Abstract
Spermatogenesis is a highly complicated process in which functional spermatozoa (haploid, 1n) are generated from primitive mitotic spermatogonia (diploid, 2n). This process involves the differentiation and transformation of several types of germ cells as spermatocytes and spermatids undergo meiosis and differentiation. Due to its sophistication and complexity, testis possesses intrinsic mechanisms to modulate and regulate different stages of germ cell development under the intimate and indirect cooperation with Sertoli and Leydig cells, respectively. Furthermore, developing germ cells must translocate from the basal to the apical (adluminal) compartment of the seminiferous epithelium. Thus, extensive junction restructuring must occur to assist germ cell movement. Within the seminiferous tubules, three principal types of junctions are found namely anchoring junctions, tight junctions, and gap junctions. Other less studied junctions are desmosome-like junctions and hemidesmosome junctions. With these varieties of junction types, testes are using different regulators to monitor junction turnover. Among the uncountable junction modulators, nitric oxide (NO) is a prominent candidate due to its versatility and extensive downstream network. NO is synthesized by nitric oxide synthase (NOS). Three traditional NOS, specified as endothelial NOS (eNOS), inducible NOS (iNOS), and neuronal NOS (nNOS), and one testis-specific nNOS (TnNOS) are found in the testis. For these, eNOS and iNOS were recently shown to have putative junction regulation properties. More important, these two NOSs likely rely on the downstream soluble guanylyl cyclase/cGMP/protein kinase G signaling pathway to regulate the structural components at the tight junctions and adherens junctions in the testes. Apart from the involvement in junction regulation, NOS/NO also participates in controlling the levels of cytokines and hormones in the testes. On the other hand, NO is playing a unique role in modulating germ cell viability and development, and indirectly acting on some aspects of male infertility and testicular pathological conditions. Thus, NOS/NO bears an irreplaceable role in maintaining the homeostasis of the microenvironment in the seminiferous epithelium via its different downstream signaling pathways.
Key words: spermatogenesis, nitric oxide, nitric oxide synthase, integral membrane proteins, testes
Introduction
Among the organs in the mammalian body, testis is one of the exceptional organs having complex cellular structures and organization. After puberty, testis functions as a sperm producing factory, generating up to millions of spermatozoa on a daily basis through the entire adulthood.1 In order to fulfill its reproductive function, testis is compartmentalized into two broad partitions, the seminiferous tubules and the inter-tubular areas (Fig. 1).2,3 In the seminiferous tubules, the epithelium is physically divided into the adluminal compartment and basal compartment by the blood-testis barrier (BTB) which is constituted by adjacent Sertoli cells near the basement membrane.4,5 Different cell types situate in specialized testicular locations. Sertoli cells and assorted germ cell types namely spermatogonia, spermatocytes, and spermatids are found in the seminiferous epithelium.6 Myoid cells locate adjacent to the tubules and Leydig cells reside in the inter-tubular space known as the interstitium.2 Each cell type performs different function, however they are communicating with each other to share the core role in sperm production during spermatogenesis. Apart from that, the male sex hormone level namely testosterone in the systemic circulation is also produced and regulated by the Leydig cells in the testis via steroidogenesis. These processes cannot be fully executed, if they are not equipped with precisely regulated interactive mechanisms during spermatogenesis. In rodents, the germ-line lineage spermatogonia, initially residing on the basement membrane of the seminiferous epithelium must differentiate into preleptotene spermatocytes, which in turn, traverse the BTB at stages VII–VIII of the epithelial cycle to gain entry into the adluminal compartment for further development7 (Fig. 1). During this event of germ cell movement, Sertoli cells also play a paramount role in determining the molecular events of germ cell development, including mitosis, meiosis, cellular differentiation and transformation.8 Sertoli cells accomplish this in part by monitoring the assembly and disassembly of inter-Sertoli junctions in the testes and partly by initiating cross-talk with germ cells.9 For instance, Sertoli cells are equipped with certain architectural machineries, such as microtubules, that interact with the movement-associated germ cell proteins (e.g., motor proteins) to provide this coordination.9 As such, premature germ cell release from the epithelium will be prohibitive. To sustain the optimal germ cell population in the testis, more than half (∼75%) of the germ cells that are produced including spermatogonia, spermatocytes and spermatids undergo apoptosis, and are phagocytosed by Sertoli cells, thereby restricting the numbers of germ cells in the seminiferous epithelium. This spontaneous removal mechanism ensures that the limited resources from Sertoli cells (note: the number of Sertoli cells in adult rats remain the same throughout adulthood) are sufficient for germ cell development, and are within the capacity of the testes, by eliminating excessive germ cells to maintain germ cell quality.
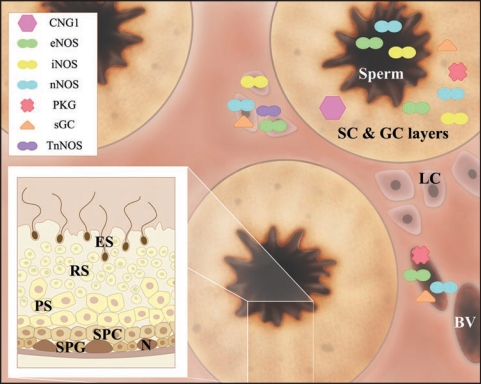
Figure 1.
Cellular localization of different NOS/NO signaling pathway components in the testes. Schematic drawing depicting the gross morphology of a testis cross-section. Seminiferous tubules are prominent structures found in the testes. Area surrounding the seminiferous tubules is termed interstitial area, which harbors Leydig cells and blood vessels. The inset diagram illustrates different germ cell layers of a seminiferous tubule. During spermatogenesis, spermatogonium migrates from the basal compartment to the adluminal compartment, during which spermatogonium differentiates into advanced germ cell types, namely preleptotene/leptotene spermatocytes, pachytene spermatocytes, round spermatids, and elongated spermatids, before spermatozoa are released into the tubule lumen. Components of the NOS/NO pathway, such as CNG1, eNOS, iNOS, nNOS, PKG, sGC, and TnNOS, are represented by the symbols tabulated. They are localized in specific locations within the testes, such as in the Sertoli cell and germ cell layers (SC & GC layers), sperm, Leydig cells (LC), and blood vessel (BV). Abbreviations used: BV, blood vessel; CNG1, cyclic nucleotide-gated channel 1; eNOS, endothelial NOS; ES, elongated spermatid; GC, germ cell; iNOS, inducible NOS; LC, Leydig cells; N, Sertoli cell nucleus; nNOS, neuronal NOS; PKG, protein kinase G; PS, pachytene spermatocyte; RS, round spermatid; N, Sertoli cell nucleus; sGC, soluble guanylyl cyclase; SC, Sertoli cells; SPC, preleptotene/leptotene spermatocyte; SPG, spermatogonium; TnNOS, testis-specific nNOS.
