Abstract
Antibody targeting of cancer is showing clinical and commercial success after much intense research and development over the last 30 years. They still have the potential to delivery long-term cures but a shift in thinking towards a cancer stem cell (CSC) model for tumor development is certain to impact on how antibodies are selected and developed, the targets they bind to and the drugs used in combination with them. CSCs have been identified from many human tumors and share many of the characteristics of normal stem cells. The ability to renew, metabolically or physically protect themselves from xenobiotics and DNA damage and the range of locomotory-related receptors expressed could explain the observations of drug resistance and radiation insensitivity leading to metastasis and patient relapse.
Targeting CSCs could be a strategy to improve the outcome of cancer therapy but this is not as simple as it seems. Targets such as CD133 and EpCAM/ESA could mark out CSCs from normal cells enabling specific intervention but indirect strategies such as interfering with the establishment of a supportive niche through anti-angiogenic or anti-stroma therapy could be more effective.
This review will outline the recent discoveries for CSCs across the major tumor types highlighting the possible molecules for intervention. Examples of antibody-directed CSC therapies and the outlook for the future development of this emerging area will be given.
Key words: antibody, targeting, cancer, stem cell, therapy
Monoclonal antibodies are clinically and commercially-established therapeutics.1,2 A great deal of progress has been made over the last 30 years in overcoming problems and translating the phenomenal amount of laboratory research into clinical products. However, antibodies or other molecular interventions against cancer do not necessarily cure. In many cases, they can increase survival and improve quality of life. So, have we been hitting the wrong targets? Certainly, receptors such as human epidermal growth factor-1 (HER1/EGFR), HER2, CD20 and growth factors such as vascular endothelial cell (VEGF) and Interleukin-6 (IL-6) are involved in the cancer process, but have we been overlooking the real culprits?
This review aims to examine the biology of cancer stem cells considering the markers defining them and their survival and will describe the new antibody-focused strategies emerging to target them for more effective treatment of cancer.
Introduction to Cancer Stem Cells (CSCs)
The ‘Seed and Soil’ theory of the English surgeon Paget, in 1889,3 significantly pre-dates the current cancer stem cell hypothesis and once again shows how many of the best theories were thought about many years ago, went generally unnoticed but were later supported by technological advances. Paget surveyed breast cancers in patients and was struck by the discrepancy between blood supply and site of metastasis in some organs. He concluded that cancer cells (‘seeds’) could only grow in congenial conditions (‘soil’). This theory contradicted the previous view that tumor cells lodged in the vasculature, and was challenged by others over the next 100 years. However, after 120 years of scrutiny, it seems that this view was correct. The ‘seed’ is now the cancer stem cell/tumor-initiating cell/progenitor cell and the ‘soil’ is made up of stroma, host factors and all the interactions within an organ which regulate angiogenesis, adhesion and migration.
The multi-step, clonal evolution nature of cancer development has been the accepted paradigm for many years with the central idea that the majority of cancer cells are tumorigenic after having accumulated key mutations.4,5 A pathway to tumorigenesis occurs whereby cells acquire six hallmarks: self sufficiency in growth signaling, insensitivity to anti-growth signaling, evasion of apoptosis, unlimited replicative potential, sustained angiogenesis and tissue invasion. Being a genetic disease, an early event is usually a defect in DNA stability, the so-called ‘caretaker’ pathway, followed by the loss of a tumor suppressor gene or activaton of an oncogene (‘gatekeeper’ pathway).4,5 The explanation that the key tumorigenic mutations occur in a few cells that can self-renew and reside in tissues long-term is a major shift in thinking and has wide-ranging implications for cancer therapy. The view that is emerging is that cancer originates from tissue stem/progenitor cells through dysregulation of the self-renewal process and that these CSCs drive tumor growth. Chemotherapy and radiotherapy intervention destroys the proliferating and differentiated cells that form the bulk of the tumor, but are largely ineffective against the relatively quiescent/dormant CSCs which have protective mechanisms for repairing DNA and counteracting cytotoxic drugs (see below). Most therapies do not target self-renewal pathways. To overcome radiation/drug resistance which leads to patient relapse, we must target the CSCs.
Cancer stem cells can represent approximately 0.1–10% of all tumor cells and their antigens are typically expressed at lower levels than the ‘established’ tumor-associated antigens (Table 1). Unlike these, the discovery of CSC antigens was not based on their overexpression but due to their presence on populations of cells which had stem cell-like properties.6–12 This made their discovery difficult. The first reports of CSC were in 1997 for acute myeloid leukaemia (AML)6 which were shown to be CD34+CD38−, similar to normal haematopoietic stem cells. The variable expression levels on CSC and often co-expression on normal stem cells has made CSC antigen distinction, as possible therapeutic targets, difficult.
Table 1.
Antibody-mediated cancer stem cells (related) therapies
| Target | Cancer | Effect (Reference) |
| CD44 | Acute myeloid leukaemia (AML) | Inhibited tumor proliferation, increased apoptosis, inhibited niche localization/protection64 |
| p-glyco-protein 1, | Pancreas, AML | Inhibited tumor growth in vivo66 |
| hyaluronate (HA) receptor | Head and neck cancer | Radio-immunotherapy inhibited tumor growth69,70 |
| Drug conjugate inhibited tumor growth but side effects71,72 | ||
| Melanoma | Inhibited tumor growth, decreased metastases, increased animal survival75 | |
| EpCAM Epidermal surface Ag | Colon, prostate | Inhibited tumor growth,84 in clinical trials85 |
| CD326, flotillin | Breast, prostate, ovarian, GI, colon | Inhibited tumor growth via ADCC mech.,87 clinical trials86 |
| CD133 Prominin-1 | Hepatocellular cancer | Potent drug conjugate in vitro80 |
| CD24 Heat-stable antigen | Colon, pancreas | Inhibited tumors in vivo via CD24 degradation97 |
| CXCR4 | Multiple melanoma | Inhibited tumor growth151 |
| Prostate | Inhibited tumor growth152 | |
| Colon | Inhibited tumor growth153 | |
| IL-4 | Colon | Sensitized tumor cells to chemotherapy102 |
| IL-6 | Prostate | Inhibited tumors in vivo, in clinical trial127 |
| PSCA | Prostate | Inhibited tumors in vivo (signaling?)120 |
| Prostate | Inhibited tumor metastases121 | |
| CD200 | Chronic lymphocytic leukaemia (CML) | Reversed immunosupression = tumor rejection133 |
| CD123 | AML, CML | scFv-pseudomonas exotoxin killed cells128 |
| DLL4 | Various (colon, breast) | Increased non-productive angiogenesis to inhibit tumor growth114 |
| Colon | Inhibited tumors with chemotherapy112 | |
| Frizzled | Colon, breast | Inhibited tumors in vivo115 |
| Wnt | Non small cell lung cancer | Inhibited tumors by increasing apoptosis116 |
| Notch | Breast | Targeted non-ligand binding site, inhibited tumors with chemotherapy108 |
| Various tumors | Targeted ligand-binding site, inhibited tumors with chemotherapy109 | |
| Patched | Pancreas | Inhibited tumor cell lines117 |
| Integrin | Various tumors | Inhibited tumors in vivo156 |
| Prostate | Inhibited tumors in vivo157 | |
| Colon | Inhibited tumors making more radiosensitive160,161 | |
| ALDH-1 | Melanoma | Inhibited tumor growth and eradicated tumors by ADCC94 |
| VEGF/VEGFR | Glioma | Inhibited tumor growth by disrupting niche?138,141,142 |
Summary of cancer stem cell markers and key antibody-mediated cancer stem cell-related therapies.
Progress in the field of stem cell biology has been hindered by difficulties in identifying, isolating and characterizing stem cells. Functional properties such as self-renewal and differentiation were used but more recently, cell surface markers such as CD133 and CD44 in combination with flow cytometry have identified subpopulations.6–12 Using mouse xenograft assays, serial transplantation of tumor cells have identified clones which are able to form tumors resembling the original tumor. Reports from different tumor models have shown that as few as one hundred CSCs were sufficient to initiate tumor formation in vivo.10 In vitro assays such as the ability to form multicellular spheroids have also facilitated studies.9
Characteristics of Cancer Stem Cells
Normal stem cells (bone marrow, hair, gastro-intestinal mucosal) recover after chemo- and radio-therapy, therefore the CSC model can explain the occurrence of patient relapse in that CSCs form a reservoir for disease recurrence after treatment. CSCs resistance to radiotherapy is primarily due to slow cell cycling, lower proliferation, increased expression of DNA repair and anti-apoptosis genes. This has been seen in CD133+ glioblastoma cells12 and CD44+/CD24− breast cancer cells.14 All these properties may explain tumor relapse in cancer patients, where radiation-resistant tumor cells regrow after radiotherapy. Radiation-treated glioblastoma tumors contain a higher level of CD133+ cells which were radio-resistant. Closer inspection of these cells found higher levels of DNA repair in pathways such as DNA checkpoint kinases (chk).13 Small molecule inhibition of chk-1 or chk-2 kinases could reverse this and make CSCs more sensitive to radiation.13,15 Notch-1/Jagged pathway defects are also related to radiation resistance.15 Radiation-induced death works by the formation of damaging reactive oxygen species,16 so a hypoxic environment which is likely to occur in solid tumor niches would result in less effective cytotoxic damage.17 This could also explain CSCs insensitivity to radiation-induced damage.
CSCs are also thought to contribute to chemotherapy resistance. Low proliferation and effective DNA repair can modulate this,15 however specific membrane proteins have been associated with CSC drug resistance.14 ATP-binding cassette (ABC, see below) drug transporters are membrane efflux, proteins pumps which are highly expressed in normal SCs providing protection from xenobiotic molecules. The ABC-B1/MDR1 and ABC-G2 pumps are also expressed in many tumors.18 The function of these receptors can be monitored by the expulsion of fluorescent molecules such as the Hoecht 33342 dye. This property allowed CSCs to be separated by flow cytometry from non-stem cells, as a so-called ‘side population’.18,19 Leukaemic side population cells, enriched for CSCs have increased ability to pump out chemotherapy drugs such as daunorubicin and mitoxanthrone.20 Such receptors are obvious targets for inhibitors, especially small molecules. However, they may cause toxicity due to their role in normal stem cells. Additionally, CSCs express metabolic enzymes such as aldehyde dehydrogenase (ALDH-1, see below) which confers resistance to cyclophosphamide in normal stem cells. ALDH-1 is amplified in leukaemic CSCs rendering them resistant to cyclophosphamide and by breast CSCs where it indicates poor prognosis.21 Blocking the function of surface expressed drug pumps may be an interesting approach in combination with chemotherapy for CSC therapy.19 Although gemcitabine is often used as first line therapy in pancreatic cancer, it has recently been shown that pancreatic CSCs may be resistant to gemcitabine.22 Glioma CSCs are resistant to several drugs including temozolamide, carboplatin, etoposide and paclitaxel.23 Akt signaling activity is thought to mediate resistance in hepatocellular CSC24 indicating an upregulation in the survival pathway.
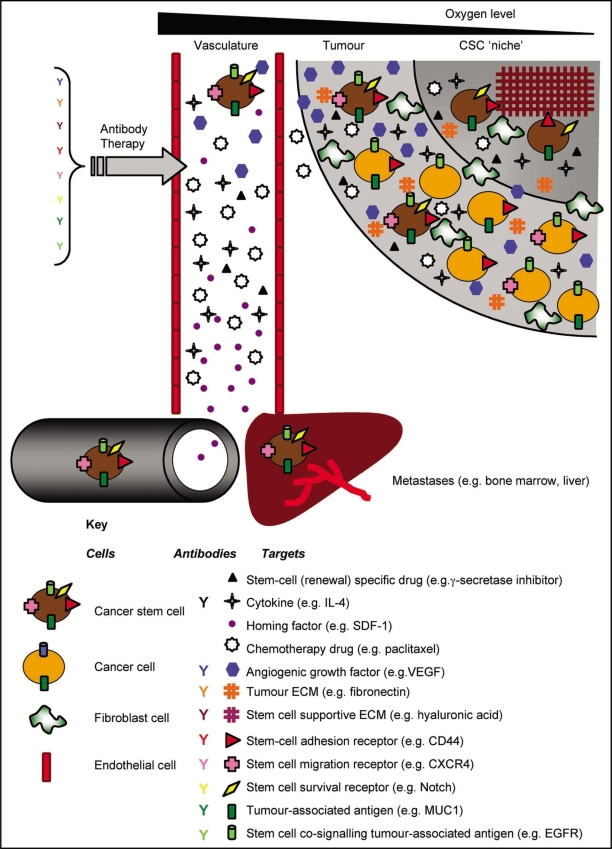
The CSC model also proposes that CSCs reside in a supportive niche, often hypoxic but of a poor vascular supply (Fig. 1). This would result in poor drug perfusion contributing to an ineffective chemotherapy response. This idea is addressed later in detail.
Figure 1.
