Abstract
Precision binding of monoclonal antibodies (mAbs) to biological targets, their relative clinical success, and expansion of indications following initial approval, are distinctive clinical features. The relatively high cost of mAbs, together with the absence of a regulatory pathway to generics, stand out as distinctive economic features. Based on both literature review and primary data collection we enumerated mAb original approvals, supplemental indications and off-label uses, assessed payer formulary management of mAbs, and determined new challenges to Medicare beneficiary access to mAbs. We found that the FDA has approved 22 mAbs and 30 supplemental indications pertaining to the originally approved mAbs. In addition, there are 46 off-label use citations in officially recognized pharmaceutical compendia. Across Part B carriers and Part D plans, we found considerable variation in terms of coverage and conditions of reimbursement related to on- and off-label uses of mAbs. Our results point to four major challenges facing mAb developers, health care providers, Medicare beneficiaries, payers and policymakers. These include administrative price controls, coverage variation, projected shift from physician- to self-administered mAbs, and comparative effectiveness. We suggest more systematic use of “coverage with evidence development” as a means of optimally addressing these challenges.
Key words: mAbs, medicare, formulary management, comparative effectiveness, off-label, reimbursement
Introduction
During the past decade or so, we have observed the “first wave of successes from the molecular targeted therapeutics.”1 Targeted therapeutics have become a mainstay, particularly in the treatment of cancer and autoimmune conditions. In evaluating mAbs, three clinical features stand out: Their ability to precisely bind to biological targets, potential to significantly improve health outcomes and propensity for expansion of indications following the original mAb approval. At the same time, two economic features also stand out: Their relatively high cost per patient per dose, and the absence of a regulatory pathway to generics.
We examined all mAb approvals in the United States, as well as supplemental and off-label indications and the evolving market dynamic for these molecules. And, we assessed formulary management by leading Medicare payers. Our findings suggested four major challenges facing mAb developers, health care providers, Medicare beneficiaries, payers and policymakers. These include: (1) administrative price controls; (2) coverage variation; (3) projected shift from physician- to self-administered mAbs; and (4) comparative effectiveness.
Clinical Picture
Clinical significance of mAbs.
MAbs are large molecules produced by genetic engineering. The ability to target specific antigens within cells, tissues and organs involved in the pathology of disease, while minimizing side effects, has underlined their popularity in clinical applications.2 Unlike conventional therapies that often offer only short-term symptomatic relief and can cause serious side effects, mAbs provide treatments with potentially greater effectiveness (i.e., extended survival and improved quality of life) and tolerability. In brief, the important characteristics that distinguish mAbs and other targeted therapeutics from chemical agents include their mode of administration (injection or infusion), relatively lengthy FDA approval process, absence of a generic pathway, and wide range of therapeutic uses.
The FDA has approved 22 mAbs for marketing (see Table 1). Furthermore, there are over 200 mAbs currently in clinical development.3
Table 1.
FDA-approved therapeutic monoclonal antibodies
| Generic name (Trade) | Approval date | First approved indication |
| Abciximab (Reopro) | 12/22/1994 | Adjunct to percutaneous transluminal coronary angioplasty or atherectomy (PTCA) for the prevention of acute cardiac ischemic complications in patients at high risk for abrupt closure of the treated coronary vessel. |
| Adalimumab (Humira) | 12/31/2002 | Reducing signs and symptoms and inhibiting the progression of the structural damage in adult patients with moderately to severly active rheumatoid arthritis who have had an inadequate response to one or more disease-modifying anti-rheumatic drugs (DMARDS). |
| Alemtuzumab (Campath-1H) | 05/07/2001 | Treatment of patients with B-cell chronic lymphocytic leukemia who have been treated with alkylating agents and who have failed fludarabine therapy. |
| Basiliximab (Simulect) | 05/12/1998 | Prophylaxis of acute organ rejection in patients receiving renal transplantation when used as part of an immunosuppressive regimen that includes cyclosporine and corticosteroids. |
| Bevacizumab (Avastin) | 02/26/2004 | First-line treatment of patients with metastatic carcinoma of the colon and rectum in combination with intravenous 5-fluorouracil-based chemotherapy. |
| Certolizumab pegol (Cimzia) | 04/22/2008 | Reducing the signs and symptoms of Crohn's disease and maintaining clinical response in adult patients with moderately to severely active disease who have had an inadequate response to conventional therapy. |
| Cetuximab (Erbitux) | 02/12/2004 | In combination with irinotecan, treatment of EGFR-expressing, metastatic colorectal carcinoma in patients who are refractory to irinotecan-based chemotherapy; administered as a single agent, treatment of EGFR-expressing, metastatic colorectal carcinoma in patients who are intolerant to irinotecan-based chemotherapy. |
| Daclizumab (Zenapax) | 12/10/1997 | Prophylaxis of acute organ rejection in patients receiving renal transplants, to be used as a part of an immunosuppressive regimen that includes cyclosporine and corticosteroids. |
| Eculizumab (Soliris) | 03/16/2007 | Treatment of paroxysmal nocturnal hemoglobinuria to reduce hemolysis. |
| Efalizumab (Raptiva) | 10/27/2003 | Treatment of adult patients (18 years or older) with chronic moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy. |
| Gemtuzumab ozogamicin (Mylotarg) | 05/17/2000 | Treatment of patients with CD33 positive acute myeloid leukemia in first relapse who are 60 years of age or older and who are not considered candidates for cytotoxic chemotherapy. |
| Ibritumomab tuixetan (Zevalin) | 02/19/2002 | Ibritumomab Tiuxetan, as part of a specific therapeutic regimen, is indicated for the treatment of patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin's lymphoma, including patients with Rituximab (Rituxan) refractory follicular non-Hodgkin's lymphoma. The therapeutic regimen includes Rituximab, Indium-111 Ibritumomab Tiuxetan and Yttrium-90 Ibritumomab Tiuxetan. |
| Infliximab (Remicade) | 08/24/1998 | Treatment of moderately to severely active Crohn's disease for the reduction of the signs and symptoms, in patients who have an inadequate response to conventional therapies; and treatment of patients with fistulizing Crohn's disease for the reduction in the number of draining enterocutaneous fistula(s). |
| Muromonab-CD3 (Orthoclone-Okt) | 06/19/1986 | Reversal of acute kidney transplant rejection. |
| Natalizumab (Tysabri) | 11/23/2004 | Treatment of patients with relapsing forms of multiple sclerosis to reduce the frequency of clinical exacerbations. |
| Omalizumab (Xolair) | 06/20/2003 | Omalizumab is indicated for adults and adolescents (12 years of age and above) with moderate to severe persistent asthma who have a positive skin test or in vitro reactivity to a perennial aeroallergen and whose symptoms are inadequately controlled with inhaled corticosteroids. |
| Palivizumab (Synagis) | 06/19/1998 | Prophylaxis of serious lower respiratory tract disease, caused by respiratory syncytial virus, in pediatric patients at high risk of RSV disease. |
| Panitumumab (Vectibix) | 09/27/2006 | Treatment of EGFR-expressing metastatic colorectal carcinoma with disease progression on or following fluoropyrimidine-, oxaliplatin- and irinotecan-containing chemotherapy regimens. |
| Ranibizumab (Lucentis) | 06/30/2006 | Treatment of patients with neovascular (wet) age-related macular degeneration. |
| Rituximab (Rituxan) | 11/26/1997 | Treatment of patients with relapsed or refractory low-grade or follicular, B-cell non-Hodgkin's lymphoma. |
| Tositumomab-I131 (Bexxar) | 06/27/2003 | Tositumomab and Iodine I 131 Tositumomab, administered as a therapeutic regimen, are indicated for the treatment of patients with CD20 positive, follicular, non-Hodgkin's lymphoma, with and without transformation, whose disease is refractory to Rituximab and has relapsed following chemotherapy. |
| Trastuzumab (Herceptin) | 09/25/1998 | Treatment of patients with metastatic breast cancer whose tumors overexpress the HER2 protein and who have received one or more chemotherapy regimens for their metastatic disease. Trastizumab in combination with paclitaxel is indicated for treatment of patients with metastatic breast cancer whose tumors overexpress HER2 protein and who have not received chemotherapy for their metastatic disease. |
Source: FDA, www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm. Accessed: May 25, 2008.
