DOI] [PubMed] [Google Scholar]
- Oken B. Endogenous event-related potentials. In: Chiappa K, editor. Evoked potentials in clinical medicine. New York: Lippincott-Raven Publishers; 1997. pp. 529–563. [Google Scholar]
- Onofrj M, Fulgente T, Nobiolio D, Malatesta G, Bazzano S, Colamartino P, Gambi D. P300 recordings in patients with bilateral temporal lobe lesions. Neurology. 1992;42:1762–1767. doi: 10.1212/wnl.42.9.1762. [DOI] [PubMed] [Google Scholar]
- Opitz B. ERP and fMRI correlates of target novelty processing. In: Polich J, editor. Detection of change: event-related Potential and fMRI findings. The Netherlands: Kluwer; 2003. pp. 117–132. [Google Scholar]
- Opitz B, Mecklinger A, von Cramon DY, Kruggel F. Combining electrophysiological and hemodynamic measures of the auditory oddball. Psychophysiology. 1999;36:142–147. doi: 10.1017/s0048577299980848. [DOI] [PubMed] [Google Scholar]
- Orlebeke JF, Kok A, Zeillemaker CW. Disinhibition and the processing of auditory stimulus intensity: an ERP-study. Pers Ind Diff. 1989;10:445–451. [Google Scholar]
- Paller K, McCarthy G, Roessler E, Allison T, Wood C. Potentials evoked in human and monkey medial temporal lobe during auditory and visual oddball paradigms. Electroencephalogr Clin Neurophysiol. 1992;84:269–279. doi: 10.1016/0168-5597(92)90008-y. [DOI] [PubMed] [Google Scholar]
- Paller K, Zola-Morgan S, Squire L, Hillyard SA. P3-like brain waves in normal monkeys and in monkeys with medial temporal lesions. Behav Neurosci. 1988;102:714–725. doi: 10.1037//0735-7044.102.5.714. [DOI] [PubMed] [Google Scholar]
- Pan J, Takahashi T, Morimot K. P300 as a measure of cognitive dysfunction from occupational and environmental insults. Environ Health Prev Med. 1999;4:103–110. doi: 10.1007/BF02932264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo J, Fox P, Raichle M. Localization of human system for sustained attention by positron emission tomography. Nature. 1991;349:61–64. doi: 10.1038/349061a0. [DOI] [PubMed] [Google Scholar]
- Pashler H. Dual-task Interference in simple tasks: data and theory. Psychol Bull. 1994;116:220–244. doi: 10.1037/0033-2909.116.2.220. [DOI] [PubMed] [Google Scholar]
- Pelosi L, Holly M, Slade T, Hayward M, Barrett G, Blumhardt LD. Event-related potential (ERP) correlates of performance of intelligence-tests. Electroencephalogr Clin Neurophysiol. 1992a;84:515–520. doi: 10.1016/0168-5597(92)90040-i. [DOI] [PubMed] [Google Scholar]
- Pelosi L, Holly M, Slade T, Hayward M, Barrett G, Blumhardt LD. Wave form variations in auditory event-related potentials-evoked by a memory-scanning task and their relationship with tests of intellectual function. Electroencephalogr Clin Neurophysiol. 1992b;84:344–352. doi: 10.1016/0168-5597(92)90087-r. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Christensen C, Ford JM, Kopell BS. Apparent response incompatibility effects on P3 latency depend on the task. Electroencephalogr Clin Neurophysiol. 1986;64:424–437. doi: 10.1016/0013-4694(86)90076-3. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM, Weller BJ, Kopell BS. ERPs to response production and inhibition. Electroencephalogr Clin Neurophysiol. 1985;60:423–434. doi: 10.1016/0013-4694(85)91017-x. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Klimesch W. Event-related desynchronization during motor behavior and visual information processing. Electroencephalogr Clin Neurophysiol Suppl. 1991;42:58–65. [PubMed] [Google Scholar]
