Abstract
Background
Alcoholics report persistent alcohol craving that is heightened by cognitive cues, stressful situations, and abstinence. The role of endogenous cannabinoids in human alcohol craving—though long suspected—remains elusive.
Materials and methods
We employed laboratory exposure to stress, alcohol cue, and neutral relaxed situations through guided imagery procedures to evoke alcohol desire and craving in healthy social drinkers (n=11) and in treatment-engaged, recently abstinent alcoholic subjects (n=12) and assessed alcohol craving, heart rate, and changes in circulating endocannabinoid levels. Subjective anxiety was also measured as a manipulation check for the procedures.
Results
In healthy social drinkers, alcohol cue imagery increased circulating levels of the endocannabinoid anandamide, whereas neutral and stress-related imagery had no such effect. Notably, baseline and response anandamide levels in these subjects were negatively and positively correlated with self-reported alcohol craving scores, respectively. Cue-induced increases in heart rate were also correlated with anandamide responses. By contrast, no imagery-induced anandamide mobilization was observed in alcoholics, whose baseline anandamide levels were markedly reduced compared to healthy drinkers and were uncorrelated to either alcohol craving or heart rate.
Conclusions
The results suggest that plasma anandamide levels provide a marker of the desire for alcohol in social drinkers, which is suppressed in recently abstinent alcoholics.
Keywords: Alcoholism, Anandamide, Addiction, Endocannabinoid, Alcohol craving
Introduction
Unlike healthy drinkers, who can moderate their desire for alcohol, alcoholics often suffer from persistent craving that is heightened by cognitive cues, stressful life events, or abstinence (Sinha 2007; Sinha et al. 2009). Theories of alcohol addiction conceptualize this loss of control as a disease state in which homeostatic mechanisms that respond to rewarding stimuli are partly replaced by maladaptive stress signals (Koob and Le Moal 2005; Sinha 2007). The neural processes underlying this transition remain incompletely understood, though animal studies suggest involvement of multiple neurotransmitter systems, including the endogenous cannabinoids anandamide (arachidonoylethanolamide) and 2-arachidonoylglycerol (2-AG) (Fattore et al. 2007). Animal experiments have implicated these lipid messengers in the response to pleasurable stimuli (Bortolato et al. 2007; Mahler et al. 2007; Rademacher and Hillard 2007; Soria-Gomez et al. 2007;) and in the modulation of stress-related behaviors (Bortolato et al. 2007; Gobbi et al. 2005; Hill et al. 2005, 2006a, b; Hohmann et al. 2005; Kathuria et al. 2003; Patel et al. 2005; Scherma et al. 2008). For example, blockade of anandamide deactivation by inhibition of the intracellular enzyme fatty-acid amide hydrolase (FAAH; Kathuria et al. 2003) restores normal body-weight gain and sucrose intake in rats subjected to chronic mild stress (Bortolato et al. 2007), while inhibition of anandamide transport reduces alcohol self-administration in rats (Cippitelli et al. 2007). Moreover, FAAH inhibition or direct anandamide injections into the nucleus accumbens stimulate feeding (Soria-Gomez et al. 2007) and enhance “liking” reactions elicited by intraoral sucrose in rats (Mahler et al. 2007). On the other hand, genetic or pharmacological blockade of FAAH activity increased alcohol preference in mice (Vinod et al. 2008). Despite this and other emerging evidence (Basavarajappa 2007), the role of the endocannabinoid system in the response to rewarding stimuli in humans remains largely unknown.
Alcohol craving can be elicited experimentally by having subjects imagine, while listening to audio-taped narratives, personalized alcohol-related or stressful life events reconstructed from prior interviews. Using this guided-imagery approach, it has been shown that (1) exposure to alcohol or drug-related imagery increases alcohol craving in healthy drinkers, alcoholics, and drug-dependent individuals (Fox et al. 2007, 2008, Sinha et al. 2003, 2009); (2) exposure to stress-related imagery elicits robust alcohol craving in alcoholics but has minimal effect in healthy drinkers (Chaplin et al. 2008; Sinha et al. 2009); and (3) exposure to neutral imagery provides an active control condition for comparison of nonspecific effects (Sinha 2007; Sinha et al. 2009)). Notably, increases in alcohol craving responses, such as those elicited by guided imagery, are predictive of relapse and treatment outcomes (Anton et al. 1996; Brady et al. 2006; Cooney et al. 1997; Sinha et al. 2006; Sinha 2007).
In addition to craving, exposure to cues and stress also evokes a series of physiological responses that include changes in heart rate, blood pressure, and skin conductance (Carter and Tiffany 1999; Sinha 2001a; Sinha et al. 2009). Furthermore, cue-related increases in heart rate are significantly associated with cue-induced alcohol craving responses in a meta-analysis of cue reactivity studies (Carter and Tiffany 1999). More recently, we have shown that this significant association is observed in social drinkers and not in abstinent alcoholics and more robustly in men than women (Chaplin et al. 2008; Sinha et al. 2009). These increased heart rate responses, which are initiated by imagery-induced activation of the autonomic nervous system, are sensed by polymodal C- and Aδ-fibers and are transmitted via spinothalamocortical connections to specific brain regions including the insular cortex, a brain region that is specifically involved in the integration of body-state information (Contreras et al. 2007; Craig 2002; Naqvi et al. 2007) and also known to play a role in the complex neural circuitry associated with drug craving (Contreras et al. 2007; Naqvi et al. 2007).
In the present study, we asked whether peripheral endocannabinoid signaling contributes to the interoceptive state experienced during craving for alcohol. To address this question, we used the guided imagery method—a widely used approach for experimental provocation of emotion, stress, mood, and craving states (e.g., Drobes and Tiffany 1997; Litt and Cooney 1999; Mayberg et al. 1999; Orr et al. 1993; Pitman et al. 1987; Sinha 2007; Sinha et al. 2009; Teasdale et al. 1999) to elicit alcohol craving in healthy social drinkers and abstinent alcoholics. Subjects were presented with stress, alcohol cue, and neutral imagery across three experimental sessions on consecutive days, one exposure per day, with order randomized and counterbalanced across subjects. Concentrations of circulating endocannabinoids were assessed at multiple time points on each day and were compared to self-reported levels of alcohol craving and changes in heart rate responses.