Cancer stem cell-related antibody targeting. Tumors are proposed to contain a CSC niche which supports the small number of CSCs through cell-matrix interactions. These CSCs, bearing receptors for adhesion and self-renewal give rise to the bulk tumor cells which also have tumor-associated antigens as well as daughter CSCs. Metastatic CSCs may arise when receptors involved in invasion and homing are expressed. Growth factors are expressed which stimulate tumor angiogenesis, migration to metastatic sites and promote proliferation and protection within existing niches. Chemotherapy drugs have poor access to the tumor. Factors which could be modulated by antibody intervention are shown as well as the use of stem-cell renewal-specific drugs for a combinatorial approach to CSC therapy. Refer to the key for the types and examples of receptors and CSC-factors.
Markers on CSCs
Acute myeloid leukaemia (AML).
Cells responsible for tumor initiation and recurrence were first discovered in AML in 1997.6 Here, CD34+CD38− leukaemic stem cells, isolated by flow cytometry, shared markers with normal haematopoietic stem/progenitor cells (HSCs). These cells had the capacity to engraft in non-obese diabetic severe combined immunodeficient (NOD-SCID) mice. A leukaemic hierarchy was discovered within the clonally heterogenous tumor population of undifferentiated myeloid blasts. Leukaemic CSCs continually replenished these tumor cells. AML CSCs are relatively quiescent and unresponsive to anti-proliferative therapies, explaining why less than 30% of patients with AML will survive. This important work raised many questions, particularly whether leukaemic CSCs, like their normal HSC counterparts, were dependent on the bone marrow stem cells niche, a supporting microenvironment. If true, new therapeutic approaches disrupting adhesion and migration to this niche could be exploited. As we will see below, this approach has yielded many examples and successes.
Breast cancer.
Breast cancer stem cells were identified using similar approaches for AML. Breast CSCs have been shown to express CD44, but have low amounts of CD24. It has been shown that as few as 100 (CD44+/CD24low) cells were able to initiate tumors in vivo,7 in contrast to 100-times more cells without this marker distribution being unable to form tumors. Breast CSCs also express the drug-metabolising enzyme, ALDH-1, like normal breast stem cells. CD44+/CD24low/lin−/ADH+ breast CSCs were found to be 10-times more tumorigenic.25 Breast CSCs were also shown to be CD44+CD24low/ESA+.26 The presence of CD44 may be related to the adhesion function required in metastasis and CD24 is a negative regulator of the chemokine receptor CXCR4, another molecule involved in breast cancer metastasis.27 These receptors are discussed below.
Pancreatic cancer.
As in breast CSCs, CD44, CD24 and ESA antigens were examined in pancreatic cancer stem cells, using primary tumor samples from patients.11 Cells with the CD44+/CD24+/ESA+ pattern (present in less than 1% of the patients' pancreatic tumor cells) had the highest tumorigenic potential whereas CD44−/CD24−/ESA− cells were at least 100-fold less tumorigenic.11 Recent studies have also identified CD133 (see below) as a marker in pancreatic CSCs present in 1–3% of tumor cells. This opens up the possibility of more than one CSC subtype. There was a 14% overlap between the CD133+ and CD44+/CD24+/ESA+ cells in pancreatic cancer but no overlap between CD44+/CD24− and CD133+ breast CSCs subpopulations.22,28 It is further possible that pancreatic CSCs possessing all four markers (CD44+/CD24+/ESA+/CD133+) are even more tumorigenic.
Prostate cancer.
Like the breast, the prostate is a hormone-regulated gland whose regeneration is regulated by androgen. Prostate stem cells appear to be present in the basal compartment and don't express androgen receptors.29 Prostate Stem Cell Antigen (PSCA, see below) has the potential to be a CSC marker but is more likely to be expressed on differentiated cells as is the androgen receptor (AR).30 Self-renewing tumor cells have been isolated from primary prostate cancers (and later from cell lines and xenografts) bearing the CD44+CD133+α2β1Hi markers (the latter being integrin receptor subunits). In mice, this subpopulation of 0.1% total prostate tumor cells had tumor-initiating capacity.29
Head and neck (squamous cell carcinoma).
As in other solid cancers, the CD44+ population of tumor cells from patients with head and neck cancer can initiate tumor growth in immunodeficient mice.31 CD44+lin− cells were shown to be able to self-renew and are predominantly basal cells that co-localize with cells expressing the stem cell survival factor bmi-1. Soluble CD44 is found as a biomarker in head and neck cancer patients, whose levels reduce upon treatment.32 Some CD44 splice variants have been identified and one was subject of an antibody-targeted therapy (see below) before the emergence of the CSC model.
Glioblastoma.
Glioblastoma CSCs have been identified primarily by the cell surface marker CD133,10 but the intracellular signaling molecule nestin has also been associated.12 CD133+ brain CSCs from patients escaped lethal radiotherapy damage by activation of DNA repair checkpoint enzymes chk-1 and chk-2. After irradiation, CD133+ cells were enriched in the population compared to CD133− due to a reduction in apoptosis.13 This points to a mechanism where CSCs upregulate their capacity for DNA repair.13 In a parallel study,33 the bone morphogenic protein (BMP) pathway was found to regulate CD133+ brain CSC differentiation. Exposure of CD133+ brain CSCs to BMP induced morphological changes, decreased proliferation and invasion. In vivo, this approach led to a reduction in tumor growth and points to a way to make such CSCs more susceptible to conventional therapy. As will be discussed later, much of the work regarding CSC niches and supportive microenvironments in solid tumors have come from studies on brain tumors.
Colon cancer.
Gastro-intestinal stem cells have been extensively studied due to their role in the structure and function of the colon crypt and the high rate of cell renewal in colonic epithelium.34 CSCs which are able to form tumors after transplanting 100–3,000 cells have been identified as CD44+ESA+.34 A subpopulation within this which were CD166+ were even more tumorigenic.36 Additional properties such as drug resistance to cyclophosphamide is mediated by expression of ALDH-1 in colon cancer exposed to chemotherapy.37
Signaling Pathways
As with cell surface markers, CSCs share various signaling characteristics with normal stem cells, but they also contain unique and disease-specific features and pathways with potential for exploitation as therapeutic targets. Normal SCs have the ability to differentiate into a variety of cell types and generate numerous daughter cells. A characteristic feature of self-renewing cells is an increase in telomerase activity, the enzyme which ensures that the length of telomeres remains constant after cell division. This means that the cells are not subject to the aging effect and maintain an infinite replication potential. As the cells differentiate the progenitor cells lose their ability to self-renew, probably from loss of telomerase activity.38 The case is similar for cancer stem cells: telomerase activation occurs in most human haematological neoplasia, indicating that telomerase activation and telomere stabilization are necessary for cancer stem cell progression.39 In addition, telomerase activity is upregulated in glioblastoma multiforme but is undetectable in human fetal neural stem cells, suggesting that telomerase is also a key component in CSC populations.40 Taken together, these studies point to a molecular model in which the telomerase molecular mechanism that helps to define normal stem cells also defines cancer stem cells.
The signaling pathways that govern normal SC proliferation are also those promoting carcinogenesis, by initiating CSC proliferation. Deregulation of signaling pathways, such as p53/p21, Notch, Sonic hedgehog (Shh) Wnt/-catenin, Bmi-1 and Hox gene family products, can lead to transformation of SCs into CSCs.41
The Notch pathway functions in determining a diverse array of cell fates and regulates many cellular processes during embryonic development and throughout adulthood. It has been associated with several human cancers, including cervical, lung, breast carcinoma and neuroblastoma.42 Direct evidence that Notch signaling is activated in a wide variety of human breast carcinomas was observed, and was accompanied by the accumulation of its cleaved form—the Notch intracellular domain (NICD)—and the expression of known downstream target genes.43 More significantly, its was shown that attenuation of Notch signaling reverted the transformed phenotype of human breast cancer cell lines, suggesting that inhibition of Notch signaling may be a therapeutic strategy for this disease (see below).
The Hedgehog molecules (e.g. Hh) are important signaling proteins in the development of embryonic stem cells and in the differentiation of many tissues. In normal tissues, the pathway has been shown to be involved in the maintenance of haematopoietic stem cells and expansion of progenitors, in postnatal and adult brain development, and in the development of skin, hair follicles and sebaceous glands.44 Hedgehog (Hh) binds to the cell-surface receptor Patched (PTCH) and signals through the Smoothened and GLI proteins. Hh overexpression leads to the unregulated growth of tissue stem cells. This drives hyperproliferation and makes the stem cells targets for genetic events that could drive them into the formation of tumor stem cells. Continued evolution of the tumor stem cells could occur to give rise to metastatic cells or further drug resistance. Components of the Hh-PTCH pathway are disrupted or overexpressed in a large number of tumors, including sporadic medulloblastomas, breast, prostate, stomach, colon and pancreatic cancers.45
The Wnt-dependent signaling pathways are conserved through evolution and control many events during embryonic development. Secreted Wnt ligands bind to Frizzled receptors and activate cascades important in development. Inappropriate regulation and activation of these cascades is associated with several pathological disorders including cancer (notably in 90% of colorectal cancer), retinopathy, and bone and cartilage disease such as arthritis. In addition, recent research suggests that Wnt signaling is also essential in stem cell self-renewal.46 The expression of Wnt proteins in the bone marrow suggests that they may influence haematopoietic stem cells (HSCs) as well. Wnt signaling has been shown to increase the expression of HoxB4 and Notch-1 genes, both of which are implicated in the specification and/or self-renewal of HSCs.47
The Bmi-1 protein also plays a crucial role in regulating the self-renewal process of SCs and CSCs. The Bmi-1 proto-oncogene takes part in haematopoietic and neural SC self-renewal maintenance48 via cyclin-dependent kinase inhibition. Lack of p16, accompanied by abnormal Bmi-1 function, promotes cell proliferation by increasing its self-renewal potential, whereas a lack of the p14 inhibitor hinders proapoptotic gene expression. These have been shown to play a role in stem cell senescence in the blood, brain and pancreatic islet cells. In patients with AML, expression of Bmi-1 is higher in AML cells than in normal bone marrow.49
The above studies provide evidence for the existence of CSCs with the following characteristics.
FIve Key Characteristics of CSCs
Only a small portion of the tumor cells in a tumor have tumorigenic potential when transplanted into immunodeficient mice.
The CSC subpopulation can be separated from other tumor cells by sorting with distinctive cell surface markers.
Tumors resulting from CSCs contain the mixed tumorigenic and non-tumorigenic cells of the original tumor.
The CSC subpopulation can be serially transplanted through multiple generations, showing that it is a self-renewing population.
CSCs tend to be resistant to conventional therapies such as radiation, hormones, cytokines and chemotherapy due to signaling and gene expression differences.
Targeting CSCs
The concept of molecular targeting of diseased cells is a rather old one50 but the tools enabling this to be done effectively were not available until the pioneering work of Köhler and Milstein51 produced murine cell lines capable of secreting defined antibodies. Many highs and lows followed antibody therapy development,1,2 but technologies such as chimerization,52 humanization,53 phage display,54 antibody engineering,2 cell-line development55 and the booming biotechnology industry have greatly facilitated their clinical and commercial success. The targets of such antibodies represent the so-called ‘low hanging fruit’ of tumor-associated antigens. Receptors such as EGFR1, HER2, CD20, CEA, EpCAM and MUC1 are highly overexpressed in the majority of cells within many tumors and were thus relatively easy to identify and characterize.56
Due to their relevance to the development of cancer, CSCs are natural targets for antibody-directed therapies. The limitations are that compared to existing antibody therapies, CSCs are typically present at very low levels. CSC markers overlap with other CSCs and normal stem cells57 and the absence of certain markers also serves to classify certain CSCs (Table 1). This means that the identification and validation of CSC antigens for the generation of antibody therapeutics is still in its infancy. There are many other markers which are not strictly CSC antigens but are molecules which support CSCs in their niche within the tumor microenvironment (see below). These of course are also targets for CSC therapies and represent an indirect approach (Fig. 1). The CSC-related markers described below are not restricted to CSCs alone therefore the goal in antibody targeting is to bring about the specific destruction of CSCs whilst sparing normal cells. Antibody parameters such as dose, affinity, epitope will be important factors to optimize in development,2,58 but more elaborate approaches such as dual-targeting may represent a new challenge for antibody developers (see below).
Targeting ‘Confirmed’ CSC Markers
The characterization of CSCs has led to a number of experimentally ‘confirmed’ markers as well as markers involved in the process. These will be described below and a general therapeutic summary is provided in Figure 1.
CD44.
