Scientific Abstract
The impaired development of joint attention is a cardinal feature of autism. Therefore, understanding the nature of joint attention is a central to research on this disorder. Joint attention may be best defined in terms of an information processing system that begins to develop by 4–6 months of age. This system integrates the parallel processing of internal information about one’s own visual attention with external information about the visual attention of other people. This type of joint encoding of information about self and other attention requires the activation of a distributed anterior and posterior cortical attention network. Genetic regulation, in conjunction with self-organizing behavioral activity guides the development of functional connectivity in this network. With practice in infancy the joint processing of self-other attention becomes automatically engaged as an executive function. It can be argued that this executive joint-attention is fundamental to human learning, as well as the development of symbolic thought, social-cognition and social-competence throughout the life span. One advantage of this parallel and distributed processing model of joint attention (PDPM) is that it directly connects theory on social pathology to a range of phenomenon in autism associated with neural connectivity, constructivist and connectionist models of cognitive development, early intervention, activity-dependent gene expression, and atypical ocular motor control.
Research on autism is increasingly multidisciplinary (Braunschweig et al.,, 2007; Dawson, 2008). However, there are currently few models of autism that bridge observations between the biological, environmental, and behavioral sciences. Constructivist and connectionist theory on neural and cognitive development may be useful in this regard (for reviews of theory see Elman, 2005; Dosenbach et al.,, 2007; Knudson, 2004; McClelland & Rogers, 2003; Munakata & McClelland, 2003; Quartz, 1999; Ramnani et al.,, 2004). Elements of constructivists and connectionist theory figure prominently in the current genetic and developmental neuroscience of autism (e.g., Courchesne & Pierce 2005; Geschwind & Levitt, 2007; Horowitz et al., 1998; Just et al., 2004; Lewis & Elman, 2008; Morrow, Yoo, Flavell, Kim et al., 2008; Wickelgren, 2005). Connectionist, neural network models of learning and behavioral impairments in autism have also been described (Cohen, 2007).
Following these leads elements of a constructivist and connectionist model have been proposed to explain why the impairment of joint attention development is a primary and cardinal behavioral feature of autism (Mundy, 2003; Mundy & Newell, 2007). The goal of this paper is to continue the development of this model in the context of a review of evidence for the central role of joint attention in autism and to describe how the model contributes to an integration of biological and behavioral phenomenon associated with this disorder. In particular, joint attention may be best conceived as an essential form of parallel and distributed social information processing that is essential to human learning. Examples of how this conceptualization fosters theoretical links between the specific social symptoms of autism with genetic, neurodevelopmental, executive and social-cognitive processes are described.
JOINT ATTENTION AND AUTISM
Numerous studies indicate that behavioral impairments of joint attention reflect processes that are central to the developmental etiology of autism (e.g., Charman, 2004; Curcio, 1977 Dawson et al., 2004; Lord et al., 2003; Loveland & Landry, 1986; Mundy et al., 1986; Sigman & Ruskin, 1999; Wetherby & Prutting, 1984). At its most basic level joint attention refers to the ability to consider information about one’s own visual attention in parallel with information about the visual attention of other people. This enables infants to socially coordinate their attention with other people, which is fundamental to social reference and social learning (Baldwin, 1995; Bruner, 1975; Striano et al., 2006,a, b).
Two types of joint attention behaviors emerge in the first months of life. Responding to Joint Attention (RJA) refers to infants’ ability to follow the direction of gaze, head posture or gestures of other people and consequently share a common social point of visual reference. Alternatively, Initiating Joint Attention (IJA) refers to infants ’ ability to spontaneously create or indicate a shared point of reference by the use of gestures, or more frequently, alternating gaze between objects or events and other people. Both of these behaviors involve social signals that designate interest in objects or events, but the former emphasizes information processing of others signals and the latter involves processes related to the volitional generation of goal-related behavior (Figure 1).
Figure 1.
Illustrations of different types of infant social attention coordination behaviors: a) Responding to Joint Attention-RJA involving following and other persons gaze and pointing gesture; b) Initiating Joint Attention-IJA involving a conventional gesture ‘pointing’ to share attention regarding a room poster, c1,2,3) IJA involving alternating eye contact to share attention with respect to a toy, d) Initiating Behavior Request involving pointing to elicit aid in obtaining an out of reach object, and e) Responding to Behavior Requests involving following an adult’s open-palm “give it to me” gesture.
The Dissociation of Joint Attention Impairment in Autism
To interpret IJA and RJA impairments in autism it is useful to recognize that they dissociate in development. Both RJA and IJA may be useful in the identification and diagnosis of autism from 18 to 24 months of age through childhood (e.g. Lord, et al., 2000; Stone, Counrod & Ousley, 1997). However, even at a young age there is considerable variability in adaptive facility with RJA expressed in children with autism where that is defined as sufficient RJA to foster language learning in early intervention (Bono, Daley & Sigman, 2004). With advances in cognitive development after in the preschool years RJA impairment tested in social interactions becomes less evident (Leekum, Hunnisett, & Moore, 1998; Mundy et al., 1994, see Figure 2). This does not mean that problems in gaze following are not evident in some individuals with autism (e.g. Wallace, Coleman, Pascalis & Bailey, 2006). Rather, there is inconsistent evidence of a robust and adaptive impairment in the ability to process the direction of gaze or respond to joint attention in all people with autism (Charwarska, Klin & Volkmar, 2003; Kylliainen & Heitanen, 2004; Nation & Penny, 2008). On the other hand, IJA deficits are observed in children with autism from preschool through adolescence, and IJA is a better diagnostic discriminator of autism than is RJA or gaze following (e.g. Charman, 2004; Dawson et al., 2004; Hobson & Hobson, 2007; Lord et al. 2000; Mundy et al., 1986; Sigman & Ruskin, 1999). Moreover, while both RJA and IJA appear to be related to language development, to our knowledge only IJA has consistently been observed to be specifically associated with social and affective symptom presentation in autism (Charman 2004; Kasari et al., 1990; Kasari et al., 2007; Lord et al., 2003; Mundy et al., 1994; Naber et al., 2008; Sigman & Ruskin, 1999).
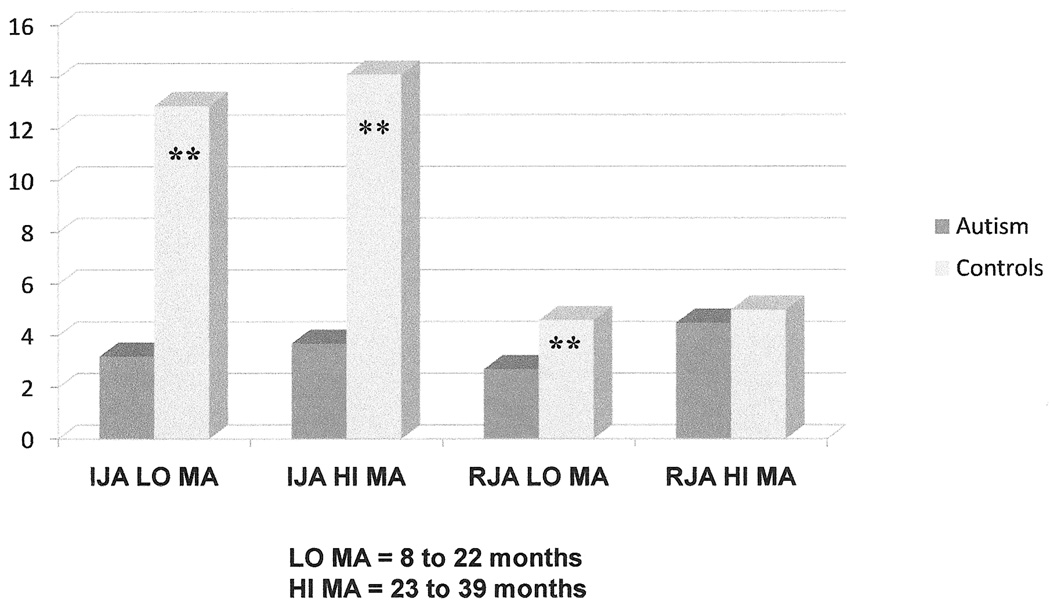
Figure 2.
Illustration of the moderating affect of mental age on diagnostic group differences on RJA versus IJA reported in Mundy et al., (1994).
This literature emphasizes that joint attention deficits are neither absolute nor uniform in autism. Moreover, where while its responsive nature may make RJA and gaze following more directly amendable to experimental research paradigms, it is equally important though perhaps more challenging to study and theorize about the role of IJA in autism (Mundy, 1995; 2003). The latter observation is by the results of intervention research, which suggest that autism involves a disturbance of the spontaneous generation of social behaviors, as much as or more than a disturbance of the perception and response to the social behaviors of others (Koegel et al., 2003). A similar interpretation has recently be put forth based on research with the infant siblings of children with autism (Zweiganbaum et al., 2005). Indeed, the significance of initiating deficits, especially IJA, is highlighted in current nosology where ‘a lack of spontaneous seeking to share enjoyment, interests, or achievements with other people, (e.g., by a lack of showing, bringing, or pointing out objects of interest to other people)’ is described as one of the four primary social symptoms of autism (APA, 1994). Furthermore, the “gold standard” diagnostic observation instrument, the Autism Diagnostic Observation Schedule (ADOS), recognizes that measures of both IJA and RJA are useful in Module 1 diagnostic algorithms for the youngest children, Module 2 for older children only includes IJA measures in its diagnostic criteria (Lord et al., 2000).
Evidence of a dissociation between IJA and RJA is also apparent in studies of typical infant development. The development of the frequent or consistent use of IJA and RJA is characterized by different growth patterns in infancy, and these domains display weak to non-significant correlations (e.g. Meltzoff & Brooks, 2008; Mundy et al., 2007; Sheinkopf et al., 2004; Slaughter & McDonald, 2003). IJA rather than RJA has also been observed to have more consistent correlations with childhood IQ (Ulvund & Smith, 1998), frontal brain activity (Caplan, Chugani, Messa, Guthrie, et al.,, 1993; Mundy et al.,, 2000); reward based behavioral goal-inhibition and selfmonitoring behaviors (Nichols et al., 2005), and caregiving (Claussen et al., 2002). The implication here is that recognizing the nature of the differences, as well as commonalities between IJA and RJA, may be at least equally important to conceptualizations of the joint attention impairments of autism.
On the Nature and Description of Joint Attention in Autism
While the topography of RJA and gaze following behavior disturbance in autism has been reasonable well described, this has not necessarily the case for IJA. For example, initiating joint attention impairments are often equated with problems with pointing and showing gestures. However, diminished alternating gaze behavior (See Fig. 1) may be a more important and useful measure of IJA impairment in autism. In one study this alternating gaze was superior to point and showing and correctly identified 94% of fifty-four preschool children with autism, mental retardation or typical development (Mundy et al., 1986). Others have observed that IJA measured with the Early Social-Communication Scales (ESCS, Seibert, Hogan & Mundy, 1982) had a sensitivity of 83% to 97% and a specificity of 63% to 67% in discriminating fifty-three 3- to 4-year-olds with autism from controls (Dawson et al., 2004). Indeed, most of the variance in ESCS total IJA scores is carried by alternating gaze behavior (Mundy et al., 2007). The IJA alternating gaze of two- year-olds also predicts 4-year-old symptom outcomes in children with autism (Charman, 2004), as well as social-cognition in typical 4-year-olds (Charman et al., 2000).
With regard to the nature of following gaze and alternating gaze impairments it has often been suggested that these joint attention impairment may be an epiphenomenon of developmental antecedent or successor processes that are considered to be more fundamental. A non-exhaustive list here includes reward-sensitivity, executive attention control, social-orienting, identification, imitation and mirror neurons, intersubjectivity, and most prominently social-cognition (e.g., Baron-Cohen, 1989; Charman, 2004; Dawson et al., 2004; Mundy, 1995; Mundy et al., 1986; Williams, 2008). This reductionism has lead to a paradox. Joint attention deficits are viewed as pivotal to the nature and treatment of autism, but not necessarily central to the endophenotype of autism. Charman (2004) recognized this paradox in noting that we often think of joint attention not as “a starting point [for autism], but merely a staging post in early social communicative development, and hence a ‘postcursor’ of earlier psychological and developmental processes … [which may] underlie the impaired development of joint attention skills in autism.” (p. 321).
