Abstract
Monoclonal antibodies (mAbs) as a class of novel oncology therapeutics are demonstrating clinical efficacy as measured by tumor response (shrinkage in tumor size), and prolongations in progression-free survival (PFS) and overall survival (OS). However, clinical benefits are often limited to when antibodies are used in combination with chemotherapy or radiation modalities, with tumor responses only seen in a fraction of patients, and improvements in PFS and OS are incremental.1 The potential of mAbs and mAb constructs has yet to be fully exploited for maximal clinical benefit. New approaches to further improve the effectiveness of these mAb therapies include (1) selection of patients who may derive the most benefit based on the molecular characteristics of their tumors; (2) improvements in biodistribution to maximize delivery of mAbs to susceptible tumor cells; and (3) optimization of antibody immune effector mechanisms such as antibody-dependent cellular cytotoxicity (ADCC).
Key words: monoclonal antibodies, solid tumors, cancer, pharmacogenomics, biodistribution, bioengineering
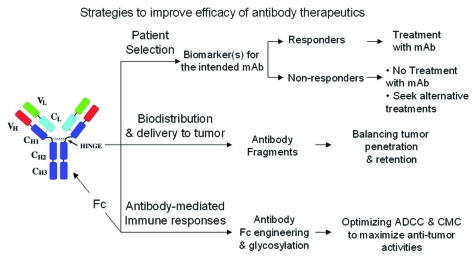
Monoclonal antibodies (mAbs) as a class of novel oncology therapeutics are demonstrating clinical efficacy as measured by tumor response (shrinkage in tumor size), and prolongations in progression-free survival (PFS) and overall survival (OS). However, clinical benefits are often limited to when antibodies are used in combination with chemotherapy or radiation modalities, with tumor responses only seen in a fraction of patients, and improvements in PFS and OS are incremental.1 The potential of mAbs and mAb constructs has yet to be fully exploited for maximal clinical benefit. New approaches to further improve the effectiveness of these mAb therapies include (1) selection of patients who may derive the most benefit based on the molecular characteristics of their tumors; (2) improvements in biodistribution to maximize delivery of mAbs to susceptible tumor cells; and (3) optimization of antibody immune effector mechanisms such as antibody-dependent cellular cytotoxicity (ADCC) (Fig. 1).
Figure 1.
Efficacy of monoclonal antibodies may be improved by selecting responding patient subpopulations, improving biodistribution and delivery of antibody to the tumor and maximizing antibody-mediated immune responses through application of protein and glyco-engineering.
Patient Selection
The selectivity and specificity of mAbs determine that these highly targeted agents block only the specific antigen proteins and associated pathways. These mAbs are best used in patients with tumors driven by the antigen proteins or pathways neutralized by the therapeutic mAbs. Therefore, it is conceivable that the best clinical benefits will derive from treating only carefully selected subpopulations of patients.
Accumulating clinical evidence has emerged in recent years supporting the critical importance of patient selection.2 A classic example is the effect of her2/neu oncogene (or its protein product HER2) expression on anti-HER2 mAb, trastuzumab (Herceptin) therapeutic efficacy.3,4 Because its efficacy was dependent on HER2 expression, Herceptin was approved in 1998 for patients with tumors evaluated to overexpress HER2 or to have HER2 gene amplification as evidenced by the HercepTest immunohistochemistry (IHC) test or the PathVysion fluorescent in situ hybridization (FISH) assay respectively. Such patient selection has limited trastuzumab use to ∼20% of breast cancer patients with HER2 overexpressing tumors, the subpopulation most likely to benefit from the anti-HER2 treatment.
Although trastuzumab was the first mAb therapy to be approved with a companion diagnostic assay, patient selection based on antigen protein expression has previously been used to support rituximab (Rituxan, an anti-CD20 antibody), treatment in CD20-positive hematological malignancies, such as non-Hodgkin's lymphoma (NHL). In contrast, it remains ambiguous whether EGFR protein expression levels are predictive of clinical responses to anti-EGFR mAb treatment.2 Potential reasons for this inconsistency may include biopsy sampling errors, poor IHC assay sensitivity, or faulty IHC reading and scoring methodology. Recent reports have indicated that EGFR gene copy number using FISH assay may be more sensitive and consistent than an IHC assay and such information successfully predicted responses in some studies. However, use of IHC and FISH assay results may still suffer from temporal differences in expression, depending upon the time the sample was obtained (at diagnosis) and when the therapy was applied (at relapse).