- Pfurtscheller G, Klimesch W. Functional topography during a visuoverbal judgment task studied with event-related desynchronization mapping. J Clin Neurophysiol. 1992;9:120–131. doi: 10.1097/00004691-199201000-00013. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 1999;110:1842–1857. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Stancak A, Neuper C. Event-related synchronization (ERS) in the alpha band: an electrophysiological correlate of cortical idling: a review. Int J Psychophysiol. 1996;24:39–46. doi: 10.1016/s0167-8760(96)00066-9. [DOI] [PubMed] [Google Scholar]
- Picton TW. The P300 wave of the human event-related potential. J Clin Neurophysiology. 1992;9:456–479. doi: 10.1097/00004691-199210000-00002. [DOI] [PubMed] [Google Scholar]
- Picton TW, Stuss DT. The component structure of the human event-related potentials. Prog Brain Res. 1980;54:17–48. doi: 10.1016/S0079-6123(08)61604-0. [DOI] [PubMed] [Google Scholar]
- Pineda JA. Are neurotransmitter systems of subcortical origin relevant to the electrogenesis of cortical ERPs? Elec Clin Neurophsyiol. 1995;44 (suppl):143–150. [PubMed] [Google Scholar]
- Pineda JA, Foote SL, Neville HJ. Effects of locus coeruleus lesions on auditory, long-latency, event-related potentials in monkey. J Neurosci. 1989;9:81–93. doi: 10.1523/JNEUROSCI.09-01-00081.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poceta SJ, Houser M, Polich J. Event-related potentials in restless legs syndrome and Parkinson’s Disease (abstract) Sleep. 2006;28:A274. [Google Scholar]
- Polich J. Normal variation of P300 from auditory stimuli. Electroencephalogr Clin Neurophysiol. 1986;65:236–240. doi: 10.1016/0168-5597(86)90059-6. [DOI] [PubMed] [Google Scholar]
- Polich J. Task difficulty, probability, and inter-stimulus interval as determinants of P300 from auditory stimuli. Electroencephalogr Clin Neurophysiol. 1987;68:311–320. doi: 10.1016/0168-5597(87)90052-9. [DOI] [PubMed] [Google Scholar]
- Polich J. Bifurcated P300 peaks: P3a and P3b revisited? J Clin Neurophysiol. 1988;5:287–294. [PubMed] [Google Scholar]
- Polich J. Habituation of P300 from auditory stimuli. Psychobiology. 1989;17:19–28. [Google Scholar]
- Polich J. P300, probability, and Interstimulus-Interval. Psychophysiology. 1990;27:396–403. doi: 10.1111/j.1469-8986.1990.tb02333.x. [DOI] [PubMed] [Google Scholar]
- Polich J. Meta-analysis of P300 normative aging studies. Psychophysiology. 1996;33:334–353. doi: 10.1111/j.1469-8986.1996.tb01058.x. [DOI] [PubMed] [Google Scholar]
- Polich J. On the relationship between, EEG and P300: individual differences, aging, and ultradian rhythms. Int J Psychophysiol. 1997;26:299–317. doi: 10.1016/s0167-8760(97)00772-1. [DOI] [PubMed] [Google Scholar]
- Polich J. Overview of P3a and P3b. In: Polich J, editor. Detection of change: event-related potential and fMRI findings. Boston, MA: Kluwer; 2003. pp. 83–98. [Google Scholar]
- Polich J. Clinical application of the P300 event-related potential. Phys Med Rehab Clinics. 2004;15:133–161. doi: 10.1016/s1047-9651(03)00109-8. [DOI] [PubMed] [Google Scholar]
- Polich J, Bloom FE. P300, alcoholism heritability, and stimulus modality. Alcohol. 1999;17:149–156. doi: 10.1016/s0741-8329(98)00047-0. [DOI] [PubMed] [Google Scholar]
- Polich J, Bondurant T. P300 sequence effects, probability, and interstimulus interval. Physiol Behav. 1997;61:843–849. doi: 10.1016/s0031-9384(96)00564-1. [DOI] [PubMed] [Google Scholar]