CD44, the cell-surface extracellular matrix receptor is probably the most established and common CSC marker. It therefore deserves some detailed description. It is a highly glycosylated, type-I transmembrane p-glycoprotein of around 90 kDa.59 Variant isoforms are expressed in a subset of cells including embryonic cells progenitor cells and cancer. Hyaluronic acid is a major glycosaminoglycan component of the extracellular matrix and CD44 binds it (as well as other matrix proteins to a lesser extent) as part of its adhesive properties. Despite lacking an intrinsic kinase domain, CD44 mediates signaling through many protein interactions ranging from matrix metalloproteases, growth factor receptors, Src/Rho kinases and transcriptional regulators. Thus CD44 can have multiple signaling functions, from proliferation, apoptosis, survival, migration and differentiation and these functions greatly depend on the cell-type CD44 is expressed on, the proteins interacting and the CD44-epitope bound.60 This somewhat accounts for the presence of CD44 on many CSCs, but having different effects, especially when modulated by antibodies. Thus CSC antigen context is important, even as far as saying that the lack of a certain receptor will define a subset of CSCs (Table 1).
Anti-CD44 antibody therapy represents the major anti-CSC approach. Just after leukaemic CSCs were discovered and characterized,6 antibodies were used to selectively induce differentiation or inhibit proliferation to eradicate them. So far, studies on normal myelopoiesis showed that CD44 binding did not lead to any undesirable side-effects.61 In the context of AML, Anti-CD44 antibodies H90 and A3D8 promoted terminal differentiation of AML blasts,62 inhibited proliferation by stabilizing p2763 and induced apoptosis.61 Then in 2006, Jin et al.64 showed that H90 anti-CD44 therapy eradicated AML CSCs showing that it interfered with transport to a stem cell supportive microenvironment (see below). Commercial development of this antibody is expected.65
Given this promising result, a number of other anti-CD44 antibodies are being developed. Young et al.66 describe H460-16-2 which was made into the humanized HuARH460-16-2 anti CD44 monoclonal antibody. A dose of 2 mg/kg caused almost 80% growth inhibition in BxPC3 pancreatic cancer xenografts and 57% tumor growth inhibition by binding to CD34+CD38− AML CSCs at a dose of 10 mg/kg.66 Increased median survival was also seen and this antibody is close to entering clinical trials. Other anti-CD44 antibodies include a panel of clones developed by Dyax Corp some of which interact with the CD44-HA interface.67
In head and neck cancer, a splice-variant of CD44, CD44v6 was targeted by monoclonal antibodies and immunoconjugates well before CD44's importance as a CSC marker was appreciated. In this cancer, the antigen is shed in the circulation, so antibody pharmacokinetics were hampered by complex formation. BIWA-4 monoclonal antibody (bivatuzumab) was developed as a stand-alone antibody,68 as radiolabelled conjugates with Rhenium-186,69 and Technicium-99,70 and microtubule inhibitor mertansine.71,72 The radio-immunotherapy trials generated moderate responses with stable disease in around half the patients studied, however, the mertansine conjugates showed severe and dose-limiting skin toxicities due to CD44v6 expression in the skin and these clinical trials were discontinued.71,72 Recent evidence suggests that CD44 may co-localize with EGFR and co-regulate CSC signaling32 increasing chemotherapy resistance in head and neck SCC.73 CD44/EFGR signaling acts through increased PKC/Akt signaling leading to increased tumor motility in melanoma by upregulating MMP and ROS.74 This opens up opportunities for combination antibody therapy with lower toxicity such as co-targeting with EGFR.
Another older study was in melanoma where monoclonal antibody GKW.A2 was used in melanoma xenografts75 which led to a decrease in tumor metastasis and increased animal survival, showing use of the strategy of disrupting the HA-CD44 interaction to alter cell proliferation and motility.
Conversely, the CD44-HA pathway may be important in tumor escape from receptor-orientated therapy.76 High expression of CD44 in JIMT-1 cells correlated with HER2 downregulation and siRNA inhibition of CD44 decreased the rate of trastuzumab-mediated HER2 internalization and trastuzumab-mediated growth inhibition. Inhibiting HA synthesis using the drug 4-methylumbelliferon (4-MU) enhanced the binding of trastuzumab to HER2, making trastuzumab therapy more effective.76
CD133.
CD133 was the first molecule of the pentaspan/prominin family of membrane proteins to be identified. It is a 120 kDa, highly glycosylated 5-span transmembrane protein.77 Its function is still unclear but may be involved in the organization of the plasma membrane. CD133 is found on CD34+ haematopoietic progenitor cells, but also one of the key markers linked with CSCs. The monoclonal antibodies AC133 and AC141 were discovered over ten years ago which recognize still as yet poorly characterized glycoprotein epitopes.78 CD133 is one of the most appropriate CSC markers in colon cancer whose expression correlates closely with low survival.98 Carter et al. conjugated the AC133 antibody to a highly potent cytotoxic drug, monomethyl auristatin, using a protease-cleavable linker. This immuno-conjugate internalized, colocalized with the lysosome and potently killed Hep3B hepatocellular cancer cells with an IC50 value of 2 ng/ml in vitro.80 In vivo tumor growth delay was seen with 3 mg/kg. They noted that Su86.86 cells were insensitive to this immuno-conjugate, even though it was internalized, but not processed in the lysosome. This differential trafficking could add another level of specificity to CSC targeting80 if this could be compared and determined effectively between CSC and normal cells.
ESA/EpCAM.
ESA/EpCAM is one of the most highly- and frequently-expressed tumor-associated antigens, being found on a wide range of epithelial cancers.81 It was the target of one the earliest approved monoclonal antibodies, edrecolomab (Panorex), which was later withdrawn due to randomized phase III clinical trials showing no overall benefit after 26 months.82 It is a type-I single-span transmembrane, calcium-independent, homophilic adhesion molecule of 39–42 kDa. It contains a large extracellular domain and 2 EGF-like repeats.83 Like many tumor antigens (such as MUC1), its normal basolateral expression pattern changes to an intense and uniform overexpression. Over the last few years, EpCAM, often called ESA and many other names (Table 1) has been ‘rediscovered’ as a CSC antigen in breast, colon, prostate and pancreatic cancers.7,9,11 In studies on colon cancer tumorigenicity, EpCAMhighCD44+ was seen as a robust marker with the CD166+ cells subpopulation being even more ‘stem-like.’8
Investigations into anti-EpCAM therapy are continuing. For example a monoclonal antibody ING1 (heMab-the first to be humanized using an alternative approach called ‘human engineering’), showed tumor suppression in colon and prostate cancer in preclinical studies84 and was well tolerated in patients given 0.6 mg/kg/week85 and adecatumumab (humanized anti-EpCAM antibody MT201 being developed by Micromet), which is well tolerated at a dose of 262 mg/m2 in patients undergoing a phase I clinical trial.86 MT201 showed potent anti-tumor activity in brain, prostate, ovarian, gastro-intestinal and colon cancer in vitro and in vivo,87 acting by what is thought to be an antibody-dependent cellular cytotoxicity (ADCC)/Complement-dependent cytotoxicity (CDC) mechanism. Recruiting effector cells to tumors using an anti-EpCAM/anti-CD3 bispecific, trifunctional antibody (catumaxomab-bispecific T-cell engager:BiTE) is also in advanced studies.88
Drug resistance proteins.
A number of membrane pumps and enzymes are expressed by normal SCs and CSCs, which contribute to their increased resilience. Aldehyde dehydrogenase-1 (ALDH-1) is an enzyme involved in alcohol metabolism, but also drug resistance. Chemotherapy drugs such as cyclophosphamide and cis-platin generate toxic aldehydes which are metabolized by ALDH-1.89 ALDH-1 also metabolizes retinal to retinoic acid a key molecule involved in cellular differentiation and stem cell self protection, hence, drug resistance.90 ALDH-1 is expressed across a number of CSC types including breast, prostate, liver and colon, often in sub-populations of CD133+ cells.35,90 ALDH-1 expression correlates with poor prognosis in breast cancer as determined from immunohistochemical analyses of almost 600 breast cancer specimens.91 Another protective mechanism that stem cells possess is the expression of ATP-binding cassette (ABC) transporters, efflux pumps which eliminate xenobiotic toxins.92 As mentioned above, this property was linked to the identification of the ‘side population’ in stem cell sorting.15,18 This led to the identification of this receptor in tumor-initiating cells from leukaemias93 and melanomas.94 The first example of antibody-inhibition of this receptor type was demonstrated by Schatton et al.94 An anti-ABCB5 antibody used to treat melanoma xenografts in mice resulted in a 10-fold reduction in tumor size and tumor eradication in 70% of the animals treated given at a dose of 0.5 mg (i.p.) twice per week. A CSC-targeting ADCC mechanism was proposed, rather than inhibition of drug resistance, which was not tested.94 There are some concerns that inhibiting the drug resistance mechanism of ABC transporters could have side effects due to their normal physiological roles.92
CD24.
CD24 is a small (31 residue) heavily glycosylated, mucin-like, cell surface, GPI-anchored protein, with multiple O- or N-linked glycosylation sites. It plays a role in cell selection/maturation in haematopoiesis and embryonic development, signaling via lyn kinase.95 It is a potential oncogene, overexpressed in 90% of colon cancers.96 An anti-CD24 monoclonal antibody at 0.3 mg given twice weekly (i.v.) inhibited tumor growth in vivo. In vitro cell killing was enhanced by many chemotherapy drugs and closer inspection of the mechanism showed tumor cell inhibition to be mediated through CD24 degradation.97
As described here, breast cancer stem cells express undetectable or low levels of CD24. However, it has been shown that CD24-P-selectin interaction is needed for rolling of breast cancer cells and elevated levels are found on invasive breast cancer suggesting a role for CD24 in metastasis even though CD44+CD24low mark breast CSCs.7 The disparity with breast CSCs needs further investigation but it may be related to a compensatory CXCR4 association with lipid rafts in CD24− cells leading to increased metastasis.98
CD20.
CD20 is highly overexpressed in over 90% of B-cell lymphomas and leukaemias99 and expressed at lower levels on normal B-cells. It is therefore not a CSC antigen in the context of haematological malignancies. However, it has been found expressed on a subpopulation of melanomas which have CSC-like properties.100 The successful treatment of lymphoma with rituximab, which works by a variety of mechanisms101 suggests that anti-CD20 antibody therapy could also be used for a solid tumor like melanoma.
Targeting CSC-Supporting Factors
Interleukin-4.
Interleukin-4 cytokine is expressed by some cancers and in colon cancer has an autocrine effect on colon CSCs. Inhibition of IL-4 by a blocking antibody was seen to sensitize colon CSCs to chemotherapy.102 Therefore, therapeutic strategies involving CSCs could be to sensitize CSCs to chemotherapy and radiotherapy by inhibiting stem-cell (“stem-ness”) properties, inhibiting their metastases as well as promoting direct cyotoxicity (Fig. 1). To complicate things a little, it is well-established that IL-4 has significant paracrine effects, playing a major role in modulating the immune response and affecting the T-helper type-1 and type-2 balance. IL-4 leads to a more immunosuppressive Th-2 immune response, which promotes tumor growth as opposed to a Th-1 polarized response which enhances cancer directed immunosurveillance.103
HER2.
The elucidation of the pathways that regulate breast stem cell renewal gives us a clearer picture of how breast cancer may evolve.104 HER2 is expressed in around 25% of human breast cancer and correlates with poor prognosis and high metastasis.105 HER2 inhibition therapies such as trastuzumab in the adjuvant setting reduced recurrence by almost 50%.106 There has been found a strong correlation between ALDH-1 expression and HER2 overexpression107 suggesting that HER2 may play a role in breast cancer by regulating or even driving the stem cell population.
Notch signaling.
Notch signaling as outlined above is a central pathway in cell renewal and development. Although not restricted to CSCs, Notch signaling intervention is emerging as a hot topic in cancer drug development.108 Oncomed Pharmaceuticals have made a lot of progress in targeting these receptors. Lewicki et al.109 describe an antibody (13M57) which binds to the non-ligand binding region of Notch which induces a 50% decrease in breast cancer xenograft tumors in combination with paclitaxel. Oncomed also have developed antibodies against the conserved ligand-binding region of Notch. Gurney et al.110 produced antibodies (90R21, 90R22, 90R29) which bind to epitopes around the ‘PCEHAGKCINT’ sequence, which is where the DLL-family of receptors bind. Tumor inhibition was seen with these antibodies which were eradicated when used in combination with paclitaxel chemotherapy. Similar results were seen in colon cancer xenografts when anti-Notch receptor monoclonal antibody (59M07) was administered at a dose of 10 mg/kg in combination with irinotecan chemotherapy.