Supplemental indications; off-label uses.
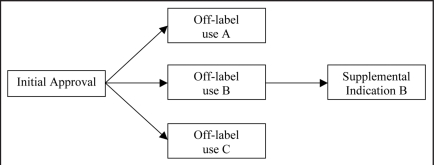
Therapeutics that have been approved for one or more indications, may be applied over time to a different stage of the same indication or a different indication altogether. Initially, such new indications are off-label. To illustrate, a cancer therapy may be approved for treatment against an advanced form of cancer that has metastasized and then be prescribed off-label for an earlier stage of the same cancer, and subsequently obtain a supplemental approval for this use (e.g., trastuzumab). MAbs have a particularly strong propensity for having supplemental indications and off-label uses.4 For example, infliximab was originally approved for Crohn's disease, but has since also been approved as a treatment for rheumatoid and psoriatic arthritis, ankylosing spondylitis and ulcerative colitis. Additionally, infliximab has off-label uses for juvenile idiopathic arthritis and uveitis. Some off-label uses ultimately get approved as supplemental indications, while others remain off-label (see Fig. 1). It has been estimated that approximately 70% of off-label uses are included in officially recognized drug compendia and therefore considered “medically accepted” and reimbursable. The investment required by biopharmaceutical firms to obtain compendia coverage may compare favorably with the time and cost associated with filing a supplementary new drug application. This applies especially towards the end of a drug's patent life. Of the 22 approved mAbs, 14 have one or more supplemental indications, 14 have one or more off-label uses, and 18 have either one or more supplemental or off-label indications (see Tables 2 and 3).
Figure 1.
Off-label uses and supplemental indications. FDA specifies a drug's initial approved indication(s). Subsequently, the drug may have multiple off-label uses, of which some may turn out to become FDA-approved supplemental indications(s).
Table 2.
FDA-approved supplemental indications for monoclonal antibodies
| Generic name (Trade) | Supplemental indications (approval date) |
| Abciximab (Repro) | • Percutaneous coronary intervention including unstable angina; adjunct (11/5/1997)a |
| Adalimumab (Humira) | • Ankylosing spondylitis (07/31/2006)a |
| • Psoriatic arthritis | |
| - improving physical function; inhibiting structural damage (11/09/2006)a | |
| - treatment (10/03/2005)b | |
| • Crohn's disease (02/27/2007)a | |
| • Plaque psoriasis (01/18/2008)a | |
| • Rheumatoid Arthritis | |
| - improving physical function (07/30/2004)b | |
| - treatment of recent diagnosis and not yet on methotrexate (10/3/2005)b | |
| • Juvenile idiopathic arthritis (02/21/2008)b | |
| Alemtuzumab (Campath-1H) | • Leukemia; monotherapy (09/19/2007)b |
| Basiliximab (Simulect) | • Kidney transplant rejection |
| - pediatric use (03/23/2001)a | |
| - IV bolus use (03/23/2001)a | |
| - Combination therapy (03/23/2001)a | |
| Bevacizumab (Avastin) | • Lung cancer; first-line combination therapy (10/11/2006)a |
| • Breast cancer; combination therapy in patients not yet receiving chemo therapy (02/22/2008)a | |
| • Colorectal cancer; second-line adjunct treatment (06/20/2006)b | |
| Cetuximab (Erbitux) | • Head and neck cancer |
| - combination therapy (03/01/2006)a | |
| - monotherapy (03/01/2006)a | |
| • Colorectal cancer; monotherapy (10/02/2007)b | |
| Daclizumab (Zenapax) | • Kidney transplant rejection; pediatric use (07/29/2002)a |
| Ibritumomab tuixetan (Zevalin) | • Non-Hodgkin's lymphoma; removed transformed B-cell non-Hodgkin's lymphoma as an indication (03/25/2008)a |
| Infliximab (Remicade) | • Rheumatoid Arthritis |
| - reducing signs and symptoms (11/10/1999)a | |
| - inhibiting structural damage (12/29/2000)b | |
| - improve physical function (02/27/2005)b | |
| - treatment in early stages not yet treated with methotrexate (09/29/2004)b | |
| • Psoriatic arthritis | |
| - treatment (05/13/2005)b | |
| - improve physical function (08/11/2006)b | |
| • Active arthritis; inhibit structural damage (08/11/2006)b | |
| • Crohn's disease | |
| - pediatric use (05/19/2006)a | |
| - reducing signs and symptoms; inducing and maintaining remission (06/28/2002)b | |
| - reducing number of draining fistulas and maintaining fistula closure fistulizing Crohn's disease (04/01/2003)b | |
| • Ankylosing Spondylitis (12/17/2004)a | |
| • Ulcerative colitis | |
| - treatment (09/15/2005)a | |
| - maintenance of clinical remission and mucosal healing (10/13/2006)b | |
| • Plaque psoriasis (09/26/2006)a | |
| Muromonab-CD3 (Orthoclone-Okt) | • Allograft rejection in kidney transplants (09/14/1992)a |
| • Allograft rejection in heart and liver transplants (06/08/1993)a | |
| Natalizumab (Tysabri) | • Crohn's disease; inducing and maintaining clinical response and remission (02/14/2008)a |
| Rituximab (Rituxan) | • Rheumatoid arthritis |
| - combination therapy (02/28/2006)a | |
| - slow progression of structural damage (01/25/2008)b | |
| • Non-Hodgkin's lymphoma; first-line combination therapy (09/29/2006)b | |
| Tositumomab-I131 (Bexxar) | • Non-Hodgkin's lymphoma (12/22/2004)b |
| Trastuzumab (Herceptin) | • Breast cancer |
| - adjuvant combination therapy (11/16/2006)b | |
| - adjuvant monotherapy (01/18/2008)b |
New Indication: New indication as noted in the FDA supplemental approval letter.
