Abstract
Engineered nanomaterials are at the leading edge of the rapidly developing nanosciences and are founding an important class of new materials with specific physicochemical properties different from bulk materials with the same compositions. The potential for nanomaterials is rapidly expanding with novel applications constantly being explored in different areas. The unique size-dependent properties of nanomaterials make them very attractive for pharmaceutical applications. Investigations of physical, chemical and biological properties of engineered nanomaterials have yielded valuable information. Cytotoxic effects of certain engineered nanomaterials towards malignant cells form the basis for one aspect of nanomedicine. It is inferred that size, three dimensional shape, hydrophobicity and electronic configurations make them an appealing subject in medicinal chemistry. Their unique structure coupled with immense scope for derivatization forms a base for exciting developments in therapeutics. This review article addresses the fate of absorption, distribution, metabolism and excretion (ADME) of engineered nanoparticles in vitro and in vivo. It updates the distinctive methodology used for studying the biopharmaceutics of nanoparticles. This review addresses the future potential and safety concerns and genotoxicity of nanoparticle formulations in general. It particularly emphasizes the effects of nanoparticles on metabolic enzymes as well as the parenteral or inhalation administration routes of nanoparticle formulations. This paper illustrates the potential of nanomedicine by discussing biopharmaceutics of fullerene derivatives and their suitability for diagnostic and therapeutic purposes. Future direction is discussed as well.
Keywords: Metabolism, engineered nanomaterials, nanomedicine, fullerenol, carbon nanotube, titanium dioxide (TiO2), silica dioxide (SiO2), magnetic nanoparticles
1. INTRODUCTION
Full-scale research and development initiatives for nanotechnology in many countries were spawned by the National Nanotechnology Initiative (NNI) in the USA (http://www.nano.gov), announced in January 2000 under Clinton’s administration [1]. The NNI stresses long-term research looking 20 to 30 years ahead, on the conviction that prioritizing basic and challenging research will lead to breakthrough technological developments. The USA’s 21st Century Nanotechnology Research and Development Act (passed in 2003) allocated almost $3.7 billion to fund nanotechnologies during 2005–2008, which excludes a substantial defense-related expenditure. Since the NNI, the American Government’s nanotechnology research funding has increased by more than ten times, starting from $116 million in 1997 to the actual budget of $1,425 million in 2007.
Chinese government is also investing and committing to nanotechnology. In the past years the number of publications on nanotechnology originating from China rose to several hundreds per year, which is exceeded only by the United States. The high number of papers at recent nanotechnology conferences in China provides evidence of vigorous research and development that is being carried out by Chinese researchers, and shows the extensive commercialization efforts being made around Shanghai and Beijing. The establishment of the National Center for Nanoscience and Technology of China at Beijing and the National Engineering Research Center for Nanotechnology at Shanghai are good indicators of the high level of interest [1]. There is special interest in combining nanotechnology with traditional Chinese medicine, because nanoformulations may greatly increase therapeutic efficacy of traditional Chinese medicine, and improve its standardization, modernization, and internationalization.
Over the last decade or so, nanomaterials have been broadly studied because of their unique size-dependent physical and chemical properties [1, 2]. Nanomaterials are substances with one or more external dimensions, or an internal structure, on the nanoscale, which could exhibit distinct novel characteristics that the same material at large size may not feature. Nanoparticles, such as carbon lattices, nanotubes, metal oxides, liposomes, micelles and polymers etc., are made of a wide variety of nanomaterials with a diameter of less than 100nm (0.1µm). Nanoparticles can be mainly divided into two groups: (I) labile nanoparticles which disintegrate into many molecular components upon application (e.g. liposomes, micelles, polymers, nanoemulsions), and (II) insoluble nanoparticles such as titanium dioxide (TiO2), silica dioxide (SiO2), fullerenes and quantum dots (QD) (e.g. carbon lattices, nanotubes, metal oxides), which remain intact. Three principal factors, the increased relative surface area, nano-scale size and the quantum effects (in some instances), distinguish the properties of nanomaterials from those of bulk materials. Due to their unique properties, applications of nanomaterials in various areas are increasing rapidly. There are many industrial and consumer products (such as cosmetics, sunscreens, paints, textiles, toothpastes, sanitary ware coatings and even food products, etc.), which contain nanomaterials and many more are still under development [3]. As nanoscience is experiencing massive investment worldwide [4], a further increase in biological and medical products developed from nanomaterials is foreseeable [5].
The accumulating investments and efforts in nanotechnology are expected to lead to new technological breakthroughs and increase in the applications of nanomaterials in medicine in the near future [6]. Engineered nanomaterials are being exploited in many different biological and medical fields due to their unequaled photo/electro-chemical and physical properties. Multifunctional nanoparticles play important roles in cancer diagnostics and therapeutics. Particles and their aggregates can be broken down to even smaller particles via milling and/or dispersion—a distinct process to generate nanoscale particles rather than solvated materials [7]. The translocation and distribution of nanoparticles in the body is size-dependent. Hence, the adverse effects of nanoparticles may not be easily predicted or derived from the known toxicity of the same chemical constituent at micro-size [8–10]. There is a need for the information on the biopharmaceutics (ADME) of these man-made nanoparticles. Dissolution, translocation, deposition or excretion is critical for nanoparticles to exert their biological effects within the body. The present paper reviews metabolic properties and pharmacological effects of bioengineered nanoparticles known today and their uses in medicine.
2. DISTINCTIVE METHODOLOGIES FOR BIOPHARMACEUTICAL STUDIES ON NANOPARTICLES
Bulk materials may show unexpected novel properties when they are miniaturized to nanosize. This spurs great interests in exploiting physicochemical consequences from the change in size of the materials and utilizing the resulting changes for applications to benefit human services. Keeping the mass unchanged, a decrease in size of particles will result in an increase in total surface area of particles [8, 11, 12] (See Table 1). This increase in surface area would cause chemical modifications at the surface and generate additional physico-chemical and /or biological activities that the corresponding large particles may not possess. Furthermore the increase in particle surface would allow insoluble, labile particles or even particle core to carry more reactive molecule species, and ultimately produce either beneficial or adverse biological effects.
Table 1.
The Correlated Proportion of Particle Size and Particle Surface Area
| Particle diameter (nm) |
Particle number (number/pound) |
Particle surface area (mm2/pound) |
|---|---|---|
| 1000 | 1015 | 3×109 |
| 100 | 1018 | 3×1010 |
| 10 | 1021 | 3×1011 |
Metabolism of many nanomaterials has not been studied in detail. Most metabolic studies on nanomaterials employed the traditional analytical methods to compare the results from the nanoscale with the non-nanoscale materials. For example, isotope labeling of nanoparticles has been broadly used to determine their distribution and translocation in vivo. Liu et al detected 59Fe, 13C, 14C, 125I-labeled carbon nanotube (CNT) for measurement of the tissue distribution and metabolism of CNT in animal model [13]. Fluorescent labeling is also a useful method to determine the location and trafficking of nanoparticles in biological systems by detecting fluorescence intensity of the fluorophors. Inductively coupled plasmamass spectroscopy (ICP-MS) is another method used for quantitative measurement of the concentration of metal nanoparticles. It is difficult to detect and quantify the metabolism of nanomaterials by a single analytical technology. The most commonly used analytical methods are based on chromatography, mass spectrometry (MS) and NMR. Although the requirements for studying the biodistribution /toxicokinetics and mutagenicity/genotoxicity of nanoparticles are similar to those used for studying conventional micro or macro sized materials, the specific characteristics of nanoparticles may require additional considerations of methodologies to measure the unique features of distribution and metabolism of nanoparticles. For example, the present genotoxicity tests for novel chemotherapeutic agents in vivo do not cover the expected target organs or tissues where nanoparticles deposit. In addition, the broadly used cell diffusion chamber incorporating a microporous membrane is a standard device for estimating percutaneous absorption of commonly-used medical and customer products. Having stated that, the conventional models may not be appropriate for estimating the unique biological capability of nanoparticles for medical application. Therefore, new methodologies to assess the metabolic pathways (penetration, dissolution, translocation, and deposition et al) of nanoparticles are needed for characterizing the properties of engineered nanomaterials.
3. IMPROVED PHARMACEUTICAL PROPERTIES OF COMPOUNDS MODIFIED BY NANOTECHNOLOGY
Currently, in the early stage of drug discovery, most of compounds formulated for in vivo experiments are frequently lipophilic or sparingly soluble in water. Typical intravenous formulations for these compounds at neutral pH require the presence of multi-solvent, surfactant, or in mixed micellar solutions, or nanosuspensions [14]. It is complicated to determine the intrinsic pharmacokinetic behavior of nanopreparation of a new drug entity from pharmacokinetic behavior of the same drug that is formulated using micro or macro form of the drug entity. The complication may result from various situations such as slow or incomplete dissolution of injected nanoparticles, precipitation of the compounds or particles in the bloodstream, delayed release of the compounds from the dosing vehicle, competition between the compounds and formulation ingredients for transport, metabolism and binding to proteins and other blood and tissue components. The strategies used in pharmaceutical industry may be applied to improve the solubility of these compounds without alteration of their properties while developing nanoformulations. The entrapped dosage of the compound in terms of loading capacity of the drug vehicle and the kinetics of release of the entrapped compound are important determinants for the success of developing a ‘non-interfering’ vehicle for the dosage formulation. If an analytical technique used to determine drug concentration in the body is sensitive enough to detect compounds at low doses, screening for formulations at relatively low concentrations may be feasible. Nanoparticles may be employed to solve this critical problem due to their unique physical, chemical, electronic, and magnetic characteristics. Some nanoformulations can be monitored by virtue of photostable optical intensity of the compounds for clinical measurement. The low concentration of compounds can be detected by the electronic, magnetic or optical signals of nanoparticles. In this way, the metabolism and pharmacokinetic behavior of these compounds can be quantitatively measured in vivo, and the pharmacokinetic behavior of the compounds can be evaluated by monitoring the nanoparticle vehicle used in the pharmaceutical preparation [15].
Conventional formulations of the highly lipophilic taxane, paclitaxel (Taxol) for intravenous use contain cremaphor as a solvent. Toxicity due to cremaphor contributes to adverse effects of this versatile anticancer drug. Abraxane is a nanoformulation of paclitaxel conjugated to nano-bead protein structure. It is noteworthy that abraxane is one in a small list of FDA approved for the treatment of breast cancer. Paclitaxel is a very effective anticancer drug. The nano-bead protein conjugated formulation increased water solubility allowing for elimination of the toxicity associated with the solvent vehicle (cremaphor) and improved therapeutic index. Nanoparticles (such as TiO2) have been used as carriers to increase the photostability of the pharmacologically active payload. The nanoparticulate multilayer of nanoparticles was used as an optical filter to protect the drug from damage by light exposure. The increased hydrophilicity of TiO2 nanoparticles increases the aqueous wettability by preventing aggregation in the dispersion medium. This increases the dissolution rate of the entrapped photoprotected cargo molecules in the nanoformulation by facilitating the maximal exposure of compounds’ surface area to the dispersion medium[16].