- Polich J, Ladish C, Bloom FE. P300 assessment of early Alzheimer’s disease. Electroencephalogr Clin Neurophysiol. 1990;77:179–189. doi: 10.1016/0168-5597(90)90036-d. [DOI] [PubMed] [Google Scholar]
- Polich J, Ladish C, Burns T. Normal variation of P300 in children: age, memory span, and head size. Int J Psychophysiol. 1990a;9:237–248. doi: 10.1016/0167-8760(90)90056-j. [DOI] [PubMed] [Google Scholar]
- Polich J, Comerchero MD. P3a from visual stimuli: typicality, task, and topography. Brain Topogr. 2003;15:141–152. doi: 10.1023/a:1022637732495. [DOI] [PubMed] [Google Scholar]
- Polich J, Corey-Bloom J. Alzheimer’s disease and P300: Review and evaluation of task and modality. Curr Alzheimer Res. 2005;2:515–525. doi: 10.2174/156720505774932214. [DOI] [PubMed] [Google Scholar]
- Polich J, Criado JR. Neuropsychology and neuropharmacology of P3a and P3b. Int J Psychophysiol. 2006;60:172–185. doi: 10.1016/j.ijpsycho.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Polich J, Heine MRD. P300 topography and modality effects from a single stimulus paradigm. Psychophysiology. 1996;33:747–752. doi: 10.1111/j.1469-8986.1996.tb02371.x. [DOI] [PubMed] [Google Scholar]
- Polich J, Alexander JE, Bauer LO, Kuperman S, Rohrbaugh J, Mozarati S, O’Connor SJ, Porjesz B, Begleiter H. P300 topography of amplitude/latency correlations. Brain Topogr. 1997;9:275–282. doi: 10.1007/BF01464482. [DOI] [PubMed] [Google Scholar]
- Polich J, Ehlers C, Otis S, Mandell A, Bloom F. P300 latency reflects the degree of cognitive decline in dementing illness. Electroencephalogr Clin Neurophysiol. 1986;63:138–144. doi: 10.1016/0013-4694(86)90007-6. [DOI] [PubMed] [Google Scholar]
- Polich J, Eischen SE, Collins GE. P300 from a single auditory stimulus. Electroencephalogr Clin Neurophysiol. 1994;92:253–261. doi: 10.1016/0168-5597(94)90068-x. [DOI] [PubMed] [Google Scholar]
- Polich J, Herbst K. P300 as a clinical assay: rationale, evaluation, and findings. Int J Psychophysiol. 2000;38:3–19. doi: 10.1016/s0167-8760(00)00127-6. [DOI] [PubMed] [Google Scholar]
- Polich J, Hoffman L. P300 and handedness: on the possible contribution of corpus callosal size. Psychophysiology. 1998;35:497–507. doi: 10.1017/s0048577298970792. [DOI] [PubMed] [Google Scholar]
- Polich J, Howard L, Starr A. P300 latency correlates with digit span. Psychophysiology. 1983;20:665–669. doi: 10.1111/j.1469-8986.1983.tb00936.x. [DOI] [PubMed] [Google Scholar]
- Polich J, Kok A. Cognitive and biological determinants of P300: an integrative review. Biol Psychol. 1995;41:103–146. doi: 10.1016/0301-0511(95)05130-9. [DOI] [PubMed] [Google Scholar]
- Polich J, Ladish C, Burns T. Normal variation of P300 in children: age, memory span, and head size. Int J Psychophysiol. 1990b;9:237–248. doi: 10.1016/0167-8760(90)90056-j. [DOI] [PubMed] [Google Scholar]
- Polich J, Margala C. P300 and probability: comparison of oddball and single-stimulus paradigms. Int J Psychophysiol. 1997;25:169–176. doi: 10.1016/s0167-8760(96)00742-8. [DOI] [PubMed] [Google Scholar]
- Polich J, Martin S. P300, cognitive capability, and personality: a correlational study of university undergraduates. Pers Ind Diff. 1992;13:533–543. [Google Scholar]
- Polich J, Squire L. P300 from amnesic patients with bilateral hippocampal lesions. Electroencephalogr Clin Neurophysiol. 1993;86:408–417. doi: 10.1016/0013-4694(93)90136-j. [DOI] [PubMed] [Google Scholar]