In order to reduce sampling error and assay real-time expression levels, minimally-invasive or non-invasive techniques for measuring antigen expression and its heterogeneity within different lesions in the same patient would be highly desirable. Circulating tumor cells may provide such an information source for a near real-time survey of the tumor cell EGFR mutation status5 as well as EGFR gene copy numbers.6 High affinity, high selectivity antigen imaging reagents have also been designed to meet this objective, and utilize both monovalent and multivalent antigen binding strategies.7,8 These imaging reagents could be used to identify antigen-expressing subpopulations. Hypotheses linking specific subpopulations to molecular phenotypes and therapeutic response require validation in Phase 1 and 2 studies, but real-time, non-invasive imaging could be used to drive selection of appropriate sub-populations of patients in pivotal Phase 3 studies.
More recently, the efficacy of mAb therapies has also been found to be dependent on the mutational status of oncogenes that are part of the pathways engaged by target antigen proteins. Among anti-EGFR mAbs, clinical efficacy of panitumumab (Vectibix) and cetuximab (Erbitux) in metastatic colorectal cancer (mCRC) has been shown to be critically dependent on the mutational status of the KRAS oncogene. Panitumumab monotherapy efficacy in mCRC is confined to patients with wild type (WT) KRAS tumors.9 Based on compelling clinical evidence, Vectibix was approved in the European Union (EU) for patients with refractory metastatic colorectal cancer with non-mutated (WT) KRAS genes. This marks the first mAb therapeutic approved with a companion genetic mutation diagnostic assay, TheraScreenK-RasCompanion Diagnostic Kit by DxS. The lack of efficacy of EGFR-targeting mAbs in patients with activating KRAS mutation is at least in part due to the underlying EGFR signaling pathway. The target antigen protein, EGFR, activates three predominant pathways including the Ras/Raf/mitogen-activated kinase (MAPK), phosphatidylinositol-3-kinase (PI-3K)/Akt and signal transducer and activator of transcription (STAT) pathways. Activating KRAS mutations, downstream of EGFR, lock the Ras/Raf/MAPK pathway in a constitutively activated state and therefore render tumors with such mutations resistant to anti-EGFR mAbs. Indeed, such activating KRAS mutations are predictive for lack of responses to cetuximab, in both monotherapy and combination settings.10–12 Furthermore, such predictive value is not limited to mCRC, but may also explain modest or no activity in patients with lung cancer treated with anti-EGFR therapeutics whose tumors harbor KRAS mutations.
These examples highlight the critical importance of prospective patient selection in the era of targeted therapy, both during clinical development of oncology mAb therapeutics and in clinical use of these novel agents.
Biodistribution and Delivery of mAb to Tumor Tissue
Biodistribution of monoclonal antibodies deep into solid tumors is both limited and inhomogeneous, with tumor uptake being only a fraction of injected dose.13 This is due to the disorganization of intratumoral blood vessels (leading to elevated interstitial pressure), tumor extracellular matrix composition, and the large molecular size of antibodies (slow diffusion of antibodies throughout the tumor).14 To uniformly penetrate a tumor, a mAb needs to diffuse half the distance away from the nearest blood vessel, typically an average of 40–100 microns, but with considerable variation around the average.15 The time for diffusion varies with the square of the distance: e.g., doubling the distance increases the diffusion time by four-fold. Large antibody molecules diffuse more slowly than small molecules. Both the intervessel distances and the interstitial pressure become greater with increasing tumor size, making it more difficult to uniformly deliver mAbs to larger tumor masses.16 Serum pharmacokinetics may or may not accurately reflect the intratumoral compartment. Clearly, optimal clinical results cannot be obtained if all susceptible tumor cells are not treated. A variety of strategies may be used to overcome limited penetration, including the use of smaller antibody constructs, or prolonged therapy at higher doses.17