4. POTENTIAL MEDICAL APPLICATIONS AND BIOLOGICAL EFFECTS OF NANOMATERIALS
Carbon nanotubes comprise an important class of nanomaterials. A hydrophilic functionalized soluble single-walled carbon nanotube (SWNT) is of special interest because of its wide use as a highly effective means of transporting molecular cargo of various sizes and types across the cell membrane to the therapeutic cellular target. SWNT has been demonstrated to carry smaller molecules into cells through a variety of energy -dependent and/or -independent processes [17–19]. Therapeutic drug molecules or diagnostic sensor molecules are usually linked covalently to functionalized SWNT through ester or disulfide bonds. Upon cellular entry, SWNTs encounter the acidic reducing environment of lysosomes or endosomes, where the disulfide and ester bonds are cleaved to selectively release their cargo [20]. There are several studies employing CNTs for nanoformulations designed for intracellular transport and controlled release of pharmaceuticals including cancer chemotherapeutic agents. For example, the full clinical potential of the highly effective anti-cancer drug is not achieved because of inadequate delivery to the targeted tumor in vivo. Lippard et al. [21] combined cisplatin with SWNTs due to their proven capacity to act as a longboat, shuttling smaller molecules across tumor cell membranes. They demonstrated that nanoformulations of SWNTs conjugated to platinum(IV) could effectively deliver a lethal dose of the anti-cancer drug inside the malignant cancer cells [21]. However, these studies have to be viewed with some caution because of potential toxicity of nanotubes [22]. The potential hazards of CNTs to humans and other biological systems was assessed by measuring DNA damage caused by CNTs in mouse embryonic stem (ES) cells [23]. Many experiments have demonstrated the adverse pulmonary effects of SWNTs in vivo after intratracheal instillation, in both rats and mice. Further studies indicate that if CNT reach the lungs, they are much more toxic than carbon black or quartz [24, 25]. It is noteworthy that the report of National Institute for Occupational Safety and Health (NIOSH) states that none or only a small fraction of the CNT enters in the lung through inhalation at the workplace [26]. SWNT induced oxidative stress, which is exemplified by the formation of free radicals, accumulation of peroxidative products, antioxidant depletion, and cytotoxicity in human keratinocytes [27, 28]. In addition, Moller et al. showed that other carbon nanomaterial such as ultrafine carbon black particles could impair phagosome transport and cause cytoskeletal dysfunctions with a transient increase of intracellular calcium signal [29].
Bioeffect of nanoparticles in the body depends not only on the amount of nanoparticles but also the size and surface of nanoparticles. It has been demonstrated that the ultrafine nanoparticles were deposited in lungs and other organs, when rats were subjected to chronic inhalation of TiO2 nanoparticles of different sizes, for 3 months. These studies showed more translocation of nanoscale TiO2 to interstitial sites and regional lymph nodes when compared to the fine TiO2 nanoparticles. By comparing carbon black or CNT particles of similar size and composition but with significant difference of specific surface area, it was found that the biological effects (inflammation, genotoxicity, and tissue damage as indicated by histopathologic examination) of nanoparticles were determined by specific surface area, not by particle mass. Similar findings were reported in earlier studies on the tumorigenic effects of other nanoparticles. It has been shown that tumor incidence was correlated better with specific surface area than with particle mass [30].
Liposomes have been used as an effective vehicle for drugs, such as for cisplatin, paclitaxel, daunomycin, doxorubicin and amphotericin et al. Nanocarriers for medical applications are made of relatively safe materials, including synthetic biodegradable polymers, lipids and polysaccharides. Administration of doxorubicin encapsulated into PEG-PE micelles increased doxorubicin accumulation and penetration in tumors, compared to the much lower levels of drug achieved with doxorubicin itself without nanotechnological modification. In vitro and in vivo pulmonary deposition of nano liposomes has been performed using Andersen Cascade Impactor and intratracheal instillation in rats, respectively. Liposomes were prepared by thin film evaporation technique and liposomal dispersion was passed through high pressure homogenizer. Nanoliposomes (NLs) were harvested by centrifugation and characterized by scanning electronic microscopy (SEM). NLs were dispersed in phosphate buffered saline (PBS) at pH 7.4. The dispersion was spray-dried and sizes of powders were measured by atomic force microscopy (AFM). In vivo studies revealed significant retention of nanoliposome-trapped medicine after 24 hours, suggesting slow clearance of NLs in biological system. The encapsulated nanoliposome provides a practical approach for direct delivery of compounds for controlled and prolonged retention at the targeted site of action. It may play a promising role as effective therapeutic drug delivery system in reducing the risk of acute and chronic toxicity [31]. Nano-sized particles are more likely to increase the bioavailability, which could result in improved chemotherapeutic effects. It is important to note that specific particle surface area may be a better indication for maximum tolerated exposure level of nanoparticles. Recent publications on the pulmonary effects of CNT confirm the intuitive assumption that nano-sized nanotube can induce a rather general non-specific pulmonary response [22, 24, 26, 32] .
5. POTENTIAL THERAPEUTIC STRATEGY BASED ON GENOTOXICITY OF NANOPARTICLES
Some of the adverse biological effects of nanoparticles in vivo have linked to the inflammatory response with exacerbation of airways disease, cardiovascular events caused by hypercoagulability or atherosclerotic plaque destabilization [33, 34]. Many of these conclusions are derived from studies on nanoscale particles that can cause inflammatory response or inflammation and these conclusions may not be extrapolatable to nanoparticles in general, and they may be less relevant for engineered nanomaterials. Nanoparticles that readily cross cellular membranes can be expected to be able to reach the nucleus and DNA, which is important when genotoxic effects are considered. For instance, functionalized SWCN have been reported to reach the cell nucleus [18, 35]. Even if nanoparticles do not go through the nuclear envelope, they would eventually have access to the nucleus in dividing cells, because the nuclear envelope dissolves during mitosis leading to cell division. For most nanoparticles, it is unknown whether they interact directly with DNA or the mitotic spindle, and what size and/or charge of nanoparticles could lead to defects in DNA transcription and chromosomal damage and aberration. However, there are indications that certain types of nanoparticles are capable of causing DNA damage.. Surface area and charge density of nanotubes are considered to be critical in determining their electrostatic complex formation with DNA [36]. Therefore, cationic functionalized CNTs are frequently used for binding to DNA and widely used for cell specific delivery of functional DNA and siRNA to specifically modify the expression of the targeted gene. The composition and the coating of nanoparticles are probably the key factors in determining their genotoxic effects. Nanotubes coated with a positively charged polyelectrolyte, functioning as a counterpart for negatively-charged DNA, have been wrapped with DNA to generate DNA sensors [37]. The property of various types of nanoparticles complexed with DNA has been utilized for cellular and nuclear delivery of DNA and oligonucleotides [38–40]. In addition, SWNT can bind with single- and double stranded DNA, and/or peptide amino acid (PAA) [41–44]. QDs, such as cadmium selenide (CdSe), have been used for tracking and monitoring biological molecular process and function in vitro and vivo. CdSe is usually capped with a shell of PEG, with biotin surface functionality for broad biological application in vivo. Water-soluble semiconductor QDs can also cause bond breakage in DNA strands due to photogenerated and surfaceoxide- generated free radicals [45].
In principle, genotoxicity of nanoparticles can be assessed using in vitro assays on mammalian cells. However, the timing of the tests has to be long enough to ensure that the nanomaterial can reach the nucleus. In the in vitro chromosome aberration test which was related to sample prep/ agglomeration state, it is necessary to examine two post-treatment metaphases by allowing two rounds of cell replication. In the cytokinesis-block micronucleus test in vitro, the treatment with nanoparticle should occur in one cell cycle without cytochalasin B (Cyt-B), followed by another cycle with the presence of Cyt-B, to examine the cells after the 2nd post-treatment mitosis. If the genotoxic effects of nanoparticles are related with inflammation, simple in vitro assays may not be adequate for demonstrating the genotoxic potential of nanoparticles. In general, the relationship between inflammation and genotoxic effects is still not well understood for many types of nanoparticles including weakly soluble nanoparticles. Depending on the endpoint of study, demonstration of an association between inflammation and genotoxic effects may require relatively long-term experiments, which may be impractical. In rats, intratracheal instillation of nanosized carbon black, fine TiO2 (anatase) and fine α-quartz (SiO2) resulted in an increase of hprt mutations in alveolar cells after 15 months treatment [30]. It appears that basic studies on the association between inflammation and genotoxicity are necessary before conclusions can be drawn on the importance of inflammation-related genotoxicity of nanoparticles. In vivo, actively dividing cells are expected to be the primary targets of genotoxic effects of nanoparticles, as genotoxicity can be fixed only when the cell divides. The widely used in vivo genotoxicity tests, the bone marrow micronucleus test and the liver UDS (Unscheduled DNA Synthesis) test, have been employed to detect DNA damaging effects of nanoparticles that reach the bone marrow and the liver, respectively, or that have systemic genotoxic effects. Currently, there is no validated standard method for assessing genotoxic effects on dividing cells associated with carcinogenesis in the expected target tissues in vivo. However, techniques such as the comet assay, micronucleus test, and gene mutation analysis in transgenic animals could probably yield valuable results for studying the effects of nanoparticles on DNA.
6. TRANSLOCATION OF NANOPARTICLES IN BIOLOGICAL SYSTEM
There is a tendency for accumulation of insoluble nanoparticles in the body and the biological effects would be dependent on the properties and dose of nanoparticles, as well as the site of accumulation. Nanoscale particles may affect movement of endogenous substances (proteins, enzymes) or exhibit increased reactions with biological molecules. Autonomic nervous system may also be a target for the adverse effects of inhaled particulates. In the in vivo evaluation of the biological effects of nanoparticles, the translocation to the systemic circulation is an important aspect. The possible translocation of nanoparticles has not been studied extensively, although the importance of nanoparticle transport and translocation in biological systems, in pharmacology and delivery of biomolecular drugs [46]. Characterizing the status of the component molecule of nanoparticle, and understanding the processes of translocation, dissolution, and possible nanoparticle-receptor interactions are key challenges to define the biological effects of nanoparticles in vivo.
Nanomaterials are expected to dissolve faster than larger sized materials of the same mass based on surface area considerations alone—although other factors such as surface curvature/roughness also play a role. In addition to phagocytosis, which is mostly for cellular ingestion of relatively large particles, translocation of insoluble particles into cells is most likely to be dependent on processes of endocytosis, mainly pinocytosis and phagocytosis, or receptor-mediated endocytosis [47]. Similarly, expulsion of nanoparticles from live cells may occur predominantly by exocytosis. Dissolution, even over the period of weeks or months, may significantly enhance clearance of nanoparticles. Diffusional movement of nanoparticles through cell membranes is likely to be limited under normal conditions [48]. More research in diffusional movement may provide a better understanding of nanoscale particle translocation processes. Several authors have reported on the movement of nanoscale particles from the lung to the blood [49]. Nemmar et al. [50] have studied the translocation of inhaled technetium (99mTc) labeled carbon nanoparticles to the blood. The authors concluded that phagocytosis by macrophages and/or endocytosis by epithelial and endothelial cells was responsible for carbon nanoparticle-translocation to the blood but other routes must also exist.
Particles up to 25µm size were found in the foot dermis of elephantiasis patients with impaired lymphatic drainage in the lower legs. These particles were located either in the phagosomes of macrophages, or in the cytoplasm of other cells. The failure of conducting lymph to the node produces a permanent deposit of particles in the dermal tissues. This indicates that particles, which penetrate normal or damaged skin, are removed via the lymphatic system of healthy people [12]. Micro-sized and even submicron sized liposomes do not easily penetrate into the viable epidermis, while nano-sized liposomes with an average diameter up to 272 nm have been found to reach into the viable epidermis and some in the dermis. Smaller sized liposomes of 116 and 71 nm have been found in higher concentrations in the dermis. Liposomal emulsion particles with diameter of 50 nm to 1 micron, have been detected in the epidermis in association with the cell membranes after topical application to human skin [51]. In subsequent studies, it has been shown that formulations containing nanoliposomal microspheres allow penetration of nanoliposome spheres into melanoma cells, and even into the nucleus.
New degradable nanomaterials such as polyanhydrides were used for preparing controlled release formulations of proteins and peptides. Some of these formulations can be utilized for delivering proteins and peptide through the skin and lungs [52]. Some conclusions regarding nanoparticles penetration of skin by nanoparticles can be drawn from the rather limited literature on the subject. Firstly, penetration through the skin barrier is dependent on particle size. Nano-sized particles are more likely to penetrate deeper into the skin than larger ones. Secondly, nanomaterials, which can dissolve, or leak from a nanoparticle (e.g. metals), or break into smaller parts (e.g. liposomes), can possibly penetrate the skin. There is no clear indication that nanoparticles, which had penetrated the skin, also entered the systemic circulation. However, it has been observed that nanoparticles can be phagositized by macrophages, Langerhans cells or other cells.