- Polich J, Starr A. Middle-, late-, and long-latency auditory evoked potentials. In: Moore EJ, editor. Bases of auditory brain-stem evoked responses. New York: Grune & Stratton; 1983. pp. 345–361. [Google Scholar]
- Polich J, Burns T. P300 from identical twins. Neuropsychologia. 1987;25:299–304. doi: 10.1016/0028-3932(87)90143-6. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Rangaswamy M, Kamarajan C, Jones KA, Padmanabhapillai A, Begleiter H. The utility of neurophysiological markers in the study of alcoholism. Clin Neurophysiol. 2005;116:993–1018. doi: 10.1016/j.clinph.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Posner MI. Attention as a cognitive neural system. Curr Dir Psychol Sci. 1992;1:11–14. [Google Scholar]
- Posner MI, Petersen S. The attention system of the human brain. Ann Rev Psychol. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Posner MI, Dehaene S. Attentional networks. Trends Neurosci. 1994;17:75–79. doi: 10.1016/0166-2236(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Potter D, Barrett K. Assessment of mild head injury with ERPs and neuropsychological tasks. J Psychophys. 1999;13:173–189. [Google Scholar]
- Potts G, Liotti M, Tucker D, Posner MI. Frontal and inferior temporal cortical activity in visual target detection: evidence from high spatially sampled event-related potentials. Brain Topogr. 1996;9:3–14. [Google Scholar]
- Pribram K, McGuinness D. Arousal, activation and effort in the control of attention. Psychol Rev. 1975;82:116–149. doi: 10.1037/h0076780. [DOI] [PubMed] [Google Scholar]
- Price RL, Smith DBD. P3(00) Wave of averaged evoked-potential: bibliography. Physiol Psychol. 1974;2:387–391. [Google Scholar]
- Pritchard WS. Psychophysiology of P300. Psychol Bull. 1981;89:506–540. [PubMed] [Google Scholar]
- Pritchard WS, Brandt ME, Shappell SA, Odell TJ, Barratt ES. P300 amplitude prestimulus EEG power relationships. Psychophysiology. 1985;22:609–610. [Google Scholar]
- Ragot R, Renault B. P300 and spatial conflict. Electroencephalogr Clin Neurophysiol. 1981;52:S135–S135. [Google Scholar]
- Ragot R, Renault B. P300 and S-R compatibility: reply. Psychophysiology. 1985;22:349–352. doi: 10.1111/j.1469-8986.1985.tb01614.x. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Rainer G. Neural mechanisms for detecting and remembering novel events. Nat Rev Neurosci. 2003;4:193–202. doi: 10.1038/nrn1052. [DOI] [PubMed] [Google Scholar]
- Ravden D, Polich J. On P300 measurement stability: habituation, intra-trial block variation, and ultradian rhythms. Biol Psychol. 1999;51:59–76. doi: 10.1016/s0301-0511(99)00015-0. [DOI] [PubMed] [Google Scholar]
- Regan D. Human brain electrophysiology: evoked potentials and evoked magnetic fields in science and medicine. New York: Elsevier; 1989. [Google Scholar]
- Reinvang I. Cognitive event-related potentials in neuropsychological assessment. Neuropsychol Rev. 1999;9:231–248. doi: 10.1023/a:1021638723486. [DOI] [PubMed] [Google Scholar]
- Renault B, Ragot R, Lesèvre N, Remond A. Onset and offset of brain events as indexes of mental chronometry. Science. 1982;215:1413–1415. doi: 10.1126/science.7063853. [DOI] [PubMed] [Google Scholar]
- Riggins BR, Polich J. Habituation of P3a from visual stimuli. Kor J Think Prob Solving. 2002;12:71–81. [Google Scholar]