A variety of smaller antibody-like constructs are available to improve tumor diffusion efficiency, including diabodies, minibodies, Fab fragments, single chain Fv domains and single chain antibodies derived from camels and llamas (reviewed in ref. 18). These constructs range in size from 15,000 to 60,000 molecular weight and generally offer faster, more homogeneous penetration of tumors at the expense of tumor retention, serum half life and ADCC functionality. Tumor retention can be improved by creating multivalent constructs.19 The electrical charge of an antibody molecule also affects its ability to penetrate tumors and antibodies which are uncharged in the physiologic pH range enter tumors more readily.20 Finally, there is an optimal affinity between an antibody and its target. Antibodies which bind too tightly may be immobilized at the tumor periphery,21 but the ultimate affinity must be high enough so that the antibody stays bound long enough to exert therapeutic effects.18,22
We believe antibody constructs and their dosing regimens should be designed with optimal tumor penetration in mind, unless the antibody is being used only to elicit a generalized immune response or to bind a soluble tumor factor such as vascular endothelial growth factor (VEGF), or is attached to a radioactive moiety with a long radiation path length. Optimal engineering requires a comprehensive preclinical and clinical program which correlates penetration into the tumor with plasma exposure and with preclinical efficacy. Mathematical models for scaling these kinds of detailed results from mouse to man are available.23 Ideally, we would like to see a histogram of the degree to which a tumor mass is penetrated as a function of distance from the nearest blood vessel, based on immunohistochemical analysis of preclinical model systems, and correlation of this data with preclinical efficacy.
Preclinical and clinical use of a labeled imaging reagent that can be reproducibly manufactured, bind to the target antigen, and readily penetrate tumors, would be of great utility to evaluate biodistribution. Affibody molecules are an example of this type of affinity ligand imaging reagent.8 Affibody ligands are 58 amino acid constructs derived from staphylococcal protein A, with random substitutions in a 13 amino acid segment leading to customized binding properties. Affibody molecules are selected to exhibit sub-nanomolar affinity and selectivity for cell surface antigens, and their small size promises to afford excellent tumor penetration and rapid plasma clearance. In support of this hypothesis, preclinical and pilot clinical data have demonstrated that radiolabeled Affibody molecules can be used for in vivo radionuclide imaging of HER2 expression in malignant tumors. Although not yet developed as imaging agents, DARPins—Designed ankyrin repeat proteins24 may also offer a non-antibody imaging ligand approach. DARPins are obtained from libraries comprised of 33 amino acids with 6 variable residue positions which, when two or three repeats are combined, leads to a large diversity of potential binding proteins with molecular weights between 10 and 20 kDa. Given the widespread availability of phage display tools, peptide synthesis capabilities, a variety of bioconjugate techniques, and sophisticated molecular modeling tools, we anticipate that a variety of useful affinity ligand imaging reagents will be engineered.
Optimal antibody biodistribution requires careful construct engineering, preclinical validation and clinical optimization of the dosing regimen. Currently, randomized clinical studies with efficacy endpoints are the only way to reliably distinguish between dosages, but in the future it may be possible to identify and validate dosing regimens by establishing receptor occupancy using imaging. A rapidly penetrating and rapidly cleared affinity ligand imaging agent could be administered both prior to treatment and after steady state mAb administration, to determine the fraction of target sites occupied by the therapeutic antibody. Such a paradigm is applicable only if the antibody binding epitope overlaps that of the affinity ligand imaging agent sufficiently to allow blocking of the imaging agent by the therapeutic antibody. Moreover, blocking experiments are complicated by antigen internalization and re-expression kinetics. Thus, preclinical feasibility studies correlated with preclinical efficacy would need to precede any clinical studies, to establish proof-of-concept for the competition experiment between therapeutic antibody and imaging reagent.
Delivery to tissues is an important issue for all therapies, but even more so for mAbs because of their large size and consequent slow diffusion. A comprehensive preclinical and clinical biodistribution program would support optimal antibody engineering and choice of dose and schedule, to maximize the delivery of antibody therapies to target cells.