There are at least three problems with this perspective. First, stable individual differences in RJA gaze following and IJA alternating gaze are well established by 8 to 9 months in typical development (Mundy et al., 2007; Venezia et al., 2004). Moreover, the onset of cortical control of following gaze likely begins in the first 3 months of life and alternating gaze between 4 to 6 months (Mundy & Vaughan van Hecke, 2008; Striano & Reid, 2006). If these observations are valid joint attention “precursors” would need to be present prior to at least 8 months of age, and quite possibly prior to the third month of life. Second, there is little evidence that the association of joint attention with the etiology or outcomes of autism is mediated by more basic antecedent or successor processes. Dawson et al., (2004) observed that neither social-orienting measures, nor empathy measures, could account for relations between IJA and language development in a large sample of children with autism. Joint attention disturbance in autism also cannot be explained in terms of affect regulation or social relatedness measured with attachment measures (Capps, Sigman & Mundy, 1994; Naber et al., 2008). Moreover, numerous regression analyses indicate that joint attention accounts for significant portions of variance in the language, symbolic play, and symptom development of children with autism above and beyond variance associated with executive functions, imitation, processing others intentions, or global measures of mental development (e.g. Charman 2004; Royers et al., 1998; Kasari et al., 2007; Naber et al., 2008; Rutherford et al., 2007; Sigman & Ruskin, 1999; Smith et al., 2007; Thurm et al., 2007; Toth et al., 2006).
A third issue is that precursor and successor process hypotheses rarely account for the dissociation of IJA and RJA (Mundy et al., 2007). Social-cognitive hypotheses suggest that RJA and IJA should be highly related because they both are precursors of a common “mentalizing” ability involved in perceiving the intentions of others (e.g., Baron-Cohen, 1995; Tomasello, 1995). Similarly, executive attention and social orienting hypotheses don’t explicitly account for why some children with autism display the capacity to disengage attention from eyes and faces in order to follow gaze on RJA tasks, but these same children rarely disengage attention from a interesting object to spontaneously alternate gaze with a social partner in interactions (e.g. Dawson et al. 2004; Mundy et al. 1994). Imitation and mirror neuron theory ostensibly emphasize the role of deficits in processing and responding to the behavior of other people in the development of autism (e.g. Williams, 2008). However, this theoretical perspective also doesn’t speak to the dissociation of RJA and IJA development in autism or typical development. Moreover, one might expect imitation and mirror neuron activity to be highly related to the responsive form of joint attention, yet some if not many children with autism display better abilities with RJA than IJA skills (e.g., Mundy et al. 1994). Why would this be the case? Could initiating deficits like IJA impairment and responding deficits like RJA impairment reflect related but unique developmental factors in autism? To begin to address this question it is important to recognize the vital but perhaps divergent roles of both forms of joint attention in human social learning (Bruner, 1975, 1995).
LEARNING AND THE IMPORTANCE OF JOINT ATTENTION
Early language learning often takes place in unstructured, incidental situations where parents spontaneously refer to a new object (Figure 3). How do infants know how to map their parents’ vocal labels to the correct parts of the environment amidst a myriad of potential referents? Baldwin (1995) suggested that they use RJA and the direction of gaze of their parent to guide them to the correct area of the environment, thereby reducing ‘referential’ mapping errors. Infants’ use of IJA also reduces the chance of referential mapping errors. IJA serves to denote something of immediate interest to the child. This helps parents to follow their child’s attention in order to provide new information in context when the child’s interest and attention is optimal for learning (Tomasello & Farrar, 1986). Hence, joint attention may be conceived of as a self-organizing system that facilitates information processing in support of social-learning (Mundy, 2003). This ‘learning function’ is fundamental to the nature of joint attention (Bruner, 1975) and continues to operate throughout our lives (e.g., Bayliss et al., 2006; Nathan et al., 2007).
Figure 3.
Illustration from Baldwin (1995) depicting the referential mapping problem encountered by infants in incidental social word learning situations.
Without the capacity for joint attention, success in many pedagogical contexts would be difficult. Imagine the school readiness problems of a five-year-old who enters kindergarten but is not facile with coordinating attention with the teacher. Similarly, children, adolescents and adults who cannot follow, initiate or join with the rapid-fire exchanges of shared attention in social interactions may be impaired in any social-learning context, as well as in their very capacity for relatedness and relationships (Mundy & Sigman, 2006).
If joint attention helps self-organize social-learning, then the more children engage in joint attention, the more optimal social-learning opportunities they help create for themselves. Indeed, the frequency with which infants engage in joint attention is positively related to their language acquisition and childhood IQ status (e.g. Mundy et al., 2007; Smith & Ulvund, 2003). Direct evidence of the links between joint attention and early learning is provided by the observation that coordinated social attention to pictures elicits electrophysiological evidence of enhanced neural activity (Striano, Reid & Hoel, 2006) and recognition memory associated with greater depth of processing in 9-month-olds (Striano, Chen, Cleveland, & Bradshaw, 2006).
So, if joint attention is basic to early learning, but IJA and RJA dissociate in development, does the dissociation of these two behavior domains have meaning for the role of joint attention in early learning. We think it does. To begin to support this conjecture we next need to review theory and research that suggests that the two forms of joint attention, IJA and RJA, develop from and involve functions of distinct neural networks (Mundy & Newell, 2007). In this review we begin to lay the foundation for the hypothesis that joint attention is a form of parallel and distributed cortical processing, and that practice with joint attention early in development serves to entrain a distributed, executive social attention that contributes to the foundations for social cognitive and symbolic processes.
The Two Neural Systems of Joint Attention and Social Cognition
Research has indicated that IJA is associated with frontal-cortical activity (Caplan et al., 1993; Henderson et al., 2004; Mundy et al., 2000; Torkildsen et al., 2008) while RJA and related behaviors are more closely tied to parietal and temporal cortical processes (e.g. Emery, 2002; Frieschen, Bayliss, & Tipper, 2007; Materna, Dicke & Thern, 2008; Mundy et al., 2000). One interpretation of these data is that joint attention involves functions of both the anterior and posterior cortical attention networks that have been described by Michael Posner and others (e.g. Posner & Rothbart, 2007).
The functions of the posterior network are common to many primates, but the anterior network is not well represented in primates other than humans (Astafiev, Shulman, Stanley & Snyder et al., 2003; Emery, 2000; Jellema, Baker, Wicker, & Perrett, D. 2000). RJA appears to be most closely associated with the posterior system that regulates relatively involuntary attention, begins to develop in the first 3 months of life, and prioritizes orienting to biologically meaningful stimuli. It is supported by neural networks of the parietal (precuneous) and superior temporal cortices (Figure 4). These neural networks are active in the perception of the eye and head orientations of others, as well as the perception of spatial relations between self, other and the environment. The posterior system is especially involved in control of orienting on a trial by trial basis, and the development of cognitive representations about the world built from information acquired through external senses (Dosenbach et al., 2007; Fuster, 2006; Cavana & Trimble, 2006).
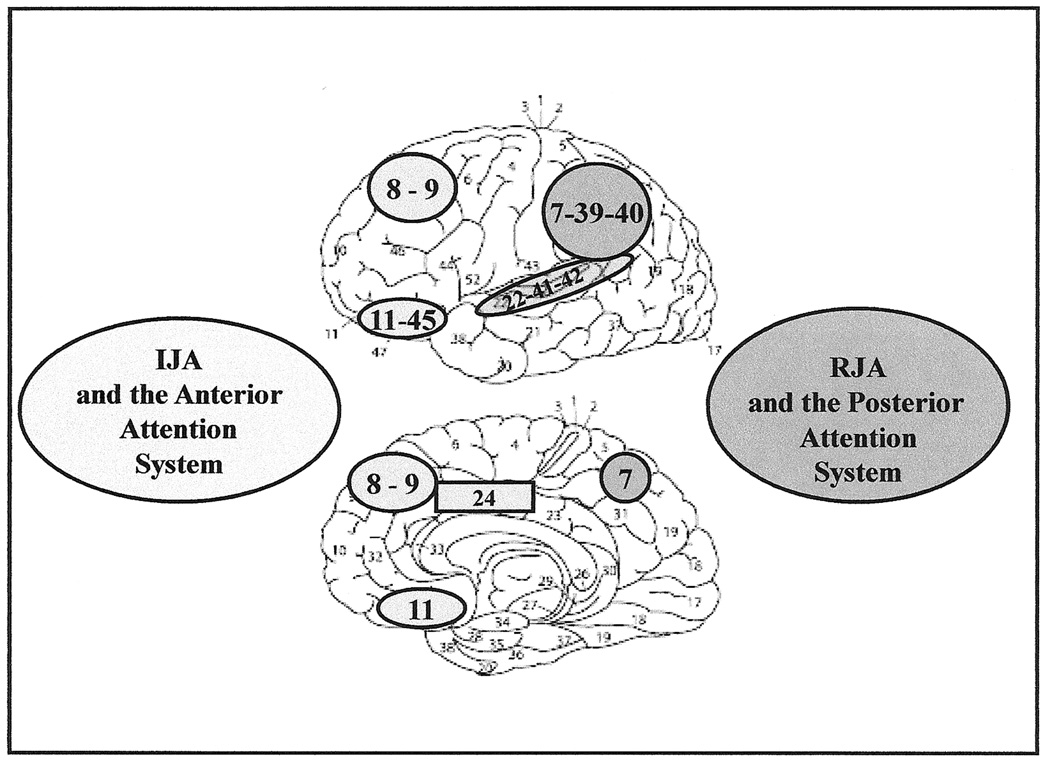
Figure 4.
Illustration from Mundy & Newell (2007) depicting the lateral (top) and medial (bottom) illustrations of Brodmann’s cytoarchitectonic areas of the cerebral cortex associated with Initiating Joint Attention and the anterior attention system, as well as RJA and the posterior attention systems. The former include areas 8 (frontal eye fields) 9 (prefrontal association cortex), 24 (anterior cingulate), 11 and 47 (orbital prefrontal association cortex). The latter include areas 7 (precuneous, posterior parietal association area), 22, 41, and 42 (superior temporal cortex) and 39 and 40 (parietal, temporal, occipital association cortex).
We hypothesize that initiating joint attention is primarily supported by the later developing anterior attention network involved in the cognitive processing, representation and regulation of self-initiated goal directed action. This network includes the components of the anterior cingulate, medial superior frontal cortex including the frontal eye fields, anterior prefrontal cortex and orbital frontal cortex (e.g. Dosenbach et al., 2007; Fuster, 2006) as well as systems of the orbital frontal and basal ganglia (caudate) especially associated with reward modulation of functions of the frontal eye fields (Dawson et al. 2004; Long & Hikosaka, 2006). The development of the intentional control of visual attention begins at about 3 to 4 months of age, when a pathway from the frontal eye fields (BA 8/9) that releases the superior colliculus from inhibition begins to be actively involved in the prospective control of saccades and visual attention (Canfield & Kirkham, 2001; Johnson, 1990). The function of this pathway may underlie four month old infants’ ability to suppress automatic visual saccades in order to respond to a second, more attractive stimulus (Johnson, 1995), and six month olds’ ability to respond to a peripheral target when central, competing stimuli are present (Atkinson, Hood, Wattam-Bell, & Braddick, 1992). We assume that the functions of this pathway also enable intentional gaze alternation between interesting events and social partners (Mundy, 2003).
Differences in the functions and developmental timing of the anterior and posterior attention networks help to explain why IJA and RJA dissociate in development (Mundy et al., 2000, 2007). However, although IJA and RJA follow distinct bio-behavioral paths of development it is also likely that they interact and integrate in development. Indeed, EEG data indicates that activation of a distributed anterior and posterior cortical system predicts IJA development in infants (Henderson et al., 2004) and fMRI data indicate that activation of a distributed anterior-posterior cortical network is associated with the experience of joint attention in adults (Williams, Waiter, Perra, Perrett, et al., 2005). A major of assumption of the parallel and distributed information processing model (PDPM) is that the integrated processing of internal information about our own visual attention with posterior processing of information about the visual attention of other people gives rise to a cognitive synthesis that is the defining feature of joint attention (Figure 5., Mundy & Newell, 2007).
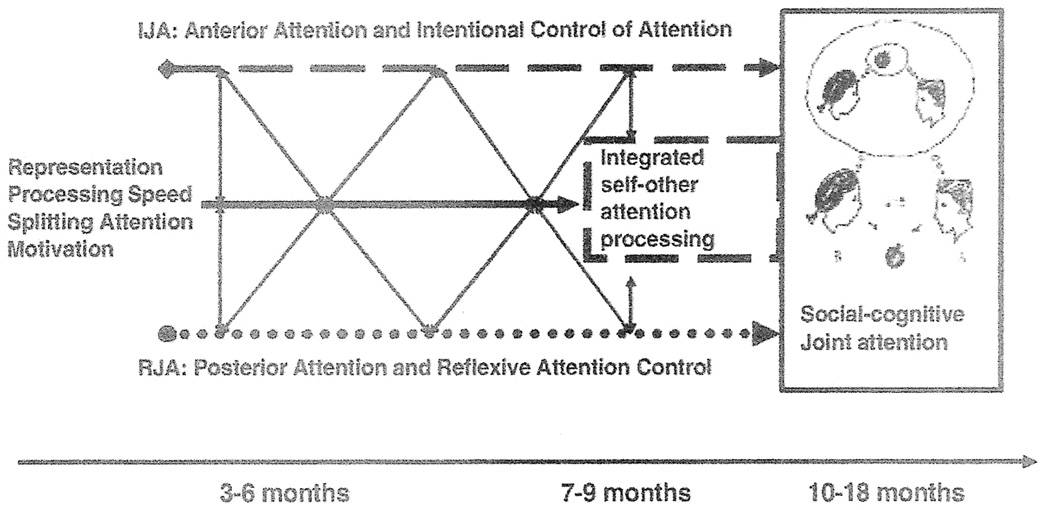
Figure 5.