- Ritter W, Simson R, Vaughan HG., Jr Event-related potential correlates of two stages of information processing in physical and semantic discrimination tasks. Psychophysiology. 1983;20:168–179. doi: 10.1111/j.1469-8986.1983.tb03283.x. [DOI] [PubMed] [Google Scholar]
- Rugg M, Pickles C, Potter D, Roberts R. Normal P300 following extensive damage to the left medial temporal lobe. J Neurol Neurosurg Psychiatry. 1991;54:217–222. doi: 10.1136/jnnp.54.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushby JA, Barry RJ, Johnstone SS. Event-related potential correlates of serial-position effects during an elaborative memory test. Int J Psychophysiology. 2002;46:13–27. doi: 10.1016/s0167-8760(02)00037-5. [DOI] [PubMed] [Google Scholar]
- Rushby JA, Barry RJ, Doherty RJ. Separation of the components of the late positive complex in an ERP dishabituation paradigm. Clin Neurophysiol. 2005;116:2363–2380. doi: 10.1016/j.clinph.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Salisbury DF, Griggs CB, Shenton ME, McCarley RW. The nogo P300 ‘anteriorization’ effect and response inhibition. Clin Neurophysiol. 2004;115:1550–1558. doi: 10.1016/j.clinph.2004.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samar VJ, Swartz KP, Raghuveer MR. Multiresolution analysis of event-related potentials by wavelet decomposition. Brain Cogn. 1995;27:398–438. doi: 10.1006/brcg.1995.1028. [DOI] [PubMed] [Google Scholar]
- Satomi K, Horai T, Kinoshita Y, Wakazono A. Hemispheric asymmetry of event-related potentials in a patient with callosal disconnection syndrome: a comparison of auditory, visual, and somatosensory modalities. Electroencephalogr Clin Neurophysiol. 1995;94:440–449. doi: 10.1016/0013-4694(94)00314-b. [DOI] [PubMed] [Google Scholar]
- Schack B, Klimesch W, Sauseng P. Phase synchronization between theta and upper alpha oscillations in a working memory task. Int J Psychophysiol. 2005;57:105–114. doi: 10.1016/j.ijpsycho.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Segalowitz S, Barnes K. The reliability of ERP components in the auditory oddball paradigm. Psychophysiology. 1993;30:451–459. doi: 10.1111/j.1469-8986.1993.tb02068.x. [DOI] [PubMed] [Google Scholar]
- Sergeant J, Geuze R, van Winsum W. Event-related desynchronization and P300. Psychophysiology. 1987;24:272–277. doi: 10.1111/j.1469-8986.1987.tb00294.x. [DOI] [PubMed] [Google Scholar]
- Simons JS, Spiers HJ. Prefrontal and medial temporal lobe interactions in long-term memory. Nat Rev Neurosci. 2003;4:637–648. doi: 10.1038/nrn1178. [DOI] [PubMed] [Google Scholar]
- Simons RF, Graham FK, Miles MA, Chen X. On the relationship of P3a and the novelty-P3. Biol Psychol. 2001;56:207–218. doi: 10.1016/s0301-0511(01)00078-3. [DOI] [PubMed] [Google Scholar]
- Smith M, Halgren E. Dissociation of recognition memory components following temporal lobe lesions. J Exp Psychol: Gen. 1989;15:50–60. doi: 10.1037//0278-7393.15.1.50. [DOI] [PubMed] [Google Scholar]
- Snyder E, Hillyard S. Long-latency evoked potentials to irrelevant, deviant stimuli. Behav Biol. 1976;16:319–331. doi: 10.1016/s0091-6773(76)91447-4. [DOI] [PubMed] [Google Scholar]
- Sokolov EN. Brain functions: neuronal mechanisms of learning and memory. Ann Rev Psychol. 1977;28:85–112. [Google Scholar]
- Soltani M, Knight RT. Neural origins of the P300. Crit Rev Neurobiol. 2000;14:199–224. [PubMed] [Google Scholar]