Expanded Indication: Expanded indication as noted in the FDA supplemental approval letter. Source: FDA, www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm. Accessed: May 26, 2008.
Table 3.
“Medically accepted”** off-label indications for FDA-approved monoclonal antibodies
| Generic name (Trade) | Off-label indications |
| Abciximab (Repro) | • Kawasaki disease |
| • Arterial thrombosis | |
| • Cardiogenic shock | |
| • Occlusive cerebrovascular disease | |
| Alemtuzumab (Campath-1H) | • Cytopenia |
| • Lymphoid hemopoietic tumor | |
| • Prolymphocytic leukemia | |
| Basiliximab (Simulect) | • Graft versus host disease |
| • Liver transplant rejection | |
| Bevacizumab (Avastin) | • Macular degeneration |
| • Diabetic macular edema | |
| • Retinal macular edema | |
| Cetuximab (Erbitux) | • Head and neck cancer; combination therapy |
| • Colorectal cancer; first-line combination therapy | |
| • Pancreatic cancer | |
| Efalizumab (Raptiva) | • Atopic dermatitis |
| Gemtuzumab ozogamicin (Mylotarg) | • Myeloid leukemia |
| • Promyelosytic leukemia | |
| Infliximab (Remicade) | • Hidradenitis supperativa |
| • Juvenile idiopathic arthritis | |
| • Rheumatoid arthritis; monotherapy | |
| • Chronic systemic onset juvenile arthritis | |
| • Uveitis; adjunct therapy | |
| • Wegener's granulomatosis; combination therapy | |
| Muromonab-CD3 (Orthoclone-Okt) | • Heart transplant rejection; prophylaxis |
| • Kidney transplant rejection; prophylaxis | |
| • Graft versus host disease | |
| Omalizumab (Xolair) | • Allergic rhinitis |
| • Peanut allergy | |
| • Latex allergy | |
| • Subcutaneous immunotherapy | |
| Panitumumab (Vectibix) | • Colorectal cancer |
| • Lung cancer | |
| Rituximab (Rituxan) | • Autoimmune hemolytic anemia |
| • Lymphoma | |
| • Chronic lymphoid leukemia; first-line combination therapy | |
| • Evan syndrome | |
| • Chronic graft versus host disease | |
| • Hodgkin's disease; monotherapy | |
| • Post-transplant lymphoproliferative disorder | |
| • Lupus erythematosis | |
| • Thrombocytopenic purpura | |
| • Waldenstrom's macroglobulinemia | |
| • Wegener's granulomatosis; combination therapy | |
| Trastuzumab (Herceptin) | • Breast cancer; adjuvant |
| • Breast cancer; neoadjuvant combination therapy |
“Medically accepted” off-label uses indicate those for which the quantity and content of the existing evidence is considered compelling enough by recognized drug compendia to demonstrate an acceptable level of safety and efficacy. Source: DrugPoints® System. STAT!Ref Online Electronic Medical Library, http://online.statref.com/document.aspx?fxid=6&docid=1. Accessed: May 26, 2008.
Market Dynamics
mAbs' clinical success has translated into commercial success. The 2006 global market for mAbs was over US$17 billion, and sales are forecast to grow annually by 14% between 2006 and 2012. In 2006, seven mAbs had blockbuster status with global sales of over US $1 billion each, accounting for over 90% of total mAb sales.
In terms of sales, mAbs are the second largest class of biologics, closing in on the number one class, broadly defined as growth factors, which include the erythropoietins. Since 2001, mAb sales have increased at an average annual rate of 35%. Growth is fueled by expansion into new indications, by virtue of often being the only available option in therapy areas with a largely unmet need, and growing (ageing) populations suffering from diseases targeted by mAbs.5 Sales growth of certain mAbs, such as infliximab and trastuzumab, has slowed because of market saturation, or increased competition.
Over the period 2008–2013, mAbs are forecast to serve as the key growth segment of the prescription pharmaceutical market, driven by expanding sales of existing products combined with sales of new products, as pharmaceutical firms are continuing to actively invest in research and development (R&D) of therapeutic mAbs. During this period, revenues are anticipated to grow annually by 15%, compared to only 2% for the overall pharmaceutical market. Four players currently hold a dominant position in the global mAb market: Genentech, Roche, Johnson & Johnson and Abbott, accounting for a combined 75% share of mAb market revenues. In addition, large pharmaceutical firms have acquired a number of small companies that focus on mAb R&D. For example, Merck, GlaxoSmithKline, Eisai and Astellas acquired Abmaxis, Domantis, Morphotek and Agensys, respectively. In addition, Cambridge Antibody Technology and MedImmune were both acquired by AstraZeneca.6
Despite their uniqueness, competition is slowly emerging among mAbs, but also between mAbs and other targeted therapeutics in several therapeutic areas. To illustrate, panitumumab has entered the market in competition with both cetuximab and bevacizumab for the treatment of colon cancer. And, infliximab and adalimumab now compete with etanercept in the treatment of rheumatoid arthritis on the basis of effectiveness, safety and cost.7 Other targeted therapeutics that compete with mAbs in the therapeutic areas of rheumatoid arthritis, cancer and Crohn's disease, include etanercept, gefitinib, erlotinib, sorafenib, lapatinib and sunitinib.
The jury is still out as to whether competition will exert downward pressure on prices, or whether mAb prices will decrease as numbers of patients eligible for treatment increase, or as indications increase. In the face of public backlash, some companies have instituted financial caps on what patients will pay per year for some of their drugs. Genentech, for instance, has placed a cap of $55,000 per year on the total cost of bevacizumab for patients below a certain income level, provided the drug is used for any of the three tumors for which it has FDA approval.8 Similarly, Amgen recently instituted an annual cap for patient co-payments on its colon cancer drug panitumumab, which it priced at a 20% discount relative to cetuximab, hoping to secure a competitive advantage.9 Amgen also developed a patient assistance program that provides panitumumab at no cost to uninsured patients with household adjusted annual gross income of up to $75,000.
mAb market access.
Given their high price tag, the market for mAbs exists primarily where health plans are able to spread the high costs across a large group of insured people.
Price controls.
Prices of mAbs are relatively high due to their relative effectiveness, a low degree of competition among mAbs, and the fact that there is not (yet) a bio-generic pathway. Comparatively high prices notwithstanding, universally applied price control measures are unlikely in the US in the short term. Nevertheless, mAbs' relatively high per patient cost implies they will be subject to considerable payer scrutiny in their coverage decision-making process. In a relatively uncompetitive market lacking generic biologics and without government-imposed price controls, health plans have few opportunities to negotiate reduced pricing, therefore other strategies must be devised to manage use.
Medicare formulary management.