7. EVALUATION OF BIOLOGICAL EFFECTS OF NANOPARTICLES
Nanoparticles, because of their ultrahigh reactive surface sites and quantum effects, are being widely used in the traditional industries, such as dyes, paints, medical diagnosis, sunscreens and cosmetics. There are certain principles for biological and medical evaluation of potential nanoparticles (See Table 2). Metal nanoparticles have found uses in biological and medical studies [53]. Increased numbers of neutrophils and phagocytes in lung fluid and the deposition of nanoparticles in alveolar cells were observed in rats and hamsters following inhalation of silver or gold nanoparticles. Acute pulmonary reaction in response to administration of nanoparticles such as carbon black, TiO2, and iron oxide revealed rapid translocation of nanoparticles across the epithelium after deposition. It was found that severe effect on renal function could occur in the metal nanoparticles-treated mice, without significant change in biochemical profile of blood. Blood-element test showed that in the rats treated with metal nanoparticles (such as Zn- or Cu), red cell distribution width, corpuscular volume and blood platelet significantly increased, and hemoglobin as well as haematocrit significantly decreased. The study indicated that metal nanoparticles could cause anemia in animal model. Besides the pathological lesions in the liver, renal, and heart tissue, only slight stomach and intestinal inflammation was evident in all the metal-treated mice, without significant pathological effects in other organs [54, 55].
Table 2.
Biological and Medical Evaluation of Nanoparticle
| Subjects of evaluation | Aspects of evaluation |
|---|---|
| Biodistribution | Whole organism, tissue and organ level |
| Localization | Different intracellular vesicle, organelles, cell level |
| Metabolic routes | Absorption, distribution, metabolism and excretion (ADME) |
| Immunological properties | IgG/IgM specific Abs, cytokine induction, T cell activation |
| In vivo degradation | Enzyme digestion, lysosomal decomposition |
| Biocompatibility | Biological environment and adverse health effect in vivo |
| Toxicological characters | Chemical composition, particle size, reactivity, structure/ properties, surface coating modification |
| Chemotherapeutic concerns | Therapeutic index of nanomedicines and their delivery systems relates with clinical administration |
| Etc ·········· | Other unclear consequence associated with nanomedicines |
TiO2 nanoparticles are widely used for a variety of applications. Pulmonary inflammatory response was observed for 20 nm TiO2, but not for 250 nm TiO2 particles [56]. In order to evaluate the biological effect of TiO2 particles, the acute biological reaction caused by nano-sized TiO2 particles in adult mice was compared with that resulting from fine TiO2 particles [56]. Due to the low toxicity, a fixed large dose of TiO2 nanosuspensions was administered to mice by a single oral gavage. TiO2 particles showed no obvious acute toxicity in 2 weeks, however, female mice showed high accumulation of the nano-sized nanoparticles in liver. In the assessment of acute biological effects of TiO2 particles, abnormal activities were not observed in these mice, and there were no cancer and carcinogenic symptoms after autopsy because of the short exposure period. Different laboratories have reported that the retention halftime of TiO2 particles in vivo was long because of inefficient and slow rate of excretion. Oberdorster et al. [57] reported that the retention halftimes of TiO2 in rat lung were 117 days for fine particles and 541 days for ultrafine particles. After intravenous injection of rats with 200–400nm TiO2 nanoparticles, about 69% of the injected TiO2 nanoparticles at 5 min and 80% at 15 min were accumulated in the rat liver [58]. The 80 nm TiO2 in vivo may directly result in the nanoparticle deposition in the liver and lead to hepatic lesion. Surprisingly, the 25 nm TiO2, the same as the fine particles, are not retained in the liver, but accumulate mainly in the spleen, kidneys, and lung tissues.
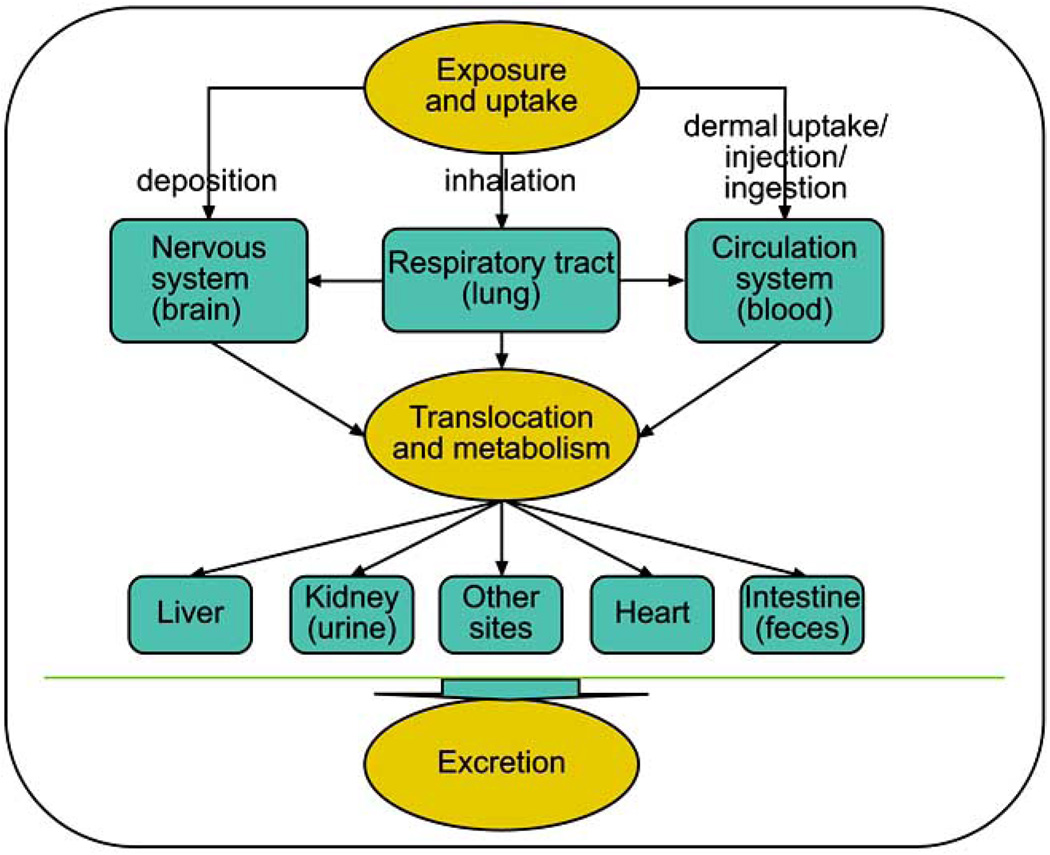
Biodistribution, penetration through tissue, phagocytosis and endocytosis of nanosized materials are all likely to have an impact on potential toxicity of nanoparticles. These processes are most likely dependent on surface characters of nanoparticles. The complex pathways involved in exposure and uptake, translocation and metabolism, and elimimnation of nanoparticles in biological systems are depicted (Fig. 1). The liver, as a main detoxification organ, is activated to eliminate the side effects induced by most of the mass ingested nanoparticles. Following oral intake, a part of these nanoparticles are excreted through the kidneys. However, the small size and difficult clearance resulted in prolonged retention of nanoparticles in vivo and damage to the liver and kidneys. The changes of serum biochemical parameters including bilirubin levels (TBIL), alkaline phosphatase (ALP), alanine aminotransferase (ALT) and aspartate aminotransferase (AST) and other pathological characteristics of liver were mostly used to evaluate the effect of nanoparticles on liver function, which was usually indicated by measurement of hydropic degeneration around the central vein and the spotty necrosis of hepatocytes. In addition, the nephrotoxicity could be assessed by measurement of activities of creatine kinase (CK), lactate dehydrogenase (LDH) and alpha-hydroxybutyrate dehydrogenase (HBDH). The myocardial damage was shown by change of serum LDH and alpha-HBDH caused by treatment with nanoparticles. For example, increase in levels of uric acid (UA), blood urea nitrogen (BUN) and creatinine were accompanied by pathological changes in the kidneys of experimental animals treated with TiO2 nanoparticles. However, there were no abnormal or pathological changes in the heart, lung, testicle (ovary), and spleen tissues. Biodistribution studies showed that TiO2 nanoparticles were retained mainly in the liver, spleen, kidneys, and lung tissues, which indicated that TiO2 particles could be transported to other tissues and organs after uptake from the gastrointestinal tract after oral dosing.
Fig. (1). Possible routes for translocation and metabolism of nanoparticles in biological system.
There are many pathways for systemic exposure, translocation and excretion of nanoparticles, which were proposed in published literatures. The exact pathways for transport and metabolism of nanoparticles are not clear. Further in vitro and in vivo investigations are necessary.
Although the levels of AST, ALT, and TBIL enzymes for liver function did not change much, the ratio of ALT/AST (as a more sensitive indicator for hepatic injury) could be measured for bioevaluation of liver function after oral ingestion of nanoparticles. The changed liver weight and the hepatocyte necrosis in the pathological examination were helpful to estimate liver injury caused by administration of nanoparticles. In addition, test of LDH in serum is often used to detect tissue alterations and diagnose heart attack, anemia, and liver diseases. Generally, high LDH level shows the myocardial lesion when combined with data for CK and alpha-HBDH, and the hepatocellular damage are expressed when combined with AST and ALT enzymes. The high LDH and alpha-HBDH enzyme levels implied that nanoparticles resulted in more myocardial damage although the pathological change was not observed in cardiac tissue. From the changes of biochemical parameters, such as ALT/AST, BUN, and LDH, it was clear that nanoparticles affected the functions of liver and kidneys in female mice. In summary, administered nanoparticles were retained mainly in liver, kidneys, spleen, and lung,. The obvious hepatic injury caused by nanoparticles could be seen by hydropic degeneration around the central vein and the spotty necrosis of hepatocytes, and the kidney damage was inferred from histopathological examination of the renal tubule and observation of swelling in the renal glomerulus [56].
8. BIOLOGICAL REACTION OF NANOPARTICLES IN RESPIRATORY SYSTEM
Because lung has extensive, location-specific defense systems such as mucociliary clearance in the upper airways and macrophage clearance in the lower, unciliated part of the respiratory tract, most of the nanoparticles in the dosage could be found in lung after the inhaled administration to rats or mice. Deposition of nanosized particles less than 0.5 µm diameter in the respiratory tract is mainly determined by diffusion. The diffusional deposition of nanosized particle during breathing is affected by: (1) dynamics of nanoparticles and their size and shape; (2) geometry of the branching airways and the alveolar structures; (3) breathing pattern, either nose or mouth breathing; and (4) airflow velocity and the residence time in the respiratory tract [59]. Some of the nanoparticles from the administered dose can remain in the airway for a long time. Nanoparticle transport from the alveolar region toward the larynx in humans is mediated through macrophages. This is a slow process even under normal conditions, and serves to eliminate only a part of the nanoparticles deposited in the peripheral lung. The remainder may accumulate in lung surfactant or interstitial fluid unless the nanoparticles are biodegradable or cleared by simple chemical dissolution. Therefore, it can lead to a different cumulative dose for a given deposited dose of nanoparticles with different durability and resistance to dissolution or degradation. The kinetics of dissolution of inhaled nanoparticles is the most important factor in determining whether a low-toxicity nanoparticle, such as amorphous silica (SiO2), could be eliminated in the epithelial lining fluid or whether nanoparticles such as carbon blacks or iron oxides are engulfed by alveolar macrophages. Thus, solubility of nanoparticles is a key driver for the mechanisms of their biological metabolism including their clearance in vivo.