An illustration of the distributed information processing system model of joint attention and social cognition, from Mundy & Newell (2007). In this model, different types of lines depict the multiple paths of joint attention development from. The posterior attention system path associated with RJA development is illustrated with a dotted line  and the anterior attention system path associated with IJA development is illustrated with a dashed line
and the anterior attention system path associated with IJA development is illustrated with a dashed line  . The central solid line in the figure depicts the developments of other processes during infancy that influence joint attention development such as representational ability, speed of processing, motivation and the executive attention control, as well as each other during infancy. The diagonal arrows connect all paths throughout early development. This reflects the dynamic and coactive nature of joint attention development whereby the maturation of attention, cognitive and affective systems interact in reciprocal cause and effect relations with experience, including the experiences children create for themselves through their own actions. Finally, the development of integrated self and other attention processing is considered to be a social attention executive function of the anterior system that emerges in the 4 to 9 months period. This is represented by the
. The central solid line in the figure depicts the developments of other processes during infancy that influence joint attention development such as representational ability, speed of processing, motivation and the executive attention control, as well as each other during infancy. The diagonal arrows connect all paths throughout early development. This reflects the dynamic and coactive nature of joint attention development whereby the maturation of attention, cognitive and affective systems interact in reciprocal cause and effect relations with experience, including the experiences children create for themselves through their own actions. Finally, the development of integrated self and other attention processing is considered to be a social attention executive function of the anterior system that emerges in the 4 to 9 months period. This is represented by the  box. The capacity to integrate and share overt aspects of attention provides a foundation for the ability to share covert aspects of attention, such as representations, and social cognition.
box. The capacity to integrate and share overt aspects of attention provides a foundation for the ability to share covert aspects of attention, such as representations, and social cognition.
The integration of information about self-attention and the attention of others is a form of parallel processing that occurs across a distributed cortical network. It is typically practiced in infancy across many daily trials and contributes to an information processing synthesis that plays a crucial role in human social cognition. This basic idea related to the notion that that human levels of self-awareness cannot develop without bidirectional interaction of information processing about self and others (e.g. Piaget, 1952). Moreover, the potential role of this type of parallel and distributed processing in human social cognition has previously been recognized (Decety & Sommerville, 2003; Keysers & Perrett, 2004). However, the hypothetical role of practice with joint attention in infancy in the development of the specific parallel and distributed processing substrates necessary for social cognition is less well recognized.
Social-Cognition, Joint Attention and the PDPM
Social-cognitive models often describe joint attention development in terms of incremental stages of knowledge about the intentionality of other people. Baron-Cohen (1995) described a sequence of cognitive modules which included the Intentionality Detector (ID), a dedicated cognitive facility that attributes goal directed behavior to objects or people, and the Eye Direction Detector (EDD) that senses and processes information about eyes. These combine to form the Shared Attention Mechanism (SAM), a cognitive module that represents self and other as attending to the same referent and attributes volitional states (intentionality) to direction of gaze of other people. As infancy ebbs the Theory of Mind Mechanism replaces SAM and enables representation of the full range of mental states of others and enables us to make sense of others behaviors.
Tomasello et al., (2005) described joint attention development in terms of three stages of what infants know about other people. In Understanding Animate Action, 3- to 8-month-old infants can perceive contingencies between their own animate actions and emotions relative to the animate actions and affect of others. However, they cannot represent the internal mental goals of others that are associated with these actions. In the next Understanding of Pursuit of Goals stage 9-month-olds become capable of shared action and attention on objects (e.g. building a block tower with parents). Tomasello et al., 2005 suggest this involves joint perception, rather than joint attention, because the social-cognitive capacity to represent others internal mental representations necessary for true joint attention is not yet available. However, this ability emerges between 12–15 months in the Understanding Choice of Plans stage. This stage is heralded when infants become truly active in initiating episodes of joint engagement by alternating their eye-contact between interesting sights and caregivers (Tomasello et al., 2005). This shift to active alternating gaze indicates infants’ appreciation that others make mental choices about alternative actions that affect their attention. Infants also now know themselves as agents that initiate collaborative activity based on their own goals. Hence, the development of “true” joint attention at this stage is revealed in the capacity to adopt two perspectives analogous to speaker-listener.
The capacity to adopt two perspectives is also assumed to be an intrinsic characteristic of symbolic representations. In this regard, Tomasello et al., (2005) argue for the truly seminal hypothesis that symbolic thought is a transformation of joint attention. They argue that symbols themselves serve to socially coordinate attention so that the intentions of the listener align with those of the speaker. In other words linguistic symbols both lead to and are dependent upon the efficient social coordination of covert mental attention to common abstract representations among people. This hypothesis fits well with the parallel and distributed information processing model of joint attention, but the PDPM places this hypothesis in a decidedly different developmental framework.
The PDPM does not emphasize functional segregation of cognitive systems implicit to modular perspectives, but instead stresses a connectionist perspective in keeping with the view that because of the “massively parallel nature of human brain networks” it is likely that cognitive “functions also emerges from the flow of information between brain areas“ (Ramnani et al., 2004, p. 613). Furthermore, cognitive development need not only be construed in terms of discontinuous changes in knowledge. It can also be modeled in terms of continuous changes in the speed, efficiency and combinations of information processing that give rise to knowledge (Hunt, 1999). As such, the PDPM describes joint attention development in terms of increased speed, efficiency and complexity of processing of information. In particular joint attention development involves increased efficiencies of processing: 1) internal information about self–referenced visual attention, 2) external information about the visual attention of other people, and 3) the integrated (parallel) processing of self-generated visual attention information with processing of information about the visual attention behavior of other people (Mundy & Newell, 2007).
Consequently, the notion that true joint attention does not emerge until requisite social-cognitive awareness emerges at 12–15 months (Tomasello et al., 2005) is not especially germane to the PDPM. Rather, the PDPM holds that the true joint processing of attention information from oneself and others begins to be practiced by infants by three to four months of age. This assertion is consistent with a growing empirical literature on the development of joint attention (D’Entremont, Hains, & Muir, 1997; Farroni, Massuccessi, & Francesca, 2002; Hood, Willen, & Driver, 1998, Morales et al., 1998, Striano & Reed, 2006; Striano & Stahl, 2005). Indeed, even the types of active alternating gaze thought to mark the onset of true joint at 12–15 months (Tomasello et al., 2005) develops no later than at 8–9 months of life, and quite possibly earlier (Mundy et al., 2007; Venezia et al., 2004).
Equally important, the PDPM assumes that joint attention is not replaced by the subsequent development of social-cognitive processes. Rather, joint attention remains an active system of information processing that supports cognition through adulthood (Mundy & Newell, 2007). As an example of this, recall the hypothesis that linguistic symbols enable the social coordination of covert attention to common mental representations across people (Tomasello et al., 2005). According to the PDPM symbolic thinking involves joint attention, but does not replace joint attention. Just as 12-month-olds can shift eye contact or use pointing to establish a common visual point of reference with other people, four-year-olds can use symbols to establish a common reference to covert mental representations with other people. Symbolic representations are often, if not always, initially encoded during the joint processing of information about the overt attention of self and the attention of others directed toward some third object or event (Adamson et al., 2004; Baldwin, 1995; Wener & Kaplan, 1963). According to the PDPM symbol acquisition may incorporate the distributed activation of self-attention and other-attention processing units, which were engaged during encoding, as part of the functional neural representational mapping of symbols. Hence, the distributed joint attention processing system may always be activated as a network encoding that contributes to the social intersubjectivity (i.e., shared experience) of symbolic thought. This element of the PDPM is based on the connectionist notion that “representations can take the form of patterns of activity distributed across processing units” that were activated during encoding (Munakata & McClelland, 2003, p. 415). Specifically, the idea is that symbol encoding involves incorporation of anterior and posterior cortical joint processing as part of the pattern of distributed activity across processing units. This, is one of the hypotheses that arises from the PDPM model hypotheses that may be open to empirical verification or falsification.
In infancy the distributed joint attention processing system is initially effortful. However, thousands of episodes of practice allow the joint information processing of self-and-other’s attention to become efficient, less effortful and even automatically activated in social engagement. As this occurs, joint attention becomes a social-executive “subroutine” that runs in support of cognitive processing in social interactions, such as learning social conventional representations (i.e. symbols), processing representations of self and others in social interactions (i.e. social cognition) and maintaining a shared focus in social interactions (i.e. social competence). By 12 months the distributed neural activation patterns associated with joint attention are part of infants’ sense of relatedness and intersubjectivity with others (Mundy & Hogan, 1994; Mundy, Kasari & Sigman, 1993). Again, though, rather than being replaced by new stages of social-cognition or symbolic development after 12 months, joint attention can be thought of as an enduring stratum of a more continuous spiral of human social-neurocognitive development that supports if not enables these later emerging human cognitive facilities (see Figure 6).
Figure 6.
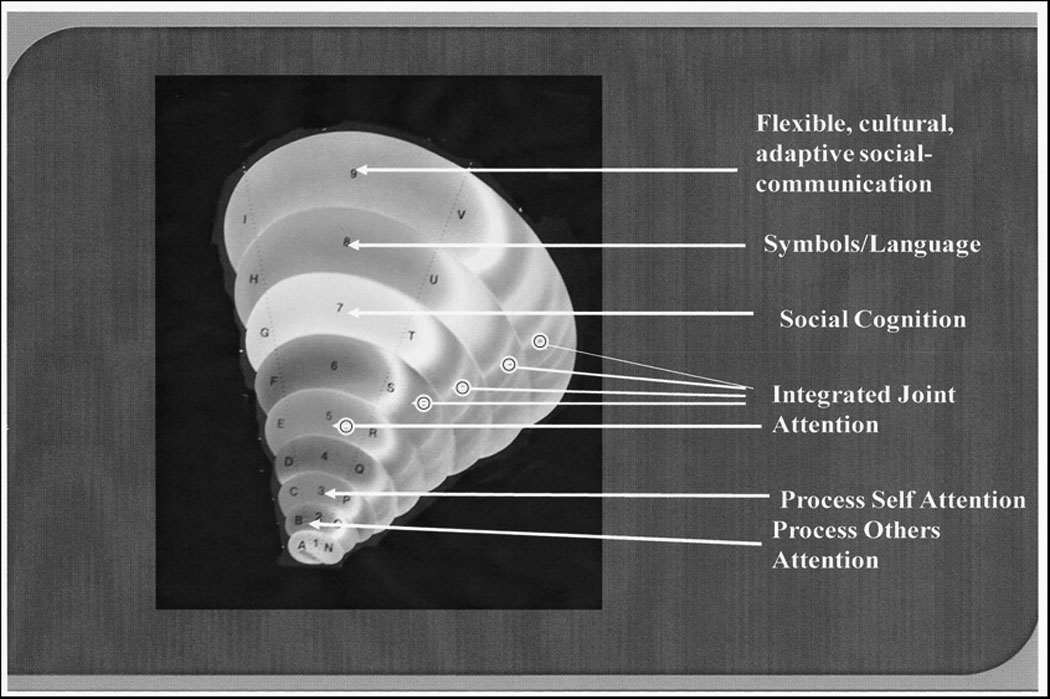
An illustration of the continuous nature of joint attention development. Development is modeled as a spiral in which the initial acquisition of the capacity for integrated processing of information about self and other attention (joint attention) remains an active but deeper layer of cognitive activity throughout life that supports symbolic thought, language and cultural social exchange. Numbers of the spiral bands represent changing phases of development. The letters on each side of the spiral bands represent change and continuity in multiple developmental factors which may impact joint attention development, such as speed of information processing, representational development, and memory.