Medicare is the largest market for mAbs, as diseases targeted by mAbs are much more prevalent among the Medicare than non-Medicare populations.10 mAbs are primarily managed by a trio consisting of the agency responsible for overseeing and administering Medicare and Medicaid, the Centers for Medicare and Medicaid Services (CMS), Medicare Part B carriers, and Medicare Part D prescription drug plans and Medicare Advantage plans.
Before implementation of the Part D prescription drug benefit in 2006, Medicare covered only a small percentage of outpatient drugs. However, the drugs it covers under Part B are used to treat patients with very serious medical conditions, such as cancer, hemophilia and rheumatoid arthritis. Approximately 5% of Part B drugs are mAbs.
MAbs are considered so-called specialty drugs. There is no cut-and-dried definition of a specialty drug, though its defining features include high cost, biotechnological derivation and need for close supervision and monitoring. As specialty drugs, mAbs fall into one of two categories based on the site and mode of administration: those that can be self-administered by the patient and those that require a health care professional to administer them in a physician's office or infusion center, corresponding to Medicare Part D and B, respectively.
About 90% of current mAbs and those in late-stage development require administration by injection or infusion, and 70% must be administered by a health care professional. Of the 22 approved MAbs, 82% are covered under Medicare Part B, and 18% under Part D. It should be noted that mAb immunosuppressants are covered under Part B for Medicare-covered transplant patients, and Part D for all other situations.11 Many plans are instituting a policy in which Medicare Part D drugs that may also be covered under Medicare Part B will require prior authorization to determine coverage. Prior authorization criteria include diagnosis criteria, which identify the indications for which the drug may be used (both FDA approved and off-label uses), prescriber criteria, which identify which health care professionals are authorized to prescribe specific drugs and drug-specific criteria, which identify approved doses, frequency of dosing, and duration of therapy.
By law, CMS, Medicare Part B carriers and Part D plans have the authority to evaluate whether medical technologies are “reasonable and necessary” and should be paid for. They must continually balance the tension between providing timely access to medical innovations and controlling costs. Between 1997 and 2006, Medicare spending on drugs covered under Part B grew on average annually at close to 20%.12 Medicare Part B drug spending now accounts for almost 5% of overall Medicare expenditures, while implementation in 2006 of Part D raised the percentage that Medicare spends on drugs and biologics to 15% of its overall budget.13 In response, Medicare carriers and Part D plans have diverged from their historical tendency to provide unconditional coverage. Most of the hundreds of thousands of health care services provided by Medicare are not subject to specific coverage policies. However, Medicare now has thousands of local coverage determinations (LCDs) conducted by regional Medicare carriers, and a growing number of national coverage determinations (NCDs) for especially contentious cases; about 10 to 15 NCDs per year. Many LCDs, but also some NCDs concern drugs and biologics. In very few instances is coverage denied outright. However, in some instances, LCDs and NCDs limit or condition coverage for certain drugs by subpopulation, specific indication and setting. There can be significant differences in the management of individual drugs and biologics by regional Medicare carriers, as our empirical analysis will demonstrate.
Methods
To inform our discussion of CMS, Part B carrier and Part D plan formulary management, we reviewed coverage of all 22 FDA-approved mAbs for all FDA-approved indications, including supplemental indications. Specifically, we examined whether a drug was covered, the time from FDA approval to reimbursement coding, and cost sharing. We could not gauge this for Part D mAbs, but for Part B mAbs we used the proxy time between approval and assignment of a so-called J-code or identifying number for reimbursement. We also examined formulary management tools at payers' disposal to control cost growth and promote appropriate prescribing patterns, including:
Indication restrictions;
Prior authorization;
Step therapy;
Quantity limits.
Here, step therapy is the practice of beginning drug therapy for a medical condition with the most cost-effective and safest drug therapy and progressing to other more costly or risky therapy, only if necessary. Our assessment was based on a review of pertinent NCDs, LCDs and Part D plan formularies.
NCD and LCD review methodology.
The CMS Coverage Database is a searchable internet-based database that archives all active LCDs and related articles as well as active and proposed NCDs and other documents. The keywords we used to search the LCD and NCD database included specific generic and trade names (e.g., “bevacizumab” and “Remicade”) for each mAb. We included all LCDs with a unique identification number that reference a specific mAb in the title. However, there were exceptions. In some instances, a contractor issued the same coverage determination for the same geographic jurisdiction as both a carrier and fiscal intermediary. Here, we only included the LCD issued by the carrier. Also, contractors may develop a single, broadly defined LCD and issue multiple “policy articles” that refine coverage for individual drugs or procedures. We identified four policy articles for four different mAbs where this was the case and treated each as an individual LCD. We examined each LCD with respect to the following formulary management tools:
Indication Restrictions—We included any stated criteria to stratify patients and determine treatment eligibility, such as platelet counts, serum levels or clinical practice guidelines that recommend utilization to particular subpopulations;
Prior authorization;
Step therapy;
Quantity limits.
For three of the four Part D mAbs, we also reviewed all 44 4-tier formularies designed by the 18 leading Medicare prescription drug plans on the CMS “formulary plan finder.”14 These were made up of 37 4-tier formularies and 7 5-tier formularies with identical cost-sharing rates in tiers 4 and 5. For the latter group, these tiers were combined and counted as 4-tier plans. Tier 4 systems have now been incorporated in 86% of plans that participate in the Medicare drug benefit, and 15% of private commercial plans.15
We also examined LCDs for mentions of off-label restrictions. LCDs may exclude off-label coverage or restrict off-label coverage to one or more “commonly accepted” off-label uses.
Results
There are approximately 60 regional Medicare Part B carriers. Table 5 shows that eleven of the carriers issued LCDs for at least one of 12 FDA-approved mAbs. Not a single LCD denied coverage for on-label indications, nor was step therapy or prior authorization applied to any mAb. Here, we assume that carriers that did not issue LCDs imposed no conditions of reimbursement. Across all 18 Part B mAbs, less than three percent of carriers imposed conditions of reimbursement. However, five of the mAbs had on-label indication restrictions applied by between one and three carriers, and two had quantity limits applied by three carriers.
Table 5.
Local coverage determinations (LCDs) issued for 12 mAbs
| MAb | Number of LCDs | IR | QL | Off-label use | |
| LC | NC | ||||
| Bevacizumab | 3 | - | - | 3 | - |
| Tositumomab | 3 | 3 | 3 | - | 3 |
| Alemtuzumab | 1 | - | - | - | 1 |
| Cetuximab | 1 | - | - | 1 | - |
| Trastuzumab | 1 | - | - | 1 | - |
| Ranibizumab | 2 | - | - | - | 2 |
| Gemtuzumab | 1 | - | - | - | 1 |
| Infliximab | 10 | 2 | - | 6 | 4 |
| Rituximab | 4 | 1 | - | 4 | - |
| Panitumumab | 1 | - | - | - | 1 |
| Omalizumab | 3 | 2 | - | - | 3 |
| Ibritumomab | 3 | 2 | 3 | - | 3 |
| Total | 33 | 10 | 6 | 15 | 18 |
IR, indication restrictions; QL, quantity limits; LC , limited coverage; NC, no coverage.