On the epithelium walls of the respiratory tract, nanoparticles first come into contact with the mucous or serous lining fluid and its surfactant layer. Therefore, the destiny of particle compounds that are soluble in this lining fluid is different from the slower dissolving or insoluble compounds [59]. Soluble nanoparticle could dissolve and often be metabolized in the lining fluid, eventually be transferred to the blood, and undergo further metabolism. In this way, particle compounds may have the potential to reach organs far from the original site of entry and to produce biological effects. On the other hand, the slower-dissolving and insoluble nanoparticles deposited on the wall of airway will only be moved by ciliated cells shuffling, coughing, and swallowing. There is evidence that a significant fraction of nanoparticles is retained in the respiratory tract. The slow dissolving and insoluble nanoparticles deposited in alveoli will be taken up by macrophages under normal physiological conditions. However, for the nanoparticles able to penetrate into interstitium, the macrophage uptake is less likely.
The alveolar macrophage plays an important role in the response of the lung to inhaled nanoparticles. Their essential function is phagocytosis and clearance of inhaled nanoparticles. The alveolar cells could produce surfactant by cellular secretion, which contain significant quantities of biotransformation enzymes, particularly cytochrome P450-dependent mono-oxygenases, which participate in detoxification reactions for overcoming nanoparticle toxicity. In addition, macrophages also carry out several other metabolic functions, such as the active uptake of endogenous and exogenous compounds, permeability functions and immunologic functions. The kinetics of nanoparticle metabolism in vivo may also be affected by differences in the pH of subcellular compartments [60]. If nanoparticle is neither soluble nor degradable in the biological environment, it is likely to have high biostability with a strong tendency for accumulation upon constant administration. In addition to the known biological effects of nanomaterials with low-solubility, disposition factors of nanoparticles may also influence their biological effects and applications in vivo.
Macrophage-mediated nanoparticle removal may be impaired especially for the young and the old people, and persons with lung diseases. As noted above, nanoparticles that escape phagocytosis by macrophages may interact with cells lining the epithelium. In general larger nanoparticles are phagotized, while smaller particles escape uptake by macrophages. The large surface area of nanoparticle presents a reactive interface with cells. Depending on the nature of their molecular surface, nanoparticles may have a greater capacity to induce or mediate adverse effects than larger particles, not only in the respiratory system, but also in the cardiovascular system, the central nervous system and the immune system [8].
9. BIOLOGICAL REACTION OF NANOPARTICLES IN NERVOUS SYSTEM
The absorption of nanoparticles in olfactory nervous system has been studied in connection with the development of drug-delivery systems. Some drug-delivery systems might circumvent the blood– brain barrier in order to enable penetration of diagnostics or medical therapeutics to the brain [61, 62]. Because of the smaller diameter, larger surface area and increased number of nanoparticles compared with non-nanoscale particles in a fixed volume and amount, nanoparticles could carry more reactive materials such as free radicals, transition metals, or chemotherapeutic compounds to the deep organs, and increase drug accumulation in body. Although ingestion is possible when dust accumulates on mucosa surfaces of the oropharynx and nasopharynx or from contaminated food, inhalation is still the most common route for exposure of nanoparticle in biological system. Nanoparticle could be translocated into the central nervous system (CNS) via the olfactory pathway. There is evidence that an increased Mn++ concentration was observed in the brain of rats after inhalation of manganese phosphate or manganese sulfate. Also, there was an increase of 13C concentration in the olfactory bulb, cerebrum and cerebellum of rat brain after exposure to 13C labeled carbon nanoparticles [63]. Severe damage to brain tissue was also observed when large mouth bass lived in the water containing 500 ppb buckyballs [64].
Nanoparticles, such as TiO2 particles, can be taken up by the olfactory bulb via the primary olfactory neurons and accumulated in the olfactory nerve layer, olfactory ventricle, and granular cell layer of the olfactory bulb. The distribution areas of larger TiO2 particles were wider than those of nano-sized TiO2 in the olfactory bulb, indicating that larger TiO2 particles entered more easily into the olfactory bulb through olfactory tract than nano-sized TiO2. This is likely because nanosized TiO2 particles might be adsorbed in the nasal cavity and/or mucosa and only a small fraction of particles were translocated into the olfactory bulb and brain through the respiratory tract and olfactory nerve system.
Because nanoparticles can be translocated to the olfactory bulb through the olfactory nerve system after nasal inhalation by mice, translocation of nanoparticles could influence the micro-distributions of Fe, Cu, and Zn in the olfactory bulb [65]. The changes in micro distribution patterns of metal would influence the normal metabolism of substance and energy in organism. In brain, Zn is one of the most prevalent trace elements and is highly enriched in the hippocampus. Zn is also known to influence the synthesis and the metabolism of proteins and nucleic acids. The concentration of trace metals in brain also shows a close relationship with Alzheimer’s disease, Parkinson’s disease and multiple sclerosis. Numbers of segmented neutrophils and lymphocytes, protein carbonyl levels, and interstitial fibrosis were higher in golden hamsters exposed to carbon black, TiO2, and SiO2 [66]. Nanoparticles do not rest at the original site after inhalation, and they could be transferred to the surrounding tissues and other areas, such as, epithelial, interstitial, and endothelial sites of lung, and enter the blood circulation [56]. Therefore, nanoparticles could migrate via the secondary and tertiary olfactory pathways to most parts of brain. The subsequent influence on neuron and brain functions could be induced by the invasion of nanoparticles.
10. METABOLIC FATE AND DIPOSITION OF FULLERENE IN BIOLOGICAL SYSTEMS
There have been very few studies on the destiny, distribution, metabolism and bioaccumulation of nanoparticles in humans. Following exposure to nanoparticles in vivo, the target organs and resultant biological responses need to be identified, and realistic exposure levels for effect assessment should be determined. These include dose response data for the target organs, and knowledge of the subcellular locations of nanoparticles, and the mechanism of their interactions at the cellular level. For ordinary chemical substances, pharmacokinetic and pharmacodynamic information to assess the effectiveness of the compound is generally already available or can be predicted on the basis of existing data from tests on analogous compounds. For nanoparticles, however, this information is scarce or even non-existent. Due to lack of information, appropriate assessment of biological effects of nanoparticles is accompanied by large uncertainties. Both pharmacodynamic and pharmacokinetic studies are necessary for various types of nanoparticles on a “case by case” basis to assess the impacts of their biological applications, before more general assumptions can be made with respect to all nanoparticles.
Although the types of nanomaterials are increasing rapidly, many are particles containing organic molecules as building materials and inorganic elements (usually metals) as cores. Fullerenes have attracted considerable attention in various disciplines of nanotechnology and nanoscience. Investigations of chemical, physical and biological properties of fullerenes have yielded promising information. The unique carbon cage structure coupled with immense scope for derivatization makes fullerenes attractive for pharmaceutical applications.
Fullerene was first reported by Dr. Kroto et al. twenty years ago [67]. Before their discovery in 1985, graphite and diamond were the only two known allotropic forms of carbon. Dr. Kroto et al.[67] discovered a novel allotrope of carbon, which they called buckminsterfullerene due to its geodesic structure. Buckminsterfullerene is well known by its shortened name fullerene. Fullerene is known with 60 carbon atoms arranged as a truncated icosahedron, with 60 vertices and 32 faces. Twelve of the 32 faces are pentagonal and 20 hexagonal. The pentagons are necessary in order to allow curvature and eventual closure of the surface. The crystal and molecular structure of C60 fullerene have been resolved using single-crystal xray diffraction methods [68]. Considerable research activities have followed after the procedures of preparing fullerene in workable quantities were developed [69]. Biological and pharmacological activities of fullerenes have been investigated for evaluating their therapeutic potential. These include anti-HIV protease activity, photodynamic DNA cleavage, free radical scavengeing action, and antimicrobial action as well as the utility of fullerenes as diagnostic agents [70–74]. Fullerene molecule can fit inside the hydrophobic cavity of HIV proteases and block the access of substrates to the catalytic site of enzyme. Fullerenes can be used as radical scavengers and antioxidants. Photosensitization of fullerenes can produce singlet oxygen in high quantum yields. This action, together with direct electron transfer from excited state of fullerene and DNA bases, can be used to cleave DNA. In addition, fullerenes have also been used as carriers for gene and drug delivery systems. Meanwhile, they are used for serum protein profiling as MELDI material for biomarker discovery through MALDITOF mass spectrometry.
Even though most fullerenes are man-made synthetic compounds, naturally occurring fullerene has also been reported in the geological environment of Shunga, a town in the lake region of Karwelia in Russia [75]. Synthetic fullerenes are produced by high temperature vaporization of solid graphitic rods by resistive heating in the presence of a few to several torr of rare gas. The soot produced by vaporization contains various levels of fullerene, depending on the vaporization conditions. However, the majority of the fullerenes produced are C60 and C70, with C60 being more abundant. The fullerenes are extracted by placing the soot with a solvent in which the fullerenes are soluble. The solution is then filtered and allowed to evaporate to yield fullerene powders.
Endohedral metallofullerenes, i.e., compounds in which a fullerene encapsulates a metal atom(s), have shown great promise for biomedical applications. Although C60 has been the most commonly studied fullerene in biological systems, few endohedral materials have been synthesized using C60 as a cage molecule because of the limited interior volume of C60. Most endohedral metallofullerenes are synthesized using C82 or higher molecular weight fullerenes, and many more derivatives of C82 fullerenes have been synthesized currently. Gd@C82 is one of the most important molecules in the metallofullerene family [76]. Gadolinium endohedral metallofullerenol (e.g., Gd@C82(OH)22) is a functionalized fullerene with gadolinium, a transition metal of lanthanide family, trapped inside fullerene cage and was originally designed as a contrast agent for magnetic resonance imaging (MRI) [72]. It has been previously reported that the chemical and physical properties of gadolinium endohedral metallofullerenols are dependent on the number and position of the hydroxyl groups on the fullerene cage [76–78]. The results have shown that modifying the outer cage of Gd@C82 with a number of hydroxyl groups alters the electronic properties of inner metal atom as well as the electron density and polarizibility of the electrons in the cage surface. Although Gd@C82(OH)22 was originally studied as a novel highly efficient contrast agent for MRI, it has been demonstrated that aggregates of Gd@C82(OH)22, Gd@C82(OH)22 nanoparticles, also inhibit tumor growth in animals[79]. It has been shown that intraperitoneal injection of Gd@C82(OH)22 nanoparticles efficiently inhibited the growth of hepatoma cells implanted subcutaneously in the legs of mice. Further studies suggested that inhibition of tumor growth involved reduction in tumor-induced oxidative stress rather than direct cytotoxicity to tumor cells [79]. The potential of Gd@C82(OH)22 nanoparticles for advances in cancer therapeutics will be discussed in following sections.
11. MEDICAL APPLICATION OF ENDOHEDRAL METALLOFULLERENE
Water-soluble endohedral metallofullerols have been extensively studied over the last decade for their potential in using endohedral metallofullerene derivatives in biological systems. Generally, the endohedral metallofullerols can be synthesized using procedures similar to C60 hydroxylation. In 2000, Hirahara et al. reported the synthesis of a multi-hydroxylated fullerene, Gd@C82(OH)n (n= 30–40), using TBAOH (tetrabutylammonium hydroxide) as a transfer agent [80]. Although available only in small quantities now, endohedral metallofullerene derivatives have demonstrated potential as a novel MRI contrast agent for diagnostic use [78], and as therapeutic agents [81, 82]. Endohedral metallofullerene contains unique features including all-carbon shell with a large surface, a hollow core capable of accommodating ions, a cage structure that protects entrapped metal ions from being released into biological system. These compounds are especially interesting because of the proven chemical reactivity of surface carbon atoms that can be used for introducing reactive functional groups for possible conjugation to additional chemotherapeutic payloads. These unique properties distinguish endohedral metallofullerenes from all other contrast agents for delivering metal ions in vivo.
11.1. Magnetic Nanoparticles as MRI Contrast Agents
Nanotechnologies based on magnetic nanoparticles (MNPs) have been applied to biological systems for diagnostic or therapeutic purposes. Magnetic resonance imaging (MRI) was employed to obtain images by magnetic characteristics of MNPs as a disturbance of the proton resonance by paramagnetic interaction with nearby MNP [83]. In addition to the use of magnetic nanoparticles as MRI contrast agents, magnetic nanoparticles can be used for magnet guided targeting of the nanoparticles themselves or of cells containing these nanoparticles for a variety of applications. Such targeting techniques are a promising approach for efficient delivery of drugs to localized disease sites, such as tumors [84]. Currently, MRI is still one of the most important diagnostic applications of magnetic nanoparticles as contrast agents.