Materials and methods
Participants
Treatment-seeking individuals (n=12; ten men, two women) meeting Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV criteria for current alcohol dependence (AD) were admitted to the Clinical Neuroscience Research Unit (CNRU) of the Connecticut Mental Health Center at Yale University for 5 weeks of inpatient treatment and research participation. The CNRU is a locked inpatient treatment research facility with no access to alcohol or drugs and limited access to visitors. Urine and breath analyzer testing were conducted regularly to ensure continued abstinence. All patients participated in specialized substance abuse treatment for 4 weeks prior to the laboratory sessions. Healthy individuals (n=11, nine men, two women), who did not meet current or lifetime DSM-IV criteria for alcohol dependence were recruited from the community via local advertisements. Healthy subjects were light social drinkers (SD; up to six drinks weekly) and had been abstinent an average of 40±24.19 days. Although average age was significantly different between social drinkers and abstinent alcoholics, age was not found to contribute significantly to anandamide responses in this study and was not correlated to circulating anandamide levels in our previous studies (Giuffrida et al. 2004; Leweke et al. 2007). Participants did not meet current DSM-IV criteria for dependence on any other psychoactive substances, with the exception of alcohol and nicotine in the AD group. In addition, individuals on medications for medical or psychiatric problems were excluded from the study. All subjects underwent a complete medical evaluation to ensure good physical health, and the study was approved by the Human Investigation Committee of the Yale University School of Medicine.
All participants completed demographic, diagnostic Structured Clinical Interview for DSM-IV-I (First et al. 1995) and alcohol and drug use assessments over the course of two to three intake sessions. Following these sessions, healthy individuals were admitted for a three-night stay to the Yale General Clinical Research Center to participate in three laboratory sessions on consecutive days. Alcoholic individuals were similarly tested in three laboratory sessions after 28 days of abstinence.
Procedures
Imagery script development procedures
Prior to the laboratory sessions, guided imagery scripts for alcohol cues, personal stressors, and neutral relaxing states were developed. Alcohol cue scripts were developed based on situations that included alcohol-related stimuli and resulted in subsequent alcohol use (e.g., buying alcohol, being at a bar, watching others drink alcohol). Alcohol-related situations that occurred in the context of negative affect or psychological distress were not allowed. Stress imagery scripts were developed from subjects’ descriptions of recent personal stressful events that were experienced as “most stressful.” “Most stressful” was determined by having the subjects rate their individual level of distress for each stressful situation on a ten-point Likert scale where “1=not at all stressful” and “10=the most stress they felt recently in their life.” Only situations rated as 8 or above were accepted as appropriate for script development. Examples include a breakup with a significant other or unemployment-related stress. Neutral scripts were developed from the participants’ individual experiences of commonly experienced neutral relaxing situations, such as a summer day relaxing at the beach or a fall day reading at the park. Details of each elicited situation were described using the Scene Construction Questionnaires, based on methods developed by Lang et al. (1980, 1983) and further adapted in our previous work with drinking samples (Chaplin et al. 2008; Fox et al. 2007; Sinha 2001b; Sinha et al. 2003, 2009).
Each script presented during the laboratory sessions was 5 min in length and was audio-taped and presented on consecutive days, one stimulus script presentation per day presented in a randomized order and counterbalanced across participants. All research staff and laboratory technicians involved in conducting the experimental procedures, including blood processing and assay analysis, were blind to the type of the imagery condition and content of the scripts assigned to each laboratory session. Subjects remained blind to the order of the imagery condition until imagery induction on each day.
Habituation and imagery training session
On a day prior to the laboratory sessions, subjects were brought into the testing room where they were acclimatized to specific aspects of the study procedures, such as intravenous insertion, and training in completing the subjective rating forms and specific training in relaxation and imagery procedures (Sinha 2001b).
Laboratory sessions
On each day, subjects abstained from breakfast and were brought into the testing room at 8:00 a.m. by the research nurse. All subjects were allowed an initial smoke break at 7:30 a.m. to address potential nicotine craving. After settling into a sitting position in a hospital bed, a heparin-treated catheter was inserted by the research nurse in the antecubital region of the subject's nonpreferred arm, in order to periodically obtain repeated blood samples during the laboratory sessions. A pulse sensor was also attached to the subject's index finger on that same arm. This was followed by a 1-h adaptation period. At 9:00 a.m., subjects were provided headphones, and the audiotape presented the instructions for the imagery procedure and the script for guided imagery. After imagery, subjects remained in the testing room for an additional 75 min for repeated measurements to examine recovery from the imagery exposure. After the last assessment at 10:30 a.m., the subject was disconnected from the apparatus and served breakfast.
Laboratory assessments
Subjective measures
Alcohol craving
Desire to drink alcohol was assessed using a ten-point visual analog scale (VAS) in which 1 was anchored at “not at all” and10at “extremely high.” Subjects rated their “desire for an alcoholic drink” in that moment. Alcohol craving was assessed prior to imagery (−5), immediately following imagery (0 time point) and at +15, +30, +45, +60, and +75 min after imagery.
Subjective anxiety
Anxiety was assessed using a ten-point VAS in which 1 was anchored at “not at all” and 10 at “extremely high” for how “anxious, tense, and/or jittery” subjects felt in that moment. Subjective anxiety was assessed prior to imagery (−5), immediately following imagery (0 time point) and at +15, +30, +45, +60, and +75 min after imagery.
Physiological measures
Heart rate
A pulse sensor was attached to the subject's finger and connected to the Dinamap Monitor to provide a continuous measure of pulse. Heart rate was averaged for the 5 min prior to imagery (as a baseline measurement), during the imagery period (averaged for the 0 time point) and then at each of the +15, +30, +45, +60, and +75 min after imagery.
Plasma preparation
To assess plasma levels of endocannabinoids, 4 ml of whole blood were collected in heparinized EDTA glass vials at two baseline timepoints (−20 and −5), immediately following imagery (0 time point) and at +15, +30, +45, +60, and +75 min after imagery for all three experimental sessions. The tubes were placed on ice immediately after blood drawing. Within 30 min of collection, the blood was centrifuged at 4°C, and 2 ml of plasma were pooled, aliquoted, and stored at −80°C until shipment to the University of California, Irvine for analysis.
Lipid extraction from plasma
Anandamide, oleoylethanolamide (OEA), and 2-arachidonoylglycerol were extracted from 0.3 ml of plasma/sample essentially as described (Giuffrida et al. 2000), with minor modifications. Proteins were precipitated by adding an equal volume of acetone (−20°C) containing deuterium-labeled internal standards for anandamide, OEA, and 2-AG, followed by centrifugation at 1,000×g for 10 min. Supernatants were collected, and residual acetone was evaporated under a stream of nitrogen. Lipids were extracted using chloroform/methanol (2:1 vol/ vol), and the organic phase was concentrated for liquid chromatograpy/mass spectrometry (LC/MS) analyses.