Seventy-five percent of the LCDs we examined pertained to off-label uses. Eight mAbs were denied coverage of off-label uses by at least one carrier. Not a single LCD allowed for all (compendium) off-label uses. In fact, at least one LCD recommended against off-label use altogether for eight of the 12 mAbs, and limited coverage to “accepted uses” for five of the 12 mAbs. Overall, however, across all 18 Part B mAbs, only three percent of carriers either denied off-label use coverage outright or restricted its use.
Further, we found that LCD policies on off-label use reimbursement differ significantly from carrier to carrier. They cited different compendia and recommended different sets of indications. These results are corroborated in a recent Tufts CSDD survey study on off-label reimbursement among 33 payers administering pharmacy benefits in the public sector. There, respondents reported divergent off-label use policies, as well as the use of a variety of sources to reach decisions on off-label reimbursement.
Note that use of Part B drugs by beneficiaries enrolled in carriers without LCDs is not entirely uncontrolled. Medicare has the authority to do post-hoc review based on general principles of medical necessity and community practice.
We found two NCDs that evaluate mAb off-label uses. First, in January 2005, CMS published an NCD for anti-cancer therapies for colorectal cancer. CMS determined that the use of irinotecan, oxaliplatin, cetuximab and bevacizumab, will be covered for off-label indications in clinical trials identified by CMS and sponsored by the National Cancer Institute.16 Medicare contractors will continue to make “reasonable and necessary” coverage determinations for medically accepted uses of off-label indications of the colorectal chemotherapeutic agents outside of the identified clinical trials. Second, in July 2002, CMS conducted a three-year review of two radiopharmaceuticals that target Non-Hodgkins lymphoma: ibritumomab and tositumomab. Based on the review, CMS decided to leave off-label uses to local contractors.17
For Part D drugs, we found that most plans covered all three mAbs, yet 16% did not cover efalizumab and omalizumab. We excluded palivizumab from our formulary analysis, as it is indicated in pediatric patients, a non-Medicare population. Nonetheless, it is placed on slightly more than half of Part D formularies.
About 85% of mAbs covered under Part D belong to the fourth (specialty) tier, and 95% were designated to co-insurance tiers. While Part B beneficiary cost sharing is 20%, Part D plans impose higher co-insurance percentages, ranging from 25% to 75%. And, for the 5% of Part D mAbs that were placed into co-payment tiers, there is a similarly high degree of variation across co-payments; from $0 to $107 per prescription. We also found that plans impose a very high percentage of restrictions on Part D mAbs (85%). Notably, as with Part B mAbs, we found no use of step therapy as a condition of reimbursement for Part D mAbs. Part D plan formularies did not include off-label uses of Part D drugs. Furthermore, none of the Part D plans report specific off-label use policies. Anecdotal evidence as well as mounting concerns among beneficiary advocates suggests that Part D plans impose more onerous restrictions on off-label uses than Part B carriers.18 When we add together the Part B and Part D mAbs, we observe that an average of 14% of carriers and plans imposed conditions of reimbursement.
For 18 Part B mAbs, we found an average delay of 14 months between FDA approval and reimbursement coding. This delay between approval and reimbursement coding usually does not lead to an actual delay in reimbursement. This is because during the phase when a specific reimbursement code is not available, carriers typically use a non-specific code for billing and reimbursement purposes. This said, temporary coding can lead to confusion on the part of providers with respect to the size and number of vials reimbursed, for example, or the fact that multiple drugs may be assigned the same code. There is no information available about delays between FDA approval of Part D mAbs and reimbursement coding.
Challenges and Policy Implications
Our findings point to four major challenges facing mAb developers, health care providers, Medicare beneficiaries, payers and policymakers:
Administrative price controls;
Variation in reimbursement policies across Medicare Part B carriers and Part D prescription drug plans;
Projected shift from physician- to self-administered mAbs; more restrictions on Part D than Part B drugs;
Comparative effectiveness.
Administrative pricing.
Though at present few government-imposed price controls are in place for biopharmaceuticals, CMS is establishing a precedent, particularly among Part B biologics. In 2003, CMS set darbepoeitin-alfa's reimbursement rate equal to epoeitin-alfa, effectively reducing payment in half. CMS justified its decision on the grounds that darbepoetin-alfa was “functionally equivalent” to epoetin-alfa. Here, functional equivalence implied both products use the same biological mechanism to produce the same clinical result, with no important differences in side effects.19 Congress barred CMS from applying functional equivalence policy to other Part B drugs and biologics. Nonetheless, depending on changing political priorities and preferences, this policy stance may soon be revisited.20
Moreover, recent administrative rulings by CMS on the prices of several high-profile drugs, medical devices and diagnostics indicate price controls are being employed more frequently. For example, in early 2008 a CMS ruling administratively set the reimbursement rates for the radiopharmaceutical mAbs ibritumomab and tositumomab at half the 2007 rate.21 CMS calculated each price based on hospital data and a series of fixed rules at its disposal. This decision caused considerable consternation among patients, hospital administrators, and the biopharmaceutical industry.22
The use of price controls may be ill advised, as they can have unintended consequences. As was the case with darbepoetin-alfa, and now applies to ibritumomab and tositumomab, CMS is the single largest purchaser of these products. As a monopsonist, it exerts significant influence on the entire market for these products. If CMS sets prices too low, hospitals and physicians may balk at providing these products to patients. On the other hand, if CMS sets prices too high, the products may be unaffordable to CMS, or increase the financial burden confronting taxpayers and those paying Medicare premiums.
Variation in payer reimbursement policies.
Given the cost pressures and the emerging intra-mAb competition, as well as between mAbs and other therapeutics, firms developing new biologics will increasingly have to differentiate their products by demonstrating their value to payers. Our study shows that payers value mAbs differently. Though mAb coverage of on-label indications is 97% across Part B carriers and Part D plans, there is variation among Part D plans. In addition, carriers and plans vary considerably in terms of the conditions they impose on mAb reimbursement. While the percentage of carriers imposing such conditions is low (3%) for Part B mAbs, it is very high among Part D plans (85%). We also observed highly variable cost sharing arrangements for mAbs.
Owing to price-inelastic demand for most mAbs, relatively high cost sharing will likely not reduce their use, but will transfer a greater share of their costs to patients.23,24 Most mAbs cost more than $1,000 per dose and most require at least six doses annually. Consequently, the majority of Medicare beneficiaries being prescribed mAbs will reach the doughnut hole or gap in coverage between the initial coverage limit ($2,500) and the catastrophic coverage threshold ($5,720). Within this gap, the beneficiary pays 100% of the cost of prescription drugs before catastrophic coverage kicks in. CMS guidelines recommend Part D cost sharing for the specialty tier to be set no higher than 25%, but higher cost sharing is allowed if it is offset by lower deductibles.