Optimization of Antibody-mediated Immune Responses
mAbs exert their anticancer effects not only via blockade of growth factor/receptor interaction and/or downregulation of oncogenic proteins (e.g., growth factor receptors) on the tumor cell surface, but also by their ability to elicit effector mechanisms of the immune system, such as antibody-dependent cellular cytotoxicity (ADCC) and complement-mediated cytotoxicity (CMC). Such immune responses are mediated by IgG antibodies through their engagement of the cellular immune system via interaction of the Fc domain of antibodies with Fcgamma receptors (FcγR's) on immune cells.25,26 Current data suggest that activating receptors, FcγRIIa and FcγRIIIa, and inactivating receptor, FcγRIIb, are most crucial to regulating antibody directed cytotoxicity.25,26 FcγR genetic polymorphisms in individual patients have now been linked to (1) the ability to mount an ADCC response, and (2) clinical outcome to not only rituximab in patients with B-cell lymphoma,27,28 but also most recently trastuzumab in patients with breast cancer,29 and cetuximab in patients with colorectal cancer.30 In addition to predicting individual patient clinical outcome, implications of such clinical findings also suggest the possibility of improving mAb efficacy by developing mAbs tailored to each patient's FcγR genetic polymorphisms. For example, it would seem that an antitumor mAb engineered to have high affinity for the activating FcγR's and low affinity for inhibitory FcγRIIb would be desirable for certain patients.25,26
Several approaches have been developed for modifying the affinity of human antibodies to the various FcγR's. These can be grouped as those techniques that modify Fc amino acid sequence and those that modify glycosylation state.25,26 Engineering of the amino acid sequence of the Fc portion of antibodies, based on knowledge gleaned from screening of libraries of Fc variants and/or from computational design based on structural knowledge, has been successful in producing antibodies with altered binding to specific FcγR's.25,26 Several of these antibodies have been evaluated in vivo and demonstrated to produce the predicted effects upon ADCC.31–33
Glycosylation, including fucosylation, sialylation and mannose structures have all been demonstrated to alter Fc binding to FcγR's and ADCC.34 Fucose content appears to be a major factor in ADCC in vitro.34,35 Removal of fucose has been demonstrated to significantly increase FcγRIIIa affinity and improve ADCC.36 Greater than 90% of recombinant immunoglobulin G's (rIgGs) produced in normal Chinese hamster ovary (CHO) cells are fucosylated; therefore several strategies have been developed to reduce fucosylation of rIgGs, including production of CHO cell lines that lack or have reduced expression of 1,6-fucosyltransferase, or in which inducible overexpression of the enzymeβ1, r-N-acetylglucosaminyltransferase III can be employed to secondarily reduce fucose content.34,35 Several rIgGs that have been engineered to contain little or no fucose are currently in clinical trials.34
Conclusions
mAbs are an established therapeutic option for patients with cancer. However, there have been only incremental gains in progression free survival and overall survival following treatment with mAbs. These incremental gains may be due to: (1) treatment of mixed populations of patients, some of whom are not able to respond to the mAb, (2) incomplete penetration of the tumor with mAb, leading to inevitable relapse (driven by cells that have not been exposed to therapy) or (3) emergence of resistance to inhibition of signaling—either by mutation, selection of pre-existing populations or feedback effects on existing signaling pathways.
Identifying responders is critical for any targeted therapy. In particular, molecular information regarding target expression or pathway activation can help to define hypotheses for identifying responders. This has been successfully achieved for mAbs against CD20, her2neu and EGFR. These responder ID hypotheses need to be confirmed clinically in early clinical studies, leading to progressive, data-driven enrichment of responders in later stage clinical development and, ultimately, clinical application.
Therapy cannot be fully effective if it is not delivered to all susceptible cells, although partial shrinkage of tumors can be achieved. Distribution of mAbs to the majority of target cells may require novel, smaller constructs, with multiple valence to facilitate tumor retention. Alternatively, it may be possible to achieve homogeneous intratumoral distribution through prolonged high dosing. Biodistribution of antibodies may, in the future, be visualized by utilizing imaging studies to monitor the ability of a mAb to block receptor sites.
While mAbs very specifically address signaling pathways, such very specific therapy may lead to selection of pre-existing resistant sub-populations, evolution of new acquired resistance or rapid adjustment of cells by feedback mechanisms within the pathways. In this regard, it is useful to also have a supplementary non-specific mechanism of cell killing, such as antibody dependent cellular cytotoxicity (ADCC). We believe increased use of protein engineering and glycoengineering to enhance ADCC will be seen in the future.