Notch ligands such as DLL1/3/4/Jagged1/2,111 which could be antagonized by antibodies are also targets. DLL4 has emerged as a good candidate with a number of monoclonal antibodies being developed against it.112,113 Vascular endothelial cells utilize Notch and Dlls, with DLL4 almost exclusive expressed on endothelia. Notch and VEGF signaling work together to regulate arterial/venous differentiation but a more complex relationship exists in terms of vasculogenesis and angiogenesis. VEGF induces Notch activation during sprout formation and upregulates DLL4 in the tip cells which in turn activates Notch in the adjacent stalk cell. Notch/DLL4 signaling provides a negative feedback loop to change the effect of VEGF on the stalk cell thereby limiting proliferation. This ensures the tip grows and the stem remains connected. This opens up the possibility that Notch/DLL4 is a drug target for tumor vasculature, since DLL4 is upregulated in tumors, in part by VEGF.113 A neutralising antibody generated by Genentech scientists against DLL4 increased angiogenesis but the new formed vessels are abnormal and dysfunctional. This led to an increase in tumor hypoxia and growth delay.114 Oncomed's 21M18 anti-DLL4 antibody caused tumor regression in C8 colon cancer tumor models in combination with 5FU and irrinotecan chemotherapy. It has also been tested in combination with anti-EGFR antibody therapy.112
Wnt-signaling.
Wnt-signaling is key in cell development and is upregulated in 50% of cancer.44 The Frizzled family of receptors activate Wnt-signaling and consequently, anti-Frizzled-6 (clone 23M2) and anti-Frizzled-5 (clone 44M13) antibodies developed by Oncomed have anti-tumor properties.115 Direct inhibition of Wnt-1 receptor function by an antibody induced tumor apoptosis in vitro.116 Anti-patched antibodies, derived from peptide immunizations, inhibited tumor cell lines panc-1 and suit-2 through hedgehog signaling disruption.117
Prostate stem cell antigen.
Prostate Stem Cell Antigen (PSCA) is a 123 residue, cysteine-rich GPI-anchored surface glycoprotein related to the Thy-1 family which is expressed on most prostate cancers-local disease and particularly metastatic prostate cancer118 as well as bladder and transitional cell tumors. PSCA overexpression correlates with tumor stage, grade and progression to the androgen-independent form which usually accompanies drug resistance.119 Despite its name, it has not been identified as a prostate CSC marker. A murine monoclonal antibody 1G8 has been used to image prostate cancer in murine models, showing high uptake (12–17% id/g in 1–4 days).120 A chimaeric form showed anti-tumor activity in a LAPC xenograft model, an effect which was put down to binding rather than Fc-mediated as a F(ab)2 also had an anti-tumor effect. This antibody has now been humanized by grafting the CDRs onto the trastuzumab Fv framework with retention of the 1 nM affinity. The humanized antibody, Hu2B3, successfully targeted PC3 prostate cancers using 124-I micro-PET imaging. The humanized antibody was more effective therapeutically than the murine form on cell lines expressing low levels of PSCA.120 Also anti-PSCA antibody therapy led to the retardation of established murine orthotopic tumors and prevented distant metastases, prolonging survival in prostate cancer suggesting it may have a role in inhibiting CSC-like functions.121
Tetraspanins (CD9).
Tetraspanins (CD9) are a family of 4-span transmembrane receptors involved in the regulation of fertilization, adhesion, motility and invasion.122 These act as molecular facilitators/adaptors through protein-protein interactions with members of the immunoglobulin superfamily, integrins and receptor tyrosine kinases. They are expressed in epithelial, endothelial and haematopoietic cells as well as some tumors, with expression being closely related to metastasis. Arius are developing a monoclonal antibody (AR40A746.2.3) which inhibits tumor growth in a variety of cancers, particularly CD9+CD34+CD38− AML CSCs [The American Association for Cancer Research conference, San Diego (2008) Abstract 3993]. CD9 is interesting in the context of solid tumors in that it associates with EGFR and co-regulates signaling, likely through EGFR internalization.122
L1CAM.
L1CAM (CD171) is a neural cell adhesion molecule, which regulates neural growth, survival, migration, axonal outgrowth and neurite extension. It is also overexpressed in gliomas and other cancers. L1CAM is a negative prognostic factor in colon cancer and its overexpression is suggested to protect cancer cells from apoptosis during chemotherapy. L1CAM is a possible target for antibody intervention because siRNA studies have shown that its downregulation in CD133+ glioma CSCs leads to these stem cells having a higher rate of apoptosis, decreased neurosphere formation and decreased tumor growth.123
Interleukin-6.
Interleukin-6 is a pleiotropic cytokine involved in many processes from tumor growth proliferation to inflammation.124 It is widely studied in prostate cancer but recent work in hepatocellular cancer (HCC) has uncovered a new role. Recently, CSCs for liver cancer were discovered125 but it is thought that the very few liver stem cells which exist may develop into HCC tumors due to aberrant TGFβ and IL-6 signaling. IL-6 may drive HCC growth from a transformed liver SC which has defective TGFβ signaling.126 Inhibiting IL6 signaling, already an approach in clinical trials in prostate cancer127 could be used against HCC CSCs.
CD123.
CD123 is the α-subunit of the IL-3 receptor found in haematopoietic cells and overexpressed in leukaemic blasts and leukaemic CSCs in AML and CML. Du et al.128 constructed three scFvs from anti-CD123 hybridomas and constructed Pseudomonas exotoxin immuntotoxins, with a KDEL retention signal. One clone, with a Kd of 3.5 nM was very potent with an IC50 of 40 ng/ml towards several CD123-positive cells lines.
CD200.
The immune response to CSCs is yet to be understood but it is likely that CSCs have active mechanisms of immune-evasion whether it is simply hiding within protected niches such as stroma to the expression of immune-regulatory factors. A hallmark of cancer is its ability to evade the immune system. In addition to the well-established immuno-suppressive molecules such as programed cell death-1 (PD-1), PD-1 ligand (PD-L1), TGFβ and CTLA-4, CD200 (Ox-2) is emerging as a candidate for CSC immunotherapy. CD200 is an immunoglobulin superfamily-related receptor playing multiple roles in autoimmunity, inflammation and the adaptive immune response.129 It is expressed in many cancers130 and is a prognostic factor in some. Recently, some CSCs were found to co-express CD200 with CD44 and CD133.131 Therefore, CD200 may be a marker for a subpopulation of CSCs which have the ability to escape the immune system, presumably by inducing a downregulation of the Th-1 immune response (such as IL-2 and IFNγ expression) promoting tumor growth.132 Blocking antibodies restored IL-2 and IFNγ expression, rebalancing the immune response from a Th-2 response and promoting tumor rejection.133
Targeting the Stem Cell Niche
Normal adult stem cells depend on cell-intrinsic and cell-extrinsic factors for homeostatic maintenance. There must be a careful balance between self-renewal vs. potential to differentiate. A specialized microenvironment consisting of cells, matrix proteins and growth factors, known as a ‘niche’ is thought to physically restrain stem cells and enable them to maintain their ‘stemness’ by providing the necessary factors.134 Niches have been identified for many mammalian stem cells and many molecules have been identified as being important in niche control of cell fate (such as Notch, Wnt, Hedgehog, BMPs and FGF). There are some major similarities between SC niches and the tumor microenvironment providing evidence for a CSC niche.135 There is increasing evidence that disruption of epithelial homeostasis where tumor cells acquire a more ‘mesenchymal’ phenotype is required for metastasis.136 This epithelial-mesenchymal transition (EMT) requires Wnt and TGFβ signaling136 and may occur in CSC niches. Thus, there may even be distinct CSC niches-a ‘primary’ CSC niche and a ‘metastatic’ CSC niche135 where CSCs undergo a transformation to a metastatic CSC (Fig. 1), with changes in gene expression.22 The presence or architecture of such a niche has not been physiologically identified, but its conception has led to a number of approaches for CSC therapy.
Anti-angiogenic therapy.
Vascular endothelial cells are critical components of the neural stem cell niche.137 This suggests that brain CSCs rely on signaling within nearby tumor vasculature to maintain a stem-like state. Disruption of this by anti-angiogenic therapy reduces ‘stemness’ and increases CSC sensitivity to chemotherapy.138 The CSC model together with the ‘seed and soil’ hypothesis includes a role for CSCs in tumor angiogenesis. Anti-angiogenic drugs (antibodies and small molecules) are well-established forms of therapy, even if the precise mechanisms of action are contentious. Anti-vasculature therapies (direct ones such as anti-VEGF antibodies-bevacizumab or indirect ones such as anti-EGFR antibodies-cetuximab or small molecules like erlotonib) can work at many different levels, from destroying vessels leading to nutrient and oxygen starvation to normalizing vessel structure to reduce tumor interstital pressure and enhance cytotoxic drug uptake.139,140 CSCs have been shown to express higher levels of VEGF in normoxic and hypoxic conditions compared to non-stem cancer cells. This leads to increased endothelial cell migration and tube formation which can be blocked by the in vivo administration of bevacizumab.141 Tumors-derived from non-CSC were unaffected by this blocking antibody. A similar approach was used by Calabrese using bevacizumab and erlotinib142 also identifying a brain CSC niche. Like normal stem cells, CSCs depend on support from the vascular and stromal niche for survival. Factors such as LIF and BDNF have been implicated in normal stem cells maintenance134,135 and could be important growth factors for intervention in CSC maintenance.
Using a mouse-specific anti-VEGFR2 monoclonal antibody administered i.p. at 100 mg/kg in combination with a low dose (metronomic) chemotherapy, the CSC population (as measured by sphere-forming units) was reduced.138 Individual therapies alone were unable to achieve this.
There are other significant angiogenic factors which could be targeted, basic FGF for example. Integrin signaling, dealt with separately below is critical in modulating the angiogenic process. We have carried out some work on a novel anti-angiogenesis humanized monoclonal antibody called huMC3 (Angiolix) directed against a unique target, lactadherin.143 Lactadherin is expressed by tumor cells and induces tumor proliferation, expansion and spread through the formation of new blood vessels.144 It was shown that this antibody could selectively block and inhibit lactadherin leading to a significant anti-cancer effect in vivo against preclinical models of human breast cancer, an effect which was enhanced when given in combination with conventional chemotherapy used for breast cancer (Deonarain et al., unpublished). More tumor-selective anti-angiogenic therapies such as this could have a greater effect on the CSC niche than the more general VEGF-directed anti-angiogenic therapies. Taken together, these approaches make it clear that targeting the supporting niche of CSCs could be a cancer therapy approach rather than direct targeting of the cells themselves and also suggested a novel and additional mechanism for anti-angiogenic therapy.139
Integrins and chemokine receptors.
The CXCR4 chemokine (Cys-X-Cys motif ) receptor is essential for the homing, retention and maintenance of haematopoietic stem cells in stromal niches in the bone marrow. Cells bearing this receptor respond to a gradient of SDF-1 (stromal derived factor-1/CXCL12) constitutively secreted by bone marrow stromal cells.145,146 The link between CSCs and metastasis is being studied intensively, particularly interactions with ECM/stromal factors. Pancreatic CSCs could be separated into CD133+/CXCR4+ and CD133+/CXCR4− which were equally tumorigenic, but antibody depletion experiments showed that the CXCR4+ cells were able to invade.21 This has therapeutic implications for the antibody-mediated inhibition of CSCs metastasis.
Leukaemia cells also express CXCR4 which is thought to induce homing of tumor cells to the favorable niche environment of the bone marrow, normally restricted to stem/progenitor cells. This environment provides factors needed to maintain stem-like features and CSCs are believed to exploit this for their survival.146 Originally studied for its role as a co-receptor for HIV infection, the CXCR4/CXCL12 interaction is now thought to play a role in immune cell trafficking. CXCR4/CXCL12 is also upregulated via HIF-1 in hypoxic conditions, in order to recruit circulating progenitor cells to sites of hypoxia for tissue repair. The overexpression of CXCR4 in many leukaemias such as acute lymphoblastic leukaemia (ALL)147 has led to the development of antagonists of CXCR4. There are concerns about potential side effects about this approach given the central role CXCR4/CXCL12 plays in haematopoiesis. CXCR4 knockout mice have defects in haematopoiesis, vascular and cardiac development.146 Inhibitors of CXCR4 may mobilize cancer or CSCs making them more sensitive to cytotoxic therapy, but this may also hurt normal HPCs. Combination with other targeted agents such as anti-CD20 (rituximab) or anti-CD52 (alemtuzumab) antibodies may counteract this. Thus mobilizing CSCs from their protected environment could be a future therapeutic approach, with the anti-CD44 therapy against AML being a good example of this.64
Chemokine-induced locomotory function is also related to solid tumor metastases.148 Organ-specific metastatic spreading of tumor cells is also mediated by the interactions between chemokines and their appropriate receptors. This was elegantly shown by Müller et al., for the CXCR4/CXCL12 in breast cancer.149 Tumor cell extravazation is thought to be the rate-limiting step in metastasis, a process which is related to leukocyte/lymphocye and HSPC extravazation. The ‘locking and docking hypothesis’ was proposed for the adhesion of the tunmor cells to endothelial lining to allow tumor cell rolling (facilitated by selectins). The following locking phase is mediated by β1 and αv integrins allowing the locomotion of tumor cells into the underlying tissue.150 Adhesion molecules such as CD24, CD34 and sialyl Lewis antigens interact with these selectins to facilitate extravazation. Anti-CXCR4 monoclonal antibodies have had anti-tumor effects in multiple myeloma tumors,149 prostate cancer150 and colon cancers.153
Integrins are another diverse family of transmembrane receptors involved in cell proliferation, differentiation, apoptosis, adhesion and migration through outside-in and inside-out signaling. They function as receptors for other adhesion molecules and components of the extra-cellular matrix.154 Integrin mutation and expression is often associated with cancer invasion and metastasis which has been studied extensively in breast and colorectal cancer.155 The presence of appropriate ligands for integrins is thought to play a role in organ-specific metastases.155 The central role played by integrins in the extravazation cascade suggests that anti-integrin strategies could work as anti-metastatic, anti-angiogenic or anti-proliferation therapies. αvβ3 and αvβ5 integrins are potential targets from their expression in various malignancies.154,155 The anti-αvβ3 integrin antibody LM609 (Vitaxin) has been shown to inhibit multiple tumor growths156 whereas an anti-β3 antibody inhibited prostate cancer growth, bone metastases but also some bone degradation.157 A phase I study showed that this antibody was well tolerated despite concerns of its effect on osteoclast function and bone homeostasis.156 The close link between integrin and chemokine receptors in cancer suggests that use of combination therapies would be beneficial, particularly as the expression of one influences the other. Integrins and chemokine receptors may act synergistically and inhibiting both the chemotactic recruitment as well as the adhesion of circulating tumor cells may work more effectively than either antagonist on its own.
