Although monoclonal antibodies (mAbs) have long been staples of biotech research and development (R&D), the study of mAb therapeutics has recently attracted a great deal of attention by the pharmaceutical industry. Interest has been piqued in part because pathways to establish three key features for approva—safety, efficacy and quality- are now well defined for mAbs, and because physicians and patients clearly accept mAbs as innovative therapeutics. A total of 22 mAbs are now approved in the US as well as in numerous other countries, and eight mAb products have global sales of over US$1 billion: infliximab ($5.3b), rituximab ($5.2b), trastuzumab ($4.0b), bevacizumab ($3.4b), adalimumab ($3.1b), cetuximab ($1.3b) ranibizumab ($1.2b), and palivizumab ($1.2b).
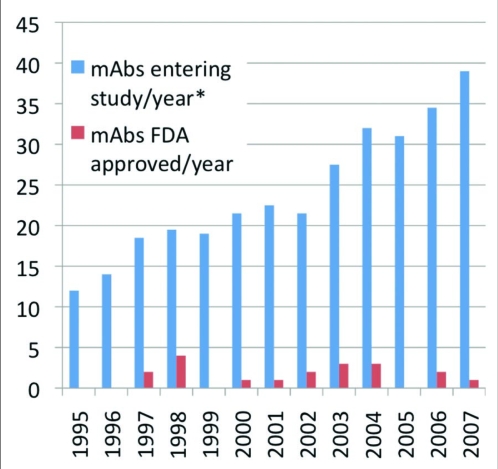
Due to these factors, the pharmaceutical industry is aggressively investing in therapeutic mAb R&D by initiating internal programs and by acquiring companies that focus on this area. The combined efforts of biotechnology and pharmaceutical firms have led to a dramatic increase in the number of molecules entering clinical study each year. This number has more than tripled since the mid-1990's, although the number gaining US approval has not yet increased (Figure 1). This lag is at least partially due to the length of the clinical and approval phases (average 6–8 years in total). The near term prospects for additional approvals are promising—4 mAb candidates were undergoing US regulatory review as of September 2008.
Figure 1.
Therapeutic monoclonal antibodies entering clinical study or approved, 1995–2007. Data presented as two-year moving average Source: Tufts Center for the Study of Drug Development
Advances in mAb discovery technology, such as grafting techniques used to make humanized mAbs and preparation of human mAbs from transgenic mice, have had an impact on the type of mAb candidates entering clinical study over time. Murine mAbs comprised nearly 80% of those entering clinical study sponsored by commercial firms in the 1980's, but less immunogenic humanized mAbs were the preferred version during the 1990's. Though the 2000's are not yet complete, human mAbs appear to be favored over other types in the current decade.
One key reason for the current interest in therapeutic mAbs is the relatively high approval success rates of the molecules. Chimeric and humanized mAbs tend to have higher success rates than those for new chemical entities. Humanized mAbs have been studied most frequently in the clinic, so they are the canonical mAb type for determination of success rates. Based on available data, humanized mAbs that entered clinical study during 1988 to 2007 had an overall success rate of 18%. When stratified by therapeutic category, the anticancer mAbs had a slightly lower success rate, while the immunological mAbs had a slightly higher rate. Only preliminary success rates for human mAbs are can be calculated because candidates have entered clinical studies in large numbers only recently and, to date, only two human mAbs have been approved. The available data on fates for human mAbs is thus limited. Preliminary results for human mAbs that entered clinical study during 1995 to 2007 suggest that, so far, the overall success rate for human mAbs is slightly lower than that for humanized versions. The rate will increase if two human mabs currently in FDA review, golimumab and ustekinumab, are approved.
As the production and use of full-size mAb therapeutics has become standard, the industry is moving toward development of next-generation mAbs with improved clinical safety and efficacy. Thus, antibody fragments comprise a nascent category of mAbs, although genetic engineering techniques were used to produce these molecules as early as 1988. As therapeutics, antibody fragments have features that could be advantages or disadvantages compared to those of full-size mAbs. Fragments are smaller and so might potentially penetrate tissues or tumors that could be invulnerable to full-size counterparts. However, fragments degrade faster in humans and have short circulating half-lives, and thus might not accumulate in sufficient quantities at the targeted site. Modes of action for antibody fragments include blocking the action of biological molecules by binding either ligand or receptor, or engaging signaling pathways by cross-linking receptors, but, unless designed otherwise, antibody fragments do not induce effector functions such as antibody-dependent cell-mediated cytotoxicty or complement-dependent cytotoxicity. Fragments are potentially easier and less costly to manufacture, though they are also more likely to form undesirable aggregates and can be less stable than full-size mAbs.
mAbs are complex molecules, yet their structure and functions are well understood. Companies can rationally design new types of therapeutic mAbs, including smaller versions (e.g., single-chain or domain antibodies), molecules with more stable structures, and mAbs that might interact with the immune system more efficiently. In addition, new targets and pathways important to the pathology of cancer, immunological, and infective diseases are being discovered. This new knowledge provides opportunities for the development of mAbs with novel modes of action. mAbs thus continue have a great deal of potential as therapeutics for a variety of indications, including diseases that are currently untreatable.
Footnotes
Previously published online as a mAbs E-publication: www.landesbioscience.com/journals/mabs/article/7645