LC/MS analyses
Anandamide, OEA, and 2-AG were fractionated using an Agilent 1100 Series LC/MS system (Palo Alto, CA, USA), equipped with a Zorbax Eclipse XDB-C18 cartridge column (4.6×50 mm i.d., 1.8 μm). We used a gradient of methanol (B) in water (A) at a flow rate of 1.5 ml min−1 (85% to 90% B in 2 min; 90% to 100% B in 1 min; isocratic 100% B for 1 min and an equilibration time of 3 min) and a column temperature of 40°C. MS analyses were performed with an electrospray ion source in the positive ionization mode. Capillary voltage was 3,000 V, and fragmentor voltage was varied from 120 to 140 V. Nitrogen gas flow was 13 l min−1 at 350°C, and nebulizer pressure was 60 psi. Sodium adducts of molecular ions ([M+Na+]) of anandamide (mass-to-charge ratio, m/z=370), OEA (m/z=348), and 2-AG (m/z=401) were quantified in the selected-ion monitoring mode using appropriate deuterium-containing internal standards. Spectra were acquired at 0.6 s/cycle and collected with Agilent Chemstation software.
Statistical analyses
Linear Mixed Effect (LME) models (Laird and Ware 1982) were implemented to analyze baseline and change from baseline measures, using the SAS software package (version 9.1, 2006; SAS Institute, Cary, NC, USA). Fixed effect factors were the between-subjects factor of group (healthy vs. alcoholic drinkers) and the within-subjects factors of imagery condition (alcohol cues, stress, and neutral) and time point (varying levels), and subjects the random effect factor. LMEs are particularly well suited when the design calls for repeated measurements within the same individual that can lead to positive correlations between measurements. Such models are also useful if data are missing, as it prevents the exclusion of subjects with missing data points (Littell et al. 1998). Spearman's Rho coefficients were used to assess correlations between anandamide measurements and alcohol craving and heart rate responses.
Results
Participants
There were no differences between the alcoholic and healthy drinking groups on gender, race, years of education, body mass index (BMI), and lifetime prevalence of psychiatric disorders. However, the alcoholics were significantly older in age and were all smokers, while the socially drinking group was younger, and only 36% were smokers (see Table 1).
Table 1.
Demographics and sample characteristics of alcohol dependent and healthy social drinkers
| Participant characteristics | Alcoholic patients (n=12) | Social drinkers (n=11) |
|---|---|---|
| Gender: number (%) male | 10 (83%) | 9 (82%) |
| Race: number (%) Caucasian | 9 (75%) | 6 (54%) |
| African American | 3 (25%) | 1 (9%) |
| Hispanic | 0 (0%) | 3 (27%) |
| Other | 0 (0%) | 1 (9%) |
| Age: mean (SD)* | 39.17 (6.9) | 28.3 (9.5) |
| Years in education: mean (SD) | 13.17 (2.0) | 14.0 (1.7) |
| Years of alcohol use: mean (SD)** | 18.42 (7.13) | 9.1 (9.9) |
| Number of smokers: N (%)** | 12 (100%) | 4 (36%) |
| Mean BMI: mean (SD) | 26.43 (4.59) | 25.77 (4.84) |
| Lifetime prevalence of psychiatric disorders: N (%) | ||
| Lifetime mood disorders | 4 (33.3%) | 2 (18%) |
| Lifetime posttraumatic stress disorder (PTSD) | 1 (9%) | 1 (9%) |
| Lifetime Anxiety Disorders (without PTSD) | 2 (18%) | 1 (9%) |
P<0.05
P<0.01
Circulating endocannabinoid levels
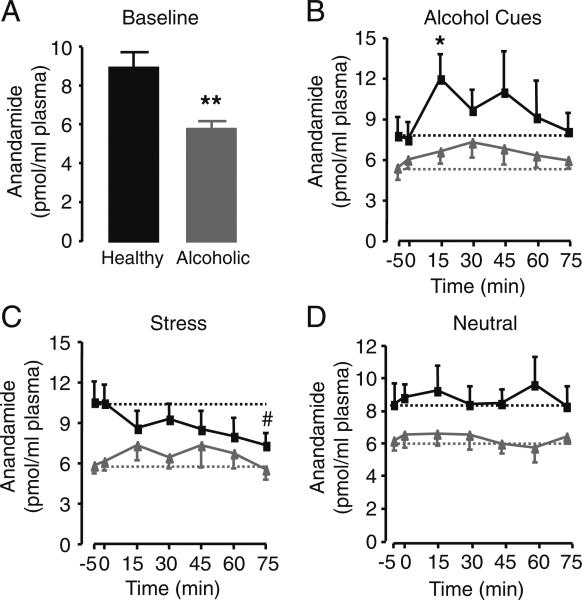
Baseline endocannabinoid levels in plasma were consistent with those reported in previous studies using similar analytical approaches (Sparling et al. 2003). Baseline plasma anandamide were markedly reduced in abstinent alcoholics compared to healthy social drinkers (F1,20=10.68, P=0.004; Fig. 1a).
Fig. 1.
a Baseline anandamide levels averaged across all imagery conditions. Black bar healthy drinkers; gray bar alcoholics; **P<0.01, F1,20=10.68, healthy drinkers vs. alcoholics. b–d Plasma anandamide levels in healthy drinkers (black squares) and abstinent alcoholics (gray triangles) measured before and after exposure to b alcohol-related, c stress-related, or d neutral imagery. t=−5 min indicates beginning, and t=0 min indicates conclusion of guided imagery. Dotted lines indicate average baseline values of anandamide for healthy drinkers (black lines) or alcoholics (gray lines) within each imagery session. *P<0.05, t=15 vs. 0 min in healthy drinkers following alcohol-related imagery. Pound sign P=0.05, t=75 vs. 0 min in healthy drinkers following stress-related imagery. Results are expressed as mean±SEM
To assess response to imagery procedures, LME analysis of change from baseline measure revealed an overall significant imagery main effect (F2,38=6.81, P=0.003) and a group × imagery interaction (F2,38=5.94, P=0.006). Simple effects analysis indicated that, in healthy drinkers, alcohol cues significantly increased plasma anandamide levels when compared to neutral or stress-related imagery (Fig. 1b–d). There was an overall effect of stress-related imagery to decrease anandamide, but no single time-point reached statistical significance (Fig. 1c). Underscoring the biochemical selectivity of this response, alcohol cues did not alter plasma concentrations of the other major endocannabinoid ligand, 2-AG, or those of OEA, a noncannabinoid analogue of anandamide (Table 1). Importantly, no alcohol cue-induced anandamide mobilization was observed in the alcoholics (Fig. 1b).