Cellular adhesion to the ECM is long known to affect radiation sensitivity. Integrins, especially β1 is upregulated in response to radiotherapy and is implicated in mediating resistance156 and correlates with poor survival in patients.159 This radio-resistance is mediated via integrin signaling via the PI3 kinase/Akt pathway.15 Treatment of MCF-7 breast cancer cells with A11b2 anti-β1 antibody re-sensitized tumor cells to radiotherapy, making a 2 Gy dose effectively an 8 Gy dose.160 Another anti-β1 antibody was used to prevent peritoneal tumor recurrences in surgery-induced adhesion of colon cancer in a rat model.161
Future Therapeutic Developments against CSCs
There has been a lot of progress in the development of small molecule drug intervention of CSC pathways. Inhibiting Notch signaling is good example with drugs like MK0752,162 and γ-secretase inhibitors163 showing anti-tumor effects. Hedgehog signaling in gliomas can be disrupted with the drug cyclopamine93 and the Wnt pathway with molecules such as PKF118-310 and ZTM000990.164 Telomerase inhibitors based on the RNA template region are in advanced clinical trials.165 Cyclosporine modulation for drug transporter inhibition has shown limited efficacy166 due to expression of these transporters in normal HSC. Most of these drugs target the renewal pathways and still require research before they can used in a truly CSC-specific way. The potent NFκB inhibitor, parthenolide did show some differentiation in cell killing between normal HSC and leukaemic CSC due to subtle signaling differences,167 as was shown when treating leukaemic CSCs and normal haematopoietic stem cells by rapamycin due to differing levels of Pten protein.168 But these are exceptional examples. Treatment with these drugs has led to the reduction in CSC properties such as a reduction in ABC drug transporter levels, an effect also seen with non-CSC tyrosine kinase inhibitor drugs such as gefitinib, erlotinib, lapatinib, sorafenib and sunitinib.15 There are many examples of antibodies against tumor antigens/factors used in combination with chemotherapy. A description of these is beyond the scope of this review but for examples see.1,169 In the context of CSCs, using the more CSC-targeted drugs (above) in combination with antibodies will be the initial way forward as already shown above. Small molecules against CSC renewal pathways in combination with non-stem cancer antigens could be a powerful combination with much lower toxicity. However, there is still the need to fully characterize the CSCs in terms of cell surface markers.
Non-antibody therapeutic proteins targeting important cancer stem cell pathways intracellularly and intranuclearly are already in development. Using the antennapedia cell-penetrating domain, already used successfully to eradicate tumor cells by restoring tumor-suppressive functions (Kousparou et al., Antennapedia ‘trojan’ peptide delivers p21 protein resulting in tumor eradication: AACR, 2008, Abstract # 4908 ) we have shown that delivering the ‘mastermind’ protein to tumors (TR4-Antennapedia-mastermind fusion protein) caused a broad based inhibition of the Notch transcriptional cascade and causes complete tumor regression in cancers with Notch activation including xenografted metastatic breast cancer stem cell lines in preclinical model without any significant side effects to the animals. (Stylianou et al., ‘The Role of Cancer Stem Cells in the Initiation and Propagation of Tumorigenesis’, AACR Special Conferences Series, 2008).
The use of combinations of antibodies to modulate two different but related tumor signaling pathways is emerging as a successful clinical approach.170,171 One can see this approach being used to target CSCs. Combination or cocktails of antibodies172 against bulk tumor targets and CSC targets could destroy the whole tumor and the resilient CSC population, preventing relapses. Or, bispecific antibodies recognising both CSC markers (which are also co-expressed on normal SCs) and tumor antigens could be used to increase the specificity of CSC targeting. This approach was successfully used by Adams et al. (unpublished, application of monoclonal antibodies in clinical oncology conference, Rhodes, 2008) who made a bispecific anti-HER2/HER2 single-chain Fv. This fusion protein was able to bind more specifically to cell lines expressing both receptors than to cell lines bearing the individual receptors. Dual targeting was also achieved for EGFR/HER2.173
Tumor architecture and stem cell niches have been discussed above but may pose additional hurdles to therapy. So-called metastasising CSCs should be accessible to systemic therapies but dormant CSCs, buried deep within poorly vascularized and hypoxic areas may be hard to get to. Direct antibody therapies may be less appropriate, but indirect or medium-distance acting therapies could find use here. Although compelling data for radio-immunotherapy (RIT) has accumulated over the years,174 its clinical uptake has been slow. RIT targeting CSCs, already shown for CD44,69,70 is likely to rejuvenate this area. Similar ideas could be put forward for diffusible cytotoxics such as targeted photodynamic therapy175 and ADEPT.176 Another approach to overcoming the heterogeneous uptake of antibody therapeutics is to use smaller derivatives of antibodies engineered to have a longer blood half-life. This could be achieved using technologies such as antibody PEGylation,177 polysialylation178 and albumin binding.179 The goal of anti-CSC therapy is to eliminate CSCs and not necessarily reduce tumor bulk as this would gradually die out as the ability to self-renew is diminished. Therefore new clinical endpoints or biomarkers will be needed such as a measurement of CSC reduction by imaging of circulating CSCs (using imaging ex vivo or intravital imaging)180 or increase in progression-free survival.
Conclusion
Although the CSC model is able to explain many of our observations concerning tumor development and therapy, there is still considerable research to be done as not all cancers can be accounted for this way, especially ones in murine models. Although clinically observed, the full clinical relevance is yet to be established. Not destroying normal stem cells is key to the success of anti-CSC therapies. A refined understanding of the signaling pathways between normal and CSCs is needed and the use of combination therapies should afford a window where this is achievable, There are already examples emerging, but dual antibody- or drug-antibody combinations would see these increase.
There is increasing evidence illustrating the commonality of CSCs in multiple tumor types. This suggests that diagnostic and therapeutic monoclonal antibodies and other proteins may be applicable across several tumor types (Table 1). In addition, because pathways of CSC self-renewal and survival appear to be preserved across different tumor types, antibody therapies developed to target CSCs may have a broad clinical application. It may thus be advantageous to develop pathway-based rather than tumor type-based approaches to CSC-based therapy (Table 1).
Clearly there is a great deal of interest and progress in research in the area of cancer stem cells CSCs. The discovery of new and unique markers on CSCs as well as CSC pathways will undoubtedly lead to new diagnostic and therapeutic antibodies as well as other CSC targeting biologicals. This is a fertile area for commercial drug development. For example Raven Biologicals have developed a library of cancer stem cells arising from many types of primary tumors and have generated more than 1,300 monoclonal antibodies, including many that target cancer stem cells and cancers. Oncomed has developed the ability to isolate and monitor tumor initiating cells using specific surface markers and flow cytometry. This has allowed their scientists to evaluate specific targets associated with biologic pathways implicated in both stem cell biology and cancer.
In summary, if the approaches and hypotheses presented in this review article prove to be correct or at least partly so, then our understanding of the onset and progression of oncogenesis as well as our possible therapeutic intervention would have been enriched and to, some extent, altered from the previously held views and practice. At the end of the day, further work and investigation in this area may lead to more effective strategies for cancer prevention, diagnosis and treatment. In view of the fact that therapeutic antibodies and other proteins directed against CSCs are close to entering clinical trials, the next decade of translational research and development in this area will undoubtedly prove to be a most exciting period.
Acknowledgements
We thank Dr. David Mann for critically reading this manuscript. M.P.D. is supported by Imperial College London, the Wellcome Trust, Cancer Research UK, The European Union FP6 program and Access Pharmaceuticals Inc., for various research programs.
Footnotes
Previously published online as a mAbs E-publication: http://www.landesbioscience.com/journals/mabs/article/7347
References
- 1.Deonarain MP. Recombinant antibodies for cancer therapy. Expert Opin Biol Ther. 2008;8:1123–1141. doi: 10.1517/14712598.8.8.1123. [DOI] [PubMed] [Google Scholar]
- 2.Carter PJ. Potent antibody therapeutics by design. Nat Rev Immunol. 2006;6:343–357. doi: 10.1038/nri1837. [DOI] [PubMed] [Google Scholar]
- 3.Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;133:571–573. [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 5.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 6.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 7.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumourigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumourigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 9.O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 10.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 11.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 12.Dirks PB. Brain tumour stem cells: bringing order to the chaos of brain cancer. J Clin Oncol. 2008;26:2916–2924. doi: 10.1200/JCO.2008.17.6792. [DOI] [PubMed] [Google Scholar]
- 13.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 14.Phillips TM, McBride WH, Pajonk F. The response of CD24(−/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst. 2006;98:1777–1785. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]
- 15.Eyler CE, Rich JN. Survival of the fittest: cancer stem cells in therapeutic resistance and angiogenesis. J Clin Oncol. 2008;26:2839–2845. doi: 10.1200/JCO.2007.15.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer. 2004;4:437–447. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- 17.Brown JM, Giaccia AJ. The unique physiology of solid tumours: opportunities (and problems) for cancer therapy. Cancer Res. 1998;58:1408–1416. [PubMed] [Google Scholar]
- 18.Kim M, Turnquist H, Jackson J, Sgagias M, Yan Y, Gong M, et al. The multidrug resistance transporter ABCG2 (breast cancer resistance protein 1) effluxes Hoechst 33342 and is overexpressed in hematopoietic stem cells. Clin Cancer Res. 2002;8:22–28. [PubMed] [Google Scholar]
- 19.Mimeault M, Hauke R, Mehta PP, Bhtra SK. Recent advances in cancer stem/progenitor cell research: therapeutic implications for overcoming resistance to the most aggressive cancer. J Cell Mol Med. 2007;11:981–1011. doi: 10.1111/j.1582-4934.2007.00088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wulf GG, Wang RY, Kuehnle I, Weidner D, Marini F, Brenner MK, et al. A leukemic stem cell with intrinsic drug efflux capacity in acute myeloid leukemia. Blood. 2001;98:1166–1173. doi: 10.1182/blood.v98.4.1166. [DOI] [PubMed] [Google Scholar]