Nitric oxide (NO) is a free radical synthesized by nitric oxide synthase (NOS). NOS is composed of two identical monomers with molecular weights ranging from 130 to 160 kDa.10,11 Three isoforms of NOS, NOS I, II, and III, are known to date, which are the alternate names for neuronal NOS (nNOS) (Mr, ∼320 kDa), inducible NOS (iNOS) (Mr, ∼260 kDa), and endothelial NOS (eNOS) (Mr, ∼270 kDa), respectively.12 On the other hand, these NOS isoforms are functionally categorized into two groups, based on their intrinsic NO production efficacy. The constitutive group of NOS includes nNOS and eNOS, whereas iNOS belongs to the inducible form. Despite of these differences, all of them execute the same enzymatic reaction by converting L-arginine into NO and L-citrulline, using the cosubstrates of O2 and nicotinamide adenine dinucleotide phosphate (NADPH). In addition, tetrahydrobiopterin (BH4), flavin adenine dinucleotide (FAD), flavin mononucleotide (FMN), heme, calcium ions, and zinc ions are other necessary cofactors of this reaction.11,13 nNOS and eNOS usually synthesize NO in nanomole range, unlike the micromole range of NO generated by iNOS. Excessive level of NO, at >1 µM, has a direct detrimental effect on the physiological system, via the production of peroxynitrile, after interacting with oxygen or superoxide.14 In contrast, low concentration of NO, at <1 µM, works as an upstream regulatory molecule, goading the downstream signaling proteins, such as soluble guanylyl cyclase (sGC) that sequentially produces cGMP and activates protein kinase G (PKG).15,16 An inimitable form of nNOS is being documented as testis-specific nNOS (TnNOS).17 In short, the multifarious effects exhibited by NO are paramount in the general body metabolism. With the identification of a unique isoform of NOS, TnNOS, in the testis, an interest to examine the role of NOS and NO in spermatogenesis and steroidogenesis is expanding. As a complement to the earlier data regarding to the localization studies of NOS/NO and their related proteins and molecules, the functions of NOS/NO in spermatogenesis and steroidogenesis are being unfolded due to the establishment of several in vivo models or in clinical situations. Results derived from these studies advance the knowledge of NOS/NO in the testes and open the gate for the diagnosis and treatment of male reproductive dysfunctions.
NOS/NO in Junction Dynamics
Germ cells undergo various stages of differentiation during spermatogenesis, and these cells must also migrate from the basal compartment to adluminal compartment during maturation in the testis. To facilitate the course of germ cell movement, coordinated junction restructuring takes place between Sertoli and germ cells, resulting in the luminal release of spermatozoa. This phenomenon seems to be simple superficially; however, the mechanism(s) underneath is perhaps one of the most complicated procedures in the mammalian body. Unlike other endothelia and epithelia with assorted junction zones, seminiferous epithelium contains hybrid junctions instead.18–20 The most imperative exposition of this heterogeneity is the BTB, which encompasses a minimal of three junction types: the tight junction (TJ), the gap junction (GJ), and the adherens junction (AJ).20 As such, the passage of preleptotene spermatocytes through the BTB depends on the dynamic changes of the junction structures at the BTB. In the testes, junctions are broadly classified into three types, the anchoring junctions, the TJ, and the GJ. The former junction is further divided into two sub-groups, the actin cytoskeleton-based AJ, known alternatively as the ectoplasmic specialization (ES),3,9 and the intermediate filament-based. Fundamental structure of junction complex consists of integral membrane or transmembrane proteins, which are indirectly linked to the underlying cytoskeletons via adaptors. In addition, peripheral regulatory proteins, such as kinases or phosphatases, are also found in the vicinity or in direct contact with these junction complexes. Thus, controlling the spatial and physical distributions of these junctions and their status are one of the important tasks for the successful generation of spermatozoa in a timely manner. Among numerous signaling molecules, NOS/NO is one of the important regulators of the junction integrity in the seminiferous epithelium.21 The roles of NOS/NO in the junction dynamics are also implicated in other endothelia and epithelia. Below are discussions of the pertinent studies of these structural components in the testes and how NOS/NO alters the junction stability and integrity by affecting integral membrane proteins, adaptors, cytoskeletons, and regulatory proteins.
Integral membrane proteins.
Three types of transmembrane proteins in the testis have been extensively studied in recent years. First, occludins, claudins, and junctional adhesion molecules (JAM) are restricted to TJ at the BTB.4,21 Second, connexins are the known GJ proteins in the testes, with their distributions from the basal to the adluminal compartment in the seminiferous epithelium.20,22 Third, different assortment of proteins in anchoring junctions.9 Anchoring junctions are further divided according to their cytoplasmic associated cytoskeletons that serve as their attachment sites. Cadherins are the prominent proteins located between cells at the sites of actin-based AJ, such as basal ES at the BTB;9 whereas integrins are found between cells and extracellular matrix at the sites of focal adhesion as well as at the Sertoli-germ cell interface at the apical ES. The structures and the components of these testicular junctions are resemblance of other epithelia in mammals. However, their relative locations are unique in the testes. For instance, ES is a testis-specific AJ type, but it is also a hybrid anchoring junction having the properties of both AJ and focal adhesion.18,19 The physiological basis of these unusual features are currently unknown but recent studies have illustrated that there are cross-talk between different junction types in the seminiferous epithelium, which is mediated by the associated adaptors and/or the underlying cytoskeletons.19,20
Adaptors.
Adaptors are proteins that originally thought to have a restricted function by maintaining junction integrity in which they link the transmembrane proteins to the underlying cytoskeletons, such as actin, microtubule, or intermediate filament. Subsequent studies in recent years have shown that adaptors are functionally much more diversified than initially conceived, such as by recruiting signaling molecules (e.g., kinases, phosphatases) to the junction site and by mediating cross-talk between different junctional protein complexes in the testes.19 These expanded functionalities of adaptors are not entirely unexpected in view of their widespread occurrence in different junctional complexes. Amidst all the currently known adaptors, ZO-1 is known for its ability to conjoin occludin and connexin and it is found in both TJ and GJ.19 As such, one junction type (e.g., TJ) can impose indirect influence to other junctions (e.g., GJ) via a common adaptor (e.g., ZO-1). This view has been validated in at least two other studies in the testis. When rats were treated with a blocking connexin peptide, disruption of connexin functions using pan-connexin peptide renders the disruption of occludin-based TJ, but not N-cadherin-based AJ in the testes.23 This proves the vulnerability of the occludin-associated complex following a dysfunction of connexin-based GJ, since pan-connexin peptide with sequence conserved among all connexins virtually blocks all connexin-associated functions in the testes.23 In addition, the structural association of γ-catenin, an AJ adaptor, and ZO-1, a TJ adaptor, is weakened after treatment of rats using adjudin, which is shown to have a role in disturbing the AJ without compromising the TJ in the testes.3,24 These findings substantiate the significance of adaptors that mediate cross-talk between different junction types in the testes. Apart from that, other functions are recently uncovered for adaptors, including their participation in cytokine signaling in the testes and immune-related activities25,26 Based on this emerging evidence, adaptors are proteins with diversified physiological functions in maintaining spermatogenesis, in particular the events of junction restructuring.