Alcohol craving and subjective anxiety responses
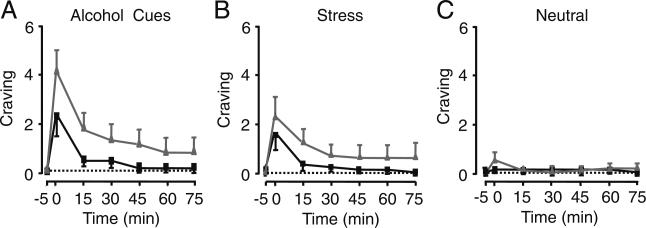
As expected from previous studies (Chaplin et al. 2008; Fox et al. 2007; Sinha et al. 2003, 2009), alcohol-related imagery robustly increased craving in both healthy drinkers (t40=2.45, P=0.019) and alcoholics (t40=7.48, P<0.0001) relative to the neutral condition, whereas stressful vs. neutral imagery elicited alcohol craving only in alcoholics (healthy drinkers, t40=1.01, P=0.32; alcoholics, t40=4.01, P=0.0003; Fig. 2). There were no increases in alcohol craving in the neutral condition in either group. As a manipulation check, we also assessed anxiety levels, and as expected and reported in our previous studies (Chaplin et al. 2008; Fox et al. 2007; Sinha et al. 2003, 2009), subjective anxiety also significantly increased with stress imagery (healthy drinkers, t40=4.40, P<0.0001; alcoholics, t40=3.51, P<0.001) and with alcohol cue imagery (healthy drinkers, t40=2.75, P<0.009; alcoholics, t40=5.24, P<0.0001) relative to neutral-relaxing imagery.
Fig. 2.
Alcohol craving in healthy drinkers (black squares) and abstinent alcoholics (gray triangles) measured before and after the following imagery conditions: a alcohol-related, b stress-related, or c neutral. t=−5 min indicates beginning, and t=0 min indicates conclusion of guided imagery. Dotted lines indicate average baseline values for craving. Results are expressed as mean±SEM; n=11 (healthy subjects) and 12 (alcoholic subjects)
Relationship between plasma anandamide and craving
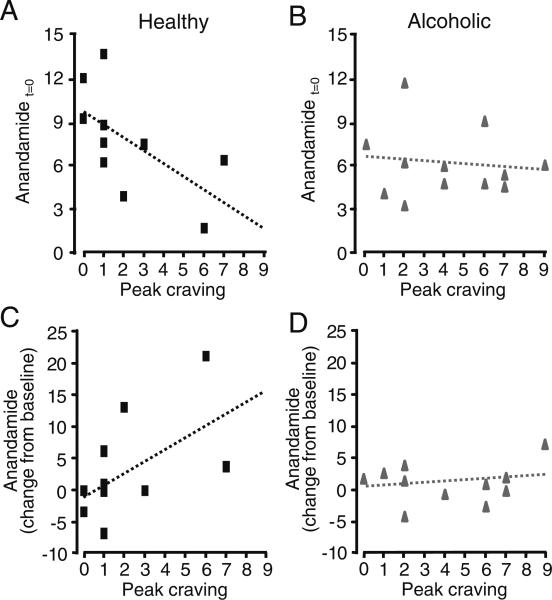
Correlation analyses revealed that, in healthy drinkers, maximal alcohol cue-induced craving was negatively correlated with anandamide levels at time=0 (rho=−0.69, P=0.027; Fig. 3a). By contrast, no such correlation was found in alcoholics (rho=−0.04, P=0.90; Fig. 3b), nor were comparable relationships present in stress and neutral imagery conditions (data not shown). Furthermore, in healthy but not alcoholic drinkers, craving was positively correlated to cumulative alcohol cue-induced anandamide elevations in plasma (average change from baseline over time=15−45 min, the period during which maximal increases in anandamide levels were observed; healthy drinkers, rho=0.64, P=0.045; alcoholics, rho=−0.039, P=0.90; Fig. 3c, d).
Fig. 3.
Correlations (scatter plots) between plasma anandamide levels and peak alcohol craving following alcohol cues, at t=0 min in a healthy drinkers (black squares), or b alcoholics (gray triangles). Scatter plots for peak craving vs. average change in anandamide over t=15−45 min in c healthy drinkers (black squares)or d alcoholics (gray triangles). Dotted lines depict linear regression lines
Relationships of plasma anandamide with heart rate
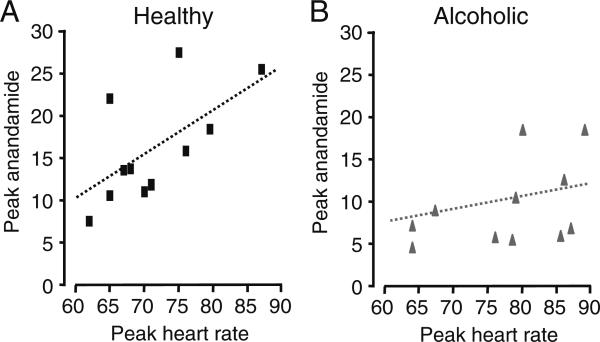
To explore the possible relationship between peripheral anandamide mobilization and autonomic activation during alcohol craving, we compared plasma anandamide levels with peak changes in heart rate. In healthy drinkers, alcohol-related imagery produced elevations in heart rate (alcohol cues vs. neutral imagery, t40=2.30, P=0.027), which were correlated to maximal elevations in plasma anandamide levels (peak heart rate vs. peak anandamide, alcohol cues imagery, rho=0.66, P=0.039; Fig. 4a). Notably, stress imagery also increased heart rate in healthy drinkers (stress vs. neutral imagery, t40=3.46, P=0.0013), but they failed to elevate plasma anandamide (Fig. 1c), and peak heart rate changes elicited by stress imagery were uncorrelated to peak anandamide levels (peak heart rate vs. peak anandamide, stressful imagery, rho=0.47, P=0.17). Overall, heart rate area under the curve (AUC) and anandamide-area under the curve response in alcohol cue but also in stress condition were significantly correlated (alcohol cue, rho=0.76, P=0.01, stressful imagery, rho=0.68, P=0.02) only in the healthy controls. There were no significant associations between neutral heart rate (AUC) and plasma anandamide (AUC). By contrast, neither alcohol cues nor stressful imagery significantly elevated heart rate in alcoholics (alcohol cues vs. neutral imagery: t40=1.87, P=0.07; stress vs. neutral imagery: t40=0.64, P=0.53), and no relationship was found between peak heart rate and plasma anandamide levels in these subjects (peak heart rate vs. peak anandamide, alcohol cue: rho=0.28, P=0.37, stressful imagery, rho=−0.32, P=0.31; Fig. 4b and data not shown).
Fig. 4.
Correlations (scatter plots) between peak plasma anandamide levels and peak heart rate following alcohol cues in a healthy drinkers (black squares)or b alcoholics (gray triangles). Dotted lines depict linear regression lines
Secondary analyses
As the alcoholics were significantly older than the controls, we repeated the LME analyses of change from baseline for anandamide levels with age as a covariate, and the findings remained significant (imagery main effect, F2,38=5.45, P=0.008; group × imagery interaction, F2,38 = 5.07, P=0.01). As all of the alcoholics were smokers, it was not possible to include smoking as a covariate in the overall LME analyses. However, we conducted an analysis to examine whether anandamide levels were different at baseline or in the LME models of change from baseline in the smoking and nonsmoking healthy drinking subgroups. There were no differences at baseline and no significant effects of smoking status on the main effect of imagery in the healthy drinking group. Although these analyses are post hoc, they suggest that the differences in age and smoking status between groups did not influence baseline anandamide levels and response of anandamide to stress, alcohol cues, and neutral imagery.