The formulary management tools currently being used appear to be rather blunt instruments, not necessarily reflective of cost, cost-effectiveness or differences in clinical effectiveness and safety profiles. Consider, for example, bevacizumab and trastuzumab, indicated for breast cancer. Both drugs cost roughly the same per treatment cycle. Bevacizumab slows the progression of metastatic breast cancer, but studies show it has no effect on overall survival.25 Despite significant differences in clinical effectiveness, payers manage both drugs similarly.
Additionally, off-label use reimbursement, especially relevant to mAbs, constitutes a major challenge.26 We have seen that Part B carriers have divergent off-label use policies. Some do not cover certain off-label uses at all, which appears to be in contravention with legislative statutes. Also, carriers cite different compendium sources for off-label use reimbursement. Legislation at the federal and state levels has attempted to minimize this variation. As a result, part of what Medicare deems permissible in terms of off-label use reimbursement is now statutorily determined:
Statutory language in Section 1861(t)(1) and (2) of the Social Security Act stipulates Medicare must reimburse off-label indications of all anti-cancer drugs covered under Part B, so long as off-label indications are included in officially recognized pharmaceutical compendia, as well as peer-reviewed literature. CMS has added ten peer-reviewed journals to be considered in making coverage determinations for off-label use of anti-cancer drugs under Part B.27 In addition, 39 states mandate payment by all Employment Retirement Income Security Act (ERISA) plans for FDA-approved cancer drugs prescribed for off-label indications, so long as they appear in officially recognized compendia.
A patchwork quilt of different laws and regulations at both the state and federal levels result in inconsistent requirements based on the type of plan, geography or class of drug. These statutory mandates reflect the persistence of inconsistent public policy measures. First, while anti-cancer drugs are included in the statutory requirements, other drugs are not. Second, while almost 80% of states have instituted mandates for ERISA plans to pay for anti-cancer drugs, non-ERISA plans are exempt. And third, in apparent conflict with the statutory language from Section 1861(t)(1), Part D regulations prohibit coverage of off-label uses that are not listed in recognized compendia, even if the use is supported by evidence in peer-reviewed clinical research. We do not know with certainty whether Part D plans have erected barriers to compendium- or peer-reviewed recommended off-label uses. However, the law contains no provisions that would prevent plans from limiting coverage to on-label uses. Indeed, beneficiary advocates maintain that coverage restrictions on off-label uses recommended in the peer-reviewed literature but not listed in the officially recognized drug compendia may lead to denial of coverage.28
Projected shift from physician- to self-administered mAbs.
Until recently, utilization and cost management of specialty drugs was not subject to oversight by a pharmacy benefit manager. As a consequence, use of specialty drugs, such as mAbs, was relatively unrestricted and unmanaged. This is changing, as our analysis demonstrates. And, this is influenced by the growth in specialty drug spending, the availability of integrated computerized data monitoring, and the shift from medical to pharmacy benefits management, for a number of specialty drugs.
Formally, Part B (medical) claims processing pairs each drug with a disease code. For example, Part B will pay for infliximab if prescribed for rheumatoid arthritis, while it would deny reimbursement of infliximab if prescribed for schizophrenia. However, as claims are submitted after a drug has been prescribed and dispensed, and not in real-time electronic format, the accurate tracking and enforcement of conditions of reimbursement is much more difficult unless a carrier authorizes a formal audit of physicians' records.
Having a drug processed through Part D (pharmacy) facilitates the use of conditions of reimbursement “at the claims processor level.”29 This is consistent with what we have seen in terms of Part D mAbs being assigned far more conditions of reimbursement than Part B mAbs. Plans, in fact, may give preferential formulary status to self-administered biologics. To illustrate, in a direct comparison between etanercept and infliximab, one plan identified etanercept as the preferred biologic because of its “fixed 25 mg dose, fixed twice-weekly dosing interval, subcutaneous route of administration, lack of mandated methotrexate co-therapy, availability through retail community pharmacies, and billing through the pharmacy claims system.”30
There is a trend towards more self-administered biologics. As this shift intensifies, there will likely be more four-tier design. Currently, approximately 90% of Part D plans have four-tier formularies. Those that do so charge an average of 33% co-insurance for specialty drugs, which is much higher than the 20% Medicare Part B co-insurance.31 Over the course of long-term treatment, self-administration and ease of use may positively impact patient compliance. However, as more mAbs move to Part D, a qualitatively different access problem we alluded to will arise having to do with lack of coverage in the doughnut hole.
Unlike Part D, Part B does not have a catastrophic cost category, limiting out-of-pocket costs to 5%. Due to Part D's catastrophic coverage, patient cost sharing for high cost drugs can be more, less or similar to Part B, depending on the full cost of the drug, and Part D plan characteristics. Since not all Part D plans cover all specialty drugs, as we have seen, patients may seek injections at a physician's office (covered under Part B) when a self-administered injectable would be medically appropriate and less costly.
Comparative effectiveness.
With Congressional legislation on comparative effectiveness being debated vigorously among lawmakers, policymakers and the public are asking difficult questions about the value of biopharmaceuticals, and comparatively expensive mAbs in particular.32 Questions raised in a New York Times feature article entitled “What does it mean to say an expensive drug works?” include:33 Is slowing the growth of tumors enough if life is not significantly prolonged or improved? How much evidence must there be before billions of dollars are spent on a drug? Who decides? When, if ever, should cost come into the equation?
When anti-cancer drugs, such as trastuzumab, are first introduced they are usually labeled for use in advanced or metastatic disease, which may only offer patients a few additional months of life. Such minor extensions of life represent a statistically valid endpoint and will satisfy regulators, but may not be sufficient for payers, who ideally want more information on a drug's comparative (cost) effectiveness. At the individual payer level, comparative effectiveness is gradually becoming a reality. For example, one prominent plan carried out its own study and concluded that omalizumab should have tight prior authorization controls on its use, because it provides “only marginal incremental clinical benefits over existing options, but at a substantially increased cost.”34 Specifically, this plan designed prior authorization criteria that require a patient to have an inadequate response to existing options (e.g., inhaled corticosteroid) and evidence of increased resource utilization, such as emergency room visits or hospitalization, prior to omalizumab being reimbursed. Several of the LCDs we reviewed appear to be based on similarly derived comparative effectiveness outcomes.
Some payers are saying they are not willing to wait until FDA approval before at least estimating a drug's comparative effectiveness.35 To meet this challenge more payer data requirements related to comparative (cost) effectiveness will need to be incorporated in clinical trial design, particularly during Phases II and III. In turn, this could lead to early termination decisions in cases of drugs with poor comparative effectiveness prospects, while in others with better estimates it may have the opposite effect. Pre-approval assessments could also serve to reduce the time delays between FDA approval and reimbursement coding, which may impede market access. Typically, it takes about one year after licensing for CMS to grant the code necessary for hospital outpatient reimbursement under Medicare. Drugs that do not yet have an HCPCS code may obtain a temporary code and therefore be reimbursable in the interim. However, it is important that a “critical path for reimbursement” be established, one that clearly delineates a set of procedures and policies that would result in a more efficient reimbursement decision-making process.36 The FDA has analogous mechanisms for designating technologies that have potentially significant impact on public health to qualify for special attention during regulatory review. It may be reasonable to assume that all or most mAbs would merit such special attention during reimbursement review.