Cytoskeletons.
Actin filaments, intermediate filaments, and microtubules are the three cytoskeletons found in mammalian cells including testes.8 Actin filaments are assembled by the polymerization of the actin monomers, whereas microtubules are largely composed of α- and β-tubulins, which form the heterodimers as the basic constituents.8,27 In spite of the identification of at least five categories of intermediate filament elements in the testes, vimentin is one of the most studied intermediate filament components in the testis.8,28 Recently, the physiological roles ascribed to cytoskeletons are rapidly expanding. In addition to their roles that provide the cellular structural support and cell motility,29 they are proven to participate in other signaling mechanisms, such as in the transcriptional regulation of genes in the nucleus.30 Remarkably, nuclear actin and myosin are among two of the vital elements.31 Other functional activities include the organization of chromosomes, and their allocation and segregation during mitosis.32 In the seminiferous epithelium, actin is intimately involved in the development of acrosomes.33 On the other hand, tubulin-based microtubules are known to serve as the track, which works in concert with motor proteins to direct the trans-epithelial movement of spermatids from basal to the apical compartment or vice versa.9 Cytoskeletons have specific locations within the seminiferous epithelium. Due to the multiplexing nature of the junctions in the testes, the underlying junctions are intimately lying in the proximity of each other or even exhibiting an overlapping position. This further implicates the possible interaction among different cytoskeletons. Studies in other epithelia have illustrated that cytoskeletons mediate cross-talk between junctions.34,35 Interestingly, there are accumulating evidence regarding the signal transduction properties of different cytoskeletal elements, such as the intermediate filament.36 Due to the coherent complex nature of junctions in the testes, much work is needed in this area to decipher the functional roles of different cytoskeletons and their interactions during spermatogenesis.
Studies of NOS/NO in the Testes
Many proteins and/or molecules are known modulators of the junction integrity and functionality in the testes25,37 including NO. NO is highly efficient in these processes due to its small molecular size and diffusible nature, making its sites of action distance away from its production sites. The major source of NO in the testes derives from macrophages found in the microvessels and in the inter-tubular compartment in the interstitium, making Leydig cells as one of the most immediate responders to NO (Fig. 1).38,39 This effect inevitably alters the metabolic activities in Leydig cells, modulating their testosterone output during steroidogenesis (see below). Indeed, testicular macrophages are prominent cellular regulators in Leydig cell physiology, due to their proximal location with Leydig cells and that they are also the cellular source of growth factors and/or cytokines.39 Apart from that, NO also influences the activity of Sertoli and germ cells within the seminiferous tubules. However, the effects exhibited are not as remarkable as those observed in Leydig cells due to the intrinsic physical distance between the interstitium and the seminiferous epithelium. However, NO released from the different forms of NOS found in the seminiferous epithelium is the major regulator of the spermatogenetic process. Several NOSs are found in different testicular cells. Three major forms of NOS and their defined locations in the testes are known to date (Table 1 and Fig. 1). Germ cells express eNOS, whereas Leydig cells produce iNOS abundantly.21 On the other hand, minor amounts of nNOS are produced by Sertoli cells. In spite of the presence of these classic NOSs, a testis-specific NOS variant has been identified and entitled as the truncated form of nNOS (TnNOS).17 Its expression is limited to Leydig cells, strongly implicating its role in steroidogenesis.40 This testicular forms of NOS seems to be an appropriate subject of an intensive investigation, however, its functions are not fully deciphered due to the lack of knockout mouse model.
Table 1.
Cellular localization of NOS signaling pathway components and their putative associated proteins in mammalian testes
| Cellular Localization* | |||||||||
| Proteins | SC | GC | AC | LC | MC | MF | EC | SP | Putative Associated Proteins† |
| CNG 1 | + | + | − | − | − | − | − | − | n.k. |
| eNOS | + | + | / | + | / | + | + | + | actin, β-catenin, eNOS, iNOS, N-cadherin, occludin, sGC, α-tubulin, vimentin |
| iNOS | + | + | / | + | + | / | − | + | actin, β-catenin, eNOS, iNOS, N-cadherin, occludin, sGC, α-tubulin, vimentin |
| nNOS | + | − | + | + | / | + | + | + | sGC, cGMP |
| PKG | + | + | + | − | − | + | + | − | β-catenin, sGC |
| sGC | + | + | + | + | + | + | + | − | actin, afadin, cadherin, catenin, connexins, eNOS, espin, iNOS, JAM, nectin, nNOS, occludin, PKC, PKG, ponsin, sGC, tubulin, vimentin, ZO-1 |
| TnNOS | − | − | − | + | − | − | − | − | n.k. |
Cellular localization was revealed by RT-PCR, IB, IHC and/or IF.
Putative associated proteins were assessed by co-IP, IHC, and/or IF. This table was prepared based on the following research articles and reviews.40–44,59,60,67–75 +, presence; −, absence; /, not positively identified; AC, acrosome; CNG 1, cyclic nucleotide-gated channel 1; co-IP, coimmunoprecipitation; EC, endothelial cells in blood vessels; eNOS, endothelial NOS; IB, immunoblot; IF, immunofluorescent microscopy; IHC, immunohistochemistry; GC, germ cells; iNOS, inducible NOS; JAM, junctional adhesion molecule; LC, Leydig cells; MC, myoid cells; MF, myofibroblasts; n.k., not known; nNOS, neuronal NOS; NOS, nitric oxide synthase; PKG, protein kinase G; SC, Sertoli cells; sGC, soluble guanylyl cyclase; RT-PCR, reverse transcription polymerase chain reaction; SP, spermatozoa; TnNOS, testis-specific nNOS; ZO-1, zonula occludens-1.