Discussion
In the present study, we used a guided imagery approach to investigate the role of peripheral endocannabinoid signaling in alcohol craving. As previously shown (Chaplin et al. 2008; Fox et al. 2007; Sinha et al. 2003, 2009), exposure to a personalized alcohol-related imagery increased craving both in healthy social drinkers and detoxified alcoholics, whereas stressful imagery produced craving only in alcoholics. In healthy drinkers, alcohol cue-induced craving was accompanied by a marked elevation in circulating levels of anandamide but not of related lipid messengers (2-AG, OEA). Plasma anandamide concentrations in these subjects were closely correlated with both craving scores and cue-induced heart rate responses, suggesting that anandamide mobilization in peripheral tissues may be a marker for the body state associated with the desire for alcohol. Although the functional significance of these findings remains uncertain, the negative relationships between plasma anandamide levels at peak cue-induced craving on the one hand and the positive relationship between peak craving scores and increases in cue-induced anandamide responses on the other lead us to speculate that peripheral anandamide might be involved in physiological processes aimed at modulating alcohol craving. Testing this hypothesis will require further experimentation, but it is interesting to note that alcoholics appear to lack the alcohol cue-induced rises in plasma anandamide levels observed in social drinkers and to display reduced baseline anandamide levels in plasma (uncorrelated to either alcohol craving or heart rate responses).
The possibility that peripheral anandamide signaling is engaged by processes involved in the modulation of alcohol craving raises several questions. The first relates to the tissue source responsible for the rise in plasma anandamide evoked by alcohol cues. Although anandamide is produced both in brain and peripheral tissues, its levels in human blood and cerebrospinal fluid show no statistical correlation (Giuffrida et al. 2004), which implies that the bulk of circulating anandamide originates in the periphery rather than the brain. This is also supported by the presence of efficient anandamide-deactivating mechanisms (transport and hydrolysis) in the microvasculature that separates brain from blood (Chen et al. 2004) and by the greater concentration of anandamide found in plasma than in cerebrospinal fluid (Giuffrida et al. 2004). However, we cannot exclude the possibility that a small fraction of peripheral anandamide may derive from a source within the central nervous system.
A second question prompted by our findings pertains to the neural mechanism(s) by which alcohol-related cues increase plasma anandamide levels. We found a positive correlation between alcohol cue-induced changes in anandamide levels and peak heart rate, which may be interpreted to suggest that alcohol cues engage the autonomic nervous system to initiate anandamide mobilization in peripheral tissues. Indeed, autonomic activation has been previously invoked to explain the ability of physical exercise to increase plasma anandamide levels in human volunteers (Sparling et al. 2003), consistent with current data that overall heart rate (AUC) and anandamide levels (AUC) during both the stress and the alcohol cue condition were significantly correlated only in controls. The observation that exposure to stressful imagery does not elevate circulating anandamide levels and peak anandamide is not correlated with peak heart rate even though stress markedly increases heart rate may be explained in the context of a growing body of evidence, which shows that stress- and cue-induced drug and alcohol craving, as well as positive and negative emotions, are associated with distinct patterns of somato-visceral activity ( Fox et al. 2007; Liu and Weiss 2002; Rainville et al. 2006; Shaham et al. 2003). On the other hand, alcoholics showed suppressed basal levels of plasma anandamide, no increases in anandamide during cue-induced imagery, and no significant associations between the suppressed anandamide levels, heart rate, and the high levels of alcohol craving in the cue or the stress condition. Chronic alcohol abuse is associated with significant autonomic dysregulation (Rechlin et al. 1996; Ingjaldsson et al. 2003; Bar et al. 2006; Kahkonen and Bondarenko 2000; Shively et al. 2007), documented during early abstinence in basal states as well as in response to stress and alcohol cue exposure in alcoholics (Thayer et al. 2006; Sinha et al. 2009). The current data indicating suppression of basal and response levels of anandamide in alcoholics is consistent with autonomic dysregulation noted in alcoholism, allowing us to speculate that an important aspect of healthy desire and craving modulation could involve sympathetic and parasympathetic balance as well as anandamide signaling, both of which are dysfunctional in early abstinent alcoholics. Clearly, future research is needed to more specifically understand the role of anandamide in autonomic regulation and in specific modulation of hedonic states.
A third question prompted by our results centers on the possible mechanism by which peripheral anandamide might influence subjective increases in alcohol craving. We do not have enough data to answer this question, but one possibility is that anandamide released in autonomic end-organs may activate CB1 receptors localized on primary C- and Aδ-fibers to modulate the activity of multimodal sensory neurons (Agarwal et al. 2007; Calignano et al. 1998, 2000). These neurons transmit somatic signals to specific cortical regions including the insular cortex via the spinothalamocortical pathway (Craig 2002), thus providing a possible route by which transient changes in peripheral anandamide production might influence interoceptive signaling in the brain. Other possibilities are that anandamide mobilization might serve as a negative feedback mechanism to oppose an increase in sympathetic outflow or that it might represent an epiphenomenon devoid of functional significance. Nevertheless, the questions outlined above can be addressed in human experimental models such as that employed in the present study. For example, the role of anandamide in craving may be differentially probed using CB1 receptor antagonists (Rinaldi-Carmona et al. 2004) and FAAH inhibitors (Kathuria et al. 2003). Additional points to be explored experimentally in the future are whether appetitive cues other than alcohol (for example, food) also increase plasma anandamide levels and whether anandamide mobilization is impaired in addictions other than alcoholism.
The hypothesis that alcohol addiction is accompanied by an allostatic disruption in peripheral anandamide mobilization leads to the prediction that drugs that normalize this signaling mechanism, such as FAAH inhibitors (Kathuria et al. 2003), might help control craving in alcoholics. This idea is apparently contradicted by animal experiments showing that CB1 receptor blockade reduces alcohol and drug cue-induced reinstatement of drug seeking (De Vries et al. 2001; Fattore et al. 2007) which have led to the suggestion that endocannabinoids facilitate, rather than modulate, alcohol craving. This interpretation may be overly simplistic, however, as it is based on the assumption that different endocannabinoids play identical roles in addiction and that such roles can be unmasked by system-wide blockade of CB1 receptors. On the contrary, multiple lines of evidence suggest that the two best-known endocannabinoids, anandamide and 2-AG, operate as highly localized messengers with functions that are often distinct (Batkai et al. 2004; Hohmann et al. 2005; Kurihara et al. 2001; Makara et al. 2005) or even antagonistic (Maccarrone et al. 2008).