Given that clinical, economic and quality-of-life data will need to be generated to support comparative effectiveness studies, do multiple data assessments make sense? Where there is discretion, drug manufacturers usually prefer to let Medicare's regional carriers make the call at the local level, rather than risk an all or nothing national determination. But, there are legislative efforts calling for consolidation of information gathering and an injection of more uniformity into third party payer reimbursement policies, preferably based on federally sponsored comparative effectiveness research.
Law and policymakers should, however, take heed and draw lessons from controversies surrounding certain decisions made by the British National Institute for Healthcare and Clinical Excellence (NICE). NICE is responsible for providing guidance to providers and National Health Service trusts in England and Wales on utilization and reimbursement of selected new technologies, including many newly approved drugs. NICE bases its decisions on rigorous analyses of comparative cost-effectiveness data. About 90% of NICE's analyses have resulted in positive recommendations. However, in the case of mAbs, NICE has recommended against or severely restricted the use of natalizumab, adalimumab, rituximab, bevacizumab and cetuximab.37 Some suggest NICE has been too rigid in its application of cost-effectiveness thresholds.38
One mechanism that has shown some promise in terms of promoting innovation, while acceding to demands on improving access and containing the growth in prescription drug expenditures, is coverage with evidence development. In certain instances, Medicare has conditionally covered promising but unproven novel technologies in indications characterized by limited treatment options.39 Medicare could more systematically pursue this policy of conditionally covering the costs of on- or off-label uses of certain new technologies, provided evidence is gathered on their risk, benefit and cost profiles in officially designated clinical trials. MAbs certainly fit the bill of “promising but unproven novel technologies.” Once the trials are completed and the data analyzed, Medicare could revisit its reimbursement policy and adjust accordingly. Such a policy envisions ways of developing more evidence on risks and benefits in actual practice for technologies that are generally shown to be safe and efficacious in clinical trials, but for which there is still insufficient information for answering practical questions, such as effects in certain subgroups of patients, effects in settings that differ from those in the trials, and risks and benefits of off-label uses.40
Table 4.
Global sales of Top 7 mAbs (2007)
| Brand name | Total sales (billions) |
| Remicade | $5.3 |
| Rituxan | $5.2 |
| Herceptin | $4.0 |
| Avastin | $3.4 |
| Humira | $3.1 |
| Erbitux | $1.3 |
| Synagis | $1.2 |
Source: Med Ad News 2008.
Table 6.
Formulary placement, conditions of reimbursement and cost-sharing on 44 prescription drug plan 4-tiered formularies for self-administered mAbs (% of plans)
| Tier placement | Coverage restrictions | |||||||
| NC | 1 | 2 | 3 | 4 | QL | PA | ST | |
| MAbs | ||||||||
| Adalimumab | - | - | 2% | - | 98% | 52% | 89% | - |
| Efalizumab | 16% | - | - | - | 84% | 48% | 66% | - |
| Omalizumab | 16% | - | 7% | 2% | 75% | 11% | 73% | - |
| Cost Sharing: | ||||||||
| Co-pay | ||||||||
| Median | $5 | $30 | $66.58 | - | ||||
| Min | $0 | $20 | $42.50 | - | ||||
| Max | $9 | $45 | $107 | - | ||||
| Co-insurance | ||||||||
| Median | 25% | 25% | 30%** | 28% | ||||
| Min | 23% | 23% | 25% | 25% | ||||
| Max | 25% | 35% | 75% | 33% | ||||
NC, not covered; QL, quantity limits; PA, prior authorization; ST, step therapy. **Mean = 39%.
Table 7.
Time between FDA approval and HCPCS* code assignment
| Generic name (Trade name) | Date of 1st FDA approval | Date 1st HCPCS code added | Delay (months) |
| Abciximab (Reopro) | 12/22/1994 | 1/1/1999 | 48 |
| Adalimumab (Humira) | 12/31/2002 | 1/1/2005 | 24 |
| Alemtuzumab (Campath-1H) | 5/7/2001 | 1/1/2003 | 19 |
| Basiliximab (Simulect) | 5/12/1998 | 7/1/2000 | 25 |
| Bevacizumab (Avastin) | 2/26/2004 | 1/1/2005 | 10 |
| Certolizumab pegol (Cimzia) | 4/22/2008 | Not yet added | Not applicable |
| Cetuximab (Erbitux) | 2/12/2004 | 1/1/2005 | 10 |
| Daclizumab (Zenapax) | 12/10/1997 | 1/1/1999 | 12 |
| Eculizumab (Soliris) | 3/16/2007 | 10/1/2007 | 7 |
| Efalizumab (Raptiva) | 10/27/2003 | 4/1/2004 | 5 |
| Gemtuzumab ozogamicin (Mylotarg) | 5/17/2000 | 1/1/2002 | 19 |
| Ibritumomab tuixetan (Zevalin) | 2/19/2002 | 1/1/2003 | 10 |
| Infliximab (Remicade) | 8/24/1998 | 1/1/2000 | 16 |
| Muromonab-CD3 (Orthoclone-Okt) | 6/19/1986 | 1/1/1988 | 18 |
| Natalizumab (Tysabri) | 11/23/2004 | 1/1/2005 | 1 |
| Omalizumab (Xolair) | 6/20/2003 | 1/1/2004 | 6 |
| Palivizumab (Synagis) | 6/19/1998 | 10/1/2001 | 39 |
| Panitumumab (Vectibix) | 9/27/2006 | 1/1/2007 | 3 |
| Ranibizumab (Lucentis) | 6/30/2006 | 1/1/2007 | 6 |
| Rituximab (Rituxan) | 11/26/1997 | 1/1/1999 | 13 |
| Tositumomab-I131 (Bexxar) | 6/27/2003 | 7/1/2003 | <1 |
| Trastuzumab (Herceptin) | 9/25/1998 | 1/1/2000 | 15 |
Healthcare Common Procedure Coding System. Sources: FDA; CMS, 2008 HCPCS file.