MRI is one of the main diagnostic tools used in medicine. MRI contrast agents are used to change the relaxation times of protons in water to provide an increased image contrast between the target tissue and the surrounding background tissues. Magnevist™ (Gd-DTPA) and Omniscan™ (Gd(DTPA-BMA)) are the most commonly used MRI contrast agents. These two agents are highly soluble in water and contain Gd, a paramagnetic metal. Gadolium is responsible for shortening the spin-lattice relaxation time, T1, of hydrogen in water and produces a brighter image in the T1 weighted MRI scan. However, there are several problems associated with the use of these agents. These agents may dissociate and release toxic Gd metal to the surrounding tissues. In addition, [Gd(DTPA)(H2O)]−2 is unstable in acidic conditions. These problems prompted the need for a new class of MRI contrast agents. The new contrast agents must meet certain requirements: (1) enhance the proton relaxation rate significantly; (2) localize in the target tissue for a period of time and (3) possess good stability and be non-toxic. Also, the new contrast agents must be water-soluble to enhance the relaxation of water protons.
Water-soluble Gd-based endohedral metallofullerenes have potential to be a new generation of MRI contrast agents. Several groups have reported that Gd@C82(OH)x, a new metallofullerol, demonstrated a proton magnetic relaxation rate (1/T1) much higher than that of the commercial compounds [85]. Since the surrounding water molecules will not directly interact with the gadolium metal inside the cage, the higher relaxation rate is suggested due to the electronic interactions between the water molecules and paramagnetic cage of the metallofullerenol. The large surface area of the paramagnetic cage interacting with numerous water molecules simultaneously via hydrogen bonding makes the spin-lattice relaxation process faster and the T1 relaxation time shorter.
11.2. Medical Application and Biological Effects of Fullerenols
Proliferation, viability, metabolism, and differentiation capacity have been measured in mesenchymal stem cell (MSC) cultured with Gd@C82 fullerenol. Gd@C82 fullerenols were taken up by cells and distributed in endosomes in the cytoplasm of MSC and macrophages as shown by light and electron microscopy. It was demonstrated that cellular labeling with Gd@C82 is feasible and can produce T1 contrast enhancement on MRI. This study suggests that further investigation of Gd fullerenols for tracking viable cells, including stem cells, is warranted.
Fullerenes are effective photosensitizers. Under photoirradiation, fullerenes can induce DNA cleavage, mutations, cancer initiation, and cytotoxicity. In rat, microsomes exposed to UV and visible light, fullerene (as a cyclodextrin complex) induced time- and concentration dependent oxidative injuries seen as lipid peroxidation and protein damage. While a slight genotoxic effect was seen in the somatic mutation and recombination test (SMART) in Drosophila at the highest concentration of fullerene tested, fullerene gave negative results in the SOS chromosome test in Escherichia coli (bacterial genotoxicity test indicating DNA damage). Fullerene dissolved in polyvinylpyrrolidone was mutagenic to Salmonella typhimurium tester strains in the presence of rat liver microsomes when irradiated by visible light. Mutation was probably due to the generation of oxidized phospholipids by the action of reactive oxygen species. The photo-induced bioactivities of fullerene were suggested to be caused by reactive oxygen species (superoxide radical and OH-) generated by electron transfer reaction of fullerene with molecular oxygen [72]. On the other hand, C60(OH)22 fullerol was found to be a potent hydroxyl radical scavenger in human breast cancer cell lines [86]. This type of water-soluble fullerols had excellent anti-oxidant capacity in cultured cortical neurons [87] and prevented hydrogen peroxide- and cumene hydroperoxide-elicited changes in rat hippocampus in vitro [88].
Intraperitoneal injection of gadolinium endohedral metallofullerenol ([Gd@C82(OH)22]n nanoparticles) could decrease activities of enzymes associated with the metabolism of reactive oxygen species (ROS) in tumor-bearing mice [79]. Gd@C82(OH)22 administration can efficiently restore the functions of damaged liver and kidney of the tumor-bearing mice. It has shown that Gd@C82(OH)22 nanoparticles were delivered to almost all types of tissues in mice after intraperitoneal administration and mainly aggregated in bone, kidneys, stomach, liver, spleen, pancreas and thymus. The fullerene cage can not be destroyed during metabolism in organisms and the internal Gd3+ was not liberated and distributed to the tissues. The presence of Gd in vivo represents the exact distribution of [Gd@C82(OH)22]n nanoparticles in vivo. Watanabe et al. found that the accumulation of Gd in liver was higher than that in tumor when they investigated the accumulation of Gd-incorporating nanoemulsion in mice tumor for neutron-capture study [89]. Polyhydroxylated fullerene derivatives could be entrapped in the reticuloendothelial system (RES) and transported to all tissues by blood circulation in vivo, and retained in bone, liver, spleen and kidney [90]. Intravenous administration of Gd@C82(OH)40 to mice resulted mainly in delivery to lung, liver, spleen and kidney [90]. The prothrombin time (PT), thrombin time (TT), active partial thromboplastin time (APTT) and fibrinogen (Fbg) in plasma were measured after [Gd@C82(OH)22]n administration. The prolongation of APTT and increased fibrinogen were observed due to [Gd@C82(OH)22]n treatment, and the PT was shortened. A possible link with fibrinogen/ fibrin and tumor growth and dissemination has been clarified using tumor-bearing fibrinogen-deficient mice. The tumor cells directly or indirectly influence the coagulation system by interacting with blood cells (monocytes, platelets, neutrophils) and vascular endothelial cells. The shortened PT and increased Fbg are indications of thrombus. Moreover, blood clotting can be accelerated not only by tissue factor, but also by thrombin–antithrombin (TAT) complexes. One might suspect the increased levels of TAT are induced by the changes of one or more coagulation factors within the cascades after injection of [Gd@C82(OH)22]n particles in mice. For mice inoculated with H22 hepatoma, which induced the metabolic imbalance of detoxification, the functions of liver and spleen of experimental mice were deteriorated. Surprisingly, in the [Gd@C82(OH)22]n-treated nude mice, hepatomegaly and splenomegaly were less.. This indicates that the liver damage was inhibited. It is in agreement with the serum enzyme profiles reported before, in which, the serum AST and ALT activities, the sensitive biochemical parameters for hepatocellular damage, were significantly decreased by i.p. injection of [Gd@C82(OH)22]n. Based on renal function tests, markers of kidney damage were restored to the normal level by nanoparticle-treatments.
The activities of hepatic superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), glutathione S-transferase (GST) and catalase were measured in mice administered with [Gd@C82(OH)22]n nanoparticles, the GSH-Px, CAT and SOD activities were downregulated after treatment with [Gd@C82(OH)22]n nanoparticles. Additionally, the levels of glutathione, protein bound thiols and malondialdehyde were also lower. Hydroxylated fullerene derivatives might be novel antioxidants in vivo. [Gd@C82(OH)22]n nanoparticles help to maintain the antioxidant-oxidant balance during tumor growth in mice. GST is a member of a family of detoxification enzymes that metabolize a variety of carcinogens by conjugating lipophilic electrophiles to GSH for excretion as mercapturic acid derivatives and thioethers and thioesters. GSH plays a vital role in the protection of cells against oxidative stress and acts as an important water-phase non-enzymatic antioxidant and an essential cofactor for antioxidant enzymes taking part in cellular redox reactions [91]. Their reduction with the [Gd@C82 (OH)22]n nanoparticle treatment could be attributed to the elimination of ROS in vivo and alleviating the burden of liver detoxification.
In addition, to measure the biological effect of [Gd@C82(OH)22]n nanoparticles as ROS scavenger in vitro, electron spin resonance (ESR) spectroscopy was used to measure direct scavenging of several physiologically relevant ROS made by [Gd@C82(OH)22]n nanoparticles and inhibitory effects on lipid peroxidation. [Gd@C82(OH)22]n nanoparticles significantly reduced the ESR signal of the stable free radical, 2,2-diphenyl-1-picryhydrazyl radical (DPPH•). Similarly, studies using ESR with spin-trapping demonstrated efficient scavenging of superoxide radical anion (O2•-), hydroxyl radical (HO•) and singlet oxygen (1O2) by [Gd@C82(OH)22]n nanoparticles. In vitro studies using liposomes prepared from bovine liver phosphatidylcholine revealed that [Gd@C82(OH)22]n nanoparticles also had a strong inhibitory effect on lipid peroxidation. Consistent with their ability to scavenge free radicals and inhibit lipid peroxidation, [Gd@C82(OH)22]n nanoparticles also protected cells subjected in vitro to oxidative stress. Studies using human lung adenocarcinoma cells or rat brain capillary endothelial cells demonstrated that [Gd@C82(OH)22]n nanoparticles reduced H2O2-induced free radical formation and mitochondrial damage. In summary, the results revealed strong free radical-scavenging activities of [Gd@C82(OH)22]n nanoparticles in vitro and in vivo. As these ROS have been implicated in the etiology of a wide range of human diseases, including cancer, the present findings demonstrated that the potent inhibition of [Gd@C82(OH)22]n nanoparticles on tumor growth likely related to the high free radical scavenging capacity. [Gd@C82(OH)22]n nanoparticles may exhibit antitumor activity by enabling recovery of hepatic and renal functions and by regulating oxidative stress in tumor cells. In the future some appropriate modifications to the unique surface of [Gd@C82 (OH)22]n nanoparticles may lead to modified properties suitable for diagnostic and therapeutic applications.
12. POTENTIAL APPLICATION OF NANOMATERIALS IN NANOMEDICINE
Nanomedicine is the science and technology of diagnosing, treating and preventing disease and improving human health. From the standpoint of nanomedicine, there is an urgent need to understand the metabolic implications of nanomatrials in relationship to specific nanoscale properties. Once nanoparticles are absorbed by the gastrointestinal tract, these particles will be transported directly to the liver via the portal vein. The liver is able to actively remove compounds from the blood. Currently, there is no evidence that hepatic elimination affects the bioavailability of absorbed nanoparticles in the body.
Although there are few studies on metabolism of nanoparticles, it is considered unlikely that inert nanoparticles such as gold and silver particles, fullerenes and CNT, can be metabolized effectively by enzymes in the body. However, it is likely that nanoparticles with functionalized groups can be metabolized. For instance, the protein cap of a functionalized QD could be cleaved by proteases [92]. Also the metallic core of QDs and other metal oxides could be sequestered by metallothionein and excreted. These enzymes, present in liver and kidney, can bind metal and restore the cellular metal homeostasis [93]. In addition, nanoparticle drug-delivery systems consisting of liposomes are able to fuse with cell membranes and enable intracellular delivery of nanoparticles. The intracellular load of nanoparticles could then be metabolized according to the normal metabolic pathways described for the conventionally formulated drugs.
Dendrimers, another kind of nanoparticles, are characterized by a combination of high degree of end-group functionality and a compact, precisely defined molecular structure. These characteristics can be used in biomedical applications for the amplification or multiplication of effects on a molecular level, or to create extremely high local concentrations of drugs, molecular labels, or probe moieties. In diagnostics, dendrimers that bear GdIII complexes are used as contrast agents in magnetic resonance imaging. DNA dendrimers have potential for routine use in high-throughput functional genomic analysis, and as DNA biosensors. Dendrimers are also being investigated for therapeutics, for example, as carriers for controlled drug delivery, in gene transfection, as well as in neutron-capture therapy.