In conclusion, it is important to note that, as the current findings are limited by a small sample size, they should be considered preliminary and clearly warrant replication. Furthermore, although the influence of smoking in the controls did not appear to affect anandamide levels in response to alcohol cue imagery, as all of the alcoholics were smokers, the role of nicotine smoking will need to be systematically explored in future studies. Despite these caveats, the results suggest that peripheral anandamide mobilization, elicited by cue-induced autonomic activation, is involved in interoceptive signaling contributing to the moderation of desire for alcohol in healthy drinkers. This signal is ostensibly suppressed in alcoholics. If replicated in a larger study, our results might point to potential new avenues for therapeutic intervention in decreasing craving and relapse susceptibility in alcohol addicts.
Acknowledgments
The present study was partially supported by NIH grants R01-AA113892 and K02-DA17232 (to R.S.) and DA-012413 and DA-022702 (to D.P.) and by the NIH Roadmap Interdisciplinary Research Consortium grant on Stress, Self Control and Addiction (UL1-DE019586) and the NIH/NCRR/CTSA Program Grant (UL1 RR024139). Support was also provided by the Department of Mental Health and Addiction Services of the State of Connecticut. We wish to thank Giuseppe Astarita, Lauren Burgeno, Saurabh Sharma, Ronak Kedia, Monica Arnold, Keri Bergquist, Kristen Siedlarz, and Helen Fox for their assistance.
Footnotes
Disclosure DP is an inventor in a patent disclosing FAAH inhibitors, owned by the University of California, Irvine and licensed to Organon Biosciences, a unit of Schering-Plough.
Contributor Information
Regina A. Mangieri, Department of Pharmacology, University of California, Irvine, 3101 Gillespie NRF, Irvine, CA 92697, USA
Kwang-Ik A. Hong, Department of Psychiatry, Yale University School of Medicine, 34 Park Street, Room S110, New Haven, CT 06519, USA
Daniele Piomelli, Department of Pharmacology, University of California, Irvine, 3101 Gillespie NRF, Irvine, CA 92697, USA e-mail: piomelli@uci.edu; Unit of Drug Discovery and Development, Italian Institute of Technology, Genoa 16136, Italy.
Rajita Sinha, Department of Psychiatry, Yale University School of Medicine, 34 Park Street, Room S110, New Haven, CT 06519, USA.
References
- Agarwal N, Pacher P, Tegeder I, Amaya F, Constantin CE, Brenner GJ, Rubino T, Michalski CW, Marsicano G, Monory K, Mackie K, Marian C, Batkai S, Parolaro D, Fischer MJ, Reeh P, Kunos G, Kress M, Lutz B, Woolf CJ, Kuner R. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat Neurosci. 2007;10(7):870–879. doi: 10.1038/nn1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton RF, Moak DH, Latham PK. The Obsessive Compulsive Drinking Scale: a new method of assessing outcome in alcoholism treatment studies. Arch Gen Psychiatry. 1996;53:225–231. doi: 10.1001/archpsyc.1996.01830030047008. [DOI] [PubMed] [Google Scholar]
- Bar KJ, Boettger MK, Neubauer R, Groteluschen M, Jochum T, Baier V, Sauer H, Voss A. Heart rate variability and sympathetic skin response in male patients suffering from acute alcohol withdrawal syndrome. Alcohol Clin Exp Res. 2006;30(9):1592–1598. doi: 10.1111/j.1530-0277.2006.00191.x. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS. The endocannabinoid signaling system: a potential target for next-generation therapeutics for alcoholism. Mini Rev Med Chem. 2007;7(8):769–779. doi: 10.2174/138955707781387920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batkai S, Pacher P, Osei-Hyiaman D, Radaeva S, Liu J, Harvey-White J, Offertaler L, Mackie K, Rudd MA, Bukoski RD, Kunos G. Endocannabinoids acting at cannabinoid-1 receptors regulate cardiovascular function in hypertension. Circulation. 2004;110(14):1996–2002. doi: 10.1161/01.CIR.0000143230.23252.D2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolato M, Mangieri RA, Fu J, Kim JH, Arguello O, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. Antidepressant-like activity of the fatty acid amide hydrolase inhibitor URB597 in a rat model of chronic mild stress. Biol Psychiatry. 2007;62(10):1103–1110. doi: 10.1016/j.biopsych.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Brady KT, Back SE, Waldrop AE, McRae AL, Anton RF, Upadhyaya HP, Saladin ME, Randall PK. Cold pressor task reactivity: predictors of alcohol use among alcohol-dependent individuals with and without comorbid posttraumatic stress disorder. Alcohol Clin Exp Res. 2006;30(6):938–946. doi: 10.1111/j.1530-0277.2006.00097.x. [DOI] [PubMed] [Google Scholar]
- Calignano A, La Rana G, Giuffrida A, Piomelli D. Control of pain initiation by endogenous cannabinoids. Nature. 1998;394(6690):277–281. doi: 10.1038/28393. [DOI] [PubMed] [Google Scholar]
- Calignano A, Katona I, Desarnaud F, Giuffrida A, La Rana G, Mackie K, Freund TF, Piomelli D. Bidirectional control of airway responsiveness by endogenous cannabinoids. Nature. 2000;408(6808):96–101. doi: 10.1038/35040576. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94(3):327–40. [PubMed] [Google Scholar]
- Chaplin TM, Hong KA, Bergquist K, Sinha R. Gender differences in response to emotional stress across subjective, behavioral, cardiovascular, neuroendocrine, and behavioral systems. Alcohol Clin Exp Res. 2008;32:1242–1250. doi: 10.1111/j.1530-0277.2008.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Hu S, Harmon SD, Moore SA, Spector AA, Fang X. Metabolism of anandamide in cerebral microvascular endothelial cells. Prostaglandins Other Lipid Mediat. 2004;73(1−2):59–72. doi: 10.1016/j.prostaglandins.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Bilbao A, Gorriti MA, Navarro M, Massi M, Piomelli D, Ciccocioppo R, Rodriguez de Fonseca F. THe anandamide transport inhibitor AM404 reduces alcohol self-administration. Eur J Neurosci. 2007;26(2):476–486. doi: 10.1111/j.1460-9568.2007.05665.x. [DOI] [PubMed] [Google Scholar]
- Contreras M, Ceric F, Torrealba F. Inactivation of the interoceptive insula disrupts drug craving and malaise induced by lithium. Science. 2007;318(5850):655–658. doi: 10.1126/science.1145590. [DOI] [PubMed] [Google Scholar]