- 21.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 Is a Marker of Normal and Malignant Human Mammary Stem Cells and a Predictor of Poor Clinical Outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dontu G, Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, et al. Distinct populations of cancer stem cells determine tumour growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, et al. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma S, Lee TK, Zheng BJ, Chan KW, Guan XY. CD133+ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene. 2008;27:1749–1758. doi: 10.1038/sj.onc.1210811. [DOI] [PubMed] [Google Scholar]
- 25.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 Is a Marker of Normal and Malignant Human Mammary Stem Cells and a Predictor of Poor Clinical Outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fillmore CM, Kuperwasser C. Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res. 2008;10:25. doi: 10.1186/bcr1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abraham BK, Fritz P, McClellan M, Hauptvogel P, Athelogou M, Brauch H. Prevalence of CD44+/CD24−/low cells in breast cancer may not be associated with clinical outcome but may favor distant metastasis. Clin Cancer Res. 2005;11:1154–1159. [PubMed] [Google Scholar]
- 28.Wright MH, Calcagno AM, Salcido CD, Carlson MD, Ambudkar SV, Varticovski L. Brca1 breast tumours contain distinct CD44+/CD24− and CD133+ cells with cancer stem cell characteristics. Breast Cancer Res. 2008;10:10. doi: 10.1186/bcr1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maitland NJ, Collins AT. Prostate cancer stem cells: a new target for therapy. J Clin Oncol. 2008;26:2862–2870. doi: 10.1200/JCO.2007.15.1472. [DOI] [PubMed] [Google Scholar]
- 30.Jalkut MW, Reiter RE. Role of prostate stem cell antigen in prostate cancer research. Curr Opin Urol. 2002;12:401–406. doi: 10.1097/00042307-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Prince ME, Ailles LE. Cancer stem cells in head and neck squamous cell cancer. J Clin Oncol. 2008;26:2871–2875. doi: 10.1200/JCO.2007.15.1613. [DOI] [PubMed] [Google Scholar]
- 32.Kawano T, Yanoma S, Nakamura Y, Shiono O, Kokatu T, Kubota A, Furukawa M, Tsukuda M. Evaluation of soluble adhesion molecules CD44 (CD44st, CD44v5, CD44v6), ICAM-1 and VCAM-1 as tumour markers in head and neck cancer. Am J Otolaryngol. 2005;26:308–313. doi: 10.1016/j.amjoto.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Piccirillo SG, Reynolds BA, Zanetti N, Lamorte G, Binda E, Broggi G, et al. Bone morphogenetic proteins inhibit the tumourigenic potential of human brain tumour-initiating cells. Nature. 2006;444:761–765. doi: 10.1038/nature05349. [DOI] [PubMed] [Google Scholar]
- 34.Boman BM, Huang E. Human colon cancer stem cells: a new paradigm in gastrointestinal oncology. J Clin Oncol. 2008;26:2828–2838. doi: 10.1200/JCO.2008.17.6941. [DOI] [PubMed] [Google Scholar]
- 35.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;444:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 36.Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci USA. 2007;104:10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dylla SJ, Beviglia L, Park IK, Chartier C, Raval J, Ngan L, et al. Colorectal cancer stem cells are enriched in xenogeneic tumours following chemotherapy. PLoS ONE. 2008;3:2428. doi: 10.1371/journal.pone.0002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huntly BJP, Gilliland DG. Leukemia stem cells and the evolution of Cancer Stem Cells. Nature Reviews Cancer. 2005;5:311–321. doi: 10.1038/nrc1592. [DOI] [PubMed] [Google Scholar]
- 39.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 40.Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, et al. Isolation and characterization of tumourigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 41.Bjerkvig R, Tysnes BB, Aboody KS, Najbauer J, Terzis AJA. Opinion: the origin of the cancer stem cell: current controversies and new insights. Nat Rev Cancer. 2005;5:899–904. doi: 10.1038/nrc1740. [DOI] [PubMed] [Google Scholar]
- 42.Radtke F, Raj K. The role of Notch in tumourigenesis: oncogene or tumour suppressor? Nat Rev Cancer. 2003;3:756–767. doi: 10.1038/nrc1186. [DOI] [PubMed] [Google Scholar]
- 43.Stylianou S, Clarke RB, Brennan K. Aberrant Activation of Notch Signaling in Human Breast Cancer. Cancer Research. 2006;66:1517–1525. doi: 10.1158/0008-5472.CAN-05-3054. [DOI] [PubMed] [Google Scholar]
- 44.Chari NS, McDonnell TJ. The sonic hedgehog signaling network in development and neoplasia. Adv Anat Pathol. 2007;14:344–352. doi: 10.1097/PAP.0b013e3180ca8a1d. [DOI] [PubMed] [Google Scholar]
- 45.Rubin LL, de Sauvage FJ. Targeting the Hedgehog pathway in cancer. Nat Rev Drug Discov. 2006;5:1026–1033. doi: 10.1038/nrd2086. [DOI] [PubMed] [Google Scholar]
- 46.Clevers H. Wnt/β-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. regulates B lymphocyte proliferation through a LEF-1 dependent mechanism. Immunity 2000; 13:15–24. [DOI] [PubMed] [Google Scholar]
- 47.Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, et al. A role for Wnt signaling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 48.Park IK, Qian D, Kiel M, Becker MW, Pihalja M, et al. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003;423:302–305. doi: 10.1038/nature01587. [DOI] [PubMed] [Google Scholar]
- 49.Sawa M, Yamamoto K, Yokozawa T, Kiyoi H, Hishida A, et al. BMI-1 is highly expressed in M0-subtype acute myeloid leukemia. Int J Hematol. 2005;82:42–44. doi: 10.1532/IJH97.05013. [DOI] [PubMed] [Google Scholar]
- 50.Ehrlich P. Physiology or medicine 1901–1921. Amsterdam: Elsevier Publishing Co.; 1967. Nobel Lectures; pp. 304–320. [Google Scholar]
- 51.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 52.Boulianne GL, Hozumi N, Shulman MJ. Production of functional chimaeric mouse/human antibody. Nature. 1984;312:643–646. doi: 10.1038/312643a0. [DOI] [PubMed] [Google Scholar]
- 53.Riechmann L, Clark M, Waldmann H, Winter G. Reshaping human antibodies for therapy. Nature. 1988;332:323–327. doi: 10.1038/332323a0. [DOI] [PubMed] [Google Scholar]
- 54.Hoogenboom HR. Selecting and screening recombinant antibody libraries. Nat Biotechnol. 2005;23:1105–1116. doi: 10.1038/nbt1126. [DOI] [PubMed] [Google Scholar]
- 55.Andersen DC, Krummen L. Recombinant protein expression for therapeutic applications. Curr Opin Biotechnol. 2002;13:117–123. doi: 10.1016/s0958-1669(02)00300-2. [DOI] [PubMed] [Google Scholar]
- 56.Mufson RA. Tumour antigen targets and tumour immunotherapy. Front Biosci. 2006;11:337–343. doi: 10.2741/1801. [DOI] [PubMed] [Google Scholar]
- 57.Yang YM, Chang JW. Current status and issues in cancer stem cell study. Cancer Invest. 2008;26:741–755. doi: 10.1080/07357900801901856. [DOI] [PubMed] [Google Scholar]
- 58.Beckman RA, Weiner LM, Davis HM. Antibody constructs in cancer therapy: protein engineering strategies to improve exposure in solid tumours. Cancer. 2007;109:170–179. doi: 10.1002/cncr.22402. [DOI] [PubMed] [Google Scholar]
- 59.Marhaba R, Zöller M. CD44 in cancer progression: adhesion, migration and growth regulation. J Mol Histol. 2004;35:211–231. doi: 10.1023/b:hijo.0000032354.94213.69. [DOI] [PubMed] [Google Scholar]
- 60.Wang SJ, Bourguignon LY. Hyaluronan and the interaction between CD44 and epidermal growth factor receptor in oncogenic signaling and chemotherapy resistance in head and neck cancer. Arch Otolaryngol Head Neck Surg. 2006;132:771–778. doi: 10.1001/archotol.132.7.771. [DOI] [PubMed] [Google Scholar]
- 61.Gadhoum Z, Delaunay J, Maquarre E, Durand L, Lancereaux V, Qi J, Robert-Lezenes J, Chomienne C, Smadja-Joffe F. The effect of anti-CD44 monoclonal antibodies on differentiation and proliferation of human acute myeloid leukemia cells. Leuk Lymphoma. 2004;45:1501–1510. doi: 10.1080/1042819042000206687. [DOI] [PubMed] [Google Scholar]
- 62.Charrad RS, Li Y, Delpech B, Balitrand N, Clay D, Jasmin C, Chomienne C, Smadja-Joffe F. Ligation of the CD44 adhesion molecule reverses blockage of differentiation in human acute myeloid leukemia. Nat Med. 1999;5:669–676. doi: 10.1038/9518. [DOI] [PubMed] [Google Scholar]
- 63.Gadhoum Z, Leibovitch MP, Qi J, Dumenil D, Durand L, Leibovitch S, Smadja-Joffe F. CD44: a new means to inhibit acute myeloid leukemia cell proliferation via p27Kip1. Blood. 2004;103:1059–1068. doi: 10.1182/blood-2003-04-1218. [DOI] [PubMed] [Google Scholar]
- 64.Jin L, Hope KJ, Zhai Q, Smadja-Joffe F, Dick JE. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med. 2006;12:1167–1174. doi: 10.1038/nm1483. [DOI] [PubMed] [Google Scholar]
- 65.Dick J. France: University Health Network; 2007. Patent US2007237761. [Google Scholar]
- 66.Young D. Arius Research Inc.; 2007. Patent WO2007098571. [Google Scholar]
- 67.Rondon I. Dyax Corp.; 2004. Patent, WO2004024750. [Google Scholar]
- 68.Verel I, Heider KH, Siegmund M, Ostermann E, Patzelt E, Sproll M, et al. Tumour targeting properties of monoclonal antibodies with different affinity for target antigen CD44V6 in nude mice bearing head-and-neck cancer xenografts. Int J Cancer. 2002;20(99):396–402. doi: 10.1002/ijc.10369. [DOI] [PubMed] [Google Scholar]
- 69.Koppe M, Schaijk F, Roos J, Leeuwen P, Heider KH, Kuthan H, Bleichrodt R. Safety, pharmacokinetics, immunogenicity and biodistribution of (186)Re-labeled humanized monoclonal antibody BIWA 4 (Bivatuzumab) in patients with early-stage breast cancer. Cancer Biother Radiopharm. 2004;19:720–729. doi: 10.1089/cbr.2004.19.720. [DOI] [PubMed] [Google Scholar]
- 70.Colnot DR, Roos JC, de Bree R, Wilhelm AJ, Kummer JA, Hanft G, et al. Safety, biodistribution, pharmacokinetics and immunogenicity of 99mTc-labeled humanized monoclonal antibody BIWA 4 (bivatuzumab) in patients with squamous cell carcinoma of the head and neck. Cancer Immunol Immunother. 2003;52:576–582. doi: 10.1007/s00262-003-0396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tijink BM, Buter J, de Bree R, Giaccone G, Lang MS, Staab A, Leemans CR, van Dongen GA. A phase I dose escalation study with anti-CD44v6 bivatuzumab mertansine in patients with incurable squamous cell carcinoma of the head and neck or esophagus. Clin Cancer Res. 2006;12:6064–6072. doi: 10.1158/1078-0432.CCR-06-0910. [DOI] [PubMed] [Google Scholar]
- 72.Riechelmann H, Sauter A, Golze W, Hanft G, Schroen C, Hoermann K, Erhardt T, Gronau S. Phase I trial with the CD44v6-targeting immunoconjugate bivatuzumab mertansine in head and neck squamous cell carcinoma. Oral Oncol. 2008;44:823–829. doi: 10.1016/j.oraloncology.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 73.Wang SJ, Bourguignon LY. Hyaluronan and the interaction between CD44 and epidermal growth factor receptor in oncogenic signaling and chemotherapy resistance in head and neck cancer. Arch Otolaryngol Head Neck Surg. 2006;132:771–778. doi: 10.1001/archotol.132.7.771. [DOI] [PubMed] [Google Scholar]
- 74.Kim Y, Lee YS, Choe J, Lee H, Kim YM, Jeoung D. CD44-epidermal growth factor receptor interaction mediates hyaluronic acid-promoted cell motility by activating protein kinase C signaling involving Akt, Rac1, Phox, reactive oxygen species, focal adhesion kinase and MMP-2. J Biol Chem. 2008;283:22513–22528. doi: 10.1074/jbc.M708319200. [DOI] [PubMed] [Google Scholar]
- 75.Guo Y, Ma J, Wang J, Che X, Narula J, Bigby M, Wu M, Sy MS. Inhibition of human melanoma growth and metastasis in vivo by anti-CD44 monoclonal antibody. Cancer Res. 1994;54:1561–1565. [PubMed] [Google Scholar]
- 76.Pályi-Krekk Z, Barok M, Isola J, Tammi M, Szöllo si J, Nagy P. Hyaluronan-induced masking of ErbB2 and CD44-enhanced trastuzumab internalisation in trastuzumab resistant breast cancer. Eur J Cancer. 2007;43:2423–2433. doi: 10.1016/j.ejca.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 77.Mizrak D, Brittan M, Alison MR. CD133: molecule of the moment. J Pathol. 2008;214:3–9. doi: 10.1002/path.2283. [DOI] [PubMed] [Google Scholar]