The ability of tracing a single molecule at both light microscopic and ultrastructural levels is a powerful tool in understanding the dynamics of cellular function. The search for optimal techniques for tracing QDs at light microscopic and ultrastructural levels is being actively pursued. QD probes are nanometer-sized semi-conductors with fluorescent properties suitable for biological imaging of the tagged molecules [94]. OD sized 5–10 nm have been suggested to be more photo stable than conventional fluorophores [95, 96]. The most commonly used ODs are Cd–Se, Zn–S, Cd–Te, Cd–S and Pb–S nanocrystals and they are all commercially available. QDs offer a favorable compromise between small fluorophores and large beads for single molecule experiments in living cells. QDs will be invaluable tools for ultrasensitive studies of the dynamics of cellular metabolism [97].
Micronised particles with a diameter of about 15 nm are known to be used in sunscreens as physical UV filter. As potential therapy, penetration of micronised Ti, Zn and Si oxides into the skin was investigated due to their small particle size. Coated nanoparticles were found in the epidermis and dermis of the treated human skin samples taken during surgery. It is suggested that nanoparticles could pass through the uppermost horny skin layer (stratum corneum) via intercellular channels and penetrate into deeper vital skin layers. In addition, TiO2 nanoparticles photoexcited by UV irradiation could suppress the growth of tumor cells implanted in nude mice. This cytotoxic effect of photoexcited TiO2 particles is associated with the generation of strong reactive oxygen species such as OH· and H2O2 on the surface of TiO2 nanoparticles. This radical generating property of TiO2 nanoparticles makes them ideal candidates for cancer treatment. The cell killing effect of TiO2 nanoparticles could be adapted to an anticancer modality by the local or regional administration of TiO2 nanoparticles to the tumor, followed by light irradiation focusing on the tumor, especially for the treatment of superficial tumors in an organ appropriate for light exposure such as skin, oral cavity, trachea, and urinary bladder. It may be possible to modify the surface characteristics of TiO2 or other nanoparticles to produce stronger and more extensive anticancer effects without troublesome side effects.
It will be important to be aware of the potential issues to safely and efficiently propel nanotechnology forward into clinical application. At the National Institute of Health, USA, the National Cancer Institute (NCI) led a National Nanotechnology Initiative and supported a Nanotechnology Characterization Laboratory to perform preclinical toxicology and other studies. In addition, the National Institute for Enviromental Health and Safety (NIEHS) also funded a Nanotechnology Safety Initiative through the National Toxicology program. It will be essential to develop a systematic program of nanotechnology for long-term safety and clinical application of nanomaterials.
CONCLUSIONS
The metabolism of nanoparticles in the body strongly depends on the surface characteristics of the nanomaterials. A threshold limited size of nanoparticles may exist for restricting the movement of nanoparticles in various parts of body. The pharmacokinetic behaviors of different types of nanoparticles require thorough investigation. The biological effects associated with different nanoparticles should be studied at the target organs, tissues and cellular levels. The assessment of biological effects in cardiopulmonary system is necessary for every new nanoparticle to be used for medical application. There is no universal “nanoparticle” to fit for all needs. Each nanomaterial should be treated individually for evaluation of biological effects. It is challenging to put together a set of high throughput and low cost tests for assessing biological effects of nanoparticles without compromising efficiency and reliability. Nanoparticles designed for drug delivery or as nanomedicine need special attention.
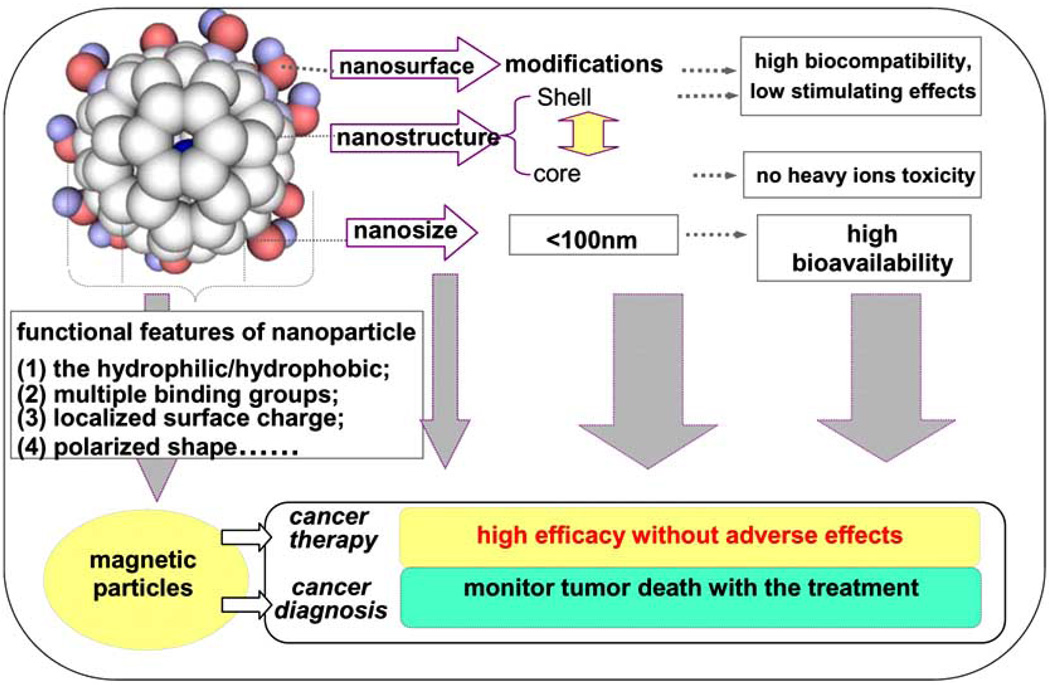
This is particularly important for nanoparticles than for larger particles because of the difference in their surface area and the resulting changes in their physico-chemical properties (Fig. 2). The available information on most nanoparticles indicates that certain nanoparticles may be genotoxic and phototoxic and photogenotoxic. There is need for more data on toxicokinetics and toxicodynamics of insoluble nanoparticles with respect to different uptake routes. There are only a few long term studies reported that are related to nanomaterials including SiO2, CNT, and TiO2. Nanoparticles may have potential for generating free radicals and exhibit oxidative tendency depending on their surface characteristics. The toxicokinetics and metabolism of nanomaterials have not been studied in detail [98]. Therefore, it is difficult to model the metabolism of nanomaterials. In particular, there is limited information about how the physico-chemical parameters of nanoparticles affect their absorption and transport across barriers of skin, gut, lungs and eye, and their entry into systemic circulation, metabolism, accumulation in secondary target organs and excretion. Because the current static imaging technology used for in vivo investigations on biodistribution of nanoparticles can not detect small fractions of nanoparticles in vascular bed, blood stream and inner organs of body, we need to develop better methods to quantitate more precisely the unique metabolism of nanoparticles in biological systems.
Fig. (2). Unique structural and surface features of nanoparticles.
This sandwich-type nanostructure carries a potent anticancer nanomedicine that may produce little side-effects to normal tissues in vivo and nearly no cytotoxicity to normal cells in vitro. The inner core with or without heavy metallic atom, the type and number of outer surrounded functional-groups can be changed to generate different series of relatively non toxic anticancer nanomedicine. The size of individual particle is at nanolevel (less than 100 nm). The paramagnetic metallic atom inside the cage can serve as an MRI contrast agent to simultaneously monitor the chemotherapeutic effects during treatment of tumor.
Acknowledgements
This work is financially supported by the Chinese Academy of Sciences (CAS) “Hundred Talents Program” (07165111ZX), MOST 2006CB705600, National Basic Research Program of China (973 Program) No. 2009CB930200, NSFC 10525524 and in part by NIH/NCRR/RCMI 2G12RR003048, NIH 5U 54CA091431, and USAMRMC W81XWH-05-1-0291 grants.
LIST OF ABBREVIATIONS
- NNI
National Nanotechnology Initiative
- ADME
Absorption, distribution, metabolism and excretion
- CNT
Carbon nanotube
- SWNT
Single-walled carbon nanotube
- ICP-MS
Inductively couple plasma-mass spectroscopy
- TiO2
Titanium dioxide
- SiO2
Silicon dioxide
- NLs
Nano-liposomes
- SEM
Scanning electronic microscopy
- AFM
Atomic force microscopy
- QD
Quantum dots
- CdSe
Cadmium selenide
- Cyt-B
Cytochalasin B
- TBIL
Total bilirubin levels
- ALP
Alkaline phosphatase
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- CK
Creatine kinase
- LDH
Lactate dehydrogenase
- HBDH
Alpha-hydroxybutyrate dehydrogenase
- UA
Uric acid
- BUN
Blood urea nitrogen
- CNS
Central nervous system
- AD
Alzheimer’s disease
- PD
Parkinson’s disease
- MS
Multiple sclerosis
- TBAOH
Tetrabutylammonium hydroxide
- SMART
Somatic mutation and recombination test
- ROS
Reactive oxygen species
- RES
Reticuloendothelial system
- SOD
Superoxide dismutase
- GSH-Px
Glutathione peroxidase
- GST
Glutathione S-transferase
- ESR
Electron spin resonance
REFERENCES
- 1.Jia L. Global governmental investment in nanotechnologies. Curr. Nanosci. 2005;3:263–266. doi: 10.2174/157341305774642957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitesides GM. The ‘right’ size in nanobiotechnology. Nat. Biotechnol. 2003;21:1161–1165. doi: 10.1038/nbt872. [DOI] [PubMed] [Google Scholar]
- 3.Seaton A, Donaldson K. Nanoscience, nanotoxicology, and the need to think small. Lancet. 2005;365:923–924. doi: 10.1016/S0140-6736(05)71061-8. [DOI] [PubMed] [Google Scholar]
- 4.Mazzola L. Commercializing nanotechnology. Nat. Biotechnol. 2003;21:1137–1143. doi: 10.1038/nbt1003-1137. [DOI] [PubMed] [Google Scholar]
- 5.Paull R, Wolfe J, Hebert P, Sinkula M. Investing in nanotechnology. Nat. Biotechnol. 2003;21:1144–1147. doi: 10.1038/nbt1003-1144. [DOI] [PubMed] [Google Scholar]
- 6.Roszek B, de Jong WH, Geertsma RE. Nanotechnology in medical applications: state-of-the-art in materials and devices. 2005 RIVM report 265001001/2005. [Google Scholar]
- 7.Jia L. Nanoparticle formulation Increases oral bioavailability of poorly soluble drugs: experimental evidences, theory, and approaches. Curr. Nanosci. 2005;3(1):237–243. doi: 10.2174/157341305774642939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oberdorster G OE, Oberdorster J. Nanotoxicology: An Emerging Discipline Evolving from Studies of Ultrafine Particles. Environ. Health Perspect. 2005b;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Jong WH, Roszek B, Geertsma RE. Nanotechnology in medical applications: possible risks for human health. 2005 RIVM report 265001002/2005. [Google Scholar]
- 10.Zhao Y, Nalwa HS. Nanotoxicology. California: American Scientific Publishers; 2007. 2007. [Google Scholar]
- 11.Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 12.Hoet PH, Bruske-Hohlfeld I, Salata OV. Nanoparticles - known and unknown health risks. J. Nanobiotechnol. 2004;2:12. doi: 10.1186/1477-3155-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y, Wang HF. Nanotechnology tackles tumours. Nat. Nanotechnol. 2007;2(1):20–21. doi: 10.1038/nnano.2006.188. [DOI] [PubMed] [Google Scholar]
- 14.Jia LW, Cerna C, Weitman S. Effect of nanonization on absorption of 301029: Ex vivo and in vivo pharmacokinetic correlations determined by LC/MS. Pharm. Res. 2002;14:1091–1096. doi: 10.1023/a:1019829622088. [DOI] [PubMed] [Google Scholar]