- Cooney NL, Litt MD, Morse PA, Bauer LO, Gaupp L. Alcohol cue reactivity, negative-mood reactivity, and relapse in treated alcoholic men. J Abnorm Psychology. 1997;106(2):243–250. doi: 10.1037//0021-843x.106.2.243. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3(8):655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Shaham Y, Homberg JR, Crombag H, Schuurman K, Dieben J, Vanderschuren LJ, Schoffelmeer AN. A cannabinoid mechanism in relapse to cocaine seeking. Nat Med. 2001;7(10):1151–1154. doi: 10.1038/nm1001-1151. [DOI] [PubMed] [Google Scholar]
- Drobes DJ, Tiffany ST. Induction of smoking urge through imaginal and in vivo procedures: physiological and self-report manifestations. J Abnorm Psychology. 1997;106(1):15–25. doi: 10.1037//0021-843x.106.1.15. [DOI] [PubMed] [Google Scholar]
- Fattore L, Spano MS, Deiana S, Melis V, Cossu G, Fadda P, Fratta W. An endocannabinoid mechanism in relapse to drug seeking: a review of animal studies and clinical perspectives. Brain Res Rev. 2007;53(1):1–16. doi: 10.1016/j.brainresrev.2006.05.003. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV. Patient edition American Psychiatric Press Inc; Washington DC: 1995. [Google Scholar]
- Fox HC, Bergquist KL, Hong KI, Sinha R. Stress-induced and alcohol cue-induced craving in recently abstinent alcohol dependent individuals. Alcohol Clin Exp Res. 2007;31(3):395–403. doi: 10.1111/j.1530-0277.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- Fox HC, Hong KI, Siedlarz K, Sinha R. Enhanced sensitivity to stress and drug/alcohol craving in abstinent cocaine-dependent individuals compared to social drinkers. Neuropsychopharmacology. 2008;33(4):796–805. doi: 10.1038/sj.npp.1301470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuffrida A, Rodriguez de Fonseca F, Piomelli D. Quantification of bioactive acylethanolamides in rat plasma by electrospray mass spectrometry. Anal Biochem. 2000;280(1):87–93. doi: 10.1006/abio.2000.4509. [DOI] [PubMed] [Google Scholar]
- Giuffrida A, Leweke FM, Gerth CW, Schreiber D, Koethe D, Faulhaber J, Klosterkotter J, Piomelli D. Cerebrospinal anandamide levels are elevated in acute schizophrenia and are inversely correlated with psychotic symptoms. Neuropsychopharmacology. 2004;29(11):2108–2114. doi: 10.1038/sj.npp.1300558. [DOI] [PubMed] [Google Scholar]
- Gobbi G, Bambico F, Mangieri R, Bortolato M, Campolongo P, Solinas M, Cassano T, Morgese M, Debonnel G, Duranti A, Tontini A, Tarzia G, Mor M, Trezza V, Goldberg SR, Cuomo V, Piomelli D. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci U S A. 2005;102(51):18620–18625. doi: 10.1073/pnas.0509591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Patel S, Carrier EJ, Rademacher DJ, Ormerod BK, Hillard CJ, Gorzalka BB. Downregulation of endocannabinoid signaling in the hippocampus following chronic unpredictable stress. Neuropsychopharmacology. 2005;30(3):508–515. doi: 10.1038/sj.npp.1300601. [DOI] [PubMed] [Google Scholar]
- Hill MN, Ho WS, Sinopoli KJ, Viau V, Hillard CJ, Gorzalka BB. Involvement of the endocannabinoid system in the ability of long-term tricyclic antidepressant treatment to suppress stress-induced activation of the hypothalamic-pituitary-adrenal axis. Neuropsychopharmacology. 2006a;31(12):2591–2599. doi: 10.1038/sj.npp.1301092. [DOI] [PubMed] [Google Scholar]
- Hill MN, Kambo JS, Sun JC, Gorzalka BB, Galea LA. Endocannabinoids modulate stress-induced suppression of hippocampal cell proliferation and activation of defensive behaviours. Eur J Neurosci. 2006b;24(7):1845–1849. doi: 10.1111/j.1460-9568.2006.05061.x. [DOI] [PubMed] [Google Scholar]
- Hohmann G, Suplita RLII, Bolton NM, Neely MH, Fegley D, Mangieri R, Krey J, Walker JM, Holmes PV, Crystal JD, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. An endocannabinoid mechanism for stress-induced analgesia. Nature. 2005;435:1108–1112. doi: 10.1038/nature03658. [DOI] [PubMed] [Google Scholar]
- Ingjaldsson JT, Laberg JC, Thayer JF. Reduced heart rate variability in chronic alcohol abuse: relationship with negative mood, chronic thought suppression, and compulsive drinking. Biol Psychiatry. 2003;54(12):1427–1436. doi: 10.1016/s0006-3223(02)01926-1. [DOI] [PubMed] [Google Scholar]
- Kahkonen S, Bondarenko BB. Cardiovascular changes in alcoholic patients during withdrawal phase. Ger J Psychiatry. 2000;3:1–6. [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, Mor M, Tarzia G, LaRana G, Calignano A, Giustino A, Tattoli M, Palmery M, Cuomo V, Piomelli D. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci. 2005;8(11):1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Kurihara J, Nishigaki M, Suzuki S, Okubo Y, Takata Y, Nakane S, Sugiura T, Waku K, Kato H. 2-Arachidonoylglycerol and anandamide oppositely modulate norepinephrine release from the rat heart sympathetic nerves. Jpn J Pharmacol. 2001;87(1):93–96. doi: 10.1254/jjp.87.93. [DOI] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38(4):963–974. [PubMed] [Google Scholar]
- Lang PJ, Kozak MJ, Miller GA, Levin DN, McLean A., Jr Emotional imagery: Conceptual structure and pattern of somatovisceral response. Psychophysiology. 1980;17(2):179–192. doi: 10.1111/j.1469-8986.1980.tb00133.x. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Levin DN, Miller GA, Kozak MJ. Fear behavior, fear imagery, and the psychophysiology of emotion: The problem of affective response integration. J Abnorm Psychology. 1983;92(3):276–306. doi: 10.1037//0021-843x.92.3.276. [DOI] [PubMed] [Google Scholar]
- Leweke FM, Giuffrida A, Koethe D, Schreiber D, Nolden BM, Kranaster L, Neatby MA, Schneider M, Gerth CW, Hellmich M, Klosterkotter J, Piomelli D. Anandamide levels in cerebrospinal fluid of first-episode schizophrenic patients: impact of cannabis use. Schizophr Res. 2007;94(1−3):29–36. doi: 10.1016/j.schres.2007.04.025. [DOI] [PubMed] [Google Scholar]
- Litt MD, Cooney NL. Inducing craving for alcohol in the laboratory. Alcohol Res Health. 1999;23(3):174–178. [PMC free article] [PubMed] [Google Scholar]
- Littell RC, Henry PR, Ammerman CB. Statistical analysis of repeated measures data using SAS procedures. J Anim Sci. 1998;76(4):1216–1231. doi: 10.2527/1998.7641216x. [DOI] [PubMed] [Google Scholar]
- Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci. 2002;22(18):7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccarrone M, Rossi S, Bari M, De Chiara V, Fezza F, Musella A, Gasperi V, Prosperetti C, Bernardi G, Finazzi-Agro A, Cravatt BF, Centonze D. Anandamide inhibits metabolism and physiological actions of 2-arachidonoylglycerol in the striatum. Nat Neurosci. 2008;11(2):152–159. doi: 10.1038/nn2042. [DOI] [PubMed] [Google Scholar]
- Mahler SV, Smith KS, Berridge KC. Endocannabinoid hedonic hotspot for sensory pleasure: anandamide in nucleus accumbens shell enhances ‘liking’ of a sweet reward. Neuropsychopharmacology. 2007;32(11):2267–2278. doi: 10.1038/sj.npp.1301376. [DOI] [PubMed] [Google Scholar]
- Makara J, Mor M, Fegley D, Szabo S, Kathuria S, Astarita G, Duranti A, Tontini A, Tarzia G, Rivara S, Freund TF, Piomelli D. Selective inhibition of 2-AG hydrolysis enhances endocannabinoid signaling in hippocampus. Nat Neurosci. 2005;8:1139–1141. doi: 10.1038/nn1521. [DOI] [PubMed] [Google Scholar]
- Mayberg H, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315(5811):531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr SP, Pitman RK, Lasko NB, Herz LR. Psychophysiological assessment of posttraumatic stress disorder imagery in World War II and Korean combat veterans. J Abnorm Psychology. 1993;102(1):152–159. doi: 10.1037//0021-843x.102.1.152. [DOI] [PubMed] [Google Scholar]
- Patel S, Roelke CT, Rademacher DJ, Hillard CJ. Inhibition of restraint stress-induced neural and behavioural activation by endogenous cannabinoid signalling. Eur J Neurosci. 2005;21(4):1057–1069. doi: 10.1111/j.1460-9568.2005.03916.x. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Orr SP, Forgue DF, de Jong JB, Claiborn JM. Psychophysiologic assessment of posttraumatic stress disorder imagery in Vietnam combat veterans. Arch Gen Psychiatry. 1987;44(11):970–975. doi: 10.1001/archpsyc.1987.01800230050009. [DOI] [PubMed] [Google Scholar]
- Rademacher DJ, Hillard CJ. Interactions between endocannabinoids and stress-induced decreased sensitivity to natural reward. Prog Neuro-psychopharmacol Biol Psychiatry. 2007;31(3):633–641. doi: 10.1016/j.pnpbp.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainville P, Bechara A, Naqvi N, Damasio AR. Basic emotions are associated with distinct patterns of cardiorespiratory activity. Int J Psychophysiol. 2006;61(1):5–18. doi: 10.1016/j.ijpsycho.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Rechlin T, Orbes I, Weis M, Kaschka WP. Autonomic cardiac abnormalities in alcohol-dependent patients admitted to a psychiatric department. Clinic Auton Res. 1996;6(2):119–122. doi: 10.1007/BF02291234. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Congy C, Martinez S, Oustric D, Perio A, Poncelet M, Maruani J, Arnone M, Finance O, Soubrie P, Le Fur G. SR147778 [5-(4-bromophenyl)-1-(2, 4-dichlorophenyl)-4-ethyl-N-(1-piperidinyl)-1H-pyr azole-3-carboxamide], a new potent and selective antagonist of the CB1 cannabinoid receptor: biochemical and pharmacological characterization. J Pharmacol Exp Ther. 2004;310(3):905–914. doi: 10.1124/jpet.104.067884. [DOI] [PubMed] [Google Scholar]
- Scherma M, Medalie J, Fratta W, Vadivel SK, Makriyannis A, Piomelli D, Mikics E, Haller J, Yasar S, Tanda G, Goldberg SR. The endogenous cannabinoid anandamide has effects on motivation and anxiety that are revealed by fatty acid amide hydrolase (FAAH) inhibition. Neuropharmacology. 2008;54(1):129–140. doi: 10.1016/j.neuropharm.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, de Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shively CA, Mictus JE, Grant KA, Goldberger AL, Bennett AJ, Willard SL. Effects of chronic moderate alcohol consumption and novel environment on heart rate variability in primates. Psychopharmacology (Berl) 2007;192:183–191. doi: 10.1007/s00213-007-0709-z. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001a;158(4):343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sinha R. Imagery procedures development manual. 2001b. Unpublished manual.
- Sinha R. The role of stress in addiction relapse. Curr Psychiatry Rep. 2007;9(5):388–295. doi: 10.1007/s11920-007-0050-6. [DOI] [PubMed] [Google Scholar]
- Sinha R, Talih M, Malison R, Anderson GA, Cooney N, Kreek M. Hypothalamic-pituitary-adrenal axis and sympathoadreno-medullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology (Berl) 2003;170:62–72. doi: 10.1007/s00213-003-1525-8. [DOI] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry. 2006;63(3):324–331. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, Siedlarz KM. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology. 2009;34:1198–1208. doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria-Gomez E, Matias I, Rueda-Orozco PE, Cisneros M, Petrosino S, Navarro L, Di Marzo V, Prospero-Garcia O. Pharmacological enhancement of the endocannabinoid system in the nucleus accumbens shell stimulates food intake and increases c-Fos expression in the hypothalamus. Br J Pharmacol. 2007;151(7):1109–1116. doi: 10.1038/sj.bjp.0707313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparling PB, Giuffrida A, Piomelli D, Rosskopf L, Dietrich A. Exercise activates the endocannabinoid system. Neuroreport. 2003;14(17):2209–2211. doi: 10.1097/00001756-200312020-00015. [DOI] [PubMed] [Google Scholar]
- Teasdale JD, Howard RJ, Cox SG, Ha Y, Brammer MJ, Williams SCR, Checkley SA. Functional MRI study of the cognitive generation of affect. Am J Psychiatry. 1999;156(2):209–215. doi: 10.1176/ajp.156.2.209. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Hall M, Sollers JJ, 3rd, Fischer JE. Alcohol use, urinary cortisol, and heart rate variability in apparently healthy men: evidence for impaired inhibitory control of the HPA axis in heavy drinkers. Int J Psychophysiol. 2006;59(3):244–250. doi: 10.1016/j.ijpsycho.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Vinod KY, Sanguino E, Yalamanchili R, Hungund BL. Manipulation of fatty acid amide hydrolase functional activity alters sensitivity and dependence to ethanol. J Neurochem. 2008;104(1):233–243. doi: 10.1111/j.1471-4159.2007.04956.x. [DOI] [PubMed] [Google Scholar]