- 78.Bidlingmaier S, Zhu X, Liu B. The utility and limitations of glycosylated human CD133 epitopes in defining cancer stem cells. J Mol Med. 2008;86:1025–1032. doi: 10.1007/s00109-008-0357-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Horst D, Kriegl L, Engel J, Kirchner T, Jung A. CD133 expression is an independent prognostic marker for low survival in colorectal cancer. Br J Cancer. 2008 doi: 10.1038/sj.bjc.6604664. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smith LM, Nesterova A, Ryan MC, Duniho S, Jonas M, Anderson M, Zabinski RF, Sutherland MK, Gerber HP, Van Orden KL, Moore PA, Ruben SM, Carter PJ. Br J Cancer. 2008;99:100–109. doi: 10.1038/sj.bjc.6604437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Went P, Vasei M, Bubendorf L, Terracciano L, Tornillo L, Riede U, et al. Frequent high-level expression of the immunotherapeutic target Ep-CAM in colon, stomach, prostate and lung cancers. Br J Cancer. 2006;94:128–135. doi: 10.1038/sj.bjc.6602924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Punt CJ, Nagy A, Douillard JY, Figer A, Skovsgaard T, Monson J, et al. Edrecolomab alone or in combination with fluorouracil and folinic acid in the adjuvant treatment of stage III colon cancer: a randomised study. Lancet. 2002;360:671–677. doi: 10.1016/S0140-6736(02)09836-7. [DOI] [PubMed] [Google Scholar]
- 83.Balzar M, Winter MJ, de Boer CJ, Litvinov SV. The biology of the 17-1A antigen (Ep-CAM) J Mol Med. 1999;77:699–712. doi: 10.1007/s001099900038. [DOI] [PubMed] [Google Scholar]
- 84.Ammons WS, Bauer RJ, Horwitz AH, Chen ZJ, Bautista E, Ruan HH, et al. In vitro and in vivo pharmacology and pharmacokinetics of a human engineered monoclonal antibody to epithelial cell adhesion molecule. Neoplasia. 2003;5:146–154. doi: 10.1016/s1476-5586(03)80006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Goel S, Bauer RJ, Desai K, Bulgaru A, Iqbal T, Strachan BK, et al. Pharmacokinetic and safety study of subcutaneously administered weekly ING-1, a human engineere monoclonal antibody targeting human EpCAM, in patients with advanced solid tumours. Ann Oncol. 2007;18:1704–1707. doi: 10.1093/annonc/mdm280. [DOI] [PubMed] [Google Scholar]
- 86.Oberneder R, Weckermann D, Ebner B, Quadt C, Kirchinger P, Raum T, et al. A phase I study with adecatumumab, a human antibody directed against epithelial cell adhesion molecule, in hormone refractory prostate cancer patients. Eur J Cancer. 2006;42:2530–2538. doi: 10.1016/j.ejca.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 87.Naundorf S, Preithner S, Mayer P, Lippold S, Wolf A, Hanakam F, et al. In vitro and in vivo activity of MT201, a fully human monoclonal antibody for pancarcinoma treatment. Int J Cancer. 2002;100:101–110. doi: 10.1002/ijc.10443. [DOI] [PubMed] [Google Scholar]
- 88.Shen J, Zhu Z. Catumaxomab, a rat/murine hybrid trifunctional bispecific monoclonal antibody for the treatment of cancer. Curr Opin Mol Ther. 2008;10:273–284. [PubMed] [Google Scholar]
- 89.Douville J, Beaulieu R, Balicki D. ALDH1 as a Functional Marker of Cancer Stem and Progenitor Cells. Stem Cells Dev. 2008 doi: 10.1089/scd.2008.0055. In press. [DOI] [PubMed] [Google Scholar]
- 90.Visus C, Ito D, Amoscato A, Maciejewska-Franczak M, Abdelsalem A, Dhir R, et al. Identification of human aldehyde dehydrogenase 1 family member A1 as a novel CD8+ T-cell-defined tumour antigen in squamous cell carcinoma of the head and neck. Cancer Res. 2007;67:10538–10545. doi: 10.1158/0008-5472.CAN-07-1346. [DOI] [PubMed] [Google Scholar]
- 91.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 Is a Marker of Normal and Malignant Human Mammary Stem Cells and a Predictor of Poor Clinical Outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lou H, Dean M. Targeted therapy for cancer stem cells: the patched pathway and ABC transporters. Oncogene. 2007;26:1357–1360. doi: 10.1038/sj.onc.1210200. [DOI] [PubMed] [Google Scholar]
- 93.Raaijmakers MH. ATP-binding-cassette transporters in hematopoietic stem cells and their utility as therapeutical targets in acute and chronic myeloid leukemia. Leukemia. 2007;21:2094–2102. doi: 10.1038/sj.leu.2404859. [DOI] [PubMed] [Google Scholar]
- 94.Schatton T, Murphy GF, Frank NY, Yamaura K, Waaga-Gasser AM, Gasser M, et al. Identification of cells initiating human melanomas. Nature. 2008;451:345–349. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kristiansen G, Sammar M, Altevogt P. Tumour biological aspects of CD24, a mucin-like adhesion molecule. J Mol Histol. 2004;35:255–262. doi: 10.1023/b:hijo.0000032357.16261.c5. [DOI] [PubMed] [Google Scholar]
- 96.Sagiv E, Memeo L, Karin A, Kazanov D, Jacob-Hirsch J, Mansukhani M, et al. CD24 is a new oncogene, early at the multistep process of colorectal cancer carcinogenesis. Gastroenterology. 2006;131:630–639. doi: 10.1053/j.gastro.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 97.Sagiv E, Starr A, Rozovski U, Khosravi R, Altevogt P, Wang T, Arber N. Targeting CD24 for treatment of colorectal and pancreatic cancer by monoclonal antibodies or small interfering RNA. Cancer Res. 2008;68:2803–2812. doi: 10.1158/0008-5472.CAN-07-6463. [DOI] [PubMed] [Google Scholar]
- 98.Schabath H, Runz S, Joumaa S, Altevogt P. CD24 affects CXCR4 function in pre-B lymphocytes and breast carcinoma cells. J Cell Sci. 2006;119:314–325. doi: 10.1242/jcs.02741. [DOI] [PubMed] [Google Scholar]
- 99.Coiffier B. Rituximab therapy in malignant lymphoma. Oncogene. 2007;26:3603–3613. doi: 10.1038/sj.onc.1210376. [DOI] [PubMed] [Google Scholar]
- 100.Zabierowski SE, Herlyn M. Melanoma stem cells: the dark seed of melanoma. J Clin Oncol. 2008;26:2890–2894. doi: 10.1200/JCO.2007.15.5465. [DOI] [PubMed] [Google Scholar]
- 101.Glennie MJ, French RR, Cragg MS, Taylor RP. Mechanisms of killing by anti-CD20 monoclonal antibodies. Mol Immunol. 2007;44:3823–3837. doi: 10.1016/j.molimm.2007.06.151. [DOI] [PubMed] [Google Scholar]
- 102.Todaro M, Alea MP, Di Stefano AB, Cammareri P, Vermeulen L, Iovino F, et al. Colon cancer stem cells dictate tumour growth and resist cell death by production of interleukin-4. Cell Stem Cell. 2007;1:389–402. doi: 10.1016/j.stem.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 103.Prokopchuk O, Liu Y, Henne-Bruns D, Kornmann M. Interleukin-4 enhances proliferation of human pancreatic cells: evidence for autocrine and paracrine actions. Br J Cancer. 2005;92:921–928. doi: 10.1038/sj.bjc.6602416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kakarala M, Wicha MS. Implications of the cancer stem-cell hypothesis for breast cancer prevention and therapy. J Clin Oncol. 2008;26:2813–2820. doi: 10.1200/JCO.2008.16.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nahta R, Yu D, Hung MC, Hortobagyi GN, Esteva FJ. Mechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol. 2006;3:269–280. doi: 10.1038/ncponc0509. [DOI] [PubMed] [Google Scholar]
- 106.Hudis CA. Trastuzumab—mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 107.Korkaya H, Paulson A, Lovino F, et al. Signalling regulates the mammary stem/progenitor cell population driving tumourigenesis and invasion. Cancer Cell. 2008 doi: 10.1038/onc.2008.207. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rizzo P, Osipo C, Foreman K, Golde T, Osborne B, Miele L. Rational targeting of Notch signaling in cancer. Oncogene. 2008;27:5124–5131. doi: 10.1038/onc.2008.226. [DOI] [PubMed] [Google Scholar]
- 109.Lewicki J. Oncomed Pharmaceuticals Inc.; 2007. Patent WO2007145840. [Google Scholar]
- 110.Gurney A. Oncomed Pharmaceuticals Inc.; 2008. Patent WO2008091641. [Google Scholar]
- 111.D'Souza B, Miyamoto A, Weinmaster G. The many facets of Notch ligands. Oncogene. 2008;27:5148–5167. doi: 10.1038/onc.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gurney A. Oncomed Pharmaceuticals Inc.; 2008. Patent WO2008042236. [Google Scholar]
- 113.Sainson RC, Harris AL. Anti-Dll4 therapy: can we block tumour growth by increasing angiogenesis? Trends Mol Med. 2007;13:389–395. doi: 10.1016/j.molmed.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 114.Ridgway J, Zhang G, Wu Y, Stawicki S, Liang WC, Chanthery Y, et al. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006;444:1083–1087. doi: 10.1038/nature05313. [DOI] [PubMed] [Google Scholar]
- 115.Gurney A. Oncomed Pharmaceuticals Inc.; 2007. Patent WO2007142711. [Google Scholar]
- 116.He B, You L, Uematsu K, Xu Z, Lee AY, Matsangou M, McCormick F, Jablons DM. A monoclonal antibody against Wnt-1 induces apoptosis in human cancer cells. Neoplasia. 2004;6:7–14. doi: 10.1016/s1476-5586(04)80048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nakamura M, Kubo M, Yanai K, Mikami Y, Ikebe M, Nagai S, et al. Anti-patched-1 antibodies suppress hedgehog signaling pathway and pancreatic cancer proliferation. Anticancer Res. 2007;27:3743–3747. [PubMed] [Google Scholar]
- 118.Reiter RE, Gu Z, Watabe T, Thomas G, Szigeti K, Davis E, et al. Prostate stem cell antigen: a cell surface marker overexpressed in prostate cancer. Proc Natl Acad Sci USA. 1998;95:1735–1740. doi: 10.1073/pnas.95.4.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gu Z, Thomas G, Yamashiro J, Shintaku IP, Dorey F, Raitano A, et al. Prostate stem cell antigen (PSCA) expression increases with high gleason score, advanced stage and bone metastasis in prostate cancer. Oncogene. 2000;19:1288–1296. doi: 10.1038/sj.onc.1203426. [DOI] [PubMed] [Google Scholar]
- 120.Olafsen T, Gu Z, Sherman MA, Leyton JV, Witkosky ME, Shively JE, et al. Targeting, imaging and therapy using a humanized antiprostate stem cell antigen (PSCA) antibody. J Immunother. 2007;30:396–405. doi: 10.1097/CJI.0b013e318031b53b. [DOI] [PubMed] [Google Scholar]
- 121.Saffran DC, Raitano AB, Hubert RS, Witte ON, Reiter RE, Jakobovits A. Anti-PSCA mAbs inhibit tumour growth and metastasis formation and prolong the survival of mice bearing human prostate cancer xenografts. Proc Natl Acad Sci USA. 2001;98:2658–2663. doi: 10.1073/pnas.051624698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Murayama Y, Shinomura Y, Oritani K, Miyagawa J, Yoshida H, Nishida M, et al. The tetraspanin CD9 modulates epidermal growth factor receptor signaling in cancer cells. J Cell Physiol. 2008;216:135–143. doi: 10.1002/jcp.21384. [DOI] [PubMed] [Google Scholar]
- 123.Bao S, Wu Q, Li Z, Sathornsumetee S, Wang H, McLendon RE, Hjelmeland AB, Rich JN. Targeting cancer stem cells through L1CAM suppresses glioma growth. Cancer Res. 2008;68:6043–6048. doi: 10.1158/0008-5472.CAN-08-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Naugler WE, Karin M. The wolf in sheep's clothing: the role of interleukin-6 in immunity, inflammation and cancer. Trends Mol Med. 2008;14:109–119. doi: 10.1016/j.molmed.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 125.Sell S, Leffert HL. Liver cancer stem cells. J Clin Oncol. 2008;26:2800–2805. doi: 10.1200/JCO.2007.15.5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tang Y, Kitisin K, Jogunoori W, Li C, Deng CX, Mueller SC, et al. Progenitor/stem cells give rise to liver cancer due to aberrant TGFbeta and IL-6 signaling. Proc Natl Acad Sci USA. 2008;105:2445–2450. doi: 10.1073/pnas.0705395105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wallner L, Dai J, Escara-Wilke J, Zhang J, Yao Z, Lu Y, et al. Inhibition of interleukin-6 with CNTO328, an anti-interleukin-6 monoclonal antibody, inhibits conversion of androgen-dependent prostate cancer to an androgen-independent phenotype in orchiectomized mice. Cancer Res. 2006;66:3087–3095. doi: 10.1158/0008-5472.CAN-05-3447. [DOI] [PubMed] [Google Scholar]
- 128.Du X, Ho M, Pastan I. New immunotoxins targeting CD123, a stem cell antigen on acute myeloid leukemia cells. J Immunother. 2007;30:607–613. doi: 10.1097/CJI.0b013e318053ed8e. [DOI] [PubMed] [Google Scholar]