In order to fully appreciate the physiological effects of NOS/NO, recent studies have extended to their effectors, which are the executioners of the NOS/NO signaling pathway. The components of the sGC/cGMP/PKG pathway are expressed in a cyclic manner during the seminiferous epithelial cycle in the testes41–43 (Table 1 and Fig. 1). Spatially, iNOS is depicted to exist in coherent locations with their associated proteins, such as sGC and PKG, as demonstrated by immunohistochemistry and immunofluorescent microscopy.41,42,44 These observations emphasize the close association of components of NOS/NO pathways in the seminiferous epithelium. A panel of junction proteins has been shown to be putative NOS-binding proteins using the techniques of coimmunoprecipitation and immunoblottings. For instance, iNOS and eNOS were shown to associate with occludin-based TJ complexes in the testes.44 Besides, these two NOS isoforms also link to the N-cadherin-based junction network.42 Additionally, sGC, the downstream effector of the NOS pathway, is structurally associated with both AJ and TJ protein complexes in the testes.43 In this context, NOS/NO is a putative regulator of junction restructuring in the testes as illustrated in other functional studies. Zinc (II) protoporphyrin-IX, a broad spectrum NOS inhibitor and a sGC inhibitor, was shown to perturb the TJ integrity in Sertoli cell cultures.44 This event also accompanied by a down-regulation in the amount of intracellular cGMP and the occludin protein steady-state levels.44 iNOS and eNOS were also shown to dissociate from the N-cadherin/β-catenin protein complexes during the Adjudin-induced AJ restructuring in the rat testes that led to germ cell depletion from the epithelium.42 Apart from that, the administration of KT-5823, a PKG inhibitor, delayed the actions of Adjudin in mediating germ cell loss from the seminiferous epithelium.42 Significantly, a tighter association of sGC with cadherin-based protein complex was observed during Adjudin-induced junction restructuring in vivo, whilst a weakened interaction was found between sGC and TJ protein complexes43 (Table 2 and Fig. 2). Based on these findings, the significance of the NOS/NO/sGC/PKG pathway in AJ dynamics in the testes is increasingly clear. In short, the NOS/NO pathway is one of the versatile mechanisms utilized by the testes to regulate junction dynamics.
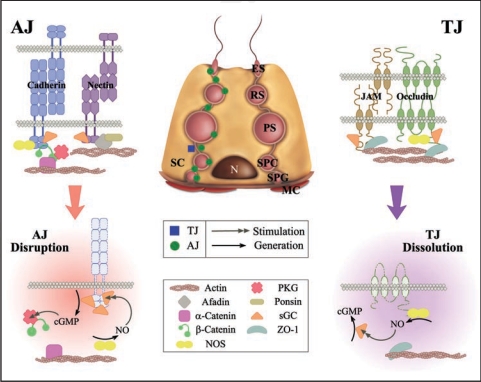
Figure 2.
The NOS/NO signaling pathways that regulate AJ and TJ dynamics in the testes. This is a simplified schematic diagram summarizing recent studies regarding the role of the NOS/NO signaling pathway that regulates junction dynamics in the testis using the Adjudin model.42–44 AJ, adherens junction; ES, elongated spermatid; JAM, junctional adhesion molecule; MC, myoid cells; N, Sertoli cell nucleus; NO, nitric oxide; NOS, nitric oxide synthase; PKG, protein kinase G; PS, pachytene spermatocyte; RS, round spermatid; SC, Sertoli cell; sGC, soluble guanylyl cyclase; SPC, preleptotene/leptotene spermatocyte; SPG, spermatogonium; TJ, tight junction; ZO-1, zonula occludens-1.
Studies of NOS/NO in Other Systems that can be the Basis of Future Studies in the Testes
As described above, NOS/NO is a novel pathway to regulate AJ and TJ dynamics in the testes. However, information regarding the ability of NOS/NO in regulating other junction types, such as gap junctions and other anchoring junctions (e.g., desmosome-like junctions), are lacking. Recent studies have shown that NO regulates connexin 35-associated gap junction coupling in neurons.45 However, it remains to be determined if there is any interaction between NO and connexins in the testes. Also, NO has a negative role in modulating integrin-linked kinase, a protein kinase related to the integrin-associated protein complex, in rat kidneys46 (Table 2). However, these results still need to be validated in the testes.
Table 2.
Participation of NOS/NO in the regulation of junctions in testes and other epithelia
| Organ or Tissue/Junction Type | NOS | Target Junction Proteins | Regulation | Selected References |
| Testis/TJ | eNOS, iNOS | n.k. | − | 44 |
| Testis/AJ | iNOS | N-Cadherin, β-Catenin | − | 42 |
| Hepatobiliary duct/GJ | iNOS | Occludin, ZO-1, ZO-2, ZO-3 | − | 76 |
| Brain/TJ | gNOS | Occludin | + | 77 |
| Vascular/GJ | gNOS | Connexin 37 | − | 78 |
| Muscular/GJ | gNOS | Connexin 43 | − | 79 |
| Neuronal/GJ | gNOS | n.k. | + | 80 |
This table only contains selected examples of NOS and the role of NO in junctions including the testes and other nongonadal tissues. Other important studies are not cited due to the page limit. +, positive regulation; −, negative regulation; nk, information not known; AJ, adherens junction; eNOS, endothelial NOS; iNOS, inducible NOS; GJ, gap junction; gNOS, studies involved the activation of general NOS activity in the systems; NO, nitric oxide; NOS, nitric oxide synthase; TJ, tight junction; ZO, zonula occludens.
NOS/NO and Spermatogenesis
NOS/NO and the hormone/cytokine/paracrine/autocrine system in the testis.
Hormones and cytokines are known to have versatile functions ranging from the modulation of endocrine systems to the fine-tuning of immune systems. Within the microenvironment in the testis, testosterone, the major male sex hormone, dominates the processes of spermatogenesis. The synthesis of testosterone by Leydig cells is under the control of luteinizing hormone (LH) released from the pituitary gland. For cytokines, they are mostly derived from the immune cells (e.g., macrophages) in the circulation and diffuse into the testicles via the blood vessels.39 Importantly, large part of the NO produced in the testes is derived from activated testicular macrophages, which are having high levels of iNOS.47 These exogenous and endogenous sources of hormones and cytokines are involved in constraining the expression of different forms of NOS in the testes, and as such, indirectly affecting the testicular NO steady-state level.21
On the other side, NO coordinates the testicular production of hormones and cytokines. Exposure of Leydig cell cultures to an NO donor, S-nitrosoglutathione can elicite an inhibition of testosterone production in vitro.47 Other studies have demonstrated that the activated macrophage-produced NO is associated with a reduction in the testosterone producing activity in Leydig cells.48 Notably, this inhibition is at least in part involved with the blockade of the P450 steroidogenic enzymes.48
In short, the bi-directional relationship manifested by NOS/NO and the hormone/cytokine level is crucial in maintaining the physiological function of the testes.
NOS/NO in germ cell development and differentiation
Germ cell apoptosis and germ cell output in the seminiferous epithelium. The number of total germ cells produced by the testes is tightly regulated in order to secure the production of viable and fertile germ cells within the supporting capacity of the Sertoli cells in the testes. In the rat, Sertoli cells begin to proliferate at day 16 post-coitus, and at birth, the number of Sertoli cells is about 1 million per testis; and by day 15 post-natal, the number of Sertoli cells rises to ∼40 million, however, proliferation ceases to occur thereafter.49 The Sertoli cell number remains relatively constant throughout adulthood.50 Thus, this fixed number of Sertoli cells cannot nurture unlimited number of germ cells generated from the primordial spermatogonia. To remove redundant and abnormal perhaps unhealthy germ cells, an elimination mechanism involving germ cell apoptosis is being utilized by the testes. More than half of the germ cells, perhaps ∼75%, particular spermatogonia and spermatocytes, undergo spontaneous apoptosis during normal spermatogenesis. Besides estrogens (e.g., 17β-estradiol) that regulate the germ cell number via apoptosis (see below) (for a review, see ref. 51), another mechanism via phagocytosis is also used by the testis. In brief, apoptotic germ cells destined to be phagocytosed by Sertoli cells are recognized via an externalized phosphatidylserine on the apoptotic germ cell surface, which, in turn, coheres the class B scavenger receptor type I (SR-BI) on Sertoli cell surface52 since Sertoli cells also serve as the scavenger in the epithelium. If the SR-BI on the Sertoli cell surface is inactivated by a monoclonal anti-SR-BI antibody, this causes an increase in residual apoptotic germ cells in the epithelium.52 Using these mechanisms, the sperm output by the testis can be finely maintained without overwhelming the Sertoli cells.