Inside-Out Processing and the PDPM
In addition to parallel and distributed processing the PDPM is distinguished from other models by its constructivist perspective on development. Rather than focusing on the development of knowledge about others, the PDPM gives equal footing to the significance of infants’ development in processing and controlling their own intentional visual behavior in modeling joint attention and social cognitive development (Mundy et al., 1993). The assumption here is that the quantity and fidelity of information neonates and young infants receive about self-intended actions (e.g. active looking) through proprioception meets or exceeds the quantity and quality of information they receive about other’s intended actions through exteroceptive information processing. Thus, infants have the opportunity to learn as much or more about intentionality from their own actions as from observing the actions of others. Thus, the PDPM suggests that joint attention is an embodied form of cognition (Feldman & Narayanan, 2004). It is a developmental constructivist process whereby processing information from one’s own body (e.g. facial and eye direction) relative to the environment provides information about intentionality that contribute to the attribution of meaning to the perception of others behaviors. We have referred to this as the “inside out” processing assumption of the PDPM (Mundy & Vaughan, 2008).
The general tenor of these assumptions is nothing new. Bates et al., (1979) suggested that a sense of self-agency was essential to joint attention. More generally Piaget (1952) argued that infants do not learn through the passive perception of objects [or others] in the world. Rather, infants take action on objects and learn from their own [causal] actions. They then modify their actions, observe changes in causal relations, and learn new things about the physical world. Thus, Piaget viewed the processing of self-initiated actions on objects as a singularly important fuel for the engines of cognitive development. This constructivist viewpoint is now a mainstay of contemporary connectionist biological principles of typical and atypical neurocognitive development (e.g., Blakemore & Frith, 2003; Elman, 2005; Mareschal et al., 2007; Meltzoff, 2007; Quartz, 1999). Moreover, just as Piaget envisioned that infants learn about the physical world from their self-generated actions on objects, it is reasonable to think that infants learn about the social world from their self-generated actions with people. One type of self-generated action that may be developmentally key in this regard is active vision.
Active Vision and the PDPM
One of the first and most vitally informative types of actions infants take involves the self-control of their looking behaviors, or active vision. The science of vision has moved away from the study of “seeing” or passive visual perception, to the study of “looking” or intentional, active-vision and attention deployment (Findlay & Gilchrist, 2003). As previously noted active vision in infancy begins to develop at 3–4 months (e.g. Canfield & Kirkham, 2001; Johnson, 1990; 1995). It is key to the goal directed selection of information to process, and can elicit contingent social behavior responses from other people, such as parental smiles, vocalizations or gaze shifts. It also is one of the first types of volitional actions that infants use to control stimulation in order to self-regulate arousal and affect (Posner & Rothbart, 2007).
Vision and looking behavior have unique properties. Vision or at least the direction of gaze provides information regarding the relative spatial location of ourselves and other people. Moreover, direction of gaze conveys the distal and proximal spatial direction of our attention to others, and visa versa. Comparable information on the spatial direction of attention is not as clearly available from the other senses. This is especially true in the first 9 months of life, and for distal rather than proximal information. The importance of spatial relevance of visual information for the development of joint attention was emphasized by Butterworth and Jarrett (1991) in their influential article “What minds have in common is space.”
Primate eyes may be specialized for social spatial attention processing (e.g. Tomasello, Hare, Lehman, Call, 2006). Frontal binocular eye positions allow for enhanced spatial processing and depth of perception through parallax perception. Intricate musculatures allow for rapid visual focus on objects that are far or near. Equally important, relatively precise information about the spatial direction of attention is available from human eyes because of the highlighted contrast between the dark coloration of the pupil and iris versus the light to white coloration of the sclera. These unique qualities of human vision have been connected to theory regarding the phylogenetic and ontogenetic emergence of social-cognition (e.g. Tomasello et al., 2006).
It is also the case, though, that the characteristics of the human eye allow the saccades of infants to be readily observed by other people and, consequently, to act as elicitors of contingent social feedback. When infants shift attention to an object their parents may pick-up and show them the object. When infants shift attention to their parents’ eyes they may also receive a vocal or physical parental response. Thus, just as the characteristics of eyes make it easier for infants to perceive the attention of others, the signal value of eyes makes the active control of vision a likely nexus of information about infants’ own developing intentional agency. A corollary here is that a sense of visual self-agency may play a role in joint attention and social cognitive development.
The notion that active vision has primacy in social development relates back to the time honored observation that visual behavior is at least as important to human social development as physical contact (Rheingold, 1966; Robson 1967). However, the contemporary literature on social-cognitive development emphasizes only the importance of the information infants gather from processing the visual attention of others (e.g. Johnson et al., 2005). It neglects the potential importance of the information infants’ process about their own active vision, and socially contingent responses.
This aspect of the PDPM offers one explanation for why activation of the frontal eye-fields (a cortical area involved in volitional saccadic control) is a consistent significant correlate of social-cognition in imaging studies (Mundy, 2003). This is because the volitional control of active vision, via the frontal eye fields, may be central to developing an integrated sense of the relations between self-attention and other-attention, which is fundamental to joint attention and social cognition. This hypothesis leads to the testable prediction that the frontal eye fields should be less active in social-cognitive processing in older congenitally blind individuals than in sighted individuals (Mundy & Newell, 2007). If true this hypothesis may also help to explain some of the developmental commonalities observed for children with autism and blind infants (Bigelow, 2003; Hobson, Lee & Brown, 1999). This is not to say though congenital blindness leads to autism. It does not. It is just that some of the early transient developmental behavioral delays expressed by children with congenital blindness bear some similarity to the more chronic early behavioral manifestations of autism.
Dynamic Systems, Integrated Processing, and the PDPM
The PDPM emphasizes inside-out processing, constructivism and the role of active vision in the development of joint attention. However, it does not maintain that the inside-out processing of self-attention is more important for social-cognitive development than out-side in processing of other’s-attention. This is because social meaning, and even conscious self-awareness, may not be derived either from processing self-attention or others’-attention in isolation (Decety & Sommerville, 2003; Keyser & Perret, 2004; Vygotsky, 1962). Ontogeny and phylogeny may be best viewed as a dynamic system that, through interactions of multiple factors, over time and experience, coalesce into higher order integrations, structures and skills (e.g. Smith & Thelen, 2003). The development of joint attention, or the joint processing of the attention of self and other, likely reflects such a dynamic system. Its pertinence derives from the developmental synthesis of parallel processing of self attention and other attention. Consequently, it is not possible to comprehensively account for the role of joint attention in typical or atypical development with research or theory that focuses only on processing of others attention, or children’s processing of their own attention.
The co-active system of joint attention begins to synergize as frontal executive functions increasingly enable attention allocation to multiple sources of information during infancy. One definition of executive functions is that they involve the transmission of bias signals throughout neural network to selectively inhibit comparatively automatic behavioral responses in favor of more volitional, planned and goal-directed ideation and action in problem solving contexts (Miller & Cohen, 2001). These bias signals act as regulators affecting attention to task relevant response execution, as well as memory retrieval, emotional evaluation, etc. The aggregate effect of these bias signals is to guide the flow of neural activity along pathways that establish the proper mappings between inputs, internal states, and outputs needed to perform a given task more efficiently (Miller & Cohen, 2001). According to this definition joint attention development may be thought of as reflecting the emergence of frontal bias signals that establish the proper mappings across: 1) outside-in posterior cortical {temporal-precuneous} processing of inputs about the attention behaviors of other people and, 2) inside-out rostral-medial frontal cortical {BA 8–9, anterior cingulate} processing of internal states and outputs related to active vision, in order to 3) engage in the types of coordinated visual reference that support significant aspects of human social learning. This mapping results in the integrated development of a distributed anterior and posterior cortical joint attention system.
It is conceivable that the early establishment of this mapping of the joint processing of attention is formative with respect to the shared neural network of representations of self-other that Decety and Grezes (2006) suggest is essential to social-cognition. The integrated processing of self and other attention is also consistent with the ‘gateway” hypothesis that suggests that a primary function of the rostral, medial frontal cortex is to switch attention between self-generated and perceptual information for adequate social-cognitive performance (Gilbert & Burgess, 2008). The processing of self-other attention information in infancy may also play a role in what Keysers and Perrett (2004) have described as a Hebbian learning model of social-cognition. Neural networks that are repeatedly active at the same time become associated, such that activity [e.g. representations] in one network triggers activity in the other (Hebb, 1949). Keysers and Perrett suggest that common activation of neural networks for processing self-generated information and information about people is fundamental to understanding the actions of others. This Hebbian learning process is fundamental to the hypothesized functions of simulation (Gordon, 1986) and mirror neurons (i.e., Decety & Summerville, 2003; Williams, 2008) that are commonly invoked in current models of social-cognitive development.
The PDPM is consistent with these ideas and specifically suggests that Hebbian mapping in social-cognition begins with integrated rostral medial frontal processing of information about self-produced visual attention and posterior processing of the visual attention of others. Moreover, the PDPM specifically operationalizes the study of development of this dynamic mapping system in terms of psychometrically sound measures of early joint attention development (Mundy et al., 2007). Indeed, IJA assessments may be relatively powerful in research on social-cognitive development and autism, because they measure variance in the development of a whole dynamic system, rather than any one of its parts alone.
Once well practiced the joint processing of attention information requires less mental effort. As basic joint attention process is mastered and its ‘effort to engage’ goes down it can become integrated as an executive function that contributes to the initial development and increasing efficiency of social-cognitive problem solving. Thus, joint attention development may be envisioned as shifting from “learning to do” phase of development at 6 to 9 months to a “learning from” phase of development in the second year of life (Mundy & Vaughan, 2008, Figure 7). In the learning from phase the capacity to attend to multiple sources of information in “triadic attention” deployment becomes more common (Scaife & Bruner, 1975). Triadic attention contexts provide infants with rich opportunities to compare information about them gleaned through processing their own volitional visual attention deployment, and the processing of the visual attention of others in reference to a common third object or event. Through simulation (Gordon, 1986) infants may begin to impute that others have intentional control over their looking behavior that is similar to their own.
Figure 7.
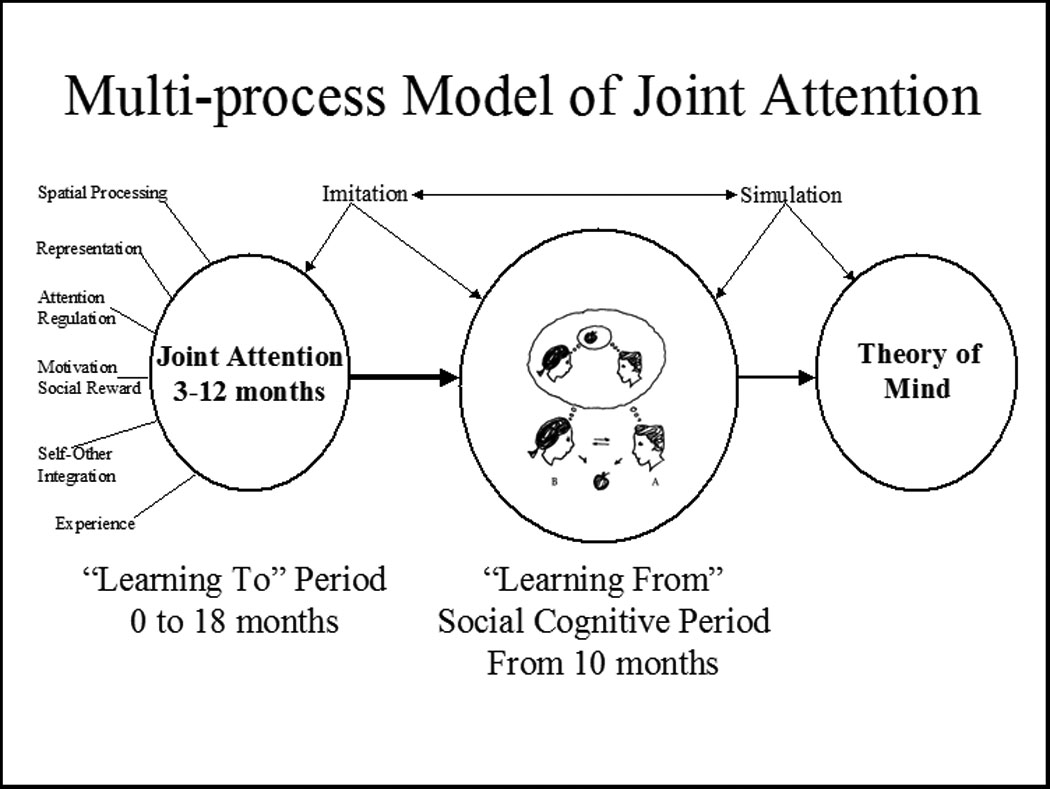
In the first year the development of joint attention involves the “learning to” integration of executive, motivation and imitation processes to support the routine, rapid and efficient (error free) execution of patterns of behavior that enable infants to coordination processing of overt aspects of visual self attention with processing of the social attention of other people. In the latter part of the first year and the second year infants can better monitor their own experiences and integrate it with information about the social partners during joint attention events. This provides a critical multi-modality source of information to the infants about the convergence and divergence of self and others experience and behavior during sharing information in social interactions. Theoretically, this provides the stage for the “learning from” phase of joint attention development. In this stage infants can control their attention to self organize and optimize information processing in social learning opportunities. The integration of anterior and posterior self-other attention processing (Fig. 5) provides a neural network that enriches encoding in social learning. The internalization of the overt joint processing of attention to the covert joint processing of attention to representations is part of an executive system that facilitates symbolic development and the social cognition. Indeed both symbolic thought and social cognition may be characterized by a transition from learning to socially coordinate overt attention to the capacity to socially coordinate covert mental representations of the attention of self and others.