Footnotes
Previously published online as a mAbs E-publication: http://www.landesbioscience.com/journals/mabs/article/7246
References
- 1.Culliton B. Promoting medical innovation while developing sound social and business policy: a conversation with Thomas G, Roberts. Health Affair. 2008;27:34–40. doi: 10.1377/hlthaff.27.1.w34. [DOI] [PubMed] [Google Scholar]
- 2.Reichert J, Wenger J. Development trends for new cancer therapeutics and vaccines. Drug Discov Today. 2008;13:30–37. doi: 10.1016/j.drudis.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Kaitin K, editor. Tufts Center for the Study of Drug Development Impact Report. 2008. Number of mAbs entering clinical study nearly tripled in last decade; p. 10. [Google Scholar]
- 4.Reichert J, Valge-Archer V. Development trends for monoclonal antibody cancer therapeutics. Nat Rev Drug Discov. 2007;6:349–356. doi: 10.1038/nrd2241. [DOI] [PubMed] [Google Scholar]
- 5.Reichert J. Trends in the development and approval of monoclonal antibodies for viral infections. BioDrugs. 2007;21:1–7. doi: 10.2165/00063030-200721010-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aggarwal S. What's fueling the biotech engine? Nat Biotechnol. 2007;25:1097–1104. doi: 10.1038/nbt1007-1097. [DOI] [PubMed] [Google Scholar]
- 7.Lipsy R. Injectable biologic case studies. J Manag Care Pharm. 2004;10:10–16. [PubMed] [Google Scholar]
- 8.Kloata G, Pollack A. Costly cancer drug offers hope, but also a dilemma. New York Times. 2008 [Google Scholar]
- 9.Farrington G, Hasson M, Garvey E, Caeser M. How to price cancer drugs. In Vivo. 2007;25:1–5. [Google Scholar]
- 10. Medicare Payment Advisory Commission: Medicare Part B drugs and oncology.
- 11. Centers for Medicare and Medicaid Services: Medicare Part B versus Part D issues.
- 12.Mullins CD, DeVries A, Van Doren Hsu, Meng F, Palumbo FB. Variability and growth in spending for outpatient specialty pharmaceuticals. Health Affair. 2005;24:1117–1127. doi: 10.1377/hlthaff.24.4.1117. [DOI] [PubMed] [Google Scholar]
- 13.Kaiser Family Foundation: Medicare spending and financing. [2008]. See http://www.amsa.org/business/Medicare%20Report.pdf.
- 14.Hoadley J, Hargrave E, Cubanski J, Neuman T. An in-depth examination of formularies and other features of Medicare drug plans. Kaiser Family Foundation. [2008]. See http://www.kff.org/medicare/7489.cfm.
- 15.Kolata G. Co-payments go way up for drugs with high prices. New York Times. 2008 [Google Scholar]
- 16.Carino T, Williams R, Colbert A, Bridger P. Medicare's coverage of colorectal cancer drugs:a case study in evidence development and policy. Health Affair. 2006;25:1231–1239. doi: 10.1377/hlthaff.25.5.1231. [DOI] [PubMed] [Google Scholar]
- 17.Keenan P, Neumann P, Phillips K. Biotechnology and Medicare's new technology policy: lessons from three case studies. Health Affair. 2006;25:1260–1269. doi: 10.1377/hlthaff.25.5.1260. [DOI] [PubMed] [Google Scholar]
- 18.Ratner M, Gura T. Off-label or off-limits? Nat Biotechnol. 2008;26:867–875. doi: 10.1038/nbt0808-867. [DOI] [PubMed] [Google Scholar]
- 19.Lichtenberg F. Did CMS' functional equivalence decision result in equitable payments? J Pharm Financ Econ Policy. 2006;15:7–20. [Google Scholar]
- 20.Kanavos P, Saka O. How should functionally equivalent drugs be reimbursed? A retrospective analysis of reimbursement for epoetin-alfa and darbepoeitin-alfa in 2001–2003 and the cost implications for CMS. Dis Manag Health Out. 2005;13:359–370. [Google Scholar]
- 21.Centers for Medicare and Medicaid Services: CMS-1392-FC Medicare program: changes to the hospital outpatient prospective payment system and CY 2008 payment rates. [2008]. See http://www.cms.hhs.gov/eRulemaking/downloads/CMS-1392-FCPC36-48.pdf.
- 22.Alter J. How Washington is nixing a cancer cure. Newsweek web exclusive. [2008]. See http://www.newsweek.com/id/70301/output/print.
- 23.Goldman D, Joyce G, Lawless G, Crown W, Willey V. Benefit design and specialty drug use. Health Affair. 2006;25:1319–1331. doi: 10.1377/hlthaff.25.5.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hargrave E, Hoadley J. Coverage and pricing of drugs that can be covered under Part B and D. MedPAC, October 2007. Medicare Payment Advisory Commission. Report to the Congress. [Google Scholar]
- 25.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. NEJM. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 26.Biotechnology Monitor & Survey 2008: Marketplace Policies, Practices and Perspectives. http://www.biotechmonitor.com/publication/index.html.
- 27.Medicare Part D drug plans should be able to consider a variety of evidence supporting off-label us, not just the standard medical compendia, a lawsuit challenging CMS interpretation argues. Pink Sheet. 2007;69:9. [Google Scholar]
- 28.Government Accounting Office: Medicare Part D: Plan sponsors' processing and CMS monitoring. [2008]. See http://www.gao.gov/new.items/d0847.pdf.
- 29.Lipsy R, Fuller M, Roski J, Mansukani S. Anticipating the future: how the emergence of innovative biologic agents impacts benefit design, utilization and provider relations. J Manag Care Pharm. 2004;10:4–9. doi: 10.18553/jmcp.2004.10.S3-a.S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silverman E. What do payers really want to see? J Commer Biotechnol. 2008;14:65–72. [Google Scholar]
- 31.Hoadley J, Hargrave E, Cubanski J, Neuman T. Kaiser Family Foundation, author. An in-depth examination of formularies and other features of Medicare drug plans. [2007]. See http://www.kff.org/medicare/7489.cfm.
- 32.Polata G, Pollack A. Costly cancer drug offers hope, but also a dilemma. New York Times. 2008 [Google Scholar]
- 33.Anand G. As costs rise, new medicines face pushback. Wall Street Journal. 2007 [Google Scholar]
- 34.Lipsy R. Injectable biologic case studies. J Manag Care Pharm. 2004;10:10–16. [PubMed] [Google Scholar]
- 35.Congressional Budget Office: Research on the comparative effectiveness of medical treatments. 2007. Dec, [2008]. See http://www.cbo.gov/ftpdocs/88xx/doc8891/12-18-ComparativeEffectiveness.pdf.
- 36.National Venture Capital Association: A proposal for a reimbursement ”Critical Path“ for CMS. [2008]. See http://www.nvca.org/pdf/CMS-critical-path.pdf.
- 37.Moran N. Priced out of the UK market. Nat Biotechnol. 2008;26:151–154. doi: 10.1038/nbt0208-151. [DOI] [PubMed] [Google Scholar]
- 38.Devlin N, Appelby J, Parkin D. Patients' views of explicit rationing: what are the implications for health service decision-making? J Health Serv Res. 2003;8:183–186. doi: 10.1258/135581903322029557. [DOI] [PubMed] [Google Scholar]
- 39.Hutton J, Trueman P, Henshall C. Coverage with evidence development: an examination of conceptual and policy issues. Int J Technol Assess. 2007;23:425–435. doi: 10.1017/S0266462307070651. [DOI] [PubMed] [Google Scholar]
- 40.Gottlieb S. Opening Pandora's pillbox: using modern information tools to improve drug safety. Health Affair. 2005;24:938–948. doi: 10.1377/hlthaff.24.4.938. [DOI] [PubMed] [Google Scholar]