Notwithstanding, uncontrolled apoptosis would disintegrate the harmonized microenvironment and the Sertoli:germ cell ratios in the seminiferous epithelium during spermatogenesis. As such, these apoptotic events can also be triggered by external stimuli or artificially induced, such as testosterone deprivation and local testicular heating,53 which are detrimental to spermatogenesis. Based on these other models, several germ cell apoptotic pathways have also been identified. At the molecular levels, caspases, especially caspase 3,53 and Fas/Fas ligand (Fas L)54 are the main regulatory molecules. These pathways are probably inter-connected and mediated by junction proteins, such as connexins, during apoptosis in the testes.23 There are reports in the literature illustrating an excessive NO level can directly trigger massive germ cell apoptosis in the testes. These are clearly depicted in the artificial spermatic vessel ligation model55 and in the mouse model of congenital cryptorchidism,56 both of which were shown to elevate NO testicular levels and uncontrolled germ cell apoptosis. The role of NO in germ cell apoptosis has been further strengthened by the observation that treatment of Hoxa 11 knockout mice having congenital cryptorchidism using L-NAME, an NOS inhibitor, to block the NO-mediated effects can grossly attenuate germ cell apoptosis.56 Normal aging process also illustrates the association of NO and apoptosis.57 In aged testes, aggravated NOS activity and an induced iNOS level were shown to accompany with high incidence of germ cell apoptosis.57 iNOS and eNOS are also postulated to participate in germ cell apoptosis in the testes.21 Specifically, eNOS has been known to be highly expressed in degenerating germ cells in comparison with other germ cell types.58,59 In addition, iNOS plays a determining role in restricting the germ cell numbers in the testes, since testes from iNOS-/- mice are heavier on average when compared to normal testes,60 and a marked reduction on germ cell apoptosis, in particular pachytene spermatocytes and round spermatids, is noted.60 These observations thus clearly illustrate the unequivocal role of NO in triggering germ cell apoptosis in the testes. Nonetheless, the downstream NO pathway(s) that regulates germ cell apoptosis is presently not known.
NOS/NO in fertility and pathogenesis.
Male fertility has always been a primary concern in human reproduction. At the molecular level, fertility partly refers to the successful fusion of male and female gametes, which depends on several factors for its completion. The quality and quantity of sperm and sperm concentrations are several of the key issues directly affecting fertilization. As mentioned earlier, apoptosis is one of the key processes that maintains the quality and quantity of germ cells in the testes.61 Thus, if apoptosis fails to control these variables, a lack of viable and fertile germ cells will be the consequence. If testes fail to produce sufficient number of healthy spermatozoa without defects, infertility is unavoidable. Several etiologies are known to goad infertility, such as varicocele, tumors, and azoospermia. Also, DNA integrity in sperm is another major determinant.62 It is known that an unbalanced levels of NO or dysregulation of NOS in the testes contribute to some of these defects (Table 3). For instance, a reduced testicular eNOS protein level was observed in patients with idiopathic azoospermia versus patients with obstructive azoospermia or varicocele and healthy individual.63 Besides, excess NO in human varicocele conditions was demonstrated to be harmful to the sperm motility.64 Despite all this, a physiological level of NO is required for successful fertilization.65 Apart from that, NO is also known to participate in sperm transport from the rete testis to the epididymis. The propelling force that transports spermatozoa to the epididymis via the rete testis is generated by the contraction and relaxation of the tunica albuginea in the testis, which, in turn, is regulated by PKG, a downstream effector of NOS/NO.66 In short, NOS/NO has diversified roles in testis pathogenesis.
Concluding Remarks and Future Perspectives
NO is a versatile molecule with diversified functions ranging from coordinating cell and blood vessel permeability to junction regulation. It is produced by four isoforms of NOS, namely eNOS, iNOS, nNOS, and TnNOS. NOS/NO is depicted to regulate disparate junction dynamics, at AJ and TJ, in the testes. However, the significance of NOS/NO in the regulation of other junction types, such as gap junctions and desmosomes, are not fully deciphered and much effort is needed to uncover the associated mechanisms and identify the proteins that are involved. Apart from that, NOS/NO is illustrated to have roles in monitoring the levels of hormones and cytokines, indirectly controlling the processes of spermatogenesis and steroidogenesis. Importantly, NOS/NO also takes part in the regulation of germ cell development and differentiation, partially via the coordination of germ cell apoptosis and maintaining the correct ratio of Sertoli:germ cells in the epithelium, securing the efficacy in the production of viable and fertile spermatozoa. As such, NOS/NO is crucial to maintain male fertility and pathogenesis. An expanded research in this area can form the foundation of identifying candidate molecules for male contraception and to understand male reproductive pathogenesis.
Acknowledgements
This work was supported in part by grants from the National Institutes of Health (NICHD U01 HD045908, and U54 HD29990 Project 3) and the CONRAD Program (CICCR CIG 01-72).
Footnotes
Previously published online as an Oxidative Medicine and Cellular Longevity E-publication: www.landesbioscience.com/journals/oximed/article/6856
Reprinted from: Molecular Mechanisms in Spermatogenesis, edited by C.Y. Cheng © 2007 Landes Bioscience.