Further attention to treating the right patients with mAbs, getting mAbs to distribute to the majority of target cells, and optimizing their non-specific effector functions may allow even greater clinical benefit with these novel agents.
Footnotes
Previously published online as a mAbs E-publication: http://www.landesbioscience.com/journals/mabs/article/7359
References
- 1.Yan L, Hsu K, Beckman RA. Antibody-based therapy for solid tumors. Cancer J. 2008;14:178–183. doi: 10.1097/PPO.0b013e318172d71a. [DOI] [PubMed] [Google Scholar]
- 2.Yan L, Beckman RA. Pharmacogenetics and pharmacogenomics in oncology therapeutic antibody development. Biotechniques. 2005;39:565–568. [PubMed] [Google Scholar]
- 3.Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639–2648. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 4.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 5.Hirsch FR, Herbst RS, Olsen C, et al. Increased EGFR gene copy number detected by fluorescent in situ hybridization predicts outcome in non-small-cell lung cancer patients treated with cetuximab and chemotherapy. J Clin Oncol. 2008;26:3351–3357. doi: 10.1200/JCO.2007.14.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maheswaran S, Sequist LV, Nagrath S, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med. 2008;359:366–377. doi: 10.1056/NEJMoa0800668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li ZB, Wu Z, Chen K, Ryu EK, Chen X. 18F-labeled BBN-RGD heterodimer for prostate cancer imaging. J Nucl Med. 2008;49:453–461. doi: 10.2967/jnumed.107.048009. [DOI] [PubMed] [Google Scholar]
- 8.Orlova A, Tolmachev V, Pehrson R, et al. Synthetic affibody molecules: a novel class of affinity ligands for molecular imaging of HER2-expressing malignant tumors. Cancer Res. 2007;67:2178–2186. doi: 10.1158/0008-5472.CAN-06-2887. [DOI] [PubMed] [Google Scholar]
- 9.Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–1634. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 10.Lievre A, Bachet JB, Le Corre D, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66:3992–3995. doi: 10.1158/0008-5472.CAN-06-0191. [DOI] [PubMed] [Google Scholar]
- 11.Bokemeyer C, Bondarenko I, Hartmann JT, et al. KRAS status and efficacy of first-line treatment of patients with metastatic colorectal cancer (mCRC) with FOLFOX with or without cetuximab: The OPUS experience. J Clin Oncol. 2008:26. [Google Scholar]
- 12.Van Cutsem E, Lang I, D'haens G, et al. KRAS status and efficacy in the first-line treatment of patients with metastatic colorectal cancer (mCRC) treated with FOLFIRI with or without cetuximab: The CRYSTAL experience. J Clin Oncol. 2008;26:2. [Google Scholar]
- 13.Sedlacek HH, Seemann G, Hoffmann D. Antibodies as carriers of cytotoxicity. Basel: Karger; 1992. [Google Scholar]
- 14.Jang SH, Wientjes MG, Lu D, Au JL. Drug delivery and transport to solid tumors. Pharm Res. 2003;20:1337–1350. doi: 10.1023/a:1025785505977. [DOI] [PubMed] [Google Scholar]
- 15.Nugent LJ, Jain RK. Extravascular diffusion in normal and neoplastic tissues. Cancer Res. 1984;44:238–244. [PubMed] [Google Scholar]
- 16.Williams LE, Bares RB, Fass J, Hauptmann S, Schumpelick V, Buell U. Uptake of radio-labeled anti-CEA antibodies in human colorectal primary tumors as a function of tumor mass. Eur J Nucl Med. 1993;20:345–347. doi: 10.1007/BF00169812. [DOI] [PubMed] [Google Scholar]
- 17.Blumenthal RD, Fand I, Sharkey RM, Boerman OC, Kashi R, Goldenberg DM. The effect of antibody protein dose on the uniformity of tumor distribution of radioantibodies: an autoradiographic study. Cancer Immunol Immunother. 1991;33:351–358. doi: 10.1007/BF01741594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beckman RA, Weiner LM, Davis HM. Antibody constructs in cancer therapy: protein engineering strategies to improve exposure in solid tumors. Cancer. 2007;109:170–179. doi: 10.1002/cncr.22402. [DOI] [PubMed] [Google Scholar]