The role of simulation in the learning from phase of joint attention development is well illustrated by a recent sequence of elegant experimental studies. Twelve-month-olds often follow the “gaze direction” of testers even if their eyes are closed. After 12 months, though, infants discriminate and follow the gaze of testers whose eyes are open, but not the gaze of testers whose eyes are closed. This suggests that infants’ understanding of the meaning of the eye gaze of others may improve in this period leading older infants to inhibit looking in the “eyes closed” condition (Brooks & Meltzoff, 2002).
To examine this interpretation Meltzoff and Brooks (2008) conducted an experimental intervention. They provided 12-month-olds with the experience of blindfolds that occluded their own looking behavior. After gaining that experience, 12-month-olds did not follow the head-turn of blindfolded testers, but did follow the head-turn and gaze of non-blindfolded testers. Meltzoff and Brooks also provided 18-month-olds with experience with blindfolds that looked opaque but were transparent when worn. After this condition the 18-month-olds reverted to following the gaze of blindfolded social partners. These data strongly suggest that the infants demonstrated inside-out learning and constructed social-cognitive awareness about the meaning of others’ gaze based on their own experience of the effects of blindfolds on active vision.
THE PARALLEL AND DISTRIBUTED PROCESSING MODEL AND AUTISM
Neural Connectivity & Activity Dependent Genes in Autism
Assumptions of the PDPM provide heuristics that bridge theory on the development of joint attention with phenomenon associated with the nature of autism. One of these is that the PDPM links theory and research on early social behavior impairments to neural connectivity models of the etiology of autism.
Over the last 10 years numerous research groups have proposed that problems in functional connectivity between brain regions contribute to the development of autism (e.g. Courchesne & Pierce 2005; Geschwind & Levitt, 2007; Horowitz et al., 1998; Just et al., 2004; Lewis & Elman, 2008; Wickelgren, 2005). However, rather than specific to autism, impaired connectivity has been posited to be central to many forms of mental retardation and developmental disorders (Dierssen & Ramakers, 2006). So, how do neurodevelopmental connectivity impairments lead to the specific social symptom impairments of autism, and how are these different from the connectivity impairments that characterize other developmental disorders?
One possibility is that mental retardation may be associated with connectivity impairments within proximal brain networks, but that autism may be characterized by distal connectivity problems (Courchesne & Pierce, 2005; Lewis & Elman, 2008). Indeed, several studies suggest that distal connectivity problems between frontal and temporal-parietal networks may be especially prominent in autism (Cherkassky, Kana, Keller & Just, 2006; Courchesne & Pierce, 2005; Wicker et al., 2008). The PDPM offers a moderately explicit developmental account of how the impairment of distal frontal-parietal pathways may have an early and robust effect specific to a disturbance of joint attention and related social symptom of autism such as a lack of spontaneously sharing experiences with other people. The PDPM’s focus on the fundamental relations between the joint processing of attention information, learning and symbolic development also provide a means for understanding why variations in the strength of the disturbance of anterior-posterior connectivity could contribute to phenotypic variability in autism such as the co-occurrence of mental retardation and or specific language impairments in some children.
The connectivity assumptions of PDPM also leads to the prediction that differences in the development of joint attention in typical and atypical children should be associated with measures of synchrony or coherence in cortical activity or development. There is some support for this, but currently available data are no more than suggestive in this regard (Mundy et al., 2000; 2003). Nevertheless, the PDPM offers a conceptual framework that emphasizes the benefits of a multidisciplinary approach to neurodevelopment, attention, connectionist network models of development and autism (Cohen, 2008; Lewis & Elman, 2008). This emphasis is in line with the recent call for a multidisciplinary examination of relations between EEG or imagining coherence in developmental as well as intervention studies of autism (Dawson, 2008).
It also may be that autism is associated with postnatal connectivity processes that are more activity dependent than is the case in mental (Morrow, Yoo, Flavell, Kim et al., 2008). Morrow et al., observed that the n expression of three genes associated with the two large deletions specific to families of children with autism (c3orf58, NHE9 and PCDH10) are regulated by neuronal activity. From this observation Morrow et al., (2008) raise the hypothesis that defects in activity-dependent gene expression may be a cause of cognitive deficits in autism. They note that these genes likely have a defined temporal course of vulnerability to atypical expression depending on the timing and quality of the young child’s postnatal activity and experience dependent processes.
The PDPM proposes that problems with initiating activity, specifically initiating joint attention activity may be especially key to understanding activity dependent alterations of gene expression associated with autism. Some evidence consistent with this proposition stems from the observation that activity of children with autism may affect and modify the attempts of caregivers to scaffold the development of joint attention in their children (Adamson et al., 2001). Indeed, if the PDPM is correct, it may be important to build an understanding of genetic influences on the typical range of expression individual differences in IJA in infant development. Little if any information currently exists on this topic. Recent observations, however, have revealed a surprising degree of variability and longitudinal stability among infants on IJA measures from 9 to 18 months of age. This suggests that these infant measures may have sufficient psychometric characteristics to make it possible to bring behavioral or metabolic genetic methods to bear in the study of sources of variance in early joint attention development (Mundy et al. 2007).
The PDPM, Visual Attention Control, and Autism
The work of several research groups indicate that basic mechanisms of visual control may play a role in autism (Brenner, Turner & Muller, 2007; Landry & Bryson, 2003; Johnson et al., 2005). Brenner et al., (2007) have noted that one of the essential issues for this line of research is to understand precisely, “how an ocular-motor system that is over-specialized for certain tasks and under-specialized for others early in life might affect later development in [social ] domains such as joint attention” (p. 1302). The PDPM offers a guide in this regard. First, it encourages the research community to recognize the possibility that joint attention may not be a “later” development, but one that begins as part of the development of volitional visual attention control by the 4th month of life. In addition, the PDPM provides one path for understanding how significantly altered early visual preferences could have a cascading effect on the development of intentional joint attention and autism (Mundy, 1995). Consider two recent studies in this regard.
McCleery et al., (2007) observed that magnocellular visual processing may remain atypically enhanced in a sample of 6-month-old infant siblings of children with autism. Similarly Karmel et al., (2008) observed visual attention patterns that are consistent with a magnocellular bias in 6-month-olds in neonatal intensive care who later received the diagnosis of autism at three years of age. The magnocellular visual system contributes to orienting based on movement and contrast-sensitivity related to small achromatic differences in brightness. This system dominates early visual orienting. However, by 2- to 4-months visually orienting becomes increasingly influenced by the parvocellular system, which contributes to orienting based on high-resolution information about shape, or low resolution information about color and shades of grey. The studies of Karmel et al., (2008) and McCleary et al., (2007) raise the possibility that a delay in the developmental shift from the magnocellular to parvocellular visual systems could alter what children with autism choose or prefer to attend to early in life.
Hypothetically, the maintenance of a magnocellular bias may lead to a relatively long standing visual preference for objects characterized by movement or achromatic contrasts such as surface edges, power lines, spinning objects, the outlines of faces, or mouth movement. Reciprocally, the decreased influence of the parvocellular system could lead to developmental delays in the emergence of a visual attention bias to targets that are socially informative but involve differentiation based on high resolution of shape and color information, such as may be involved in the distal processing of eyes and facial expressions. Thus, the alteration of visual preferences during early critical periods of development could degrade the establishment of the dynamic system of internal information processing about active looking, relative to contingent social feedback, and information about the attention of other people (Mundy 1995; Mundy & Burnette, 2005). Moreover, if magnocelluar guidance bias and connectivity impairments are orthogonal processes in the etiology of autism, combinations of varying levels of their effects could present as phenotypic differences in joint attention processing and social symptom expression, such as some children having difficulty in disengaging object attention and others have difficulty flexibly integrating aspects of social attention. Both problems could lead to a significant common pathway of developmental disturbance through disruption of the dynamic development of the joint attention system.
Early Intervention, Learning, Motivation and the PDPM
The PDPM may also help to explain why joint attention is a pivotal skill in early intervention for children with autism (Bruinsma, Koegel & Koegel, 2004; Charman, 2004; Mundy & Crowson, 1997). Improvements in pivotal skills, by definition, lead to positive changes in a broad array of other problematic behaviors. This appears to be the case with joint attention. It can be improved with early intervention (e.g., Kasari et al., 2006, 2007; Peirce & Schreibman, 1995; Rocha, et al., 2007; Yoder & Stone, 2006). Equally important, joint attention improvement has collateral benefits on language, cognitive and social development (Kasari et al., 2007; Jones, Carr & Feely, 2006; Whalen & Schreibman, 2006). The level of expression of joint attention behaviors at the beginning of intervention may also mediate responsiveness to early intervention in children with autism (Bono et al., 2004; Yoder & Stone, 2006).
According to the PDPM joint attention is a pivotal skill in autism because its improvement has multiple effects on social learning. Recall that joint attention facilitates the self-organization of information processing to optimize incidental as well as structured social learning opportunities (Baldwin, 1995). Hence, impairment in joint attention may be viewed as part of a broader social constructivist learning disturbance in autism. By the same token effective intervention likely improves social constructivist learning in autism.
Second, the PDPM proposes that joint attention serves as a foundation for social cognitive development. Social-cognitive development is defined in terms of advances in the comparative processing of information about self and other, rather than singularly in terms of changes in knowledge about intentionality. Following connectionist cognitive theory (McClelland & Rogers, 2003; Otten et al., 2001) an assumption of the PDPM is that whenever semantic information is acquired during social learning, it is also encoded in parallel with the activation of a frontal-temporal-parietal neural joint attention network that maps relations between representations of information about self-directed attention and information about the attention of other people. Thus, every time we process information in social learning we encode it as an activation pattern in a distributed semantic network in conjunction with an activation pattern of the anterior-posterior cortical joint attention network (see Fig. 4 & Fig. 5). Recall that deeper information processing and learning occurs best in the context of the simultaneous activation of multiple neural networks during encoding (Otten et al., 2001). If so, joint attention may lead to deeper processing because it adds activation of the distributed social attention network (a form of episodic encoding) to the network activation associated more directly with semantic information. This conjecture provides one interpretation of the observation that joint attention facilitates depth of processing in 9 month olds (Striano et al., 2006a,b). It also suggests that part of the learning disability of autism occurs because children with this disorder do not reap the full benefits of encoding semantic information in conjunction with episodic memory encoded within the integrated processing of self and other attention. This, in turn, may help to explain the attenuation of self-referenced memory effects in autism (Henderson et al., in press).
Third, a related proposition of the PDPM is that as overt joint attention becomes an increasingly internalized (representational) process it supports the social coordination of covert mental attention to cognitive representations. The coordination of covert attention among people to a specific mental representation (or categorical exemplar) is an essential element of symbolic thought (Tomasello et al., 2005). The PDPM assumes that months of practice of the social coordination of overt attention (i.e. joint attention) in the first years of life is required before we can internalized and transformed this function to the social coordination of covert mental attention to common representations signified by symbols. Thus, symbolic thought processes incorporates, indeed may require the activation of the self-other joint attention system. Joint attention on the other hand does not necessarily involve symbolic process (Mundy et al., 1987). Consistent with this observation joint attention is a unique predictor of pretend play development in children with autism relative to measures of imitation or executive functions (Rutherford et al., 2007). Also symbolic play intervention may have a greater impact on joint attention development than joint attention intervention may have on symbolic play development in young children with autism (Kasari et al. 2006).
Fourth the joint processing of attention information also plays a fundamental role in social cognition defined in terms of the development of knowledge about intentions in self and other (Mundy & Newell, 2007). The assumption here is that when infants or primates practice monitoring others attention (RJA), statistical learning ultimately leads to the associative rule ‘where others’ eyes go, their behavior follows’ (Jellema, Baker, Wicker, & Perrett, D., 2000). Similarly, anterior monitoring or self-awareness of control of visual attention likely leads awareness of the self-referenced associative rule that ‘where my eyes go, my intended behavior follows’ (Mundy & Newell, 2007). An integration of the development of these ‘concepts’ leads to the logical cognitive output ‘where others eyes go → their intended behavior follows’, which is a building block of social cognitive development (Mundy & Newell, 2007). Social-cognition of this kind is thought to enable new and more efficient levels of social or cultural learning, which is atypical in autism.