References
- 1.Johnson AD, Gomes WR, Vandemark NL. The Testis Volume 1: Development, Anatomy, and Physiology. New York: Academic Press; 1970. [Google Scholar]
- 2.Russell L, Ettlin RA, Sinha Hikim AP, et al. Histological and Histopathological Evaluation of the Testis. Clearwater: Cache River. 1990:1–52. [Google Scholar]
- 3.Mruk DD, Cheng CY. Cell-cell interactions at the ectoplasmic specialization in the testis. Trends Endocrinol Metab. 2004;15:439–447. doi: 10.1016/j.tem.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Wong CH, Cheng CY. The blood-testis barrier: Its biology, regulation, and physiological role in spermatogenesis. Curr Top Dev Biol. 2005;71:263–296. doi: 10.1016/S0070-2153(05)71008-5. [DOI] [PubMed] [Google Scholar]
- 5.Parreira GG, Melo RCN, Russell L. Relationship of Sertoli-Sertoli tight junctions to ectoplasmic specialization in conventional and en face views. Biol Reprod. 2002;67:1232–1241. doi: 10.1095/biolreprod67.4.1232. [DOI] [PubMed] [Google Scholar]
- 6.Russell LD. Sertoli-germ cell interrelations: A review. Gamete Res. 1980;3:179–202. [Google Scholar]
- 7.Russell L. Movement of spermatocytes from the basal to the adluminal compartment of the rat testis. Amer J Anat. 1977;148:313–328. doi: 10.1002/aja.1001480303. [DOI] [PubMed] [Google Scholar]
- 8.Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev. 2004;25:747–806. doi: 10.1210/er.2003-0022. [DOI] [PubMed] [Google Scholar]
- 9.Lee NPY, Cheng CY. Ectoplasmic specialization, a testis-specific cell-cell actin-based adherens junction type: Is this a potential target for male contraceptive development? Human Reprod Update. 2004;10:349–369. doi: 10.1093/humupd/dmh026. [DOI] [PubMed] [Google Scholar]
- 10.Stuehr DJ. Structure-function aspects in the nitric oxide synthases. Annu Rev Pharmacol Toxicol. 1997;37:339–359. doi: 10.1146/annurev.pharmtox.37.1.339. [DOI] [PubMed] [Google Scholar]
- 11.Bogdan C. Nitric oxide and the regulation of gene expression. Trends Cell Biol. 2001;11:66–75. doi: 10.1016/s0962-8924(00)01900-0. [DOI] [PubMed] [Google Scholar]
- 12.Forstermann U, Gath I, Schwarz P, et al. Isoforms of nitric oxide synthase: Properties, cellular distribution and expressional control. Biochem Pharmacol. 1995;50:1321–1332. doi: 10.1016/0006-2952(95)00181-6. [DOI] [PubMed] [Google Scholar]
- 13.Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: Structure, function and inhibition. Biochem J. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis KL, Martin E, Turko IV, et al. Novel effects of nitric oxide. Annu Rev Pharmacol Toxicol. 2001;41:203–336. doi: 10.1146/annurev.pharmtox.41.1.203. [DOI] [PubMed] [Google Scholar]
- 15.Hanafy KA, Krumenacker JS, Murad F. NO, nitrotyrosine, and cyclic GMP in signal transduction. Medical Science Monitor. 2001;7:801–819. [PubMed] [Google Scholar]
- 16.Hofmann F, Ammendola A, Schlossmann J. Rising behind NO: cGMP-dependent protein kinases. J Cell Sc. 2000;113:1671–1676. doi: 10.1242/jcs.113.10.1671. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Goligorsky MS, Lin M, et al. A novel, testis-specific mRNA transcript encoding an NH2-terminal truncated nitric oxide synthase. J Biol Chem. 1997;272:11392–11401. [PubMed] [Google Scholar]
- 18.Schneeberger EE, Lynch RD. The tight junction: A multifunctional complex. Am J Physiol Cell Physiol. 2004;286:C1213–C1228. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- 19.Lee NPY, Cheng CY. Mini-review: Adaptors, junction dynamics, and spermatogenesis. Biol Reprod. 2004;71:392–404. doi: 10.1095/biolreprod.104.027268. [DOI] [PubMed] [Google Scholar]
- 20.Lee NPY, Yeung WSB, Luk JM. Junction interaction in the seminiferous epithelium: Regulatory roles of connexin-based gap junction. Front Biosci. 2007;12:1552–1562. doi: 10.2741/2168. [DOI] [PubMed] [Google Scholar]
- 21.Lee NPY, Cheng CY. Nitric oxide/nitric oxide synthase, spermatogenesis, and tight junction dynamics. Biol Reprod. 2004;70:267–276. doi: 10.1095/biolreprod.103.021329. [DOI] [PubMed] [Google Scholar]
- 22.Pointis G, Segretain D. Role of connexin-based gap junction channels in testis. Trends Endocrinol Metab. 2005;16:300–306. doi: 10.1016/j.tem.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Lee NPY, Leung KW, Wo JY, et al. Blockage of testicular connexins induced apoptosis in rat seminiferous epithelium. Apoptosis. 2006;11:1215–1229. doi: 10.1007/s10495-006-6981-2. [DOI] [PubMed] [Google Scholar]
- 24.Yan HH, Cheng CY. Blood-testis barrier dynamics are regulated by an engagement/disengagement mechanism between tight and adherens junctions via peripheral adaptors. Proc Natl Acad Sci USA. 2005;102:11722–11727. doi: 10.1073/pnas.0503855102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xia W, Mruk DD, Lee WM, et al. Cytokines and junction restructuring during spermatogenesis-a lession to learn from the testis. Cytokine Growth Factor Rev. 2005;16:469–493. doi: 10.1016/j.cytogfr.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Togni M, Lindquist J, Gerber A, et al. The role of adaptor proteins in lymphocyte activation. Mol Immunol. 2004;41:615–630. doi: 10.1016/j.molimm.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 27.Nogales E. Structural insights into microtubule function. Annu Rev Biophys Biomol Struct. 2001;30:397–420. doi: 10.1146/annurev.biophys.30.1.397. [DOI] [PubMed] [Google Scholar]
- 28.Helfand BT, Chang L, Goldman RD. The dynamic and motile properties of intermediate filaments. Annu Rev Cell Dev Biol. 2003;19:445–467. doi: 10.1146/annurev.cellbio.19.111401.092306. [DOI] [PubMed] [Google Scholar]
- 29.Small JV, Resch GP. The comings and goings of actin: Coupling protrusion and retraction in cell motility. Curr Opin Cell Biol. 2005;17:517–523. doi: 10.1016/j.ceb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 30.Pederson T, Aebi U. Nuclear actin extends, with no contraction in sight. Mol Biol Cell. 2005;16:5055–5060. doi: 10.1091/mbc.E05-07-0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Lanerolle P, Johnson T, Hofmann WA. Actin and myosin I in the nucleus: What next? Nat Struct Mol Biol. 2005;12:742–746. doi: 10.1038/nsmb983. [DOI] [PubMed] [Google Scholar]