- 15.Bittner B, Mountfield RJ. Intravenous administration of poorly soluble new drug entities in early drug discovery: the potential impact of formulation on pharmacokinetic parameters. Curr. Opin. Drug Discov. Dev. 2002;5:59–71. [PubMed] [Google Scholar]
- 16.Li N, Kommireddy DS, Lvov Y, Liebenberg W, Tiedt LR, De Villiers MM. Nanoparticle multilayers: surface modification of photosensitive drug microparticles for increased stability and in vitro bioavailability. J. Nanosci. Nanotechnol. 2006;6:3252–3260. doi: 10.1166/jnn.2006.421. [DOI] [PubMed] [Google Scholar]
- 17.Kam NW, O’Connell M, Wisdom JA, Dai H. Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction. Proc. Natl. Acad. Sci. USA. 2005;102:11600–11605. doi: 10.1073/pnas.0502680102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pantarotto D, Briand JP, Prato M, Bianco A. Translocation of bioactive peptides across cell membranes by carbon nanotubes. Chem. Commun. (Camb.) 2004;1:16–17. doi: 10.1039/b311254c. [DOI] [PubMed] [Google Scholar]
- 19.Kostarelos KLL, Pastorin G, Wu W, Wieckowski S, Luangsivilay J, Godefroy S, Pantarotto D, Briand J, Muller S, Prato M, Bianco A. Cellular uptake of functionalized carbon nanotubes is independent of functional group and cell type. Nat. Nanotechnol. 2007;2:108–113. doi: 10.1038/nnano.2006.209. [DOI] [PubMed] [Google Scholar]
- 20.Arunachalam B, Phan UT, Geuze HJ, Cresswell P. Enzymatic reduction of disulfide bonds in lysosomes: characterization of a gamma-interferon-inducible lysosomal thiol reductase (GILT) Proc. Natl. Acad. Sci. USA. 2000;97:745–750. doi: 10.1073/pnas.97.2.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feazell RP, Nakayama-Ratchford N, Dai H, Lippard SJ. Soluble single-walled carbon nanotubes as longboat delivery systems for platinum(IV) anticancer drug design. J. Am. Chem. Soc. 2007;129:8438–8439. doi: 10.1021/ja073231f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Service RF. American Chemical Society meeting. Nanomaterials show signs of toxicity. Science. 2003;300:243. doi: 10.1126/science.300.5617.243a. [DOI] [PubMed] [Google Scholar]
- 23.Zhu L, Chang DW, Dai L, Hong Y. DNA damage induced by multiwalled carbon nanotubes in mouse embryonic stem cells. Nano. Lett. 2007;7:3592–3597. doi: 10.1021/nl071303v. [DOI] [PubMed] [Google Scholar]
- 24.Lam CW, James JT, McCluskey R, Hunter RL. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol. Sci. 2004;77:126–134. doi: 10.1093/toxsci/kfg243. [DOI] [PubMed] [Google Scholar]
- 25.Liu A, Sun K, Yang J, Zhao D. Toxicological effects of multi-wall carbon nanotubes in rats. J. Nanopart. Res. 2008 [Google Scholar]
- 26.Maynard AD, Baron PA, Foley M, Shvedova AA, Kisin ER, Castranova V. Exposure to carbon nanotube material: aerosol release during the handling of unrefined single-walled carbon nanotube material. J. Toxicol. Environ. Health A. 2004;67:87–107. doi: 10.1080/15287390490253688. [DOI] [PubMed] [Google Scholar]
- 27.Shedova AA, Castranova V, Kisin ER, Schwegler-Berry D, Murray AR, Gandelsman VZ, M, A, Baron P. Exposure to carbon nanotube material: assessment of nanotube cytotoxicity using human keratinocyte cells. J. Toxicol. Environ. Health A. 2003;66:1909–1926. doi: 10.1080/713853956. [DOI] [PubMed] [Google Scholar]
- 28.Deng XJ, G, Wang H, Sun H, Wang X, Yang S, Wang T, Liu Y. Translocation and fate of multi-walled carbon nanotubes in vivo. Carbon. 2007;45:1419–1424. [Google Scholar]
- 29.Moller W, Brown DM, Kreyling WG, Stone V. Ultrafine particles cause cytoskeletal dysfunctions in macrophages: role of intracellular calcium. Part. Fibre Toxicol. 2005;2:7. doi: 10.1186/1743-8977-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Driscoll KE, Deyo LC, Carter JM, Howard BW, Hassenbein DG, Bertram TA. Effects of particle exposure and particle-elicited inflammatory cells on mutation in rat alveolar epithelial cells. Carcinogenesis. 1997;18:423–430. doi: 10.1093/carcin/18.2.423. [DOI] [PubMed] [Google Scholar]
- 31.Chougule M, Padhi B, Misra A. Nano-liposomal dry powder inhaler of tacrolimus: preparation, characterization, and pulmonary pharmacokinetics. Int. J. Nanomed. 2007;2:675–688. [PMC free article] [PubMed] [Google Scholar]
- 32.Warheit DB, Laurence BR, Reed KL, Roach DH, Reynolds GA, Webb TR. Comparative pulmonary toxicity assessment of single-wall carbon nanotubes in rats. Toxicol. Sci. 2004;77:117–125. doi: 10.1093/toxsci/kfg228. [DOI] [PubMed] [Google Scholar]
- 33.Pope CA, 3rd, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109:71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- 34.Borm PJ, Schins RP, Albrecht C. Inhaled particles and lung cancer, part B: paradigms and risk assessment. Int. J. Cancer. 2004;110:3–14. doi: 10.1002/ijc.20064. [DOI] [PubMed] [Google Scholar]
- 35.Pantarotto D, Singh R, McCarthy D, Erhardt M, Briand JP, Prato M, Kostarelos K, Bianco A. Functionalized carbon nanotubes for plasmid DNA gene delivery. Angew. Chem. Int. Ed. Engl. 2004;43:5242–5246. doi: 10.1002/anie.200460437. [DOI] [PubMed] [Google Scholar]
- 36.Singh R, Pantarotto D, McCarthy D, Chaloin O, Hoebeke J, Partidos CD, Briand JP, Prato M, Bianco A, Kostarelos K. Binding and condensation of plasmid DNA onto functionalized carbon nanotubes: toward the construction of nanotube-based gene delivery vectors. J. Am. Chem. Soc. 2005;127:4388–4396. doi: 10.1021/ja0441561. [DOI] [PubMed] [Google Scholar]
- 37.He P, Bayachou M. Layer-by-layer fabrication and characterization of DNA-wrapped single-walled carbon nanotube particles. Langmuir. 2005;21:6086–6092. doi: 10.1021/la050581b. [DOI] [PubMed] [Google Scholar]
- 38.Tan Y, Whitmore M, Li S, Frederik P, Huang L. LPD nanoparticles–novel nonviral vector for efficient gene delivery. Methods Mol. Med. 2002;69:73–81. doi: 10.1385/1-59259-141-8:073. [DOI] [PubMed] [Google Scholar]
- 39.Gemeinhart RA, Luo D, Saltzman WM. Cellular fate of a modular DNA delivery system mediated by silica nanoparticles. Biotechnol. Prog. 2005;21:532–537. doi: 10.1021/bp049648w. [DOI] [PubMed] [Google Scholar]
- 40.Bejjani RA, BenEzra D, Cohen H, Rieger J, Andrieu C, Jeanny JC, Gollomb G, Behar-Cohen FF. Nanoparticles for gene delivery to retinal pigment epithelial cells. Mol. Vis. 2005;11:124–132. [PubMed] [Google Scholar]
- 41.Zheng M, Jagota A, Semke ED, Diner BA, McLean RS, Lustig SR, Richardson RE, Tassi NG. DNA-assisted dispersion and separation of carbon nanotubes. Nat. Mater. 2003;2:338–342. doi: 10.1038/nmat877. [DOI] [PubMed] [Google Scholar]
- 42.Rajendra J, Baxendale M, Dit Rap LG, Rodger A. Flow linear dichroism to probe binding of aromatic molecules and DNA to single-walled carbon nanotubes. J. Am. Chem. Soc. 2004;126:11182–11188. doi: 10.1021/ja048720j. [DOI] [PubMed] [Google Scholar]
- 43.Rajendra J, Rodger A. The binding of single-stranded DNA and PNA to single-walled carbon nanotubes probed by flow linear dichroism. Chemistry. 2005;11:4841–4847. doi: 10.1002/chem.200500093. [DOI] [PubMed] [Google Scholar]
- 44.Audette GF, van Schaik J, Hazes B, Irvin RT. DNA-binding protein nanotubes. Nano. Lett. 2004;4:1897–1902. [Google Scholar]
- 45.Green M, Howman E. Semiconductor quantum dots and free radical induced DNA nicking. Chem. Commun. (Camb) 2005;7(1):121–123. doi: 10.1039/b413175d. [DOI] [PubMed] [Google Scholar]
- 46.Florence AT, Hussain N. Transcytosis of nanoparticle and dendrimer delivery systems: evolving vistas. Adv. Drug. Deliv. Rev. 2001;50(Suppl 1):S69–S89. doi: 10.1016/s0169-409x(01)00184-3. [DOI] [PubMed] [Google Scholar]
- 47.Li WC, Ye C, Wei T, Zhao Y, Lao F, Chen Z, Meng H, Gao Y, Yuan H, Xing G, Zhao F, Chai Z, Zhao Y, Yang F, Han D, Tang X, Zhang Y. The translocation of Fullerenic nanoparticles into Iysosome via the pathway of clathrin-mediated endocytosis. Nanotechnology. 2008;19:145102–145114. doi: 10.1088/0957-4484/19/14/145102. [DOI] [PubMed] [Google Scholar]
- 48.Heckel K, Kiefmann R, Dorger M, Stoeckelhuber M, Goetz AE. Colloidal gold particles as a new in vivo marker of early acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2004;287:L867–L878. doi: 10.1152/ajplung.00078.2004. [DOI] [PubMed] [Google Scholar]
- 49.Nemmar A, Hoet PH, Vanquickenborne B, Dinsdale D, Thomeer M, Hoylaerts MF, Vanbilloen H, Mortelmans L, Nemery B. Passage of inhaled particles into the blood circulation in humans. Circulation. 2002;105:411–414. doi: 10.1161/hc0402.104118. [DOI] [PubMed] [Google Scholar]
- 50.Nemmar A, Vanbilloen H, Hoylaerts MF, Hoet PH, Verbruggen A, Nemery B. Passage of intratracheally instilled ultrafine particles from the lung into the systemic circulation in hamster. Am. J. Respir. Crit. Care Med. 2001;164:1665–1668. doi: 10.1164/ajrccm.164.9.2101036. [DOI] [PubMed] [Google Scholar]
- 51.Verma DD, Verma S, Blume G, Fahr A. Particle size of liposomes influences dermal delivery of substances into skin. Int. J. Pharm. 2003;258:141–151. doi: 10.1016/s0378-5173(03)00183-2. [DOI] [PubMed] [Google Scholar]
- 52.Langer R. Biomaterials in drug delivery and tissue engineering: one laboratory’s experience. Acc. Chem. Res. 2000;33:94–101. doi: 10.1021/ar9800993. [DOI] [PubMed] [Google Scholar]