- Sommer W, Leuthold H, Matt J. The expectancies that govern P300 are mostly automatic and unconscious. Behav Brain Sci. 1998;21:149–150. [Google Scholar]
- Sommer W, Matt J, Leuthold H. Consciousness of attention and expectancy as reflected in event-related potentials and reaction times. J Exp Psychol: Learn Mem Cogn. 1990;16:902–915. doi: 10.1037//0278-7393.16.5.902. [DOI] [PubMed] [Google Scholar]
- Spencer K, Dien J, Donchin E. Spatiotemporal analysis of the late ERP responses to deviant stimuli. Psychophysiology. 2001;38:343–358. [PubMed] [Google Scholar]
- Spencer K, Polich J. Post-stimulus EEG spectral analysis and P300: attention, task, and probability. Psychophysiology. 1999;36:220–232. [PubMed] [Google Scholar]
- Spencer KM, Dien J, Donchin E. A componential analysis of the ERP elicited by novel events using a dense electrode array. Psychophysiology. 1999;36:409–414. doi: 10.1017/s0048577299981180. [DOI] [PubMed] [Google Scholar]
- Squire L, Kandel E. Memory from mind to molecules. New York: Scientific American Library; 1999. [Google Scholar]
- Squires K, Petuchowski S, Wickens C, Donchin E. Effects of stimulus sequence on event related potentials: comparison of visual and auditory sequences. Percept Psychophys. 1977;22:31–40. [Google Scholar]
- Squires KC, Wickens C, Squires NK, Donchin E. Effect of stimulus sequence on waveform of cortical event-related potential. Science. 1976;193:1142–1146. doi: 10.1126/science.959831. [DOI] [PubMed] [Google Scholar]
- Squires N, Squires K, Hillyard S. Two varieties of long-latency positive waves evoked by unpredictable auditory stimuli in man. Electroencephalogr Clin Neurophysiol. 1975;38:387–401. doi: 10.1016/0013-4694(75)90263-1. [DOI] [PubMed] [Google Scholar]
- Stampfer H, Başar E. Does frequency-analysis lead to better understanding of endogenous evoked-potentials. Int J Neurosci. 1986;29:189–189. doi: 10.3109/00207458508985616. [DOI] [PubMed] [Google Scholar]
- Stanzione P, Fattapposta F, Guiunti P. P300 variations in Parkinsonian patients before and during dopaminergic monotherapy: A suggested dopamine component in P300. Electroencephalogr Clin Neurophysiol. 1991;80:446–453. doi: 10.1016/0168-5597(91)90093-d. [DOI] [PubMed] [Google Scholar]
- Stassen HH, Bomben G, Propping P. Genetic-aspects of the EEG: an Investigation into the within-pair similarity of monozygotic and dizygotic twins with a new method of analysis. Electroencephalogr Clin Neurophysiol. 1987;66:489–501. doi: 10.1016/0013-4694(87)90095-2. [DOI] [PubMed] [Google Scholar]
- Stelmack RM, Houlihan M. Event-related potentials, personality, and intelligence: concepts, issues, and evidence. In: Saklofske DH, Zaidner M, editors. International handbook of personality and intelligence. New York: Plenum Press; 1994. pp. 349–365. [Google Scholar]
- Stenberg G. Personality and the EEG: arousal and emotional arousability. Pers Ind Diff. 1992;13:1097–1113. [Google Scholar]
- Stenberg G. Extraversion and the P300 in a visual classification task. Pers Ind Diff. 1994;16:543–560. [Google Scholar]
- Strüber D, Polich J. P300 and slow wave from oddball and single-stimulus visual tasks: inter-stimulus interval effects. Int J Psychophysiol. 2002;45:187–196. doi: 10.1016/s0167-8760(02)00071-5. [DOI] [PubMed] [Google Scholar]
- Sutton S. P300—Thirteen years later. In: Begleiter H, editor. Evoked brain potentials and behavior. New York: Plenum Press; 1979. pp. 107–125. [Google Scholar]