- 32.McIntosh JR, Grishchuk EL, West RR. Chromosome-microtubule interactions during mitosis. Annu Rev Cell Dev Biol. 2002;18:193–219. doi: 10.1146/annurev.cellbio.18.032002.132412. [DOI] [PubMed] [Google Scholar]
- 33.Breitbart H, Cohen G, Rubinstein S. Role of actin cytoskeleton in mammalian sperm capacitation and the acrosome reaction. Reproduction. 2005;129:263–268. doi: 10.1530/rep.1.00269. [DOI] [PubMed] [Google Scholar]
- 34.Chou YH, Helfand BT, Goldman RD. New horizons in cytoskeletal dynamics: Transport of intermediate filaments along microtubule tracks. Curr Opin Cell Biol. 2001;13:106–109. doi: 10.1016/s0955-0674(00)00181-2. [DOI] [PubMed] [Google Scholar]
- 35.Chang L, Goldman RD. Intermediate filaments mediate cytoskeletal crosstalk. Nat Rev Mol Cell Biol. 2004;5:601–613. doi: 10.1038/nrm1438. [DOI] [PubMed] [Google Scholar]
- 36.Helfand BT, Chou YH, Shumaker DK, et al. Intermediate filament proteins participate in signal transduction. Trends Cell Biol. 2005;15:568–570. doi: 10.1016/j.tcb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 37.Wong CH, Cheng CY. Mitogen-activated protein kinases, adherens junction dynamics, and spermatogenesis: A review of recent data. Dev Biol. 2005;286:1–15. doi: 10.1016/j.ydbio.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 38.Hutson JC. Physiologic interactions between macrophages and Leydig cells. Exp Biol Med. 2006;231:1–7. doi: 10.1177/153537020623100101. [DOI] [PubMed] [Google Scholar]
- 39.Hales DB. Testicular macrophage modulation of Leydig cell steroidogenesis. J Reprod Immunol. 2002;57:3–18. doi: 10.1016/s0165-0378(02)00020-7. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Newton DC, Miller TL, et al. An alternative promoter of the human neuronal nitric oxide synthase gene is expressed specifically in Leydig cells. Am J Pathol. 2002;160:369–380. doi: 10.1016/S0002-9440(10)64380-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi F, Wang T. Stage- and cell-specific expression of soluble guanylyl cyclase alpha and beta subunits, cGMP-dependent protein kinase I alpha and beta, and cyclic nucleotide-gated channel subunit 1 in the rat testis. J Androl. 2005;26:258–263. doi: 10.1002/j.1939-4640.2005.tb01093.x. [DOI] [PubMed] [Google Scholar]
- 42.Lee NPY, Mruk DD, Wong CH, et al. Regulation of Sertoli-germ cell adherens junction dynamics in the testis via the nitric oxide synthase (NOS)/cGMP/protein kinase G (PRKG)/β-catenin (CATNB) signaling pathway: An in vitro and in vivo study. Biol Reprod. 2005;73:458–471. doi: 10.1095/biolreprod.105.040766. [DOI] [PubMed] [Google Scholar]
- 43.Sarkar O, Xia W, Mruk DD. Adjudin-mediated junction restructuring in the seminiferous epithelium leads to displacement of soluble guanylate cyclase from adherens junctions. J Cell Physiol. 2006;208:175–187. doi: 10.1002/jcp.20651. [DOI] [PubMed] [Google Scholar]
- 44.Lee NPY, Cheng CY. Regulation of Sertoli cell tight junction in the rat testis via the nitric oxide synthase/soluble guanylate cyclase/cGMP/protein kinase G signaling pathway: An in vitro study. Endocrinology. 2003;144:3114–3129. doi: 10.1210/en.2002-0167. [DOI] [PubMed] [Google Scholar]
- 45.Patel LS, Mitchell CK, Dubinsky WP, et al. Regulation of gap junction coupling through the neuronal connexin Cx35 by nitric oxide and cGMP. Cell Commun Adhes. 2006;13:41–54. doi: 10.1080/15419060600631474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beck KF, Walpen S, Eberhardt W, Pfeilschifter J. Downregulation of integrin-linked kinase mRNA expression by nitric oxide in rat glomerular mesangial cells. Life Sci. 2001;69:2945–2955. doi: 10.1016/s0024-3205(01)01403-5. [DOI] [PubMed] [Google Scholar]
- 47.Weissman BA, Niu E, Ge R, et al. Paracrine modulation of androgen synthesis in rat Leydig cells by nitric oxide. J Androl. 2005;26:369–378. doi: 10.2164/jandrol.04178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pomerantz DK, Pitelka V. Nitric oxide is a mediator of the inhibitory effect of activated macrophages on production of androgen by the Leydig cell of the mouse. Endocrinology. 1998;139:922–931. doi: 10.1210/endo.139.3.5773. [DOI] [PubMed] [Google Scholar]
- 49.Orth JM. Proliferation of Sertoli cells in fetal and postnatal rats: A quantitative autoradiographic study. Anat Rec. 1982;203:485–492. doi: 10.1002/ar.1092030408. [DOI] [PubMed] [Google Scholar]
- 50.Wang ZX, Wreford NG, de Kretser DM. Determination of Sertoli cell numbers in the developing rat testis by stereological methods. Int J Androl. 1989;12:58–64. doi: 10.1111/j.1365-2605.1989.tb01285.x. [DOI] [PubMed] [Google Scholar]
- 51.Shaha C. Estrogen and spermatogenesis. In: Cheng CY, editor. Molecular Mechanisms in Spermatogenesis. Austin: Landes Bioscience; 2007. in press. [Google Scholar]
- 52.Nakanishi Y, Shiratsuchi A. Phagocytic removal of apoptotic spermatogenic cells by Sertoli cells: Mechanisms and consequences. Biol Pharm Bull. 2004;27:13–16. doi: 10.1248/bpb.27.13. [DOI] [PubMed] [Google Scholar]
- 53.Sinha Hikim AP, Lue Y, Diaz-Romero M, et al. Deciphering the pathways of germ cell apoptosis in the testis. J Steroid Biochem Mol Biol. 2003;85:175–182. doi: 10.1016/s0960-0760(03)00193-6. [DOI] [PubMed] [Google Scholar]
- 54.Koji T. Male germ cell death in mouse testes: Possible involvement of Fas and Fas ligand. Med Electron Microsc. 2001;34:213–222. doi: 10.1007/s007950100018. [DOI] [PubMed] [Google Scholar]
- 55.Taneli F, Vatansever S, Ulman C, et al. The effect of spermatic vessel ligation on testicular nitric oxide levels and germ cell-specific apoptosis in rat testis. Acta Histochem. 2005;106:459–466. doi: 10.1016/j.acthis.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 56.DeFoor WR, Kuan CY, Pinkerton M, et al. Modulation of germ cell apoptosis with a nitric oxide synthase inhibitor in a murine model of congenital cryptorchidism. J Urol. 2004;172:1731–1735. doi: 10.1097/01.ju.0000138846.56399.de. [DOI] [PubMed] [Google Scholar]