- 53.Meng H, Chen Z, Xing G, Yuan H, Chen C, Zhao F, Zhang C, Zhao Y. Ultrahigh reactivity provokes nanotoxicity: explanation of oral toxicity of nano-copper particles. Toxicol. Lett. 2007;175:102–110. doi: 10.1016/j.toxlet.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 54.Wang B, Feng WY, Wang TC, Jia G, Wang M, Shi JW, Zhang F, Zhao YL, Chai ZF. Acute toxicity of nano-and micro-scale zinc powder in healthy adult mice. Toxicol. Lett. 2006;161:115–123. doi: 10.1016/j.toxlet.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 55.Chen Z, Meng H, Xing G, Chen C, Zhao Y, Jia G, Wang T, Yuan H, Ye C, Zhao F, Chai Z, Zhu C, Fang X, Ma B, Wan L. Acute toxicological effects of copper nanoparticles in vivo. Toxicol. Lett. 2006;163:109–120. doi: 10.1016/j.toxlet.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 56.Wang J, Zhou G, Chen C, Yu H, Wang T, Ma Y, Jia G, Gao Y, Li B, Sun J, Li Y, Jiao F, Zhao Y, Chai Z. Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol. Lett. 2007;168:176–185. doi: 10.1016/j.toxlet.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 57.Oberdorster G, Ferin J, Lehnert BE. Correlation between particle size, in vivo particle persistence, and lung injury. Environ. Health Perspect. 1994;102 Suppl. 5:173–179. doi: 10.1289/ehp.102-1567252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huggins CB, Froehlich JP. High concentration of injected titanium dioxide in abdominal lymph nodes. J. Exp. Med. 1966;124:1099–1106. doi: 10.1084/jem.124.6.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kreyling WG, Semmler-Behnke M, Moller W. Ultrafine particle-lung interactions: does size matter? J. Aerosol. Med. 2006;19:74–83. doi: 10.1089/jam.2006.19.74. [DOI] [PubMed] [Google Scholar]
- 60.Borm P, Klaessig FC, Landry TD, Moudgil B, Pauluhn J, Thomas K, Trottier R, Wood S. Research strategies for safety evaluation of nanomaterials, part V: role of dissolution in biological fate and effects of nanoscale particles. Toxicol. Sci. 2006;90:23–32. doi: 10.1093/toxsci/kfj084. [DOI] [PubMed] [Google Scholar]
- 61.Olivier JC. Drug transport to brain with targeted nanoparticles. NeuroRx. 2005;2:108–119. doi: 10.1602/neurorx.2.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boffetta P, Soutar A, Cherrie JW, Granath F, Andersen A, Anttila A, Blettner M, Gaborieau V, Klug SJ, Langard S, Luce D, Merletti F, Miller B, Mirabelli D, Pukkala E, Adami HO, Weiderpass E. Mortality among workers employed in the titanium dioxide production industry in Europe. Cancer. Causes Control. 2004;15:697–706. doi: 10.1023/B:CACO.0000036188.23970.22. [DOI] [PubMed] [Google Scholar]
- 63.Oberdorster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling W, Cox C. Translocation of inhaled ultrafine particles to the brain. Inhal. Toxicol. 2004;16:437–445. doi: 10.1080/08958370490439597. [DOI] [PubMed] [Google Scholar]
- 64.Oberdorster E. Manufactured nanomaterials (fullerenes, C60) induce oxidative stress in the brain of juvenile largemouth bass. Environ. Health Perspect. 2004;112:1058–1062. doi: 10.1289/ehp.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang JC, Yu H, Sun J, Li B, Li Y, Gao Y, Chai Z, He W, Huang Y, Zhao Y. Distribution of TiO2 particles in the olfactory bulb of mice after nasal inhalation using microbeam SRXRF mapping techniques. J. Radioanal. Nucl. Chem. 2007;272(3):527–531. [Google Scholar]
- 66.Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kroto HW, HJR, O’Brien SC, Curl RF, Smalley RE. C60: Buckminsterfullerene. Nature. 1985;318:162–163. [Google Scholar]
- 68.Liu S, Lu YJ, Kappes MM, Ibers JA. The Structure of the C60 Molecule: X-Ray Crystal Structure Determination of a Twin at 110 K. Science. 1991;254:408–410. doi: 10.1126/science.254.5030.408. [DOI] [PubMed] [Google Scholar]
- 69.Kratschmer W, LDL, Fostiropoulos K, Huffman DR. Solid C60: a new form of carbon. Nature. 1990;347:354–357. [Google Scholar]
- 70.Qiao R, Roberts AP, Mount AS, Klaine SJ, Ke PC. Translocation of C60 and its derivatives across a lipid bilayer. Nano. Lett. 2007;7:614–619. doi: 10.1021/nl062515f. [DOI] [PubMed] [Google Scholar]
- 71.Satoh M, Takayanagi I. Pharmacological studies on fullerene (C60), a novel carbon allotrope, and its derivatives. J. Pharmacol. Sci. 2006;100:513–518. doi: 10.1254/jphs.cpj06002x. [DOI] [PubMed] [Google Scholar]
- 72.Yamakoshi Y, Umezawa N, Ryu A, Arakane K, Miyata N, Goda Y, Masumizu T, Nagano T. Active oxygen species generated from photoexcited fullerene (C60) as potential medicines: O2-* versus 1O2. J. Am. Chem. Soc. 2003;125:12803–12809. doi: 10.1021/ja0355574. [DOI] [PubMed] [Google Scholar]
- 73.Zhao X, Striolo A, Cummings PT. C60 binds to and deforms nucleotides. Biophys. J. 2005;89:3856–3862. doi: 10.1529/biophysj.105.064410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Friedman SHD, DL, Sijbesma RP, Srdanov G, Wudl F, Kenyon GL. Inhibitionof HIV-1 Protease by Fullerene Derivatives: Model Building Studies and Experimental Verification. J. Am. Chem. Soc. 1993;115:6505–6509. [Google Scholar]
- 75.Buseck PR, Tsipursky SJ, Hettich R. Fullerenes from the Geological Environment. Science. 1992;257:215–217. doi: 10.1126/science.257.5067.215. [DOI] [PubMed] [Google Scholar]
- 76.Tang J, Xing G, Zhao F, Yuan H, Zhao Y. Modulation of structural and electronic properties of fullerene and metallofullerenes by surface chemical modifications. J. Nanosci. Nanotechnol. 2007;7:1085–1101. doi: 10.1166/jnn.2007.301. [DOI] [PubMed] [Google Scholar]
- 77.Tang J, Xing G, Zhao Y, Jing L, Gao X, Cheng Y, Yuan H, Zhao F, Chen Z, Meng H, Zhang H, Qian H, Su R, Ibrahim K. Periodical variation of electronic properties in polyhydroxylated metallofullerene materials. Adv. Mater. 2006;18:1458–1462. [Google Scholar]
- 78.Chen C, Xing G, Wang J, Zhao Y, Li B, Tang J, Jia G, Wang T, Sun J, Xing L, Yuan H, Gao Y, Meng H, Chen Z, Zhao F, Chai Z, Fang X. Antioxidative function and biodistribution of [Gd@C82(OH)22]n nanoparticles in tumor-bearing mice. Nano. Lett. 2005;5:2050–2057. [Google Scholar]
- 79.Wang J, Chen C, Li B, Yu H, Zhao Y, Sun J, Li Y, Xing G, Yuan H, Tang J, Chen Z, Meng H, Gao Y, Ye C, Chai Z, Zhu C, Ma B, Fang X, Wan L. One-dimensional metallofullerene crystal generated inside single-walled carbon nanotubes. Biochem. Pharmacol. 2006;71:872–881. [Google Scholar]
- 80.Hirahara K, Suenaga K, Bandow S, Kato H, Okazaki T, Shinohara H, Iijima S. In vivo studies of fullerene-based materials using endohedral metallofullerene radiotracers. Phys. Rev. Lett. 2000;85:5384–5387. doi: 10.1103/PhysRevLett.85.5384. [DOI] [PubMed] [Google Scholar]
- 81.Cagle DW, Kennel SJ, Mirzadeh S, Alford JM, Wilson LJ. The structural determination of endohedral metallofullerene Gd@C(82) by XANES. Proc. Natl. Acad. Sci. USA. 1999;96:5182–5187. doi: 10.1073/pnas.96.9.5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu L, Gao B, Chu W, Chen D, Hu T, Wang C, Dunsch L, Marcelli A, Luo Y, Wu Z. Magnetic Nanoparticles for Cancer Therapy. Chem. Commun. (Camb) 2008;28(4):474–476. doi: 10.1039/b714603e. [DOI] [PubMed] [Google Scholar]
- 83.Goya GFGV, Ibarra MR. Magnetically modulated therapeutic systems. Curr. Nanosci. 2008;4(1):1–16. [Google Scholar]
- 84.Häfeli UIJP. Antioxidant, prooxidant and cytotoxic activity of hydroxylated resveratrol analogues: structure-activity relationship. Int. J. Pharm. 2004;277:19–24. doi: 10.1016/j.bcp.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 85.Murias M, Jager W, Handler N, Erker T, Horvath Z, Szekeres T, Nohl H, Gille L. A biologically effective fullerene (C60) derivative with superoxide dismutase mimetic properties. Biochem. Pharmacol. 2005;69:903–912. doi: 10.1016/j.bcp.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 86.Zhu X, Zhu L, Li Y, Duan Z, Chen W, Alvarez PJ. Developmental toxicity in zebrafish (Danio rerio) embryos after exposure to manufactured nanomaterials: buckminsterfullerene aggregates (nC60) and fullerol. Environ. Toxicol. Chem. 2007;26:976–979. doi: 10.1897/06-583.1. [DOI] [PubMed] [Google Scholar]
- 87.Dugan LL, Gabrielsen JK, Yu SP, Lin TS, Choi DW. Buckminsterfullerenol free radical scavengers reduce excitotoxic and apoptotic death of cultured cortical neurons. Neurobiol. Dis. 1996;3:129–135. doi: 10.1006/nbdi.1996.0013. [DOI] [PubMed] [Google Scholar]
- 88.Tsai MC, Chen YH, Chiang LY. Polyhydroxylated C60, fullerenol, a novel free-radical trapper, prevented hydrogen peroxide- and cumene hydroperoxide-elicited changes in rat hippocampus in vitro. J. Pharm. Pharmacol. 1997;49:438–445. doi: 10.1111/j.2042-7158.1997.tb06821.x. [DOI] [PubMed] [Google Scholar]
- 89.Watanabe T, Ichikawa H, Fukumori Y. Tumor accumulation of gadolinium in lipid-nanoparticles intravenously injected for neutron-capture therapy of cancer. Eur. J. Pharm. Biopharm. 2002;54:119–124. doi: 10.1016/s0939-6411(02)00085-1. [DOI] [PubMed] [Google Scholar]
- 90.Mikawa M, Kato H, Okumura M, Narazaki M, Kanazawa Y, Miwa N, Shinohara H. Paramagnetic water-soluble metallofullerenes having the highest relaxivity for MRI contrast agents. Bioconjug. Chem. 2001;12:510–514. doi: 10.1021/bc000136m. [DOI] [PubMed] [Google Scholar]
- 91.Sies H. Glutathione and its role in cellular functions. Free. Radic. Biol. Med. 1999;27:916–921. doi: 10.1016/s0891-5849(99)00177-x. [DOI] [PubMed] [Google Scholar]
- 92.Hardman R. A toxicologic review of quantum dots: toxicity depends on physicochemical and environmental factors. Environ. Health Perspect. 2006;114:165–172. doi: 10.1289/ehp.8284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Coyle P, Philcox JC, Carey LC, Rofe AM. Metallothionein: the multipurpose protein. Cell. Mol. Life Sci. 2002;59:627–647. doi: 10.1007/s00018-002-8454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yacoby A, Heiblum M, Mahalu D, Shtrikman H. Coherence and Phase Sensitive Measurements in a Quantum Dot. Phys. Rev. Lett. 1995;74:4047–4050. doi: 10.1103/PhysRevLett.74.4047. [DOI] [PubMed] [Google Scholar]
- 95.Chan WC, Maxwell DJ, Gao X, Bailey RE, Han M, Nie S. Luminescent quantum dots for multiplexed biological detection and imaging. Curr. Opin. Biotechnol. 2002;13:40–46. doi: 10.1016/s0958-1669(02)00282-3. [DOI] [PubMed] [Google Scholar]
- 96.Wu X, Liu H, Liu J, Haley KN, Treadway JA, Larson JP, Ge N, Peale F, Bruchez MP. Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots. Nat. Biotechnol. 2003;21:41–46. doi: 10.1038/nbt764. [DOI] [PubMed] [Google Scholar]
- 97.Dahan M, Levi S, Luccardini C, Rostaing P, Riveau B, Triller A. Diffusion dynamics of glycine receptors revealed by single-quantum dot tracking. Science. 2003;302:442–445. doi: 10.1126/science.1088525. [DOI] [PubMed] [Google Scholar]
- 98.Chen Z, Meng H, Xing G, Chen C, Zhao Y. Toxicological and biological effects of nanomaterials. Int. J. Nanotechnol. 2007;4(12):179–196. [Google Scholar]