- Sutton S, Braren M, Zubin J, John E. Evoked potential correlates of stimulus uncertainty. Science. 1965;150:1187–1188. doi: 10.1126/science.150.3700.1187. [DOI] [PubMed] [Google Scholar]
- Suwazono S, Machado L, Knight R. Predictive value of novel stimuli modifies visual event-related potentials and behavior. Clin Neurophysiol. 2000;111:29–39. doi: 10.1016/s1388-2457(99)00186-8. [DOI] [PubMed] [Google Scholar]
- Takeshita S, Ogura C. Effect of the dopamine D2 antagonist sulpiride on event-related potentials and its relation to the law of the initial value. Int J Psychophysiol. 1994;16:99–106. doi: 10.1016/0167-8760(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Tarkka IM, Stokic DS. Source localization of P300 from oddball, single stimulus, and omitted-stimulus paradigms. Brain Topogr. 1998;11:141–151. doi: 10.1023/a:1022258606418. [DOI] [PubMed] [Google Scholar]
- Trenkwalder C, Winkelmann J. Pathophysiology of the restless legs syndrome. In: Chokroverty S, Hening WA, Walters AS, editors. Sleep and movement disorders. Philadelphia: Butterworth/Henemann; 2003. pp. 322–332. [Google Scholar]
- Urbach TP, Kutas M. The intractability of scaling scalp distributions to infer neuroelectric sources. Psychophysiology. 2002;39:791–808. doi: 10.1111/1469-8986.3960791. [DOI] [PubMed] [Google Scholar]
- van Beijsterveldt CE, van Baal GC. Twin and family studies of the human electroencephalogram: a review and meta-analysis. Biol Psychol. 2002;61:111–138. doi: 10.1016/s0301-0511(02)00055-8. [DOI] [PubMed] [Google Scholar]
- Verbaten M, Huyben M, Kemner C. Processing capacity and the frontal P3. Int J Psychophysiol. 1997;25:237–248. doi: 10.1016/s0167-8760(96)00748-9. [DOI] [PubMed] [Google Scholar]
- Verleger R. Event-related potentials and cognition: a critique of the context-updating hypothesis and an alternative interpretation of P3. Behav Brain Sci. 1988;11:343–356. [Google Scholar]
- Verleger R. On the utility of P3 latency as an index of mental chronometry. Psychophysiology. 1997;34:131–156. doi: 10.1111/j.1469-8986.1997.tb02125.x. [DOI] [PubMed] [Google Scholar]
- Verleger R. Event-related EEG potential research in neurological patients. In: Zani A, Proverbio AM, editors. The cognitive electrophysiology of mind and brain. Amsterdam: Academic Press; 2003. pp. 309–341. [Google Scholar]
- Verleger R, Ja^kowskis P, Wascher E. Evidence for an integrative role of P3b in linking reaction to perception. J Psychophysiol. 2005;19:150–150. [Google Scholar]
- Verleger R, Heide W, Butt C, Kömpf D. Reduction of P3b in patients with temporo-parietal lesions. Cogn Brain Res. 1994;2:103–116. doi: 10.1016/0926-6410(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Vogel F, Schalt E, Krüger J. The electroencephalogram (EEG) as a research tool in human behavior genetics: psychological examination in healthy males with various inherited EEG variants. I. Rationale of the study, material, methods. Hum Genet. 1979b;47:1–45. doi: 10.1007/BF00295569. [DOI] [PubMed] [Google Scholar]
- Vogel F, Schalt E, Krüger J. The electroencephalogram (EEG) as a research tool in human behavior genetics: psychological examination in healthy males with various inherited EEG variants. II. Results. Hum Genet. 1979a;47:47–80. doi: 10.1007/BF00295570. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM. One-year test-retest reliability of auditory ERPs in young and old adults. Int J Psychophysiol. 2002;46:29–40. doi: 10.1016/s0167-8760(02)00039-9. [DOI] [PubMed] [Google Scholar]