- 19.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 20.Melkko S, Halin C, Borsi L, Zardi L, Neri D. An antibody-calmodulin fusion protein reveals a functional dependence between macromolecular isoelectric point and tumor targeting performance. Int J Radiat Oncol Biol Phys. 2002;54:1485–1490. doi: 10.1016/s0360-3016(02)03927-5. [DOI] [PubMed] [Google Scholar]
- 21.Fujimori K, Covell DG, Fletcher JE, Weinstein JN. A modeling analysis of monoclonal antibody percolation through tumors: a binding-site barrier. J Nucl Med. 1990;31:1191–1198. [PubMed] [Google Scholar]
- 22.Graff CP, Wittrup KD. Theoretical analysis of antibody targeting of tumor spheroids: importance of dosage for penetration, and affinity for retention. Cancer Res. 2003;63:1288–1296. [PubMed] [Google Scholar]
- 23.Khawli LA, Biela B, Hu P, Epstein AL. Comparison of recombinant derivatives of chimeric TNT-3 antibody for the radioimaging of solid tumors. Hybrid Hybridomics. 2003;22:1–9. doi: 10.1089/153685903321538026. [DOI] [PubMed] [Google Scholar]
- 24.Stumpp MT, Amstutz P. DARPins: a true alternative to antibodies. Curr Opin Drug Discov Devel. 2007;10:153–159. [PubMed] [Google Scholar]
- 25.Desjarlais JR, Lazar GA, Zhukovsky EA, Chu SY. Optimizing engagement of the immune system by anti-tumor antibodies: an engineer's perspective. Drug Discov Today. 2007;12:898–910. doi: 10.1016/j.drudis.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 26.Presta LG. Molecular engineering and design of therapeutic antibodies. Curr Opin Immunol. 2008 doi: 10.1016/j.coi.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 27.Cartron G, Dacheux L, Salles G, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754–758. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- 28.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 2003;21:3940–3947. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 29.Musolino A, Naldi N, Bortesi B, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol. 2008;26:1789–1796. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- 30.Zhang W, Gordon M, Schultheis AM, et al. FCGR2A and FCGR3A polymorphisms associated with clinical outcome of epidermal growth factor receptor expressing metastatic colorectal cancer patients treated with single-agent cetuximab. J Clin Oncol. 2007;25:3712–3718. doi: 10.1200/JCO.2006.08.8021. [DOI] [PubMed] [Google Scholar]
- 31.Dall'Acqua WF, Kiener PA, Wu H. Properties of human IgG1s engineered for enhanced binding to the neonatal Fc receptor (FcRn) J Biol Chem. 2006;281:23514–23524. doi: 10.1074/jbc.M604292200. [DOI] [PubMed] [Google Scholar]
- 32.Lazar GA, Dang W, Karki S, et al. Engineered antibody Fc variants with enhanced effector function. Proc Natl Acad Sci USA. 2006;103:4005–4010. doi: 10.1073/pnas.0508123103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stavenhagen JB, Gorlatov S, Tuaillon N, et al. Fc optimization of therapeutic antibodies enhances their ability to kill tumor cells in vitro and controls tumor expansion in vivo via low-affinity activating Fcgamma receptors. Cancer Res. 2007;67:8882–8890. doi: 10.1158/0008-5472.CAN-07-0696. [DOI] [PubMed] [Google Scholar]
- 34.Raju TS. Terminal sugars of Fc glycans influence antibody effector functions of IgGs. Curr Opin Immunol. 2008 doi: 10.1016/j.coi.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 35.Satoh M, Iida S, Shitara K. Non-fucosylated therapeutic antibodies as next-generation therapeutic antibodies. Expert Opin Biol Ther. 2006;6:1161–1173. doi: 10.1517/14712598.6.11.1161. [DOI] [PubMed] [Google Scholar]
- 36.Shields RL, Lai J, Keck R, et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J Biol Chem. 2002;277:26733–26740. doi: 10.1074/jbc.M202069200. [DOI] [PubMed] [Google Scholar]