Finally, the constructivist assumptions of the PDPM stress that motivation factors are part of a crucial fifth path of association between joint attention and social learning. Initiating joint attention requires “choosing” between behavior goals, such as fixated looking at an event, or alternating looking to the event and another person. Choice among behavior goals is thought to involve frontal and medial cortical processing of the relative reward associated with different goals (e.g. Holroyd & Coles, 2002). Therefore, IJA impairment in autism may be related to deficits in bio-behavioral processes associated with reward sensitivity and motivation (Dawson, 2008; Kasari et al., 1990; Mundy, 1995). Alternatively, the less volitional characteristics of the posterior attention system suggest that motivation processes may be less likely to contribute to RJA or gaze following problems in the development of autism (Mundy, 1995).
The motivational tendencies involved in IJA deficits in autism could be expressed in one of three basic patterns. Social stimuli could be aversive for children with autism and, therefore, these children less frequently spontaneously look to others to share interest in objects or events. However, the aversion hypothesis is complicated by observations of behaviors indicative of relatively intact caregiver-attachment in many children with autism and a willingness to engage in playful physical interactions with strangers (e.g. Mundy et al., 1986; Sigman & Underer, 1984). On the other hand, social stimuli may not be aversive. Rather, they may simply not be as rewarding as may be the case in typical development leading to a relative attenuation of social-orienting and joint attention early in the life of children with autism (Dawson et al., 1998; Mundy, 1995). Finally, social stimuli could have a positive valence for children with autism, but be overshadowed by an atypically strong visual preference that make objects rather that social elements of the world more “interesting” (Karmel et al., 2008; McCleary et al., 2006; Mundy & Crowson, 1997).
The construction of effective empirical approaches to address these alternatives is one of the current, outstanding challenges in the science of autism (Dawson, 2008; Koegel et al., 2003). Research on joint attention in relation to motivation and the perceived valence of objects in adults (Bayliss et al., 2006) offers one potential route for developmental and functional neurocognitive studies on this topic. Bayliss et al. (2006) have shown that looking pictures with another person lends a more positive perceived valence to the pictures than when looking at pictures in conjunction with directional arrows. First it could be useful to determine it this phenomenon is evident among people with autism or related to symptom presentation. If not present, or if individual differences are related to symptom presentation, it may be possible and useful to examine the cortical and/or striatal systems involved in preference for stimuli viewed in joint attention versus non-joint attention conditions in people with autism versus comparison groups. Alternatively, the literature on early intervention may be the best source of information on motivation and its role in joint attention development in autism. Early interventions studies offer some of the most systematic investigations to date of how to structure social engagements with young children with autism to modify and increase their motivation to initiate episodes of shared attention and shared experience with others (e.g., Kasari et al., 2006, 2007).
In conclusion, only in its most expansive or grandiose interpretation can the PDPM be viewed as an explanatory model of joint attention, or autism. Nevertheless, the PDPM does serve a purpose. It presents a new perspective on joint attention that suggests its impairment in autism is more than an epiphenomenon associated with other fundamental precursor or successor processes. The alternative perspective of the PDPM can be summed up in terms of several general principles. First, autism is as much about impairments in self-generated constructivist activity, as it is about problems in perceiving or responding to the behavior of others. Hence, we need to consider the neurodevelopments involved in initiating behavior and attention control, as well as those involved in perceiving and responding to the behaviors of others to understand this disorder (Mundy, 2003). Second, joint attention and social-cognition are forms of information processing that give rise to knowledge, but their development may not be wholly defined in terms of stages of knowledge acquisition. Third, joint attention is a form of parallel processing because it involves the conjoint perception and analysis of information about self attention and the attention of other people. This conjoint analysis of information involves distributed processing across an anterior cortical system for processing internal information about goal directed attention, and a posterior cortical system for processing external information about the attention related behavior of other people. This observation encourages the adoption of a multidisciplinary approach to better link constructivist, connectionist neuroscience with parallel and distributed cognitive processing models to better understand the specific nature of the social impairments of autism.
Acknowledgments
The research and theory development reported in this paper were supported by NIH Grants HD 38052, MH 071273 as well as the Lisa Capps Chair Endowment Fund to the UC Davis MIND Institute and School of Education.
References
- American Psychiatric Association. Diagnostic and statistical manual on mental disorders. 4th ed. Washington, DC: Author; 1994. [Google Scholar]
- Adamson L, Bakeman R, Dekner D. The development of symbol infused joint engagement. Child Development. 2004;75:1171–1187. doi: 10.1111/j.1467-8624.2004.00732.x. [DOI] [PubMed] [Google Scholar]
- Adamson L, McArthur D, Markov Y, Dunbar B, Bakeman R. Autism and joint attention: Young children’s responses to maternal bids. Applied Developmental Psychology. 2001;22:439–453. [Google Scholar]
- Astafiev S, Shulman G, Stanley C, Snyder A, Essen D, Corbetta M. Functional organization of human intraparietal and frontal cortex for attending, looking and pointing. J. of Neuroscience. 2003;23:4689–4699. doi: 10.1523/JNEUROSCI.23-11-04689.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson J, Hood B, Wattam-Bell J, Braddick O. Changes in infants’ ability to switch attention in the first three months of life. Perception. 1992;21:643–653. doi: 10.1068/p210643. [DOI] [PubMed] [Google Scholar]
- Baldwin D. Understanding the link between joint attention and language. In: Moore C, Dunham P, editors. Joint Attention: Its origins and role in development. Hillsdale, NJ: Erlbaum; 1995. pp. 131–158. [Google Scholar]
- Baron-Cohen S. Joint attention deficits in autism: Towards a cognitive analysis. Development and Psychopathology. 1989;3:185–190. [Google Scholar]
- Baron-Cohen S. Mindblindness. Cambridge, MA: MIT Press; 1995. [Google Scholar]
- Bates E. Language and context: The acquisition of performatives. New York: Academic Press; 1976. [Google Scholar]
- Bates E, Benigni L, Bretherton I, Camaioni L, Volterrra V. The emergence of symbols: Cognition and communication in infancy. New York: Academic Press; 1979. [Google Scholar]
- Bayliss A, Paul M, Cannon P, Tipper S. Gaze cuing and affective judgments of objects. I like what you look at. Psychonomic Bulletin and Review. 2006;13:1061–1066. doi: 10.3758/bf03213926. [DOI] [PubMed] [Google Scholar]
- Bigelow A. The development of joint attention in blind infants. Development & Psychopathology. 2003;15:259–275. doi: 10.1017/s0954579403000142. [DOI] [PubMed] [Google Scholar]
- Blakemore S, Frith C. Self awareness and action. Current Opinion in Neurobiology. 2003;13:219–224. doi: 10.1016/s0959-4388(03)00043-6. [DOI] [PubMed] [Google Scholar]
- Bono M, Daley T, Sigman M. Joint attention moderates the relation between intervention and language development in young children with autism. Journal of Autism and Related Disorders. 2004;34:495–505. doi: 10.1007/s10803-004-2545-x. [DOI] [PubMed] [Google Scholar]
- Brenner L, Turner K, Muller R. Eye movement and visual search: Are there elementary abnormalities in autism. Journal of Autism and Developmental Disorders. 2007;37:1289–1309. doi: 10.1007/s10803-006-0277-9. [DOI] [PubMed] [Google Scholar]
- Bruinsma Y, Koegle R, Koegle L. Joint attention and children with autism: a review of the literature. Mental Retardation and Developmental Disabilities Research Reviews. 2004;10:169–175. doi: 10.1002/mrdd.20036. [DOI] [PubMed] [Google Scholar]
- Brooks R, Meltzoff A. The importance of eyes: How infants interpret adult looking behavior. Developmental Psychology. 2002;38:958–966. doi: 10.1037//0012-1649.38.6.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner JS. From communication to language: A psychological perspective. Cognition. 1975;3:255–287. [Google Scholar]
- Bruner JS. From joint attention to the meeting of minds: An Introduction. In: Moore C, Dunham PJ, editors. Joint Attention: Its Origins and Role in Development. Hillsdale, NJ: Lawrence Erlbaum, Asso.; 1995. pp. 1–14. [Google Scholar]
- Butterworth G, Jarrett N. What minds have in common is space: Spatial mechanisms in serving joint visual attention in infancy. British Journal of Developmental Psychology. 1991;9:55–72. [Google Scholar]
- Canfield R, Kirkham N. Infant cortical development and the prospective control of saccadic eye movements. Infancy. 2001;2:197–211. [Google Scholar]
- Caplan R, Chugani H, Messa C, Guthrie D, Sigman M, Traversay J, et al. Hemispherectomy for early onset intractable seizures: presurgical cerebral glucose metabolism and postsurgical nonverbal communication patterns. Developmental Medicine and Child Neurology. 1993;35:574–581. doi: 10.1111/j.1469-8749.1993.tb11695.x. [DOI] [PubMed] [Google Scholar]
- Cavanna A, Trimble M. The precuneous: a review of its functional anatomy and behavioural correlates. Brain. 2006;10:1–20. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Charman T. Why is joint attention a pivotal skill in autism? Philosophical Transactions of the Royal Society of London. 2004;358:315–324. doi: 10.1098/rstb.2002.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charman T, Baron-Cohen S, Swettenham J, Baird G, Cox A, Drew A. Testing joint attention, imitation, and play infancy precursors to language and theory of mind. Cognitive Development. 2000;15:481–498. [Google Scholar]
- Charwarska K, Klin A, Volkmar F. Automatic attention cuing through eye movement in 2-year-old-children with autism. Child Development. 2003;74:1108–1122. doi: 10.1111/1467-8624.00595. [DOI] [PubMed] [Google Scholar]
- Cherkassky V, Kana R, Keller T, Just M. Functional connectivity in the baseline resting state network in autism. Neuroreport. 2006;17:1687–1690. doi: 10.1097/01.wnr.0000239956.45448.4c. [DOI] [PubMed] [Google Scholar]
- Claussen A, Mundy P, Malik S, Willoughby J. Joint attention and disorganized attachment status in at risk infants. Development and Psychopathology. 2002;14:279–292. doi: 10.1017/s0954579402002055. [DOI] [PubMed] [Google Scholar]
- Cohen I. A neural network model of autism: implication for theory and treatment. In: Mareschal D, Sylvain S, Westerman G, editors. Neuroconstructivism II: Perspectives and Prospects. New York, NY: Oxford University Press; 2007. pp. 231–264. [Google Scholar]
- Courschesne E, Pierce K. Why the frontal cortex in autism might be talking only to itself: Local over connectivity but long distance disconnection. Current Opinion in Neurology. 2005;15:225–230. doi: 10.1016/j.conb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Curcio F. Sensorimotor functioning and communication in mute autistic children. Journal of Autism and Developmental Disorders. 1978;8:281–292. doi: 10.1007/BF01539631. [DOI] [PubMed] [Google Scholar]
- Dawson G. Early behavioral intervention, brain plasticity and the prevention of autism spectrum disorders. Development & Psychopathology. 2008;20:775–804. doi: 10.1017/S0954579408000370. [DOI] [PubMed] [Google Scholar]
- Dawson G, Meltzoff A, Osterling J, Rinalidi J, Brown E. Children with autism fail to orient to naturally occurring social stimuli. Journal of Autism and Developmental Disorders. 1998;28:479–485. doi: 10.1023/a:1026043926488. [DOI] [PubMed] [Google Scholar]
- Dawson G, Toth K, Abbott R, Osterling J, Munson J, Estes A, et al. Early social attention impairments in autism: Social orienting, joint attention, and attention in autism. Developmental Psychology. 2004;40:271–283. doi: 10.1037/0012-1649.40.2.271. [DOI] [PubMed] [Google Scholar]