- Wang L, Kuroiwa Y, Li M, Kamitani T, Wang J, Takahashi T, Suzuki Y, Ikegami T, Matsubara S. The correlation between P300 alterations and regional cerebral blood flow in non-demented Parkinson’s disease. Neurosci Lett. 2000;282:133–136. doi: 10.1016/s0304-3940(00)00886-7. [DOI] [PubMed] [Google Scholar]
- Ward LM. Synchronous neural oscillations and cognitive processes. Trends Cogn Sci. 2003;7:553–559. doi: 10.1016/j.tics.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Wickens C, Kramer A, Vanasse L, Donchin E. Performance of concurrent tasks: a psychophysiological analysis of the reciprocity of information-processing resources. Science. 1983;221:1080–1082. doi: 10.1126/science.6879207. [DOI] [PubMed] [Google Scholar]
- Wijers AA, Mulder G, Okita T, Mulder LJ. Event-related potentials during memory search and selective attention to letter size and conjunctions of letter size and color. Psychophysiology. 1989;26:529–547. doi: 10.1111/j.1469-8986.1989.tb00706.x. [DOI] [PubMed] [Google Scholar]
- Witelson S. Cognitive neuroanatomy: a new era. Neurology. 1992;42:709–713. doi: 10.1212/wnl.42.4.709. [DOI] [PubMed] [Google Scholar]
- Woods DL, Courchesne E. The recovery functions of auditory event-related potentials during split-second discriminations. Electroencephalogr Clin Neurophysiol. 1986;65:304–315. doi: 10.1016/0168-5597(86)90009-2. [DOI] [PubMed] [Google Scholar]
- Yago E, Escera C, Alho K, Giard M, Serra-Grabulosa J. Spatiotemporal dynamics of the auditory novelty-P3 event-related brain potential. Cogn Brain Res. 2003;16:383–390. doi: 10.1016/s0926-6410(03)00052-1. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Knight RT. Effects of temporal-parietal lesions on the somatosensory P3 to lower limb stimulation. Electroencephalogr Clin Neurophysiol. 1992;84:139–148. doi: 10.1016/0168-5597(92)90018-7. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Knight RT. P300 generation by novel somatosensory stimuli. Electroencephalogr Clin Neurophysiol. 1991;78:50–55. doi: 10.1016/0013-4694(91)90018-y. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Hale LA, D’Esposito M, Knight RT. Rapid prefrontal-hippocampal habituation to novel events. J Neuroscience. 2004;24:5356–5363. doi: 10.1523/JNEUROSCI.4587-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Tsuchiya H, Yamagata S, Toyoda G, Kobayashi S. Event-related brain potentials in response to novel sounds in dementia. Clin Neurophysiol. 2000;111:195–203. doi: 10.1016/s1388-2457(99)00228-x. [DOI] [PubMed] [Google Scholar]
- Yordanova J, Devrim M, Kolev V, Ademoglu A, Demiralp T. Multiple time-frequency components account for the complex functional reactivity of P300. Neuroreport. 2000;11:1097–1103. doi: 10.1097/00001756-200004070-00038. [DOI] [PubMed] [Google Scholar]
- Yordanova J, Kolev V. Event-related alpha oscillations are functionally associated with P300 during information processing. Neuroreport. 1998a;9:3159–3164. doi: 10.1097/00001756-199810050-00007. [DOI] [PubMed] [Google Scholar]
- Yordanova J, Kolev V. Single-sweep analysis of the theta frequency band during an auditory oddball task. Psychophysiology. 1998b;35:116–126. [PubMed] [Google Scholar]
- Yordanova J, Kolev V, Polich J. P300 and alpha event-related desynchronization (ERD) Psychophysiology. 2001;38:143–152. [PubMed] [Google Scholar]