- 129.Kawasaki BT, Farrar WL. Cancer stem cells, CD200 and immunoevasion. Trends Immunol. 2008;29:464–468. doi: 10.1016/j.it.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 130.Siva A, Xin H, Qin F, Oltean D, Bowdish KS, Kretz-Rommel A. Immune modulation by melanoma and ovarian tumour cells through expression of the immunosuppressive molecule CD200. Cancer Immunol Immunother. 2008;57:987–996. doi: 10.1007/s00262-007-0429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kawasaki BT, Mistree T, Hurt EM, Kalathur M, Farrar WL. Co-expression of the toleragenic glycoprotein, CD200, with markers for cancer stem cells. Biochem Biophys Res Commun. 2007;364:778–782. doi: 10.1016/j.bbrc.2007.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ossendorp F, Mengedé E, Camps M, Filius R, Melief CJ. Specific T helper cell requirement for optimal induction of cytotoxic T lymphocytes against major histocompatibility complex class II negative tumours. J Exp Med. 1998;187:693–702. doi: 10.1084/jem.187.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.McWhirter JR, Kretz-Rommel A, Saven A, Maruyama T, Potter KN, Mockridge CI, et al. Antibodies selected from combinatorial libraries block a tumour antigen that plays a key role in immunomodulation. Proc Natl Acad Sci USA. 2006;103:1041–1046. doi: 10.1073/pnas.0510081103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li L, Xie T. Stem cell niche: structure and function. Annu Rev Cell Dev Biol. 2005;21:605–631. doi: 10.1146/annurev.cellbio.21.012704.131525. [DOI] [PubMed] [Google Scholar]
- 135.Sneddon JB, Werb Z. Location, location, location: the cancer stem cell niche. Cell Stem Cell. 2007;1:607–611. doi: 10.1016/j.stem.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 137.Riquelme PA, Drapeau E, Doetsch F. Brain micro-ecologies: neural stem cell niches in the adult mammalian brain. Philos Trans R Soc Lond B Biol Sci. 2008;363:123–137. doi: 10.1098/rstb.2006.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Folkins C, Man S, Xu P, Shaked Y, Hicklin DJ, Kerbel RS. Anticancer therapies combining antiangiogenic and tumour cell cytotoxic effects reduce the tumour stem-like cell fraction in glioma xenograft tumours. Cancer Res. 2007;67:3560–3564. doi: 10.1158/0008-5472.CAN-06-4238. [DOI] [PubMed] [Google Scholar]
- 139.Jain RK. Lessons from multidisciplinary translational trials on anti-angiogenic therapy of cancer. Nat Rev Cancer. 2008;8:309–316. doi: 10.1038/nrc2346. [DOI] [PubMed] [Google Scholar]
- 140.Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 141.Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, Hjelmeland AB, Shi Q, et al. Stem cell-like glioma cells promote tumour angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66:7843–7848. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- 142.Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, et al. A perivascular niche for brain tumour stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 143.Ceriani RL, Blank EW, Couto JR, Peterson JA. Biological activity of two humanized antibodies against two different breast cancer antigens and comparison to their original murine forms. Cancer Res. 1995;55:5852–5856. [PubMed] [Google Scholar]
- 144.Neutzner M, Lopez T, Feng X, Bergmann-Leitner ES, Leitner WW, Udey MC. MFG-E8/lactadherin promotes tumour growth in an angiogenesis-dependent transgenic mouse model of multistage carcinogenesis. Cancer Res. 2007;67:6777–6785. doi: 10.1158/0008-5472.CAN-07-0165. [DOI] [PubMed] [Google Scholar]
- 145.Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med. 1997;185:111–120. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Burger JA, Bürkle A. The CXCR4 chemokine receptor in acute and chronic leukaemia: a marrow homing receptor and potential therapeutic target. Br J Haematol. 2007;137:288–296. doi: 10.1111/j.1365-2141.2007.06590.x. [DOI] [PubMed] [Google Scholar]
- 147.Corcione A, Arduino N, Ferretti E, Pistorio A, Spinelli M, Ottonello L, et al. Chemokine receptor expression and function in childhood acute lymphoblastic leukemia of B-lineage. Leuk Res. 2006;30:365–372. doi: 10.1016/j.leukres.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 148.Dittmar T, Heyder C, Gloria-Maercker E, Hatzmann W, Zänker KS. Adhesion molecules and chemokines: the navigation system for circulating tumour (stem) cells to metastasize in an organ-specific manner. Clin Exp Metastasis. 2008;25:11–32. doi: 10.1007/s10585-007-9095-5. [DOI] [PubMed] [Google Scholar]
- 149.Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 150.Honn KV, Tang DG. Adhesion molecules and tumour cell interaction with endothelium and subendothelial matrix. Cancer Metastasis Rev. 1992;11:353–375. doi: 10.1007/BF01307187. [DOI] [PubMed] [Google Scholar]
- 151.Alsayed Y, Ngo H, Runnels J, Leleu X, Singha UK, Pitsillides CM, et al. Mechanisms of regulation of CXCR4/SDF-1 (CXCL12)-dependent migration and homing in multiple myeloma. Blood. 2007;109:2708–2717. doi: 10.1182/blood-2006-07-035857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sun YX, Schneider A, Jung Y, Wang J, Dai J, Wang J, et al. Skeletal localization and neutralization of the SDF-1(CXCL12)/CXCR4 axis blocks prostate cancer metastasis and growth in osseous sites in vivo. J Bone Miner Res. 2005;20:318–329. doi: 10.1359/JBMR.041109. [DOI] [PubMed] [Google Scholar]
- 153.Ottaiano A, di Palma A, Napolitano M, Pisano C, Pignata S, Tatangelo F, et al. Inhibitory effects of anti-CXCR4 antibodies on human colon cancer cells. Cancer Immunol Immunother. 2005;54:781–791. doi: 10.1007/s00262-004-0636-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Huveneers S, Truong H, Danen HJ. Integrins: signaling, disease and therapy. Int J Radiat Biol. 2007;83:743–751. doi: 10.1080/09553000701481808. [DOI] [PubMed] [Google Scholar]
- 155.Ramsay AG, Marshall JF, Hart IR. Integrin trafficking and its role in cancer metastasis. Cancer Metastasis Rev. 2007;26:567–578. doi: 10.1007/s10555-007-9078-7. [DOI] [PubMed] [Google Scholar]
- 156.Gutheil JC, Campbell TN, Pierce PR, Watkins JD, Huse WD, Bodkin DJ, Cheresh DA. Targeted antiangiogenic therapy for cancer using Vitaxin: a humanized monoclonal antibody to the integrin alphavbeta3. Clin Cancer Res. 2000;6:3056–3061. [PubMed] [Google Scholar]
- 157.Nemeth JA, Cher ML, Zhou Z, Mullins C, Bhagat S, Trikha M. Inhibition of alpha(v)beta3 integrin reduces angiogenesis, bone turnover and tumour cell proliferation in experimental prostate cancer bone metastases. Clin Exp Metastasis. 2003;20:413–420. doi: 10.1023/a:1025461507027. [DOI] [PubMed] [Google Scholar]
- 158.Cordes N, Seidler J, Durzok R, Geinitz H, Brakebusch C. beta1-integrin-mediated signaling essentially contributes to cell survival after radiation-induced genotoxic injury. Oncogene. 2006;25:1378–1390. doi: 10.1038/sj.onc.1209164. [DOI] [PubMed] [Google Scholar]
- 159.Yao ES, Zhang H, Chen YY, Lee B, Chew K, Moore D, Park C. Increased beta1 integrin is associated with decreased survival in invasive breast cancer. Cancer Res. 2007;67:659–664. doi: 10.1158/0008-5472.CAN-06-2768. [DOI] [PubMed] [Google Scholar]
- 160.Park CC, Zhang HJ, Yao ES, Park CJ, Bissell MJ. Beta1 integrin inhibition dramatically enhances radiotherapy efficacy in human breast cancer xenografts. Cancer Res. 2008;68:4398–4405. doi: 10.1158/0008-5472.CAN-07-6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Oosterling SJ, van der Bij GJ, Bögels M, ten Raa S, Post JA, Meijer GA, Beelen RH, van Egmond M. Anti-beta1 integrin antibody reduces surgery-induced adhesion of colon carcinoma cells to traumatized peritoneal surfaces. Ann Surg. 2008;247:85–94. doi: 10.1097/SLA.0b013e3181588583. [DOI] [PubMed] [Google Scholar]
- 162.Nickoloff BJ, Osborne BA, Miele L. Notch signaling as a therapeutic target in cancer: a new approach to the development of cell fate modifying agents. Oncogene. 2003;22:6598–6608. doi: 10.1038/sj.onc.1206758. [DOI] [PubMed] [Google Scholar]
- 163.van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton DJ, Radtke F, Clevers H. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 164.Barker N, Clevers H. Mining the Wnt pathway for cancer therapeutics. Nat Rev Drug Discov. 2006;5:997–1014. doi: 10.1038/nrd2154. [DOI] [PubMed] [Google Scholar]
- 165.Harley CB. Telomerase and cancer therapeutics. Nat Rev Cancer. 2008;7:7. doi: 10.1038/nrc2275. [DOI] [PubMed] [Google Scholar]
- 166.List AF, Kopecky KJ, Willman CL, Head DR, Persons DL, Slovak ML, et al. Benefit of cyclosporine modulation of drug resistance in patients with poor-risk acute myeloid leukemia: a Southwest Oncology Group study. Blood. 2001;98:3212–3220. doi: 10.1182/blood.v98.12.3212. [DOI] [PubMed] [Google Scholar]
- 167.Guzman ML, Rossi RM, Karnischky L, Li X, Peterson DR, Howard DS, Jordan CT. The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells. Blood. 2005;105:4163–4169. doi: 10.1182/blood-2004-10-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yilmaz OH, Valdez R, Theisen BK, Guo W, Ferguson DO, Wu H, Morrison SJ. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- 169.Sandler A, Herbst R. Combining targeted agents: blocking the epidermal growth factor and vascular endothelial growth factor pathways. Clin Cancer Res. 2006;12:4421–4425. doi: 10.1158/1078-0432.CCR-06-0796. [DOI] [PubMed] [Google Scholar]
- 170.Lin CC, Calvo E, Papadopoulos KP, Patnaik A, Sarantopoulos J, Mita AC, et al. Phase I study of cetuximab, erlotinib and bevacizumab in patients with advanced solid tumours. Cancer Chemother Pharmacol. 2008 doi: 10.1007/s00280-008-0811-x. In press. [DOI] [PubMed] [Google Scholar]
- 171.Hainsworth JD, Vazquez ER, Spigel DR, Raefsky E, Bearden JD, Saez RA, Greco FA. Combination therapy with fludarabine and rituximab followed by alemtuzumab in the first-line treatment of patients with chronic lymphocytic leukemia or small lymphocytic lymphoma: a phase 2 trial of the Minnie Pearl Cancer Research Network. Cancer. 2008;112:1288–1295. doi: 10.1002/cncr.23271. [DOI] [PubMed] [Google Scholar]
- 172.Logtenberg T. Antibody cocktails: next-generation biopharmaceuticals with improved potency. Trends Biotechnol. 2007;25:390–394. doi: 10.1016/j.tibtech.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 173.Reid A, Vidal L, Shaw H, de Bono J. Dual inhibition of ErbB1 (EGFR/HER1) and ErbB2 (HER2/neu) Eur J Cancer. 2007;43:481–489. doi: 10.1016/j.ejca.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 174.Goldenberg DM, Sharkey RM. Advances in cancer therapy with radiolabeled monoclonal antibodies. Q J Nucl Med Mol Imaging. 2006;50:248–264. [PubMed] [Google Scholar]
- 175.Bhatti M, Yahioglu G, Milgrom LR, Garcia-Maya M, Chester KA, Deonarain MP. Targeted photodynamic therapy with multiply-loaded recombinant antibody fragments. Int J Cancer. 2008;122:1155–1163. doi: 10.1002/ijc.23206. [DOI] [PubMed] [Google Scholar]
- 176.Bagshawe KD, Sharma SK, Begent RH. Antibody-directed enzyme prodrug therapy (ADEPT) for cancer. Expert Opin Biol Ther. 2004;4:1777–1789. doi: 10.1517/14712598.4.11.1777. [DOI] [PubMed] [Google Scholar]
- 177.Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov. 2003;2:214–221. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 178.Constantinou A, Epenetos AA, Hreczuk-Hirst D, Jain S, Deonarain MP. Modulation of antibody pharmacokinetics by chemical polysialylation. Bioconjug Chem. 2008;19:643–650. doi: 10.1021/bc700319r. [DOI] [PubMed] [Google Scholar]
- 179.Dennis MS, Jin H, Dugger D, Yang R, McFarland L, Ogasawara A, et al. Imaging tumors with an albumin-binding Fab, a novel tumor-targeting agent. Cancer Res. 2007;67:254–261. doi: 10.1158/0008-5472.CAN-06-2531. [DOI] [PubMed] [Google Scholar]
- 180.Hart LS, El-Deiry WS. Invincible, but not invisible: imaging approaches toward in vivo detection of cancer stem cells. J Clin Oncol. 2008;26:2901–2910. doi: 10.1200/JCO.2008.16.9573. [DOI] [PubMed] [Google Scholar]