- Dawson G, Webb S, Schellenberg G, Dager S, Friedman S, Ayland E, et al. Defining the broader phenotype of autism: Genetic, brain, and behavioral perspectives. Development and Psychopathology. 2002;14:581–612. doi: 10.1017/s0954579402003103. [DOI] [PubMed] [Google Scholar]
- Dierson M, Ramakers G. Dendritic pathology in mental retardation: form molecular genetics to neurobiology. Genes, Brain and Behavior. 2006;5:48–60. doi: 10.1111/j.1601-183X.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- Decety J, Sommerville J. Shared representations between self and other: a social cognitive neuroscience view. Trends in Cognitive Sciences. 2003;7:527–533. doi: 10.1016/j.tics.2003.10.004. [DOI] [PubMed] [Google Scholar]
- D’Entremont B, Hains S, Muir D. A demonstration of gaze following in 3-to 6-month-olds. Infant Behavior and Development. 1997;20:569–572. [Google Scholar]
- Dosenbach N, Fair D, Miezin F, Cohen A, Wenger K, et al. Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman J. Connectionist models of cognitive development: where next. Trends in Cognitive Science. 2005;9:111–117. doi: 10.1016/j.tics.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Emery N. The eyes have it: the neuroethology, function, and evolution of social gaze. Neuroscience and Biobehavioral Reviews. 2000;24:581–604. doi: 10.1016/s0149-7634(00)00025-7. [DOI] [PubMed] [Google Scholar]
- Farroni T, Massaccesi S, Francesca S. Can the direction of gaze of another person shift the attention of a neonate? Giornole-Italiano-di-Psicologia. 2002;29:857–864. [Google Scholar]
- Feldman J, Narayanan S. Embodied meaning in a neural theory of language. Brain & Language. 2004;89:385–392. doi: 10.1016/S0093-934X(03)00355-9. [DOI] [PubMed] [Google Scholar]
- Findlay J, Gilchrist I. Active vision: The psychology of looking and seeing. New York, NY: Oxford University Press; 2003. [Google Scholar]
- Frieschen A, Bayliss A, Tipper S. Gaze cueing of attention: Visual attention, social cognition and individual differences. Psychological Bulletin. 2007;133:694–724. doi: 10.1037/0033-2909.133.4.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster J. The cognit: A network model of cortical representation. International Journal of Psychophysiology. 2006;60:125–132. doi: 10.1016/j.ijpsycho.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Geswind D, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Current Opinion in Neurobiology. 2007;17:103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Gilbert S, Burgess P. Social and nonsocial functions of rostral prefrontal cortex: Implications for education. Mind, Brain & Education. 2008;2:148–156. [Google Scholar]
- Gordon R. Folk psychology as simulation. Mind and Language. 1986;1:158–171. [Google Scholar]
- Griffith E, Pennington B, Wehner E, Rogers S. Executive functions in young children with autism. Child Development. 1999;70:817–832. doi: 10.1111/1467-8624.00059. [DOI] [PubMed] [Google Scholar]
- Henderson H, Zahka N, Kojkowski N, Inge A, Schwarz C, Hileman C, et al. Self referenced memory, social cognition and symptom presentation in autism. Journal of Child Psychology & Psychiatry. doi: 10.1111/j.1469-7610.2008.02059.x. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L, Yoder P, Yale M, McDuffie A. Getting the point: Electrophysiological correlates of protodeclarative pointing. International Journal of Developmental Neuroscience. 2002;20:449–458. doi: 10.1016/s0736-5748(02)00038-2. [DOI] [PubMed] [Google Scholar]
- Hebb D. The Organization of Behaviour. New York, NY: Wiley; 1949. [Google Scholar]
- Hobson J, Hobson RP. Identification: The missing link between joint attention and imitation. Development and Psychopathology. 2007;19:411–431. doi: 10.1017/S0954579407070204. [DOI] [PubMed] [Google Scholar]
- Hobson RP, Lee A, Brown R. Autism and congenital blindness. Journal of Autism and Developmental Disorders. 1999;12:45–66. doi: 10.1023/a:1025918616111. [DOI] [PubMed] [Google Scholar]
- Holroyd C, Coles M. The neural basis of human error processing: Reinforcement learning, dopamine and the error related negativity. Psychological Review. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Hood B, Willen J, Driver J. Adult’s eyes trigger shifts of visual attention in human infants. Psychological Science. 1998;9:131–134. [Google Scholar]
- Horwitz B, Rumsey J, Grady C, Rapoport S. The cerebral metabolic landscape in autism. Intercorrelations of regional glucose utilization. Archives of Neurology. 1988;45:749–755. doi: 10.1001/archneur.1988.00520310055018. [DOI] [PubMed] [Google Scholar]
- Jellema T, Baker C, Wicker B, Perrett D. Neural representation for the perception of intentionality of actions. Brain and Cognition. 2000;44:280–302. doi: 10.1006/brcg.2000.1231. [DOI] [PubMed] [Google Scholar]
- Johnson M. Cortical maturation and the development of visual attention in early infancy. Journal of Cognitive Neuroscience. 1990;2:81–95. doi: 10.1162/jocn.1990.2.2.81. [DOI] [PubMed] [Google Scholar]
- Johnson M. The inhibition of automatic saccades in early infancy. Developmental Psychobiology. 1995;28:281–291. doi: 10.1002/dev.420280504. [DOI] [PubMed] [Google Scholar]
- Johnson M, Griffin R, Csibra G, Halit H, Farronni T, et al. The emergence of the social brain network: Evidence from typical and atypical development. Development and Psychopathology. 2005;17:599–619. doi: 10.1017/S0954579405050297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E, Carr E, Feeley K. Multiple effects of joint attention intervention for children with autism. Behavior Modification. 2006;30:782–834. doi: 10.1177/0145445506289392. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky V, Keller T, Minshew N. Cortical activation and synchronization during sentence comprehension in high functioning autism: evidence of underconnectivity. Brain. 2004;127:1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Karmel B, Gardner J, Swensen L, Lennon E, London E. Contrasts of Medical and Behavioral Data from NICU Infants Suspect and Non-Suspect for Autism Spectrum Disorder (ASD). Paper presented at the International Conference on Infant Studies; Vancouver. 2008. Mar, [Google Scholar]
- Kasari C, Sigman M, Mundy P, Yirmiya N. Affective sharing in the context of joint attention interactions of normal, autistic, and mentally retarded children. Journal of Autism and Developmental Disorders. 1990;20:87–100. doi: 10.1007/BF02206859. [DOI] [PubMed] [Google Scholar]
- Kasari C, Freeman S, Paparella T. Joint attention and symbolic play in young children with autism: a randomized controlled intervention study. Journal of Child Psychology and Psychiatry. 2006;47:611–620. doi: 10.1111/j.1469-7610.2005.01567.x. [DOI] [PubMed] [Google Scholar]
- Kasari C, Freeman S, Paparella T. The UCLA RCT on Play and Joint Attention. Paper presented at the Biennial Conference of the Society for Research on Child Development; Boston, MA. 2007. Apr, [Google Scholar]
- Keysers C, Perrett D. Demystifying social-cognition: A Hebbian perspective. Trends in Cognitive Science. 2006;8:501–507. doi: 10.1016/j.tics.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Kylliainen A, Hietanen J. Attention orienting by another’s gaze direction in children with autism. Journal of Child Psychology and Psychiatry. 2004;45:435–444. doi: 10.1111/j.1469-7610.2004.00235.x. [DOI] [PubMed] [Google Scholar]
- Knudson E. Sensitive periods in the development of the brain and behavior. Journal of Cognitive Neuroscience. 2004;16:1412–1425. doi: 10.1162/0898929042304796. [DOI] [PubMed] [Google Scholar]
- Koegel L, Carter C, Koegel R. Teaching children with autism self-initiations as a pivotal response. Topics in Language Disorders. 2003;23:134–145. [Google Scholar]
- Landry R, Bryson S. Impaired disengagement of attention in young children with autism. Journal of Child psychology and Psychiatry. 2004;45:115–1122. doi: 10.1111/j.1469-7610.2004.00304.x. [DOI] [PubMed] [Google Scholar]
- Leekham S, Hunnisett E, Moore C. Targets and cues: Gaze following in children with autism. Journal of Child Psychology and Psychiatry. 1998;39:951–962. [PubMed] [Google Scholar]
- Lewis J, Elman J. Growth-related neural organization and the autism phenotype: a test of the hypothesis that altered brain growth leads to altered connectivity. Developmental Science. 2008;11:135–155. doi: 10.1111/j.1467-7687.2007.00634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lond D, Hikosaka O. Comparison of reward modulation in the frontal eye field and caudate of the macaque. Journal of Neuroscience. 2006;26:6695–6703. doi: 10.1523/JNEUROSCI.0836-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Floody H, Anderson D, Pickles A. Social engagement in very young children with autism: Differences across contexts. Tampa, FL: The Society for Research in Child Development; 2003. Apr, [Google Scholar]
- Lord C, Lambrecht L, Cook E, Leventhal B, DiLavore P, et al. The Autism Diagnostic Observation Schedule-Generic: A standard measure of social communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Loveland K, Landry S. Joint attention and language in autism and developmental language delay. Journal of Autism and Developmental Disorders. 1986;16:335–349. doi: 10.1007/BF01531663. [DOI] [PubMed] [Google Scholar]
- Mareschal D, Johnson M, Sirois S, Spratling S, Thomas M, Wasserman G. Neuroconstructivism I: How the brain constructs cognition. New York, NY: Oxford University Press; 2007. [Google Scholar]
- Materna S, Dicke P, Thern P. Dissociable roles of the superior-temporal sulcus and the intraparietal sulcus in joint attention: A functional magnetic resonance imaging study. Journal of Cognitive Neuroscience. 2008;20:108–119. doi: 10.1162/jocn.2008.20.1.108. [DOI] [PubMed] [Google Scholar]
- McClelland J, Rogers T. The parallel distributed processing approach to semantic cognition. Nature Reviews: Neuroscience. 2003;4:310–322. doi: 10.1038/nrn1076. [DOI] [PubMed] [Google Scholar]
- McCleery J, Allman E, Carver L, Dobkins K. Abnormal magnocellular pathway visual processing in infants at risk for autism. Biological Psychiatry. 2007;62:1007–1014. doi: 10.1016/j.biopsych.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Meltzoff A. ‘Like me’: a foundation for social cognition. Developmental Science. 2007;10:126–134. doi: 10.1111/j.1467-7687.2007.00574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff A, Brooks R. Self experiences a mechanism for learning about others: A training study in social cognition. Developmental Psychology. 2008;44:1–9. doi: 10.1037/a0012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E, Cohen J. An integrative theory of prefrontal cortex functioning. Annual Review of Neurosciences. 2001;24:167–2002. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Morales M, Mundy P, Rojas J. Following the direction of gaze and language development in 6-month olds. Infant Behavior and Development. 1998;21:373–377. [Google Scholar]
- Morrow E, Yoo S, Flavell S, Kim T, Lin Y. Identifying autism loci and genes by tracing recent shared ancestry. Science. 2008;32:218–225. doi: 10.1126/science.1157657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munakata Y, McClelland J. Connectionist models of development. Developmental Science. 2003;6:413–429. [Google Scholar]
- Mundy P. Joint attention and social-emotional approach behavior in children with autism. Development and Psychopathology. 1995;7:63–82. [Google Scholar]
- Mundy P. The neural basis of social impairments in autism: the role of the dorsal medial-frontal cortex and anterior cingulate system. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2003;44:793–809. doi: 10.1111/1469-7610.00165. [DOI] [PubMed] [Google Scholar]
- Mundy P. Joint Attention and Our Sharing Minds. New York: Guilford Publications, Inc.; (in preparation). [Google Scholar]
- Mundy P, Burnette C. Joint attention and neurodevelopment. In: Volkmar F, Klin A, Paul R, editors. Handbook of Autism and Pervasive Developmental Disorders. 3rd ed. Hoboken, NJ: John Wiley; 2005. pp. 650–681. [Google Scholar]
- Mundy P, Card J, Fox N. EEG correlates of the development of infant joint attention skills. Developmental Psychobiology. 2000;36:325–338. [PubMed] [Google Scholar]
- Mundy P, Crowson M. Joint attention and early social communication: Implications for research on intervention with autism. Journal of Autism and Developmental Disorders. 1997;27:653–676. doi: 10.1023/a:1025802832021. [DOI] [PubMed] [Google Scholar]
- Mundy P, Fox N, Card Joint attention, EEG coherence and early vocabulary development. Developmental Science. 2003;6:48–54. [Google Scholar]