- 57.Sinha Hikim AP, Vera Y, Vernet D, et al. Involvement of nitric oxide-mediated intrinsic pathway signaling in age-related increase in germ cell apoptosis in male Brown-Norway rats. J Gerontol A Biol Sci Med Sci. 2005;60:702–708. doi: 10.1093/gerona/60.6.702. [DOI] [PubMed] [Google Scholar]
- 58.Zini A, Abitbol J, Girardi SK, et al. Germ cell apoptosis and endothelial nitric oxide synthase (eNOS) expression following ischemia-reperfusion injury to testis. Arch Androl. 1998;41:57–65. doi: 10.3109/01485019808988547. [DOI] [PubMed] [Google Scholar]
- 59.Zini A, O'Bryan MK, Magid MS, et al. Immunohistochemical localization of endothelial nitric oxide synthase in human testis, epididymis, and vas deferens suggests a possible role for nitric oxide in spermatogenesis, sperm maturation, and programmed cell death. Biol Reprod. 1996;55:935–941. doi: 10.1095/biolreprod55.5.935. [DOI] [PubMed] [Google Scholar]
- 60.Lue Y, Hikim APS, Wang C, et al. Functional role of inducible nitric oxide synthase in the induction of male germ cell apoptosis, regulation of sperm number, and determination of testes size: Evidence from null mutant mice. Endocrinology. 2003;144:3092–3100. doi: 10.1210/en.2002-0142. [DOI] [PubMed] [Google Scholar]
- 61.Sakkas D, Seli E, Manicardi GC, et al. The presence of abnormal spermatozoa in the ejaculate: Did apoptosis fail? Hum Fertil. 2004;7:99–103. doi: 10.1080/14647270410001720464. [DOI] [PubMed] [Google Scholar]
- 62.O'Brien J, Zini A. Sperm DNA integrity and male infertility. Urology. 2005;65:16–22. doi: 10.1016/j.urology.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 63.Fujisawa M, Yamanaka K, Tanaka H, et al. Expression of endothelial nitric oxide synthase in the Sertoli cells of men with infertility of various causes. BJU Int. 2001;87:85–88. doi: 10.1046/j.1464-410x.2001.00986.x. [DOI] [PubMed] [Google Scholar]
- 64.Kisa U, Basar MM, Ferhat M, et al. Testicular tissue nitric oxide and thiobarbituric acid reactive substance levels: Evaluation with respect to the pathogenesis of varicocele. Urol Res. 2004;32:196–199. doi: 10.1007/s00240-004-0401-2. [DOI] [PubMed] [Google Scholar]
- 65.Kim BH, Kim CH, Jung KY, et al. Involvement of nitric oxide during in vitro fertilization and early embryonic development in mice. Arch Pharm Res. 2004;27:86–93. doi: 10.1007/BF02980052. [DOI] [PubMed] [Google Scholar]
- 66.Middendorff R, Muller D, Mewe M, et al. The tunica albuginea of the human testis is characterized by complex contraction and relaxation activities regulated by cyclic GMP. J Clin Endocrinol Metab. 2002;87:3486–3499. doi: 10.1210/jcem.87.7.8696. [DOI] [PubMed] [Google Scholar]
- 67.Meroni SB, Suburo AM, Cigorraga SB. Interleukin-1β regulates nitric oxide production and γ-glutamyl transpeptidase activity in Sertoli cells. J Androl. 2000;21:855–861. [PubMed] [Google Scholar]
- 68.Burnett AL, Ricker DD, Chamness SL, et al. Localization of nitric oxide synthase in the reproductive organs of the male rat. Biol Reprod. 1995;52:1–7. doi: 10.1095/biolreprod52.1.1. [DOI] [PubMed] [Google Scholar]
- 69.Herrero MB, Perez Martinez S, Viggiano JM, et al. Localization by indirect immunofluorescence of nitric oxide synthase in mouse and human spermatozoa. Reprod Fertil Dev. 1996;8:931–934. doi: 10.1071/rd9960931. [DOI] [PubMed] [Google Scholar]
- 70.Middendorff R, Muller D, Wichers S, et al. Evidence for production and functional activity of nitric oxide in seminiferous tubules and blood vessels of the human testis. J Clin Endocrinol Metabol. 1997;82:4154–4161. doi: 10.1210/jcem.82.12.4432. [DOI] [PubMed] [Google Scholar]
- 71.Davidoff MS, Middendorff R, Mayer B, et al. Nitric oxide synthase (NOS-I) in Leydig cells of the human testis. Arch Histol Cytol. 1995;58:17–30. doi: 10.1679/aohc.58.17. [DOI] [PubMed] [Google Scholar]
- 72.Herrero MB, Goin JC, Boquet M, et al. The nitric oxide synthase of mouse spermatozoa. FEBS Letts. 1997;411:39–42. doi: 10.1016/s0014-5793(97)00570-x. [DOI] [PubMed] [Google Scholar]
- 73.O'Bryan MK, Schlatt S, Gerdprasert O, et al. Inducible nitric oxide synthase in the rat testis: Evidence for potential roles in both normal function and inflammation-mediated infertility. Biol Reprod. 2000;63:1285–1293. doi: 10.1095/biolreprod63.5.1285. [DOI] [PubMed] [Google Scholar]
- 74.O'Bryan MK, Zini A, Cheng CY, et al. Human sperm endothelial nitric oxide synthase expression: Correlation with sperm motility. Fertil Steril. 1998;70:1143–1147. doi: 10.1016/s0015-0282(98)00382-3. [DOI] [PubMed] [Google Scholar]
- 75.Davidoff MS, Middendorff R, Mayer B, et al. Nitric oxide/cGMP pathway components in the Leydig cells of the human testis. Cell Tissue Res. 1997;287:161–170. doi: 10.1007/s004410050742. [DOI] [PubMed] [Google Scholar]
- 76.Han X, Fink MP, Uchiyama T, et al. Increased iNOS activity is essential for hepatic epithelial tight junction dysfunction in endotoxemic mice. Am J Physiol Gastrointest Liver Physiol. 2004;286:G126–G136. doi: 10.1152/ajpgi.00231.2003. [DOI] [PubMed] [Google Scholar]
- 77.Yamagata K, Tagami M, Takenaga F, et al. Hypoxia-induced changes in tight junction permeability of brain capillary endothelial cells are associated with IL-1beta and nitric oxide. Neurobiol Dis. 2004;17:491–499. doi: 10.1016/j.nbd.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 78.Kameritsch P, Khandoga N, Nagel W, et al. Nitric oxide specifically reduces the permeability of Cx37-containing gap junctions to small molecules. J Cell Physiol. 2005;203:233–242. doi: 10.1002/jcp.20218. [DOI] [PubMed] [Google Scholar]
- 79.Roh CR, Heo JH, Yang SH, et al. Regulation of connexin 43 by nitric oxide in primary uterine myocytes from term pregnant women. Am J Obstet Gynecol. 2002;187:434–440. doi: 10.1067/mob.2002.123600. [DOI] [PubMed] [Google Scholar]
- 80.O'Donnell P, Grace AA. Cortical afferents modulate striatal gap junction permeability via nitric oxide. Neuroscience. 1997;76:1–5. doi: 10.1016/s0306-4522(96)00433-2. [DOI] [PubMed] [Google Scholar]
- 81.Sezer C, Koksal IT, Usta MF, et al. Relationship between mast cell and iNOS expression in testicular tissue associated with infertility. Arch Androl. 2005;51:149–158. doi: 10.1080/014850190518161. [DOI] [PubMed] [Google Scholar]