- Mundy P, Hogan A. Intersubjectivity, joint attention and autistic developmental pathology. In: Cicchetti D, Toth S, editors. Rochester Symposium of Developmental Psychopathology, Vol. 5, A developmental perspective on the self and its disorders. Hillsdale, N.J: Lawrence Erlbaum; 1994. pp. 1–30. [Google Scholar]
- Mundy P, Kasari C, Sigman M. Nonverbal communication, affective sharing, and intersubjectivity. Infant Behavior and Development. 1992;15:377–381. [Google Scholar]
- Mundy P, Newell L. Attention, joint attention and social cognition. Current Directions in Psychological Science. 2007;16:269–274. doi: 10.1111/j.1467-8721.2007.00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy P, Sigman M. Theoretical implications of joint attention deficits in autism. Development and Psychopathology. 1989;1:173–184. [Google Scholar]
- Mundy P, Sigman M. joint attention, social competence and developmental psychopathology. In: Cicchetti D, Cohen D, editors. Developmental Psychopathology: Vol. 1. Theory and Methods. 2nd ed. Hoboken, NJ: Wiley; 2006. pp. 293–332. [Google Scholar]
- Mundy P, Sigman M, Kasari C. The autistic person’s theory of mind and early nonverbal joint attention deficits. In: Baron-Cohen S, Tager- Flusberg H, Cohen D, Volkmar F, editors. Understanding other minds: Perspectives from autism. Oxford, UK: Oxford University Press; 1993. pp. 181–201. [Google Scholar]
- Mundy P, Sigman M, Kasari C. Joint attention, developmental level, and symptom presentation in children with autism. Development and Psychopathology. 1994;6:389–401. [Google Scholar]
- Mundy P, Sigman M, Ungerer J, Sherman T. Defining the social deficits of autism: The contribution of nonverbal communication measures. Journal of Child Psychology and Psychiatry. 1986;27:657–669. doi: 10.1111/j.1469-7610.1986.tb00190.x. [DOI] [PubMed] [Google Scholar]
- Mundy P, Sigman M, Ungerer J, Sherman T. Nonverbal communication and play correlates of language development in autistic children. Journal of Autism and Developmental Disorders. 1987;17:349–364. doi: 10.1007/BF01487065. [DOI] [PubMed] [Google Scholar]
- Mundy P, Block J, Vaughan Van Hecke A, Delgadoa C, Venezia Parlade M, Pomares Y. Individual differences and the development of infant joint attention. Child Development. 2007;78:938–954. doi: 10.1111/j.1467-8624.2007.01042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy P, Vaughan Van Hecke A. Neural Systems, Gaze Following and the Development of Joint Attention. In: Nelson C, Luciana M, editors. Handbook of Developmental Cognitive Neuroscience. New York: Oxford University Press; 2008. pp. 819–837. [Google Scholar]
- Naber F, Bakermans-Kranenburg M, van Ijzendoorn M, Dietz C, Daalen E, et al. Joint attention development in toddlers with autism. European Child & Adolescent Psychiatry. 2008;17:143–152. doi: 10.1007/s00787-007-0648-6. [DOI] [PubMed] [Google Scholar]
- Nation K, Penny S. Sensitivity to eye gaze in autism: Is it normal? Is it automatic? Is it social? Development and Psychopathology. 2008;20:79–97. doi: 10.1017/S0954579408000047. [DOI] [PubMed] [Google Scholar]
- Nathan M, Eilam B, Kim S. To disagree we must all agree: How intersubjectivity structures and perpetuates discourse in a mathematics classroom. Journal of Learning Sciences. 2007;16:523–563. [Google Scholar]
- Nichols KE, Fox N, Mundy P. Joint attention, self-recognition and neurocognitive functioning. Infancy. 2005;7:35–51. doi: 10.1207/s15327078in0701_4. [DOI] [PubMed] [Google Scholar]
- Otten L, Henson R, Rugg M. Depth of processing effects on neural correlates of memory encoding. Brain. 2001;125:399–412. doi: 10.1093/brain/124.2.399. [DOI] [PubMed] [Google Scholar]
- Pierce K, Schreibman L. Increasing complex social behaviors in children with autism: Effects of peer implemented pivotal response training. Journal of Applied Behavior Analysis. 1995;28:285–295. doi: 10.1901/jaba.1995.28-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piaget J. The origins of intelligence in children. New York, NY: Norton; 1952. [Google Scholar]
- Posner M, Rothbart M. Research on attention networks as a model for the integration of psychological science. Annual Review of Psychology. 2007;58:1–23. doi: 10.1146/annurev.psych.58.110405.085516. [DOI] [PubMed] [Google Scholar]
- Quartz S. The Constructivist Brain. Trends in Cognitive Science. 1999;3:48–57. doi: 10.1016/s1364-6613(98)01270-4. [DOI] [PubMed] [Google Scholar]
- Ramnani N, Behrens T, Penny W, Matthews P. New approaches for exploring anatomical and functional connectivity in the human brain. Biological Psychiatry. 2004;56:613–619. doi: 10.1016/j.biopsych.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Rheingold H. The development of social behavior in the human infant. Monographs of the Society for Research in Child Development. 1966;31:1–17. [PubMed] [Google Scholar]
- Robson K. The role of eye-to-eye contact in maternal infant attachment. Journal of Child Psychology and Psychiatry. 1967;8:13–25. doi: 10.1111/j.1469-7610.1967.tb02176.x. [DOI] [PubMed] [Google Scholar]
- Rocha M, Schriebman L, Stahmer A. Effectiveness of training parents to teach joint attention with children with autism. Journal of Early Intervention. 2007;29:154–172. [Google Scholar]
- Royers H, Van Oost P, Bothutne S. Immediate imitation and joint attention in young children with autism. Development and Psychopathology. 1998;10:441–450. doi: 10.1017/s0954579498001680. [DOI] [PubMed] [Google Scholar]
- Rutherford M, Young G, Hepburn S, Rogers S. A longitudinal study of pretend play in Autism. Journal of Autism and Developmental Disorders. 2008;37:1024–1039. doi: 10.1007/s10803-006-0240-9. [DOI] [PubMed] [Google Scholar]
- Scaife M, Bruner J. The capacity for joint visual attention in the infant. Nature. 1975;253:265–266. doi: 10.1038/253265a0. [DOI] [PubMed] [Google Scholar]
- Sheinkopf S, Mundy P, Claussen A, Willoughby J. Infant joint attention skill and preschool behavioral outcomes in at-risk children. Development and Psychopathology. 2004;16:273–293. doi: 10.1017/s0954579404044517. [DOI] [PubMed] [Google Scholar]
- Seibert JM, Hogan AE, Mundy PC. Assessing interactional competencies: The Early Social- Communication Scales. Infant Mental Health Journal. 1982;3:244–258. [Google Scholar]
- Sigman M, McGovern C. Improvements in cognitive and language skills from preschool to adolescence in autism. Journal of Autism and Developmental Disorders. 2005;35:15–23. doi: 10.1007/s10803-004-1027-5. [DOI] [PubMed] [Google Scholar]
- Sigman M, Ruskin E. Continuity and change in the social competence of children with autism, Down syndrome, and developmental delays. Monographs of the Society for Research in Child Development. 1999;64 doi: 10.1111/1540-5834.00002. (1, Serial No. 256). [DOI] [PubMed] [Google Scholar]
- Sigman M, Ungerer J. Attachment behaviors in autistic children. Journal of Autism and Related Disabilities. 1984;14:231–244. doi: 10.1007/BF02409576. [DOI] [PubMed] [Google Scholar]
- Slaughter V, McConnell D. Emergence of joint attention: Relationships between gaze following, social referencing, imitation, and naming in infancy. Journal of Genetic Psychology. 2003;164:54–71. doi: 10.1080/00221320309597503. [DOI] [PubMed] [Google Scholar]
- Smith V, Mirenda P, Zaidman-Zait A. Predictors of expressive vocabulary growth in children with autism. Journal of Speech,Language and Hearing Research. 2007;50:149–160. doi: 10.1044/1092-4388(2007/013). [DOI] [PubMed] [Google Scholar]
- Smith L, Thelen E. Development as a dynamic system. Trends in Cognitive Science. 2003;7:343–348. doi: 10.1016/s1364-6613(03)00156-6. [DOI] [PubMed] [Google Scholar]
- Smith L, Ulvund L. The role of joint attention in later development among preterm children: Linkages between early and middle childhood. Social Development. 2003;12:222–234. [Google Scholar]
- Striano T, Stahl D. Sensitivity to triadic attention in early infancy. Developmental Science. 2005;8:333–343. doi: 10.1111/j.1467-7687.2005.00421.x. [DOI] [PubMed] [Google Scholar]
- Striano T, Chen X, Cleveland A, Bradshaw S. Joint attention social cues influence infant learning. European Journal of Developmental Psychology. 2006a;3:289–299. [Google Scholar]
- Striano T, Reid V, Hoel S. Neural mechanisms of joint attention in infancy. European Journal of Neuroscience. 2006b;23:2819–2823. doi: 10.1111/j.1460-9568.2006.04822.x. [DOI] [PubMed] [Google Scholar]
- Stone W, Coonrod E, Ousley C. Brief Report: Screening tool for autism in two year olds (STAT): Development and preliminary data. Journal of Autism and Developmental Disorders. 1997;30:607–612. doi: 10.1023/a:1005647629002. [DOI] [PubMed] [Google Scholar]
- Thurm A, Lord C, Lee L, Newschaffer C. Predictors of language acquisition in preschool children with Autism Spectrum Disorders. Journal of Autism and Developmental Disorders. 2007;37:1721–1734. doi: 10.1007/s10803-006-0300-1. [DOI] [PubMed] [Google Scholar]
- Tomasello M, Carpenter M, Call J, Behne T, Moll H. Understanding sharing intentions: The origins of cultural cognition. Brain and Behavior Sciences. 2005;28:675–690. doi: 10.1017/S0140525X05000129. [DOI] [PubMed] [Google Scholar]
- Tomasello M, Farrar MJ. Joint attention and early language. Child Development. 1986;57:1454–1463. [PubMed] [Google Scholar]
- Tomasello M, Hare B, Lehman H, Call J. Reliance on head versus eyes in the gaze following of great apes and humans: the cooperative eyes hypothesis. Journal of Human Evolution. 2006;52:314–320. doi: 10.1016/j.jhevol.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Torkildsen J, Thormodsen R, Syvensen G, Smith L, Lingren M. Brain correlates of nonverbal communicative comprehension in 20–24 month olds. Paper presented at the International Conference on Infant Studies; Vancouver, BC, Canada. 2008. Mar, [Google Scholar]
- Toth K, Munson J, Meltzoff A, Dawson G. Early predictors of communication development in young children with Autism Spectrum Disorders: joint attention, imitation and toy play. Journal of Autism and Developmental Disorders. 2006;36:993–1005. doi: 10.1007/s10803-006-0137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulvund S, Smith L. The predictive validity of nonverbal communicative skills in infants with perinatal hazards. Infant Behavior and Development. 1996;19:441–449. [Google Scholar]
- Venezia M, Messinger D, Thorp D, Mundy P. Timing changes: The development of anticipatory smiling. Infancy. 2004;6:397–406. doi: 10.1207/s15327078in0603_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vygotsky L. Thought and Language. Cambridge, MA: M.I.T. Press; 1962. [Google Scholar]
- Wallace S, Coleman M, Pascalis O, Bailey A. A study of impaired judgment of eye-gaze direction and related face processing deficits in autism spectrum disorders. Perception. 2006;35:1651–1664. doi: 10.1068/p5442. [DOI] [PubMed] [Google Scholar]
- Werner H, Kaplan B. Symbol formation. Oxford: Wiley; 1963. [Google Scholar]
- Wetherby A, Prutting C. Profiles of communicative and cognitive-social abilities in autistic children. Journal of Speech and Hearing Research. 1984;27:367–377. doi: 10.1044/jshr.2703.364. [DOI] [PubMed] [Google Scholar]
- Whalen C, Schreibman L. The collateral effects of joint attention training on social initiations, positive affect, imitation, and spontaneous speech for young children with Autism. Journal of Autism and Developmental Disorders. 2006;36:655–664. doi: 10.1007/s10803-006-0108-z. [DOI] [PubMed] [Google Scholar]
- Wickelgren I. Autistic brains out of synch. Science. 2005;24:1856–1858. doi: 10.1126/science.308.5730.1856. [DOI] [PubMed] [Google Scholar]
- Wicker B, Fanlupt P, Hubert B, Tardif C, Gepner, & Derulle C. Abnormal cerebral effective connectivity during explicit emotional processing in adults with Autism Spectrum Disorders. Social-Cognitive Affective Neuroscience. 2008;3:135–143. doi: 10.1093/scan/nsn007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J, Waiter G, Perra OA, Perrett D, Murray A, Whitten A. An fMRI study of joint attention experience. NeuroImage. 2005;25:133–140. doi: 10.1016/j.neuroimage.2004.10.047. [DOI] [PubMed] [Google Scholar]
- Williams J. Self-other relations in social development and autism: Multiple roles for mirror neurons and other brain bases. Autism Research. 2008;1:73–90. doi: 10.1002/aur.15. [DOI] [PubMed] [Google Scholar]
- Yoder P, Stone W. Randomized Comparison of Two Communication Interventions for Preschoolers With Autism Spectrum Disorders. Journal of Consulting and Clinical Psychology. 2006;74:426–435. doi: 10.1037/0022-006X.74.3.426. [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. International Journal of Neuroscience. 2005;23:143152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]